User login
Confluent annular lesion on the dorsum of the hand
A 48-year-old man came to the office with a pruritic lesion that had been on the dorsum of his hand for 2 months. He said that the lesion began as 2 flesh-colored papules that had coalesced to form a larger lesion.
He denied any recent trauma, foreign travel, insect bites, disseminated rashes, or systemic symptoms associated with the appearance of the lesion. His medical history was unremarkable, and he indicated that he’d otherwise been feeling well.
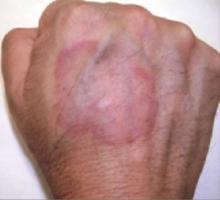
His physical exam was significant for a 6 cm, violaceous annular confluent plaque with a firm, slightly raised border (FIGURE 1). There was no visible scale.
FIGURE 1
Violaceous lesion on the hand
What is your diagnosis?
How would you manage this condition?
Diagnosis: Granuloma annulare
Granuloma annulare is a benign inflammatory skin condition classically described as annular papules and plaques. The lesions typically range from skin-colored to violaceous in appearance. There is no racial predilection for this condition.
There are 4 variants of granuloma annulare:
- The localized form—which our patient had—accounts for approximately 75% of cases.1 Lesions are typically found on the lateral or dorsal surfaces of the hands and feet. Most cases of localized granuloma annulare occur in young adult women.2
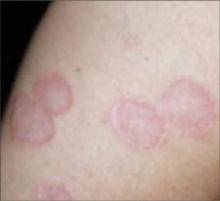
- The generalized (disseminated) form involves a number of lesions, and thus, is more widespread (FIGURE 2). Lesions tend to appear on the extremities and the trunk.
- The perforating form is rare, and occurs in both localized and generalized granuloma annulare. Papules may develop into lesions that exude a thick and creamy or clear and viscous fluid.
- The subcutaneous form presents as asymptomatic solitary lesions or in clusters that are most commonly found on the lower extremities—often on pretibial areas. The lesions with this form of granuloma annulare are deeper than the localized form, so there is more swelling, and less surface definition.
Anecdotal reports link condition to diabetes
Although the cause of granuloma annulare is unknown, it has been linked with trauma, thyroid disease, viral infection, malignancy, and diabetes mellitus. Some reports suggest that the lesions are the result of a delayed type hypersensitivity reaction. Of note: Our patient had type 2 diabetes mellitus, something we only discovered while doing a work-up for his lesion. Though largely based on anecdote and case reports, retrospective studies have supported this association.3,4
More recently however, a small case-control study failed to reveal any significant correlation between the 2.5 At this time, there is no indication to screen for diabetes in an otherwise asymptomatic patient.
FIGURE 2
Generalized granuloma annulare
Differential Dx includes ringworm, Lyme disease
Localized granuloma annulare must be differentiated from the most common annular lesion, tinea corporis, as well as other annular lesions, including necrobiosis lipoidica and erythema migrans.
- Tinea corporis, or ringworm, is a superficial fungal infection of the skin. Similar to granuloma annulare, patients present with a gradually enlarging annular, well-demarcated papular lesion. Ringworm can be distinguished from granuloma annulare by noting the presence of scale, and by performing a potassium hydroxide slide preparation, which would reveal septate hyphae.
- Necrobiosis lipoidica classically presents as annular violaceous plaques on the anterior legs, but may appear on the arms, hands, feet, or scalp. As the lesion expands, the advancing border becomes red and the central area typically develops a waxy yellow surface, with prominent telangiectasias. This lesion is generally flat or atrophic, and does not have the same raised border as is seen in granuloma annulare.
- Erythema migrans is the characteristic rash of early localized Lyme disease. This classic “bull’s-eye” rash is an annular lesion with concentric redness and expanding central clearing. Erythema migrans typically appears 7 to 10 days after a patient has been bitten by an infected tick. The classic red lesion starts as a small papule at the site of inoculation, while the expanding ring remains flat and lacks the papular appearance of granuloma annulare.
While uncommon, cutaneous sarcoidosis should also be considered in the differential diagnosis. Cutaneous manifestations occur in up to 20% of patients with sarcoidosis.2 The most common presentation is a maculopapular eruption involving the face. Lesions may also be nodular or plaque-like. Biopsy is necessary for diagnosis.
Clinical presentation typically clinches it
Diagnosis of granuloma annulare is often made by its characteristic clinical presentation. If the diagnosis is unclear, a skin biopsy may be needed. Histologic exam will reveal histiocytes in surrounding dermal tissue with increased mucin deposition.
Cosmetic concerns?
Localized lesions that are asymptomatic are often left to resolve spontaneously. If there are cosmetic concerns, or if there is significant pruritus, treatment options include intralesional steroid injection into the raised border with triamcinolone, occlusion therapy with clobetasol propionate, or liquid nitrogen therapy.
One small study of 31 patients with localized granuloma annulare showed resolution after 1 treatment with liquid nitrogen in 81% of patients.6 Topical steroids alone do not produce significant results.5 Other agents, including UV light and systemic medications, are available for the generalized form, however, none are curative and relapses are common.
No treatment for lesion; Metformin for diabetes
Our patient chose to have no treatment for his granuloma annulare, but we did put him on metformin for his diabetes. At a 3-month follow-up visit, our patient’s lesion was unchanged in appearance.
Although the disease course is variable, 50% of patients with localized granuloma annulare will see spontaneous resolution within 2 years without scarring.7
1. Nopper A, Markus R, Esterly N. When it’s not ring-worm: annular lesions of childhood. Pediatr Ann. 1998;27:136-148.
2. Habif TP. Clinical Dermatology. 4th ed. St louis, mo: mosby, Inc; 2004.
3. Studer EM, Calza AM, Saurat JH. Precipitating factors and associated diseases in 84 patients with granuloma annulare: a retrospective study. Dermatology. 1996;193:364-368.
