User login
2011 Update on fertility
Impaired fertility is no small problem. According to the Centers for Disease Control and Prevention (CDC), it affects 7.3 million women 15 to 44 years old in the United States alone, or approximately 10% of the female population of reproductive age.1
Not long ago, there was little to be done about the problem. Today, however, we have many tools and tactics at our disposal, and another CDC statistic demonstrates their efficacy: Fewer than one third of women who have received medical intervention for impaired fertility in the past year continue to experience the problem.1
In this article, we highlight three recent developments in fertility:
- We know more about the effects of obesity on fecundity, and more about how to increase the likelihood of pregnancy and live birth in obese women
- The development of in vitro fertilization (IVF) more than 30 years ago represents a significant achievement and vast benefit to mankind, noted the Nobel Committee in awarding the 2010 prize for Physiology or Medicine to Robert Edwards, PhD
- Ovarian hyperstimulation syndrome after controlled ovarian stimulation cannot be avoided completely—but its likelihood can be reduced significantly through careful assessment of the patient and a cautious approach to ovarian stimulation.
Be mindful of the effects of obesity
on a woman’s reproductive function
Obese patients are almost three times as likely as women of normal weight to be infertile. Polycystic ovarian syndrome (PCOS) is generally unmasked or exacerbated, or both, by obesity, and the hyperandrogenicity associated with PCOS can cause ovulatory dysfunction. The hypothalamic-pituitary-ovarian (HPO) axis is also affected by overweight and obesity, resulting in oligo-ovulation in 30% to 47% of women.2 Some studies suggest that fecundity may be reduced in ovulatory obese women as well as those with ovulatory dysfunction.2 Most obese women are not infertile, however.
Once pregnancy is achieved, the risk of miscarriage is elevated in obese women (odds ratio [OR] ~1.67), and the live birth rate is lower (OR ~0.75), compared with women of normal weight.2–4 Obese women also have an elevated risk of miscarriage after egg donation (OR ~1.52) and ovulation induction (OR ~5.11). There is no evidence that the rate of miscarriage is increased after IVF, compared with other treatments.
The diagnosis of infertility is difficult in obese patients because the pelvic examination is less informative, although ultrasonography (US) is usually helpful.5 In addition, obesity can blur the distinction between PCOS and HPO axis-related oligo-ovulation. Laparoscopy and other diagnostic interventions are performed less frequently in obese women, and complications of diagnostic laparoscopy are higher in this population.3
Take the initiative in recommending weight loss
As health-care providers, we need to be more proactive in recommending lifestyle changes for obese women so that they lose weight before pregnancy. Women who have infertility are usually very motivated to conceive; as a result, they may also be motivated to lose weight. Caloric restriction, increased physical activity, behavioral modification, and professional expertise are all essential for successful weight loss.2 Even a reduction as small as 5% to 10% of body weight can have clinical benefit.2,4,5
Metformin is an additional option. When combined with a low-calorie diet, metformin may lead to weight loss, restore ovulation, and improve fecundity in women who have PCOS.2
Bariatric surgery is now commonly reserved for women whose body weight is 45 kg or more above normal. Bariatric surgery can improve the altered hormone profile, including elevated thyroid-stimulating hormone (TSH), of obese women. It also appears to improve fecundity and reduce pregnancy-associated complications. However, it is not always successful and can have complications of its own.
What can you offer to obese patients who experience infertility?
- Clomiphene citrate is the most commonly used ovarian-stimulation agent for oligo-ovulation that arises from PCOS or HPO-axis disruption; it is most effective in patients of normal weight.4,6 The protocols associated with clomiphene administration in obese patients are similar to those for women of normal weight; so are results, although the pregnancy rate is not as high in obese women.
- Gonadotropins are effective ovarian-stimulation drugs that are used in hypothalamic hypogonadal patients as well as after failed treatment with clomiphene citrate. Gonadotropins can be effective even in very obese patients; the dosage increases with body mass index (BMI).4,6
- Metformin reduces insulin resistance in women who have PCOS. By itself, metformin is ineffective at inducing ovulation and has not proved to increase the pregnancy rate when it is added to clomiphene.5 Nevertheless, it is commonly given at a daily dosage of 1,000 to 2,000 mg to women who have hyperinsulinemia, and it may reduce the miscarriage rate in women who have PCOS.
- Other medications that have been used to enhance ovulation in obese women include dexamethasone to reduce elevated androgen levels, bromocryptine for elevated prolactin levels, and thyroid hormone for hypothyroidism.
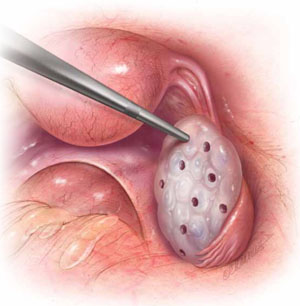
FOR WOMEN WHO HAVE PCOS
In laparoscopic ovarian drilling, an insulated needle unipolar electrode is inserted in the ovary perpendicular to the surface to create 6 to 12 evenly spaced punctures using 40 watts of coagulating current for 2 seconds at each point. The mechanism of action is unclear, but it is thought that the destruction of androgen-producing stroma is responsible for the reduction in testosterone level, increase in follicle-stimulating hormone (FSH), and return to FSH cyclicity in 80% of cases.
