User login
What every ObGyn should know about Supreme Court rulings in the recent term
The most recently concluded term of the US Supreme Court, which began on October 1, 2018, yielded a number of decisions of interest to health care professionals and to ObGyns in particular. Although the term was viewed by some observers as less consequential than other recent terms, a review of the cases decided paints a picture of a more important term than some commentators expected.
When the term began, the Court had only 8 justices—1 short of a full bench: Judge Brett Kavanaugh had not yet been confirmed by the Senate. He was confirmed on October 6, by a 50-48 vote, and Justice Kavanaugh immediately joined the Court and began to hear and decide cases.
Increasingly, important decisions affect medical practice
From the nature of practice (abortion), to payment for service (Medicare reimbursement), resolution of disputes (arbitration), and fraud and abuse (the federal False Claims Act), the decisions of the Court will have an impact on many areas of medical practice. Organized medicine increasingly has recognized the significance of the work of the Court; nowhere has this been more clearly demonstrated than with amicus curiae (friend of the court) briefs filed by medical organizations.
Amicus curiae briefs. These briefs are filed by persons or organizations not a party to a case the Court is hearing. Their legitimate purpose is to inform the Court of 1) special information within the expertise of the amicus (or amici, plural) or 2) consequences of the decision that might not be apparent from arguments made by the parties to the case. Sometimes, the Court cites amicus briefs for having provided important information about the case.
Filing amicus briefs is time-consuming and expensive; organizations do not file them for trivial reasons. Organizations frequently join together to file a joint brief, to share expenses and express to the Court a stronger position.
Three categories of health professionals file amicus briefs in ObGyn-related cases:
- Major national organizations, often representing broad interests of health care professions or institutions (the American Medical Association [AMA], the Association of American Medical Colleges, and the American Hospital Association [AHA]), have filed a number of amicus briefs over the years.
- Specialty boards increasingly file amicus briefs. For example, the American College of Obstetricians and Gynecologists (ACOG) and the American Society for Reproductive Medicine have filed briefs related to abortion issues.
- In reproductive issues, the American Association of Pro-Life Obstetricians and Gynecologists, the American College of Pediatricians, and the Christian Medical & Dental Associations have been active amicus filers—frequently taking positions different than, even inconsistent with, amicus briefs filed by major specialty boards.
Amicus briefs filed by medical associations provide strong clues to what is important to clinicians. We have looked at such briefs to help us identify topics and cases from the just-concluded term that can be of particular interest to you.
Continue to: Surveying the shadow docket...
Surveying the shadow docket. As part of our review of the past term, we also looked at the so-called shadow docket, which includes decisions regarding writs of certiorari (which cases it agrees to hear); stays (usually delaying implementation of a law); or denials of stays. (Persuading the Court to hear a case is not easy: It hears approximately 70 cases per year out of as many as 7,000 applications to be heard.)
Abortion ruling
At stake. A number of states recently enacted a variety of provisions that might make an abortion more difficult to obtain. Some of the cases challenging these restrictions are making their way through lower courts, and one day might be argued before the Supreme Court. However, the Court has not (yet) agreed to hear the substance of many new abortion-related provisions.
Box v Planned Parenthood of Indiana and Kentucky, Inc.
The Court decided only 1 abortion restriction case this term.1 The Indiana law in question included 2 provisions that the Court considered:
Disposal of remains. The law regulated the manner in which abortion providers can dispose of fetal remains (ie, they cannot be treated as “infectious and pathologic waste”).
Motivation for seeking abortion. The Indiana law makes it illegal for an abortion provider to perform an abortion when the provider knows that the mother is seeking that abortion “solely” because of the fetus’s race, sex, diagnosis of Down syndrome, disability, or related characteristics.
Final rulings. The Court held that the disposal-of-remains provision is constitutional. The provision is “rationally related to the state’s interest in proper disposal of fetal remains.”2 Planned Parenthood had not raised the issue of whether the law might impose an undue burden on a woman’s right to obtain an abortion, so the Court did not decide that issue.
The Court did not consider the constitutionality of the part of the law proscribing certain reasons for seeking an otherwise legal abortion; instead, it awaits lower courts’ review of the issue. Justice Clarence Thomas wrote an extensive concurring opinion suggesting that this law is intended to avoid abortion to achieve eugenic goals.3
Key developments from the shadow docket
The Court issued a stay preventing a Louisiana statute that requires physicians who perform abortions to have admitting privileges at a nearby hospital from going into effect, pending the outcome of litigation about that law.4 Four dissenters noted that all 4 physicians who perform abortions in Louisiana have such privileges. Chief Justice Roberts was the fifth vote to grant the stay. This case likely will make its way back to the Court, as will a number of other state laws being adopted. The issue may be back as soon as the term just starting.
The Court is also considering whether to take another Indiana case, Box v Planned Parenthood of Indiana and Kentucky, Inc. (Box II). This case involves an Indiana ultrasonography viewing option as part of the abortion consent process.5
The Court declined to hear cases from Louisiana and Kansas in which the states had cut off Medicaid funding to Planned Parenthood. Lower courts had stopped the implementation of those laws.6 The legal issue was whether private parties, as opposed to the federal government, had standing to bring the case. For now, the decision of the lower courts to stop implementation of the funding cutoff is in effect. There is a split in the Circuit Courts on the issue, however, making it likely that the Supreme Court will have to resolve it sooner or later.
Health care organizations have filed a number of amicus briefs in these and other cases involving new abortion regulations. ACOG and others filed a brief opposing a Louisiana law that requires abortion providers to have admitting privileges at a nearby facility,7 and a brief opposing a similar Oklahoma law.8 The Association of Pro-Life Obstetricians and Gynecologists and others filed amicus curiae briefs in Box II9 and in an Alabama case involving so-called dismemberment abortion.10
Continue to: Medicare payments...
Medicare payments
Azar, Secretary of Health and Human Services v Allina Health Services, et al11
This case drew interest—and many amicus briefs—from health care providers, including the AMA and the AHA.12,13 There was good reason for their interest: First, the case involved more than $3 billion in reimbursements; second, it represented a potentially important precedent about the rights of providers and patients to comment on Medicare reimbursement changes. The question involved the technical calculation of additional payments made to institutions that serve a disproportionate number of low-income patients (known as Medicare Fractions).
At stake. The issue was a statutory requirement for a 60-day public notice and comment period for rules that “change a substantive legal standard” governing the scope of benefits, eligibility, or payment for services.14 In 2014, the Secretary of the Department of Health and Human Services (HHS) in the Obama administration posted a spreadsheet announcing Medicare fractions rates for hospitals (for 2012)—without formal notice or comment regarding the formula used. (The spreadsheet listed what each qualifying institution would receive, but it was based on a formula that, as noted, had not been subject to public notice and comment.) The AMA and AHA briefs emphasized the importance of a notice and comment period, especially when Medicare reimbursement is involved.
Final ruling. The Court held that the HHS process violated the notice and comment provision, thereby invalidating the policy underlying the so-called spreadsheet reimbursement. The decision was significant: This was a careful statutory interpretation of the 60-day notice and comment period, not the reimbursement policy itself. Presumably, had the HHS Secretary provided for sufficient notice and comment, the formula used would have met the requirements for issuing reimbursement formulas.
Key points. Hospitals will collectively receive $3 or $4 billion as a consequence of the ruling. Perhaps more importantly, the decision signals that HHS is going to have to take seriously the requirement that it publish Medicare-related reimbursement policies for the 60-day period.
A number of diverse cases ruled on by the Supreme Court are worth mentioning. The Court:
- allowed the President to move various funds from the US Department of Defense into accounts from which the money could be used to build a portion of a wall along the southern US border.1
- essentially killed the "citizenship question" on the census form. Technically, the Court sent the issue back to the Commerce Department for better justification for including the question (the announced reasons appeared to be pretextual).2
- changed, perhaps substantially, the deference that courts give to federal agencies in interpreting regulations.3
- upheld, in 2 cases, treaty rights of Native Americans to special treatment on Indian Lands4,5; the Court held that treaties ordinarily should be interpreted as the tribe understood them at the time they were signed. (These were 5 to 4 decisions; the split in the Court leaves many unanswered questions.)
- made it easier for landowners to file suit in federal court when they claim that the state has "taken" their property without just compensation.6
- held that a refusal of the US Patent and Trademark Office to register "immoral" or "scandalous" trademarks infringes on the First Amendment. (The petitioner sought to register "FUCT" as a trademark for a line of clothing.)7
- allowed an antitrust case by iPhone users against Apple to go forward. At issue: the claim that Apple monopolizes the retail market for apps by requiring buyers to obtain apps from Apple.8
- held that, if a drunk-driving suspect who has been taken into custody is, or becomes, unconscious, the "reasonable search" provision of the Fourth Amendment generally does not prevent a state from taking a blood specimen without a warrant. (Wisconsin had a specific "implied consent" law, by which someone receiving a driving license consents to a blood draw.9)
- decided numerous capital punishment cases. In many ways, this term seemed to be a "capital term." Issues involved in these cases have split the Court; it is reasonable to expect that the divide will endure through upcoming terms.