4. Muhlemann MF. Localized granuloma annulare is associated with insulin-dependent diabetes mellitus. Br J Dermatol. 1984;111:325-329.
5. Nebesio CL, Lewis C, Chuang TY. Lack of an association between granuloma annulare and type 2 diabetes mellitus. Br J Dermatol. 2002;146:122-124.
6. Blume-peytavi U, Zouboulis CH, Jacobi H, Scholz A, Bisson S, Orfanos CE. Successful outcome of cryosurgery in patients with granuloma annulare. Br J Dermatol. 1994;130:494-497.
7. Smith MD, Downie JB, DiCostanzo D. Granuloma annulare. Int J Dermatol. 1997;36:326-333.
A 48-year-old man came to the office with a pruritic lesion that had been on the dorsum of his hand for 2 months. He said that the lesion began as 2 flesh-colored papules that had coalesced to form a larger lesion.
He denied any recent trauma, foreign travel, insect bites, disseminated rashes, or systemic symptoms associated with the appearance of the lesion. His medical history was unremarkable, and he indicated that he’d otherwise been feeling well.
His physical exam was significant for a 6 cm, violaceous annular confluent plaque with a firm, slightly raised border (FIGURE 1). There was no visible scale.
FIGURE 1
Violaceous lesion on the hand
What is your diagnosis?
How would you manage this condition?
Diagnosis: Granuloma annulare
Granuloma annulare is a benign inflammatory skin condition classically described as annular papules and plaques. The lesions typically range from skin-colored to violaceous in appearance. There is no racial predilection for this condition.
There are 4 variants of granuloma annulare:
- The localized form—which our patient had—accounts for approximately 75% of cases.1 Lesions are typically found on the lateral or dorsal surfaces of the hands and feet. Most cases of localized granuloma annulare occur in young adult women.2
- The generalized (disseminated) form involves a number of lesions, and thus, is more widespread (FIGURE 2). Lesions tend to appear on the extremities and the trunk.
- The perforating form is rare, and occurs in both localized and generalized granuloma annulare. Papules may develop into lesions that exude a thick and creamy or clear and viscous fluid.
- The subcutaneous form presents as asymptomatic solitary lesions or in clusters that are most commonly found on the lower extremities—often on pretibial areas. The lesions with this form of granuloma annulare are deeper than the localized form, so there is more swelling, and less surface definition.
Anecdotal reports link condition to diabetes
Although the cause of granuloma annulare is unknown, it has been linked with trauma, thyroid disease, viral infection, malignancy, and diabetes mellitus. Some reports suggest that the lesions are the result of a delayed type hypersensitivity reaction. Of note: Our patient had type 2 diabetes mellitus, something we only discovered while doing a work-up for his lesion. Though largely based on anecdote and case reports, retrospective studies have supported this association.3,4
More recently however, a small case-control study failed to reveal any significant correlation between the 2.5 At this time, there is no indication to screen for diabetes in an otherwise asymptomatic patient.
FIGURE 2
Generalized granuloma annulare
Differential Dx includes ringworm, Lyme disease
Localized granuloma annulare must be differentiated from the most common annular lesion, tinea corporis, as well as other annular lesions, including necrobiosis lipoidica and erythema migrans.
- Tinea corporis, or ringworm, is a superficial fungal infection of the skin. Similar to granuloma annulare, patients present with a gradually enlarging annular, well-demarcated papular lesion. Ringworm can be distinguished from granuloma annulare by noting the presence of scale, and by performing a potassium hydroxide slide preparation, which would reveal septate hyphae.
- Necrobiosis lipoidica classically presents as annular violaceous plaques on the anterior legs, but may appear on the arms, hands, feet, or scalp. As the lesion expands, the advancing border becomes red and the central area typically develops a waxy yellow surface, with prominent telangiectasias. This lesion is generally flat or atrophic, and does not have the same raised border as is seen in granuloma annulare.
- Erythema migrans is the characteristic rash of early localized Lyme disease. This classic “bull’s-eye” rash is an annular lesion with concentric redness and expanding central clearing. Erythema migrans typically appears 7 to 10 days after a patient has been bitten by an infected tick. The classic red lesion starts as a small papule at the site of inoculation, while the expanding ring remains flat and lacks the papular appearance of granuloma annulare.
While uncommon, cutaneous sarcoidosis should also be considered in the differential diagnosis. Cutaneous manifestations occur in up to 20% of patients with sarcoidosis.2 The most common presentation is a maculopapular eruption involving the face. Lesions may also be nodular or plaque-like. Biopsy is necessary for diagnosis.
Clinical presentation typically clinches it
Diagnosis of granuloma annulare is often made by its characteristic clinical presentation. If the diagnosis is unclear, a skin biopsy may be needed. Histologic exam will reveal histiocytes in surrounding dermal tissue with increased mucin deposition.
Cosmetic concerns?
Localized lesions that are asymptomatic are often left to resolve spontaneously. If there are cosmetic concerns, or if there is significant pruritus, treatment options include intralesional steroid injection into the raised border with triamcinolone, occlusion therapy with clobetasol propionate, or liquid nitrogen therapy.
One small study of 31 patients with localized granuloma annulare showed resolution after 1 treatment with liquid nitrogen in 81% of patients.6 Topical steroids alone do not produce significant results.5 Other agents, including UV light and systemic medications, are available for the generalized form, however, none are curative and relapses are common.
No treatment for lesion; Metformin for diabetes
Our patient chose to have no treatment for his granuloma annulare, but we did put him on metformin for his diabetes. At a 3-month follow-up visit, our patient’s lesion was unchanged in appearance.
Although the disease course is variable, 50% of patients with localized granuloma annulare will see spontaneous resolution within 2 years without scarring.7
A 48-year-old man came to the office with a pruritic lesion that had been on the dorsum of his hand for 2 months. He said that the lesion began as 2 flesh-colored papules that had coalesced to form a larger lesion.