- Among obese women who have oligo-ovulation, ovarian drilling (FIGURE) is generally reserved for patients who have not responded to clomiphene or gonadotropins. This procedure has proved to be as effective as clomiphene administration, with the advantage that it does not increase the risk of multiple gestation and leads to longstanding improvement in one third of patients.5 Ovarian drilling is a modification of ovarian wedge resection for women who have PCOS. The mechanism of action is not clear, but it is thought that destruction of adrogen-producing stroma causes an immediate reduction in testosterone, an increase in follicle-stimulating hormone (FSH), and a return to FSH cyclicity in 80% of cases. These effects can persist for several years, and a pregnancy rate of approximately 60% can be attained in less than 6 months. Clomiphene-resistant women may be more responsive to the drug after ovarian drilling, and the risk of ovarian hyperstimulation appears to be reduced. Ovarian drilling is less effective in obese women than in women of normal weight. Complications include adhesions around the ovary and reduced ovarian reserve.
- Assisted reproductive technology (ART) is sometimes used in this population, but it is less likely to lead to pregnancy and live birth, for unknown reasons.2,3 We inform obese women that a BMI below 30 is desirable before ART.
Overall, the management of infertility in obese women is extremely challenging because of its multiple causes—many of which are still not well understood. However, the profound implications of obesity for all aspects of reproduction make it imperative that we pay more attention to identification and treatment of obesity in the infertile population.
IVF is cited by Nobel Committee for its “benefit to mankind”
On December 10, 2010, Robert Edwards, PhD, was awarded the Nobel Prize in Physiology or Medicine for his innovative and pioneering work to create IVF. In presenting the award, the Committee noted that Professor Edwards’ work “represents a monumental medical advance that can truly be said to confer the greatest benefit to mankind.”
Professor Edwards is the embryologist who performed the basic science and laboratory work, along with Dr. Patrick Steptoe, who provided clinical care, which led to the birth of the world’s first IVF baby, Louise Brown, on July 25, 1978. Since then, IVF has become the most successful treatment for infertility and is available in more than 100 countries. The delivery rate for each single IVF attempt is about 25% globally; it more than doubles in selected patients who have a good prognosis. In some countries, almost 5% of all births arise from IVF; in the United States, that figure is about 1%. The International Committee Monitoring ART (ICMART) estimates that more than 4 million babies have been born from IVF around the world.
Prof. Robert Edwards (left) holds the infant Louise Brown
Early road was rocky
Despite the broad and significant success of IVF, Professor Edwards struggled for years against scientific and social opposition. His research was opposed by some on ethical and religious grounds, and the United Kingdom refused to fund some of his early work. The initial lack of support for IVF has transformed into acceptance as infertility has been recognized as a disease by many governments and the World Health Organization (WHO). In addition, the Centers for Disease Control and Prevention (CDC) has recognized infertility as a public health issue.
Nevertheless, most insurers still provide inadequate IVF coverage because of misperceptions about infertility and lack of recognition that it is a disease that globally affects 9% of all women of reproductive age, with male-partner sperm problems contributing to the problem in about 50% of cases.
IVF technologies achieve many goals
Over the past 32 years, IVF has revolutionized reproductive medicine and the treatment of infertility and brought an entirely new science to human reproduction. Specific IVF technologies that have changed the face of medicine include:
- intracytoplasmic sperm injection (ICSI) to treat male factor infertility
- cryopreservation or vitrification of sperm, eggs, and embryos to allow optimal results from IVF and to help cancer patients have babies after treatment
- preimplantation genetic diagnosis (PGD) to prevent major genetic diseases. (For more on PGD, see the January 2009 “Update on Prenatal Counseling” in our archive at www.obgmanagement.com.)
Much scientific research is now directed toward assessing the quality of embryos so that the live birth rate can be increased at the same time that multiple births are reduced. Advances in PGD and stem cell research show great promise for the future of human reproduction and the management of diseases of all organ systems.
The Nobel Committee’s recognition of Professor Robert Edwards’s extraordinary and visionary accomplishments marks the highest global acknowledgement of the efficacy, safety, and applicability of IVF, as well as its great promise for the future. All physicians should be aware of how this powerful technology can be used in caring for their patients.
Ovarian hyperstimulation can be tempered
through strategic management
Controlled ovarian stimulation is pharmacotherapy of the ovaries to produce more than one oocyte in non-ART cycles or to produce multiple oocytes for retrieval at follicular aspiration.7 Ovarian hyperstimulation syndrome (OHSS) is an iatrogenic, and potentially serious, complication of controlled ovarian stimulation. With vigilant management, however, its risks and sequelae can be reduced.
Best approach: Prevent OHSS
To reduce a woman’s risk of OHSS, identify her risk factors and employ the appropriate prevention strategies. The list of potential risk factors includes:
- age <33 years
- PCOS or its features
- high antral follicle count
- history of OHSS
- high basal anti-müllerian hormone level
- robust response to ovarian stimulation (≥18 follicles or estrogen level of 5,000 ng/dL, or both).
Once that patient’s risk is established, steps can be taken to judiciously manage her cycle and reduce the likelihood that she will develop OHSS.
