User login
The telltale heart: What HDL reveals about a woman’s risk
JUDY’S CASE
“WHAT’S MY RISK?”
At her periodic gynecologic exam, Judy, a 58-year-old white woman, expresses concern about her heart health in light of her 62-year-old sister’s recent heart attack. Further questioning reveals that her father died of a heart attack at age 56, and her brother, 52, has high blood pressure and adult-onset diabetes.
Judy has not experienced any symptoms of cardiovascular disease, such as chest pain or tightness with exertion, and she doesn’t smoke. The last time her cholesterol was checked was about 15 years earlier, at which time it was “OK.” She admits she is not very physically active and has gained about 30 lb over the past 10 years. Her vital signs and physical exam are entirely normal except for borderline high blood pressure at 140/90 mm Hg, weight of 170 lb (height: 68 inches), and a waist circumference of 37 inches.
What is her cardiovascular risk?
Judy has physical findings suggestive of metabolic syndrome in addition to the significant family history of cardiovascular disease (CVD). Although her current risk of a cardiac event appears to be low, she is likely to deteriorate if the hypertension and excess body weight are not addressed.
To predict her level of risk more precisely, the physician asks Judy to return in 2 weeks for a blood pressure recheck, and instructs her to perform additional readings on her own. The clinician also refers Judy for a fasting lipid panel and blood glucose level.
In women, CVD is especially lethal
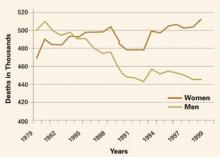
Contrary to earlier assumptions, CVD now appears to be more lethal in women than in men and warrants aggressive efforts to prevent it and manage risk factors (FIGURE). This article focuses on improving high-density lipoprotein (HDL) cholesterol, a key factor in women at risk.
This approach differs slightly from the National Cholesterol Education Program Adult Treatment Panel III (NCEP/ATP III) guidelines, which target low-density lipoprotein (LDL) cholesterol as the primary lipid risk factor for intervention.1
FIGURE Women surpass men in CVD deaths
Over the past 25 years, the trend in deaths attributable to cardiovascular disease has been upward for women, while it is shifting downward for men.
Why focus on HDL?
Improvement in HDL is the strongest predictor of reduced risk for cardiac events. So says an analysis of 17 major lipid intervention trials between 1987 and 2003 (n = 44,170).2
Another study3 demonstrated that HDL cholesterol is the stronger predictor of CVD risk in women. In addition, several large observational studies have shown that increasing HDL cholesterol by 1 mg/dL in men reduces CVD risk by 2%, whereas increasing HDL in women by 1 mg/dL decreases CVD risk by 3%.4
Higher HDL levels may explain later onset of CVD in women. The advantage that women have over men in CVD risk prior to menopause seems to be at least partly due to their higher levels of HDL cholesterol: The average HDL level in women is 56 mg/dL, versus 46 mg/dL in men. This advantage begins to disappear after menopause, presumably because of changes in hormone levels and decreasing HDL-2 cholesterol, the most cardioprotective HDL fraction.
Other reasons to focus on HDL. In addition to the increased CVD risk associated with menopause, women of all ages in the United States have an increasing tendency—at almost epidemic rates—toward obesity and metabolic syndrome, which are known CVD risk factors.5 The dyslipidemia seen with those conditions is characterized by low HDL cholesterol and high triglycerides. Thus, maintaining healthy HDL levels is clearly an important goal for overall cardiovascular health, especially in women.
In addition, HDL is the key lipoprotein associated with reverse cholesterol transport, the process of carrying excess cholesterol from peripheral tissues to the liver for catabolism.
JUDY’S CASE
METABOLIC SYNDROME CONFIRMED
At her return visit, Judy’s blood pressure remains 140/90 mm Hg, with outside readings ranging from 135 to 145 mm Hg systolic and 85 to 95 mm Hg diastolic. Her laboratory tests reveal fasting blood glucose of 115 mg/dL, total cholesterol of 220 mg/dL, LDL cholesterol of 120 mg/dL, HDL cholesterol of 35 mg/dL, and triglycerides of 300 mg/dL.
What are her key heart-health issues?
Individualized management—recommended by NCEP/ATP III guidelines— should focus on reversing the metabolic defects of insulin resistance: hypertension, obesity, glucose intolerance, and dyslipidemia.
At present, Judy’s 10-year risk of a cardiac event is 4% by Framingham risk scoring, but it is likely to increase if the metabolic syndrome is not addressed.
How to preserve healthy HDL levels
Diet and regular aerobic exercise are recommended by the NCEP/ATP III guidelines, especially for obese patients with metabolic syndrome. However, dietary changes do not raise HDL cholesterol. In fact, dieting to lose weight will almost certainly lower HDL levels. That’s because people who eat a diet very low in fat tend to replace the fat calories with carbohydrates, and a low-fat, high-carbohydrate diet will reduce all cholesterol fractions—but HDL cholesterol will decrease disproportionately.6
Recommend complex carbohydrates and moderate fat intake
To avoid excessive reduction in HDL cholesterol, any weight-loss diet should involve moderate fat intake (35% of total calories) and emphasize monounsaturated fats such as olive or canola oil, along with complex carbohydrates found in fruits, vegetables, and whole grains.7 After weight loss is stabilized, HDL cholesterol tends to increase somewhat, but may not return all the way to baseline.
New guidelines on how to intervene
Most women who die suddenly from coronary heart disease had no previous symptoms, and that makes prevention all the more important.
Though it hasn’t been long since the first woman-centered recommendations on preventing cardiovascular disease,19 our understanding of preventive interventions has improved enormously. Thus, the American Heart Association convened a new expert panel to review and, where necessary, revise the original guidelines.20 Here are some of the highlights.
Forget “haves” and “have-nots”
Lori Mosca and the other members of the expert panel observe that “the concept of CVD as a categorical, ‘have-or-have-not’ condition has been replaced with a growing appreciation for the existence of a continuum of CVD risk.”20
For example, the low-risk category (<10% risk of a coronary heart disease event in the next 10 years, according to the Framingham Risk Score for women) would include women with optimal levels of risk factors and a heart-healthy lifestyle, as well as women with metabolic syndrome but no other risk factors. In contrast, women with established coronary heart disease, diabetes mellitus, or chronic kidney disease would fall into the high-risk group (>20% risk). Those with multiple risk factors or markedly elevated levels of a single risk factor fall somewhere in between.
The 5 lifestyle laws
Women are advised to consistently:
- eat plenty of fruits and vegetables, whole grains, and low-fat or nonfat dairy items
- avoid smoking and smokeless tobacco
- perform at least 30 minutes of moderate-intensity activity, such as brisk walking, most days
- achieve and maintain a body mass index between 18.5 and 25.9 kg/m2 and a waist circumference less than 35 inches
- consider omega-3 fatty acid supplementation, especially if risk is high. The guidelines extol the benefits of fish, which contain omega-3 fatty acids, but warn against consumption of fish likely to contain unsafe levels of mercury (shark, sword-fish, king mackerel, and tilefish) by gravidas and women of reproductive age.
BP and cholesterol control
- Blood pressure. Maintain at or below 120/80 mm Hg through lifestyle approaches, or drug therapy when BP is 140/90 mm Hg or higher.
- Dietary fat and cholesterol. Keep saturated fat intake to less than 7% of calories, and cholesterol below 200 mg/dL with high risk or elevated LDL.
- LDL-cholesterol. Use drug therapy (preferably statins) if risk is high and LDL is below 100 mg/dL. Add lifestyle adjustments if risk is high and LDL cholesterol is at or above 100 mg/dL.
What not to take to prevent CVD
The guidelines warn against prescribing—or continuing—estrogen-progestin therapy to prevent CVD. Nor should other forms of menopausal hormone therapy, such as unopposed estrogen, be prescribed or continued unless new findings indicate a beneficial role.
Also discouraged are antioxidant vitamin supplements to prevent CVD.
Look for depression
Especially women with CVD should be referred or treated when depression is found, as it can hamper a woman’s efforts at prevention.
The full text of the guidelines can be viewed at
http://circ.ahajournals.org/cgi/content/full/109/5/672.
Regular exercise raises HDL
One way to avoid an overall reduction in HDL levels with weight loss is to prescribe exercise in conjunction with it. Regular exercise is an excellent way to raise HDL cholesterol levels. Studies suggest that, for women, the volume of exercise is more important than intensity. Thus, women should strive to walk 7 to 14 miles per week or otherwise expend approximately 1,200 to 1,600 Kcal.8,9
Regular exercise is recommended as one of the NCEP/ATP III therapeutic lifestyle changes that directly benefit cardiovascular health and blood pressure and help the individual maintain optimal body weight. Although exercise raises HDL levels, it does so more easily in men than in women. HDL cholesterol increases in men with both intensity and volume of exercise.10 Women appear to derive slightly less of a benefit, perhaps because their HDL levels are already generally higher.
In women, studies have reported an increase in HDL cholesterol as high as 15% for both premenopausal and post-menopausal women who follow moderate exercise regimens—and the lower the baseline HDL level, the greater the increase.8
Exercise level was inversely associated with cardiovascular risk in the Women’s Health Initiative,9 which followed a cohort of 73,743 women prospectively for an average of 3.2 years. Women at the highest quintile of exercise level reduced their CVD risk by 53% over the lowest quintile, and moderate activity such as walking led to risk reductions similar to those involving more vigorous activities.
Recommend alcohol—or not?
Moderate alcohol consumption—1 to 3 drinks per day, or 15 to 45 g ethanol— improves lipoprotein levels and reduces CVD risk. The flavonoids in red wines also reduce platelet aggregation and clotting tendencies. However, advising a patient to consume alcohol to reduce her CVD risk is controversial; many physicians are reluctant to do so due to obvious concerns over toxicity and dependency.
Response to alcohol may vary by menopausal status. In 1 study,11 pre-menopausal women decreased their LDL cholesterol by 12% (P=.01) after 3 glasses of wine daily for 3 weeks, but there was no significant increase in HDL levels.
In the same study, postmenopausal women increased HDL levels by 12% (P=.03), but their LDL cholesterol decreased only minimally.11
Another study12 in postmenopausal women who consumed 0, 1, or 2 drinks daily (0, 15, or 30 g ethanol) for 8 weeks showed a significant decrease of 4% in LDL cholesterol at 1 drink (15 g), but no significant further decrease at 2 drinks (30 g). In the same study, HDL increased non-significantly at 1 drink (15 g), but increased significantly (5%) at 2 drinks (30 g). There may be a threshold of 30 g ethanol daily for postmenopausal women to benefit significantly from alcohol consumption.
JUDY’S CASE
LIFESTYLE RECOMMENDATIONS
The foundation of Judy’s management should be the triad of weight loss, exercise, and a diet that limits refined carbohydrates and emphasizes fiber, fruits and vegetables, whole grains, and healthy fats (ie, olive, canola, and fish oils).
In prescribing exercise, emphasize quantity over intensity, such as 14 miles of walking weekly or 60 to 90 minutes daily of aerobic activity.
Since she has no history of cardiac symptoms, and low-intensity, high-quantity exercise is advised, she does not need a screening cardiac stress test before beginning her regimen.
Pantethine
Pantethine is an over-the-counter (OTC) nutritional supplement that combines the B vitamin pantothenic acid with the amino acid cysteamine. Although pantothenic acid alone has no lipid-lowering effects, pantethine does: It lowers LDL cholesterol, very-low-density lipoprotein (VLDL) cholesterol, and triglycerides, and raises HDL levels. The exact mechanism of action is unknown, but it is thought to involve primarily the effects of cysteamine on lipid metabolism in the liver.
Recommended dose. At 600 to 900 mg daily, the typical response is an increase of up to 18% in HDL cholesterol and reductions in LDL cholesterol, VLDL cholesterol, and triglycerides of 13%, 20%, and 26%, respectively.13
In a recent US study, pantethine significantly raised HDL-2 cholesterol, the more cardioprotective fraction of HDL, and increased the particle size of LDL cholesterol, rendering it less atherogenic.14
Pantethine also reduces visceral fat. The same study14 reported a significant reduction in visceral fat, a component of metabolic syndrome, as measured by computerized dual-energy x-ray absorptiometry in individuals taking 900 mg daily for 6 weeks. This reduction appears to be primarily an effect of pantothenic acid and had been demonstrated in animal studies.
No side effects. Pantethine is exceptionally well tolerated, with virtually no side effects. An occasional person will experience gastrointestinal upset and diarrhea, which resolve when the drug is discontinued.
Recommend formulations that contain Pantesin (Daiichi Fine Chemicals, New York City), a pharmaceutical grade of pantethine, because extensive research confirms the benefits I have described. Otherwise, make sure the manufacturer is a reputable company, with scientific studies to support its claims of safety, efficacy, and bioavailability.
Nicotinic acid
Nicotinic acid (NA), another B vitamin available OTC as a nutritional supplement, is the most effective agent for raising HDL levels.
Recommended dose. A divided dose of 3,000 mg daily of the immediate-release form of NA can raise HDL levels as much as 30%. Unfortunately, that form of NA has a high incidence of adverse effects, such as flushing, itching, and GI upset.15
Sustained-release NA is generally well tolerated, but somewhat less effective at raising HDL. A lower dose of sustained-release NA (1,000 to 1,500 mg daily) is recommended to avoid liver toxicity and typically leads to HDL increases as high as 20%.15
NA offers additional benefits to the lipid profile, typically lowering LDL cholesterol by 20%, triglycerides by 15%, and lipoprotein(a) by 15%.15
Monitor all forms of NA with liver enzymes, blood glucose, and uric acid because treatment can elevate these markers.
Give NA preparations extra scrutiny. The general caveats about quality and bioavailability apply especially to NA. Nonprescription products on the market that contain nicotinamide and inositol hexanicotinate are advertised as “no-flush” niacin because they do not cause cutaneous side effects, but they also have no lipid benefits.
Cost. NA is relatively inexpensive, and good-quality sustained-release products such as Endur-acin (Endurance Products, Tigard, Ore) can be purchased OTC for 12 cents per 500-mg tablet or a daily cost of 24 to 36 cents.16
Sustained-release NA is also available by prescription (Niaspan, Kos Pharmaceuticals, Cranbury, NJ), but it is considerably more expensive ($1.98 per 500-mg tablet, or $3.96 to $5.94 daily) and has not been shown to be more effective or better tolerated than the better OTC products.16
JUDY’S CASE
RAISING HDL WITHOUT DRUGS
Judy’s lipid abnormalities would benefit from a supplement of pantethine (300 mg thrice daily) or NA (1,000 mg thrice daily of plain/quick release or 500 mg twice daily of sustained release to reduce flushing side effects).
Her greatest risk with her current lipid levels is low HDL, and the best agent to raise these levels is NA.
Pantethine also raises HDL and selectively improves the HDL-2 subfraction, which is most cardioprotective. Both supplements also improve the other lipid abnormalities.
In addition, referral to a dietician for detailed instruction in food choices and preparation may be useful.
Pharmacologic therapies
Hormone replacement therapy (HRT) is no longer recommended to prevent CVD and should be given only for menopausal symptoms during the perimenopausal years—despite the fact that estrogen has been shown to raise HDL cholesterol by 8% in postmenopausal women.17
Estrogens increase HDL by increasing production of apoA-1, the main lipoprotein in HDL, and by decreasing HDL catabolism by inhibiting hepatic lipase. The 17-hydroxyprogesterone component of HRT given to women with an intact uterus to prevent endometrial cancer has no significant lipid effects.
Paradoxically, the increased CVD risk found in the HRT arm of the Women’s Health Initiative17 was in the face of improved HDL (8%) and LDL (–14%) cholesterol and appeared to be the result of increased thrombotic events.