References
- Donald J. Trump, President of the United States, et al. v Sierra Club, et al. 588 US 19A60 (2019).
- Department of Commerce et al. v New York et al. 18 996 (2018).
- Kisor v Wilkie, Secretary of Veterans Affairs. 18 15 (2018).
- Washington State Department of Licensing v Cougar Den, Inc. 16 1498 (2018).
- Herrera v Wyoming. 17 532 (2018).
- Knick v Township of Scott, Pennsylvania, et al. 17 647 (2018).
- Iancu, Under Secretary of Commerce for Intellectual Property and Director, Patent and Trademark Office v Brunetti. 18 302 (2018).
- Apple Inc. v Pepper et al. 17 204 (2018).
- Mitchell v Wisconsin. 18 6210 (2018).
Liability under the False Claims Act
The False Claims Act (FCA) protects the federal government from fraudulent claims for payment and for shoddy goods and services. It incentivizes (by a percentage of recovery) private parties to bring cases to enforce the law.15 (Of course, the federal government also enforces the Act.)
At stake. The FCA has been of considerable concern to the AHA, the Association of American Medical Colleges, and other health care organizations—understandably so.16 As the AHA informed the Court in an amicus brief, “The prevalence of [FCA] cases has ballooned over the past three decades.... These suits disproportionately target healthcare entities.... Of the 767 new FCA cases filed in 2018, for example, 506 involved healthcare defendants.”17
Final ruling. The Court considered an ambiguity in the statute of limitations for these actions and the Court unanimously ruled to permit an extended time in which qui tam actions (private actions under the law) can be filed.18
Key points. As long a period as 10 years can pass between the time an FCA violation occurs and an action is brought. This decision is likely to increase the number of FCA actions against health care providers because the case can be filed many years after the conduct that gave rise to the complaint.
Continue to: Registering sex offenders...
Registering sex offenders
The Court upheld the constitutionality of the federal Sex Offender Registration and Notification Act (SORNA).19 Sex offenders must register and periodically report, in person, to law enforcement in every state in which the offender works, studies, or resides.
At stake. The case involved the applicability of SORNA registration obligations to those convicted of sex offenses before SORNA was adopted (pre-Act offenders).20 The court upheld registration requirements for pre-Act offenders.
Former Justice Stevens, the longest-living and third-longest-serving Supreme Court justice, died in July 2019 at 99 years of age. He was appointed to the Court in 1975 by President Ford and served until his retirement in 2010, when he was 90. Stevens had recently published a memoir, The Making of a Justice: Reflections on My First 94 Years.
Stevens's judicial philosophy generally is described as having changed over the course of his 35 years of service: He was viewed as becoming more liberal. He was a justice of enduring kindness and integrity. It is possible to find people who disagree with him, but almost impossible to find anyone who disliked him. He was continuously committed to the law and justice in the United States.
Arbitration
The Court continued its practice of deciding at least one case each term that emphasizes that federal law requires that courts rather strictly enforce agreements to arbitrate (instead of to litigate) future disputes.21 In another case, the Court ruled that there can be “class” or “joint” arbitration only if the agreement to arbitrate a dispute clearly permits such class arbitration.22
Pharma’s liability regarding product risk
The Court somewhat limited the liability of pharmaceutical companies for failing to provide adequate warning about the risk that their products pose. The case against Merck involved 500 patients who took denosumab (Fosamax) and suffered atypical femoral fractures.23
At stake. Because prescribing information (in which warnings are provided) must be approved by the US Food and Drug Administration (FDA), the legal test is: Would the FDA have refused to approve a change in the warning if Merck had “fully informed the FDA of the justifications for the warning” required by state law to avoid liability?24,25 Lower-court judges (not juries) will be expected to apply this test in the future.
The doctor and the death penalty
The Court has established a rule that, when a prisoner facing capital punishment objects to a form of execution because it is too painful, he has to propose an alternative that is reasonably available. In one case,26 a physician, an expert witness for the prisoner, did not answer some essential relative-pain questions (ie, would one procedure be more painful than another?).
At stake. The AMA filed an amicus brief in this case, indicating that it is unethical for physicians to participate in an execution. The brief noted that “testimony used to determine which method of execution would reduce physical suffering would constitute physician participation in capital punishment and would be unethical.”27
The expert witness’s failure to answer the question on relative pain had the unfortunate result of reducing the likelihood that the prisoner would prevail in his request for an alternative method of execution.
Analysis
Despite obvious disagreements about big issues (notably, abortion and the death penalty) the Court maintained a courteous and civil demeanor—something not always seen nowadays in other branches of government. Here are facts about the Court’s term just concluded:
- The Court issued 72 merits opinions (about average).
- Only 39% of decisions were unanimous (compared with the average of 49% in recent terms).
- On the other hand, 26% of decisions were split 5 to 4 (compared with a 10% recent average).
- In those 5 to 4 decisions, Justices were in the majority as follows28: Justice Gorsuch, 65%; Justice Kavanaugh, 61%; Justice Thomas, 60%; Chief Justice Roberts and Justices Ginsburg and Alito, each 55%; Justice Breyer, 50%; and Justices Sotomayor and Kagan each at 45%.
- There were 57 dissenting opinions—up from 48 in the previous term.
- What is referred to as “the liberal-conservative split” might seem more profound than it really is: “Every conservative member of the court at some point voted to form a majority with the liberal justices. And every liberal at least once left behind all of his or her usual voting partners to join the conservatives.”29
Continue to: Last, it was a year of personal health issues for...
Last, it was a year of personal health issues for the Court: Justice Ginsburg had a diagnosis of lung cancer and was absent, following surgery, in January. Of retired Justices, Sandra Day O’Connor suffers from dementia and former Justice John Paul Stevens died.
In closing
The Court has accepted approximately 50 cases for the current term, which began on October 7. The first 2 days of the term were spent on arguments about, first, whether a state can abolish the insanity defense and, second, whether nondiscrimination laws (“based on sex”) prohibit discrimination based on sexual orientation or transgender status. Cases also will deal with Patient Protection and Affordable Care Act payments to providers; the Deferred Action for Childhood Arrivals, or DACA; the death penalty; and international child custody disputes. The Court will be accepting more cases for several months. It promises to be a very interesting term.
- Box v Planned Parenthood of Indiana and Kentucky, Inc. 587 US 18 483 (2019).
- Box v Planned Parenthood of Indiana and Kentucky, Inc., at 2.
- Box v Planned Parenthood of Indiana and Kentucky, Inc., Justice Thomas concurring.
- June Medical Services, LLC, et al. v Rebekah Gee, Secretary, Louisiana Department of Health and Hospitals. 586 US 18A774 (2019).
- Box v Planned Parenthood of Indiana and Kentucky, Inc. Docket 18-1019.
- Rebekah Gee, Secretary, Louisiana Department of Health and Hospitals v Planned Parenthood of Gulf Coast, Inc., et al. 586 US 17 1492 (2018).
- June Medical Services L.L.C., et al., Petitioners, v Rebekah Gee, Secretary, Louisiana Department of Health and Hospitals. No. 18-1323. Brief of Amici Curiae American College of Obstetricians and Gynecologists, American Academy of Family Physicians, American Academy of Pediatrics, American College of Nurse-Midwives, American College of Osteopathic Obstetricians and Gynecologists, American College of Physicians, American Society for Reproductive Medicine, National Association of Nurse Practitioners in Women's Health, North American Society for Pediatric and Adolescent Gynecology, and Society For Maternal-Fetal Medicine, Amicus Curiae in Support of Petitioners. May 2019.
- Planned Parenthood of Kansas & Eastern Oklahoma, et al., Petitioners, v Larry Jegley, et al., Respondents. No. 17-935. Brief Amici Curiae of American College of Obstetricians and Gynecologists and American Public Health Association as Amici Curiae in Support of Petitioners. February 1, 2018.
- Box v Planned Parenthood of Indiana & Kentucky. No. 18-1019. Brief Amici Curiae of American Association of Pro-Life Obstetricians & Gynecologists, American College of Pediatricians, Care Net, Christian Medical Association, Heartbeat International, Inc., and National Institute Of Family & Life Advocates in Support of Petitioners. March 6, 2019.
- Steven T. Marshall, et al., Petitioners, v West Alabama Women's Center, et al., Respondents. No. 18-837. Brief of Amici Curiae American Association of Pro-Life Obstetricians & Gynecologists and American College of Pediatricians, in Support of Petitioners. January 18, 2019.
