User login
Which factors increase the risk of an infant becoming an overweight child?
Variables that increase the risk of overweight in childhood include formula feeding, high birth weight, high rate of weight gain in the first 4 months of life, low socioeconomic status, and maternal obesity (strength of recommendation [SOR]: A, systematic reviews and consistent cohort studies). No single risk factor predicts overweight, and not all infants with risk factors become overweight children.
Evidence summary
The Centers for Disease Control and Prevention defines overweight in children as weight-for-length greater than the 95th percentile for sex in children younger than 24 months and body mass index (BMI) greater than the 95th percentile for age and sex in children >24 months.
Breastfeeding is protective
Breastfed infants are less likely to be over-weight later in life than infants fed formula. A meta-analysis of 9 studies found that 7 showed a significantly lower risk of overweight among children who were breastfed (odds ratio [OR]=0.78; 95% confidence interval [CI], 0.71-0.85).1
Four of the studies demonstrated that longer duration of breastfeeding offered greater protection than shorter duration. Two of the 4 studies defined longer duration as more than 6 months, 1 defined it as more than 3 months, and 1 examined breastfeeding for periods of less than 1 week, 1 week to 1 month, 2 to 3 months, 4 to 6 months, 7 to 9 months, and longer than 9 months, showing a duration-dependent decrease in risk. The other studies in the meta-analysis evaluated never-breastfed vs ever-breastfed infants.1
Higher birth weight increases risk
Several meta-analyses report that birth weight is an early risk factor for later overweight. One found a positive association between birth weight and over-weight in childhood in 9 of 11 studies.2 Another meta-analysis found a positive association in 25 of 28 studies that examined birth weight and BMI in childhood.3 These descriptive meta-analyses didn’t calculate pooled odds ratios (ORs) because of heterogeneity of the ages included and methods used to measure obesity.
A high rate of weight gain in infancy is also a risk factor for later overweight. One descriptive meta-analysis reported that 13 of 15 studies found a positive association between weight gain in the first year of life and overweight later in childhood, although overall OR and relative risk weren’t reported.4 A large cohort study found that each 100 g per month increase in weight gain above the mean (820 g per month) during the first 4 months of life increased the odds of overweight at 7 years of age by 38% (OR=1.38; 95% CI, 1.32-1.44).5
Socioeconomic status is a factor
Low socioeconomic status in infancy or early childhood increases the risk of childhood overweight, perhaps because of less breastfeeding and more smoking, among other factors.6,7 Socioeconomic status was determined using the International Standard Classification of Occupations; children whose parents worked at unskilled manual labor jobs or were unemployed were considered in the lowest socioeconomic group.6,7
A Brazilian study found that children born in the lowest socioeconomic group had BMI measurements at 18 years of age that were an average of 1.21 kg/m2 higher than children in the highest socioeconomic group (P<.05). The study controlled for birth weight, maternal smoking, gestational age, and level of schooling eventually achieved by the child.8
Maternal overweight or obesity during the child’s infancy also increases the risk of childhood overweight.9,10 Infants of obese parents were more likely to be overweight at 7 years, compared with children whose mothers were normal weight (OR=10.44; 95% CI, 5.11-21.23).9
Recommendations
The American Academy of Pediatrics (AAP) cites prevention of overweight as a potential benefit of breastfeeding.11 The American Academy of Family Physicians notes that obese mothers should be especially encouraged to breastfeed.12 The American Medical Association-AAP Expert Panel recommends breastfeeding; safe, free movement; and no television for infants to decrease the risk of later over-weight.13
1. Arenz S, Ruckerl R, Koletzko B, et al. Breast-feeding and childhood obesity—a systematic review. Int J Obes Relat Metab Disord. 2004;28:1247-1256.
2. Parsons TJ, Power C, Logan S, et al. Childhood predictors of adult obesity: a systematic review. Int J Obes Relat Metab Disord. 1999;23(suppl 8):S1-S107.
3. Rogers I. EURO-BLCS Study Group. The influence of birthweight and intrauterine environment on adiposity and fat distribution in later life. Int J Obes Relat Metab Disord. 2003;27:755-777.
4. Monteiro PO, Victora CG. Rapid growth in infancy and childhood and obesity in later life—a systematic review. Obes Rev. 2005;6:143-154.
5. Stettler N, Zemel BS, Kumanyika S, et al. Infant weight gain and childhood overweight status in a multicenter, cohort study. Pediatrics. 2002;109:194-199.
6. Bergmann KE, Bergmann RL, Von Kries R, et al. Early determinants of childhood overweight and adiposity in a birth cohort study: role of breast-feeding. Int J Obes Relat Metab Disord. 2003;27:162-172.
7. Dubois L, Girard M. Early determinants of over-weight at 4.5 years in a population-based longitudinal study. Int J Obes. 2006;30:610-617.
8. Goldani MZ, Haeffner LS, Agranonik M, et al. Do early life factors influence body mass index in adolescents? Braz J Med Biol Res. 2007;40:1231-1236.
9. Reilly JJ, Armstrong J, Dorosty AR, et al. Early life risk factors for obesity in childhood: cohort study. BMJ. 2005;330-1357.
10. Whitaker RC. Predicting preschooler obesity at birth: the role of maternal obesity in early pregnancy. Pediatrics. 2004;114:e29-e36.
11. Gartner LM, Morton J, Lawrence RA, et al. for the American Academy of Pediatrics Section on Breastfeeding Breastfeeding and the use of human milk. Pediatrics. 2005;115:496-506.
12. American Academy of Family Physicians. Breast-feeding, family physicians supporting (position paper). Available at: www.aafp.org/online/en/home/policy/policies/b/breastfeedingpositionpaper.html. Accessed February 12, 2008.
13. Barlow SE. The Expert Committee. Expert Committee recommendations regarding the prevention, assessment, and treatment of child and adolescent overweight and obesity: summary report. Pediatrics. 2007;120(suppl 4):S164-S192.
Variables that increase the risk of overweight in childhood include formula feeding, high birth weight, high rate of weight gain in the first 4 months of life, low socioeconomic status, and maternal obesity (strength of recommendation [SOR]: A, systematic reviews and consistent cohort studies). No single risk factor predicts overweight, and not all infants with risk factors become overweight children.
Evidence summary
The Centers for Disease Control and Prevention defines overweight in children as weight-for-length greater than the 95th percentile for sex in children younger than 24 months and body mass index (BMI) greater than the 95th percentile for age and sex in children >24 months.
Breastfeeding is protective
Breastfed infants are less likely to be over-weight later in life than infants fed formula. A meta-analysis of 9 studies found that 7 showed a significantly lower risk of overweight among children who were breastfed (odds ratio [OR]=0.78; 95% confidence interval [CI], 0.71-0.85).1
Four of the studies demonstrated that longer duration of breastfeeding offered greater protection than shorter duration. Two of the 4 studies defined longer duration as more than 6 months, 1 defined it as more than 3 months, and 1 examined breastfeeding for periods of less than 1 week, 1 week to 1 month, 2 to 3 months, 4 to 6 months, 7 to 9 months, and longer than 9 months, showing a duration-dependent decrease in risk. The other studies in the meta-analysis evaluated never-breastfed vs ever-breastfed infants.1
Higher birth weight increases risk
Several meta-analyses report that birth weight is an early risk factor for later overweight. One found a positive association between birth weight and over-weight in childhood in 9 of 11 studies.2 Another meta-analysis found a positive association in 25 of 28 studies that examined birth weight and BMI in childhood.3 These descriptive meta-analyses didn’t calculate pooled odds ratios (ORs) because of heterogeneity of the ages included and methods used to measure obesity.
A high rate of weight gain in infancy is also a risk factor for later overweight. One descriptive meta-analysis reported that 13 of 15 studies found a positive association between weight gain in the first year of life and overweight later in childhood, although overall OR and relative risk weren’t reported.4 A large cohort study found that each 100 g per month increase in weight gain above the mean (820 g per month) during the first 4 months of life increased the odds of overweight at 7 years of age by 38% (OR=1.38; 95% CI, 1.32-1.44).5
Socioeconomic status is a factor
Low socioeconomic status in infancy or early childhood increases the risk of childhood overweight, perhaps because of less breastfeeding and more smoking, among other factors.6,7 Socioeconomic status was determined using the International Standard Classification of Occupations; children whose parents worked at unskilled manual labor jobs or were unemployed were considered in the lowest socioeconomic group.6,7
A Brazilian study found that children born in the lowest socioeconomic group had BMI measurements at 18 years of age that were an average of 1.21 kg/m2 higher than children in the highest socioeconomic group (P<.05). The study controlled for birth weight, maternal smoking, gestational age, and level of schooling eventually achieved by the child.8
Maternal overweight or obesity during the child’s infancy also increases the risk of childhood overweight.9,10 Infants of obese parents were more likely to be overweight at 7 years, compared with children whose mothers were normal weight (OR=10.44; 95% CI, 5.11-21.23).9
Recommendations
The American Academy of Pediatrics (AAP) cites prevention of overweight as a potential benefit of breastfeeding.11 The American Academy of Family Physicians notes that obese mothers should be especially encouraged to breastfeed.12 The American Medical Association-AAP Expert Panel recommends breastfeeding; safe, free movement; and no television for infants to decrease the risk of later over-weight.13
Variables that increase the risk of overweight in childhood include formula feeding, high birth weight, high rate of weight gain in the first 4 months of life, low socioeconomic status, and maternal obesity (strength of recommendation [SOR]: A, systematic reviews and consistent cohort studies). No single risk factor predicts overweight, and not all infants with risk factors become overweight children.
Evidence summary
The Centers for Disease Control and Prevention defines overweight in children as weight-for-length greater than the 95th percentile for sex in children younger than 24 months and body mass index (BMI) greater than the 95th percentile for age and sex in children >24 months.