Prevention strategies include:
- lowering the dosage of gonadotropin (consider a gonadotropin-releasing hormone [GnRH] antagonist protocol)
- coasting cycles until the estradiol level plateaus or decreases (reduce the dosage of human chorionic gonadotropin [hCG], use a GnRH agonist trigger for antagonist cycles, and avoid using hCG for luteal support)
- using an insulin-sensitizing agent such as metformin
- cryopreserving embryos for transfer at a later date (consider in vitro maturation instead of standard IVF [experimental]).8
Proposed clinical grading system for OHSS
| Criteria | How would OHSS be graded? | ||
|---|---|---|---|
| Mild | Moderate | Severe | |
| Objective findings | |||
| Fluid in pouch of Douglas | |||
| Fluid around uterus (major pelvis) | |||
| Fluid around intestinal loops | |||
| Hematocrit >45% | |||
| White blood cells >15,000/mm3 | ±* | ||
| Low urine output <600 mL/24 h | ±* | ||
| Creatinine >1.5 mg/dL | ±* | ± | |
| Elevated transaminases | ±* | ± | |
| Clotting disorder | ±† | ||
| Pleural effusion | ±† | ||
| Subjective findings | |||
| Abdominal distention | |||
| Pelvic discomfort | |||
| Breathing disorder | ±** | ±** | |
| Acute pain | ±** | ±** | ±** |
| Nausea and vomiting | ± | ± | ± |
| Ovarian enlargement | |||
| Pregnancy occurrence | ± | ± | |
| Note: ± indicates that the finding may or may not be present. | |||
| * If two of these are present, consider hospitalization | |||
| † If present, consider intensive care | |||
| ** If present, consider hospitalization | |||
| SOURCE: Humaidan P, et al.8 | |||
OHSS has usually been classified according to the signs and symptoms present.9 However, Humaidan and colleagues recently presented a new classification system for OHSS that is also based on objective vaginal US and laboratory parameters, as well as volume of fluid shifts (TABLE).8
The most highly effective strategies for reducing OHSS include use of a GnRh antagonist protocol and use of a GnRH agonist as a trigger. Other prevention strategies, such as metformin administration and cryopreservation of embryos, can further reduce the risk of severe OHSS. Although absolute prevention is impossible, surveillance for risk factors and careful clinical management by all physicians, including, when appropriate, referral to specialists, can reduce the incidence and severity of this dangerous complication.
We want to hear from you! Tell us what you think.
1. Chandra A, Martinez GM, Mosher WD, Abma JC, Jones J. Fertility, Family Planning, and Reproductive Health of US Women: Data from the 2002 National Survey of Family Growth. National Center for Health Statistics. Vital and Health Statistics. 2005;23:25. www.cdc.gov/nchs/data/series/sr_23/sr23_025.pdf. Published December 2005. Accessed January 5, 2011.
2. Practice Committee of the American Society for Reproductive Medicine. Obesity and reproduction: an educational bulletin. Fertil Steril. 2008;90(suppl 5):S21-29.
3. Lash MM, Armstrong A. Impact of obesity on women’s health. Fertil Steril. 2009;91(5):1712-1716.
4. Davies M. Symposium: Diet, nutrition and exercise in reproduction. Evidence for effects of weight on reproduction in women. Reprod BioMed Online. 2006;12(5):552-561.
5. Loret de Mola, JR. Obesity and its relationship to infertility in men and women. Obstet Gynecol Clin N Am. 2009;36(2):333-346.
6. Parihar M. Obesity and infertility. Rev Gynaecol Practic. 2003;3:120-126.
7. Zegers-Hochschild F, Adamson GD, de Mouzon J, et al. for ICMART and WHO. International Committee for Monitoring Assisted Reproductive Technology (ICMART) and the World Health Organization (WHO) revised glossary of ART technology, 2009. Fertil Steril. 2009;92(5):1520-1524.
8. Humaidan P, Quartarolo J, Papanikolaou E. Preventing ovarian hyperstimulation syndrome: guidance for the clinician. Fertil Steril. 2010;94(2):389-400.
9. Practice Committee of the American Society for Reproductive Medicine. Ovarian hyperstimulation syndrome. Fertil Steril. 2008;90(suppl 5):S188-193.
Impaired fertility is no small problem. According to the Centers for Disease Control and Prevention (CDC), it affects 7.3 million women 15 to 44 years old in the United States alone, or approximately 10% of the female population of reproductive age.1
Not long ago, there was little to be done about the problem. Today, however, we have many tools and tactics at our disposal, and another CDC statistic demonstrates their efficacy: Fewer than one third of women who have received medical intervention for impaired fertility in the past year continue to experience the problem.1
In this article, we highlight three recent developments in fertility:
- We know more about the effects of obesity on fecundity, and more about how to increase the likelihood of pregnancy and live birth in obese women
- The development of in vitro fertilization (IVF) more than 30 years ago represents a significant achievement and vast benefit to mankind, noted the Nobel Committee in awarding the 2010 prize for Physiology or Medicine to Robert Edwards, PhD
- Ovarian hyperstimulation syndrome after controlled ovarian stimulation cannot be avoided completely—but its likelihood can be reduced significantly through careful assessment of the patient and a cautious approach to ovarian stimulation.
Be mindful of the effects of obesity
on a woman’s reproductive function
Obese patients are almost three times as likely as women of normal weight to be infertile. Polycystic ovarian syndrome (PCOS) is generally unmasked or exacerbated, or both, by obesity, and the hyperandrogenicity associated with PCOS can cause ovulatory dysfunction. The hypothalamic-pituitary-ovarian (HPO) axis is also affected by overweight and obesity, resulting in oligo-ovulation in 30% to 47% of women.2 Some studies suggest that fecundity may be reduced in ovulatory obese women as well as those with ovulatory dysfunction.2 Most obese women are not infertile, however.