Further analysis of Women’s Health Initiative data showed that women who were still within 10 years of menopause actually did experience a reduction in CVD risk. That observation, along with some primate research on HRT, suggests that the risk or benefit of HRT with respect to CVD may be a matter of timing. See “Emerging hypothesis may explain estrogen paradox”
At the 2004 American Heart Association meeting, Dr. Thomas Clarkson, who has conducted primate research on hormone replacement therapy (HRT) and the risk of cardiovascular disease (CVD), described the emerging hypothesis that may explain the HRT paradox of increased CVD risk despite evidence of benefit in observational studies.21
Estrogen is a potent upregulator of matrix metal-loproteases. These proteolytic enzymes are a normal constituent of the endometrium and important in its remodeling with each menstrual cycle. Matrix metal-loproteases are also found in the fibrous cap that seals off the necrotic core of an advanced atheroma from the vascular lumen. Most perimenopausal women have enjoyed estrogen’s protective effect throughout their reproductive years and thus have developedlittle advanced atherosclerotic disease.
Ten or more years past menopause, however, clinically significant atherosclerotic lesions often develop in women who have been without the protection of estrogen. If estrogen is added at this stage, it causes increased matrix metalloprotease activity in the fibrous cap of any atheroma. This proteolytic activity can destabilize the fibrous cap, allowing necrotic core material to come in contact with the bloodstream.
This necrotic material is highly thrombogenic and stimulates clot formation in the vascular lumen. Clarkson demonstrated this process in research on perimenopausal and postmenopausal primates.
This hypothesis will soon be tested in a clinical trial in humans; CVD risk is expected to decrease by 50% to 70% in women who have taken HRT continuously from perimenopause.
Statins and other HDL-modifying drugs
The statins are a first choice for LDL reduction, but lead to only modest increases (4%–10%) in HDL levels.18 Fibric acid derivatives are somewhat more effective; they lead to increases in HDL of 6% to 18%. Both types of drugs are considerably more expensive than OTC NA and not as effective at raising HDL.
Combining statins with other agents that raise HDL has been tried: The NA–statin combination can be effective in managing dyslipidemias of both LDL and HDL. Fibric acid derivatives should not be used with statins because of the increased risk of serious myopathies.18
JUDY’S CASE
THERAPEUTIC TARGETS
Judy’s goals should include:
- reducing blood pressure to less than 120/80 mm Hg,
- decreasing weight to about 140 lb,
- achieving a waist measurement of less than 35 inches,
- reducing fasting blood glucose to less than 100 mg/dL,
- decreasing triglycerides to less than 150 mg/dL, total cholesterol to less than 200 mg/dL, and LDL levels to less than 100 mg/dL, and
- raising HDL to more than 45 mg/dL.
All these parameters should be checked every 3 months until the goals are achieved.
If Judy fails to progress or develops symptoms of heart disease, she should be referred to a cardiologist for further evaluation and more aggressive treatment.
The author reports no conflicts relevant to this article.
1. Executive Summary of the Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection Evaluation and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III). JAMA. 2001;285:2486-2497.
2. Alsheikh-Ali A, Abourjaily HM, Stanek EJ, et al. Increases in HDL-cholesterol are the strongest predictors of risk reduction in lipid intervention trials. Circulation. 2004;110(17 suppl 3):III-813.
3. Jacobs DR, Jr, Meban IL, Bangdiwala SI, et al. High density lipoprotein cholesterol as a predictor of cardiovascular disease mortality in men and women: the follow-up study of the Lipid Research Clinics Prevalence Study. Am J Epidemiol. 1990;131:32-47.
4. Gordon DJ, Probstfiel JL, Garrison RJ, et al. High-density lipoprotein cholesterol and cardiovascular disease: four prospective American studies. Circulation. 1989;79:8-15.
5. Hu Fb, Willet WC, Li T, et al. Adiposity as compared with physical activity in predicting mortality among women. N Engl J Med. 2004;351:2694-2703.
6. Mensink RP, Zock PL, Kester A, et al. Effects of dietary fatty acids and carbohydrates on the ratio of serum total to HDL cholesterol and on serum lipids and apolipoproteins: a meta-analysis of 60 controlled trials. Am J Clin Nutr. 2003;77:1146-1155.
7. Pereira MA, Swain J, Goldfine AB, et al. Effects of a low-glycemic load diet on resting energy expenditure and heart disease risk factors during weight loss. JAMA. 2004;292:2482-2490.
8. Kokkinos PF, Holland JC, Pittaras AE, et al. Cardiorespiratory fitness and coronary heart disease risk factor association in women. J Am Coll Cardiol. 1995;26:358-364.
9. Manson JE, Greenland P, LaCroix AZ, et al. Walking compared with vigorous exercise for the prevention of cardiovascular events in women. N Engl J Med. 2002;347:716-725.
10. Kokkinos PE, Fernhall B. Physical activity and high density lipoprotein cholesterol levels: what is the relationship? Sports Med. 1999;28:307-314.
11. Van Der Gaag MS, Sterksma A, Schaafsma G, et al. Moderate alcohol consumption lowers risk factors for cardiovascular disease in postmenopausal women fed a controlled diet. Am J Clin Nutr. 2002;75:593-599.
12. Baer DJ, Judd JT, Clevidence BA, et al. Moderate alcohol consumption and changes in postprandial lipoproteins of premenopausal and postmenopausal women: a diet-controlled randomized intervention study. J Womens Health Gend Based Med. 2000;9:607-616.
13. Pins JJ, Keenan JM. Pantethine: a new option in managing dyslipidemia. J Am College Nutr. 2005 [in press].
14. Pins JJ, First S, Shamliyan T, Keenan J. Pantethine beneficially affects apolipoprotein A-1, apolipoprotein B, low-density lipoprotein particle size but not high-sensitivity C-reactive protein in a dyslipidemic population. Circulation. 2004;110(17 suppl 3):III-778.
15. Crouse JR, 3rd. New developments in the use of niacin for treatment of hyperlipidemia: new considerations in the use of an old drug. Coron Artery Dis. 1996;7:321-326.
16. Retail pharmacy quote. Walgreen’s Pharmacy, Minneapolis, Minn. January 2005.
17. Risks and benefits of estrogen plus progestin in healthy post-menopausal women: principal results from the Women’s Health Initiative randomized controlled trial. JAMA. 2002;288:321-333.
18. Hersberger M, von Eckardstein A. Low high-density lipoprotein cholesterol: physical background, clinical importance and drug treatment. Drugs. 2003;63:1907-1945.
19. Mosca L, Grundy SM, Judelson D, et al. Guide to preventive cardiology for women. AHA/ACC scientific statement, consensus panel statement. irculation. 1999;99:2480-2484.
20. Mosca L, Appel LJ, Benjamin EJ, et al. AHA Guidelines: evidence-based guidelines for cardiovascular disease prevention in women. Circulation. 2004;109:672-693.
21. Clarkson T. Women’s Heart Health Symposium. Presented at the American Heart Association annual meeting, Nov 9, 2004, New Orleans.
JUDY’S CASE
“WHAT’S MY RISK?”
At her periodic gynecologic exam, Judy, a 58-year-old white woman, expresses concern about her heart health in light of her 62-year-old sister’s recent heart attack. Further questioning reveals that her father died of a heart attack at age 56, and her brother, 52, has high blood pressure and adult-onset diabetes.
Judy has not experienced any symptoms of cardiovascular disease, such as chest pain or tightness with exertion, and she doesn’t smoke. The last time her cholesterol was checked was about 15 years earlier, at which time it was “OK.” She admits she is not very physically active and has gained about 30 lb over the past 10 years. Her vital signs and physical exam are entirely normal except for borderline high blood pressure at 140/90 mm Hg, weight of 170 lb (height: 68 inches), and a waist circumference of 37 inches.
What is her cardiovascular risk?
Judy has physical findings suggestive of metabolic syndrome in addition to the significant family history of cardiovascular disease (CVD). Although her current risk of a cardiac event appears to be low, she is likely to deteriorate if the hypertension and excess body weight are not addressed.
To predict her level of risk more precisely, the physician asks Judy to return in 2 weeks for a blood pressure recheck, and instructs her to perform additional readings on her own. The clinician also refers Judy for a fasting lipid panel and blood glucose level.
In women, CVD is especially lethal
Contrary to earlier assumptions, CVD now appears to be more lethal in women than in men and warrants aggressive efforts to prevent it and manage risk factors (FIGURE). This article focuses on improving high-density lipoprotein (HDL) cholesterol, a key factor in women at risk.
This approach differs slightly from the National Cholesterol Education Program Adult Treatment Panel III (NCEP/ATP III) guidelines, which target low-density lipoprotein (LDL) cholesterol as the primary lipid risk factor for intervention.1
FIGURE Women surpass men in CVD deaths
Over the past 25 years, the trend in deaths attributable to cardiovascular disease has been upward for women, while it is shifting downward for men.
Why focus on HDL?
Improvement in HDL is the strongest predictor of reduced risk for cardiac events. So says an analysis of 17 major lipid intervention trials between 1987 and 2003 (n = 44,170).2
Another study3 demonstrated that HDL cholesterol is the stronger predictor of CVD risk in women. In addition, several large observational studies have shown that increasing HDL cholesterol by 1 mg/dL in men reduces CVD risk by 2%, whereas increasing HDL in women by 1 mg/dL decreases CVD risk by 3%.4
Higher HDL levels may explain later onset of CVD in women. The advantage that women have over men in CVD risk prior to menopause seems to be at least partly due to their higher levels of HDL cholesterol: The average HDL level in women is 56 mg/dL, versus 46 mg/dL in men. This advantage begins to disappear after menopause, presumably because of changes in hormone levels and decreasing HDL-2 cholesterol, the most cardioprotective HDL fraction.
Other reasons to focus on HDL. In addition to the increased CVD risk associated with menopause, women of all ages in the United States have an increasing tendency—at almost epidemic rates—toward obesity and metabolic syndrome, which are known CVD risk factors.5 The dyslipidemia seen with those conditions is characterized by low HDL cholesterol and high triglycerides. Thus, maintaining healthy HDL levels is clearly an important goal for overall cardiovascular health, especially in women.
In addition, HDL is the key lipoprotein associated with reverse cholesterol transport, the process of carrying excess cholesterol from peripheral tissues to the liver for catabolism.
JUDY’S CASE
METABOLIC SYNDROME CONFIRMED
At her return visit, Judy’s blood pressure remains 140/90 mm Hg, with outside readings ranging from 135 to 145 mm Hg systolic and 85 to 95 mm Hg diastolic. Her laboratory tests reveal fasting blood glucose of 115 mg/dL, total cholesterol of 220 mg/dL, LDL cholesterol of 120 mg/dL, HDL cholesterol of 35 mg/dL, and triglycerides of 300 mg/dL.
What are her key heart-health issues?
Individualized management—recommended by NCEP/ATP III guidelines— should focus on reversing the metabolic defects of insulin resistance: hypertension, obesity, glucose intolerance, and dyslipidemia.
At present, Judy’s 10-year risk of a cardiac event is 4% by Framingham risk scoring, but it is likely to increase if the metabolic syndrome is not addressed.
How to preserve healthy HDL levels
Diet and regular aerobic exercise are recommended by the NCEP/ATP III guidelines, especially for obese patients with metabolic syndrome. However, dietary changes do not raise HDL cholesterol. In fact, dieting to lose weight will almost certainly lower HDL levels. That’s because people who eat a diet very low in fat tend to replace the fat calories with carbohydrates, and a low-fat, high-carbohydrate diet will reduce all cholesterol fractions—but HDL cholesterol will decrease disproportionately.6
Recommend complex carbohydrates and moderate fat intake
To avoid excessive reduction in HDL cholesterol, any weight-loss diet should involve moderate fat intake (35% of total calories) and emphasize monounsaturated fats such as olive or canola oil, along with complex carbohydrates found in fruits, vegetables, and whole grains.7 After weight loss is stabilized, HDL cholesterol tends to increase somewhat, but may not return all the way to baseline.
New guidelines on how to intervene
Most women who die suddenly from coronary heart disease had no previous symptoms, and that makes prevention all the more important.
Though it hasn’t been long since the first woman-centered recommendations on preventing cardiovascular disease,19 our understanding of preventive interventions has improved enormously. Thus, the American Heart Association convened a new expert panel to review and, where necessary, revise the original guidelines.20 Here are some of the highlights.
Forget “haves” and “have-nots”
Lori Mosca and the other members of the expert panel observe that “the concept of CVD as a categorical, ‘have-or-have-not’ condition has been replaced with a growing appreciation for the existence of a continuum of CVD risk.”20
For example, the low-risk category (<10% risk of a coronary heart disease event in the next 10 years, according to the Framingham Risk Score for women) would include women with optimal levels of risk factors and a heart-healthy lifestyle, as well as women with metabolic syndrome but no other risk factors. In contrast, women with established coronary heart disease, diabetes mellitus, or chronic kidney disease would fall into the high-risk group (>20% risk). Those with multiple risk factors or markedly elevated levels of a single risk factor fall somewhere in between.
The 5 lifestyle laws
Women are advised to consistently:
- eat plenty of fruits and vegetables, whole grains, and low-fat or nonfat dairy items
- avoid smoking and smokeless tobacco
- perform at least 30 minutes of moderate-intensity activity, such as brisk walking, most days
- achieve and maintain a body mass index between 18.5 and 25.9 kg/m2 and a waist circumference less than 35 inches
- consider omega-3 fatty acid supplementation, especially if risk is high. The guidelines extol the benefits of fish, which contain omega-3 fatty acids, but warn against consumption of fish likely to contain unsafe levels of mercury (shark, sword-fish, king mackerel, and tilefish) by gravidas and women of reproductive age.
BP and cholesterol control
- Blood pressure. Maintain at or below 120/80 mm Hg through lifestyle approaches, or drug therapy when BP is 140/90 mm Hg or higher.
- Dietary fat and cholesterol. Keep saturated fat intake to less than 7% of calories, and cholesterol below 200 mg/dL with high risk or elevated LDL.
- LDL-cholesterol. Use drug therapy (preferably statins) if risk is high and LDL is below 100 mg/dL. Add lifestyle adjustments if risk is high and LDL cholesterol is at or above 100 mg/dL.
What not to take to prevent CVD
The guidelines warn against prescribing—or continuing—estrogen-progestin therapy to prevent CVD. Nor should other forms of menopausal hormone therapy, such as unopposed estrogen, be prescribed or continued unless new findings indicate a beneficial role.
Also discouraged are antioxidant vitamin supplements to prevent CVD.
Look for depression
Especially women with CVD should be referred or treated when depression is found, as it can hamper a woman’s efforts at prevention.
The full text of the guidelines can be viewed at
http://circ.ahajournals.org/cgi/content/full/109/5/672.
Regular exercise raises HDL
One way to avoid an overall reduction in HDL levels with weight loss is to prescribe exercise in conjunction with it. Regular exercise is an excellent way to raise HDL cholesterol levels. Studies suggest that, for women, the volume of exercise is more important than intensity. Thus, women should strive to walk 7 to 14 miles per week or otherwise expend approximately 1,200 to 1,600 Kcal.8,9
Regular exercise is recommended as one of the NCEP/ATP III therapeutic lifestyle changes that directly benefit cardiovascular health and blood pressure and help the individual maintain optimal body weight. Although exercise raises HDL levels, it does so more easily in men than in women. HDL cholesterol increases in men with both intensity and volume of exercise.10 Women appear to derive slightly less of a benefit, perhaps because their HDL levels are already generally higher.
In women, studies have reported an increase in HDL cholesterol as high as 15% for both premenopausal and post-menopausal women who follow moderate exercise regimens—and the lower the baseline HDL level, the greater the increase.8
Exercise level was inversely associated with cardiovascular risk in the Women’s Health Initiative,9 which followed a cohort of 73,743 women prospectively for an average of 3.2 years. Women at the highest quintile of exercise level reduced their CVD risk by 53% over the lowest quintile, and moderate activity such as walking led to risk reductions similar to those involving more vigorous activities.
Recommend alcohol—or not?
Moderate alcohol consumption—1 to 3 drinks per day, or 15 to 45 g ethanol— improves lipoprotein levels and reduces CVD risk. The flavonoids in red wines also reduce platelet aggregation and clotting tendencies. However, advising a patient to consume alcohol to reduce her CVD risk is controversial; many physicians are reluctant to do so due to obvious concerns over toxicity and dependency.