He denied any recent trauma, foreign travel, insect bites, disseminated rashes, or systemic symptoms associated with the appearance of the lesion. His medical history was unremarkable, and he indicated that he’d otherwise been feeling well.
His physical exam was significant for a 6 cm, violaceous annular confluent plaque with a firm, slightly raised border (FIGURE 1). There was no visible scale.
FIGURE 1
Violaceous lesion on the hand
What is your diagnosis?
How would you manage this condition?
Diagnosis: Granuloma annulare
Granuloma annulare is a benign inflammatory skin condition classically described as annular papules and plaques. The lesions typically range from skin-colored to violaceous in appearance. There is no racial predilection for this condition.
There are 4 variants of granuloma annulare:
- The localized form—which our patient had—accounts for approximately 75% of cases.1 Lesions are typically found on the lateral or dorsal surfaces of the hands and feet. Most cases of localized granuloma annulare occur in young adult women.2
- The generalized (disseminated) form involves a number of lesions, and thus, is more widespread (FIGURE 2). Lesions tend to appear on the extremities and the trunk.
- The perforating form is rare, and occurs in both localized and generalized granuloma annulare. Papules may develop into lesions that exude a thick and creamy or clear and viscous fluid.
- The subcutaneous form presents as asymptomatic solitary lesions or in clusters that are most commonly found on the lower extremities—often on pretibial areas. The lesions with this form of granuloma annulare are deeper than the localized form, so there is more swelling, and less surface definition.
Anecdotal reports link condition to diabetes
Although the cause of granuloma annulare is unknown, it has been linked with trauma, thyroid disease, viral infection, malignancy, and diabetes mellitus. Some reports suggest that the lesions are the result of a delayed type hypersensitivity reaction. Of note: Our patient had type 2 diabetes mellitus, something we only discovered while doing a work-up for his lesion. Though largely based on anecdote and case reports, retrospective studies have supported this association.3,4
More recently however, a small case-control study failed to reveal any significant correlation between the 2.5 At this time, there is no indication to screen for diabetes in an otherwise asymptomatic patient.
FIGURE 2
Generalized granuloma annulare
Differential Dx includes ringworm, Lyme disease
Localized granuloma annulare must be differentiated from the most common annular lesion, tinea corporis, as well as other annular lesions, including necrobiosis lipoidica and erythema migrans.
- Tinea corporis, or ringworm, is a superficial fungal infection of the skin. Similar to granuloma annulare, patients present with a gradually enlarging annular, well-demarcated papular lesion. Ringworm can be distinguished from granuloma annulare by noting the presence of scale, and by performing a potassium hydroxide slide preparation, which would reveal septate hyphae.
- Necrobiosis lipoidica classically presents as annular violaceous plaques on the anterior legs, but may appear on the arms, hands, feet, or scalp. As the lesion expands, the advancing border becomes red and the central area typically develops a waxy yellow surface, with prominent telangiectasias. This lesion is generally flat or atrophic, and does not have the same raised border as is seen in granuloma annulare.
- Erythema migrans is the characteristic rash of early localized Lyme disease. This classic “bull’s-eye” rash is an annular lesion with concentric redness and expanding central clearing. Erythema migrans typically appears 7 to 10 days after a patient has been bitten by an infected tick. The classic red lesion starts as a small papule at the site of inoculation, while the expanding ring remains flat and lacks the papular appearance of granuloma annulare.
While uncommon, cutaneous sarcoidosis should also be considered in the differential diagnosis. Cutaneous manifestations occur in up to 20% of patients with sarcoidosis.2 The most common presentation is a maculopapular eruption involving the face. Lesions may also be nodular or plaque-like. Biopsy is necessary for diagnosis.
Clinical presentation typically clinches it
Diagnosis of granuloma annulare is often made by its characteristic clinical presentation. If the diagnosis is unclear, a skin biopsy may be needed. Histologic exam will reveal histiocytes in surrounding dermal tissue with increased mucin deposition.
Cosmetic concerns?
Localized lesions that are asymptomatic are often left to resolve spontaneously. If there are cosmetic concerns, or if there is significant pruritus, treatment options include intralesional steroid injection into the raised border with triamcinolone, occlusion therapy with clobetasol propionate, or liquid nitrogen therapy.
One small study of 31 patients with localized granuloma annulare showed resolution after 1 treatment with liquid nitrogen in 81% of patients.6 Topical steroids alone do not produce significant results.5 Other agents, including UV light and systemic medications, are available for the generalized form, however, none are curative and relapses are common.
No treatment for lesion; Metformin for diabetes
Our patient chose to have no treatment for his granuloma annulare, but we did put him on metformin for his diabetes. At a 3-month follow-up visit, our patient’s lesion was unchanged in appearance.
Although the disease course is variable, 50% of patients with localized granuloma annulare will see spontaneous resolution within 2 years without scarring.7
1. Nopper A, Markus R, Esterly N. When it’s not ring-worm: annular lesions of childhood. Pediatr Ann. 1998;27:136-148.
2. Habif TP. Clinical Dermatology. 4th ed. St louis, mo: mosby, Inc; 2004.
3. Studer EM, Calza AM, Saurat JH. Precipitating factors and associated diseases in 84 patients with granuloma annulare: a retrospective study. Dermatology. 1996;193:364-368.
4. Muhlemann MF. Localized granuloma annulare is associated with insulin-dependent diabetes mellitus. Br J Dermatol. 1984;111:325-329.
5. Nebesio CL, Lewis C, Chuang TY. Lack of an association between granuloma annulare and type 2 diabetes mellitus. Br J Dermatol. 2002;146:122-124.