Once pregnancy is achieved, the risk of miscarriage is elevated in obese women (odds ratio [OR] ~1.67), and the live birth rate is lower (OR ~0.75), compared with women of normal weight.2–4 Obese women also have an elevated risk of miscarriage after egg donation (OR ~1.52) and ovulation induction (OR ~5.11). There is no evidence that the rate of miscarriage is increased after IVF, compared with other treatments.
The diagnosis of infertility is difficult in obese patients because the pelvic examination is less informative, although ultrasonography (US) is usually helpful.5 In addition, obesity can blur the distinction between PCOS and HPO axis-related oligo-ovulation. Laparoscopy and other diagnostic interventions are performed less frequently in obese women, and complications of diagnostic laparoscopy are higher in this population.3
Take the initiative in recommending weight loss
As health-care providers, we need to be more proactive in recommending lifestyle changes for obese women so that they lose weight before pregnancy. Women who have infertility are usually very motivated to conceive; as a result, they may also be motivated to lose weight. Caloric restriction, increased physical activity, behavioral modification, and professional expertise are all essential for successful weight loss.2 Even a reduction as small as 5% to 10% of body weight can have clinical benefit.2,4,5
Metformin is an additional option. When combined with a low-calorie diet, metformin may lead to weight loss, restore ovulation, and improve fecundity in women who have PCOS.2
Bariatric surgery is now commonly reserved for women whose body weight is 45 kg or more above normal. Bariatric surgery can improve the altered hormone profile, including elevated thyroid-stimulating hormone (TSH), of obese women. It also appears to improve fecundity and reduce pregnancy-associated complications. However, it is not always successful and can have complications of its own.
What can you offer to obese patients who experience infertility?
- Clomiphene citrate is the most commonly used ovarian-stimulation agent for oligo-ovulation that arises from PCOS or HPO-axis disruption; it is most effective in patients of normal weight.4,6 The protocols associated with clomiphene administration in obese patients are similar to those for women of normal weight; so are results, although the pregnancy rate is not as high in obese women.
- Gonadotropins are effective ovarian-stimulation drugs that are used in hypothalamic hypogonadal patients as well as after failed treatment with clomiphene citrate. Gonadotropins can be effective even in very obese patients; the dosage increases with body mass index (BMI).4,6
- Metformin reduces insulin resistance in women who have PCOS. By itself, metformin is ineffective at inducing ovulation and has not proved to increase the pregnancy rate when it is added to clomiphene.5 Nevertheless, it is commonly given at a daily dosage of 1,000 to 2,000 mg to women who have hyperinsulinemia, and it may reduce the miscarriage rate in women who have PCOS.
- Other medications that have been used to enhance ovulation in obese women include dexamethasone to reduce elevated androgen levels, bromocryptine for elevated prolactin levels, and thyroid hormone for hypothyroidism.

FOR WOMEN WHO HAVE PCOS
In laparoscopic ovarian drilling, an insulated needle unipolar electrode is inserted in the ovary perpendicular to the surface to create 6 to 12 evenly spaced punctures using 40 watts of coagulating current for 2 seconds at each point. The mechanism of action is unclear, but it is thought that the destruction of androgen-producing stroma is responsible for the reduction in testosterone level, increase in follicle-stimulating hormone (FSH), and return to FSH cyclicity in 80% of cases.
- Among obese women who have oligo-ovulation, ovarian drilling (FIGURE) is generally reserved for patients who have not responded to clomiphene or gonadotropins. This procedure has proved to be as effective as clomiphene administration, with the advantage that it does not increase the risk of multiple gestation and leads to longstanding improvement in one third of patients.5 Ovarian drilling is a modification of ovarian wedge resection for women who have PCOS. The mechanism of action is not clear, but it is thought that destruction of adrogen-producing stroma causes an immediate reduction in testosterone, an increase in follicle-stimulating hormone (FSH), and a return to FSH cyclicity in 80% of cases. These effects can persist for several years, and a pregnancy rate of approximately 60% can be attained in less than 6 months. Clomiphene-resistant women may be more responsive to the drug after ovarian drilling, and the risk of ovarian hyperstimulation appears to be reduced. Ovarian drilling is less effective in obese women than in women of normal weight. Complications include adhesions around the ovary and reduced ovarian reserve.
- Assisted reproductive technology (ART) is sometimes used in this population, but it is less likely to lead to pregnancy and live birth, for unknown reasons.2,3 We inform obese women that a BMI below 30 is desirable before ART.
Overall, the management of infertility in obese women is extremely challenging because of its multiple causes—many of which are still not well understood. However, the profound implications of obesity for all aspects of reproduction make it imperative that we pay more attention to identification and treatment of obesity in the infertile population.
IVF is cited by Nobel Committee for its “benefit to mankind”
On December 10, 2010, Robert Edwards, PhD, was awarded the Nobel Prize in Physiology or Medicine for his innovative and pioneering work to create IVF. In presenting the award, the Committee noted that Professor Edwards’ work “represents a monumental medical advance that can truly be said to confer the greatest benefit to mankind.”
