User login
2019 at a glance: Hem-onc U.S. drug approvals
The rapid development and identification of novel drugs has translated into innovative therapies in hematology and oncology. The aim of this piece is to present newly approved drugs and expanded indications to serve as a reference guide for practicing clinicians.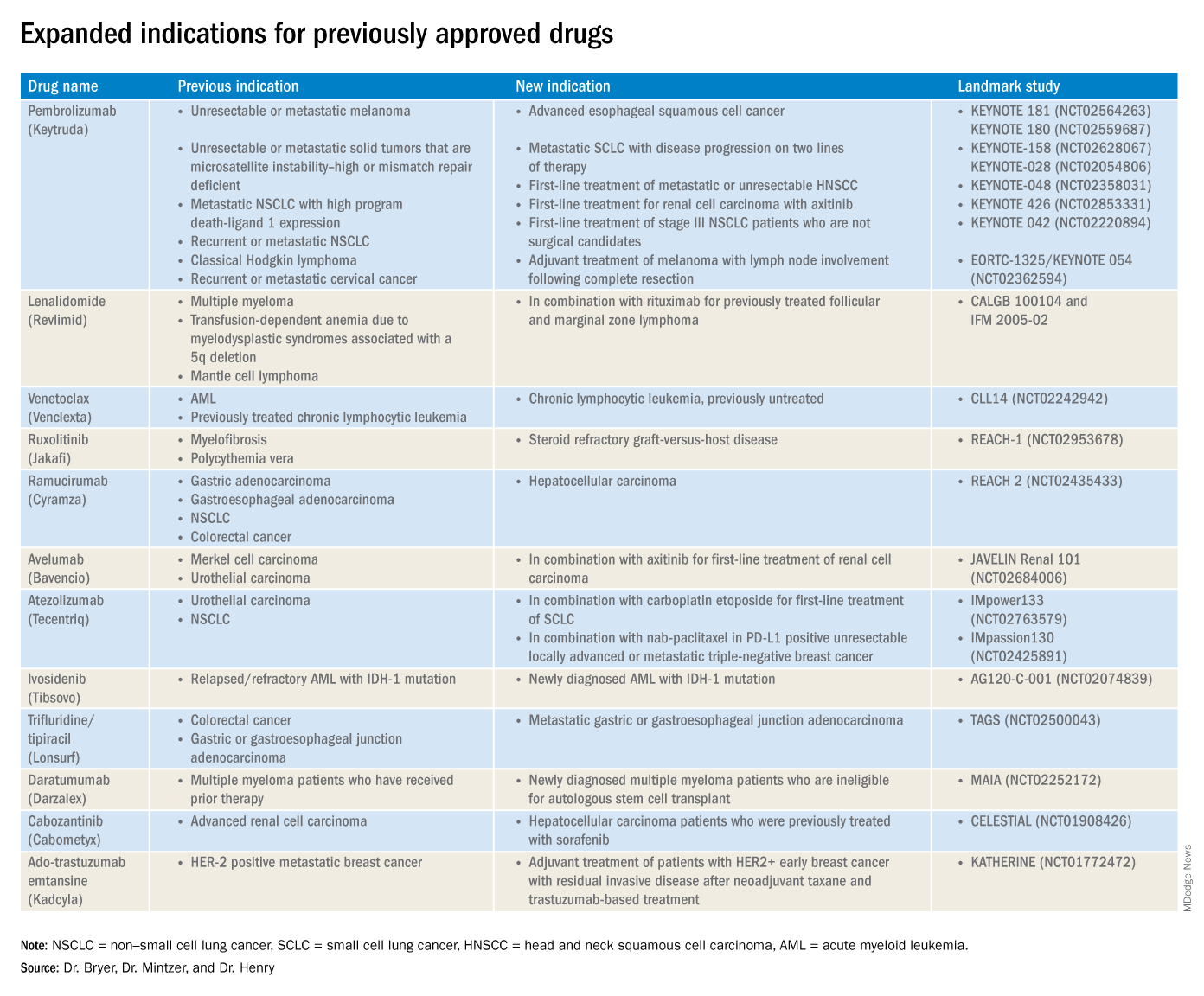
This article reviews therapies that were newly approved so far in 2019, as well as those previously approved whose indications were expanded this past year. The list highlights the most clinically important approvals, as well as adverse events that are unique or especially severe.
New approvals
Fedratinib (Inrebic)
Class: JAK2 and FLT3 selective kinase inhibitor.
Disease: Intermediate or high-risk primary or secondary (postpolycythemia vera or postessential thrombocythemia) myelofibrosis.
Dose: 400 mg orally once daily, with or without food.
Adverse events (AEs): Black box warning: Fatal encephalopathy, including Wernicke’s (thiamine level monitoring suggested).
Trials: In JAKARTA (NCT01437787), 37% of patients achieved a 35% or greater reduction in spleen volume and 40% received a 50% or greater reduction in myelofibrosis-related symptoms. In Jakarta-2, there was a 55% spleen response in patients resistant or intolerant to ruxolitinib.
Entrectinib (Rozlytrek)
Class: Tropomyosin receptor tyrosine kinase inhibitor.
Disease: Solid tumors that have a neurotrophic tyrosine receptor kinase (NTRK) gene fusion and for ROS-1 positive non–small cell lung cancer (NSCLC).
Dose: 600 mg orally once daily.
AEs: Heart failure, QT prolongation, skeletal fractures, hepatotoxicity, central nervous system effects, and hyperuricemia.
Trial: ALKA, STARTRK-1 (NCT02097810) and STARTRK-2 (NCT02568267): Overall response rate of 57% for NTRK positive patients; response rate of 77% in ROS-1 positive NSCLC.
Pexidartinib (Turalio)
Class: Small molecule tyrosine kinase inhibitor targeting CSF1R.
Disease: Symptomatic tenosynovial giant cell tumor.
Dose: 400 mg orally twice daily without food.
AEs: Black box warning on hepatotoxicity.
Trial: ENLIVEN (NCT02371369): Overall response rate of 38% at 25 weeks, with a 15% complete response rate and a 23% partial response rate.
Darolutamide (Nubeqa)
Class: Androgen receptor inhibitor.
Disease: Nonmetastatic castration-resistant prostate cancer.
Dose: 600 mg orally twice daily with food with concomitant androgen deprivation therapy.