- Azar, Secretary of Health and Human Services v Allina Health Services, et al. 17 1484 (2018).
- Alex M. Azar, II, Secretary of Health and Human Services, Petitioner, v Allina Health Services, et al., Respondents. Brief of the American Hospital Association, Federation of American Hospitals, and Association of American Medical Colleges as Amici Curiae in Support of Respondents. December 2018.
- Alex M. Azar, II, Secretary of Health and Human Services, Petitioner, v Allina Health Services, et al., Respondents. Brief of Amici Curiae American Medical Association and Medical Society of the District of Columbia Amici Curiae in Support of Respondents. December 2018.
- 42 U. S. C. §1395hh. https://uscode.house.gov/view.xhtml?req=(title:42%20section:1395hh%20edition:prelim). Accessed October 22, 2019.
- The False Claims Act: a primer. Washington DC: US Department of Justice. www.justice.gov/sites/default/files/civil/legacy/2011/04/22/C-FRAUDS_FCA_Primer.pdf. Accessed October 18, 2019.
- Universal Health Services, Inc., v United States and Commonwealth of Massachusetts ex rel. Julio Escobar and Carmen Correa. Brief of the American Hospital Association, Federation of American Hospitals, and Association of American Medical Colleges Amici Curiae in Support of Petitioner. No. 15-7. January 2016.
- Intermountain Health Care, Inc., et al., Petitioners, v United States ex rel. Gerald Polukoff, et al., Respondents. No. 18-911. Brief of the American Hospital Association and Federation of American Hospitals as Amici Curiae in Support of Petitioners. February 13, 2019.
- Cochise Consultancy, Inc., et al., v United States ex rel. Hunt. 18 315 (2018).
- 34 U.S.C. §20901 et seq. [Chapter 209--Child Protection and Safety.] https://uscode.house.gov/view.xhtml?path=/prelim@title34/subtitle2/chapter209&edition=prelim. Accessed October 17, 2019.
- Gundy v United States. 17 6086 (2018).
- Henry Schein, Inc., et al., v Archer & White Sales, Inc. 17 1272 (2018).
- Lamps Plus, Inc., et al., v Varela. 17 988 (2018).
- Merck Sharp & Dohme Corp. v Albrecht et al. 17 290 (2018).
- Merck Sharp & Dohme Corp. v Albrecht et al. 17 290 (2018) at 13-14.
- Wyeth v Levine, 555 US 555, 571 (2009).
- Russell Bucklew, Petitioner, v Anne L. Precythe, Director, Missouri Department of Corrections, et al., Respondents. 17 8151 (2018).
- Russell Bucklew, Petitioner, v Anne L. Precythe, Director, Missouri Department of Corrections, et al., Respondents. 17 8151 (2018). American Medical Association, Amicus Curiae Brief, in Support of Neither Party. July 23, 2018.
- Final stat pack for October term 2018. SCOTUSblog.com. June 28, 2019. https://www.scotusblog.com/wp-content/uploads/2019/07/StatPack_OT18-7_8_19.pdf. Accessed October 17, 2019.
- Barnes R. They're not 'wonder twins': Gorsuch, Kavanaugh shift the Supreme Court, but their differences are striking. Washington Post, June 28, 2019.
The most recently concluded term of the US Supreme Court, which began on October 1, 2018, yielded a number of decisions of interest to health care professionals and to ObGyns in particular. Although the term was viewed by some observers as less consequential than other recent terms, a review of the cases decided paints a picture of a more important term than some commentators expected.
When the term began, the Court had only 8 justices—1 short of a full bench: Judge Brett Kavanaugh had not yet been confirmed by the Senate. He was confirmed on October 6, by a 50-48 vote, and Justice Kavanaugh immediately joined the Court and began to hear and decide cases.
Increasingly, important decisions affect medical practice
From the nature of practice (abortion), to payment for service (Medicare reimbursement), resolution of disputes (arbitration), and fraud and abuse (the federal False Claims Act), the decisions of the Court will have an impact on many areas of medical practice. Organized medicine increasingly has recognized the significance of the work of the Court; nowhere has this been more clearly demonstrated than with amicus curiae (friend of the court) briefs filed by medical organizations.
Amicus curiae briefs. These briefs are filed by persons or organizations not a party to a case the Court is hearing. Their legitimate purpose is to inform the Court of 1) special information within the expertise of the amicus (or amici, plural) or 2) consequences of the decision that might not be apparent from arguments made by the parties to the case. Sometimes, the Court cites amicus briefs for having provided important information about the case.
Filing amicus briefs is time-consuming and expensive; organizations do not file them for trivial reasons. Organizations frequently join together to file a joint brief, to share expenses and express to the Court a stronger position.
Three categories of health professionals file amicus briefs in ObGyn-related cases:
- Major national organizations, often representing broad interests of health care professions or institutions (the American Medical Association [AMA], the Association of American Medical Colleges, and the American Hospital Association [AHA]), have filed a number of amicus briefs over the years.
- Specialty boards increasingly file amicus briefs. For example, the American College of Obstetricians and Gynecologists (ACOG) and the American Society for Reproductive Medicine have filed briefs related to abortion issues.
- In reproductive issues, the American Association of Pro-Life Obstetricians and Gynecologists, the American College of Pediatricians, and the Christian Medical & Dental Associations have been active amicus filers—frequently taking positions different than, even inconsistent with, amicus briefs filed by major specialty boards.
Amicus briefs filed by medical associations provide strong clues to what is important to clinicians. We have looked at such briefs to help us identify topics and cases from the just-concluded term that can be of particular interest to you.
Continue to: Surveying the shadow docket...
Surveying the shadow docket. As part of our review of the past term, we also looked at the so-called shadow docket, which includes decisions regarding writs of certiorari (which cases it agrees to hear); stays (usually delaying implementation of a law); or denials of stays. (Persuading the Court to hear a case is not easy: It hears approximately 70 cases per year out of as many as 7,000 applications to be heard.)
Abortion ruling
At stake. A number of states recently enacted a variety of provisions that might make an abortion more difficult to obtain. Some of the cases challenging these restrictions are making their way through lower courts, and one day might be argued before the Supreme Court. However, the Court has not (yet) agreed to hear the substance of many new abortion-related provisions.
Box v Planned Parenthood of Indiana and Kentucky, Inc.
The Court decided only 1 abortion restriction case this term.1 The Indiana law in question included 2 provisions that the Court considered:
Disposal of remains. The law regulated the manner in which abortion providers can dispose of fetal remains (ie, they cannot be treated as “infectious and pathologic waste”).
Motivation for seeking abortion. The Indiana law makes it illegal for an abortion provider to perform an abortion when the provider knows that the mother is seeking that abortion “solely” because of the fetus’s race, sex, diagnosis of Down syndrome, disability, or related characteristics.
Final rulings. The Court held that the disposal-of-remains provision is constitutional. The provision is “rationally related to the state’s interest in proper disposal of fetal remains.”2 Planned Parenthood had not raised the issue of whether the law might impose an undue burden on a woman’s right to obtain an abortion, so the Court did not decide that issue.
The Court did not consider the constitutionality of the part of the law proscribing certain reasons for seeking an otherwise legal abortion; instead, it awaits lower courts’ review of the issue. Justice Clarence Thomas wrote an extensive concurring opinion suggesting that this law is intended to avoid abortion to achieve eugenic goals.3
Key developments from the shadow docket
The Court issued a stay preventing a Louisiana statute that requires physicians who perform abortions to have admitting privileges at a nearby hospital from going into effect, pending the outcome of litigation about that law.4 Four dissenters noted that all 4 physicians who perform abortions in Louisiana have such privileges. Chief Justice Roberts was the fifth vote to grant the stay. This case likely will make its way back to the Court, as will a number of other state laws being adopted. The issue may be back as soon as the term just starting.
The Court is also considering whether to take another Indiana case, Box v Planned Parenthood of Indiana and Kentucky, Inc. (Box II). This case involves an Indiana ultrasonography viewing option as part of the abortion consent process.5
The Court declined to hear cases from Louisiana and Kansas in which the states had cut off Medicaid funding to Planned Parenthood. Lower courts had stopped the implementation of those laws.6 The legal issue was whether private parties, as opposed to the federal government, had standing to bring the case. For now, the decision of the lower courts to stop implementation of the funding cutoff is in effect. There is a split in the Circuit Courts on the issue, however, making it likely that the Supreme Court will have to resolve it sooner or later.
Health care organizations have filed a number of amicus briefs in these and other cases involving new abortion regulations. ACOG and others filed a brief opposing a Louisiana law that requires abortion providers to have admitting privileges at a nearby facility,7 and a brief opposing a similar Oklahoma law.8 The Association of Pro-Life Obstetricians and Gynecologists and others filed amicus curiae briefs in Box II9 and in an Alabama case involving so-called dismemberment abortion.10
Continue to: Medicare payments...