Professor Edwards is the embryologist who performed the basic science and laboratory work, along with Dr. Patrick Steptoe, who provided clinical care, which led to the birth of the world’s first IVF baby, Louise Brown, on July 25, 1978. Since then, IVF has become the most successful treatment for infertility and is available in more than 100 countries. The delivery rate for each single IVF attempt is about 25% globally; it more than doubles in selected patients who have a good prognosis. In some countries, almost 5% of all births arise from IVF; in the United States, that figure is about 1%. The International Committee Monitoring ART (ICMART) estimates that more than 4 million babies have been born from IVF around the world.

Prof. Robert Edwards (left) holds the infant Louise Brown
Early road was rocky
Despite the broad and significant success of IVF, Professor Edwards struggled for years against scientific and social opposition. His research was opposed by some on ethical and religious grounds, and the United Kingdom refused to fund some of his early work. The initial lack of support for IVF has transformed into acceptance as infertility has been recognized as a disease by many governments and the World Health Organization (WHO). In addition, the Centers for Disease Control and Prevention (CDC) has recognized infertility as a public health issue.
Nevertheless, most insurers still provide inadequate IVF coverage because of misperceptions about infertility and lack of recognition that it is a disease that globally affects 9% of all women of reproductive age, with male-partner sperm problems contributing to the problem in about 50% of cases.
IVF technologies achieve many goals
Over the past 32 years, IVF has revolutionized reproductive medicine and the treatment of infertility and brought an entirely new science to human reproduction. Specific IVF technologies that have changed the face of medicine include:
- intracytoplasmic sperm injection (ICSI) to treat male factor infertility
- cryopreservation or vitrification of sperm, eggs, and embryos to allow optimal results from IVF and to help cancer patients have babies after treatment
- preimplantation genetic diagnosis (PGD) to prevent major genetic diseases. (For more on PGD, see the January 2009 “Update on Prenatal Counseling” in our archive at www.obgmanagement.com.)
Much scientific research is now directed toward assessing the quality of embryos so that the live birth rate can be increased at the same time that multiple births are reduced. Advances in PGD and stem cell research show great promise for the future of human reproduction and the management of diseases of all organ systems.
The Nobel Committee’s recognition of Professor Robert Edwards’s extraordinary and visionary accomplishments marks the highest global acknowledgement of the efficacy, safety, and applicability of IVF, as well as its great promise for the future. All physicians should be aware of how this powerful technology can be used in caring for their patients.
Ovarian hyperstimulation can be tempered
through strategic management
Controlled ovarian stimulation is pharmacotherapy of the ovaries to produce more than one oocyte in non-ART cycles or to produce multiple oocytes for retrieval at follicular aspiration.7 Ovarian hyperstimulation syndrome (OHSS) is an iatrogenic, and potentially serious, complication of controlled ovarian stimulation. With vigilant management, however, its risks and sequelae can be reduced.
Best approach: Prevent OHSS
To reduce a woman’s risk of OHSS, identify her risk factors and employ the appropriate prevention strategies. The list of potential risk factors includes:
- age <33 years
- PCOS or its features
- high antral follicle count
- history of OHSS
- high basal anti-müllerian hormone level
- robust response to ovarian stimulation (≥18 follicles or estrogen level of 5,000 ng/dL, or both).
Once that patient’s risk is established, steps can be taken to judiciously manage her cycle and reduce the likelihood that she will develop OHSS.
Prevention strategies include:
- lowering the dosage of gonadotropin (consider a gonadotropin-releasing hormone [GnRH] antagonist protocol)
- coasting cycles until the estradiol level plateaus or decreases (reduce the dosage of human chorionic gonadotropin [hCG], use a GnRH agonist trigger for antagonist cycles, and avoid using hCG for luteal support)
- using an insulin-sensitizing agent such as metformin
- cryopreserving embryos for transfer at a later date (consider in vitro maturation instead of standard IVF [experimental]).8
Proposed clinical grading system for OHSS
| Criteria | How would OHSS be graded? | ||
|---|---|---|---|
| Mild | Moderate | Severe | |
| Objective findings | |||
| Fluid in pouch of Douglas | |||
| Fluid around uterus (major pelvis) | |||
| Fluid around intestinal loops | |||
| Hematocrit >45% | * | ||
| White blood cells >15,000/mm3 | ±* | ||
| Low urine output <600 mL/24 h | ±* | ||
| Creatinine >1.5 mg/dL | ±* | ± | |
| Elevated transaminases | ±* | ± | |
| Clotting disorder | ±† | ||
| Pleural effusion | ±† | ||
| Subjective findings | |||
| Abdominal distention | |||
| Pelvic discomfort | |||
| Breathing disorder | ±** | ±** | |
| Acute pain | ±** | ±** | ±** |
| Nausea and vomiting | ± | ± | ± |
| Ovarian enlargement | |||
| Pregnancy occurrence | ± | ± | |
| Note: ± indicates that the finding may or may not be present. | |||
| * If two of these are present, consider hospitalization | |||
| † If present, consider intensive care | |||
| ** If present, consider hospitalization | |||
| SOURCE: Humaidan P, et al.8 | |||
OHSS has usually been classified according to the signs and symptoms present.9 However, Humaidan and colleagues recently presented a new classification system for OHSS that is also based on objective vaginal US and laboratory parameters, as well as volume of fluid shifts (TABLE).8
The most highly effective strategies for reducing OHSS include use of a GnRh antagonist protocol and use of a GnRH agonist as a trigger. Other prevention strategies, such as metformin administration and cryopreservation of embryos, can further reduce the risk of severe OHSS. Although absolute prevention is impossible, surveillance for risk factors and careful clinical management by all physicians, including, when appropriate, referral to specialists, can reduce the incidence and severity of this dangerous complication.