AEs: Fatigue, extremity pain, and rash.
Trial: ARAMIS (NCT02200614): Median metastasis free survival was 40.4 months for patients with darolutamide, compared with 18.4 months for controls.
Selinexor (Xpovio)
Class: Reversible inhibitor of nuclear export of tumor suppressor proteins, growth regulators, and mRNAs of oncogenic proteins.
Disease: Relapsed or refractory multiple myeloma. Indicated for patients who have received at least four prior therapies, including at least two immunomodulatory agents and an anti-CD38 monoclonal antibody.
Dose: 80 mg orally in combination with oral dexamethasone on days 1 and 3 of each week.
AEs: Thrombocytopenia, fatigue, pancytopenia, and hyponatremia.
Trial: STORM (NCT02336815): Overall response rate 25.3% with a median time to first response of 4 weeks and 3.8-month median duration of response.
Polatuzumab vedotin-piiq (Polivy)
Class: CD79b-directed antibody-drug conjugate.
Disease: Relapsed or refractory diffuse large B-cell lymphoma. Indicated for patients who have had at least two prior therapies.
Dose: 1.8 mg/kg intravenous infusion every 21 days for six cycles in combination with bendamustine and a rituximab product.
AEs: Pancytopenia, peripheral neuropathy.
Trial: GO29365 (NCT02257567): Complete response rate was 40% for polatuzumab vedotin-piiq plus bendamustine/rituximab, compared with 18% with bendamustine/rituximab alone.*
Caplacizumab-yhdp (Cablivi)
Class: Monoclonal antibody fragment directed against von Willebrand factor.
Disease: Thrombotic thrombocytopenic purpura.
Dose: 11 mg IV initially, then daily subcutaneously; in combination with plasma exchange and immunosuppressive therapy.
AEs: Epistaxis, headache, and gingival bleeding.
Trial: Hercules trial (NCT02553317): More rapid normalization of platelets, lower incidence of composite TTP-related death, and lower rate of recurrence when added to plasma exchange and steroids.
Alpelisib (Piqray)
Class: Phosphatidylinositol-3-kinase (PI3K) inhibitor.
Disease: Hormone receptor positive HER2-negative PIK3CA-mutated, advanced or metastatic breast cancer.
Dose: 300 mg orally once daily with food with concomitant fulvestrant.
AEs: Hyperglycemia, pancytopenia.
Trial: SOLAR-1 (NCT02437318): 11-month progression-free survival among patients treated with alpelisib and fulvestrant, compared with 5.7 months in fulvestrant alone control arm; overall response rate of 36% versus 16%, respectively.
Erdafitinib (Balversa)
Class: Fibroblast growth factor receptor kinase inhibitor.
Disease: Locally advanced or metastatic urothelial carcinoma with FGFR3 or FGFR2 mutations.
Dose: 8 mg orally once daily, with or without food.
AEs: Ocular disorders including retinopathy or retinal detachment.
Trial: BLC2001 (NCT02365597): Objective response rate of 32.2%, with a complete response in 2.3% of patients and partial response in 29.9% of patients.
Biosimilar approvals
Trastuzumab and hyaluronidase-oysk (Herceptin Hylecta)
Biosimilar to: Trastuzumab.
Indication: HER2-overexpressing breast cancer.
Dr. Bryer is a resident in the department of internal medicine at the University of Pennsylvania, Philadelphia. Dr. Mintzer is chief of hematology-oncology at Pennsylvania Hospital and professor of medicine at the University of Pennsylvania. Dr. Henry is a hematologist-oncologist at Pennsylvania Hospital and professor of medicine at the University of Pennsylvania.
*Correction, 11/7/2019: An earlier version of this article misstated the drug combination in the GO29365 trial.
The rapid development and identification of novel drugs has translated into innovative therapies in hematology and oncology. The aim of this piece is to present newly approved drugs and expanded indications to serve as a reference guide for practicing clinicians.
This article reviews therapies that were newly approved so far in 2019, as well as those previously approved whose indications were expanded this past year. The list highlights the most clinically important approvals, as well as adverse events that are unique or especially severe.
New approvals
Fedratinib (Inrebic)
Class: JAK2 and FLT3 selective kinase inhibitor.
Disease: Intermediate or high-risk primary or secondary (postpolycythemia vera or postessential thrombocythemia) myelofibrosis.
Dose: 400 mg orally once daily, with or without food.
Adverse events (AEs): Black box warning: Fatal encephalopathy, including Wernicke’s (thiamine level monitoring suggested).
Trials: In JAKARTA (NCT01437787), 37% of patients achieved a 35% or greater reduction in spleen volume and 40% received a 50% or greater reduction in myelofibrosis-related symptoms. In Jakarta-2, there was a 55% spleen response in patients resistant or intolerant to ruxolitinib.
Entrectinib (Rozlytrek)
Class: Tropomyosin receptor tyrosine kinase inhibitor.
Disease: Solid tumors that have a neurotrophic tyrosine receptor kinase (NTRK) gene fusion and for ROS-1 positive non–small cell lung cancer (NSCLC).
Dose: 600 mg orally once daily.
AEs: Heart failure, QT prolongation, skeletal fractures, hepatotoxicity, central nervous system effects, and hyperuricemia.
Trial: ALKA, STARTRK-1 (NCT02097810) and STARTRK-2 (NCT02568267): Overall response rate of 57% for NTRK positive patients; response rate of 77% in ROS-1 positive NSCLC.
Pexidartinib (Turalio)
Class: Small molecule tyrosine kinase inhibitor targeting CSF1R.
Disease: Symptomatic tenosynovial giant cell tumor.
Dose: 400 mg orally twice daily without food.
AEs: Black box warning on hepatotoxicity.
Trial: ENLIVEN (NCT02371369): Overall response rate of 38% at 25 weeks, with a 15% complete response rate and a 23% partial response rate.
Darolutamide (Nubeqa)
Class: Androgen receptor inhibitor.
Disease: Nonmetastatic castration-resistant prostate cancer.
Dose: 600 mg orally twice daily with food with concomitant androgen deprivation therapy.
AEs: Fatigue, extremity pain, and rash.
Trial: ARAMIS (NCT02200614): Median metastasis free survival was 40.4 months for patients with darolutamide, compared with 18.4 months for controls.