Response to alcohol may vary by menopausal status. In 1 study,11 pre-menopausal women decreased their LDL cholesterol by 12% (P=.01) after 3 glasses of wine daily for 3 weeks, but there was no significant increase in HDL levels.
In the same study, postmenopausal women increased HDL levels by 12% (P=.03), but their LDL cholesterol decreased only minimally.11
Another study12 in postmenopausal women who consumed 0, 1, or 2 drinks daily (0, 15, or 30 g ethanol) for 8 weeks showed a significant decrease of 4% in LDL cholesterol at 1 drink (15 g), but no significant further decrease at 2 drinks (30 g). In the same study, HDL increased non-significantly at 1 drink (15 g), but increased significantly (5%) at 2 drinks (30 g). There may be a threshold of 30 g ethanol daily for postmenopausal women to benefit significantly from alcohol consumption.
JUDY’S CASE
LIFESTYLE RECOMMENDATIONS
The foundation of Judy’s management should be the triad of weight loss, exercise, and a diet that limits refined carbohydrates and emphasizes fiber, fruits and vegetables, whole grains, and healthy fats (ie, olive, canola, and fish oils).
In prescribing exercise, emphasize quantity over intensity, such as 14 miles of walking weekly or 60 to 90 minutes daily of aerobic activity.
Since she has no history of cardiac symptoms, and low-intensity, high-quantity exercise is advised, she does not need a screening cardiac stress test before beginning her regimen.
Pantethine
Pantethine is an over-the-counter (OTC) nutritional supplement that combines the B vitamin pantothenic acid with the amino acid cysteamine. Although pantothenic acid alone has no lipid-lowering effects, pantethine does: It lowers LDL cholesterol, very-low-density lipoprotein (VLDL) cholesterol, and triglycerides, and raises HDL levels. The exact mechanism of action is unknown, but it is thought to involve primarily the effects of cysteamine on lipid metabolism in the liver.
Recommended dose. At 600 to 900 mg daily, the typical response is an increase of up to 18% in HDL cholesterol and reductions in LDL cholesterol, VLDL cholesterol, and triglycerides of 13%, 20%, and 26%, respectively.13
In a recent US study, pantethine significantly raised HDL-2 cholesterol, the more cardioprotective fraction of HDL, and increased the particle size of LDL cholesterol, rendering it less atherogenic.14
Pantethine also reduces visceral fat. The same study14 reported a significant reduction in visceral fat, a component of metabolic syndrome, as measured by computerized dual-energy x-ray absorptiometry in individuals taking 900 mg daily for 6 weeks. This reduction appears to be primarily an effect of pantothenic acid and had been demonstrated in animal studies.
No side effects. Pantethine is exceptionally well tolerated, with virtually no side effects. An occasional person will experience gastrointestinal upset and diarrhea, which resolve when the drug is discontinued.
Recommend formulations that contain Pantesin (Daiichi Fine Chemicals, New York City), a pharmaceutical grade of pantethine, because extensive research confirms the benefits I have described. Otherwise, make sure the manufacturer is a reputable company, with scientific studies to support its claims of safety, efficacy, and bioavailability.
Nicotinic acid
Nicotinic acid (NA), another B vitamin available OTC as a nutritional supplement, is the most effective agent for raising HDL levels.
Recommended dose. A divided dose of 3,000 mg daily of the immediate-release form of NA can raise HDL levels as much as 30%. Unfortunately, that form of NA has a high incidence of adverse effects, such as flushing, itching, and GI upset.15
Sustained-release NA is generally well tolerated, but somewhat less effective at raising HDL. A lower dose of sustained-release NA (1,000 to 1,500 mg daily) is recommended to avoid liver toxicity and typically leads to HDL increases as high as 20%.15
NA offers additional benefits to the lipid profile, typically lowering LDL cholesterol by 20%, triglycerides by 15%, and lipoprotein(a) by 15%.15
Monitor all forms of NA with liver enzymes, blood glucose, and uric acid because treatment can elevate these markers.
Give NA preparations extra scrutiny. The general caveats about quality and bioavailability apply especially to NA. Nonprescription products on the market that contain nicotinamide and inositol hexanicotinate are advertised as “no-flush” niacin because they do not cause cutaneous side effects, but they also have no lipid benefits.
Cost. NA is relatively inexpensive, and good-quality sustained-release products such as Endur-acin (Endurance Products, Tigard, Ore) can be purchased OTC for 12 cents per 500-mg tablet or a daily cost of 24 to 36 cents.16
Sustained-release NA is also available by prescription (Niaspan, Kos Pharmaceuticals, Cranbury, NJ), but it is considerably more expensive ($1.98 per 500-mg tablet, or $3.96 to $5.94 daily) and has not been shown to be more effective or better tolerated than the better OTC products.16
JUDY’S CASE
RAISING HDL WITHOUT DRUGS
Judy’s lipid abnormalities would benefit from a supplement of pantethine (300 mg thrice daily) or NA (1,000 mg thrice daily of plain/quick release or 500 mg twice daily of sustained release to reduce flushing side effects).
Her greatest risk with her current lipid levels is low HDL, and the best agent to raise these levels is NA.
Pantethine also raises HDL and selectively improves the HDL-2 subfraction, which is most cardioprotective. Both supplements also improve the other lipid abnormalities.
In addition, referral to a dietician for detailed instruction in food choices and preparation may be useful.
Pharmacologic therapies
Hormone replacement therapy (HRT) is no longer recommended to prevent CVD and should be given only for menopausal symptoms during the perimenopausal years—despite the fact that estrogen has been shown to raise HDL cholesterol by 8% in postmenopausal women.17
Estrogens increase HDL by increasing production of apoA-1, the main lipoprotein in HDL, and by decreasing HDL catabolism by inhibiting hepatic lipase. The 17-hydroxyprogesterone component of HRT given to women with an intact uterus to prevent endometrial cancer has no significant lipid effects.
Paradoxically, the increased CVD risk found in the HRT arm of the Women’s Health Initiative17 was in the face of improved HDL (8%) and LDL (–14%) cholesterol and appeared to be the result of increased thrombotic events.
Further analysis of Women’s Health Initiative data showed that women who were still within 10 years of menopause actually did experience a reduction in CVD risk. That observation, along with some primate research on HRT, suggests that the risk or benefit of HRT with respect to CVD may be a matter of timing. See “Emerging hypothesis may explain estrogen paradox”
At the 2004 American Heart Association meeting, Dr. Thomas Clarkson, who has conducted primate research on hormone replacement therapy (HRT) and the risk of cardiovascular disease (CVD), described the emerging hypothesis that may explain the HRT paradox of increased CVD risk despite evidence of benefit in observational studies.21
Estrogen is a potent upregulator of matrix metal-loproteases. These proteolytic enzymes are a normal constituent of the endometrium and important in its remodeling with each menstrual cycle. Matrix metal-loproteases are also found in the fibrous cap that seals off the necrotic core of an advanced atheroma from the vascular lumen. Most perimenopausal women have enjoyed estrogen’s protective effect throughout their reproductive years and thus have developedlittle advanced atherosclerotic disease.
Ten or more years past menopause, however, clinically significant atherosclerotic lesions often develop in women who have been without the protection of estrogen. If estrogen is added at this stage, it causes increased matrix metalloprotease activity in the fibrous cap of any atheroma. This proteolytic activity can destabilize the fibrous cap, allowing necrotic core material to come in contact with the bloodstream.
This necrotic material is highly thrombogenic and stimulates clot formation in the vascular lumen. Clarkson demonstrated this process in research on perimenopausal and postmenopausal primates.
This hypothesis will soon be tested in a clinical trial in humans; CVD risk is expected to decrease by 50% to 70% in women who have taken HRT continuously from perimenopause.
Statins and other HDL-modifying drugs
The statins are a first choice for LDL reduction, but lead to only modest increases (4%–10%) in HDL levels.18 Fibric acid derivatives are somewhat more effective; they lead to increases in HDL of 6% to 18%. Both types of drugs are considerably more expensive than OTC NA and not as effective at raising HDL.
Combining statins with other agents that raise HDL has been tried: The NA–statin combination can be effective in managing dyslipidemias of both LDL and HDL. Fibric acid derivatives should not be used with statins because of the increased risk of serious myopathies.18
JUDY’S CASE
THERAPEUTIC TARGETS
Judy’s goals should include:
- reducing blood pressure to less than 120/80 mm Hg,
- decreasing weight to about 140 lb,
- achieving a waist measurement of less than 35 inches,
- reducing fasting blood glucose to less than 100 mg/dL,
- decreasing triglycerides to less than 150 mg/dL, total cholesterol to less than 200 mg/dL, and LDL levels to less than 100 mg/dL, and
- raising HDL to more than 45 mg/dL.
All these parameters should be checked every 3 months until the goals are achieved.
If Judy fails to progress or develops symptoms of heart disease, she should be referred to a cardiologist for further evaluation and more aggressive treatment.
The author reports no conflicts relevant to this article.
JUDY’S CASE
“WHAT’S MY RISK?”
At her periodic gynecologic exam, Judy, a 58-year-old white woman, expresses concern about her heart health in light of her 62-year-old sister’s recent heart attack. Further questioning reveals that her father died of a heart attack at age 56, and her brother, 52, has high blood pressure and adult-onset diabetes.
Judy has not experienced any symptoms of cardiovascular disease, such as chest pain or tightness with exertion, and she doesn’t smoke. The last time her cholesterol was checked was about 15 years earlier, at which time it was “OK.” She admits she is not very physically active and has gained about 30 lb over the past 10 years. Her vital signs and physical exam are entirely normal except for borderline high blood pressure at 140/90 mm Hg, weight of 170 lb (height: 68 inches), and a waist circumference of 37 inches.
What is her cardiovascular risk?
Judy has physical findings suggestive of metabolic syndrome in addition to the significant family history of cardiovascular disease (CVD). Although her current risk of a cardiac event appears to be low, she is likely to deteriorate if the hypertension and excess body weight are not addressed.
To predict her level of risk more precisely, the physician asks Judy to return in 2 weeks for a blood pressure recheck, and instructs her to perform additional readings on her own. The clinician also refers Judy for a fasting lipid panel and blood glucose level.
In women, CVD is especially lethal
Contrary to earlier assumptions, CVD now appears to be more lethal in women than in men and warrants aggressive efforts to prevent it and manage risk factors (FIGURE). This article focuses on improving high-density lipoprotein (HDL) cholesterol, a key factor in women at risk.
This approach differs slightly from the National Cholesterol Education Program Adult Treatment Panel III (NCEP/ATP III) guidelines, which target low-density lipoprotein (LDL) cholesterol as the primary lipid risk factor for intervention.1
FIGURE Women surpass men in CVD deaths
Over the past 25 years, the trend in deaths attributable to cardiovascular disease has been upward for women, while it is shifting downward for men.
Why focus on HDL?
Improvement in HDL is the strongest predictor of reduced risk for cardiac events. So says an analysis of 17 major lipid intervention trials between 1987 and 2003 (n = 44,170).2
Another study3 demonstrated that HDL cholesterol is the stronger predictor of CVD risk in women. In addition, several large observational studies have shown that increasing HDL cholesterol by 1 mg/dL in men reduces CVD risk by 2%, whereas increasing HDL in women by 1 mg/dL decreases CVD risk by 3%.4
Higher HDL levels may explain later onset of CVD in women. The advantage that women have over men in CVD risk prior to menopause seems to be at least partly due to their higher levels of HDL cholesterol: The average HDL level in women is 56 mg/dL, versus 46 mg/dL in men. This advantage begins to disappear after menopause, presumably because of changes in hormone levels and decreasing HDL-2 cholesterol, the most cardioprotective HDL fraction.
Other reasons to focus on HDL. In addition to the increased CVD risk associated with menopause, women of all ages in the United States have an increasing tendency—at almost epidemic rates—toward obesity and metabolic syndrome, which are known CVD risk factors.5 The dyslipidemia seen with those conditions is characterized by low HDL cholesterol and high triglycerides. Thus, maintaining healthy HDL levels is clearly an important goal for overall cardiovascular health, especially in women.
In addition, HDL is the key lipoprotein associated with reverse cholesterol transport, the process of carrying excess cholesterol from peripheral tissues to the liver for catabolism.
JUDY’S CASE
METABOLIC SYNDROME CONFIRMED
At her return visit, Judy’s blood pressure remains 140/90 mm Hg, with outside readings ranging from 135 to 145 mm Hg systolic and 85 to 95 mm Hg diastolic. Her laboratory tests reveal fasting blood glucose of 115 mg/dL, total cholesterol of 220 mg/dL, LDL cholesterol of 120 mg/dL, HDL cholesterol of 35 mg/dL, and triglycerides of 300 mg/dL.
What are her key heart-health issues?
Individualized management—recommended by NCEP/ATP III guidelines— should focus on reversing the metabolic defects of insulin resistance: hypertension, obesity, glucose intolerance, and dyslipidemia.
At present, Judy’s 10-year risk of a cardiac event is 4% by Framingham risk scoring, but it is likely to increase if the metabolic syndrome is not addressed.
How to preserve healthy HDL levels
Diet and regular aerobic exercise are recommended by the NCEP/ATP III guidelines, especially for obese patients with metabolic syndrome. However, dietary changes do not raise HDL cholesterol. In fact, dieting to lose weight will almost certainly lower HDL levels. That’s because people who eat a diet very low in fat tend to replace the fat calories with carbohydrates, and a low-fat, high-carbohydrate diet will reduce all cholesterol fractions—but HDL cholesterol will decrease disproportionately.6
Recommend complex carbohydrates and moderate fat intake
To avoid excessive reduction in HDL cholesterol, any weight-loss diet should involve moderate fat intake (35% of total calories) and emphasize monounsaturated fats such as olive or canola oil, along with complex carbohydrates found in fruits, vegetables, and whole grains.7 After weight loss is stabilized, HDL cholesterol tends to increase somewhat, but may not return all the way to baseline.
New guidelines on how to intervene
Most women who die suddenly from coronary heart disease had no previous symptoms, and that makes prevention all the more important.
Though it hasn’t been long since the first woman-centered recommendations on preventing cardiovascular disease,19 our understanding of preventive interventions has improved enormously. Thus, the American Heart Association convened a new expert panel to review and, where necessary, revise the original guidelines.20 Here are some of the highlights.
Forget “haves” and “have-nots”
Lori Mosca and the other members of the expert panel observe that “the concept of CVD as a categorical, ‘have-or-have-not’ condition has been replaced with a growing appreciation for the existence of a continuum of CVD risk.”20
For example, the low-risk category (<10% risk of a coronary heart disease event in the next 10 years, according to the Framingham Risk Score for women) would include women with optimal levels of risk factors and a heart-healthy lifestyle, as well as women with metabolic syndrome but no other risk factors. In contrast, women with established coronary heart disease, diabetes mellitus, or chronic kidney disease would fall into the high-risk group (>20% risk). Those with multiple risk factors or markedly elevated levels of a single risk factor fall somewhere in between.
The 5 lifestyle laws
Women are advised to consistently:
- eat plenty of fruits and vegetables, whole grains, and low-fat or nonfat dairy items
- avoid smoking and smokeless tobacco
- perform at least 30 minutes of moderate-intensity activity, such as brisk walking, most days
- achieve and maintain a body mass index between 18.5 and 25.9 kg/m2 and a waist circumference less than 35 inches
- consider omega-3 fatty acid supplementation, especially if risk is high. The guidelines extol the benefits of fish, which contain omega-3 fatty acids, but warn against consumption of fish likely to contain unsafe levels of mercury (shark, sword-fish, king mackerel, and tilefish) by gravidas and women of reproductive age.
BP and cholesterol control
- Blood pressure. Maintain at or below 120/80 mm Hg through lifestyle approaches, or drug therapy when BP is 140/90 mm Hg or higher.
- Dietary fat and cholesterol. Keep saturated fat intake to less than 7% of calories, and cholesterol below 200 mg/dL with high risk or elevated LDL.