We want to hear from you! Tell us what you think.
Impaired fertility is no small problem. According to the Centers for Disease Control and Prevention (CDC), it affects 7.3 million women 15 to 44 years old in the United States alone, or approximately 10% of the female population of reproductive age.1
Not long ago, there was little to be done about the problem. Today, however, we have many tools and tactics at our disposal, and another CDC statistic demonstrates their efficacy: Fewer than one third of women who have received medical intervention for impaired fertility in the past year continue to experience the problem.1
In this article, we highlight three recent developments in fertility:
- We know more about the effects of obesity on fecundity, and more about how to increase the likelihood of pregnancy and live birth in obese women
- The development of in vitro fertilization (IVF) more than 30 years ago represents a significant achievement and vast benefit to mankind, noted the Nobel Committee in awarding the 2010 prize for Physiology or Medicine to Robert Edwards, PhD
- Ovarian hyperstimulation syndrome after controlled ovarian stimulation cannot be avoided completely—but its likelihood can be reduced significantly through careful assessment of the patient and a cautious approach to ovarian stimulation.
Be mindful of the effects of obesity
on a woman’s reproductive function
Obese patients are almost three times as likely as women of normal weight to be infertile. Polycystic ovarian syndrome (PCOS) is generally unmasked or exacerbated, or both, by obesity, and the hyperandrogenicity associated with PCOS can cause ovulatory dysfunction. The hypothalamic-pituitary-ovarian (HPO) axis is also affected by overweight and obesity, resulting in oligo-ovulation in 30% to 47% of women.2 Some studies suggest that fecundity may be reduced in ovulatory obese women as well as those with ovulatory dysfunction.2 Most obese women are not infertile, however.
Once pregnancy is achieved, the risk of miscarriage is elevated in obese women (odds ratio [OR] ~1.67), and the live birth rate is lower (OR ~0.75), compared with women of normal weight.2–4 Obese women also have an elevated risk of miscarriage after egg donation (OR ~1.52) and ovulation induction (OR ~5.11). There is no evidence that the rate of miscarriage is increased after IVF, compared with other treatments.
The diagnosis of infertility is difficult in obese patients because the pelvic examination is less informative, although ultrasonography (US) is usually helpful.5 In addition, obesity can blur the distinction between PCOS and HPO axis-related oligo-ovulation. Laparoscopy and other diagnostic interventions are performed less frequently in obese women, and complications of diagnostic laparoscopy are higher in this population.3
Take the initiative in recommending weight loss
As health-care providers, we need to be more proactive in recommending lifestyle changes for obese women so that they lose weight before pregnancy. Women who have infertility are usually very motivated to conceive; as a result, they may also be motivated to lose weight. Caloric restriction, increased physical activity, behavioral modification, and professional expertise are all essential for successful weight loss.2 Even a reduction as small as 5% to 10% of body weight can have clinical benefit.2,4,5
Metformin is an additional option. When combined with a low-calorie diet, metformin may lead to weight loss, restore ovulation, and improve fecundity in women who have PCOS.2
Bariatric surgery is now commonly reserved for women whose body weight is 45 kg or more above normal. Bariatric surgery can improve the altered hormone profile, including elevated thyroid-stimulating hormone (TSH), of obese women. It also appears to improve fecundity and reduce pregnancy-associated complications. However, it is not always successful and can have complications of its own.
What can you offer to obese patients who experience infertility?
- Clomiphene citrate is the most commonly used ovarian-stimulation agent for oligo-ovulation that arises from PCOS or HPO-axis disruption; it is most effective in patients of normal weight.4,6 The protocols associated with clomiphene administration in obese patients are similar to those for women of normal weight; so are results, although the pregnancy rate is not as high in obese women.
- Gonadotropins are effective ovarian-stimulation drugs that are used in hypothalamic hypogonadal patients as well as after failed treatment with clomiphene citrate. Gonadotropins can be effective even in very obese patients; the dosage increases with body mass index (BMI).4,6
- Metformin reduces insulin resistance in women who have PCOS. By itself, metformin is ineffective at inducing ovulation and has not proved to increase the pregnancy rate when it is added to clomiphene.5 Nevertheless, it is commonly given at a daily dosage of 1,000 to 2,000 mg to women who have hyperinsulinemia, and it may reduce the miscarriage rate in women who have PCOS.
- Other medications that have been used to enhance ovulation in obese women include dexamethasone to reduce elevated androgen levels, bromocryptine for elevated prolactin levels, and thyroid hormone for hypothyroidism.

FOR WOMEN WHO HAVE PCOS
In laparoscopic ovarian drilling, an insulated needle unipolar electrode is inserted in the ovary perpendicular to the surface to create 6 to 12 evenly spaced punctures using 40 watts of coagulating current for 2 seconds at each point. The mechanism of action is unclear, but it is thought that the destruction of androgen-producing stroma is responsible for the reduction in testosterone level, increase in follicle-stimulating hormone (FSH), and return to FSH cyclicity in 80% of cases.