Selinexor (Xpovio)
Class: Reversible inhibitor of nuclear export of tumor suppressor proteins, growth regulators, and mRNAs of oncogenic proteins.
Disease: Relapsed or refractory multiple myeloma. Indicated for patients who have received at least four prior therapies, including at least two immunomodulatory agents and an anti-CD38 monoclonal antibody.
Dose: 80 mg orally in combination with oral dexamethasone on days 1 and 3 of each week.
AEs: Thrombocytopenia, fatigue, pancytopenia, and hyponatremia.
Trial: STORM (NCT02336815): Overall response rate 25.3% with a median time to first response of 4 weeks and 3.8-month median duration of response.
Polatuzumab vedotin-piiq (Polivy)
Class: CD79b-directed antibody-drug conjugate.
Disease: Relapsed or refractory diffuse large B-cell lymphoma. Indicated for patients who have had at least two prior therapies.
Dose: 1.8 mg/kg intravenous infusion every 21 days for six cycles in combination with bendamustine and a rituximab product.
AEs: Pancytopenia, peripheral neuropathy.
Trial: GO29365 (NCT02257567): Complete response rate was 40% for polatuzumab vedotin-piiq plus bendamustine/rituximab, compared with 18% with bendamustine/rituximab alone.*
Caplacizumab-yhdp (Cablivi)
Class: Monoclonal antibody fragment directed against von Willebrand factor.
Disease: Thrombotic thrombocytopenic purpura.
Dose: 11 mg IV initially, then daily subcutaneously; in combination with plasma exchange and immunosuppressive therapy.
AEs: Epistaxis, headache, and gingival bleeding.
Trial: Hercules trial (NCT02553317): More rapid normalization of platelets, lower incidence of composite TTP-related death, and lower rate of recurrence when added to plasma exchange and steroids.
Alpelisib (Piqray)
Class: Phosphatidylinositol-3-kinase (PI3K) inhibitor.
Disease: Hormone receptor positive HER2-negative PIK3CA-mutated, advanced or metastatic breast cancer.
Dose: 300 mg orally once daily with food with concomitant fulvestrant.
AEs: Hyperglycemia, pancytopenia.
Trial: SOLAR-1 (NCT02437318): 11-month progression-free survival among patients treated with alpelisib and fulvestrant, compared with 5.7 months in fulvestrant alone control arm; overall response rate of 36% versus 16%, respectively.
Erdafitinib (Balversa)
Class: Fibroblast growth factor receptor kinase inhibitor.
Disease: Locally advanced or metastatic urothelial carcinoma with FGFR3 or FGFR2 mutations.
Dose: 8 mg orally once daily, with or without food.
AEs: Ocular disorders including retinopathy or retinal detachment.
Trial: BLC2001 (NCT02365597): Objective response rate of 32.2%, with a complete response in 2.3% of patients and partial response in 29.9% of patients.
Biosimilar approvals
Trastuzumab and hyaluronidase-oysk (Herceptin Hylecta)
Biosimilar to: Trastuzumab.
Indication: HER2-overexpressing breast cancer.
Dr. Bryer is a resident in the department of internal medicine at the University of Pennsylvania, Philadelphia. Dr. Mintzer is chief of hematology-oncology at Pennsylvania Hospital and professor of medicine at the University of Pennsylvania. Dr. Henry is a hematologist-oncologist at Pennsylvania Hospital and professor of medicine at the University of Pennsylvania.
*Correction, 11/7/2019: An earlier version of this article misstated the drug combination in the GO29365 trial.
The rapid development and identification of novel drugs has translated into innovative therapies in hematology and oncology. The aim of this piece is to present newly approved drugs and expanded indications to serve as a reference guide for practicing clinicians.
This article reviews therapies that were newly approved so far in 2019, as well as those previously approved whose indications were expanded this past year. The list highlights the most clinically important approvals, as well as adverse events that are unique or especially severe.
New approvals
Fedratinib (Inrebic)
Class: JAK2 and FLT3 selective kinase inhibitor.
Disease: Intermediate or high-risk primary or secondary (postpolycythemia vera or postessential thrombocythemia) myelofibrosis.
Dose: 400 mg orally once daily, with or without food.
Adverse events (AEs): Black box warning: Fatal encephalopathy, including Wernicke’s (thiamine level monitoring suggested).
Trials: In JAKARTA (NCT01437787), 37% of patients achieved a 35% or greater reduction in spleen volume and 40% received a 50% or greater reduction in myelofibrosis-related symptoms. In Jakarta-2, there was a 55% spleen response in patients resistant or intolerant to ruxolitinib.
Entrectinib (Rozlytrek)
Class: Tropomyosin receptor tyrosine kinase inhibitor.
Disease: Solid tumors that have a neurotrophic tyrosine receptor kinase (NTRK) gene fusion and for ROS-1 positive non–small cell lung cancer (NSCLC).
Dose: 600 mg orally once daily.
AEs: Heart failure, QT prolongation, skeletal fractures, hepatotoxicity, central nervous system effects, and hyperuricemia.
Trial: ALKA, STARTRK-1 (NCT02097810) and STARTRK-2 (NCT02568267): Overall response rate of 57% for NTRK positive patients; response rate of 77% in ROS-1 positive NSCLC.
Pexidartinib (Turalio)
Class: Small molecule tyrosine kinase inhibitor targeting CSF1R.
Disease: Symptomatic tenosynovial giant cell tumor.
Dose: 400 mg orally twice daily without food.
AEs: Black box warning on hepatotoxicity.
Trial: ENLIVEN (NCT02371369): Overall response rate of 38% at 25 weeks, with a 15% complete response rate and a 23% partial response rate.
Darolutamide (Nubeqa)
Class: Androgen receptor inhibitor.
Disease: Nonmetastatic castration-resistant prostate cancer.
Dose: 600 mg orally twice daily with food with concomitant androgen deprivation therapy.
AEs: Fatigue, extremity pain, and rash.
Trial: ARAMIS (NCT02200614): Median metastasis free survival was 40.4 months for patients with darolutamide, compared with 18.4 months for controls.
Selinexor (Xpovio)
Class: Reversible inhibitor of nuclear export of tumor suppressor proteins, growth regulators, and mRNAs of oncogenic proteins.