6. Blume-peytavi U, Zouboulis CH, Jacobi H, Scholz A, Bisson S, Orfanos CE. Successful outcome of cryosurgery in patients with granuloma annulare. Br J Dermatol. 1994;130:494-497.
7. Smith MD, Downie JB, DiCostanzo D. Granuloma annulare. Int J Dermatol. 1997;36:326-333.
1. Nopper A, Markus R, Esterly N. When it’s not ring-worm: annular lesions of childhood. Pediatr Ann. 1998;27:136-148.
2. Habif TP. Clinical Dermatology. 4th ed. St louis, mo: mosby, Inc; 2004.
3. Studer EM, Calza AM, Saurat JH. Precipitating factors and associated diseases in 84 patients with granuloma annulare: a retrospective study. Dermatology. 1996;193:364-368.
4. Muhlemann MF. Localized granuloma annulare is associated with insulin-dependent diabetes mellitus. Br J Dermatol. 1984;111:325-329.
5. Nebesio CL, Lewis C, Chuang TY. Lack of an association between granuloma annulare and type 2 diabetes mellitus. Br J Dermatol. 2002;146:122-124.
6. Blume-peytavi U, Zouboulis CH, Jacobi H, Scholz A, Bisson S, Orfanos CE. Successful outcome of cryosurgery in patients with granuloma annulare. Br J Dermatol. 1994;130:494-497.
7. Smith MD, Downie JB, DiCostanzo D. Granuloma annulare. Int J Dermatol. 1997;36:326-333.
Bullous eruption on the posterior thigh
A healthy 11-year-old girl visited her family physician with a lesion on her right posterior thigh. The lesion was a 1-cm plaque that was tender, firm, erythematous, and indurated, with a central pustule. It had been present for 3 days; it was noticed by the patient after returning from a camping trip in southeastern Pennsylvania. The pustular area was incised, drained, and cultured, and the patient was started on cephalexin.
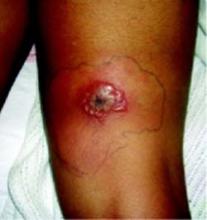
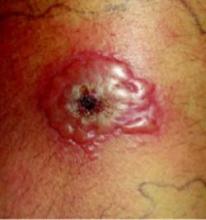
Two days later, the lesion did not improve, showing increased induration, erythema, and blistering. The patient went to the emergency department with an 8 cm by 6 cm coalescence of thin-walled vesicles and bullae with surrounding erythema (FIGURES 1 AND 2). A thick, honey-yellow adherent crust covered the eroded center of the lesion. The girl’s temperature was 37.1°C, and she reported no burning, pain, or pruritus. She had full range of motion of her right hip and knee, and no lymphadenopathy was detected. Her white blood cell count was normal; blood and wound cultures were taken.
FIGURE 1
Bullous eruption on the thigh
FIGURE 2
Close-up
What is the most likely diagnosis?
How would you empirically treat this condition?
Diagnosis: Bullous impetigo, caused by methicillin resistant S aureus
Impetigo is a highly contagious superficial skin infection, with peak incidence among children aged 2 to 6 years.1,2 Nonbullous impetigo (70% of cases) is caused by Staphylococcus aureus or betahemolytic Streptococcus.3 Bullous impetigo is almost always caused by S aureus. Epidermolytic toxins produced by phage group II strains cause loss of cell adhesion in the stratum granulosum due to proteolytic attack of desmoglein 1, resulting in bullae.4
Bullous impetigo may occur after minor skin injury, such as an insect bite, abrasion, or dermatitis. Lesions generally start as small vesicles on the face, buttocks, extremities, or perineum, and may progress to a coalescence of thin-roofed bullae. The flaccid bullae rupture easily, draining serous or purulent fluid.
Lesions are usually painless, and systemic findings are rare. Lymphadenopathy is rare in bullous impetigo but common in nonbullous impetigo. The disease is generally self-limited and complications are uncommon. However, ecthyma (ulcerative impetigo) may result from an untreated impetigo infection.5
Differential diagnosis
The differential diagnosis for bullous impetigo is broad, and may include allergic contact dermatitis, herpes simplex, herpes zoster, pemphigus foliaceus, bullous pemphigoid, pemphigus vulgaris, and (in this case specifically) erythema migrans.
Allergic contact dermatitis is a delayed hypersensitivity reaction, usually caused by skin contact with an allergen. Lesions can be vesicular, edematous, erythematous, and pruritic. In this case, the patient did not have allergen exposure or a pruritic lesion.
Herpes zoster is a reactivation of the varicella zoster virus, characterized by stabbing, neuritic pain in a dermatomal distribution. Clear vesicles on an erythematous, edematous base distributed along a dermatome constitutes the classic appearance. This was not the case with this patient.
Pemphigus foliaceous is an autoimmune intraepidermal blistering disease with lesions occurring on the face, scalp, chest, and upper back.5 Intact blisters are not commonly seen. The vesicle roof is very thin and ruptures easily, forming broad areas of crust. Skin biopsy reveals intraepidermal bulla or acantholysis in the upper epidermis.
Pemphigus vulgaris is also an autoimmune blistering disease that affects the skin and mucous membranes. It is generally seen among patients aged >40 years.
Bullous pemphigoid is an autoimmune disorder presenting with chronic eruption of erythematous, papular, urticaria lesions often evolving into bullae. Childhood cases are rare. Biopsy of the lesions demonstrates subepidermal bulla with an infiltration of eosinophils within the dermis.5
Erythema migrans with central vesiculation must be considered given the patient’s camping trip. Recent evidence shows that erythema migrans with central redness accounts for most cases in areas endemic for Lyme disease. Only 10% of the patients with early Lyme disease show the classic bulls-eye lesion with concentric erythematous rings and central clearing. Vesiculation can occur in up to 30% of lesions.6
Staphylococcus aureus and antibiotic resistance
As many as 61% of community-acquired methicillin-resistant S aureus (MRSA) infections are initially treated only with beta-lactam antibiotics, to which they are resistant.7 Risk factors for community-acquired MRSA infection include day-care attendance, recent hospitalization, recent antibiotic use, chronic illness, and frequent health care visits.8 A growing number of cases are reported among patients without risk factors.