- LDL-cholesterol. Use drug therapy (preferably statins) if risk is high and LDL is below 100 mg/dL. Add lifestyle adjustments if risk is high and LDL cholesterol is at or above 100 mg/dL.
What not to take to prevent CVD
The guidelines warn against prescribing—or continuing—estrogen-progestin therapy to prevent CVD. Nor should other forms of menopausal hormone therapy, such as unopposed estrogen, be prescribed or continued unless new findings indicate a beneficial role.
Also discouraged are antioxidant vitamin supplements to prevent CVD.
Look for depression
Especially women with CVD should be referred or treated when depression is found, as it can hamper a woman’s efforts at prevention.
The full text of the guidelines can be viewed at
http://circ.ahajournals.org/cgi/content/full/109/5/672.
Regular exercise raises HDL
One way to avoid an overall reduction in HDL levels with weight loss is to prescribe exercise in conjunction with it. Regular exercise is an excellent way to raise HDL cholesterol levels. Studies suggest that, for women, the volume of exercise is more important than intensity. Thus, women should strive to walk 7 to 14 miles per week or otherwise expend approximately 1,200 to 1,600 Kcal.8,9
Regular exercise is recommended as one of the NCEP/ATP III therapeutic lifestyle changes that directly benefit cardiovascular health and blood pressure and help the individual maintain optimal body weight. Although exercise raises HDL levels, it does so more easily in men than in women. HDL cholesterol increases in men with both intensity and volume of exercise.10 Women appear to derive slightly less of a benefit, perhaps because their HDL levels are already generally higher.
In women, studies have reported an increase in HDL cholesterol as high as 15% for both premenopausal and post-menopausal women who follow moderate exercise regimens—and the lower the baseline HDL level, the greater the increase.8
Exercise level was inversely associated with cardiovascular risk in the Women’s Health Initiative,9 which followed a cohort of 73,743 women prospectively for an average of 3.2 years. Women at the highest quintile of exercise level reduced their CVD risk by 53% over the lowest quintile, and moderate activity such as walking led to risk reductions similar to those involving more vigorous activities.
Recommend alcohol—or not?
Moderate alcohol consumption—1 to 3 drinks per day, or 15 to 45 g ethanol— improves lipoprotein levels and reduces CVD risk. The flavonoids in red wines also reduce platelet aggregation and clotting tendencies. However, advising a patient to consume alcohol to reduce her CVD risk is controversial; many physicians are reluctant to do so due to obvious concerns over toxicity and dependency.
Response to alcohol may vary by menopausal status. In 1 study,11 pre-menopausal women decreased their LDL cholesterol by 12% (P=.01) after 3 glasses of wine daily for 3 weeks, but there was no significant increase in HDL levels.
In the same study, postmenopausal women increased HDL levels by 12% (P=.03), but their LDL cholesterol decreased only minimally.11
Another study12 in postmenopausal women who consumed 0, 1, or 2 drinks daily (0, 15, or 30 g ethanol) for 8 weeks showed a significant decrease of 4% in LDL cholesterol at 1 drink (15 g), but no significant further decrease at 2 drinks (30 g). In the same study, HDL increased non-significantly at 1 drink (15 g), but increased significantly (5%) at 2 drinks (30 g). There may be a threshold of 30 g ethanol daily for postmenopausal women to benefit significantly from alcohol consumption.
JUDY’S CASE
LIFESTYLE RECOMMENDATIONS
The foundation of Judy’s management should be the triad of weight loss, exercise, and a diet that limits refined carbohydrates and emphasizes fiber, fruits and vegetables, whole grains, and healthy fats (ie, olive, canola, and fish oils).
In prescribing exercise, emphasize quantity over intensity, such as 14 miles of walking weekly or 60 to 90 minutes daily of aerobic activity.
Since she has no history of cardiac symptoms, and low-intensity, high-quantity exercise is advised, she does not need a screening cardiac stress test before beginning her regimen.
Pantethine
Pantethine is an over-the-counter (OTC) nutritional supplement that combines the B vitamin pantothenic acid with the amino acid cysteamine. Although pantothenic acid alone has no lipid-lowering effects, pantethine does: It lowers LDL cholesterol, very-low-density lipoprotein (VLDL) cholesterol, and triglycerides, and raises HDL levels. The exact mechanism of action is unknown, but it is thought to involve primarily the effects of cysteamine on lipid metabolism in the liver.
Recommended dose. At 600 to 900 mg daily, the typical response is an increase of up to 18% in HDL cholesterol and reductions in LDL cholesterol, VLDL cholesterol, and triglycerides of 13%, 20%, and 26%, respectively.13
In a recent US study, pantethine significantly raised HDL-2 cholesterol, the more cardioprotective fraction of HDL, and increased the particle size of LDL cholesterol, rendering it less atherogenic.14
Pantethine also reduces visceral fat. The same study14 reported a significant reduction in visceral fat, a component of metabolic syndrome, as measured by computerized dual-energy x-ray absorptiometry in individuals taking 900 mg daily for 6 weeks. This reduction appears to be primarily an effect of pantothenic acid and had been demonstrated in animal studies.
No side effects. Pantethine is exceptionally well tolerated, with virtually no side effects. An occasional person will experience gastrointestinal upset and diarrhea, which resolve when the drug is discontinued.
Recommend formulations that contain Pantesin (Daiichi Fine Chemicals, New York City), a pharmaceutical grade of pantethine, because extensive research confirms the benefits I have described. Otherwise, make sure the manufacturer is a reputable company, with scientific studies to support its claims of safety, efficacy, and bioavailability.
Nicotinic acid
Nicotinic acid (NA), another B vitamin available OTC as a nutritional supplement, is the most effective agent for raising HDL levels.
Recommended dose. A divided dose of 3,000 mg daily of the immediate-release form of NA can raise HDL levels as much as 30%. Unfortunately, that form of NA has a high incidence of adverse effects, such as flushing, itching, and GI upset.15
Sustained-release NA is generally well tolerated, but somewhat less effective at raising HDL. A lower dose of sustained-release NA (1,000 to 1,500 mg daily) is recommended to avoid liver toxicity and typically leads to HDL increases as high as 20%.15
NA offers additional benefits to the lipid profile, typically lowering LDL cholesterol by 20%, triglycerides by 15%, and lipoprotein(a) by 15%.15
Monitor all forms of NA with liver enzymes, blood glucose, and uric acid because treatment can elevate these markers.
Give NA preparations extra scrutiny. The general caveats about quality and bioavailability apply especially to NA. Nonprescription products on the market that contain nicotinamide and inositol hexanicotinate are advertised as “no-flush” niacin because they do not cause cutaneous side effects, but they also have no lipid benefits.
Cost. NA is relatively inexpensive, and good-quality sustained-release products such as Endur-acin (Endurance Products, Tigard, Ore) can be purchased OTC for 12 cents per 500-mg tablet or a daily cost of 24 to 36 cents.16
Sustained-release NA is also available by prescription (Niaspan, Kos Pharmaceuticals, Cranbury, NJ), but it is considerably more expensive ($1.98 per 500-mg tablet, or $3.96 to $5.94 daily) and has not been shown to be more effective or better tolerated than the better OTC products.16
JUDY’S CASE
RAISING HDL WITHOUT DRUGS
Judy’s lipid abnormalities would benefit from a supplement of pantethine (300 mg thrice daily) or NA (1,000 mg thrice daily of plain/quick release or 500 mg twice daily of sustained release to reduce flushing side effects).
Her greatest risk with her current lipid levels is low HDL, and the best agent to raise these levels is NA.
Pantethine also raises HDL and selectively improves the HDL-2 subfraction, which is most cardioprotective. Both supplements also improve the other lipid abnormalities.
In addition, referral to a dietician for detailed instruction in food choices and preparation may be useful.
Pharmacologic therapies
Hormone replacement therapy (HRT) is no longer recommended to prevent CVD and should be given only for menopausal symptoms during the perimenopausal years—despite the fact that estrogen has been shown to raise HDL cholesterol by 8% in postmenopausal women.17
Estrogens increase HDL by increasing production of apoA-1, the main lipoprotein in HDL, and by decreasing HDL catabolism by inhibiting hepatic lipase. The 17-hydroxyprogesterone component of HRT given to women with an intact uterus to prevent endometrial cancer has no significant lipid effects.
Paradoxically, the increased CVD risk found in the HRT arm of the Women’s Health Initiative17 was in the face of improved HDL (8%) and LDL (–14%) cholesterol and appeared to be the result of increased thrombotic events.
Further analysis of Women’s Health Initiative data showed that women who were still within 10 years of menopause actually did experience a reduction in CVD risk. That observation, along with some primate research on HRT, suggests that the risk or benefit of HRT with respect to CVD may be a matter of timing. See “Emerging hypothesis may explain estrogen paradox”
At the 2004 American Heart Association meeting, Dr. Thomas Clarkson, who has conducted primate research on hormone replacement therapy (HRT) and the risk of cardiovascular disease (CVD), described the emerging hypothesis that may explain the HRT paradox of increased CVD risk despite evidence of benefit in observational studies.21
Estrogen is a potent upregulator of matrix metal-loproteases. These proteolytic enzymes are a normal constituent of the endometrium and important in its remodeling with each menstrual cycle. Matrix metal-loproteases are also found in the fibrous cap that seals off the necrotic core of an advanced atheroma from the vascular lumen. Most perimenopausal women have enjoyed estrogen’s protective effect throughout their reproductive years and thus have developedlittle advanced atherosclerotic disease.
Ten or more years past menopause, however, clinically significant atherosclerotic lesions often develop in women who have been without the protection of estrogen. If estrogen is added at this stage, it causes increased matrix metalloprotease activity in the fibrous cap of any atheroma. This proteolytic activity can destabilize the fibrous cap, allowing necrotic core material to come in contact with the bloodstream.
This necrotic material is highly thrombogenic and stimulates clot formation in the vascular lumen. Clarkson demonstrated this process in research on perimenopausal and postmenopausal primates.
This hypothesis will soon be tested in a clinical trial in humans; CVD risk is expected to decrease by 50% to 70% in women who have taken HRT continuously from perimenopause.
Statins and other HDL-modifying drugs
The statins are a first choice for LDL reduction, but lead to only modest increases (4%–10%) in HDL levels.18 Fibric acid derivatives are somewhat more effective; they lead to increases in HDL of 6% to 18%. Both types of drugs are considerably more expensive than OTC NA and not as effective at raising HDL.
Combining statins with other agents that raise HDL has been tried: The NA–statin combination can be effective in managing dyslipidemias of both LDL and HDL. Fibric acid derivatives should not be used with statins because of the increased risk of serious myopathies.18
JUDY’S CASE
THERAPEUTIC TARGETS
Judy’s goals should include:
- reducing blood pressure to less than 120/80 mm Hg,
- decreasing weight to about 140 lb,
- achieving a waist measurement of less than 35 inches,
- reducing fasting blood glucose to less than 100 mg/dL,
- decreasing triglycerides to less than 150 mg/dL, total cholesterol to less than 200 mg/dL, and LDL levels to less than 100 mg/dL, and
- raising HDL to more than 45 mg/dL.
All these parameters should be checked every 3 months until the goals are achieved.
If Judy fails to progress or develops symptoms of heart disease, she should be referred to a cardiologist for further evaluation and more aggressive treatment.
The author reports no conflicts relevant to this article.
1. Executive Summary of the Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection Evaluation and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III). JAMA. 2001;285:2486-2497.
2. Alsheikh-Ali A, Abourjaily HM, Stanek EJ, et al. Increases in HDL-cholesterol are the strongest predictors of risk reduction in lipid intervention trials. Circulation. 2004;110(17 suppl 3):III-813.
3. Jacobs DR, Jr, Meban IL, Bangdiwala SI, et al. High density lipoprotein cholesterol as a predictor of cardiovascular disease mortality in men and women: the follow-up study of the Lipid Research Clinics Prevalence Study. Am J Epidemiol. 1990;131:32-47.
4. Gordon DJ, Probstfiel JL, Garrison RJ, et al. High-density lipoprotein cholesterol and cardiovascular disease: four prospective American studies. Circulation. 1989;79:8-15.
5. Hu Fb, Willet WC, Li T, et al. Adiposity as compared with physical activity in predicting mortality among women. N Engl J Med. 2004;351:2694-2703.
6. Mensink RP, Zock PL, Kester A, et al. Effects of dietary fatty acids and carbohydrates on the ratio of serum total to HDL cholesterol and on serum lipids and apolipoproteins: a meta-analysis of 60 controlled trials. Am J Clin Nutr. 2003;77:1146-1155.
7. Pereira MA, Swain J, Goldfine AB, et al. Effects of a low-glycemic load diet on resting energy expenditure and heart disease risk factors during weight loss. JAMA. 2004;292:2482-2490.
8. Kokkinos PF, Holland JC, Pittaras AE, et al. Cardiorespiratory fitness and coronary heart disease risk factor association in women. J Am Coll Cardiol. 1995;26:358-364.
9. Manson JE, Greenland P, LaCroix AZ, et al. Walking compared with vigorous exercise for the prevention of cardiovascular events in women. N Engl J Med. 2002;347:716-725.
10. Kokkinos PE, Fernhall B. Physical activity and high density lipoprotein cholesterol levels: what is the relationship? Sports Med. 1999;28:307-314.
11. Van Der Gaag MS, Sterksma A, Schaafsma G, et al. Moderate alcohol consumption lowers risk factors for cardiovascular disease in postmenopausal women fed a controlled diet. Am J Clin Nutr. 2002;75:593-599.
12. Baer DJ, Judd JT, Clevidence BA, et al. Moderate alcohol consumption and changes in postprandial lipoproteins of premenopausal and postmenopausal women: a diet-controlled randomized intervention study. J Womens Health Gend Based Med. 2000;9:607-616.
13. Pins JJ, Keenan JM. Pantethine: a new option in managing dyslipidemia. J Am College Nutr. 2005 [in press].
14. Pins JJ, First S, Shamliyan T, Keenan J. Pantethine beneficially affects apolipoprotein A-1, apolipoprotein B, low-density lipoprotein particle size but not high-sensitivity C-reactive protein in a dyslipidemic population. Circulation. 2004;110(17 suppl 3):III-778.
15. Crouse JR, 3rd. New developments in the use of niacin for treatment of hyperlipidemia: new considerations in the use of an old drug. Coron Artery Dis. 1996;7:321-326.
16. Retail pharmacy quote. Walgreen’s Pharmacy, Minneapolis, Minn. January 2005.
17. Risks and benefits of estrogen plus progestin in healthy post-menopausal women: principal results from the Women’s Health Initiative randomized controlled trial. JAMA. 2002;288:321-333.
18. Hersberger M, von Eckardstein A. Low high-density lipoprotein cholesterol: physical background, clinical importance and drug treatment. Drugs. 2003;63:1907-1945.
19. Mosca L, Grundy SM, Judelson D, et al. Guide to preventive cardiology for women. AHA/ACC scientific statement, consensus panel statement. irculation. 1999;99:2480-2484.
20. Mosca L, Appel LJ, Benjamin EJ, et al. AHA Guidelines: evidence-based guidelines for cardiovascular disease prevention in women. Circulation. 2004;109:672-693.
21. Clarkson T. Women’s Heart Health Symposium. Presented at the American Heart Association annual meeting, Nov 9, 2004, New Orleans.
1. Executive Summary of the Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection Evaluation and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III). JAMA. 2001;285:2486-2497.
2. Alsheikh-Ali A, Abourjaily HM, Stanek EJ, et al. Increases in HDL-cholesterol are the strongest predictors of risk reduction in lipid intervention trials. Circulation. 2004;110(17 suppl 3):III-813.
3. Jacobs DR, Jr, Meban IL, Bangdiwala SI, et al. High density lipoprotein cholesterol as a predictor of cardiovascular disease mortality in men and women: the follow-up study of the Lipid Research Clinics Prevalence Study. Am J Epidemiol. 1990;131:32-47.
4. Gordon DJ, Probstfiel JL, Garrison RJ, et al. High-density lipoprotein cholesterol and cardiovascular disease: four prospective American studies. Circulation. 1989;79:8-15.