Disease: Relapsed or refractory multiple myeloma. Indicated for patients who have received at least four prior therapies, including at least two immunomodulatory agents and an anti-CD38 monoclonal antibody.
Dose: 80 mg orally in combination with oral dexamethasone on days 1 and 3 of each week.
AEs: Thrombocytopenia, fatigue, pancytopenia, and hyponatremia.
Trial: STORM (NCT02336815): Overall response rate 25.3% with a median time to first response of 4 weeks and 3.8-month median duration of response.
Polatuzumab vedotin-piiq (Polivy)
Class: CD79b-directed antibody-drug conjugate.
Disease: Relapsed or refractory diffuse large B-cell lymphoma. Indicated for patients who have had at least two prior therapies.
Dose: 1.8 mg/kg intravenous infusion every 21 days for six cycles in combination with bendamustine and a rituximab product.
AEs: Pancytopenia, peripheral neuropathy.
Trial: GO29365 (NCT02257567): Complete response rate was 40% for polatuzumab vedotin-piiq plus bendamustine/rituximab, compared with 18% with bendamustine/rituximab alone.*
Caplacizumab-yhdp (Cablivi)
Class: Monoclonal antibody fragment directed against von Willebrand factor.
Disease: Thrombotic thrombocytopenic purpura.
Dose: 11 mg IV initially, then daily subcutaneously; in combination with plasma exchange and immunosuppressive therapy.
AEs: Epistaxis, headache, and gingival bleeding.
Trial: Hercules trial (NCT02553317): More rapid normalization of platelets, lower incidence of composite TTP-related death, and lower rate of recurrence when added to plasma exchange and steroids.
Alpelisib (Piqray)
Class: Phosphatidylinositol-3-kinase (PI3K) inhibitor.
Disease: Hormone receptor positive HER2-negative PIK3CA-mutated, advanced or metastatic breast cancer.
Dose: 300 mg orally once daily with food with concomitant fulvestrant.
AEs: Hyperglycemia, pancytopenia.
Trial: SOLAR-1 (NCT02437318): 11-month progression-free survival among patients treated with alpelisib and fulvestrant, compared with 5.7 months in fulvestrant alone control arm; overall response rate of 36% versus 16%, respectively.
Erdafitinib (Balversa)
Class: Fibroblast growth factor receptor kinase inhibitor.
Disease: Locally advanced or metastatic urothelial carcinoma with FGFR3 or FGFR2 mutations.
Dose: 8 mg orally once daily, with or without food.
AEs: Ocular disorders including retinopathy or retinal detachment.
Trial: BLC2001 (NCT02365597): Objective response rate of 32.2%, with a complete response in 2.3% of patients and partial response in 29.9% of patients.
Biosimilar approvals
Trastuzumab and hyaluronidase-oysk (Herceptin Hylecta)
Biosimilar to: Trastuzumab.
Indication: HER2-overexpressing breast cancer.
Dr. Bryer is a resident in the department of internal medicine at the University of Pennsylvania, Philadelphia. Dr. Mintzer is chief of hematology-oncology at Pennsylvania Hospital and professor of medicine at the University of Pennsylvania. Dr. Henry is a hematologist-oncologist at Pennsylvania Hospital and professor of medicine at the University of Pennsylvania.
*Correction, 11/7/2019: An earlier version of this article misstated the drug combination in the GO29365 trial.
2018 at a glance: Recently approved therapies in oncology