Breastfeeding is protective
Breastfed infants are less likely to be over-weight later in life than infants fed formula. A meta-analysis of 9 studies found that 7 showed a significantly lower risk of overweight among children who were breastfed (odds ratio [OR]=0.78; 95% confidence interval [CI], 0.71-0.85).1
Four of the studies demonstrated that longer duration of breastfeeding offered greater protection than shorter duration. Two of the 4 studies defined longer duration as more than 6 months, 1 defined it as more than 3 months, and 1 examined breastfeeding for periods of less than 1 week, 1 week to 1 month, 2 to 3 months, 4 to 6 months, 7 to 9 months, and longer than 9 months, showing a duration-dependent decrease in risk. The other studies in the meta-analysis evaluated never-breastfed vs ever-breastfed infants.1
Higher birth weight increases risk
Several meta-analyses report that birth weight is an early risk factor for later overweight. One found a positive association between birth weight and over-weight in childhood in 9 of 11 studies.2 Another meta-analysis found a positive association in 25 of 28 studies that examined birth weight and BMI in childhood.3 These descriptive meta-analyses didn’t calculate pooled odds ratios (ORs) because of heterogeneity of the ages included and methods used to measure obesity.
A high rate of weight gain in infancy is also a risk factor for later overweight. One descriptive meta-analysis reported that 13 of 15 studies found a positive association between weight gain in the first year of life and overweight later in childhood, although overall OR and relative risk weren’t reported.4 A large cohort study found that each 100 g per month increase in weight gain above the mean (820 g per month) during the first 4 months of life increased the odds of overweight at 7 years of age by 38% (OR=1.38; 95% CI, 1.32-1.44).5
Socioeconomic status is a factor
Low socioeconomic status in infancy or early childhood increases the risk of childhood overweight, perhaps because of less breastfeeding and more smoking, among other factors.6,7 Socioeconomic status was determined using the International Standard Classification of Occupations; children whose parents worked at unskilled manual labor jobs or were unemployed were considered in the lowest socioeconomic group.6,7
A Brazilian study found that children born in the lowest socioeconomic group had BMI measurements at 18 years of age that were an average of 1.21 kg/m2 higher than children in the highest socioeconomic group (P<.05). The study controlled for birth weight, maternal smoking, gestational age, and level of schooling eventually achieved by the child.8
Maternal overweight or obesity during the child’s infancy also increases the risk of childhood overweight.9,10 Infants of obese parents were more likely to be overweight at 7 years, compared with children whose mothers were normal weight (OR=10.44; 95% CI, 5.11-21.23).9
Recommendations
The American Academy of Pediatrics (AAP) cites prevention of overweight as a potential benefit of breastfeeding.11 The American Academy of Family Physicians notes that obese mothers should be especially encouraged to breastfeed.12 The American Medical Association-AAP Expert Panel recommends breastfeeding; safe, free movement; and no television for infants to decrease the risk of later over-weight.13
1. Arenz S, Ruckerl R, Koletzko B, et al. Breast-feeding and childhood obesity—a systematic review. Int J Obes Relat Metab Disord. 2004;28:1247-1256.
2. Parsons TJ, Power C, Logan S, et al. Childhood predictors of adult obesity: a systematic review. Int J Obes Relat Metab Disord. 1999;23(suppl 8):S1-S107.
3. Rogers I. EURO-BLCS Study Group. The influence of birthweight and intrauterine environment on adiposity and fat distribution in later life. Int J Obes Relat Metab Disord. 2003;27:755-777.
4. Monteiro PO, Victora CG. Rapid growth in infancy and childhood and obesity in later life—a systematic review. Obes Rev. 2005;6:143-154.
5. Stettler N, Zemel BS, Kumanyika S, et al. Infant weight gain and childhood overweight status in a multicenter, cohort study. Pediatrics. 2002;109:194-199.
6. Bergmann KE, Bergmann RL, Von Kries R, et al. Early determinants of childhood overweight and adiposity in a birth cohort study: role of breast-feeding. Int J Obes Relat Metab Disord. 2003;27:162-172.
7. Dubois L, Girard M. Early determinants of over-weight at 4.5 years in a population-based longitudinal study. Int J Obes. 2006;30:610-617.
8. Goldani MZ, Haeffner LS, Agranonik M, et al. Do early life factors influence body mass index in adolescents? Braz J Med Biol Res. 2007;40:1231-1236.
9. Reilly JJ, Armstrong J, Dorosty AR, et al. Early life risk factors for obesity in childhood: cohort study. BMJ. 2005;330-1357.
10. Whitaker RC. Predicting preschooler obesity at birth: the role of maternal obesity in early pregnancy. Pediatrics. 2004;114:e29-e36.
11. Gartner LM, Morton J, Lawrence RA, et al. for the American Academy of Pediatrics Section on Breastfeeding Breastfeeding and the use of human milk. Pediatrics. 2005;115:496-506.
12. American Academy of Family Physicians. Breast-feeding, family physicians supporting (position paper). Available at: www.aafp.org/online/en/home/policy/policies/b/breastfeedingpositionpaper.html. Accessed February 12, 2008.
13. Barlow SE. The Expert Committee. Expert Committee recommendations regarding the prevention, assessment, and treatment of child and adolescent overweight and obesity: summary report. Pediatrics. 2007;120(suppl 4):S164-S192.
1. Arenz S, Ruckerl R, Koletzko B, et al. Breast-feeding and childhood obesity—a systematic review. Int J Obes Relat Metab Disord. 2004;28:1247-1256.
2. Parsons TJ, Power C, Logan S, et al. Childhood predictors of adult obesity: a systematic review. Int J Obes Relat Metab Disord. 1999;23(suppl 8):S1-S107.
3. Rogers I. EURO-BLCS Study Group. The influence of birthweight and intrauterine environment on adiposity and fat distribution in later life. Int J Obes Relat Metab Disord. 2003;27:755-777.
4. Monteiro PO, Victora CG. Rapid growth in infancy and childhood and obesity in later life—a systematic review. Obes Rev. 2005;6:143-154.
5. Stettler N, Zemel BS, Kumanyika S, et al. Infant weight gain and childhood overweight status in a multicenter, cohort study. Pediatrics. 2002;109:194-199.
6. Bergmann KE, Bergmann RL, Von Kries R, et al. Early determinants of childhood overweight and adiposity in a birth cohort study: role of breast-feeding. Int J Obes Relat Metab Disord. 2003;27:162-172.
7. Dubois L, Girard M. Early determinants of over-weight at 4.5 years in a population-based longitudinal study. Int J Obes. 2006;30:610-617.
8. Goldani MZ, Haeffner LS, Agranonik M, et al. Do early life factors influence body mass index in adolescents? Braz J Med Biol Res. 2007;40:1231-1236.
9. Reilly JJ, Armstrong J, Dorosty AR, et al. Early life risk factors for obesity in childhood: cohort study. BMJ. 2005;330-1357.
10. Whitaker RC. Predicting preschooler obesity at birth: the role of maternal obesity in early pregnancy. Pediatrics. 2004;114:e29-e36.
11. Gartner LM, Morton J, Lawrence RA, et al. for the American Academy of Pediatrics Section on Breastfeeding Breastfeeding and the use of human milk. Pediatrics. 2005;115:496-506.
12. American Academy of Family Physicians. Breast-feeding, family physicians supporting (position paper). Available at: www.aafp.org/online/en/home/policy/policies/b/breastfeedingpositionpaper.html. Accessed February 12, 2008.
13. Barlow SE. The Expert Committee. Expert Committee recommendations regarding the prevention, assessment, and treatment of child and adolescent overweight and obesity: summary report. Pediatrics. 2007;120(suppl 4):S164-S192.
Evidence-based answers from the Family Physicians Inquiries Network
When to suggest this OC alternative
Recommend continuous or extended use of the transvaginal contraceptive ring to women who want fewer days of menstrual bleeding and have trouble remembering to, or prefer not to, take a daily pill. If breakthrough bleeding is troublesome, suggest a 4-day ring-free interval.1
Strength of recommendation
B: Based on a single randomized controlled trial (RCT) with <80% follow up.
Sulak PJ, Smith V, Coffee A, et al. Frequency and management of breakthrough bleeding with continuous use of the transvaginal contraceptive ring: a randomized controlled trial. Obstet Gynecol. 2008;112:563-571.
ILLUSTRATIVE CASE
Case 1: A healthy 25-year-old woman comes to see you because she’s worried about getting pregnant. She’s been on an extended-cycle oral contraceptive (OC) for several months and is happy to have her period only once every 3 months, but she frequently forgets to take her pill.
What can you offer that will give her the benefits of an extended-cycle OC, without the risk of pregnancy she incurs each time she misses a pill?
Case 2: You started a healthy 18-year-old on the transvaginal ring 6 months ago. After counseling, she opted for continuous cycling, so she inserts a new ring right after she removes the old one, on the same date each month. Although she likes the ring, she’s disturbed by a recent increase in breakthrough bleeding. What can you recommend to decrease the bleeding?
Clinicians have long known that the traditional 21/7 OC cycle is not necessary for safety or efficacy. More recently, many women have been happy to learn that there is no physiologic reason to have a monthly period when they’re using combination hormonal contraception. They’re also happy to discover that fewer periods often mean fewer premenstrual mood swings, episodes of painful cramping, and instances of other troublesome symptoms.
The transvaginal ring is often overlooked
The US Food and Drug Administration (FDA) has approved 2 combination OCs for extended-cycle use and 1 for continuous use. But any monophasic OC can be used off-label for extended or continuous cycling to decrease bleeding frequency. So, too, can the transvaginal contraceptive ring (NuvaRing), an infrequently used contraceptive. (According to 1 study, just 5.7% of US women using contraception used either the ring or the patch.2 ) The ring has been studied for extended use,3 but does not have FDA approval for longer-term regimens.
NuvaRing is a flexible, transparent device that contains the progestin etonogestrel and ethinyl estradiol. The manufacturer recommends a 21/7 cycle, inserting the ring in the vagina and leaving it in place for 3 weeks, removing it for 1 week, and then inserting a new ring.4 The ring is well suited to women who have no contraindications to hormonal contraception but have difficulty remembering to take a pill every day—or simply prefer the convenience of less frequent dosing.