5. Hu Fb, Willet WC, Li T, et al. Adiposity as compared with physical activity in predicting mortality among women. N Engl J Med. 2004;351:2694-2703.
6. Mensink RP, Zock PL, Kester A, et al. Effects of dietary fatty acids and carbohydrates on the ratio of serum total to HDL cholesterol and on serum lipids and apolipoproteins: a meta-analysis of 60 controlled trials. Am J Clin Nutr. 2003;77:1146-1155.
7. Pereira MA, Swain J, Goldfine AB, et al. Effects of a low-glycemic load diet on resting energy expenditure and heart disease risk factors during weight loss. JAMA. 2004;292:2482-2490.
8. Kokkinos PF, Holland JC, Pittaras AE, et al. Cardiorespiratory fitness and coronary heart disease risk factor association in women. J Am Coll Cardiol. 1995;26:358-364.
9. Manson JE, Greenland P, LaCroix AZ, et al. Walking compared with vigorous exercise for the prevention of cardiovascular events in women. N Engl J Med. 2002;347:716-725.
10. Kokkinos PE, Fernhall B. Physical activity and high density lipoprotein cholesterol levels: what is the relationship? Sports Med. 1999;28:307-314.
11. Van Der Gaag MS, Sterksma A, Schaafsma G, et al. Moderate alcohol consumption lowers risk factors for cardiovascular disease in postmenopausal women fed a controlled diet. Am J Clin Nutr. 2002;75:593-599.
12. Baer DJ, Judd JT, Clevidence BA, et al. Moderate alcohol consumption and changes in postprandial lipoproteins of premenopausal and postmenopausal women: a diet-controlled randomized intervention study. J Womens Health Gend Based Med. 2000;9:607-616.
13. Pins JJ, Keenan JM. Pantethine: a new option in managing dyslipidemia. J Am College Nutr. 2005 [in press].
14. Pins JJ, First S, Shamliyan T, Keenan J. Pantethine beneficially affects apolipoprotein A-1, apolipoprotein B, low-density lipoprotein particle size but not high-sensitivity C-reactive protein in a dyslipidemic population. Circulation. 2004;110(17 suppl 3):III-778.
15. Crouse JR, 3rd. New developments in the use of niacin for treatment of hyperlipidemia: new considerations in the use of an old drug. Coron Artery Dis. 1996;7:321-326.
16. Retail pharmacy quote. Walgreen’s Pharmacy, Minneapolis, Minn. January 2005.
17. Risks and benefits of estrogen plus progestin in healthy post-menopausal women: principal results from the Women’s Health Initiative randomized controlled trial. JAMA. 2002;288:321-333.
18. Hersberger M, von Eckardstein A. Low high-density lipoprotein cholesterol: physical background, clinical importance and drug treatment. Drugs. 2003;63:1907-1945.
19. Mosca L, Grundy SM, Judelson D, et al. Guide to preventive cardiology for women. AHA/ACC scientific statement, consensus panel statement. irculation. 1999;99:2480-2484.
20. Mosca L, Appel LJ, Benjamin EJ, et al. AHA Guidelines: evidence-based guidelines for cardiovascular disease prevention in women. Circulation. 2004;109:672-693.
21. Clarkson T. Women’s Heart Health Symposium. Presented at the American Heart Association annual meeting, Nov 9, 2004, New Orleans.
Oat Ingestion Reduces Systolic and Diastolic Blood Pressure in Patients with Mild or Borderline Hypertension: A Pilot Trial
OBJECTIVES: We assessed the short-term antihypertensive effects of soluble fiber-rich whole oat cereals when added to a standard American diet. In addition, multiple assessments of insulin sensitivity were conducted.
STUDY DESIGN: This was a randomized, controlled, parallel-group pilot study designed to compare an oat cereal group (standardized to 5.52 g/day beta-glucan) to a low-fiber cereal control group (less than 1.0 g/day total fiber) over 6 weeks.
POPULATION: A total of 18 hypertensive and hyperinsulinemic ( ≥10 μU/mL) men and women completed the trial.
OUTCOMES MEASURED: Primary study outcomes were changes in systolic blood pressure (SBP) and diastolic blood pressure (DBP). Secondary outcomes included blood lipid, fasting glucose, and insulin levels and side effects related to elevated blood pressure and increased dietary fiber intake.
RESULTS: The oat cereal group experienced a 7.5 mm Hg reduction in SBP (P < .01) and a 5.5 mm Hg reduction in DBP (P < .02), while there was virtually no change in either SBP or DBP in the control group. In the oat cereal group, a trend was observed for a lower total insulin response to a glucose load, suggesting improved insulin sensitivity. However, this could not be confirmed using estimates from the Bergman Minimal Model, perhaps because of our small sample size. As expected and reported in previous trials, the oats group experienced a significant reduction in both total cholesterol (9%) and low-density lipoprotein cholesterol (14%).
CONCLUSIONS: The addition of oat cereals to the normal diet of persons with hypertension significantly reduces both systolic and diastolic blood pressure. Soluble fiber-rich whole oats may be an effective dietary therapy in the prevention and adjunct treatment of hypertension.
Interest is growing in the use of nonpharmacologic methods for the prevention and management of hypertension. Specifically, the effect of dietary fiber on the incidence and treatment of hypertension has been explored. Epidemiologic studies show that the amount of dietary fiber ingested is inversely related to the incidence of hypertension as well as to systolic blood pressure (SBP) and diastolic blood pressure (DBP) in both hypertensive and normotensive patients.1–5 The results obtained from clinical trials, however, are inconsistent; some report modest blood pressure reductions after increased fiber intake,6–12 while others fail to demonstrate any effect of dietary fiber on blood pressure.13–16 Some animal trials17,18 and human trials19,20 have shown a consistent lowering of blood pressure upon consumption of larger amounts of soluble fiber, suggesting that the antihypertensive effects of fiber may be caused by the soluble fraction and that these effects may be contingent upon the intake of a sufficiently large quantity.
Hypertension often occurs in association with obesity, impaired glucose tolerance, and dyslipidemia. Hyperinsulinemia and insulin resistance are thought to be key pathogenic links among these disturbances.21–23 Studies show that soluble fiber from oats reduces both postprandial blood glucose and insulin levels.24–27 Therefore, we conducted the following pilot trial to investigate the antihypertensive and insulin-modifying effects of oat cereal supplementation in a population of mild and borderline hyperinsulinemic and hypertensive men and women.
Methods
Study protocol
The study participants in this 6-week, randomized, parallel-group, active-controlled pilot trial were recruited by means of local community screenings and mass media advertising. The study protocol was reviewed and approved by the University of Minnesota Institutional Review Board. All participants provided informed consent before official enrollment in the study. One hundred nine men and women aged 20 to 70 were screened for eligibility with a physical exam, medical history, and chemistry and lipid profile (see Table 1 for exclusion criteria). Only generally healthy, untreated hypertensives with average SBP of 130 to 160 mm Hg and DBP of 85 to 100 mm Hg and with at least 1 reading greater than 140/90 as well as moderately elevated levels of fasting insulin (≥10μU/mL) were considered for enrollment. Participants were determined to be eligible only after 2 sets of hypertensive (SBP > 130, DBP > 85) baseline blood pressure readings had been taken 7 days apart and only if all inclusion criteria were fulfilled.
Ultimately, 22 men and women were randomized to either an oat cereal treatment group (standardized to 5.52 g/day beta-glucan) or a low fiber cereal control group (<1 g/day total fiber). Four of these individuals (1 in the treatment and 3 in the control group) discontinued participation because of time constraints. Eighteen healthy, nonsmoking men and women aged 27 to 59 years (44 ± 18; mean, SD) completed the trial. Cereal treatments were isocaloric. Participants were instructed to consume all their cereal (treatment, 137 g; control, 146 g) daily for the next 6 weeks but were allowed to prepare and consume the cereal however and whenever they wished.
Cereal compliance was determined by participant self-report in a daily cereal calendar. In addition, dietary intake was reviewed both at baseline and at the end of the 6-week intervention, using 3-day food records. Side effect data were gathered from participants at baseline and the end of the intervention. Side effects were assessed via a questionnaire consisting of 21 items relating to potential side effects from increased fiber intake (eg, loose stools, flatulence) or hypertension (eg, headaches, dizziness). Participants reported the frequency at which they experienced these side effects on a scale ranging from “never” to “very frequently” (event occurring once or more per day). Each response was assigned a numerical value. Prestudy and post study averages were used in analyses.
Blood Pressure, Plasma Lipid Concentrations, Glucose Metabolism, and Insulin Sensitivity
Blood pressure was measured weekly for each participant for the duration of the study. Each participant reported to the Hypertension and Cholesterol Research Clinic located at the University of Minnesota Medical School at approximately the same time for each blood pressure reading. All readings were obtained in the morning after participants had rested quietly, seated, for at least 5 minutes in an examination room. An examiner who was blinded to the treatment groups took readings on the right arm using a mercury column sphygmomanometer (Korotkoff phase V for DBP). Standard cuff size was used unless upper arm circumference exceeded 31 cm, in which case the examiner used a large cuff with 15 x 35-cm bladders. Measurements were repeated 4 times in 2-minute intervals. The mean of the last 3 readings was calculated and used in analyses.
To determine plasma lipid concentrations (total, high-density lipoprotein [HDL], and low-density lipoprotein [LDL] cholesterol and triglycerides), pretreatment and posttreatment blood samples were drawn. A 75-g, 3-hour oral glucose-tolerance test (OGTT) was administered before and after treatment to assess participants’ glucose tolerance and insulin response. Whole blood sampling occurred at -30, 0, 30, 60, 90, 120, 150, and 180 minutes. A measure of insulin sensitivity was assessed within 48 hours after the OGTT by means of the modified frequently sampled intravenous glucose tolerance test (FSIGT).28 The glucose and insulin data derived from this test were used to calculate the insulin sensitivity index (SI) employing the minimal-model method developed by Bergman.29
Statistical methods
Reported results are expressed in terms of means ± SD or means SE. Student’s t test for independent samples was used to compare the 2 treatment groups at baseline and to compare mean change scores between the 2 groups. Additionally, area-under-the-curve analyses were performed to compare OGTT insulin curves. All analyses were performed on data from an intent-to-treat population, which included all randomized participants. Statistical tests were 2 sided, performed at the 5% level of significance, and conducted with Statistical Analysis System software (SAS Institute, Cary, N.C.).
Results
No statistically significant differences in baseline characteristics occurred between the groups, although this comparison is limited by the small sample size Table 2. LDL cholesterol and total cholesterol levels and blood pressure were somewhat higher in the treatment group. The blood pressure measurements in the treatment group resulted in an average SBP of 143 ± 3.7 mm Hg before intervention and 135 ± 2.6 mm Hg after intervention (an average of the last 2 study visits, P < .01) Table 3. No significant change in SBP was observed in the control group. A significant difference between the treatment and control groups was observed for the change in SBP (P < .02). DBP dropped from 93 ± 1.9 mm Hg to 87 ± 2.2 mm Hg after the oat fiber intervention (P = .02), with no significant change in the control group (P = .94). A borderline significant trend was noted for the change scores of DBP between groups (P = .055).
Changes in fasting insulin, insulin sensitivity (SI), and insulin curves derived from the oral glucose tolerance tests were assessed. Fasting insulin values Table 3 were taken from the OGTT (preglucose infusion values). Neither the control group (P = 1.00) nor the treatment group (P = .753) showed a significant change in fasting insulin levels. The Bergman minimal model method was used to estimate insulin sensitivity and showed no significant change in either group. Area-under-the-curve analysis of the insulin data derived from the OGTTs before and after treatment with oat cereal (Figure 1 and Figure 2) suggested a trend toward significance in terms of less insulin required to clear a glucose load (top of graphs, P = .093), with no significant changes in the control group (bottom).
Total cholesterol concentrations dropped 16.2 ± 6.3 mg/dL in the oat cereal group (P = .030), with a slight (nonsignificant) increase in the control group (P = .48). Additionally, a comparison of the changes in total cholesterol between the 2 groups revealed a significant mean difference of 21.1 ± 9.1 mg/dL (P = .035). LDL cholesterol was also reduced significantly after the oat cereal intervention by 15.8 ± 5.9 mg/dL (P = .025). The nonsignificant increase in LDL cholesterol in the control group (P = .231) combined with the significant reduction in the treatment group resulted in a significant difference between the groups after intervention (P < .015). Neither group experienced significant changes in HDL cholesterol or triglyceride concentrations.
An analysis of the side effect data showed no significant difference in the occurrence of side effects between groups. There was an overall decrease in the frequency of dietary fiber-related and hypertension-related side effects in both groups, with a more substantial reduction occurring in the oat cereal group (P = .11). Total body weight did not change significantly in either group. Additionally, both groups were very compliant (approximately 90%) in terms of cereal consumption Table 3.
Discussion
The results of this pilot study suggest that the inclusion of oats into the standard American diet of people with borderline or mild hypertension may reduce both SBP and DBP. In persons consuming 5.52 g/day of beta-glucan soluble fiber from oat cereal for 6 weeks, we found a statistically and clinically significant decrease in both SBP and DBP (7.5 mm Hg and 5.5 mm Hg, respectively) and a trend toward improved OGTT-determined insulin sensitivity. These findings warrant a large-scale clinical trial to explore further the relationship between whole-grain oat consumption and blood pressure, especially considering the limitations of this pilot study.
As with all small-scale trials, this one lacked sufficient power to detect true changes in both primary and secondary outcome variables. It is possible that regression to the mean explains at least part of the treatment effect, since participants in the oats group began the study with higher SBP, DBP, and LDL cholesterol levels than controls. In addition, it is possible that the reported blood pressure changes could have been caused by “other” undetected dietary change made by members of the oats group. Future trials will need to collect and analyze dietary data carefully; feeding trials should be considered. Such dietary analyses may indicate that certain micronutrients partially explain the hypotensive effects of whole-grain oat consumption. The DASH trial and others have consistently demonstrated that diets rich in certain micronutrients can reduce blood pressure.30,31
Soluble fiber-rich oat cereals may affect blood pressure by modulating changes in insulin metabolism. The mechanism of action is thought to involve the slowed absorption of macronutrients from the gut, resulting in a flattening of the postprandial glycemic curve.29 These lower postprandial blood glucose levels elicit a lower insulin response to accommodate its clearance from the plasma. This process may lead to improved insulin sensitivity if the lower circulating insulin levels lead eventually to upregulation of the insulin receptors in peripheral tissues. A recent animal trial demonstrated that soluble fiber feeding improved insulin sensitivity by increasing skeletal muscle plasma membrane GLUT-4 content.32 Findings in this pilot suggest that over time, oat ingestion may reduce the amount of insulin needed to clear a glucose load. However, the study was underpowered to detect significant differences in more sensitive measures of insulin resistance. The causal mechanistic relationship among whole grain oat consumption, blood pressure, and insulin resistance might be best studied using a long-term feeding study design.
Alternate mechanisms, such as attenuation in endothelial function, may have affected blood pressure responses in this study.33 Drugs specific to endothelial cell receptors mediating vasodilation are known to lower blood pressure.34 Moreover, plasma cholesterol reductions are associated with improvements in endothelium-mediated vasodilation.35,36 In addition, preliminary evidence in animals supports a direct relationship between changes in plasma cholesterol concentrations and blood pressure.37 In the present study, plasma cholesterol levels were significantly reduced in participants who ingested whole grain oat-based cereals compared to a more refined grain wheat, corn, and rice control. Thus, it is possible that the blood pressure reduction observed in the subjects consuming oats resulted in part from improved endothelial function due to a drop in plasma cholesterol. Additional research is needed to fully investigate this pathway.
From a practical standpoint, improvements in SBP and DBP such as those observed in this study would be a useful contribution to the clinical management of hypertension. The cereal feeding intervention was well tolerated. Participants were very compliant for the 6-week treatment period. Substantial improvements in blood lipids could serve as an added incentive for patients to maintain long-term compliance with feeding recommendations.18,19 Since treatment of hypertension is a lifelong process for most patients, future studies would need to assess the effectiveness of oat cereals to maintain blood pressure benefits over a longer time. Such studies may need to consider dietary options such as soluble fiber-rich fruits in addition to cereal consumption in efforts to deliver the desired quantity of soluble fiber. Future trials will have to investigate the antihypertensive effect of whole oats in other populations, such as people with diabetes, and to study not only surrogate endpoints such as blood pressure but also patient-oriented outcomes such as mortality and morbidity.