Advances in genomics and technology perpetually change and improve therapies in oncology. Enhanced comprehension of cellular signaling, division, and replication has created a platform to selectively restrict neoplastic growth while preserving the integrity of benign cells.
This article reviews therapies that were newly approved in 2018, as well as those previously approved whose indications were expanded this past year. The list highlights the most clinically important approvals, as well as adverse events that are unique or especially severe.
Click on the PDF above to download the full article and charts in an easy-to-print format.
Apalutamide (Erleada)
Class: Androgen receptor inhibitor.
Disease: Nonmetastatic castration-resistant prostate cancer.
Dose: 240 mg orally, once daily.
Adverse Events (AEs): Hyperkalemia and increased risks of seizures, falls, and fractures.
Phase 3 SPARTAN trial (NCT01946204): 40.5-month metastasis-free survival rate, compared with 16.2 months in the placebo group.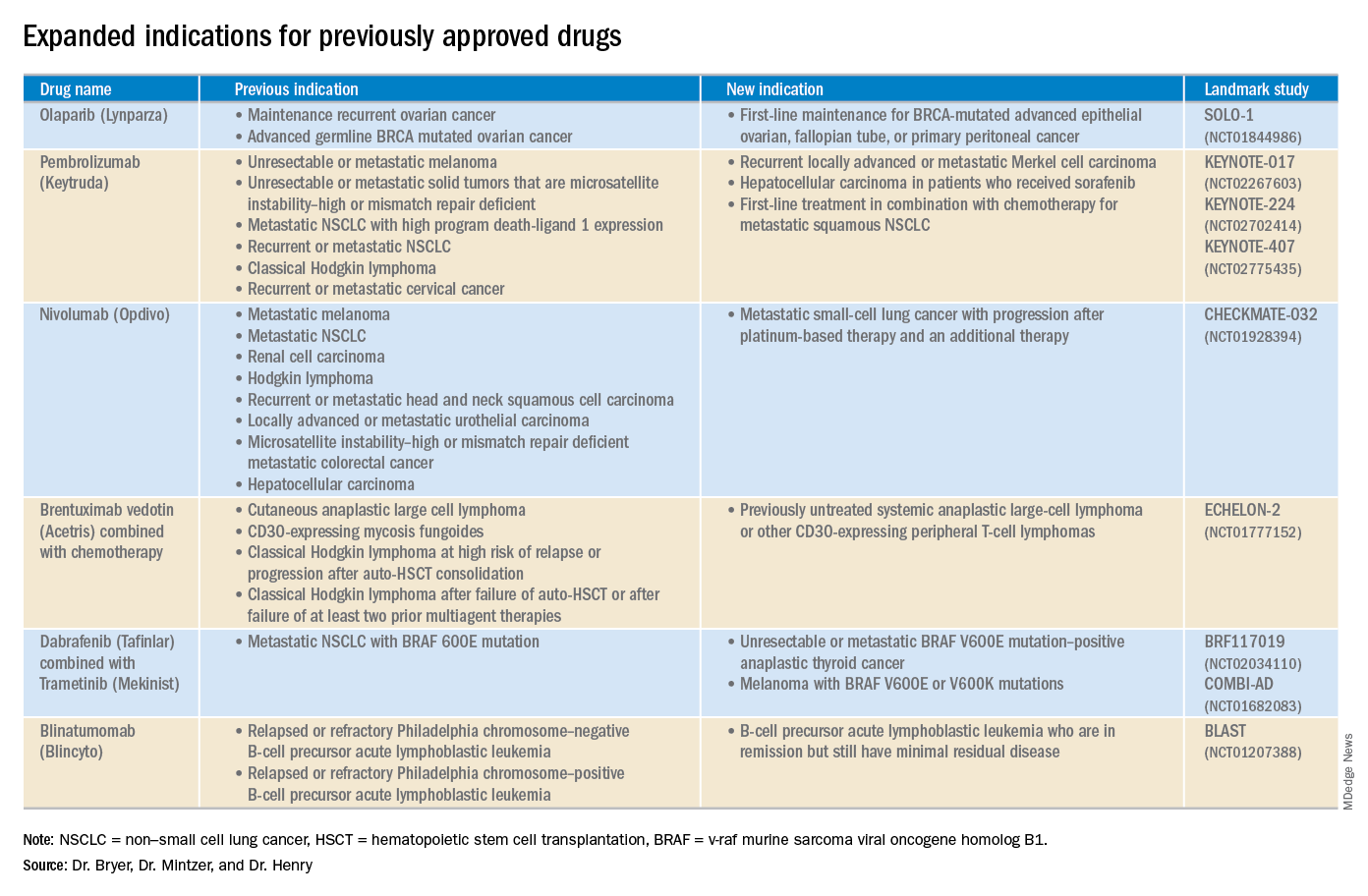
Cemiplimab (Libtayo)
Class: Antibody against programmed cell death protein-1 (PD-1).
Disease: Metastatic cutaneous squamous cell carcinoma (CSCC) or locally advanced CSCC that is ineligible for curative surgery/radiation.
Dose: 350 mg intravenous infusion every 3 weeks.
AEs: Pneumonitis, autoimmune myocarditis, hepatitis, and aseptic meningitis.
1423 and 1540 trials (NCT02383212 and NCT02760498): 47.2% of patients who received cemiplimab had complete disappearance of the tumor or a decrease in tumor size.
Dacomitinib (Vizimpro)
Class: Second-generation tyrosine kinase inhibitor.
Disease: Metastatic non–small cell lung cancer (NSCLC) with epidermal growth factor receptor (EGFR) exon 19 deletion or exon 21 L858R substitution mutation.
Dose: 45 mg orally once daily.
AEs: Dermatotoxicity and diarrhea.
ARCHER1050 trial (NCT01774721): Patients who received dacomitinib demonstrated an improved overall survival, with a median of 34.1 months, compared with 26.8 months with gefitinib.
Duvelisib (Copiktra)
Class: Dual inhibitor of phosphatidylinositol 3-kinase delta and gamma.
Disease: Relapsed or refractory chronic lymphocytic leukemia (CLL), small lymphocytic lymphoma, or relapsed or refractory follicular lymphoma after at least two prior systemic therapies.
Dose: 25 mg orally twice daily.
AEs: Infection, diarrhea or colitis, and pneumonia.
Phase 3 DUO trial (NCT02004522): Progression-free survival in the duvelisib arm was 7.3 months longer than that in the ofatumumab arm. The overall response rate for patients receiving duvelisib was 78%, compared with 39% for those receiving ofatumumab.
Gilteritinib (Xospata)
Class: Inhibits the FLT3 internal tandem duplication (ITD) and FLT3 tyrosine kinase domain (TKD).
Disease: Relapsed or refractory acute myeloid leukemia (AML) with an FLT3 mutation.
Dose: 120 mg orally daily.
ADMIRAL trial (NCT02421939): 21% of the patients who received gilteritinib exhibited complete remission or complete remission with partial hematologic recovery.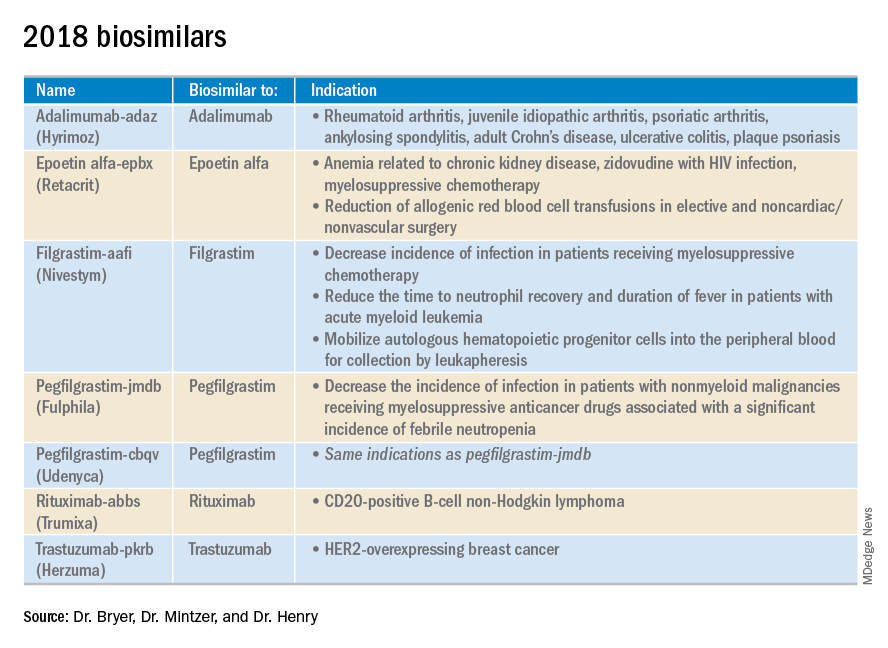
Glasdegib (Daurismo)
Class: Hedgehog pathway inhibitor.
Disease: Adults over age 75 years with newly diagnosed AML and other medical comorbidities that preclude them from intensive chemotherapy.
Dose: The recommended dose is 100 mg orally continuously in 28-day cycles.