Medicare payments
Azar, Secretary of Health and Human Services v Allina Health Services, et al11
This case drew interest—and many amicus briefs—from health care providers, including the AMA and the AHA.12,13 There was good reason for their interest: First, the case involved more than $3 billion in reimbursements; second, it represented a potentially important precedent about the rights of providers and patients to comment on Medicare reimbursement changes. The question involved the technical calculation of additional payments made to institutions that serve a disproportionate number of low-income patients (known as Medicare Fractions).
At stake. The issue was a statutory requirement for a 60-day public notice and comment period for rules that “change a substantive legal standard” governing the scope of benefits, eligibility, or payment for services.14 In 2014, the Secretary of the Department of Health and Human Services (HHS) in the Obama administration posted a spreadsheet announcing Medicare fractions rates for hospitals (for 2012)—without formal notice or comment regarding the formula used. (The spreadsheet listed what each qualifying institution would receive, but it was based on a formula that, as noted, had not been subject to public notice and comment.) The AMA and AHA briefs emphasized the importance of a notice and comment period, especially when Medicare reimbursement is involved.
Final ruling. The Court held that the HHS process violated the notice and comment provision, thereby invalidating the policy underlying the so-called spreadsheet reimbursement. The decision was significant: This was a careful statutory interpretation of the 60-day notice and comment period, not the reimbursement policy itself. Presumably, had the HHS Secretary provided for sufficient notice and comment, the formula used would have met the requirements for issuing reimbursement formulas.
Key points. Hospitals will collectively receive $3 or $4 billion as a consequence of the ruling. Perhaps more importantly, the decision signals that HHS is going to have to take seriously the requirement that it publish Medicare-related reimbursement policies for the 60-day period.
A number of diverse cases ruled on by the Supreme Court are worth mentioning. The Court:
- allowed the President to move various funds from the US Department of Defense into accounts from which the money could be used to build a portion of a wall along the southern US border.1
- essentially killed the "citizenship question" on the census form. Technically, the Court sent the issue back to the Commerce Department for better justification for including the question (the announced reasons appeared to be pretextual).2
- changed, perhaps substantially, the deference that courts give to federal agencies in interpreting regulations.3
- upheld, in 2 cases, treaty rights of Native Americans to special treatment on Indian Lands4,5; the Court held that treaties ordinarily should be interpreted as the tribe understood them at the time they were signed. (These were 5 to 4 decisions; the split in the Court leaves many unanswered questions.)
- made it easier for landowners to file suit in federal court when they claim that the state has "taken" their property without just compensation.6
- held that a refusal of the US Patent and Trademark Office to register "immoral" or "scandalous" trademarks infringes on the First Amendment. (The petitioner sought to register "FUCT" as a trademark for a line of clothing.)7
- allowed an antitrust case by iPhone users against Apple to go forward. At issue: the claim that Apple monopolizes the retail market for apps by requiring buyers to obtain apps from Apple.8
- held that, if a drunk-driving suspect who has been taken into custody is, or becomes, unconscious, the "reasonable search" provision of the Fourth Amendment generally does not prevent a state from taking a blood specimen without a warrant. (Wisconsin had a specific "implied consent" law, by which someone receiving a driving license consents to a blood draw.9)
- decided numerous capital punishment cases. In many ways, this term seemed to be a "capital term." Issues involved in these cases have split the Court; it is reasonable to expect that the divide will endure through upcoming terms.
References
- Donald J. Trump, President of the United States, et al. v Sierra Club, et al. 588 US 19A60 (2019).
- Department of Commerce et al. v New York et al. 18 996 (2018).
- Kisor v Wilkie, Secretary of Veterans Affairs. 18 15 (2018).
- Washington State Department of Licensing v Cougar Den, Inc. 16 1498 (2018).
- Herrera v Wyoming. 17 532 (2018).
- Knick v Township of Scott, Pennsylvania, et al. 17 647 (2018).
- Iancu, Under Secretary of Commerce for Intellectual Property and Director, Patent and Trademark Office v Brunetti. 18 302 (2018).
- Apple Inc. v Pepper et al. 17 204 (2018).
- Mitchell v Wisconsin. 18 6210 (2018).
Liability under the False Claims Act
The False Claims Act (FCA) protects the federal government from fraudulent claims for payment and for shoddy goods and services. It incentivizes (by a percentage of recovery) private parties to bring cases to enforce the law.15 (Of course, the federal government also enforces the Act.)
At stake. The FCA has been of considerable concern to the AHA, the Association of American Medical Colleges, and other health care organizations—understandably so.16 As the AHA informed the Court in an amicus brief, “The prevalence of [FCA] cases has ballooned over the past three decades.... These suits disproportionately target healthcare entities.... Of the 767 new FCA cases filed in 2018, for example, 506 involved healthcare defendants.”17
Final ruling. The Court considered an ambiguity in the statute of limitations for these actions and the Court unanimously ruled to permit an extended time in which qui tam actions (private actions under the law) can be filed.18
Key points. As long a period as 10 years can pass between the time an FCA violation occurs and an action is brought. This decision is likely to increase the number of FCA actions against health care providers because the case can be filed many years after the conduct that gave rise to the complaint.
Continue to: Registering sex offenders...
Registering sex offenders
The Court upheld the constitutionality of the federal Sex Offender Registration and Notification Act (SORNA).19 Sex offenders must register and periodically report, in person, to law enforcement in every state in which the offender works, studies, or resides.
At stake. The case involved the applicability of SORNA registration obligations to those convicted of sex offenses before SORNA was adopted (pre-Act offenders).20 The court upheld registration requirements for pre-Act offenders.
Former Justice Stevens, the longest-living and third-longest-serving Supreme Court justice, died in July 2019 at 99 years of age. He was appointed to the Court in 1975 by President Ford and served until his retirement in 2010, when he was 90. Stevens had recently published a memoir, The Making of a Justice: Reflections on My First 94 Years.
Stevens's judicial philosophy generally is described as having changed over the course of his 35 years of service: He was viewed as becoming more liberal. He was a justice of enduring kindness and integrity. It is possible to find people who disagree with him, but almost impossible to find anyone who disliked him. He was continuously committed to the law and justice in the United States.
Arbitration
The Court continued its practice of deciding at least one case each term that emphasizes that federal law requires that courts rather strictly enforce agreements to arbitrate (instead of to litigate) future disputes.21 In another case, the Court ruled that there can be “class” or “joint” arbitration only if the agreement to arbitrate a dispute clearly permits such class arbitration.22
Pharma’s liability regarding product risk
The Court somewhat limited the liability of pharmaceutical companies for failing to provide adequate warning about the risk that their products pose. The case against Merck involved 500 patients who took denosumab (Fosamax) and suffered atypical femoral fractures.23
At stake. Because prescribing information (in which warnings are provided) must be approved by the US Food and Drug Administration (FDA), the legal test is: Would the FDA have refused to approve a change in the warning if Merck had “fully informed the FDA of the justifications for the warning” required by state law to avoid liability?24,25 Lower-court judges (not juries) will be expected to apply this test in the future.
The doctor and the death penalty
The Court has established a rule that, when a prisoner facing capital punishment objects to a form of execution because it is too painful, he has to propose an alternative that is reasonably available. In one case,26 a physician, an expert witness for the prisoner, did not answer some essential relative-pain questions (ie, would one procedure be more painful than another?).
At stake. The AMA filed an amicus brief in this case, indicating that it is unethical for physicians to participate in an execution. The brief noted that “testimony used to determine which method of execution would reduce physical suffering would constitute physician participation in capital punishment and would be unethical.”27
The expert witness’s failure to answer the question on relative pain had the unfortunate result of reducing the likelihood that the prisoner would prevail in his request for an alternative method of execution.
Analysis
Despite obvious disagreements about big issues (notably, abortion and the death penalty) the Court maintained a courteous and civil demeanor—something not always seen nowadays in other branches of government. Here are facts about the Court’s term just concluded:
- The Court issued 72 merits opinions (about average).
- Only 39% of decisions were unanimous (compared with the average of 49% in recent terms).
- On the other hand, 26% of decisions were split 5 to 4 (compared with a 10% recent average).
- In those 5 to 4 decisions, Justices were in the majority as follows28: Justice Gorsuch, 65%; Justice Kavanaugh, 61%; Justice Thomas, 60%; Chief Justice Roberts and Justices Ginsburg and Alito, each 55%; Justice Breyer, 50%; and Justices Sotomayor and Kagan each at 45%.
- There were 57 dissenting opinions—up from 48 in the previous term.
- What is referred to as “the liberal-conservative split” might seem more profound than it really is: “Every conservative member of the court at some point voted to form a majority with the liberal justices. And every liberal at least once left behind all of his or her usual voting partners to join the conservatives.”29
Continue to: Last, it was a year of personal health issues for...