While the ring has been found to be effective and tolerable when used without a hormone-free interval—28-, 49-, 91- and 364-day dosing has been studied—breakthrough bleeding or spotting is a frequent side effect of extended-cycle hormonal contraception. In 1 study, 43% of women on a 49-day ring cycle experienced breakthrough bleeding, compared with 16% of those on a 28-day cycle.3
High satisfaction, low risk
Nonetheless, women who use the transvaginal ring often report high satisfaction. One study found that 61% of women were very satisfied with this method of contraception, compared with 34% of triphasic OC users (P<.003).5 The risk of pregnancy (1-2 pregnancies per 100 women-years of use, according to the manufacturer4 ) and the risk of venous thromboembolism (10-30 in 100,000 vs 4-11 in 100,000 nonpregnant women who are not using hormonal contraception) are comparable to that of women using OCs.6,7 The risk of other severe side effects associated with the vaginal ring is comparable to that of OCs, as well.
STUDY SUMMARY: An effective option that women used post-trial
This RCT recruited women between the ages of 18 and 45 years who had been using combination hormonal contraceptives—OCs, the transdermal patch, or the transvaginal ring. All had been on a 21/7 cycle for at least 2 months. Exclusion criteria included a body mass index ≥38 kg/m2, smoking >10 cigarettes per day, use of other estrogen- or phytoestrogen-containing products, and the presence of ovarian cysts >2.5 cm or endometrial thickness >8 mm. Women who wanted to get pregnant within a year were also excluded.
The study began with a baseline phase during which participants completed one 21/7 cycle with the ring for those using the ring prior to the study or two 21/7 cycles for those using the pill or patch prior to the study. Daily flow was assessed during this initial phase, using a scale of 0 to 4, with 4 being the heaviest. Women who completed this phase and wanted to continue using the ring (N=74) were then randomized into 2 groups (n=37) for the 6-month extended phase.
Group 1 was assigned to use the contraceptive ring with no hormone-free days. Participants were instructed to replace the rings monthly, on the same calendar day of the month. Group 2 also used the ring on a continuous basis with monthly replacement, but those who experienced breakthrough bleeding for more than 5 days were permitted to remove the ring for 4 days. Women in both groups kept a daily diary of ring usage, degree of menstrual flow, and symptomatology, including pelvic pain, headaches, and mood.
Most subjects were white (76%), nonsmokers (84%), and unmarried (68% in Group 1 and 57% in Group 2), with an average age of 28 to 29 years. Eight patients (22%) in Group 1 withdrew from the study prior to completing the 6-month extended phase, 4 of them because of side effects. Only 1 woman withdrew from Group 2, because of plans for pregnancy. No one became pregnant while using the ring.
Hormone-free interval reduced bleeding. In Group 1, the average daily flow score was slightly reduced with continuous use (from 0.33 during the 21/7 baseline phase to 0.21 in the 6-month extended phase), but researchers reported no significant difference in flow-free days. On average, 85% of the days were flow-free in the 21/7 phase, vs 89% in the extended phase.
In Group 2, flow-free days increased, from 83% in the baseline phase to 95% in the extended phase, and average flow scores fell from 0.38 to 0.17.
Overall, the 65 participants who completed 6 months of continuous ring use had fewer bleeding days per month—1.8 days, on average, vs 3.3 days during the initial 21/7 phase, but more days of spotting per month (2.5 vs 1.8 days). There was no difference between Groups 1 and 2 in pelvic pain, headache, or mood scores, and no significant difference in headache or mood scores between the baseline and continuous phases of the trial. Pelvic pain scores were lower during the extended phase, however—0.18 vs 0.32 on a scale of 0 to 10.
A high continuation rate. After the 6-month extended phase, 57 of the 65 remaining participants chose to continue using the ring for contraception, on a continuous dosing basis—a continuation rate of 88%. But more than half of the women who chose to stick with the ring (57%) decided not to take advantage of the 4-day hormone-free interval to manage breakthrough bleeding or spotting, regardless of original group assignment.
WHAT’S NEW?: The ring moves further mainstream
Continuous or extended use of the transvaginal ring may be a new idea for many patients—and physicians. But the idea may catch on in light of this study’s findings. Given the high rate of unwanted pregnancy in the United States, many women may benefit from a contraceptive that is as safe and effective as an OC but doesn’t involve a daily pill.
CAVEATS: Side effects, off-label concerns
In 2005, Oddsson et al found that women who used the ring reported more vaginitis and more leukorrhea than women who used OCs; conversely, they reported less nausea and less acne. Other side effects that are common to hormonal contraceptives, such as headache and weight gain, occurred at similar rates among women using the ring and OCs.6
However, the high proportion of patients who elected to keep using the ring at the end of the study by Sulak et al suggests that its side effects are acceptable.1 As with all contraception, however, patient preference is a key consideration. The study population was highly motivated, particularly since women who had difficulty with this means of contraception dropped out after the baseline phase of the trial.
Off-label use. Pharmacokinetic research involving the contraceptive ring has shown that hormone levels required to protect against pregnancy persist for at least 35 days after it is placed in the vagina.8 The manufacturer has data only to confirm contraceptive efficacy for up to 28 days and therefore does not recommend use beyond 4 weeks.4
This study highlights another off-label issue: Women in Group 2, who were allowed to remove the ring for 4 day-intervals to decrease breakthrough bleeding, were instructed to reinsert the same ring after 4 days and keep it in place until the next scheduled replacement date. But the manufacturer does not recommend reinsertion of a ring that has been out of the body for more than 3 hours. In my practice (KR), women are generally unwilling to store and replace a ring, preferring to place a new one after removal for more than a few hours.
Should she try continuous use?
Changing the ring on the same date each month may boost adherence for some women. Funding. The research was funded by an unrestricted educational grant from Organon, Inc, the manufacturer of NuvaRing, which included salary support for 5 of the 6 authors. The published study gives no additional information about the involvement of the pharmaceutical company.
Contraindications, drug interactions. As with other combined hormonal contraceptives, women who have a history of venous thromboembolism, headaches with focal neurological symptoms, severe hypertension, breast or endometrial cancer, or liver disease, and smokers older than 35 years should not use the contraceptive ring.4 In addition, women need to be aware that a number of medications—griseofulvin, rifampin, phenytoin, carbamazepine, and herbal products containing St. John’s Wort, among others—may reduce the effectiveness of the contraceptive ring.4
CHALLENGES TO IMPLEMENTATION: Going off-label isn’t for everyone
When it comes to choosing a contraceptive method, patient preference is paramount. Some women may not be comfortable inserting or removing the ring and should be counseled on other forms of contraception. Women who prefer to bleed every month should not use extended cycling. Similarly, some physicians may not be comfortable recommending an off-label use of the ring.
Those who are comfortable making the recommendation should be prepared to educate patients about this method of contraception and to discuss the benefits of extended or continuous use of the ring. For some women, the memory-triggering mechanism of changing the ring on the same date each month may boost adherence. For others, replacing the ring every 28 days may be acceptable—again, depending on patient preference.
Acknowledgements
The PURLs Surveillance System is supported in part by Grant Number UL1RR024999 from the National Center for Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center for Research Resources or the National Institutes of Health.
PURLs methodology
This study was selected and evaluated using FPIN’s Priority Updates from the Research Literature (PURLs) Surveillance System methodology. The criteria and findings leading to the selection of this study as a PURL can be accessed at www.jfponline.com/purls.
1. Sulak PJ, Smith V, Coffee A, et al. Frequency and management of breakthrough bleeding with continuous use of the transvaginal contraceptive ring: a randomized controlled trial. Obstet Gynecol. 2008;112:563-571.
2. Frost JJ, Singh S, Finer LB. US women’s one-year contraceptive use patterns, 2004. Perspect Sex Reprod Health. 2007;39:48-55.
3. Miller L, Verhoeven C, Hout J. Extended regimens of the contraceptive vaginal ring: a randomized trial. Obstet Gynecol. 2005;106:473-482.
4. NuvaRing (etonogestrel/ethinyl estradiol vaginal ring) [prescribing information]. Roseland, NJ: Organon USA Inc; June 2008. Available at: http://www.spfiles.com/pinuvaring.pdf. Accessed March 10, 2009.
5. Schafer JE, Osborne LM, Davis AR, et al. Acceptability and satisfaction using Quick Start with the contraceptive vaginal ring versus an oral contraceptive. Contraception. 2006;73:488-492.
6. Oddsson K, Leifels-Fischer B, de Melo NR, et al. Efficacy and safety of a contraceptive vaginal ring (NuvaRing) compared with a combined oral contraceptive: a 1-year randomized trial. Contraception. 2005;71:176-182.
7. Wilks JF. Hormonal birth control and pregnancy: a comparative analysis of thromboembolic risk. Ann Pharmacother. 2003;37:912-916.
8. Timmer C, Mulders T. Pharmacokinetics of etonogestrel and ethinylestradiol released from a combined contraceptive vaginal ring. Clin Pharmacokinet. 2000;39:233-242.
Recommend continuous or extended use of the transvaginal contraceptive ring to women who want fewer days of menstrual bleeding and have trouble remembering to, or prefer not to, take a daily pill. If breakthrough bleeding is troublesome, suggest a 4-day ring-free interval.1
Strength of recommendation
B: Based on a single randomized controlled trial (RCT) with <80% follow up.
Sulak PJ, Smith V, Coffee A, et al. Frequency and management of breakthrough bleeding with continuous use of the transvaginal contraceptive ring: a randomized controlled trial. Obstet Gynecol. 2008;112:563-571.
ILLUSTRATIVE CASE
Case 1: A healthy 25-year-old woman comes to see you because she’s worried about getting pregnant. She’s been on an extended-cycle oral contraceptive (OC) for several months and is happy to have her period only once every 3 months, but she frequently forgets to take her pill.
What can you offer that will give her the benefits of an extended-cycle OC, without the risk of pregnancy she incurs each time she misses a pill?