AE: QT prolongation and embryo-fetal toxicity
Phase 2 BRIGHT 1003 trial (NCT01546038): 3.9-month overall survival advantage for glasdegib plus cytarabine, compared with cytarabine alone. Overall, 15% of the glasdegib plus low dose cytarabine arm achieved complete remission, compared with the 1% complete remission rate in patients who received cytarabine alone.
Iobenguane I 131 (Azedra)
Class: Radiopharmaceutical agent; induces cell death within the noradrenaline transporter.
Disease: Iobenguane scan–positive, unresectable, locally advanced or metastatic pheochromocytoma or paraganglioma
Dose: Initial intravenous dosimetric dose, followed by two therapeutic doses.
AE: Pancytopenia and elevated international normalized ratio (INR).
IB12B trial (NCT00874614): One-quarter of patients receiving this therapy had at least a 50% reduction in the dose and number of antihypertensives for at least 6 months; almost all patients had a tumor response.
Ivosidenib (Tibsovo)
Class: Small-molecule inhibitor of mutant isocitrate dehydrogenase (IDH1).
Disease: Refractory AML and an IDH1 mutation
Dose: 500 mg orally daily.
AG120-C-001 trial (NCT02074839): Overall response rate of 41.6% in patients who received ivosidenib, with a 30.4% rate of complete remission or complete remission with partial hematologic recovery.
Larotrectinib (Vitrakvi)
Class: Oral tyrosine kinase inhibitor.
Disease: Advanced solid tumors harboring a neurotrophic tyrosine receptor kinase (NTRK) gene fusion.
Dose: 100 mg orally twice daily.
LOXO-TRK-14001, SCOUT, and NAVIGATE trials (NCT02122913, NCT02637687, and NCT02576431): Patients who received larotrectinib had durable responses regardless of patient age, tumor type, and fusion status.
Lutetium Lu 177 dotatate (Lutathera)
Class: Radiolabeled somatostatin analogue.
Disease: Somatostatin receptor–positive gastroenteropancreatic neuroendocrine tumors (GEP-NETs).
Dose: Intravenous infusion 7.4 GBq (200 mCi) every 8 weeks for a total of four doses.
NETTER-1 trial (NCT01578239): 65% of adults who received lutetium Lu 177 showed improved progression-free survival at 20 months, compared with just 10.8% in the control group.
Mogamulizumab (Poteligeo)
Class: Monoclonal antibody that binds to a protein (CC chemokine receptor type 4).
Disease: Relapsed or refractory mycosis fungoides or Sézary syndrome.
Dose: Intravenous infusion 1 mg/kg.
AE: Dermatologic toxicity.
MAVORIC trial (NCT01728805): Patients who received mogamulizumab had improved progression-free survival (median 7.7 months), compared with those taking vorinostat (median 3.1 months).
Moxetumomab pasudotox-tdfk (Lumoxiti)
Class: CD22-directed cytotoxin fused with a fragment of Pseudomonas exotoxin A.
Disease: Relapsed or refractory hairy cell leukemia previously treated with at least two prior systemic therapies, including a purine nucleoside analogue.
Dose: Intravenously as 0.04 mg/kg.
AE: Hemolytic uremic syndrome.
1053 trial (NCT01829711): 30% of the patients who received moxetumomab pasudotox-tdfk had a durable complete response confirmed by maintenance hematologic remission.
Talazoparib (Talzenna)
Class: Poly (ADP-ribose) polymerase (PARP) inhibitor.
Disease: gBRCAm HER2-negative locally advanced or metastatic breast cancer.
Dose: 1 mg orally per day.
EMBRACA trial (NCT01945775): Patients who received talazoparib demonstrated significantly longer progression-free survival, with a median of 8.6 months versis 5.6 months in the control arm.
Dr. Bryer is a resident in the department of internal medicine at the University of Pennsylvania, Philadelphia. Dr. Mentzer is chief of hematology-oncology at Pennsylvania Hospital and professor of medicine at the University of Pennsylvania. Dr. Henry is a hematologist-oncologist at Pennsylvania Hospital and a professor of medicine at the University of Pennsylvania.
Advances in genomics and technology perpetually change and improve therapies in oncology. Enhanced comprehension of cellular signaling, division, and replication has created a platform to selectively restrict neoplastic growth while preserving the integrity of benign cells.
This article reviews therapies that were newly approved in 2018, as well as those previously approved whose indications were expanded this past year. The list highlights the most clinically important approvals, as well as adverse events that are unique or especially severe.
Click on the PDF above to download the full article and charts in an easy-to-print format.
Apalutamide (Erleada)
Class: Androgen receptor inhibitor.
Disease: Nonmetastatic castration-resistant prostate cancer.
Dose: 240 mg orally, once daily.
Adverse Events (AEs): Hyperkalemia and increased risks of seizures, falls, and fractures.
Phase 3 SPARTAN trial (NCT01946204): 40.5-month metastasis-free survival rate, compared with 16.2 months in the placebo group.
Cemiplimab (Libtayo)
Class: Antibody against programmed cell death protein-1 (PD-1).
Disease: Metastatic cutaneous squamous cell carcinoma (CSCC) or locally advanced CSCC that is ineligible for curative surgery/radiation.
Dose: 350 mg intravenous infusion every 3 weeks.
AEs: Pneumonitis, autoimmune myocarditis, hepatitis, and aseptic meningitis.
1423 and 1540 trials (NCT02383212 and NCT02760498): 47.2% of patients who received cemiplimab had complete disappearance of the tumor or a decrease in tumor size.
Dacomitinib (Vizimpro)
Class: Second-generation tyrosine kinase inhibitor.
Disease: Metastatic non–small cell lung cancer (NSCLC) with epidermal growth factor receptor (EGFR) exon 19 deletion or exon 21 L858R substitution mutation.
Dose: 45 mg orally once daily.
AEs: Dermatotoxicity and diarrhea.
ARCHER1050 trial (NCT01774721): Patients who received dacomitinib demonstrated an improved overall survival, with a median of 34.1 months, compared with 26.8 months with gefitinib.
Duvelisib (Copiktra)
Class: Dual inhibitor of phosphatidylinositol 3-kinase delta and gamma.
Disease: Relapsed or refractory chronic lymphocytic leukemia (CLL), small lymphocytic lymphoma, or relapsed or refractory follicular lymphoma after at least two prior systemic therapies.