Last, it was a year of personal health issues for the Court: Justice Ginsburg had a diagnosis of lung cancer and was absent, following surgery, in January. Of retired Justices, Sandra Day O’Connor suffers from dementia and former Justice John Paul Stevens died.
In closing
The Court has accepted approximately 50 cases for the current term, which began on October 7. The first 2 days of the term were spent on arguments about, first, whether a state can abolish the insanity defense and, second, whether nondiscrimination laws (“based on sex”) prohibit discrimination based on sexual orientation or transgender status. Cases also will deal with Patient Protection and Affordable Care Act payments to providers; the Deferred Action for Childhood Arrivals, or DACA; the death penalty; and international child custody disputes. The Court will be accepting more cases for several months. It promises to be a very interesting term.
The most recently concluded term of the US Supreme Court, which began on October 1, 2018, yielded a number of decisions of interest to health care professionals and to ObGyns in particular. Although the term was viewed by some observers as less consequential than other recent terms, a review of the cases decided paints a picture of a more important term than some commentators expected.
When the term began, the Court had only 8 justices—1 short of a full bench: Judge Brett Kavanaugh had not yet been confirmed by the Senate. He was confirmed on October 6, by a 50-48 vote, and Justice Kavanaugh immediately joined the Court and began to hear and decide cases.
Increasingly, important decisions affect medical practice
From the nature of practice (abortion), to payment for service (Medicare reimbursement), resolution of disputes (arbitration), and fraud and abuse (the federal False Claims Act), the decisions of the Court will have an impact on many areas of medical practice. Organized medicine increasingly has recognized the significance of the work of the Court; nowhere has this been more clearly demonstrated than with amicus curiae (friend of the court) briefs filed by medical organizations.
Amicus curiae briefs. These briefs are filed by persons or organizations not a party to a case the Court is hearing. Their legitimate purpose is to inform the Court of 1) special information within the expertise of the amicus (or amici, plural) or 2) consequences of the decision that might not be apparent from arguments made by the parties to the case. Sometimes, the Court cites amicus briefs for having provided important information about the case.
Filing amicus briefs is time-consuming and expensive; organizations do not file them for trivial reasons. Organizations frequently join together to file a joint brief, to share expenses and express to the Court a stronger position.
Three categories of health professionals file amicus briefs in ObGyn-related cases:
- Major national organizations, often representing broad interests of health care professions or institutions (the American Medical Association [AMA], the Association of American Medical Colleges, and the American Hospital Association [AHA]), have filed a number of amicus briefs over the years.
- Specialty boards increasingly file amicus briefs. For example, the American College of Obstetricians and Gynecologists (ACOG) and the American Society for Reproductive Medicine have filed briefs related to abortion issues.
- In reproductive issues, the American Association of Pro-Life Obstetricians and Gynecologists, the American College of Pediatricians, and the Christian Medical & Dental Associations have been active amicus filers—frequently taking positions different than, even inconsistent with, amicus briefs filed by major specialty boards.
Amicus briefs filed by medical associations provide strong clues to what is important to clinicians. We have looked at such briefs to help us identify topics and cases from the just-concluded term that can be of particular interest to you.
Continue to: Surveying the shadow docket...
Surveying the shadow docket. As part of our review of the past term, we also looked at the so-called shadow docket, which includes decisions regarding writs of certiorari (which cases it agrees to hear); stays (usually delaying implementation of a law); or denials of stays. (Persuading the Court to hear a case is not easy: It hears approximately 70 cases per year out of as many as 7,000 applications to be heard.)
Abortion ruling
At stake. A number of states recently enacted a variety of provisions that might make an abortion more difficult to obtain. Some of the cases challenging these restrictions are making their way through lower courts, and one day might be argued before the Supreme Court. However, the Court has not (yet) agreed to hear the substance of many new abortion-related provisions.
Box v Planned Parenthood of Indiana and Kentucky, Inc.
The Court decided only 1 abortion restriction case this term.1 The Indiana law in question included 2 provisions that the Court considered:
Disposal of remains. The law regulated the manner in which abortion providers can dispose of fetal remains (ie, they cannot be treated as “infectious and pathologic waste”).
Motivation for seeking abortion. The Indiana law makes it illegal for an abortion provider to perform an abortion when the provider knows that the mother is seeking that abortion “solely” because of the fetus’s race, sex, diagnosis of Down syndrome, disability, or related characteristics.
Final rulings. The Court held that the disposal-of-remains provision is constitutional. The provision is “rationally related to the state’s interest in proper disposal of fetal remains.”2 Planned Parenthood had not raised the issue of whether the law might impose an undue burden on a woman’s right to obtain an abortion, so the Court did not decide that issue.
The Court did not consider the constitutionality of the part of the law proscribing certain reasons for seeking an otherwise legal abortion; instead, it awaits lower courts’ review of the issue. Justice Clarence Thomas wrote an extensive concurring opinion suggesting that this law is intended to avoid abortion to achieve eugenic goals.3
Key developments from the shadow docket
The Court issued a stay preventing a Louisiana statute that requires physicians who perform abortions to have admitting privileges at a nearby hospital from going into effect, pending the outcome of litigation about that law.4 Four dissenters noted that all 4 physicians who perform abortions in Louisiana have such privileges. Chief Justice Roberts was the fifth vote to grant the stay. This case likely will make its way back to the Court, as will a number of other state laws being adopted. The issue may be back as soon as the term just starting.
The Court is also considering whether to take another Indiana case, Box v Planned Parenthood of Indiana and Kentucky, Inc. (Box II). This case involves an Indiana ultrasonography viewing option as part of the abortion consent process.5
The Court declined to hear cases from Louisiana and Kansas in which the states had cut off Medicaid funding to Planned Parenthood. Lower courts had stopped the implementation of those laws.6 The legal issue was whether private parties, as opposed to the federal government, had standing to bring the case. For now, the decision of the lower courts to stop implementation of the funding cutoff is in effect. There is a split in the Circuit Courts on the issue, however, making it likely that the Supreme Court will have to resolve it sooner or later.
Health care organizations have filed a number of amicus briefs in these and other cases involving new abortion regulations. ACOG and others filed a brief opposing a Louisiana law that requires abortion providers to have admitting privileges at a nearby facility,7 and a brief opposing a similar Oklahoma law.8 The Association of Pro-Life Obstetricians and Gynecologists and others filed amicus curiae briefs in Box II9 and in an Alabama case involving so-called dismemberment abortion.10
Continue to: Medicare payments...
Medicare payments
Azar, Secretary of Health and Human Services v Allina Health Services, et al11
This case drew interest—and many amicus briefs—from health care providers, including the AMA and the AHA.12,13 There was good reason for their interest: First, the case involved more than $3 billion in reimbursements; second, it represented a potentially important precedent about the rights of providers and patients to comment on Medicare reimbursement changes. The question involved the technical calculation of additional payments made to institutions that serve a disproportionate number of low-income patients (known as Medicare Fractions).
At stake. The issue was a statutory requirement for a 60-day public notice and comment period for rules that “change a substantive legal standard” governing the scope of benefits, eligibility, or payment for services.14 In 2014, the Secretary of the Department of Health and Human Services (HHS) in the Obama administration posted a spreadsheet announcing Medicare fractions rates for hospitals (for 2012)—without formal notice or comment regarding the formula used. (The spreadsheet listed what each qualifying institution would receive, but it was based on a formula that, as noted, had not been subject to public notice and comment.) The AMA and AHA briefs emphasized the importance of a notice and comment period, especially when Medicare reimbursement is involved.
Final ruling. The Court held that the HHS process violated the notice and comment provision, thereby invalidating the policy underlying the so-called spreadsheet reimbursement. The decision was significant: This was a careful statutory interpretation of the 60-day notice and comment period, not the reimbursement policy itself. Presumably, had the HHS Secretary provided for sufficient notice and comment, the formula used would have met the requirements for issuing reimbursement formulas.
Key points. Hospitals will collectively receive $3 or $4 billion as a consequence of the ruling. Perhaps more importantly, the decision signals that HHS is going to have to take seriously the requirement that it publish Medicare-related reimbursement policies for the 60-day period.
A number of diverse cases ruled on by the Supreme Court are worth mentioning. The Court:
- allowed the President to move various funds from the US Department of Defense into accounts from which the money could be used to build a portion of a wall along the southern US border.1
- essentially killed the "citizenship question" on the census form. Technically, the Court sent the issue back to the Commerce Department for better justification for including the question (the announced reasons appeared to be pretextual).2
- changed, perhaps substantially, the deference that courts give to federal agencies in interpreting regulations.3
- upheld, in 2 cases, treaty rights of Native Americans to special treatment on Indian Lands4,5; the Court held that treaties ordinarily should be interpreted as the tribe understood them at the time they were signed. (These were 5 to 4 decisions; the split in the Court leaves many unanswered questions.)