Community-acquired MRSA isolates are usually genetically different from nosocomial isolates, and have been relatively susceptible to non–beta-lactam antibiotics. These strains vary substantially, however, and it is important to check the susceptibility of the isolate.
Virulent new strains of S aureus are infecting children—these strains have a novel transpeptidase, which offers them a mechanism of resistance to beta-lactams different from hospital-and community-acquired types.
Awareness of the local antimicrobial susceptibility patterns of community S aureus isolates is also helpful. Oral antibiotics that have been successful include clindamycin, minocycline, doxycycline, and trimethoprim-sulfamethoxazole. Cephalexin has no therapeutic value in treating community-acquired MRSA.
Preventing disease spread in the patient and contacts
Preventive efforts should be directed at patients with recurrent episodes of MRSA skin abscesses. Metabolic and immunologic screening should be performed to rule out underlying disease processes causing increased risk for infection. In most cases these test results are normal, and patients with recurrent MRSA skin abscesses should also be empirically treated for presumed nasal carriage of MRSA.
Mupirocin ointment (Bactroban) should be applied to the nares twice daily for 5 days in an effort to prevent recurrent self-inoculation and lateral transmission of MRSA.
Patients and families should also be instructed in hygienic measures such as daily changing of underwear and personal use only of towels, washcloths, and sleepwear. Fingernails should be kept short and clean. Open insect bites or superficial skin abrasions should be kept clean and covered. Benefit from the daily use of antimicrobial soaps is controversial.
Empiric treatment of impetigo: Consider a culture for MRSA
For localized impetigo, topical therapy with mupirocin 2% ointment 3 times a day for 10 days is usually adequate. A 10-day course of oral antibiotic therapy with dicloxacillin or cephalexin is indicated in more widespread impetigo presumed to be methicillin-sensitive S aureus. Azithromycin (Zithromax) or clarithromycin (Biaxin) may be given to patients allergic to penicillin.
However, it is becoming increasingly important to consider community-acquired methicillin-resistant S aureus species in cases such as this that do not respond to traditional therapy. Hence, culture and sensitivity of all suspicious lesions is highly suggested.
Patient’s treatment and recovery
In this case, the patient was diagnosed with bullous impetigo and admitted to the hospital. She was started on intravenous clindamycin at 380 mg (30 mg/kg) every 8 hours. Clindamycin was chosen because most cases of community-acquired MRSA in this geographic area are resistant to trimethoprim-sulfamethoxazole and susceptible to clindamycin.
Although doxycycline would have covered both community-acquired MRSA and Lyme disease, we were less suspicious of Lyme given the physical exam of the patient, and we were reluctant to start this patient on doxycycline due to the fact she did not have complete maturation of her dentition.
Within 24 hours of intravenous clindamycin, the lesion was markedly improved and the culture confirmed that the MRSA was sensitive to clindamycin. She was discharged on oral clindamycin at 375 mg 3 times daily, to complete a 14-day course of therapy. The lesion was completely resolved without recurrence within 2 weeks.
CORRESPONDING AUTHOR
Richard P. Usatine, MD, University of Texas Health Science Center at San Antonio, Department of Family and Community Medicine, MC 7794, 7703 Floyd Curl Drive, San Antonio, TX 78229-3900. E-mail: usatine@uthscsa.edu
1. Dagan R. Impetigo in children: changing epidemiology and new treatments. Pediatric Annals 1993;22:235-240.
2. Bruijnzeels MA, van Suijlekom-Smit LW, van der Velden J, van der Wouden JC. The child in general practice. Dutch national survey of morbidity and interventions in general practice. Rotterdam: Erasmus University Rotterdam, 1993.
3. Allen CH, Patel M, Endom E. Primary bacterial infections of the skin and soft tissues changes in epidemiology and management. Clin Ped Emerg Med 2004;5:246-255.
Amagai M, Matsuyoshi N, Wang ZH, et al. Toxin in bullous impetigo and staphylococcal scalded skin syndrome targets desmoglein 1. Nat Med 2000;6:1275.-
5. Habif TP. Skin Disease Diagnosis and Treatment. 2nd ed. Philadelphia, Pa: Elsevier-Mosby 2005;136-141.
6. Smith RP, Schoen RT, Rahn DW, et al. Clinical characteristics and treatment outcome of early Lyme disease in patients with microbiologically confirmed erythema migrans. Ann Intern Med 2002;136:477-479.
7. Naimi TS, LeDell KH, Como-Sabetti K, et al. Comparison of community-and health care-associated methicillin-resistant Staphylococcus aureus infection. JAMA 2003;290:2976.-
8. Cohen P. Community-acquired methicillin-resistant staphylococcus aureus: skin infection presenting as an axillary abscess with cellulites in a college athlete. Skin Med 2005;4:115-117.
A healthy 11-year-old girl visited her family physician with a lesion on her right posterior thigh. The lesion was a 1-cm plaque that was tender, firm, erythematous, and indurated, with a central pustule. It had been present for 3 days; it was noticed by the patient after returning from a camping trip in southeastern Pennsylvania. The pustular area was incised, drained, and cultured, and the patient was started on cephalexin.
Two days later, the lesion did not improve, showing increased induration, erythema, and blistering. The patient went to the emergency department with an 8 cm by 6 cm coalescence of thin-walled vesicles and bullae with surrounding erythema (FIGURES 1 AND 2). A thick, honey-yellow adherent crust covered the eroded center of the lesion. The girl’s temperature was 37.1°C, and she reported no burning, pain, or pruritus. She had full range of motion of her right hip and knee, and no lymphadenopathy was detected. Her white blood cell count was normal; blood and wound cultures were taken.