Case 2: You started a healthy 18-year-old on the transvaginal ring 6 months ago. After counseling, she opted for continuous cycling, so she inserts a new ring right after she removes the old one, on the same date each month. Although she likes the ring, she’s disturbed by a recent increase in breakthrough bleeding. What can you recommend to decrease the bleeding?
Clinicians have long known that the traditional 21/7 OC cycle is not necessary for safety or efficacy. More recently, many women have been happy to learn that there is no physiologic reason to have a monthly period when they’re using combination hormonal contraception. They’re also happy to discover that fewer periods often mean fewer premenstrual mood swings, episodes of painful cramping, and instances of other troublesome symptoms.
The transvaginal ring is often overlooked
The US Food and Drug Administration (FDA) has approved 2 combination OCs for extended-cycle use and 1 for continuous use. But any monophasic OC can be used off-label for extended or continuous cycling to decrease bleeding frequency. So, too, can the transvaginal contraceptive ring (NuvaRing), an infrequently used contraceptive. (According to 1 study, just 5.7% of US women using contraception used either the ring or the patch.2 ) The ring has been studied for extended use,3 but does not have FDA approval for longer-term regimens.
NuvaRing is a flexible, transparent device that contains the progestin etonogestrel and ethinyl estradiol. The manufacturer recommends a 21/7 cycle, inserting the ring in the vagina and leaving it in place for 3 weeks, removing it for 1 week, and then inserting a new ring.4 The ring is well suited to women who have no contraindications to hormonal contraception but have difficulty remembering to take a pill every day—or simply prefer the convenience of less frequent dosing.
While the ring has been found to be effective and tolerable when used without a hormone-free interval—28-, 49-, 91- and 364-day dosing has been studied—breakthrough bleeding or spotting is a frequent side effect of extended-cycle hormonal contraception. In 1 study, 43% of women on a 49-day ring cycle experienced breakthrough bleeding, compared with 16% of those on a 28-day cycle.3
High satisfaction, low risk
Nonetheless, women who use the transvaginal ring often report high satisfaction. One study found that 61% of women were very satisfied with this method of contraception, compared with 34% of triphasic OC users (P<.003).5 The risk of pregnancy (1-2 pregnancies per 100 women-years of use, according to the manufacturer4 ) and the risk of venous thromboembolism (10-30 in 100,000 vs 4-11 in 100,000 nonpregnant women who are not using hormonal contraception) are comparable to that of women using OCs.6,7 The risk of other severe side effects associated with the vaginal ring is comparable to that of OCs, as well.
STUDY SUMMARY: An effective option that women used post-trial
This RCT recruited women between the ages of 18 and 45 years who had been using combination hormonal contraceptives—OCs, the transdermal patch, or the transvaginal ring. All had been on a 21/7 cycle for at least 2 months. Exclusion criteria included a body mass index ≥38 kg/m2, smoking >10 cigarettes per day, use of other estrogen- or phytoestrogen-containing products, and the presence of ovarian cysts >2.5 cm or endometrial thickness >8 mm. Women who wanted to get pregnant within a year were also excluded.
The study began with a baseline phase during which participants completed one 21/7 cycle with the ring for those using the ring prior to the study or two 21/7 cycles for those using the pill or patch prior to the study. Daily flow was assessed during this initial phase, using a scale of 0 to 4, with 4 being the heaviest. Women who completed this phase and wanted to continue using the ring (N=74) were then randomized into 2 groups (n=37) for the 6-month extended phase.
Group 1 was assigned to use the contraceptive ring with no hormone-free days. Participants were instructed to replace the rings monthly, on the same calendar day of the month. Group 2 also used the ring on a continuous basis with monthly replacement, but those who experienced breakthrough bleeding for more than 5 days were permitted to remove the ring for 4 days. Women in both groups kept a daily diary of ring usage, degree of menstrual flow, and symptomatology, including pelvic pain, headaches, and mood.
Most subjects were white (76%), nonsmokers (84%), and unmarried (68% in Group 1 and 57% in Group 2), with an average age of 28 to 29 years. Eight patients (22%) in Group 1 withdrew from the study prior to completing the 6-month extended phase, 4 of them because of side effects. Only 1 woman withdrew from Group 2, because of plans for pregnancy. No one became pregnant while using the ring.
Hormone-free interval reduced bleeding. In Group 1, the average daily flow score was slightly reduced with continuous use (from 0.33 during the 21/7 baseline phase to 0.21 in the 6-month extended phase), but researchers reported no significant difference in flow-free days. On average, 85% of the days were flow-free in the 21/7 phase, vs 89% in the extended phase.
In Group 2, flow-free days increased, from 83% in the baseline phase to 95% in the extended phase, and average flow scores fell from 0.38 to 0.17.
Overall, the 65 participants who completed 6 months of continuous ring use had fewer bleeding days per month—1.8 days, on average, vs 3.3 days during the initial 21/7 phase, but more days of spotting per month (2.5 vs 1.8 days). There was no difference between Groups 1 and 2 in pelvic pain, headache, or mood scores, and no significant difference in headache or mood scores between the baseline and continuous phases of the trial. Pelvic pain scores were lower during the extended phase, however—0.18 vs 0.32 on a scale of 0 to 10.
A high continuation rate. After the 6-month extended phase, 57 of the 65 remaining participants chose to continue using the ring for contraception, on a continuous dosing basis—a continuation rate of 88%. But more than half of the women who chose to stick with the ring (57%) decided not to take advantage of the 4-day hormone-free interval to manage breakthrough bleeding or spotting, regardless of original group assignment.
WHAT’S NEW?: The ring moves further mainstream
Continuous or extended use of the transvaginal ring may be a new idea for many patients—and physicians. But the idea may catch on in light of this study’s findings. Given the high rate of unwanted pregnancy in the United States, many women may benefit from a contraceptive that is as safe and effective as an OC but doesn’t involve a daily pill.
CAVEATS: Side effects, off-label concerns
In 2005, Oddsson et al found that women who used the ring reported more vaginitis and more leukorrhea than women who used OCs; conversely, they reported less nausea and less acne. Other side effects that are common to hormonal contraceptives, such as headache and weight gain, occurred at similar rates among women using the ring and OCs.6
However, the high proportion of patients who elected to keep using the ring at the end of the study by Sulak et al suggests that its side effects are acceptable.1 As with all contraception, however, patient preference is a key consideration. The study population was highly motivated, particularly since women who had difficulty with this means of contraception dropped out after the baseline phase of the trial.
Off-label use. Pharmacokinetic research involving the contraceptive ring has shown that hormone levels required to protect against pregnancy persist for at least 35 days after it is placed in the vagina.8 The manufacturer has data only to confirm contraceptive efficacy for up to 28 days and therefore does not recommend use beyond 4 weeks.4
This study highlights another off-label issue: Women in Group 2, who were allowed to remove the ring for 4 day-intervals to decrease breakthrough bleeding, were instructed to reinsert the same ring after 4 days and keep it in place until the next scheduled replacement date. But the manufacturer does not recommend reinsertion of a ring that has been out of the body for more than 3 hours. In my practice (KR), women are generally unwilling to store and replace a ring, preferring to place a new one after removal for more than a few hours.
Should she try continuous use?
Changing the ring on the same date each month may boost adherence for some women. Funding. The research was funded by an unrestricted educational grant from Organon, Inc, the manufacturer of NuvaRing, which included salary support for 5 of the 6 authors. The published study gives no additional information about the involvement of the pharmaceutical company.
Contraindications, drug interactions. As with other combined hormonal contraceptives, women who have a history of venous thromboembolism, headaches with focal neurological symptoms, severe hypertension, breast or endometrial cancer, or liver disease, and smokers older than 35 years should not use the contraceptive ring.4 In addition, women need to be aware that a number of medications—griseofulvin, rifampin, phenytoin, carbamazepine, and herbal products containing St. John’s Wort, among others—may reduce the effectiveness of the contraceptive ring.4
CHALLENGES TO IMPLEMENTATION: Going off-label isn’t for everyone
When it comes to choosing a contraceptive method, patient preference is paramount. Some women may not be comfortable inserting or removing the ring and should be counseled on other forms of contraception. Women who prefer to bleed every month should not use extended cycling. Similarly, some physicians may not be comfortable recommending an off-label use of the ring.
Those who are comfortable making the recommendation should be prepared to educate patients about this method of contraception and to discuss the benefits of extended or continuous use of the ring. For some women, the memory-triggering mechanism of changing the ring on the same date each month may boost adherence. For others, replacing the ring every 28 days may be acceptable—again, depending on patient preference.
Acknowledgements
The PURLs Surveillance System is supported in part by Grant Number UL1RR024999 from the National Center for Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center for Research Resources or the National Institutes of Health.
PURLs methodology
This study was selected and evaluated using FPIN’s Priority Updates from the Research Literature (PURLs) Surveillance System methodology. The criteria and findings leading to the selection of this study as a PURL can be accessed at www.jfponline.com/purls.
Recommend continuous or extended use of the transvaginal contraceptive ring to women who want fewer days of menstrual bleeding and have trouble remembering to, or prefer not to, take a daily pill. If breakthrough bleeding is troublesome, suggest a 4-day ring-free interval.1
Strength of recommendation
B: Based on a single randomized controlled trial (RCT) with <80% follow up.
Sulak PJ, Smith V, Coffee A, et al. Frequency and management of breakthrough bleeding with continuous use of the transvaginal contraceptive ring: a randomized controlled trial. Obstet Gynecol. 2008;112:563-571.
ILLUSTRATIVE CASE
Case 1: A healthy 25-year-old woman comes to see you because she’s worried about getting pregnant. She’s been on an extended-cycle oral contraceptive (OC) for several months and is happy to have her period only once every 3 months, but she frequently forgets to take her pill.
What can you offer that will give her the benefits of an extended-cycle OC, without the risk of pregnancy she incurs each time she misses a pill?
Case 2: You started a healthy 18-year-old on the transvaginal ring 6 months ago. After counseling, she opted for continuous cycling, so she inserts a new ring right after she removes the old one, on the same date each month. Although she likes the ring, she’s disturbed by a recent increase in breakthrough bleeding. What can you recommend to decrease the bleeding?