1. He J, Klag M, Whelton P, et al. Oats and buckwheat intakes and cardiovascular disease risk factors in an ethnic minority of China. Am J Clin Nutr 1995;61:366-72.
2. Lichtenstein M, Burr M, Fehily A, Yarnell J. Heart rate, employment status, and prevalent ischemic heart disease confound relation between cereal fibre intake and blood pressure. J Epidemiol Community Health 1986;40:330-3.
3. Ascherio A, Rimm E, Giovannucci E, et al. A prospective study of nutritional factors and hypertension among US men. Circulation 1992;86:1475-84.
4. Hallfrisch J, Tobin J, Muller D, Andres R. Fiber intake, age, and other coronary risk factors in men of the Baltimore Longitudinal Study (1959-1975). J Gerontol 1988;43:M64-8.
5. Ascherio A, Hennekens C, Willett W, et al. Prospective study of nutritional factors, blood pressure, and hypertension among US women. Hypertension 1996;27:1065-72.
6. Schlamowitz P, Halberg T, Warnoe O, Wilstrup F, Ryttig K. Treatment of mild to moderate hypertension with dietary fiber. Lancet 1987;2:622-3.
7. Eliasson K, Ryttig K, Hylander B, Rossner S. A dietary fiber supplement in the treatment of mild hypertension. A randomized, double-blind, placebo-controlled trial. J Hypertens 1992;10:195-9.
8. Ryttig K, Tellnes G, Haegh L, Boe E, Fagerthun H. A dietary fiber supplement and weight maintenance after weight reduction: a randomized, double-blind, placebo-controlled long-term trial. Int J Obes 1989;13:165-71.
9. Dodson P, Stephenson J, Dodson L, et al. Randomised blind controlled trial of a high fiber, low fat and low sodium dietary regimen in mild essential hypertension. J Hum Hypertens 1989;3:197-202.
10. Little P, Girling G, Hasler A, Trafford A. A controlled trial of a low sodium, low fat, high fibre diet in treated hypertensive patients: effect on antihypertensive drug requirement in clinical practice. J Hum Hypertens 1991;5:175-81.
11. Rossner S, Andersson I, Ryttig K. Effects of a dietary fiber supplement to a weight reduction program on blood pressure. Acta Med Scand 1988;223:353-7.
12. Sandstrom B, Marckmann P, Bindslev N. An eight-month controlled study of a low-fat high-fiber diet: effects on blood lipids and blood pressure in healthy young subjects. Eur J Clin Nutr 1992;46:95-109.
13. Kestin M, Moss R, Clifton P. Comparative effects of three cereal brans on plasma lipids, blood pressure, and glucose metabolism in mildly hypercholesterolemic men. Am J Clin Nutr 1990;52:661-6.
14. Swain J, Rouse I, Curley C, Sacks F. Comparison of the effects of oat bran and low-fiber wheat on serum lipoprotein levels and blood pressure. N Engl J Med 1990;322:147-52.
15. Sciarrone S, Beilin L, Rouse I, Rogers P. A factorial study of salt restriction and a low-fat/high-fibre diet in hypertensive subjects. J Hypertens 1992;10:287-98.
16. Margetts B, Beilin L, Vandongen R, Armstrong B. A randomized controlled trial of the effect of dietary fiber on blood pressure. Clin Sci 1987;72:343-50.
17. el Zein M, Areas J, Knapka J, et al. Influence of oat bran on sucrose-induced blood pressure elevations in SHR. Life Sci 1990;47:1121-8.
18. Gondal J, MacArthy P, Myers A, Preuss H. Effects of dietary sucrose and fibers on blood pressure in hypertensive rats. Clin Nephrol 1996;45:163-8.
19. Krotkiewski M. Effect of guar gum on the arterial blood pressure. Acta Med Scand 1987;222:43-9.
20. Singh R, Rastogi S, Singh N, Ghosh S, Gupta S, Niaz M. Can guava fruit intake decrease blood pressure and blood lipids? J Hum Hypertens 1993;7:33-8.
21. Weidmann P, de Courten M, Bohlen L. Insulin resistance, hyperinsulinemia and hypertension. J Hypertens 1993;11:S27-38.
22. Tuck M. Obesity, the sympathetic nervous system, and essential hypertension. Hypertension 1992;19:I67-77.
23. Sowers J. Insulin resistance, hyperinsulinemia, dyslipidemia, hypertension, and accelerated atherosclerosis. J Clin Pharmacol 1992;32:529-35.
24. Braaten J, Wood P, Scott F, Riedel K, Poste L, Collins M. Oat gum lowers glucose and insulin after an oral glucose load. Am J Clin Nutr 1991;53:1425-30.
25. Braaten J, Scott F, Wood P, et al. High ß-glucan oat bran and oat gum reduce postprandial blood glucose and insulin in subjects with and without type 2 diabetes. Diabet Med 1994;11:312-8.
26. Hallfrisch J, Scholfield D, Behall K. Diets containing soluble oat extracts improve glucose and insulin responses of moderately hypercholesterolemic men and women. Am J Clin Nutr 1995;61:379-84.
27. Fukagawa N, Anderson J, Hageman G, Young V, Minaker K. High-carbohydrate, high-fiber diets increase peripheral insulin sensitivity in healthy young and old adults. Am J Clin Nutr 1990;52:524-8.
28. Welch S, Gebhart S, Bergman R, Phillips L. Minimal model analysis of IVGTT-derived insulin sensitivity in diabetic subjects. J Clin Endocrinol Metab 1990;71:1508-18.
29. Bergman R, Ider Y, Bowden C, Cobelli C. Quantitative estimation of insulin sensitivity. Am J Physiol 1979;236:E667-77.
30. Appel LJ, Moore TJ, Obarzanek E, et al. A clinical trial on the effects of dietary patterns on blood pressure. N Engl J Med 1997;336:1117-24.
31. Appel LJ. The role of diet in the prevention and treatment of hypertension. Curr Atheroscler Rep 2000;2:521-8.
32. Song YJ, Sawamura M, Ikeda K, Igawa S, Yamori Y. Soluble dietary fiber improves insulin sensitivity by increasing muscle GLUT-4 content in stroke-prone spontaneously hypertensive rats. Clin Exp Pharm Physiol 2000;27:41-5.
33. Taddei S, Salvetti A. Pathogenic factors in hypertension. Endothelial Factors. Clin Exp Hypertens 1996;18:323-35.
34. Krum H, Viskoper R, Lacourciere Y, Budde B, Charlon V. The effect of an endothelium-receptor antagonist, Bosentan, on blood pressure in patients with essential hypertension. N Engl J Med 1998;338:784-90.
35. Anderson T, Meredith I, Yeung A. The effect of cholesterol-lowering and antioxidant therapy on endothelium-dependent coronary vasomotion. N Engl J Med 1995;332:488-93.
36. Vogel R, Corretti M, Plotnick G. Changes in flow-mediated brachial artery vasoactivity with lowering of desirable cholesterol levels in healthy middle-aged men. Am J Cardiol 1996;77:37-40.
37. Crago M, West S, Hoeprich K, Michaelis K, McKenzie J. Effects of hyperlipidemia on blood pressure and coronary blood flow in swine. FASEB J 1998;12:A238.-
To submit a letter to the editor on this topic, click here: jfp@fammed.uc.edu.
OBJECTIVES: We assessed the short-term antihypertensive effects of soluble fiber-rich whole oat cereals when added to a standard American diet. In addition, multiple assessments of insulin sensitivity were conducted.
STUDY DESIGN: This was a randomized, controlled, parallel-group pilot study designed to compare an oat cereal group (standardized to 5.52 g/day beta-glucan) to a low-fiber cereal control group (less than 1.0 g/day total fiber) over 6 weeks.
POPULATION: A total of 18 hypertensive and hyperinsulinemic ( ≥10 μU/mL) men and women completed the trial.
OUTCOMES MEASURED: Primary study outcomes were changes in systolic blood pressure (SBP) and diastolic blood pressure (DBP). Secondary outcomes included blood lipid, fasting glucose, and insulin levels and side effects related to elevated blood pressure and increased dietary fiber intake.
RESULTS: The oat cereal group experienced a 7.5 mm Hg reduction in SBP (P < .01) and a 5.5 mm Hg reduction in DBP (P < .02), while there was virtually no change in either SBP or DBP in the control group. In the oat cereal group, a trend was observed for a lower total insulin response to a glucose load, suggesting improved insulin sensitivity. However, this could not be confirmed using estimates from the Bergman Minimal Model, perhaps because of our small sample size. As expected and reported in previous trials, the oats group experienced a significant reduction in both total cholesterol (9%) and low-density lipoprotein cholesterol (14%).
CONCLUSIONS: The addition of oat cereals to the normal diet of persons with hypertension significantly reduces both systolic and diastolic blood pressure. Soluble fiber-rich whole oats may be an effective dietary therapy in the prevention and adjunct treatment of hypertension.
Interest is growing in the use of nonpharmacologic methods for the prevention and management of hypertension. Specifically, the effect of dietary fiber on the incidence and treatment of hypertension has been explored. Epidemiologic studies show that the amount of dietary fiber ingested is inversely related to the incidence of hypertension as well as to systolic blood pressure (SBP) and diastolic blood pressure (DBP) in both hypertensive and normotensive patients.1–5 The results obtained from clinical trials, however, are inconsistent; some report modest blood pressure reductions after increased fiber intake,6–12 while others fail to demonstrate any effect of dietary fiber on blood pressure.13–16 Some animal trials17,18 and human trials19,20 have shown a consistent lowering of blood pressure upon consumption of larger amounts of soluble fiber, suggesting that the antihypertensive effects of fiber may be caused by the soluble fraction and that these effects may be contingent upon the intake of a sufficiently large quantity.
Hypertension often occurs in association with obesity, impaired glucose tolerance, and dyslipidemia. Hyperinsulinemia and insulin resistance are thought to be key pathogenic links among these disturbances.21–23 Studies show that soluble fiber from oats reduces both postprandial blood glucose and insulin levels.24–27 Therefore, we conducted the following pilot trial to investigate the antihypertensive and insulin-modifying effects of oat cereal supplementation in a population of mild and borderline hyperinsulinemic and hypertensive men and women.
Methods
Study protocol
The study participants in this 6-week, randomized, parallel-group, active-controlled pilot trial were recruited by means of local community screenings and mass media advertising. The study protocol was reviewed and approved by the University of Minnesota Institutional Review Board. All participants provided informed consent before official enrollment in the study. One hundred nine men and women aged 20 to 70 were screened for eligibility with a physical exam, medical history, and chemistry and lipid profile (see Table 1 for exclusion criteria). Only generally healthy, untreated hypertensives with average SBP of 130 to 160 mm Hg and DBP of 85 to 100 mm Hg and with at least 1 reading greater than 140/90 as well as moderately elevated levels of fasting insulin (≥10μU/mL) were considered for enrollment. Participants were determined to be eligible only after 2 sets of hypertensive (SBP > 130, DBP > 85) baseline blood pressure readings had been taken 7 days apart and only if all inclusion criteria were fulfilled.
Ultimately, 22 men and women were randomized to either an oat cereal treatment group (standardized to 5.52 g/day beta-glucan) or a low fiber cereal control group (<1 g/day total fiber). Four of these individuals (1 in the treatment and 3 in the control group) discontinued participation because of time constraints. Eighteen healthy, nonsmoking men and women aged 27 to 59 years (44 ± 18; mean, SD) completed the trial. Cereal treatments were isocaloric. Participants were instructed to consume all their cereal (treatment, 137 g; control, 146 g) daily for the next 6 weeks but were allowed to prepare and consume the cereal however and whenever they wished.
Cereal compliance was determined by participant self-report in a daily cereal calendar. In addition, dietary intake was reviewed both at baseline and at the end of the 6-week intervention, using 3-day food records. Side effect data were gathered from participants at baseline and the end of the intervention. Side effects were assessed via a questionnaire consisting of 21 items relating to potential side effects from increased fiber intake (eg, loose stools, flatulence) or hypertension (eg, headaches, dizziness). Participants reported the frequency at which they experienced these side effects on a scale ranging from “never” to “very frequently” (event occurring once or more per day). Each response was assigned a numerical value. Prestudy and post study averages were used in analyses.
Blood Pressure, Plasma Lipid Concentrations, Glucose Metabolism, and Insulin Sensitivity
Blood pressure was measured weekly for each participant for the duration of the study. Each participant reported to the Hypertension and Cholesterol Research Clinic located at the University of Minnesota Medical School at approximately the same time for each blood pressure reading. All readings were obtained in the morning after participants had rested quietly, seated, for at least 5 minutes in an examination room. An examiner who was blinded to the treatment groups took readings on the right arm using a mercury column sphygmomanometer (Korotkoff phase V for DBP). Standard cuff size was used unless upper arm circumference exceeded 31 cm, in which case the examiner used a large cuff with 15 x 35-cm bladders. Measurements were repeated 4 times in 2-minute intervals. The mean of the last 3 readings was calculated and used in analyses.
To determine plasma lipid concentrations (total, high-density lipoprotein [HDL], and low-density lipoprotein [LDL] cholesterol and triglycerides), pretreatment and posttreatment blood samples were drawn. A 75-g, 3-hour oral glucose-tolerance test (OGTT) was administered before and after treatment to assess participants’ glucose tolerance and insulin response. Whole blood sampling occurred at -30, 0, 30, 60, 90, 120, 150, and 180 minutes. A measure of insulin sensitivity was assessed within 48 hours after the OGTT by means of the modified frequently sampled intravenous glucose tolerance test (FSIGT).28 The glucose and insulin data derived from this test were used to calculate the insulin sensitivity index (SI) employing the minimal-model method developed by Bergman.29
Statistical methods
Reported results are expressed in terms of means ± SD or means SE. Student’s t test for independent samples was used to compare the 2 treatment groups at baseline and to compare mean change scores between the 2 groups. Additionally, area-under-the-curve analyses were performed to compare OGTT insulin curves. All analyses were performed on data from an intent-to-treat population, which included all randomized participants. Statistical tests were 2 sided, performed at the 5% level of significance, and conducted with Statistical Analysis System software (SAS Institute, Cary, N.C.).
Results
No statistically significant differences in baseline characteristics occurred between the groups, although this comparison is limited by the small sample size Table 2. LDL cholesterol and total cholesterol levels and blood pressure were somewhat higher in the treatment group. The blood pressure measurements in the treatment group resulted in an average SBP of 143 ± 3.7 mm Hg before intervention and 135 ± 2.6 mm Hg after intervention (an average of the last 2 study visits, P < .01) Table 3. No significant change in SBP was observed in the control group. A significant difference between the treatment and control groups was observed for the change in SBP (P < .02). DBP dropped from 93 ± 1.9 mm Hg to 87 ± 2.2 mm Hg after the oat fiber intervention (P = .02), with no significant change in the control group (P = .94). A borderline significant trend was noted for the change scores of DBP between groups (P = .055).
Changes in fasting insulin, insulin sensitivity (SI), and insulin curves derived from the oral glucose tolerance tests were assessed. Fasting insulin values Table 3 were taken from the OGTT (preglucose infusion values). Neither the control group (P = 1.00) nor the treatment group (P = .753) showed a significant change in fasting insulin levels. The Bergman minimal model method was used to estimate insulin sensitivity and showed no significant change in either group. Area-under-the-curve analysis of the insulin data derived from the OGTTs before and after treatment with oat cereal (Figure 1 and Figure 2) suggested a trend toward significance in terms of less insulin required to clear a glucose load (top of graphs, P = .093), with no significant changes in the control group (bottom).