FIGURE 1
Bullous eruption on the thigh
FIGURE 2
Close-up
What is the most likely diagnosis?
How would you empirically treat this condition?
Diagnosis: Bullous impetigo, caused by methicillin resistant S aureus
Impetigo is a highly contagious superficial skin infection, with peak incidence among children aged 2 to 6 years.1,2 Nonbullous impetigo (70% of cases) is caused by Staphylococcus aureus or betahemolytic Streptococcus.3 Bullous impetigo is almost always caused by S aureus. Epidermolytic toxins produced by phage group II strains cause loss of cell adhesion in the stratum granulosum due to proteolytic attack of desmoglein 1, resulting in bullae.4
Bullous impetigo may occur after minor skin injury, such as an insect bite, abrasion, or dermatitis. Lesions generally start as small vesicles on the face, buttocks, extremities, or perineum, and may progress to a coalescence of thin-roofed bullae. The flaccid bullae rupture easily, draining serous or purulent fluid.
Lesions are usually painless, and systemic findings are rare. Lymphadenopathy is rare in bullous impetigo but common in nonbullous impetigo. The disease is generally self-limited and complications are uncommon. However, ecthyma (ulcerative impetigo) may result from an untreated impetigo infection.5
Differential diagnosis
The differential diagnosis for bullous impetigo is broad, and may include allergic contact dermatitis, herpes simplex, herpes zoster, pemphigus foliaceus, bullous pemphigoid, pemphigus vulgaris, and (in this case specifically) erythema migrans.
Allergic contact dermatitis is a delayed hypersensitivity reaction, usually caused by skin contact with an allergen. Lesions can be vesicular, edematous, erythematous, and pruritic. In this case, the patient did not have allergen exposure or a pruritic lesion.
Herpes zoster is a reactivation of the varicella zoster virus, characterized by stabbing, neuritic pain in a dermatomal distribution. Clear vesicles on an erythematous, edematous base distributed along a dermatome constitutes the classic appearance. This was not the case with this patient.
Pemphigus foliaceous is an autoimmune intraepidermal blistering disease with lesions occurring on the face, scalp, chest, and upper back.5 Intact blisters are not commonly seen. The vesicle roof is very thin and ruptures easily, forming broad areas of crust. Skin biopsy reveals intraepidermal bulla or acantholysis in the upper epidermis.
Pemphigus vulgaris is also an autoimmune blistering disease that affects the skin and mucous membranes. It is generally seen among patients aged >40 years.
Bullous pemphigoid is an autoimmune disorder presenting with chronic eruption of erythematous, papular, urticaria lesions often evolving into bullae. Childhood cases are rare. Biopsy of the lesions demonstrates subepidermal bulla with an infiltration of eosinophils within the dermis.5
Erythema migrans with central vesiculation must be considered given the patient’s camping trip. Recent evidence shows that erythema migrans with central redness accounts for most cases in areas endemic for Lyme disease. Only 10% of the patients with early Lyme disease show the classic bulls-eye lesion with concentric erythematous rings and central clearing. Vesiculation can occur in up to 30% of lesions.6
Staphylococcus aureus and antibiotic resistance
As many as 61% of community-acquired methicillin-resistant S aureus (MRSA) infections are initially treated only with beta-lactam antibiotics, to which they are resistant.7 Risk factors for community-acquired MRSA infection include day-care attendance, recent hospitalization, recent antibiotic use, chronic illness, and frequent health care visits.8 A growing number of cases are reported among patients without risk factors.
Community-acquired MRSA isolates are usually genetically different from nosocomial isolates, and have been relatively susceptible to non–beta-lactam antibiotics. These strains vary substantially, however, and it is important to check the susceptibility of the isolate.
Virulent new strains of S aureus are infecting children—these strains have a novel transpeptidase, which offers them a mechanism of resistance to beta-lactams different from hospital-and community-acquired types.
Awareness of the local antimicrobial susceptibility patterns of community S aureus isolates is also helpful. Oral antibiotics that have been successful include clindamycin, minocycline, doxycycline, and trimethoprim-sulfamethoxazole. Cephalexin has no therapeutic value in treating community-acquired MRSA.
Preventing disease spread in the patient and contacts
Preventive efforts should be directed at patients with recurrent episodes of MRSA skin abscesses. Metabolic and immunologic screening should be performed to rule out underlying disease processes causing increased risk for infection. In most cases these test results are normal, and patients with recurrent MRSA skin abscesses should also be empirically treated for presumed nasal carriage of MRSA.
Mupirocin ointment (Bactroban) should be applied to the nares twice daily for 5 days in an effort to prevent recurrent self-inoculation and lateral transmission of MRSA.
Patients and families should also be instructed in hygienic measures such as daily changing of underwear and personal use only of towels, washcloths, and sleepwear. Fingernails should be kept short and clean. Open insect bites or superficial skin abrasions should be kept clean and covered. Benefit from the daily use of antimicrobial soaps is controversial.
Empiric treatment of impetigo: Consider a culture for MRSA
For localized impetigo, topical therapy with mupirocin 2% ointment 3 times a day for 10 days is usually adequate. A 10-day course of oral antibiotic therapy with dicloxacillin or cephalexin is indicated in more widespread impetigo presumed to be methicillin-sensitive S aureus. Azithromycin (Zithromax) or clarithromycin (Biaxin) may be given to patients allergic to penicillin.
However, it is becoming increasingly important to consider community-acquired methicillin-resistant S aureus species in cases such as this that do not respond to traditional therapy. Hence, culture and sensitivity of all suspicious lesions is highly suggested.