Clinicians have long known that the traditional 21/7 OC cycle is not necessary for safety or efficacy. More recently, many women have been happy to learn that there is no physiologic reason to have a monthly period when they’re using combination hormonal contraception. They’re also happy to discover that fewer periods often mean fewer premenstrual mood swings, episodes of painful cramping, and instances of other troublesome symptoms.
The transvaginal ring is often overlooked
The US Food and Drug Administration (FDA) has approved 2 combination OCs for extended-cycle use and 1 for continuous use. But any monophasic OC can be used off-label for extended or continuous cycling to decrease bleeding frequency. So, too, can the transvaginal contraceptive ring (NuvaRing), an infrequently used contraceptive. (According to 1 study, just 5.7% of US women using contraception used either the ring or the patch.2 ) The ring has been studied for extended use,3 but does not have FDA approval for longer-term regimens.
NuvaRing is a flexible, transparent device that contains the progestin etonogestrel and ethinyl estradiol. The manufacturer recommends a 21/7 cycle, inserting the ring in the vagina and leaving it in place for 3 weeks, removing it for 1 week, and then inserting a new ring.4 The ring is well suited to women who have no contraindications to hormonal contraception but have difficulty remembering to take a pill every day—or simply prefer the convenience of less frequent dosing.
While the ring has been found to be effective and tolerable when used without a hormone-free interval—28-, 49-, 91- and 364-day dosing has been studied—breakthrough bleeding or spotting is a frequent side effect of extended-cycle hormonal contraception. In 1 study, 43% of women on a 49-day ring cycle experienced breakthrough bleeding, compared with 16% of those on a 28-day cycle.3
High satisfaction, low risk
Nonetheless, women who use the transvaginal ring often report high satisfaction. One study found that 61% of women were very satisfied with this method of contraception, compared with 34% of triphasic OC users (P<.003).5 The risk of pregnancy (1-2 pregnancies per 100 women-years of use, according to the manufacturer4 ) and the risk of venous thromboembolism (10-30 in 100,000 vs 4-11 in 100,000 nonpregnant women who are not using hormonal contraception) are comparable to that of women using OCs.6,7 The risk of other severe side effects associated with the vaginal ring is comparable to that of OCs, as well.
STUDY SUMMARY: An effective option that women used post-trial
This RCT recruited women between the ages of 18 and 45 years who had been using combination hormonal contraceptives—OCs, the transdermal patch, or the transvaginal ring. All had been on a 21/7 cycle for at least 2 months. Exclusion criteria included a body mass index ≥38 kg/m2, smoking >10 cigarettes per day, use of other estrogen- or phytoestrogen-containing products, and the presence of ovarian cysts >2.5 cm or endometrial thickness >8 mm. Women who wanted to get pregnant within a year were also excluded.
The study began with a baseline phase during which participants completed one 21/7 cycle with the ring for those using the ring prior to the study or two 21/7 cycles for those using the pill or patch prior to the study. Daily flow was assessed during this initial phase, using a scale of 0 to 4, with 4 being the heaviest. Women who completed this phase and wanted to continue using the ring (N=74) were then randomized into 2 groups (n=37) for the 6-month extended phase.
Group 1 was assigned to use the contraceptive ring with no hormone-free days. Participants were instructed to replace the rings monthly, on the same calendar day of the month. Group 2 also used the ring on a continuous basis with monthly replacement, but those who experienced breakthrough bleeding for more than 5 days were permitted to remove the ring for 4 days. Women in both groups kept a daily diary of ring usage, degree of menstrual flow, and symptomatology, including pelvic pain, headaches, and mood.
Most subjects were white (76%), nonsmokers (84%), and unmarried (68% in Group 1 and 57% in Group 2), with an average age of 28 to 29 years. Eight patients (22%) in Group 1 withdrew from the study prior to completing the 6-month extended phase, 4 of them because of side effects. Only 1 woman withdrew from Group 2, because of plans for pregnancy. No one became pregnant while using the ring.
Hormone-free interval reduced bleeding. In Group 1, the average daily flow score was slightly reduced with continuous use (from 0.33 during the 21/7 baseline phase to 0.21 in the 6-month extended phase), but researchers reported no significant difference in flow-free days. On average, 85% of the days were flow-free in the 21/7 phase, vs 89% in the extended phase.
In Group 2, flow-free days increased, from 83% in the baseline phase to 95% in the extended phase, and average flow scores fell from 0.38 to 0.17.
Overall, the 65 participants who completed 6 months of continuous ring use had fewer bleeding days per month—1.8 days, on average, vs 3.3 days during the initial 21/7 phase, but more days of spotting per month (2.5 vs 1.8 days). There was no difference between Groups 1 and 2 in pelvic pain, headache, or mood scores, and no significant difference in headache or mood scores between the baseline and continuous phases of the trial. Pelvic pain scores were lower during the extended phase, however—0.18 vs 0.32 on a scale of 0 to 10.
A high continuation rate. After the 6-month extended phase, 57 of the 65 remaining participants chose to continue using the ring for contraception, on a continuous dosing basis—a continuation rate of 88%. But more than half of the women who chose to stick with the ring (57%) decided not to take advantage of the 4-day hormone-free interval to manage breakthrough bleeding or spotting, regardless of original group assignment.
WHAT’S NEW?: The ring moves further mainstream
Continuous or extended use of the transvaginal ring may be a new idea for many patients—and physicians. But the idea may catch on in light of this study’s findings. Given the high rate of unwanted pregnancy in the United States, many women may benefit from a contraceptive that is as safe and effective as an OC but doesn’t involve a daily pill.
CAVEATS: Side effects, off-label concerns
In 2005, Oddsson et al found that women who used the ring reported more vaginitis and more leukorrhea than women who used OCs; conversely, they reported less nausea and less acne. Other side effects that are common to hormonal contraceptives, such as headache and weight gain, occurred at similar rates among women using the ring and OCs.6
However, the high proportion of patients who elected to keep using the ring at the end of the study by Sulak et al suggests that its side effects are acceptable.1 As with all contraception, however, patient preference is a key consideration. The study population was highly motivated, particularly since women who had difficulty with this means of contraception dropped out after the baseline phase of the trial.
Off-label use. Pharmacokinetic research involving the contraceptive ring has shown that hormone levels required to protect against pregnancy persist for at least 35 days after it is placed in the vagina.8 The manufacturer has data only to confirm contraceptive efficacy for up to 28 days and therefore does not recommend use beyond 4 weeks.4
This study highlights another off-label issue: Women in Group 2, who were allowed to remove the ring for 4 day-intervals to decrease breakthrough bleeding, were instructed to reinsert the same ring after 4 days and keep it in place until the next scheduled replacement date. But the manufacturer does not recommend reinsertion of a ring that has been out of the body for more than 3 hours. In my practice (KR), women are generally unwilling to store and replace a ring, preferring to place a new one after removal for more than a few hours.
Should she try continuous use?
Changing the ring on the same date each month may boost adherence for some women. Funding. The research was funded by an unrestricted educational grant from Organon, Inc, the manufacturer of NuvaRing, which included salary support for 5 of the 6 authors. The published study gives no additional information about the involvement of the pharmaceutical company.
Contraindications, drug interactions. As with other combined hormonal contraceptives, women who have a history of venous thromboembolism, headaches with focal neurological symptoms, severe hypertension, breast or endometrial cancer, or liver disease, and smokers older than 35 years should not use the contraceptive ring.4 In addition, women need to be aware that a number of medications—griseofulvin, rifampin, phenytoin, carbamazepine, and herbal products containing St. John’s Wort, among others—may reduce the effectiveness of the contraceptive ring.4
CHALLENGES TO IMPLEMENTATION: Going off-label isn’t for everyone
When it comes to choosing a contraceptive method, patient preference is paramount. Some women may not be comfortable inserting or removing the ring and should be counseled on other forms of contraception. Women who prefer to bleed every month should not use extended cycling. Similarly, some physicians may not be comfortable recommending an off-label use of the ring.
Those who are comfortable making the recommendation should be prepared to educate patients about this method of contraception and to discuss the benefits of extended or continuous use of the ring. For some women, the memory-triggering mechanism of changing the ring on the same date each month may boost adherence. For others, replacing the ring every 28 days may be acceptable—again, depending on patient preference.
Acknowledgements
The PURLs Surveillance System is supported in part by Grant Number UL1RR024999 from the National Center for Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center for Research Resources or the National Institutes of Health.
PURLs methodology
This study was selected and evaluated using FPIN’s Priority Updates from the Research Literature (PURLs) Surveillance System methodology. The criteria and findings leading to the selection of this study as a PURL can be accessed at www.jfponline.com/purls.
1. Sulak PJ, Smith V, Coffee A, et al. Frequency and management of breakthrough bleeding with continuous use of the transvaginal contraceptive ring: a randomized controlled trial. Obstet Gynecol. 2008;112:563-571.
2. Frost JJ, Singh S, Finer LB. US women’s one-year contraceptive use patterns, 2004. Perspect Sex Reprod Health. 2007;39:48-55.
3. Miller L, Verhoeven C, Hout J. Extended regimens of the contraceptive vaginal ring: a randomized trial. Obstet Gynecol. 2005;106:473-482.
4. NuvaRing (etonogestrel/ethinyl estradiol vaginal ring) [prescribing information]. Roseland, NJ: Organon USA Inc; June 2008. Available at: http://www.spfiles.com/pinuvaring.pdf. Accessed March 10, 2009.
5. Schafer JE, Osborne LM, Davis AR, et al. Acceptability and satisfaction using Quick Start with the contraceptive vaginal ring versus an oral contraceptive. Contraception. 2006;73:488-492.
6. Oddsson K, Leifels-Fischer B, de Melo NR, et al. Efficacy and safety of a contraceptive vaginal ring (NuvaRing) compared with a combined oral contraceptive: a 1-year randomized trial. Contraception. 2005;71:176-182.