- made it easier for landowners to file suit in federal court when they claim that the state has "taken" their property without just compensation.6
- held that a refusal of the US Patent and Trademark Office to register "immoral" or "scandalous" trademarks infringes on the First Amendment. (The petitioner sought to register "FUCT" as a trademark for a line of clothing.)7
- allowed an antitrust case by iPhone users against Apple to go forward. At issue: the claim that Apple monopolizes the retail market for apps by requiring buyers to obtain apps from Apple.8
- held that, if a drunk-driving suspect who has been taken into custody is, or becomes, unconscious, the "reasonable search" provision of the Fourth Amendment generally does not prevent a state from taking a blood specimen without a warrant. (Wisconsin had a specific "implied consent" law, by which someone receiving a driving license consents to a blood draw.9)
- decided numerous capital punishment cases. In many ways, this term seemed to be a "capital term." Issues involved in these cases have split the Court; it is reasonable to expect that the divide will endure through upcoming terms.
References
- Donald J. Trump, President of the United States, et al. v Sierra Club, et al. 588 US 19A60 (2019).
- Department of Commerce et al. v New York et al. 18 996 (2018).
- Kisor v Wilkie, Secretary of Veterans Affairs. 18 15 (2018).
- Washington State Department of Licensing v Cougar Den, Inc. 16 1498 (2018).
- Herrera v Wyoming. 17 532 (2018).
- Knick v Township of Scott, Pennsylvania, et al. 17 647 (2018).
- Iancu, Under Secretary of Commerce for Intellectual Property and Director, Patent and Trademark Office v Brunetti. 18 302 (2018).
- Apple Inc. v Pepper et al. 17 204 (2018).
- Mitchell v Wisconsin. 18 6210 (2018).
Liability under the False Claims Act
The False Claims Act (FCA) protects the federal government from fraudulent claims for payment and for shoddy goods and services. It incentivizes (by a percentage of recovery) private parties to bring cases to enforce the law.15 (Of course, the federal government also enforces the Act.)
At stake. The FCA has been of considerable concern to the AHA, the Association of American Medical Colleges, and other health care organizations—understandably so.16 As the AHA informed the Court in an amicus brief, “The prevalence of [FCA] cases has ballooned over the past three decades.... These suits disproportionately target healthcare entities.... Of the 767 new FCA cases filed in 2018, for example, 506 involved healthcare defendants.”17
Final ruling. The Court considered an ambiguity in the statute of limitations for these actions and the Court unanimously ruled to permit an extended time in which qui tam actions (private actions under the law) can be filed.18
Key points. As long a period as 10 years can pass between the time an FCA violation occurs and an action is brought. This decision is likely to increase the number of FCA actions against health care providers because the case can be filed many years after the conduct that gave rise to the complaint.
Continue to: Registering sex offenders...
Registering sex offenders
The Court upheld the constitutionality of the federal Sex Offender Registration and Notification Act (SORNA).19 Sex offenders must register and periodically report, in person, to law enforcement in every state in which the offender works, studies, or resides.
At stake. The case involved the applicability of SORNA registration obligations to those convicted of sex offenses before SORNA was adopted (pre-Act offenders).20 The court upheld registration requirements for pre-Act offenders.
Former Justice Stevens, the longest-living and third-longest-serving Supreme Court justice, died in July 2019 at 99 years of age. He was appointed to the Court in 1975 by President Ford and served until his retirement in 2010, when he was 90. Stevens had recently published a memoir, The Making of a Justice: Reflections on My First 94 Years.
Stevens's judicial philosophy generally is described as having changed over the course of his 35 years of service: He was viewed as becoming more liberal. He was a justice of enduring kindness and integrity. It is possible to find people who disagree with him, but almost impossible to find anyone who disliked him. He was continuously committed to the law and justice in the United States.
Arbitration
The Court continued its practice of deciding at least one case each term that emphasizes that federal law requires that courts rather strictly enforce agreements to arbitrate (instead of to litigate) future disputes.21 In another case, the Court ruled that there can be “class” or “joint” arbitration only if the agreement to arbitrate a dispute clearly permits such class arbitration.22
Pharma’s liability regarding product risk
The Court somewhat limited the liability of pharmaceutical companies for failing to provide adequate warning about the risk that their products pose. The case against Merck involved 500 patients who took denosumab (Fosamax) and suffered atypical femoral fractures.23
At stake. Because prescribing information (in which warnings are provided) must be approved by the US Food and Drug Administration (FDA), the legal test is: Would the FDA have refused to approve a change in the warning if Merck had “fully informed the FDA of the justifications for the warning” required by state law to avoid liability?24,25 Lower-court judges (not juries) will be expected to apply this test in the future.
The doctor and the death penalty
The Court has established a rule that, when a prisoner facing capital punishment objects to a form of execution because it is too painful, he has to propose an alternative that is reasonably available. In one case,26 a physician, an expert witness for the prisoner, did not answer some essential relative-pain questions (ie, would one procedure be more painful than another?).
At stake. The AMA filed an amicus brief in this case, indicating that it is unethical for physicians to participate in an execution. The brief noted that “testimony used to determine which method of execution would reduce physical suffering would constitute physician participation in capital punishment and would be unethical.”27
The expert witness’s failure to answer the question on relative pain had the unfortunate result of reducing the likelihood that the prisoner would prevail in his request for an alternative method of execution.
Analysis
Despite obvious disagreements about big issues (notably, abortion and the death penalty) the Court maintained a courteous and civil demeanor—something not always seen nowadays in other branches of government. Here are facts about the Court’s term just concluded:
- The Court issued 72 merits opinions (about average).
- Only 39% of decisions were unanimous (compared with the average of 49% in recent terms).
- On the other hand, 26% of decisions were split 5 to 4 (compared with a 10% recent average).
- In those 5 to 4 decisions, Justices were in the majority as follows28: Justice Gorsuch, 65%; Justice Kavanaugh, 61%; Justice Thomas, 60%; Chief Justice Roberts and Justices Ginsburg and Alito, each 55%; Justice Breyer, 50%; and Justices Sotomayor and Kagan each at 45%.
- There were 57 dissenting opinions—up from 48 in the previous term.
- What is referred to as “the liberal-conservative split” might seem more profound than it really is: “Every conservative member of the court at some point voted to form a majority with the liberal justices. And every liberal at least once left behind all of his or her usual voting partners to join the conservatives.”29
Continue to: Last, it was a year of personal health issues for...
Last, it was a year of personal health issues for the Court: Justice Ginsburg had a diagnosis of lung cancer and was absent, following surgery, in January. Of retired Justices, Sandra Day O’Connor suffers from dementia and former Justice John Paul Stevens died.
In closing
The Court has accepted approximately 50 cases for the current term, which began on October 7. The first 2 days of the term were spent on arguments about, first, whether a state can abolish the insanity defense and, second, whether nondiscrimination laws (“based on sex”) prohibit discrimination based on sexual orientation or transgender status. Cases also will deal with Patient Protection and Affordable Care Act payments to providers; the Deferred Action for Childhood Arrivals, or DACA; the death penalty; and international child custody disputes. The Court will be accepting more cases for several months. It promises to be a very interesting term.
- Box v Planned Parenthood of Indiana and Kentucky, Inc. 587 US 18 483 (2019).
- Box v Planned Parenthood of Indiana and Kentucky, Inc., at 2.
- Box v Planned Parenthood of Indiana and Kentucky, Inc., Justice Thomas concurring.
- June Medical Services, LLC, et al. v Rebekah Gee, Secretary, Louisiana Department of Health and Hospitals. 586 US 18A774 (2019).
- Box v Planned Parenthood of Indiana and Kentucky, Inc. Docket 18-1019.
- Rebekah Gee, Secretary, Louisiana Department of Health and Hospitals v Planned Parenthood of Gulf Coast, Inc., et al. 586 US 17 1492 (2018).
- June Medical Services L.L.C., et al., Petitioners, v Rebekah Gee, Secretary, Louisiana Department of Health and Hospitals. No. 18-1323. Brief of Amici Curiae American College of Obstetricians and Gynecologists, American Academy of Family Physicians, American Academy of Pediatrics, American College of Nurse-Midwives, American College of Osteopathic Obstetricians and Gynecologists, American College of Physicians, American Society for Reproductive Medicine, National Association of Nurse Practitioners in Women's Health, North American Society for Pediatric and Adolescent Gynecology, and Society For Maternal-Fetal Medicine, Amicus Curiae in Support of Petitioners. May 2019.