Dose: 25 mg orally twice daily.
AEs: Infection, diarrhea or colitis, and pneumonia.
Phase 3 DUO trial (NCT02004522): Progression-free survival in the duvelisib arm was 7.3 months longer than that in the ofatumumab arm. The overall response rate for patients receiving duvelisib was 78%, compared with 39% for those receiving ofatumumab.
Gilteritinib (Xospata)
Class: Inhibits the FLT3 internal tandem duplication (ITD) and FLT3 tyrosine kinase domain (TKD).
Disease: Relapsed or refractory acute myeloid leukemia (AML) with an FLT3 mutation.
Dose: 120 mg orally daily.
ADMIRAL trial (NCT02421939): 21% of the patients who received gilteritinib exhibited complete remission or complete remission with partial hematologic recovery.
Glasdegib (Daurismo)
Class: Hedgehog pathway inhibitor.
Disease: Adults over age 75 years with newly diagnosed AML and other medical comorbidities that preclude them from intensive chemotherapy.
Dose: The recommended dose is 100 mg orally continuously in 28-day cycles.
AE: QT prolongation and embryo-fetal toxicity
Phase 2 BRIGHT 1003 trial (NCT01546038): 3.9-month overall survival advantage for glasdegib plus cytarabine, compared with cytarabine alone. Overall, 15% of the glasdegib plus low dose cytarabine arm achieved complete remission, compared with the 1% complete remission rate in patients who received cytarabine alone.
Iobenguane I 131 (Azedra)
Class: Radiopharmaceutical agent; induces cell death within the noradrenaline transporter.
Disease: Iobenguane scan–positive, unresectable, locally advanced or metastatic pheochromocytoma or paraganglioma
Dose: Initial intravenous dosimetric dose, followed by two therapeutic doses.
AE: Pancytopenia and elevated international normalized ratio (INR).
IB12B trial (NCT00874614): One-quarter of patients receiving this therapy had at least a 50% reduction in the dose and number of antihypertensives for at least 6 months; almost all patients had a tumor response.
Ivosidenib (Tibsovo)
Class: Small-molecule inhibitor of mutant isocitrate dehydrogenase (IDH1).
Disease: Refractory AML and an IDH1 mutation
Dose: 500 mg orally daily.
AG120-C-001 trial (NCT02074839): Overall response rate of 41.6% in patients who received ivosidenib, with a 30.4% rate of complete remission or complete remission with partial hematologic recovery.
Larotrectinib (Vitrakvi)
Class: Oral tyrosine kinase inhibitor.
Disease: Advanced solid tumors harboring a neurotrophic tyrosine receptor kinase (NTRK) gene fusion.
Dose: 100 mg orally twice daily.
LOXO-TRK-14001, SCOUT, and NAVIGATE trials (NCT02122913, NCT02637687, and NCT02576431): Patients who received larotrectinib had durable responses regardless of patient age, tumor type, and fusion status.
Lutetium Lu 177 dotatate (Lutathera)
Class: Radiolabeled somatostatin analogue.
Disease: Somatostatin receptor–positive gastroenteropancreatic neuroendocrine tumors (GEP-NETs).
Dose: Intravenous infusion 7.4 GBq (200 mCi) every 8 weeks for a total of four doses.
NETTER-1 trial (NCT01578239): 65% of adults who received lutetium Lu 177 showed improved progression-free survival at 20 months, compared with just 10.8% in the control group.
Mogamulizumab (Poteligeo)
Class: Monoclonal antibody that binds to a protein (CC chemokine receptor type 4).
Disease: Relapsed or refractory mycosis fungoides or Sézary syndrome.
Dose: Intravenous infusion 1 mg/kg.
AE: Dermatologic toxicity.
MAVORIC trial (NCT01728805): Patients who received mogamulizumab had improved progression-free survival (median 7.7 months), compared with those taking vorinostat (median 3.1 months).
Moxetumomab pasudotox-tdfk (Lumoxiti)
Class: CD22-directed cytotoxin fused with a fragment of Pseudomonas exotoxin A.
Disease: Relapsed or refractory hairy cell leukemia previously treated with at least two prior systemic therapies, including a purine nucleoside analogue.
Dose: Intravenously as 0.04 mg/kg.
AE: Hemolytic uremic syndrome.
1053 trial (NCT01829711): 30% of the patients who received moxetumomab pasudotox-tdfk had a durable complete response confirmed by maintenance hematologic remission.
Talazoparib (Talzenna)
Class: Poly (ADP-ribose) polymerase (PARP) inhibitor.
Disease: gBRCAm HER2-negative locally advanced or metastatic breast cancer.
Dose: 1 mg orally per day.
EMBRACA trial (NCT01945775): Patients who received talazoparib demonstrated significantly longer progression-free survival, with a median of 8.6 months versis 5.6 months in the control arm.
Dr. Bryer is a resident in the department of internal medicine at the University of Pennsylvania, Philadelphia. Dr. Mentzer is chief of hematology-oncology at Pennsylvania Hospital and professor of medicine at the University of Pennsylvania. Dr. Henry is a hematologist-oncologist at Pennsylvania Hospital and a professor of medicine at the University of Pennsylvania.
Advances in genomics and technology perpetually change and improve therapies in oncology. Enhanced comprehension of cellular signaling, division, and replication has created a platform to selectively restrict neoplastic growth while preserving the integrity of benign cells.
This article reviews therapies that were newly approved in 2018, as well as those previously approved whose indications were expanded this past year. The list highlights the most clinically important approvals, as well as adverse events that are unique or especially severe.
Click on the PDF above to download the full article and charts in an easy-to-print format.
Apalutamide (Erleada)
Class: Androgen receptor inhibitor.
Disease: Nonmetastatic castration-resistant prostate cancer.
Dose: 240 mg orally, once daily.
Adverse Events (AEs): Hyperkalemia and increased risks of seizures, falls, and fractures.
Phase 3 SPARTAN trial (NCT01946204): 40.5-month metastasis-free survival rate, compared with 16.2 months in the placebo group.
Cemiplimab (Libtayo)
Class: Antibody against programmed cell death protein-1 (PD-1).
Disease: Metastatic cutaneous squamous cell carcinoma (CSCC) or locally advanced CSCC that is ineligible for curative surgery/radiation.