Total cholesterol concentrations dropped 16.2 ± 6.3 mg/dL in the oat cereal group (P = .030), with a slight (nonsignificant) increase in the control group (P = .48). Additionally, a comparison of the changes in total cholesterol between the 2 groups revealed a significant mean difference of 21.1 ± 9.1 mg/dL (P = .035). LDL cholesterol was also reduced significantly after the oat cereal intervention by 15.8 ± 5.9 mg/dL (P = .025). The nonsignificant increase in LDL cholesterol in the control group (P = .231) combined with the significant reduction in the treatment group resulted in a significant difference between the groups after intervention (P < .015). Neither group experienced significant changes in HDL cholesterol or triglyceride concentrations.
An analysis of the side effect data showed no significant difference in the occurrence of side effects between groups. There was an overall decrease in the frequency of dietary fiber-related and hypertension-related side effects in both groups, with a more substantial reduction occurring in the oat cereal group (P = .11). Total body weight did not change significantly in either group. Additionally, both groups were very compliant (approximately 90%) in terms of cereal consumption Table 3.
Discussion
The results of this pilot study suggest that the inclusion of oats into the standard American diet of people with borderline or mild hypertension may reduce both SBP and DBP. In persons consuming 5.52 g/day of beta-glucan soluble fiber from oat cereal for 6 weeks, we found a statistically and clinically significant decrease in both SBP and DBP (7.5 mm Hg and 5.5 mm Hg, respectively) and a trend toward improved OGTT-determined insulin sensitivity. These findings warrant a large-scale clinical trial to explore further the relationship between whole-grain oat consumption and blood pressure, especially considering the limitations of this pilot study.
As with all small-scale trials, this one lacked sufficient power to detect true changes in both primary and secondary outcome variables. It is possible that regression to the mean explains at least part of the treatment effect, since participants in the oats group began the study with higher SBP, DBP, and LDL cholesterol levels than controls. In addition, it is possible that the reported blood pressure changes could have been caused by “other” undetected dietary change made by members of the oats group. Future trials will need to collect and analyze dietary data carefully; feeding trials should be considered. Such dietary analyses may indicate that certain micronutrients partially explain the hypotensive effects of whole-grain oat consumption. The DASH trial and others have consistently demonstrated that diets rich in certain micronutrients can reduce blood pressure.30,31
Soluble fiber-rich oat cereals may affect blood pressure by modulating changes in insulin metabolism. The mechanism of action is thought to involve the slowed absorption of macronutrients from the gut, resulting in a flattening of the postprandial glycemic curve.29 These lower postprandial blood glucose levels elicit a lower insulin response to accommodate its clearance from the plasma. This process may lead to improved insulin sensitivity if the lower circulating insulin levels lead eventually to upregulation of the insulin receptors in peripheral tissues. A recent animal trial demonstrated that soluble fiber feeding improved insulin sensitivity by increasing skeletal muscle plasma membrane GLUT-4 content.32 Findings in this pilot suggest that over time, oat ingestion may reduce the amount of insulin needed to clear a glucose load. However, the study was underpowered to detect significant differences in more sensitive measures of insulin resistance. The causal mechanistic relationship among whole grain oat consumption, blood pressure, and insulin resistance might be best studied using a long-term feeding study design.
Alternate mechanisms, such as attenuation in endothelial function, may have affected blood pressure responses in this study.33 Drugs specific to endothelial cell receptors mediating vasodilation are known to lower blood pressure.34 Moreover, plasma cholesterol reductions are associated with improvements in endothelium-mediated vasodilation.35,36 In addition, preliminary evidence in animals supports a direct relationship between changes in plasma cholesterol concentrations and blood pressure.37 In the present study, plasma cholesterol levels were significantly reduced in participants who ingested whole grain oat-based cereals compared to a more refined grain wheat, corn, and rice control. Thus, it is possible that the blood pressure reduction observed in the subjects consuming oats resulted in part from improved endothelial function due to a drop in plasma cholesterol. Additional research is needed to fully investigate this pathway.
From a practical standpoint, improvements in SBP and DBP such as those observed in this study would be a useful contribution to the clinical management of hypertension. The cereal feeding intervention was well tolerated. Participants were very compliant for the 6-week treatment period. Substantial improvements in blood lipids could serve as an added incentive for patients to maintain long-term compliance with feeding recommendations.18,19 Since treatment of hypertension is a lifelong process for most patients, future studies would need to assess the effectiveness of oat cereals to maintain blood pressure benefits over a longer time. Such studies may need to consider dietary options such as soluble fiber-rich fruits in addition to cereal consumption in efforts to deliver the desired quantity of soluble fiber. Future trials will have to investigate the antihypertensive effect of whole oats in other populations, such as people with diabetes, and to study not only surrogate endpoints such as blood pressure but also patient-oriented outcomes such as mortality and morbidity.
OBJECTIVES: We assessed the short-term antihypertensive effects of soluble fiber-rich whole oat cereals when added to a standard American diet. In addition, multiple assessments of insulin sensitivity were conducted.
STUDY DESIGN: This was a randomized, controlled, parallel-group pilot study designed to compare an oat cereal group (standardized to 5.52 g/day beta-glucan) to a low-fiber cereal control group (less than 1.0 g/day total fiber) over 6 weeks.
POPULATION: A total of 18 hypertensive and hyperinsulinemic ( ≥10 μU/mL) men and women completed the trial.
OUTCOMES MEASURED: Primary study outcomes were changes in systolic blood pressure (SBP) and diastolic blood pressure (DBP). Secondary outcomes included blood lipid, fasting glucose, and insulin levels and side effects related to elevated blood pressure and increased dietary fiber intake.
RESULTS: The oat cereal group experienced a 7.5 mm Hg reduction in SBP (P < .01) and a 5.5 mm Hg reduction in DBP (P < .02), while there was virtually no change in either SBP or DBP in the control group. In the oat cereal group, a trend was observed for a lower total insulin response to a glucose load, suggesting improved insulin sensitivity. However, this could not be confirmed using estimates from the Bergman Minimal Model, perhaps because of our small sample size. As expected and reported in previous trials, the oats group experienced a significant reduction in both total cholesterol (9%) and low-density lipoprotein cholesterol (14%).
CONCLUSIONS: The addition of oat cereals to the normal diet of persons with hypertension significantly reduces both systolic and diastolic blood pressure. Soluble fiber-rich whole oats may be an effective dietary therapy in the prevention and adjunct treatment of hypertension.
Interest is growing in the use of nonpharmacologic methods for the prevention and management of hypertension. Specifically, the effect of dietary fiber on the incidence and treatment of hypertension has been explored. Epidemiologic studies show that the amount of dietary fiber ingested is inversely related to the incidence of hypertension as well as to systolic blood pressure (SBP) and diastolic blood pressure (DBP) in both hypertensive and normotensive patients.1–5 The results obtained from clinical trials, however, are inconsistent; some report modest blood pressure reductions after increased fiber intake,6–12 while others fail to demonstrate any effect of dietary fiber on blood pressure.13–16 Some animal trials17,18 and human trials19,20 have shown a consistent lowering of blood pressure upon consumption of larger amounts of soluble fiber, suggesting that the antihypertensive effects of fiber may be caused by the soluble fraction and that these effects may be contingent upon the intake of a sufficiently large quantity.
Hypertension often occurs in association with obesity, impaired glucose tolerance, and dyslipidemia. Hyperinsulinemia and insulin resistance are thought to be key pathogenic links among these disturbances.21–23 Studies show that soluble fiber from oats reduces both postprandial blood glucose and insulin levels.24–27 Therefore, we conducted the following pilot trial to investigate the antihypertensive and insulin-modifying effects of oat cereal supplementation in a population of mild and borderline hyperinsulinemic and hypertensive men and women.
Methods
Study protocol
The study participants in this 6-week, randomized, parallel-group, active-controlled pilot trial were recruited by means of local community screenings and mass media advertising. The study protocol was reviewed and approved by the University of Minnesota Institutional Review Board. All participants provided informed consent before official enrollment in the study. One hundred nine men and women aged 20 to 70 were screened for eligibility with a physical exam, medical history, and chemistry and lipid profile (see Table 1 for exclusion criteria). Only generally healthy, untreated hypertensives with average SBP of 130 to 160 mm Hg and DBP of 85 to 100 mm Hg and with at least 1 reading greater than 140/90 as well as moderately elevated levels of fasting insulin (≥10μU/mL) were considered for enrollment. Participants were determined to be eligible only after 2 sets of hypertensive (SBP > 130, DBP > 85) baseline blood pressure readings had been taken 7 days apart and only if all inclusion criteria were fulfilled.
Ultimately, 22 men and women were randomized to either an oat cereal treatment group (standardized to 5.52 g/day beta-glucan) or a low fiber cereal control group (<1 g/day total fiber). Four of these individuals (1 in the treatment and 3 in the control group) discontinued participation because of time constraints. Eighteen healthy, nonsmoking men and women aged 27 to 59 years (44 ± 18; mean, SD) completed the trial. Cereal treatments were isocaloric. Participants were instructed to consume all their cereal (treatment, 137 g; control, 146 g) daily for the next 6 weeks but were allowed to prepare and consume the cereal however and whenever they wished.
Cereal compliance was determined by participant self-report in a daily cereal calendar. In addition, dietary intake was reviewed both at baseline and at the end of the 6-week intervention, using 3-day food records. Side effect data were gathered from participants at baseline and the end of the intervention. Side effects were assessed via a questionnaire consisting of 21 items relating to potential side effects from increased fiber intake (eg, loose stools, flatulence) or hypertension (eg, headaches, dizziness). Participants reported the frequency at which they experienced these side effects on a scale ranging from “never” to “very frequently” (event occurring once or more per day). Each response was assigned a numerical value. Prestudy and post study averages were used in analyses.
Blood Pressure, Plasma Lipid Concentrations, Glucose Metabolism, and Insulin Sensitivity
Blood pressure was measured weekly for each participant for the duration of the study. Each participant reported to the Hypertension and Cholesterol Research Clinic located at the University of Minnesota Medical School at approximately the same time for each blood pressure reading. All readings were obtained in the morning after participants had rested quietly, seated, for at least 5 minutes in an examination room. An examiner who was blinded to the treatment groups took readings on the right arm using a mercury column sphygmomanometer (Korotkoff phase V for DBP). Standard cuff size was used unless upper arm circumference exceeded 31 cm, in which case the examiner used a large cuff with 15 x 35-cm bladders. Measurements were repeated 4 times in 2-minute intervals. The mean of the last 3 readings was calculated and used in analyses.
To determine plasma lipid concentrations (total, high-density lipoprotein [HDL], and low-density lipoprotein [LDL] cholesterol and triglycerides), pretreatment and posttreatment blood samples were drawn. A 75-g, 3-hour oral glucose-tolerance test (OGTT) was administered before and after treatment to assess participants’ glucose tolerance and insulin response. Whole blood sampling occurred at -30, 0, 30, 60, 90, 120, 150, and 180 minutes. A measure of insulin sensitivity was assessed within 48 hours after the OGTT by means of the modified frequently sampled intravenous glucose tolerance test (FSIGT).28 The glucose and insulin data derived from this test were used to calculate the insulin sensitivity index (SI) employing the minimal-model method developed by Bergman.29
Statistical methods
Reported results are expressed in terms of means ± SD or means SE. Student’s t test for independent samples was used to compare the 2 treatment groups at baseline and to compare mean change scores between the 2 groups. Additionally, area-under-the-curve analyses were performed to compare OGTT insulin curves. All analyses were performed on data from an intent-to-treat population, which included all randomized participants. Statistical tests were 2 sided, performed at the 5% level of significance, and conducted with Statistical Analysis System software (SAS Institute, Cary, N.C.).
Results
No statistically significant differences in baseline characteristics occurred between the groups, although this comparison is limited by the small sample size Table 2. LDL cholesterol and total cholesterol levels and blood pressure were somewhat higher in the treatment group. The blood pressure measurements in the treatment group resulted in an average SBP of 143 ± 3.7 mm Hg before intervention and 135 ± 2.6 mm Hg after intervention (an average of the last 2 study visits, P < .01) Table 3. No significant change in SBP was observed in the control group. A significant difference between the treatment and control groups was observed for the change in SBP (P < .02). DBP dropped from 93 ± 1.9 mm Hg to 87 ± 2.2 mm Hg after the oat fiber intervention (P = .02), with no significant change in the control group (P = .94). A borderline significant trend was noted for the change scores of DBP between groups (P = .055).
Changes in fasting insulin, insulin sensitivity (SI), and insulin curves derived from the oral glucose tolerance tests were assessed. Fasting insulin values Table 3 were taken from the OGTT (preglucose infusion values). Neither the control group (P = 1.00) nor the treatment group (P = .753) showed a significant change in fasting insulin levels. The Bergman minimal model method was used to estimate insulin sensitivity and showed no significant change in either group. Area-under-the-curve analysis of the insulin data derived from the OGTTs before and after treatment with oat cereal (Figure 1 and Figure 2) suggested a trend toward significance in terms of less insulin required to clear a glucose load (top of graphs, P = .093), with no significant changes in the control group (bottom).
Total cholesterol concentrations dropped 16.2 ± 6.3 mg/dL in the oat cereal group (P = .030), with a slight (nonsignificant) increase in the control group (P = .48). Additionally, a comparison of the changes in total cholesterol between the 2 groups revealed a significant mean difference of 21.1 ± 9.1 mg/dL (P = .035). LDL cholesterol was also reduced significantly after the oat cereal intervention by 15.8 ± 5.9 mg/dL (P = .025). The nonsignificant increase in LDL cholesterol in the control group (P = .231) combined with the significant reduction in the treatment group resulted in a significant difference between the groups after intervention (P < .015). Neither group experienced significant changes in HDL cholesterol or triglyceride concentrations.
An analysis of the side effect data showed no significant difference in the occurrence of side effects between groups. There was an overall decrease in the frequency of dietary fiber-related and hypertension-related side effects in both groups, with a more substantial reduction occurring in the oat cereal group (P = .11). Total body weight did not change significantly in either group. Additionally, both groups were very compliant (approximately 90%) in terms of cereal consumption Table 3.
Discussion
The results of this pilot study suggest that the inclusion of oats into the standard American diet of people with borderline or mild hypertension may reduce both SBP and DBP. In persons consuming 5.52 g/day of beta-glucan soluble fiber from oat cereal for 6 weeks, we found a statistically and clinically significant decrease in both SBP and DBP (7.5 mm Hg and 5.5 mm Hg, respectively) and a trend toward improved OGTT-determined insulin sensitivity. These findings warrant a large-scale clinical trial to explore further the relationship between whole-grain oat consumption and blood pressure, especially considering the limitations of this pilot study.
As with all small-scale trials, this one lacked sufficient power to detect true changes in both primary and secondary outcome variables. It is possible that regression to the mean explains at least part of the treatment effect, since participants in the oats group began the study with higher SBP, DBP, and LDL cholesterol levels than controls. In addition, it is possible that the reported blood pressure changes could have been caused by “other” undetected dietary change made by members of the oats group. Future trials will need to collect and analyze dietary data carefully; feeding trials should be considered. Such dietary analyses may indicate that certain micronutrients partially explain the hypotensive effects of whole-grain oat consumption. The DASH trial and others have consistently demonstrated that diets rich in certain micronutrients can reduce blood pressure.30,31
Soluble fiber-rich oat cereals may affect blood pressure by modulating changes in insulin metabolism. The mechanism of action is thought to involve the slowed absorption of macronutrients from the gut, resulting in a flattening of the postprandial glycemic curve.29 These lower postprandial blood glucose levels elicit a lower insulin response to accommodate its clearance from the plasma. This process may lead to improved insulin sensitivity if the lower circulating insulin levels lead eventually to upregulation of the insulin receptors in peripheral tissues. A recent animal trial demonstrated that soluble fiber feeding improved insulin sensitivity by increasing skeletal muscle plasma membrane GLUT-4 content.32 Findings in this pilot suggest that over time, oat ingestion may reduce the amount of insulin needed to clear a glucose load. However, the study was underpowered to detect significant differences in more sensitive measures of insulin resistance. The causal mechanistic relationship among whole grain oat consumption, blood pressure, and insulin resistance might be best studied using a long-term feeding study design.