- Among obese women who have oligo-ovulation, ovarian drilling (FIGURE) is generally reserved for patients who have not responded to clomiphene or gonadotropins. This procedure has proved to be as effective as clomiphene administration, with the advantage that it does not increase the risk of multiple gestation and leads to longstanding improvement in one third of patients.5 Ovarian drilling is a modification of ovarian wedge resection for women who have PCOS. The mechanism of action is not clear, but it is thought that destruction of adrogen-producing stroma causes an immediate reduction in testosterone, an increase in follicle-stimulating hormone (FSH), and a return to FSH cyclicity in 80% of cases. These effects can persist for several years, and a pregnancy rate of approximately 60% can be attained in less than 6 months. Clomiphene-resistant women may be more responsive to the drug after ovarian drilling, and the risk of ovarian hyperstimulation appears to be reduced. Ovarian drilling is less effective in obese women than in women of normal weight. Complications include adhesions around the ovary and reduced ovarian reserve.
- Assisted reproductive technology (ART) is sometimes used in this population, but it is less likely to lead to pregnancy and live birth, for unknown reasons.2,3 We inform obese women that a BMI below 30 is desirable before ART.
Overall, the management of infertility in obese women is extremely challenging because of its multiple causes—many of which are still not well understood. However, the profound implications of obesity for all aspects of reproduction make it imperative that we pay more attention to identification and treatment of obesity in the infertile population.
IVF is cited by Nobel Committee for its “benefit to mankind”
On December 10, 2010, Robert Edwards, PhD, was awarded the Nobel Prize in Physiology or Medicine for his innovative and pioneering work to create IVF. In presenting the award, the Committee noted that Professor Edwards’ work “represents a monumental medical advance that can truly be said to confer the greatest benefit to mankind.”
Professor Edwards is the embryologist who performed the basic science and laboratory work, along with Dr. Patrick Steptoe, who provided clinical care, which led to the birth of the world’s first IVF baby, Louise Brown, on July 25, 1978. Since then, IVF has become the most successful treatment for infertility and is available in more than 100 countries. The delivery rate for each single IVF attempt is about 25% globally; it more than doubles in selected patients who have a good prognosis. In some countries, almost 5% of all births arise from IVF; in the United States, that figure is about 1%. The International Committee Monitoring ART (ICMART) estimates that more than 4 million babies have been born from IVF around the world.

Prof. Robert Edwards (left) holds the infant Louise Brown
Early road was rocky
Despite the broad and significant success of IVF, Professor Edwards struggled for years against scientific and social opposition. His research was opposed by some on ethical and religious grounds, and the United Kingdom refused to fund some of his early work. The initial lack of support for IVF has transformed into acceptance as infertility has been recognized as a disease by many governments and the World Health Organization (WHO). In addition, the Centers for Disease Control and Prevention (CDC) has recognized infertility as a public health issue.
Nevertheless, most insurers still provide inadequate IVF coverage because of misperceptions about infertility and lack of recognition that it is a disease that globally affects 9% of all women of reproductive age, with male-partner sperm problems contributing to the problem in about 50% of cases.
IVF technologies achieve many goals
Over the past 32 years, IVF has revolutionized reproductive medicine and the treatment of infertility and brought an entirely new science to human reproduction. Specific IVF technologies that have changed the face of medicine include:
- intracytoplasmic sperm injection (ICSI) to treat male factor infertility
- cryopreservation or vitrification of sperm, eggs, and embryos to allow optimal results from IVF and to help cancer patients have babies after treatment
- preimplantation genetic diagnosis (PGD) to prevent major genetic diseases. (For more on PGD, see the January 2009 “Update on Prenatal Counseling” in our archive at www.obgmanagement.com.)
Much scientific research is now directed toward assessing the quality of embryos so that the live birth rate can be increased at the same time that multiple births are reduced. Advances in PGD and stem cell research show great promise for the future of human reproduction and the management of diseases of all organ systems.
The Nobel Committee’s recognition of Professor Robert Edwards’s extraordinary and visionary accomplishments marks the highest global acknowledgement of the efficacy, safety, and applicability of IVF, as well as its great promise for the future. All physicians should be aware of how this powerful technology can be used in caring for their patients.
Ovarian hyperstimulation can be tempered
through strategic management
Controlled ovarian stimulation is pharmacotherapy of the ovaries to produce more than one oocyte in non-ART cycles or to produce multiple oocytes for retrieval at follicular aspiration.7 Ovarian hyperstimulation syndrome (OHSS) is an iatrogenic, and potentially serious, complication of controlled ovarian stimulation. With vigilant management, however, its risks and sequelae can be reduced.
Best approach: Prevent OHSS
To reduce a woman’s risk of OHSS, identify her risk factors and employ the appropriate prevention strategies. The list of potential risk factors includes:
- age <33 years
- PCOS or its features
- high antral follicle count
- history of OHSS
- high basal anti-müllerian hormone level
- robust response to ovarian stimulation (≥18 follicles or estrogen level of 5,000 ng/dL, or both).
Once that patient’s risk is established, steps can be taken to judiciously manage her cycle and reduce the likelihood that she will develop OHSS.