- Planned Parenthood of Kansas & Eastern Oklahoma, et al., Petitioners, v Larry Jegley, et al., Respondents. No. 17-935. Brief Amici Curiae of American College of Obstetricians and Gynecologists and American Public Health Association as Amici Curiae in Support of Petitioners. February 1, 2018.
- Box v Planned Parenthood of Indiana & Kentucky. No. 18-1019. Brief Amici Curiae of American Association of Pro-Life Obstetricians & Gynecologists, American College of Pediatricians, Care Net, Christian Medical Association, Heartbeat International, Inc., and National Institute Of Family & Life Advocates in Support of Petitioners. March 6, 2019.
- Steven T. Marshall, et al., Petitioners, v West Alabama Women's Center, et al., Respondents. No. 18-837. Brief of Amici Curiae American Association of Pro-Life Obstetricians & Gynecologists and American College of Pediatricians, in Support of Petitioners. January 18, 2019.
- Azar, Secretary of Health and Human Services v Allina Health Services, et al. 17 1484 (2018).
- Alex M. Azar, II, Secretary of Health and Human Services, Petitioner, v Allina Health Services, et al., Respondents. Brief of the American Hospital Association, Federation of American Hospitals, and Association of American Medical Colleges as Amici Curiae in Support of Respondents. December 2018.
- Alex M. Azar, II, Secretary of Health and Human Services, Petitioner, v Allina Health Services, et al., Respondents. Brief of Amici Curiae American Medical Association and Medical Society of the District of Columbia Amici Curiae in Support of Respondents. December 2018.
- 42 U. S. C. §1395hh. https://uscode.house.gov/view.xhtml?req=(title:42%20section:1395hh%20edition:prelim). Accessed October 22, 2019.
- The False Claims Act: a primer. Washington DC: US Department of Justice. www.justice.gov/sites/default/files/civil/legacy/2011/04/22/C-FRAUDS_FCA_Primer.pdf. Accessed October 18, 2019.
- Universal Health Services, Inc., v United States and Commonwealth of Massachusetts ex rel. Julio Escobar and Carmen Correa. Brief of the American Hospital Association, Federation of American Hospitals, and Association of American Medical Colleges Amici Curiae in Support of Petitioner. No. 15-7. January 2016.
- Intermountain Health Care, Inc., et al., Petitioners, v United States ex rel. Gerald Polukoff, et al., Respondents. No. 18-911. Brief of the American Hospital Association and Federation of American Hospitals as Amici Curiae in Support of Petitioners. February 13, 2019.
- Cochise Consultancy, Inc., et al., v United States ex rel. Hunt. 18 315 (2018).
- 34 U.S.C. §20901 et seq. [Chapter 209--Child Protection and Safety.] https://uscode.house.gov/view.xhtml?path=/prelim@title34/subtitle2/chapter209&edition=prelim. Accessed October 17, 2019.
- Gundy v United States. 17 6086 (2018).
- Henry Schein, Inc., et al., v Archer & White Sales, Inc. 17 1272 (2018).
- Lamps Plus, Inc., et al., v Varela. 17 988 (2018).
- Merck Sharp & Dohme Corp. v Albrecht et al. 17 290 (2018).
- Merck Sharp & Dohme Corp. v Albrecht et al. 17 290 (2018) at 13-14.
- Wyeth v Levine, 555 US 555, 571 (2009).
- Russell Bucklew, Petitioner, v Anne L. Precythe, Director, Missouri Department of Corrections, et al., Respondents. 17 8151 (2018).
- Russell Bucklew, Petitioner, v Anne L. Precythe, Director, Missouri Department of Corrections, et al., Respondents. 17 8151 (2018). American Medical Association, Amicus Curiae Brief, in Support of Neither Party. July 23, 2018.
- Final stat pack for October term 2018. SCOTUSblog.com. June 28, 2019. https://www.scotusblog.com/wp-content/uploads/2019/07/StatPack_OT18-7_8_19.pdf. Accessed October 17, 2019.
- Barnes R. They're not 'wonder twins': Gorsuch, Kavanaugh shift the Supreme Court, but their differences are striking. Washington Post, June 28, 2019.
- Box v Planned Parenthood of Indiana and Kentucky, Inc. 587 US 18 483 (2019).
- Box v Planned Parenthood of Indiana and Kentucky, Inc., at 2.
- Box v Planned Parenthood of Indiana and Kentucky, Inc., Justice Thomas concurring.
- June Medical Services, LLC, et al. v Rebekah Gee, Secretary, Louisiana Department of Health and Hospitals. 586 US 18A774 (2019).
- Box v Planned Parenthood of Indiana and Kentucky, Inc. Docket 18-1019.
- Rebekah Gee, Secretary, Louisiana Department of Health and Hospitals v Planned Parenthood of Gulf Coast, Inc., et al. 586 US 17 1492 (2018).
- June Medical Services L.L.C., et al., Petitioners, v Rebekah Gee, Secretary, Louisiana Department of Health and Hospitals. No. 18-1323. Brief of Amici Curiae American College of Obstetricians and Gynecologists, American Academy of Family Physicians, American Academy of Pediatrics, American College of Nurse-Midwives, American College of Osteopathic Obstetricians and Gynecologists, American College of Physicians, American Society for Reproductive Medicine, National Association of Nurse Practitioners in Women's Health, North American Society for Pediatric and Adolescent Gynecology, and Society For Maternal-Fetal Medicine, Amicus Curiae in Support of Petitioners. May 2019.
- Planned Parenthood of Kansas & Eastern Oklahoma, et al., Petitioners, v Larry Jegley, et al., Respondents. No. 17-935. Brief Amici Curiae of American College of Obstetricians and Gynecologists and American Public Health Association as Amici Curiae in Support of Petitioners. February 1, 2018.
- Box v Planned Parenthood of Indiana & Kentucky. No. 18-1019. Brief Amici Curiae of American Association of Pro-Life Obstetricians & Gynecologists, American College of Pediatricians, Care Net, Christian Medical Association, Heartbeat International, Inc., and National Institute Of Family & Life Advocates in Support of Petitioners. March 6, 2019.
- Steven T. Marshall, et al., Petitioners, v West Alabama Women's Center, et al., Respondents. No. 18-837. Brief of Amici Curiae American Association of Pro-Life Obstetricians & Gynecologists and American College of Pediatricians, in Support of Petitioners. January 18, 2019.
- Azar, Secretary of Health and Human Services v Allina Health Services, et al. 17 1484 (2018).
- Alex M. Azar, II, Secretary of Health and Human Services, Petitioner, v Allina Health Services, et al., Respondents. Brief of the American Hospital Association, Federation of American Hospitals, and Association of American Medical Colleges as Amici Curiae in Support of Respondents. December 2018.
- Alex M. Azar, II, Secretary of Health and Human Services, Petitioner, v Allina Health Services, et al., Respondents. Brief of Amici Curiae American Medical Association and Medical Society of the District of Columbia Amici Curiae in Support of Respondents. December 2018.
- 42 U. S. C. §1395hh. https://uscode.house.gov/view.xhtml?req=(title:42%20section:1395hh%20edition:prelim). Accessed October 22, 2019.
- The False Claims Act: a primer. Washington DC: US Department of Justice. www.justice.gov/sites/default/files/civil/legacy/2011/04/22/C-FRAUDS_FCA_Primer.pdf. Accessed October 18, 2019.
- Universal Health Services, Inc., v United States and Commonwealth of Massachusetts ex rel. Julio Escobar and Carmen Correa. Brief of the American Hospital Association, Federation of American Hospitals, and Association of American Medical Colleges Amici Curiae in Support of Petitioner. No. 15-7. January 2016.
- Intermountain Health Care, Inc., et al., Petitioners, v United States ex rel. Gerald Polukoff, et al., Respondents. No. 18-911. Brief of the American Hospital Association and Federation of American Hospitals as Amici Curiae in Support of Petitioners. February 13, 2019.
- Cochise Consultancy, Inc., et al., v United States ex rel. Hunt. 18 315 (2018).
- 34 U.S.C. §20901 et seq. [Chapter 209--Child Protection and Safety.] https://uscode.house.gov/view.xhtml?path=/prelim@title34/subtitle2/chapter209&edition=prelim. Accessed October 17, 2019.
- Gundy v United States. 17 6086 (2018).
- Henry Schein, Inc., et al., v Archer & White Sales, Inc. 17 1272 (2018).
- Lamps Plus, Inc., et al., v Varela. 17 988 (2018).
- Merck Sharp & Dohme Corp. v Albrecht et al. 17 290 (2018).
- Merck Sharp & Dohme Corp. v Albrecht et al. 17 290 (2018) at 13-14.
- Wyeth v Levine, 555 US 555, 571 (2009).
- Russell Bucklew, Petitioner, v Anne L. Precythe, Director, Missouri Department of Corrections, et al., Respondents. 17 8151 (2018).