Patient’s treatment and recovery
In this case, the patient was diagnosed with bullous impetigo and admitted to the hospital. She was started on intravenous clindamycin at 380 mg (30 mg/kg) every 8 hours. Clindamycin was chosen because most cases of community-acquired MRSA in this geographic area are resistant to trimethoprim-sulfamethoxazole and susceptible to clindamycin.
Although doxycycline would have covered both community-acquired MRSA and Lyme disease, we were less suspicious of Lyme given the physical exam of the patient, and we were reluctant to start this patient on doxycycline due to the fact she did not have complete maturation of her dentition.
Within 24 hours of intravenous clindamycin, the lesion was markedly improved and the culture confirmed that the MRSA was sensitive to clindamycin. She was discharged on oral clindamycin at 375 mg 3 times daily, to complete a 14-day course of therapy. The lesion was completely resolved without recurrence within 2 weeks.
CORRESPONDING AUTHOR
Richard P. Usatine, MD, University of Texas Health Science Center at San Antonio, Department of Family and Community Medicine, MC 7794, 7703 Floyd Curl Drive, San Antonio, TX 78229-3900. E-mail: usatine@uthscsa.edu
A healthy 11-year-old girl visited her family physician with a lesion on her right posterior thigh. The lesion was a 1-cm plaque that was tender, firm, erythematous, and indurated, with a central pustule. It had been present for 3 days; it was noticed by the patient after returning from a camping trip in southeastern Pennsylvania. The pustular area was incised, drained, and cultured, and the patient was started on cephalexin.
Two days later, the lesion did not improve, showing increased induration, erythema, and blistering. The patient went to the emergency department with an 8 cm by 6 cm coalescence of thin-walled vesicles and bullae with surrounding erythema (FIGURES 1 AND 2). A thick, honey-yellow adherent crust covered the eroded center of the lesion. The girl’s temperature was 37.1°C, and she reported no burning, pain, or pruritus. She had full range of motion of her right hip and knee, and no lymphadenopathy was detected. Her white blood cell count was normal; blood and wound cultures were taken.
FIGURE 1
Bullous eruption on the thigh
FIGURE 2
Close-up
What is the most likely diagnosis?
How would you empirically treat this condition?
Diagnosis: Bullous impetigo, caused by methicillin resistant S aureus
Impetigo is a highly contagious superficial skin infection, with peak incidence among children aged 2 to 6 years.1,2 Nonbullous impetigo (70% of cases) is caused by Staphylococcus aureus or betahemolytic Streptococcus.3 Bullous impetigo is almost always caused by S aureus. Epidermolytic toxins produced by phage group II strains cause loss of cell adhesion in the stratum granulosum due to proteolytic attack of desmoglein 1, resulting in bullae.4
Bullous impetigo may occur after minor skin injury, such as an insect bite, abrasion, or dermatitis. Lesions generally start as small vesicles on the face, buttocks, extremities, or perineum, and may progress to a coalescence of thin-roofed bullae. The flaccid bullae rupture easily, draining serous or purulent fluid.
Lesions are usually painless, and systemic findings are rare. Lymphadenopathy is rare in bullous impetigo but common in nonbullous impetigo. The disease is generally self-limited and complications are uncommon. However, ecthyma (ulcerative impetigo) may result from an untreated impetigo infection.5
Differential diagnosis
The differential diagnosis for bullous impetigo is broad, and may include allergic contact dermatitis, herpes simplex, herpes zoster, pemphigus foliaceus, bullous pemphigoid, pemphigus vulgaris, and (in this case specifically) erythema migrans.
Allergic contact dermatitis is a delayed hypersensitivity reaction, usually caused by skin contact with an allergen. Lesions can be vesicular, edematous, erythematous, and pruritic. In this case, the patient did not have allergen exposure or a pruritic lesion.
Herpes zoster is a reactivation of the varicella zoster virus, characterized by stabbing, neuritic pain in a dermatomal distribution. Clear vesicles on an erythematous, edematous base distributed along a dermatome constitutes the classic appearance. This was not the case with this patient.
Pemphigus foliaceous is an autoimmune intraepidermal blistering disease with lesions occurring on the face, scalp, chest, and upper back.5 Intact blisters are not commonly seen. The vesicle roof is very thin and ruptures easily, forming broad areas of crust. Skin biopsy reveals intraepidermal bulla or acantholysis in the upper epidermis.
Pemphigus vulgaris is also an autoimmune blistering disease that affects the skin and mucous membranes. It is generally seen among patients aged >40 years.
Bullous pemphigoid is an autoimmune disorder presenting with chronic eruption of erythematous, papular, urticaria lesions often evolving into bullae. Childhood cases are rare. Biopsy of the lesions demonstrates subepidermal bulla with an infiltration of eosinophils within the dermis.5
Erythema migrans with central vesiculation must be considered given the patient’s camping trip. Recent evidence shows that erythema migrans with central redness accounts for most cases in areas endemic for Lyme disease. Only 10% of the patients with early Lyme disease show the classic bulls-eye lesion with concentric erythematous rings and central clearing. Vesiculation can occur in up to 30% of lesions.6
Staphylococcus aureus and antibiotic resistance
As many as 61% of community-acquired methicillin-resistant S aureus (MRSA) infections are initially treated only with beta-lactam antibiotics, to which they are resistant.7 Risk factors for community-acquired MRSA infection include day-care attendance, recent hospitalization, recent antibiotic use, chronic illness, and frequent health care visits.8 A growing number of cases are reported among patients without risk factors.
Community-acquired MRSA isolates are usually genetically different from nosocomial isolates, and have been relatively susceptible to non–beta-lactam antibiotics. These strains vary substantially, however, and it is important to check the susceptibility of the isolate.
Virulent new strains of S aureus are infecting children—these strains have a novel transpeptidase, which offers them a mechanism of resistance to beta-lactams different from hospital-and community-acquired types.