7. Wilks JF. Hormonal birth control and pregnancy: a comparative analysis of thromboembolic risk. Ann Pharmacother. 2003;37:912-916.
8. Timmer C, Mulders T. Pharmacokinetics of etonogestrel and ethinylestradiol released from a combined contraceptive vaginal ring. Clin Pharmacokinet. 2000;39:233-242.
1. Sulak PJ, Smith V, Coffee A, et al. Frequency and management of breakthrough bleeding with continuous use of the transvaginal contraceptive ring: a randomized controlled trial. Obstet Gynecol. 2008;112:563-571.
2. Frost JJ, Singh S, Finer LB. US women’s one-year contraceptive use patterns, 2004. Perspect Sex Reprod Health. 2007;39:48-55.
3. Miller L, Verhoeven C, Hout J. Extended regimens of the contraceptive vaginal ring: a randomized trial. Obstet Gynecol. 2005;106:473-482.
4. NuvaRing (etonogestrel/ethinyl estradiol vaginal ring) [prescribing information]. Roseland, NJ: Organon USA Inc; June 2008. Available at: http://www.spfiles.com/pinuvaring.pdf. Accessed March 10, 2009.
5. Schafer JE, Osborne LM, Davis AR, et al. Acceptability and satisfaction using Quick Start with the contraceptive vaginal ring versus an oral contraceptive. Contraception. 2006;73:488-492.
6. Oddsson K, Leifels-Fischer B, de Melo NR, et al. Efficacy and safety of a contraceptive vaginal ring (NuvaRing) compared with a combined oral contraceptive: a 1-year randomized trial. Contraception. 2005;71:176-182.
7. Wilks JF. Hormonal birth control and pregnancy: a comparative analysis of thromboembolic risk. Ann Pharmacother. 2003;37:912-916.
8. Timmer C, Mulders T. Pharmacokinetics of etonogestrel and ethinylestradiol released from a combined contraceptive vaginal ring. Clin Pharmacokinet. 2000;39:233-242.
Copyright © 2009 The Family Physicians Inquiries Network.
All rights reserved.
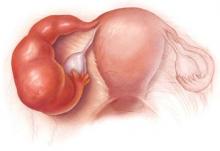
Azithromycin for PID beats doxycycline on all counts
Outpatient treatment of patients with mild pelvic inflammatory disease, using 1 g of azithromycin weekly for 2 weeks, combined with 250 mg of ceftriaxone intramuscularly on the first day, is superior to the current recommended treatment with doxycycline plus ceftriaxone.1
Strength of recommendation (SOR)
A: Single well-designed RCT
Savaris RF, Teixeira LM, Torres TG, Edelweiss MI, Moncada J, Schachter J. Comparing ceftriaxone plus azithromycin or doxycycline for pelvic inflammatory disease: a randomized controlled trial. Obstet Gynecol 2007;110:53–60.
Illustrative Case
An otherwise healthy, sexually active 21-year-old woman complains of pelvic pain for a week and yellow vaginal discharge. The history and physical exam are consistent with mild, uncomplicated pelvic inflammatory disease (PID).
You believe outpatient therapy is appropriate in this case and wonder if there is a better alternative to doxycycline, particularly given the challenges of adherence to the recommended 14-day course of treatment.
Background: 2 doses or 28 doses?
In the real world, we know that adherence is better when patients have to take 2 pills than when they have to take 28 pills. For most women with mild, uncomplicated PID, outpatient treatment is appropriate2 and a shorter treatment course is related to better adherence.3 Azithromycin can be given in 2 single doses a week apart, with few side effects, and its spectrum of activity is similar to that of doxycycline,4 which requires a 14-day regimen of 2 pills daily. Earlier studies of azithromycin for PID, however, were not designed specifically for outpatient treatment, or had methodologic bias.5 Thus, the evidence has been insufficient to recommend it.
PID affects about 1 million women in the US each year, and can cause pain, scarring of the fallopian tubes, and infertility.
Current recommendations. The Centers for Disease Control and Prevention (CDC) recommends oral doxycycline 100 mg twice daily for 14 days, along with a second- or third-generation cephalosporin administered parenterally, for mild PID in ambulatory patients.5 Metronizadole can be added at the provider’s discretion. The CDC no longer routinely recommends fluoroquinolones for PID because of gonococcal resistance.6 Dynamed, PEPID PCP, and UpToDate all cite CDC guidelines. Dynamed also notes results of the article reviewed here, though treatment recommendations were not changed.7-9
A simpler approach to pelvic inflammatory disease
Patients will likely find it easier to take 1 azithromycin pill initially and 1 pill a week later, than to take a doxycycline pill twice daily for 14 days. lf so, then the advantage of azithromycin could be greater than reported in this study
Clinical context: First comparison study, first outpatient study
The study by Savaris and colleagues is the first comparison study of azithromycin and doxycycline for PID, and the first study of outpatient treatment of PID with azithromycin.
An earlier study reported that women with PID who were prescribed doxycycline took an average of 70% of the total doses, and fewer than half took it twice daily as directed.3
Azithromycin is known to be effective for treatment of Chlamydia trachomatis cervical infections,4 and single-dose azithromycin has been also been shown to have better compliance than multidose therapy for Chlamydia infection.10
We identified only 1 prior randomized controlled trial of azithromycin for treatment of PID. That trial reported that intravenous azithromycin followed by oral azithromycin with or without metronidazole is effective in the treatment of PID.11
Study summary: Azithromycin cure rate 90%, doxycycline 72%
This randomized, double-blind, controlled study evaluated the effectiveness of azithromycin plus ceftriaxone in the treatment of mild, uncomplicated PID compared to doxycycline plus ceftriaxone, in outpatients.
Patients
The study enrolled 133 women who presented to an emergency department with PID diagnosed by the following clinical criteria:
- pelvic pain for less than 30 days
- pelvic organ (adnexal or cervical) tenderness on physical examination
- cervical leukorrhea or mucopurulent cervicitis.
Method
The women were randomized into 2 groups, and both groups received 250 mg of ceftriaxone intramuscularly.
- The control group received 100 mg of doxycycline twice daily for 2 weeks.
- The study group received 1 g of azithromycin by mouth weekly for 2 weeks and a placebo twice daily for 2 weeks to maintain blinding.
Outcomes
The primary outcome was clinical cure after 2 weeks of treatment. Clinical cure was defined as an improvement in pain scale ratings by 70%. Failure was defined as worsening of pain, lack of improvement of pain, or need for additional antibiotic therapy, hospitalization, or surgery.
Of the 133 women randomized, 13 (9 from the azithromycin group and 4 from the doxycycline group) were found to have diagnoses other than PID after randomization.
Intention-to-treat analysis was performed for the remaining 120 participants. In the azithromycin group, 56/62 (90.3%; 95% confidence interval [CI], 0.80–0.96) women were classified as clinically cured, versus 42/58 (72.4%; 95% CI, 0.58–0.82) in the doxycycline group.
Adverse events. Except for oral intolerance to the first dose of medication, which was similar in both groups, adverse events were not reported.
Adherence similar in both groups
Adherence to the study protocol was similar in both groups. The study authors concluded that azithromycin was superior to doxycycline even though the adherence in the doxycycline group was good.
What’s new?: Better adherence is the probable bonus
This RCT shows that azithromycin treatment of PID in an ambulatory population is superior to doxycycline even when there is excellent compliance with taking doxycycline (unlike the reality of clinical practice). The patients in this RCT adhered well to the protocol, so it does not provide a realistic head-to-head comparison of treatment completion.
Real-world adherence
In actual practice, we speculate that taking 2 pills 1 week apart will be much easier for patients than taking 2 pills every day for 14 days. The literature on compliance would predict that to be the case. If true, then the advantage of azithromycin over doxycycline would be even greater than reported in this study.
Caveats: Apply these findings in similar cases only
This study addresses ambulatory treatment of mild, uncomplicated PID, and results should only be extrapolated to similar cases.
Azithromycin should not be prescribed to patients with an allergy to macrolide antibiotics.
One of the study authors received azithromycin donated by Pfizer for other research; however, Pfizer did not sponsor this study.
Challenges to implementation: Cost of the prescription
Prescription cost may be a consideration for patients without insurance, although azithromycin has been shown to be cost-effective in treatment of Chlamydia.12
Reminding patients to take the second dose
Some patients may have difficulty remembering to take the second dose a week after the first dose.
A follow-up visit, reminder phone call, or suggestion to “mark this on your calendar” may help enhance adherence.
PURLs methodology
This study was selected and evaluated using the Family Physician Inquiries Network’s Priority Updates from the Research Literature Surveillance System (PURLs) methodology. The criteria and findings leading to the selection of this study as a PURL can be accessed here.
1. Savaris RF, Teixeira LM, Torres TG, Edelweiss MI, Moncada J, Schachter J. Comparing ceftriaxone plus azithromycin or doxycycline for pelvic inflammatory disease: a randomized controlled trial. Obstet Gynecol 2007;110:53-60.
2. Ness RB, Soper DE, Holley RL, et al. Effectiveness of inpatient and outpatient treatment strategies for women with pelvic inflammatory disease: results from the pelvic inflammatory disease evaluation and clinical health (PEACH) randomized trial. Am J Obstet Gynecol 2002;186:929-937.
3. Dunbar-Jacob J, Sereika SM, Foley SM, Bass DC, Ness RG. Adherence to oral therapies in pelvic inflammatory disease. J Womens Health 2004;13:285-291.
4. Lau CY, Qureshi AK. Azithromycin versus doxycycline for genital chlamydial infections: a metaanalysis of randomized clinical trials. Sex Transm Dis 2002;29:497-502.
5. Workowski KA, Berman SM. Sexually transmitted diseases treatment guidelines, 2006. Centers for Disease Control and Prevention. MMWR Recomm Rep 2006;55(RR-11):1-94.Available at: www.cdc.gov/std/treatment/2006/updated-regimens.htm. Accessed on November 14, 2007.