Dose: 350 mg intravenous infusion every 3 weeks.
AEs: Pneumonitis, autoimmune myocarditis, hepatitis, and aseptic meningitis.
1423 and 1540 trials (NCT02383212 and NCT02760498): 47.2% of patients who received cemiplimab had complete disappearance of the tumor or a decrease in tumor size.
Dacomitinib (Vizimpro)
Class: Second-generation tyrosine kinase inhibitor.
Disease: Metastatic non–small cell lung cancer (NSCLC) with epidermal growth factor receptor (EGFR) exon 19 deletion or exon 21 L858R substitution mutation.
Dose: 45 mg orally once daily.
AEs: Dermatotoxicity and diarrhea.
ARCHER1050 trial (NCT01774721): Patients who received dacomitinib demonstrated an improved overall survival, with a median of 34.1 months, compared with 26.8 months with gefitinib.
Duvelisib (Copiktra)
Class: Dual inhibitor of phosphatidylinositol 3-kinase delta and gamma.
Disease: Relapsed or refractory chronic lymphocytic leukemia (CLL), small lymphocytic lymphoma, or relapsed or refractory follicular lymphoma after at least two prior systemic therapies.
Dose: 25 mg orally twice daily.
AEs: Infection, diarrhea or colitis, and pneumonia.
Phase 3 DUO trial (NCT02004522): Progression-free survival in the duvelisib arm was 7.3 months longer than that in the ofatumumab arm. The overall response rate for patients receiving duvelisib was 78%, compared with 39% for those receiving ofatumumab.
Gilteritinib (Xospata)
Class: Inhibits the FLT3 internal tandem duplication (ITD) and FLT3 tyrosine kinase domain (TKD).
Disease: Relapsed or refractory acute myeloid leukemia (AML) with an FLT3 mutation.
Dose: 120 mg orally daily.
ADMIRAL trial (NCT02421939): 21% of the patients who received gilteritinib exhibited complete remission or complete remission with partial hematologic recovery.
Glasdegib (Daurismo)
Class: Hedgehog pathway inhibitor.
Disease: Adults over age 75 years with newly diagnosed AML and other medical comorbidities that preclude them from intensive chemotherapy.
Dose: The recommended dose is 100 mg orally continuously in 28-day cycles.
AE: QT prolongation and embryo-fetal toxicity
Phase 2 BRIGHT 1003 trial (NCT01546038): 3.9-month overall survival advantage for glasdegib plus cytarabine, compared with cytarabine alone. Overall, 15% of the glasdegib plus low dose cytarabine arm achieved complete remission, compared with the 1% complete remission rate in patients who received cytarabine alone.
Iobenguane I 131 (Azedra)
Class: Radiopharmaceutical agent; induces cell death within the noradrenaline transporter.
Disease: Iobenguane scan–positive, unresectable, locally advanced or metastatic pheochromocytoma or paraganglioma
Dose: Initial intravenous dosimetric dose, followed by two therapeutic doses.
AE: Pancytopenia and elevated international normalized ratio (INR).
IB12B trial (NCT00874614): One-quarter of patients receiving this therapy had at least a 50% reduction in the dose and number of antihypertensives for at least 6 months; almost all patients had a tumor response.
Ivosidenib (Tibsovo)
Class: Small-molecule inhibitor of mutant isocitrate dehydrogenase (IDH1).
Disease: Refractory AML and an IDH1 mutation
Dose: 500 mg orally daily.
AG120-C-001 trial (NCT02074839): Overall response rate of 41.6% in patients who received ivosidenib, with a 30.4% rate of complete remission or complete remission with partial hematologic recovery.
Larotrectinib (Vitrakvi)
Class: Oral tyrosine kinase inhibitor.
Disease: Advanced solid tumors harboring a neurotrophic tyrosine receptor kinase (NTRK) gene fusion.
Dose: 100 mg orally twice daily.
LOXO-TRK-14001, SCOUT, and NAVIGATE trials (NCT02122913, NCT02637687, and NCT02576431): Patients who received larotrectinib had durable responses regardless of patient age, tumor type, and fusion status.
Lutetium Lu 177 dotatate (Lutathera)
Class: Radiolabeled somatostatin analogue.
Disease: Somatostatin receptor–positive gastroenteropancreatic neuroendocrine tumors (GEP-NETs).
Dose: Intravenous infusion 7.4 GBq (200 mCi) every 8 weeks for a total of four doses.
NETTER-1 trial (NCT01578239): 65% of adults who received lutetium Lu 177 showed improved progression-free survival at 20 months, compared with just 10.8% in the control group.
Mogamulizumab (Poteligeo)
Class: Monoclonal antibody that binds to a protein (CC chemokine receptor type 4).
Disease: Relapsed or refractory mycosis fungoides or Sézary syndrome.
Dose: Intravenous infusion 1 mg/kg.
AE: Dermatologic toxicity.
MAVORIC trial (NCT01728805): Patients who received mogamulizumab had improved progression-free survival (median 7.7 months), compared with those taking vorinostat (median 3.1 months).
Moxetumomab pasudotox-tdfk (Lumoxiti)
Class: CD22-directed cytotoxin fused with a fragment of Pseudomonas exotoxin A.
Disease: Relapsed or refractory hairy cell leukemia previously treated with at least two prior systemic therapies, including a purine nucleoside analogue.
Dose: Intravenously as 0.04 mg/kg.
AE: Hemolytic uremic syndrome.
1053 trial (NCT01829711): 30% of the patients who received moxetumomab pasudotox-tdfk had a durable complete response confirmed by maintenance hematologic remission.
Talazoparib (Talzenna)
Class: Poly (ADP-ribose) polymerase (PARP) inhibitor.
Disease: gBRCAm HER2-negative locally advanced or metastatic breast cancer.
Dose: 1 mg orally per day.
EMBRACA trial (NCT01945775): Patients who received talazoparib demonstrated significantly longer progression-free survival, with a median of 8.6 months versis 5.6 months in the control arm.
Dr. Bryer is a resident in the department of internal medicine at the University of Pennsylvania, Philadelphia. Dr. Mentzer is chief of hematology-oncology at Pennsylvania Hospital and professor of medicine at the University of Pennsylvania. Dr. Henry is a hematologist-oncologist at Pennsylvania Hospital and a professor of medicine at the University of Pennsylvania.