Alternate mechanisms, such as attenuation in endothelial function, may have affected blood pressure responses in this study.33 Drugs specific to endothelial cell receptors mediating vasodilation are known to lower blood pressure.34 Moreover, plasma cholesterol reductions are associated with improvements in endothelium-mediated vasodilation.35,36 In addition, preliminary evidence in animals supports a direct relationship between changes in plasma cholesterol concentrations and blood pressure.37 In the present study, plasma cholesterol levels were significantly reduced in participants who ingested whole grain oat-based cereals compared to a more refined grain wheat, corn, and rice control. Thus, it is possible that the blood pressure reduction observed in the subjects consuming oats resulted in part from improved endothelial function due to a drop in plasma cholesterol. Additional research is needed to fully investigate this pathway.
From a practical standpoint, improvements in SBP and DBP such as those observed in this study would be a useful contribution to the clinical management of hypertension. The cereal feeding intervention was well tolerated. Participants were very compliant for the 6-week treatment period. Substantial improvements in blood lipids could serve as an added incentive for patients to maintain long-term compliance with feeding recommendations.18,19 Since treatment of hypertension is a lifelong process for most patients, future studies would need to assess the effectiveness of oat cereals to maintain blood pressure benefits over a longer time. Such studies may need to consider dietary options such as soluble fiber-rich fruits in addition to cereal consumption in efforts to deliver the desired quantity of soluble fiber. Future trials will have to investigate the antihypertensive effect of whole oats in other populations, such as people with diabetes, and to study not only surrogate endpoints such as blood pressure but also patient-oriented outcomes such as mortality and morbidity.
1. He J, Klag M, Whelton P, et al. Oats and buckwheat intakes and cardiovascular disease risk factors in an ethnic minority of China. Am J Clin Nutr 1995;61:366-72.
2. Lichtenstein M, Burr M, Fehily A, Yarnell J. Heart rate, employment status, and prevalent ischemic heart disease confound relation between cereal fibre intake and blood pressure. J Epidemiol Community Health 1986;40:330-3.
3. Ascherio A, Rimm E, Giovannucci E, et al. A prospective study of nutritional factors and hypertension among US men. Circulation 1992;86:1475-84.
4. Hallfrisch J, Tobin J, Muller D, Andres R. Fiber intake, age, and other coronary risk factors in men of the Baltimore Longitudinal Study (1959-1975). J Gerontol 1988;43:M64-8.
5. Ascherio A, Hennekens C, Willett W, et al. Prospective study of nutritional factors, blood pressure, and hypertension among US women. Hypertension 1996;27:1065-72.
6. Schlamowitz P, Halberg T, Warnoe O, Wilstrup F, Ryttig K. Treatment of mild to moderate hypertension with dietary fiber. Lancet 1987;2:622-3.
7. Eliasson K, Ryttig K, Hylander B, Rossner S. A dietary fiber supplement in the treatment of mild hypertension. A randomized, double-blind, placebo-controlled trial. J Hypertens 1992;10:195-9.
8. Ryttig K, Tellnes G, Haegh L, Boe E, Fagerthun H. A dietary fiber supplement and weight maintenance after weight reduction: a randomized, double-blind, placebo-controlled long-term trial. Int J Obes 1989;13:165-71.
9. Dodson P, Stephenson J, Dodson L, et al. Randomised blind controlled trial of a high fiber, low fat and low sodium dietary regimen in mild essential hypertension. J Hum Hypertens 1989;3:197-202.
10. Little P, Girling G, Hasler A, Trafford A. A controlled trial of a low sodium, low fat, high fibre diet in treated hypertensive patients: effect on antihypertensive drug requirement in clinical practice. J Hum Hypertens 1991;5:175-81.
11. Rossner S, Andersson I, Ryttig K. Effects of a dietary fiber supplement to a weight reduction program on blood pressure. Acta Med Scand 1988;223:353-7.
12. Sandstrom B, Marckmann P, Bindslev N. An eight-month controlled study of a low-fat high-fiber diet: effects on blood lipids and blood pressure in healthy young subjects. Eur J Clin Nutr 1992;46:95-109.
13. Kestin M, Moss R, Clifton P. Comparative effects of three cereal brans on plasma lipids, blood pressure, and glucose metabolism in mildly hypercholesterolemic men. Am J Clin Nutr 1990;52:661-6.
14. Swain J, Rouse I, Curley C, Sacks F. Comparison of the effects of oat bran and low-fiber wheat on serum lipoprotein levels and blood pressure. N Engl J Med 1990;322:147-52.
15. Sciarrone S, Beilin L, Rouse I, Rogers P. A factorial study of salt restriction and a low-fat/high-fibre diet in hypertensive subjects. J Hypertens 1992;10:287-98.
16. Margetts B, Beilin L, Vandongen R, Armstrong B. A randomized controlled trial of the effect of dietary fiber on blood pressure. Clin Sci 1987;72:343-50.
17. el Zein M, Areas J, Knapka J, et al. Influence of oat bran on sucrose-induced blood pressure elevations in SHR. Life Sci 1990;47:1121-8.
18. Gondal J, MacArthy P, Myers A, Preuss H. Effects of dietary sucrose and fibers on blood pressure in hypertensive rats. Clin Nephrol 1996;45:163-8.
19. Krotkiewski M. Effect of guar gum on the arterial blood pressure. Acta Med Scand 1987;222:43-9.
20. Singh R, Rastogi S, Singh N, Ghosh S, Gupta S, Niaz M. Can guava fruit intake decrease blood pressure and blood lipids? J Hum Hypertens 1993;7:33-8.
21. Weidmann P, de Courten M, Bohlen L. Insulin resistance, hyperinsulinemia and hypertension. J Hypertens 1993;11:S27-38.
22. Tuck M. Obesity, the sympathetic nervous system, and essential hypertension. Hypertension 1992;19:I67-77.
23. Sowers J. Insulin resistance, hyperinsulinemia, dyslipidemia, hypertension, and accelerated atherosclerosis. J Clin Pharmacol 1992;32:529-35.
24. Braaten J, Wood P, Scott F, Riedel K, Poste L, Collins M. Oat gum lowers glucose and insulin after an oral glucose load. Am J Clin Nutr 1991;53:1425-30.
25. Braaten J, Scott F, Wood P, et al. High ß-glucan oat bran and oat gum reduce postprandial blood glucose and insulin in subjects with and without type 2 diabetes. Diabet Med 1994;11:312-8.
26. Hallfrisch J, Scholfield D, Behall K. Diets containing soluble oat extracts improve glucose and insulin responses of moderately hypercholesterolemic men and women. Am J Clin Nutr 1995;61:379-84.
27. Fukagawa N, Anderson J, Hageman G, Young V, Minaker K. High-carbohydrate, high-fiber diets increase peripheral insulin sensitivity in healthy young and old adults. Am J Clin Nutr 1990;52:524-8.
28. Welch S, Gebhart S, Bergman R, Phillips L. Minimal model analysis of IVGTT-derived insulin sensitivity in diabetic subjects. J Clin Endocrinol Metab 1990;71:1508-18.
29. Bergman R, Ider Y, Bowden C, Cobelli C. Quantitative estimation of insulin sensitivity. Am J Physiol 1979;236:E667-77.
30. Appel LJ, Moore TJ, Obarzanek E, et al. A clinical trial on the effects of dietary patterns on blood pressure. N Engl J Med 1997;336:1117-24.
31. Appel LJ. The role of diet in the prevention and treatment of hypertension. Curr Atheroscler Rep 2000;2:521-8.
32. Song YJ, Sawamura M, Ikeda K, Igawa S, Yamori Y. Soluble dietary fiber improves insulin sensitivity by increasing muscle GLUT-4 content in stroke-prone spontaneously hypertensive rats. Clin Exp Pharm Physiol 2000;27:41-5.
33. Taddei S, Salvetti A. Pathogenic factors in hypertension. Endothelial Factors. Clin Exp Hypertens 1996;18:323-35.
34. Krum H, Viskoper R, Lacourciere Y, Budde B, Charlon V. The effect of an endothelium-receptor antagonist, Bosentan, on blood pressure in patients with essential hypertension. N Engl J Med 1998;338:784-90.
35. Anderson T, Meredith I, Yeung A. The effect of cholesterol-lowering and antioxidant therapy on endothelium-dependent coronary vasomotion. N Engl J Med 1995;332:488-93.
36. Vogel R, Corretti M, Plotnick G. Changes in flow-mediated brachial artery vasoactivity with lowering of desirable cholesterol levels in healthy middle-aged men. Am J Cardiol 1996;77:37-40.
37. Crago M, West S, Hoeprich K, Michaelis K, McKenzie J. Effects of hyperlipidemia on blood pressure and coronary blood flow in swine. FASEB J 1998;12:A238.-
To submit a letter to the editor on this topic, click here: jfp@fammed.uc.edu.
1. He J, Klag M, Whelton P, et al. Oats and buckwheat intakes and cardiovascular disease risk factors in an ethnic minority of China. Am J Clin Nutr 1995;61:366-72.
2. Lichtenstein M, Burr M, Fehily A, Yarnell J. Heart rate, employment status, and prevalent ischemic heart disease confound relation between cereal fibre intake and blood pressure. J Epidemiol Community Health 1986;40:330-3.
3. Ascherio A, Rimm E, Giovannucci E, et al. A prospective study of nutritional factors and hypertension among US men. Circulation 1992;86:1475-84.
4. Hallfrisch J, Tobin J, Muller D, Andres R. Fiber intake, age, and other coronary risk factors in men of the Baltimore Longitudinal Study (1959-1975). J Gerontol 1988;43:M64-8.
5. Ascherio A, Hennekens C, Willett W, et al. Prospective study of nutritional factors, blood pressure, and hypertension among US women. Hypertension 1996;27:1065-72.
6. Schlamowitz P, Halberg T, Warnoe O, Wilstrup F, Ryttig K. Treatment of mild to moderate hypertension with dietary fiber. Lancet 1987;2:622-3.
7. Eliasson K, Ryttig K, Hylander B, Rossner S. A dietary fiber supplement in the treatment of mild hypertension. A randomized, double-blind, placebo-controlled trial. J Hypertens 1992;10:195-9.
8. Ryttig K, Tellnes G, Haegh L, Boe E, Fagerthun H. A dietary fiber supplement and weight maintenance after weight reduction: a randomized, double-blind, placebo-controlled long-term trial. Int J Obes 1989;13:165-71.
9. Dodson P, Stephenson J, Dodson L, et al. Randomised blind controlled trial of a high fiber, low fat and low sodium dietary regimen in mild essential hypertension. J Hum Hypertens 1989;3:197-202.
10. Little P, Girling G, Hasler A, Trafford A. A controlled trial of a low sodium, low fat, high fibre diet in treated hypertensive patients: effect on antihypertensive drug requirement in clinical practice. J Hum Hypertens 1991;5:175-81.
11. Rossner S, Andersson I, Ryttig K. Effects of a dietary fiber supplement to a weight reduction program on blood pressure. Acta Med Scand 1988;223:353-7.
12. Sandstrom B, Marckmann P, Bindslev N. An eight-month controlled study of a low-fat high-fiber diet: effects on blood lipids and blood pressure in healthy young subjects. Eur J Clin Nutr 1992;46:95-109.
13. Kestin M, Moss R, Clifton P. Comparative effects of three cereal brans on plasma lipids, blood pressure, and glucose metabolism in mildly hypercholesterolemic men. Am J Clin Nutr 1990;52:661-6.
14. Swain J, Rouse I, Curley C, Sacks F. Comparison of the effects of oat bran and low-fiber wheat on serum lipoprotein levels and blood pressure. N Engl J Med 1990;322:147-52.
15. Sciarrone S, Beilin L, Rouse I, Rogers P. A factorial study of salt restriction and a low-fat/high-fibre diet in hypertensive subjects. J Hypertens 1992;10:287-98.
16. Margetts B, Beilin L, Vandongen R, Armstrong B. A randomized controlled trial of the effect of dietary fiber on blood pressure. Clin Sci 1987;72:343-50.
17. el Zein M, Areas J, Knapka J, et al. Influence of oat bran on sucrose-induced blood pressure elevations in SHR. Life Sci 1990;47:1121-8.
18. Gondal J, MacArthy P, Myers A, Preuss H. Effects of dietary sucrose and fibers on blood pressure in hypertensive rats. Clin Nephrol 1996;45:163-8.
19. Krotkiewski M. Effect of guar gum on the arterial blood pressure. Acta Med Scand 1987;222:43-9.
20. Singh R, Rastogi S, Singh N, Ghosh S, Gupta S, Niaz M. Can guava fruit intake decrease blood pressure and blood lipids? J Hum Hypertens 1993;7:33-8.
21. Weidmann P, de Courten M, Bohlen L. Insulin resistance, hyperinsulinemia and hypertension. J Hypertens 1993;11:S27-38.
22. Tuck M. Obesity, the sympathetic nervous system, and essential hypertension. Hypertension 1992;19:I67-77.
23. Sowers J. Insulin resistance, hyperinsulinemia, dyslipidemia, hypertension, and accelerated atherosclerosis. J Clin Pharmacol 1992;32:529-35.
24. Braaten J, Wood P, Scott F, Riedel K, Poste L, Collins M. Oat gum lowers glucose and insulin after an oral glucose load. Am J Clin Nutr 1991;53:1425-30.
25. Braaten J, Scott F, Wood P, et al. High ß-glucan oat bran and oat gum reduce postprandial blood glucose and insulin in subjects with and without type 2 diabetes. Diabet Med 1994;11:312-8.
26. Hallfrisch J, Scholfield D, Behall K. Diets containing soluble oat extracts improve glucose and insulin responses of moderately hypercholesterolemic men and women. Am J Clin Nutr 1995;61:379-84.
27. Fukagawa N, Anderson J, Hageman G, Young V, Minaker K. High-carbohydrate, high-fiber diets increase peripheral insulin sensitivity in healthy young and old adults. Am J Clin Nutr 1990;52:524-8.
28. Welch S, Gebhart S, Bergman R, Phillips L. Minimal model analysis of IVGTT-derived insulin sensitivity in diabetic subjects. J Clin Endocrinol Metab 1990;71:1508-18.
29. Bergman R, Ider Y, Bowden C, Cobelli C. Quantitative estimation of insulin sensitivity. Am J Physiol 1979;236:E667-77.
30. Appel LJ, Moore TJ, Obarzanek E, et al. A clinical trial on the effects of dietary patterns on blood pressure. N Engl J Med 1997;336:1117-24.
31. Appel LJ. The role of diet in the prevention and treatment of hypertension. Curr Atheroscler Rep 2000;2:521-8.
32. Song YJ, Sawamura M, Ikeda K, Igawa S, Yamori Y. Soluble dietary fiber improves insulin sensitivity by increasing muscle GLUT-4 content in stroke-prone spontaneously hypertensive rats. Clin Exp Pharm Physiol 2000;27:41-5.
33. Taddei S, Salvetti A. Pathogenic factors in hypertension. Endothelial Factors. Clin Exp Hypertens 1996;18:323-35.
34. Krum H, Viskoper R, Lacourciere Y, Budde B, Charlon V. The effect of an endothelium-receptor antagonist, Bosentan, on blood pressure in patients with essential hypertension. N Engl J Med 1998;338:784-90.
35. Anderson T, Meredith I, Yeung A. The effect of cholesterol-lowering and antioxidant therapy on endothelium-dependent coronary vasomotion. N Engl J Med 1995;332:488-93.
36. Vogel R, Corretti M, Plotnick G. Changes in flow-mediated brachial artery vasoactivity with lowering of desirable cholesterol levels in healthy middle-aged men. Am J Cardiol 1996;77:37-40.
37. Crago M, West S, Hoeprich K, Michaelis K, McKenzie J. Effects of hyperlipidemia on blood pressure and coronary blood flow in swine. FASEB J 1998;12:A238.-
To submit a letter to the editor on this topic, click here: jfp@fammed.uc.edu.