6. Centers for Disease Control and Prevention. Update to CDC sexually transmitted diseases treatment guidelines, 2006: fluoroquinolones no longer recommended for treatment of gonococcal infections. MMWR Morb Mortal Wkly Rep 2007;56(14):332-336.Available at: www.cdc.gov/mmwr/preview/mmwrhtml/mm5614a3.htm. Accessed on November 14, 2007.
7. Pelvic inflammatory disease. In: Dynamed [online database]. Available at: www.dynamicmedical.com. Accessed on August 30, 2007.
8. Pelvic inflammatory disease. In: PEPID-PCP [online database]. Available at: www.pepidonline.com. Accessed on August 30, 2007.
9. Hynes N. Treatment of pelvic inflammatory disease. In: UptoDate [online database]. Available at: www.utdol.com. Accessed on August 30, 2007.
10. Adair CD, Gunter M, Stovall TG, McElroy G, Veille JC, Ernest JM. Chlamydia in pregnancy: a randomized trial of azithromycin and erythromycin. Obstet Gynecol 1998;91:165-168.
11. Bevan CD, Ridgway GL, Rothermel CD. Efficacy and safety of azithromycin as monotherapy or combined with metronidazole compared with two standard multidrug regimens for the treatment of acute pelvic inflammatory disease. J Int Med Res 2003;31:45-54.
12. Magid D, Douglas JM, Schwartz JS. Doxycycline compared with azithromycin for treating women with genital Chlamydia trachomatis infections: an incremental cost-effectiveness analysis. Ann Intern Med 1996;124:389-399.
Outpatient treatment of patients with mild pelvic inflammatory disease, using 1 g of azithromycin weekly for 2 weeks, combined with 250 mg of ceftriaxone intramuscularly on the first day, is superior to the current recommended treatment with doxycycline plus ceftriaxone.1
Strength of recommendation (SOR)
A: Single well-designed RCT
Savaris RF, Teixeira LM, Torres TG, Edelweiss MI, Moncada J, Schachter J. Comparing ceftriaxone plus azithromycin or doxycycline for pelvic inflammatory disease: a randomized controlled trial. Obstet Gynecol 2007;110:53–60.
Illustrative Case
An otherwise healthy, sexually active 21-year-old woman complains of pelvic pain for a week and yellow vaginal discharge. The history and physical exam are consistent with mild, uncomplicated pelvic inflammatory disease (PID).
You believe outpatient therapy is appropriate in this case and wonder if there is a better alternative to doxycycline, particularly given the challenges of adherence to the recommended 14-day course of treatment.
Background: 2 doses or 28 doses?
In the real world, we know that adherence is better when patients have to take 2 pills than when they have to take 28 pills. For most women with mild, uncomplicated PID, outpatient treatment is appropriate2 and a shorter treatment course is related to better adherence.3 Azithromycin can be given in 2 single doses a week apart, with few side effects, and its spectrum of activity is similar to that of doxycycline,4 which requires a 14-day regimen of 2 pills daily. Earlier studies of azithromycin for PID, however, were not designed specifically for outpatient treatment, or had methodologic bias.5 Thus, the evidence has been insufficient to recommend it.
PID affects about 1 million women in the US each year, and can cause pain, scarring of the fallopian tubes, and infertility.
Current recommendations. The Centers for Disease Control and Prevention (CDC) recommends oral doxycycline 100 mg twice daily for 14 days, along with a second- or third-generation cephalosporin administered parenterally, for mild PID in ambulatory patients.5 Metronizadole can be added at the provider’s discretion. The CDC no longer routinely recommends fluoroquinolones for PID because of gonococcal resistance.6 Dynamed, PEPID PCP, and UpToDate all cite CDC guidelines. Dynamed also notes results of the article reviewed here, though treatment recommendations were not changed.7-9
A simpler approach to pelvic inflammatory disease
Patients will likely find it easier to take 1 azithromycin pill initially and 1 pill a week later, than to take a doxycycline pill twice daily for 14 days. lf so, then the advantage of azithromycin could be greater than reported in this study
Clinical context: First comparison study, first outpatient study
The study by Savaris and colleagues is the first comparison study of azithromycin and doxycycline for PID, and the first study of outpatient treatment of PID with azithromycin.
An earlier study reported that women with PID who were prescribed doxycycline took an average of 70% of the total doses, and fewer than half took it twice daily as directed.3
Azithromycin is known to be effective for treatment of Chlamydia trachomatis cervical infections,4 and single-dose azithromycin has been also been shown to have better compliance than multidose therapy for Chlamydia infection.10
We identified only 1 prior randomized controlled trial of azithromycin for treatment of PID. That trial reported that intravenous azithromycin followed by oral azithromycin with or without metronidazole is effective in the treatment of PID.11
Study summary: Azithromycin cure rate 90%, doxycycline 72%
This randomized, double-blind, controlled study evaluated the effectiveness of azithromycin plus ceftriaxone in the treatment of mild, uncomplicated PID compared to doxycycline plus ceftriaxone, in outpatients.
Patients
The study enrolled 133 women who presented to an emergency department with PID diagnosed by the following clinical criteria:
- pelvic pain for less than 30 days
- pelvic organ (adnexal or cervical) tenderness on physical examination
- cervical leukorrhea or mucopurulent cervicitis.
Method
The women were randomized into 2 groups, and both groups received 250 mg of ceftriaxone intramuscularly.
- The control group received 100 mg of doxycycline twice daily for 2 weeks.
- The study group received 1 g of azithromycin by mouth weekly for 2 weeks and a placebo twice daily for 2 weeks to maintain blinding.
Outcomes
The primary outcome was clinical cure after 2 weeks of treatment. Clinical cure was defined as an improvement in pain scale ratings by 70%. Failure was defined as worsening of pain, lack of improvement of pain, or need for additional antibiotic therapy, hospitalization, or surgery.
Of the 133 women randomized, 13 (9 from the azithromycin group and 4 from the doxycycline group) were found to have diagnoses other than PID after randomization.
Intention-to-treat analysis was performed for the remaining 120 participants. In the azithromycin group, 56/62 (90.3%; 95% confidence interval [CI], 0.80–0.96) women were classified as clinically cured, versus 42/58 (72.4%; 95% CI, 0.58–0.82) in the doxycycline group.
Adverse events. Except for oral intolerance to the first dose of medication, which was similar in both groups, adverse events were not reported.
Adherence similar in both groups
Adherence to the study protocol was similar in both groups. The study authors concluded that azithromycin was superior to doxycycline even though the adherence in the doxycycline group was good.
What’s new?: Better adherence is the probable bonus
This RCT shows that azithromycin treatment of PID in an ambulatory population is superior to doxycycline even when there is excellent compliance with taking doxycycline (unlike the reality of clinical practice). The patients in this RCT adhered well to the protocol, so it does not provide a realistic head-to-head comparison of treatment completion.
Real-world adherence
In actual practice, we speculate that taking 2 pills 1 week apart will be much easier for patients than taking 2 pills every day for 14 days. The literature on compliance would predict that to be the case. If true, then the advantage of azithromycin over doxycycline would be even greater than reported in this study.
Caveats: Apply these findings in similar cases only
This study addresses ambulatory treatment of mild, uncomplicated PID, and results should only be extrapolated to similar cases.
Azithromycin should not be prescribed to patients with an allergy to macrolide antibiotics.
One of the study authors received azithromycin donated by Pfizer for other research; however, Pfizer did not sponsor this study.
Challenges to implementation: Cost of the prescription
Prescription cost may be a consideration for patients without insurance, although azithromycin has been shown to be cost-effective in treatment of Chlamydia.12
Reminding patients to take the second dose
Some patients may have difficulty remembering to take the second dose a week after the first dose.
A follow-up visit, reminder phone call, or suggestion to “mark this on your calendar” may help enhance adherence.
PURLs methodology
This study was selected and evaluated using the Family Physician Inquiries Network’s Priority Updates from the Research Literature Surveillance System (PURLs) methodology. The criteria and findings leading to the selection of this study as a PURL can be accessed here.
Outpatient treatment of patients with mild pelvic inflammatory disease, using 1 g of azithromycin weekly for 2 weeks, combined with 250 mg of ceftriaxone intramuscularly on the first day, is superior to the current recommended treatment with doxycycline plus ceftriaxone.1
Strength of recommendation (SOR)
A: Single well-designed RCT
Savaris RF, Teixeira LM, Torres TG, Edelweiss MI, Moncada J, Schachter J. Comparing ceftriaxone plus azithromycin or doxycycline for pelvic inflammatory disease: a randomized controlled trial. Obstet Gynecol 2007;110:53–60.
Illustrative Case
An otherwise healthy, sexually active 21-year-old woman complains of pelvic pain for a week and yellow vaginal discharge. The history and physical exam are consistent with mild, uncomplicated pelvic inflammatory disease (PID).
You believe outpatient therapy is appropriate in this case and wonder if there is a better alternative to doxycycline, particularly given the challenges of adherence to the recommended 14-day course of treatment.
Background: 2 doses or 28 doses?
In the real world, we know that adherence is better when patients have to take 2 pills than when they have to take 28 pills. For most women with mild, uncomplicated PID, outpatient treatment is appropriate2 and a shorter treatment course is related to better adherence.3 Azithromycin can be given in 2 single doses a week apart, with few side effects, and its spectrum of activity is similar to that of doxycycline,4 which requires a 14-day regimen of 2 pills daily. Earlier studies of azithromycin for PID, however, were not designed specifically for outpatient treatment, or had methodologic bias.5 Thus, the evidence has been insufficient to recommend it.
PID affects about 1 million women in the US each year, and can cause pain, scarring of the fallopian tubes, and infertility.