Prevention strategies include:
- lowering the dosage of gonadotropin (consider a gonadotropin-releasing hormone [GnRH] antagonist protocol)
- coasting cycles until the estradiol level plateaus or decreases (reduce the dosage of human chorionic gonadotropin [hCG], use a GnRH agonist trigger for antagonist cycles, and avoid using hCG for luteal support)
- using an insulin-sensitizing agent such as metformin
- cryopreserving embryos for transfer at a later date (consider in vitro maturation instead of standard IVF [experimental]).8
Proposed clinical grading system for OHSS
| Criteria | How would OHSS be graded? | ||
|---|---|---|---|
| Mild | Moderate | Severe | |
| Objective findings | |||
| Fluid in pouch of Douglas | |||
| Fluid around uterus (major pelvis) | |||
| Fluid around intestinal loops | |||
| Hematocrit >45% | * | ||
| White blood cells >15,000/mm3 | ±* | ||
| Low urine output <600 mL/24 h | ±* | ||
| Creatinine >1.5 mg/dL | ±* | ± | |
| Elevated transaminases | ±* | ± | |
| Clotting disorder | ±† | ||
| Pleural effusion | ±† | ||
| Subjective findings | |||
| Abdominal distention | |||
| Pelvic discomfort | |||
| Breathing disorder | ±** | ±** | |
| Acute pain | ±** | ±** | ±** |
| Nausea and vomiting | ± | ± | ± |
| Ovarian enlargement | |||
| Pregnancy occurrence | ± | ± | |
| Note: ± indicates that the finding may or may not be present. | |||
| * If two of these are present, consider hospitalization | |||
| † If present, consider intensive care | |||
| ** If present, consider hospitalization | |||
| SOURCE: Humaidan P, et al.8 | |||
OHSS has usually been classified according to the signs and symptoms present.9 However, Humaidan and colleagues recently presented a new classification system for OHSS that is also based on objective vaginal US and laboratory parameters, as well as volume of fluid shifts (TABLE).8
The most highly effective strategies for reducing OHSS include use of a GnRh antagonist protocol and use of a GnRH agonist as a trigger. Other prevention strategies, such as metformin administration and cryopreservation of embryos, can further reduce the risk of severe OHSS. Although absolute prevention is impossible, surveillance for risk factors and careful clinical management by all physicians, including, when appropriate, referral to specialists, can reduce the incidence and severity of this dangerous complication.
We want to hear from you! Tell us what you think.
1. Chandra A, Martinez GM, Mosher WD, Abma JC, Jones J. Fertility, Family Planning, and Reproductive Health of US Women: Data from the 2002 National Survey of Family Growth. National Center for Health Statistics. Vital and Health Statistics. 2005;23:25. www.cdc.gov/nchs/data/series/sr_23/sr23_025.pdf. Published December 2005. Accessed January 5, 2011.
2. Practice Committee of the American Society for Reproductive Medicine. Obesity and reproduction: an educational bulletin. Fertil Steril. 2008;90(suppl 5):S21-29.
3. Lash MM, Armstrong A. Impact of obesity on women’s health. Fertil Steril. 2009;91(5):1712-1716.
4. Davies M. Symposium: Diet, nutrition and exercise in reproduction. Evidence for effects of weight on reproduction in women. Reprod BioMed Online. 2006;12(5):552-561.
5. Loret de Mola, JR. Obesity and its relationship to infertility in men and women. Obstet Gynecol Clin N Am. 2009;36(2):333-346.
6. Parihar M. Obesity and infertility. Rev Gynaecol Practic. 2003;3:120-126.
7. Zegers-Hochschild F, Adamson GD, de Mouzon J, et al. for ICMART and WHO. International Committee for Monitoring Assisted Reproductive Technology (ICMART) and the World Health Organization (WHO) revised glossary of ART technology, 2009. Fertil Steril. 2009;92(5):1520-1524.
8. Humaidan P, Quartarolo J, Papanikolaou E. Preventing ovarian hyperstimulation syndrome: guidance for the clinician. Fertil Steril. 2010;94(2):389-400.
9. Practice Committee of the American Society for Reproductive Medicine. Ovarian hyperstimulation syndrome. Fertil Steril. 2008;90(suppl 5):S188-193.
1. Chandra A, Martinez GM, Mosher WD, Abma JC, Jones J. Fertility, Family Planning, and Reproductive Health of US Women: Data from the 2002 National Survey of Family Growth. National Center for Health Statistics. Vital and Health Statistics. 2005;23:25. www.cdc.gov/nchs/data/series/sr_23/sr23_025.pdf. Published December 2005. Accessed January 5, 2011.
2. Practice Committee of the American Society for Reproductive Medicine. Obesity and reproduction: an educational bulletin. Fertil Steril. 2008;90(suppl 5):S21-29.
3. Lash MM, Armstrong A. Impact of obesity on women’s health. Fertil Steril. 2009;91(5):1712-1716.
4. Davies M. Symposium: Diet, nutrition and exercise in reproduction. Evidence for effects of weight on reproduction in women. Reprod BioMed Online. 2006;12(5):552-561.
5. Loret de Mola, JR. Obesity and its relationship to infertility in men and women. Obstet Gynecol Clin N Am. 2009;36(2):333-346.
6. Parihar M. Obesity and infertility. Rev Gynaecol Practic. 2003;3:120-126.
7. Zegers-Hochschild F, Adamson GD, de Mouzon J, et al. for ICMART and WHO. International Committee for Monitoring Assisted Reproductive Technology (ICMART) and the World Health Organization (WHO) revised glossary of ART technology, 2009. Fertil Steril. 2009;92(5):1520-1524.
8. Humaidan P, Quartarolo J, Papanikolaou E. Preventing ovarian hyperstimulation syndrome: guidance for the clinician. Fertil Steril. 2010;94(2):389-400.
9. Practice Committee of the American Society for Reproductive Medicine. Ovarian hyperstimulation syndrome. Fertil Steril. 2008;90(suppl 5):S188-193.