Awareness of the local antimicrobial susceptibility patterns of community S aureus isolates is also helpful. Oral antibiotics that have been successful include clindamycin, minocycline, doxycycline, and trimethoprim-sulfamethoxazole. Cephalexin has no therapeutic value in treating community-acquired MRSA.
Preventing disease spread in the patient and contacts
Preventive efforts should be directed at patients with recurrent episodes of MRSA skin abscesses. Metabolic and immunologic screening should be performed to rule out underlying disease processes causing increased risk for infection. In most cases these test results are normal, and patients with recurrent MRSA skin abscesses should also be empirically treated for presumed nasal carriage of MRSA.
Mupirocin ointment (Bactroban) should be applied to the nares twice daily for 5 days in an effort to prevent recurrent self-inoculation and lateral transmission of MRSA.
Patients and families should also be instructed in hygienic measures such as daily changing of underwear and personal use only of towels, washcloths, and sleepwear. Fingernails should be kept short and clean. Open insect bites or superficial skin abrasions should be kept clean and covered. Benefit from the daily use of antimicrobial soaps is controversial.
Empiric treatment of impetigo: Consider a culture for MRSA
For localized impetigo, topical therapy with mupirocin 2% ointment 3 times a day for 10 days is usually adequate. A 10-day course of oral antibiotic therapy with dicloxacillin or cephalexin is indicated in more widespread impetigo presumed to be methicillin-sensitive S aureus. Azithromycin (Zithromax) or clarithromycin (Biaxin) may be given to patients allergic to penicillin.
However, it is becoming increasingly important to consider community-acquired methicillin-resistant S aureus species in cases such as this that do not respond to traditional therapy. Hence, culture and sensitivity of all suspicious lesions is highly suggested.
Patient’s treatment and recovery
In this case, the patient was diagnosed with bullous impetigo and admitted to the hospital. She was started on intravenous clindamycin at 380 mg (30 mg/kg) every 8 hours. Clindamycin was chosen because most cases of community-acquired MRSA in this geographic area are resistant to trimethoprim-sulfamethoxazole and susceptible to clindamycin.
Although doxycycline would have covered both community-acquired MRSA and Lyme disease, we were less suspicious of Lyme given the physical exam of the patient, and we were reluctant to start this patient on doxycycline due to the fact she did not have complete maturation of her dentition.
Within 24 hours of intravenous clindamycin, the lesion was markedly improved and the culture confirmed that the MRSA was sensitive to clindamycin. She was discharged on oral clindamycin at 375 mg 3 times daily, to complete a 14-day course of therapy. The lesion was completely resolved without recurrence within 2 weeks.
CORRESPONDING AUTHOR
Richard P. Usatine, MD, University of Texas Health Science Center at San Antonio, Department of Family and Community Medicine, MC 7794, 7703 Floyd Curl Drive, San Antonio, TX 78229-3900. E-mail: usatine@uthscsa.edu
1. Dagan R. Impetigo in children: changing epidemiology and new treatments. Pediatric Annals 1993;22:235-240.
2. Bruijnzeels MA, van Suijlekom-Smit LW, van der Velden J, van der Wouden JC. The child in general practice. Dutch national survey of morbidity and interventions in general practice. Rotterdam: Erasmus University Rotterdam, 1993.
3. Allen CH, Patel M, Endom E. Primary bacterial infections of the skin and soft tissues changes in epidemiology and management. Clin Ped Emerg Med 2004;5:246-255.
Amagai M, Matsuyoshi N, Wang ZH, et al. Toxin in bullous impetigo and staphylococcal scalded skin syndrome targets desmoglein 1. Nat Med 2000;6:1275.-
5. Habif TP. Skin Disease Diagnosis and Treatment. 2nd ed. Philadelphia, Pa: Elsevier-Mosby 2005;136-141.
6. Smith RP, Schoen RT, Rahn DW, et al. Clinical characteristics and treatment outcome of early Lyme disease in patients with microbiologically confirmed erythema migrans. Ann Intern Med 2002;136:477-479.
7. Naimi TS, LeDell KH, Como-Sabetti K, et al. Comparison of community-and health care-associated methicillin-resistant Staphylococcus aureus infection. JAMA 2003;290:2976.-
8. Cohen P. Community-acquired methicillin-resistant staphylococcus aureus: skin infection presenting as an axillary abscess with cellulites in a college athlete. Skin Med 2005;4:115-117.
1. Dagan R. Impetigo in children: changing epidemiology and new treatments. Pediatric Annals 1993;22:235-240.
2. Bruijnzeels MA, van Suijlekom-Smit LW, van der Velden J, van der Wouden JC. The child in general practice. Dutch national survey of morbidity and interventions in general practice. Rotterdam: Erasmus University Rotterdam, 1993.
3. Allen CH, Patel M, Endom E. Primary bacterial infections of the skin and soft tissues changes in epidemiology and management. Clin Ped Emerg Med 2004;5:246-255.
Amagai M, Matsuyoshi N, Wang ZH, et al. Toxin in bullous impetigo and staphylococcal scalded skin syndrome targets desmoglein 1. Nat Med 2000;6:1275.-
5. Habif TP. Skin Disease Diagnosis and Treatment. 2nd ed. Philadelphia, Pa: Elsevier-Mosby 2005;136-141.
6. Smith RP, Schoen RT, Rahn DW, et al. Clinical characteristics and treatment outcome of early Lyme disease in patients with microbiologically confirmed erythema migrans. Ann Intern Med 2002;136:477-479.
7. Naimi TS, LeDell KH, Como-Sabetti K, et al. Comparison of community-and health care-associated methicillin-resistant Staphylococcus aureus infection. JAMA 2003;290:2976.-
8. Cohen P. Community-acquired methicillin-resistant staphylococcus aureus: skin infection presenting as an axillary abscess with cellulites in a college athlete. Skin Med 2005;4:115-117.