Current recommendations. The Centers for Disease Control and Prevention (CDC) recommends oral doxycycline 100 mg twice daily for 14 days, along with a second- or third-generation cephalosporin administered parenterally, for mild PID in ambulatory patients.5 Metronizadole can be added at the provider’s discretion. The CDC no longer routinely recommends fluoroquinolones for PID because of gonococcal resistance.6 Dynamed, PEPID PCP, and UpToDate all cite CDC guidelines. Dynamed also notes results of the article reviewed here, though treatment recommendations were not changed.7-9
A simpler approach to pelvic inflammatory disease
Patients will likely find it easier to take 1 azithromycin pill initially and 1 pill a week later, than to take a doxycycline pill twice daily for 14 days. lf so, then the advantage of azithromycin could be greater than reported in this study
Clinical context: First comparison study, first outpatient study
The study by Savaris and colleagues is the first comparison study of azithromycin and doxycycline for PID, and the first study of outpatient treatment of PID with azithromycin.
An earlier study reported that women with PID who were prescribed doxycycline took an average of 70% of the total doses, and fewer than half took it twice daily as directed.3
Azithromycin is known to be effective for treatment of Chlamydia trachomatis cervical infections,4 and single-dose azithromycin has been also been shown to have better compliance than multidose therapy for Chlamydia infection.10
We identified only 1 prior randomized controlled trial of azithromycin for treatment of PID. That trial reported that intravenous azithromycin followed by oral azithromycin with or without metronidazole is effective in the treatment of PID.11
Study summary: Azithromycin cure rate 90%, doxycycline 72%
This randomized, double-blind, controlled study evaluated the effectiveness of azithromycin plus ceftriaxone in the treatment of mild, uncomplicated PID compared to doxycycline plus ceftriaxone, in outpatients.
Patients
The study enrolled 133 women who presented to an emergency department with PID diagnosed by the following clinical criteria:
- pelvic pain for less than 30 days
- pelvic organ (adnexal or cervical) tenderness on physical examination
- cervical leukorrhea or mucopurulent cervicitis.
Method
The women were randomized into 2 groups, and both groups received 250 mg of ceftriaxone intramuscularly.
- The control group received 100 mg of doxycycline twice daily for 2 weeks.
- The study group received 1 g of azithromycin by mouth weekly for 2 weeks and a placebo twice daily for 2 weeks to maintain blinding.
Outcomes
The primary outcome was clinical cure after 2 weeks of treatment. Clinical cure was defined as an improvement in pain scale ratings by 70%. Failure was defined as worsening of pain, lack of improvement of pain, or need for additional antibiotic therapy, hospitalization, or surgery.
Of the 133 women randomized, 13 (9 from the azithromycin group and 4 from the doxycycline group) were found to have diagnoses other than PID after randomization.
Intention-to-treat analysis was performed for the remaining 120 participants. In the azithromycin group, 56/62 (90.3%; 95% confidence interval [CI], 0.80–0.96) women were classified as clinically cured, versus 42/58 (72.4%; 95% CI, 0.58–0.82) in the doxycycline group.
Adverse events. Except for oral intolerance to the first dose of medication, which was similar in both groups, adverse events were not reported.
Adherence similar in both groups
Adherence to the study protocol was similar in both groups. The study authors concluded that azithromycin was superior to doxycycline even though the adherence in the doxycycline group was good.
What’s new?: Better adherence is the probable bonus
This RCT shows that azithromycin treatment of PID in an ambulatory population is superior to doxycycline even when there is excellent compliance with taking doxycycline (unlike the reality of clinical practice). The patients in this RCT adhered well to the protocol, so it does not provide a realistic head-to-head comparison of treatment completion.
Real-world adherence
In actual practice, we speculate that taking 2 pills 1 week apart will be much easier for patients than taking 2 pills every day for 14 days. The literature on compliance would predict that to be the case. If true, then the advantage of azithromycin over doxycycline would be even greater than reported in this study.
Caveats: Apply these findings in similar cases only
This study addresses ambulatory treatment of mild, uncomplicated PID, and results should only be extrapolated to similar cases.
Azithromycin should not be prescribed to patients with an allergy to macrolide antibiotics.
One of the study authors received azithromycin donated by Pfizer for other research; however, Pfizer did not sponsor this study.
Challenges to implementation: Cost of the prescription
Prescription cost may be a consideration for patients without insurance, although azithromycin has been shown to be cost-effective in treatment of Chlamydia.12
Reminding patients to take the second dose
Some patients may have difficulty remembering to take the second dose a week after the first dose.
A follow-up visit, reminder phone call, or suggestion to “mark this on your calendar” may help enhance adherence.
PURLs methodology
This study was selected and evaluated using the Family Physician Inquiries Network’s Priority Updates from the Research Literature Surveillance System (PURLs) methodology. The criteria and findings leading to the selection of this study as a PURL can be accessed here.
1. Savaris RF, Teixeira LM, Torres TG, Edelweiss MI, Moncada J, Schachter J. Comparing ceftriaxone plus azithromycin or doxycycline for pelvic inflammatory disease: a randomized controlled trial. Obstet Gynecol 2007;110:53-60.
2. Ness RB, Soper DE, Holley RL, et al. Effectiveness of inpatient and outpatient treatment strategies for women with pelvic inflammatory disease: results from the pelvic inflammatory disease evaluation and clinical health (PEACH) randomized trial. Am J Obstet Gynecol 2002;186:929-937.
3. Dunbar-Jacob J, Sereika SM, Foley SM, Bass DC, Ness RG. Adherence to oral therapies in pelvic inflammatory disease. J Womens Health 2004;13:285-291.
4. Lau CY, Qureshi AK. Azithromycin versus doxycycline for genital chlamydial infections: a metaanalysis of randomized clinical trials. Sex Transm Dis 2002;29:497-502.
5. Workowski KA, Berman SM. Sexually transmitted diseases treatment guidelines, 2006. Centers for Disease Control and Prevention. MMWR Recomm Rep 2006;55(RR-11):1-94.Available at: www.cdc.gov/std/treatment/2006/updated-regimens.htm. Accessed on November 14, 2007.
6. Centers for Disease Control and Prevention. Update to CDC sexually transmitted diseases treatment guidelines, 2006: fluoroquinolones no longer recommended for treatment of gonococcal infections. MMWR Morb Mortal Wkly Rep 2007;56(14):332-336.Available at: www.cdc.gov/mmwr/preview/mmwrhtml/mm5614a3.htm. Accessed on November 14, 2007.
7. Pelvic inflammatory disease. In: Dynamed [online database]. Available at: www.dynamicmedical.com. Accessed on August 30, 2007.
8. Pelvic inflammatory disease. In: PEPID-PCP [online database]. Available at: www.pepidonline.com. Accessed on August 30, 2007.
9. Hynes N. Treatment of pelvic inflammatory disease. In: UptoDate [online database]. Available at: www.utdol.com. Accessed on August 30, 2007.
10. Adair CD, Gunter M, Stovall TG, McElroy G, Veille JC, Ernest JM. Chlamydia in pregnancy: a randomized trial of azithromycin and erythromycin. Obstet Gynecol 1998;91:165-168.
11. Bevan CD, Ridgway GL, Rothermel CD. Efficacy and safety of azithromycin as monotherapy or combined with metronidazole compared with two standard multidrug regimens for the treatment of acute pelvic inflammatory disease. J Int Med Res 2003;31:45-54.
12. Magid D, Douglas JM, Schwartz JS. Doxycycline compared with azithromycin for treating women with genital Chlamydia trachomatis infections: an incremental cost-effectiveness analysis. Ann Intern Med 1996;124:389-399.
1. Savaris RF, Teixeira LM, Torres TG, Edelweiss MI, Moncada J, Schachter J. Comparing ceftriaxone plus azithromycin or doxycycline for pelvic inflammatory disease: a randomized controlled trial. Obstet Gynecol 2007;110:53-60.
2. Ness RB, Soper DE, Holley RL, et al. Effectiveness of inpatient and outpatient treatment strategies for women with pelvic inflammatory disease: results from the pelvic inflammatory disease evaluation and clinical health (PEACH) randomized trial. Am J Obstet Gynecol 2002;186:929-937.
3. Dunbar-Jacob J, Sereika SM, Foley SM, Bass DC, Ness RG. Adherence to oral therapies in pelvic inflammatory disease. J Womens Health 2004;13:285-291.
4. Lau CY, Qureshi AK. Azithromycin versus doxycycline for genital chlamydial infections: a metaanalysis of randomized clinical trials. Sex Transm Dis 2002;29:497-502.
5. Workowski KA, Berman SM. Sexually transmitted diseases treatment guidelines, 2006. Centers for Disease Control and Prevention. MMWR Recomm Rep 2006;55(RR-11):1-94.Available at: www.cdc.gov/std/treatment/2006/updated-regimens.htm. Accessed on November 14, 2007.
6. Centers for Disease Control and Prevention. Update to CDC sexually transmitted diseases treatment guidelines, 2006: fluoroquinolones no longer recommended for treatment of gonococcal infections. MMWR Morb Mortal Wkly Rep 2007;56(14):332-336.Available at: www.cdc.gov/mmwr/preview/mmwrhtml/mm5614a3.htm. Accessed on November 14, 2007.
7. Pelvic inflammatory disease. In: Dynamed [online database]. Available at: www.dynamicmedical.com. Accessed on August 30, 2007.
8. Pelvic inflammatory disease. In: PEPID-PCP [online database]. Available at: www.pepidonline.com. Accessed on August 30, 2007.
9. Hynes N. Treatment of pelvic inflammatory disease. In: UptoDate [online database]. Available at: www.utdol.com. Accessed on August 30, 2007.
10. Adair CD, Gunter M, Stovall TG, McElroy G, Veille JC, Ernest JM. Chlamydia in pregnancy: a randomized trial of azithromycin and erythromycin. Obstet Gynecol 1998;91:165-168.
11. Bevan CD, Ridgway GL, Rothermel CD. Efficacy and safety of azithromycin as monotherapy or combined with metronidazole compared with two standard multidrug regimens for the treatment of acute pelvic inflammatory disease. J Int Med Res 2003;31:45-54.
12. Magid D, Douglas JM, Schwartz JS. Doxycycline compared with azithromycin for treating women with genital Chlamydia trachomatis infections: an incremental cost-effectiveness analysis. Ann Intern Med 1996;124:389-399.
Copyright © 2007 The Family Physicians Inquiries Network.
All rights reserved.