- Russell Bucklew, Petitioner, v Anne L. Precythe, Director, Missouri Department of Corrections, et al., Respondents. 17 8151 (2018). American Medical Association, Amicus Curiae Brief, in Support of Neither Party. July 23, 2018.
- Final stat pack for October term 2018. SCOTUSblog.com. June 28, 2019. https://www.scotusblog.com/wp-content/uploads/2019/07/StatPack_OT18-7_8_19.pdf. Accessed October 17, 2019.
- Barnes R. They're not 'wonder twins': Gorsuch, Kavanaugh shift the Supreme Court, but their differences are striking. Washington Post, June 28, 2019.
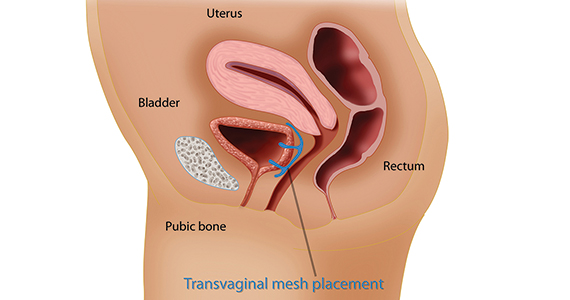
The mesh mess, enmeshed in controversy
CASE Complications with mesh placement for SUI
A 47-year-old woman (G4 P3013) presents 5 months posthysterectomy with evidence of urinary tract infection (UTI). Escherichia coli is isolated, and she responds to antibiotic therapy.
Her surgical history includes a mini-sling procedure using a needleless device and mesh placement in order to correct progressive worsening of loss of urine when coughing and sneezing. She also reported slight pelvic pain, dysuria, and urgency upon urination at that time. After subsequent development of pelvic organ prolapse (POP), she underwent the vaginal hysterectomy.
Following her UTI treatment, a host of problems occur for the patient, including pelvic pain and dyspareunia. Her male partner reports “feeling something during sex,” especially at the anterior vaginal wall. A plain radiograph of the abdomen identifies a 2 cm x 2 cm stone over the vaginal mesh. In consultation with female pelvic medicine and reconstructive surgery subspecialists, lithotripsy is performed, with the stone fragmented. The patient remains symptomatic, however.
The mesh is noted to be eroding through the vaginal wall. An attempt is made to excise the mesh, initially via transuretheral resection, then through a laparoscopic approach. Due to the mesh being embedded in the tissue, however, an open approach is undertaken. Extensive excision of the mesh and stone fragments is performed. Postoperatively, the patient reports “dry vagina,” with no other genitourinary complaints.
The patient sues. She sues the mesh manufacturer. She also seeks to sue the gynecologist who placed the sling and vaginal mesh (as she says she was not informed of “all the risks” of vaginal mesh placement. She is part of a class action lawsuit, along with thousands of other women.
WHAT’S THE VERDICT?
The device manufacturer settled out of court with the class action suit. (The gynecologist was never formally a defendant because the patient/plaintiff was advised to “drop the physician from the suit.”) The attorneys representing the class action received 40% of the award plus presented costs for the representation. The class as a whole received a little more than 50% of the negotiated award. The patient in this case received $60,000.
Medical background
Stress urinary incontinence (SUI) is a prevalent condition; it affects 35% of women.1 Overall, 80% of women aged 80 or younger will undergo some form of surgery for POP during their lifetime.2 The pathophysiology of SUI includes urethral hypermobility and intrinsic sphincter deficiency.3
Surgical correction for urinary incontinence: A timeline
Use of the gracilis muscle flap to surgically correct urinary incontinence was introduced in 1907. This technique has been replaced by today’s more common Burch procedure, which was first described in 1961. Surgical mesh use dates back to the 1950s, when it was primarily used for abdominal hernia repair. Tension-free tape was introduced in 1995.4-6
Continue to: In the late 1990s the US Food and Drug Administration...
In the late 1990s the US Food and Drug Administration (FDA) permitted use of the first transvaginal meshes, which were designed to treat SUI—the midurethral sling. These mesh slings were so successful that similar meshes were developed to treat POP.7 Almost immediately there were problems with the new POP devices, and 3 years later Boston Scientific recalled its device.8 Nonetheless, the FDA cleared more than 150 devices using surgical mesh for urogynecologic indications (FIGURE).9

Mesh complications
Managing complications from intravesical mesh is a clinically challenging problem. Bladder perforation, stone formation, and penetration through the vagina can occur. Bladder-related complications can manifest as recurrent UTIs and obstructive urinary symptoms, especially in association with stone formation. From the gynecologic perspective, the more common complications with mesh utilization are pelvic pain, groin pain, dyspareunia, contracture and scarring of mesh, and narrowing of the vaginal canal.10 Mesh erosion problems will occur in an estimated 10% to 25% of transvaginal mesh POP implants.11
In 2008, a comparison of transvaginal mesh to native tissue repair (suture-based) or other (biologic) grafts was published.12 The bottom line: there is insufficient evidence to suggest that transvaginal mesh significantly improves outcomes for both posterior and apical defects.
Legal background
Mesh used for surgical purposes is a medical device, which legally is a product—a special product to be sure, but a product nonetheless. Products are subject to product liability rules. Mesh is also subject to an FDA regulatory system. We will briefly discuss products liability and the regulation of devices, both of which have played important roles in mesh-related injuries.
Products liability
As a general matter, defective products subject their manufacturer and seller to liability. There are several legal theories regarding product liability: negligence (in which the defect was caused through carelessness), breach of warranty or guarantee (in addition to express warranties, there are a number of implied warranties for products, including that it is fit for its intended purpose), and strict liability (there was a defect in the product, but it may not have been because of negligence). The product may be defective in the way it was designed, manufactured, or packaged, or it may be defective because adequate instructions and warning were not given to consumers.
Of course, not every product involved in an injury is defective—most automobile accidents, for example, are not the result of any defect in the automobile. In medicine, almost no product (device or pharmaceutical) is entirely safe. In some ways they are unavoidably unsafe and bound to cause some injuries. But when injuries are caused by a defect in the product (design or manufacturing defect or failure to warn), then there may be products liability. Most products liability cases arise under state law.
FDA’s device regulations
Both drugs and medical devices are subject to FDA review and ordinarily require some form of FDA clearance before they may be marketed. In the case of devices, the FDA has 3 classes, with an increase in risk to the user from Class I to III. Various levels of FDA review are required before marketing of the device is permitted, again with the intensity of review increasing from I to III as follows:
- Class I devices pose the least risk, have the least regulation, and are subject to general controls (ie, manufacturing and marketing practices).
- Class II devices pose slightly higher risks and are subject to special controls in addition to the criteria for Class I.
- Class III devices pose the most risk to patients and require premarket approval (scientific review and studies are required to ensure efficacy and safety).13
Continue to: There are a number of limits on manufacturer liability for defective devices...
There are a number of limits on manufacturer liability for defective devices. For Class III devices, the thorough FDA review of the safety of a device may limit the ability of an injured patient to sue based on the state product liability laws.14 For the most part, this “preemption” of state law has not played a major role in mesh litigation because they were initially classified as Class II devices which did not require or include a detailed FDA review.15
The duty to warn of the dangers and risk of medical devices means that manufacturers (or sellers) of devices are obligated to inform health care providers and other medical personnel of the risks. Unlike other manufacturers, device manufacturers do not have to directly warn consumers—because physicians deal directly with patients and prescribe the devices. Therefore, the health care providers, rather than the manufacturers, are obligated to inform the patient.16 This is known as the learned intermediary rule. Manufacturers may still be liable for failure to warn if they do not convey to health care providers proper warnings.
Manufacturers and sellers are not the only entities that may be subject to liability caused by medical devices. Hospitals or other entities that stock and care for devices are responsible for maintaining the safety and functionality of devices in their care.
Health care providers also may be responsible for injuries from medical devices. Generally, that liability is based on negligence. Negligence may relate to selecting an improper device, installing or using it incorrectly, or failing to give the patient adequate information (or informed consent) about the device and alternatives to it.17
A look at the mesh mess
There are a lot of distressing problems and professional disappointments in dissecting the “mesh mess,” including a failure of the FDA to regulate effectively, the extended sale and promotion of intrinsic sphincter deficiency mesh products, the improper use of mesh by physicians even after the risks were known, and, in some instances, the taking advantage of injured patients by attorneys and businesses.18 A lot of finger pointing also has occurred.19 We will recount some of the lowlights of this unfortunate tale.
Continue to: The FDA, in the 1990s, classified the first POP and SUI mesh...
The FDA, in the 1990s, classified the first POP and SUI mesh as Class II after deciding these products were “substantially equivalent” to older surgical meshes. This, of course, proved not to be the case.20 The FDA started receiving thousands of reports of adverse events and, in 2008, warned physicians to be vigilant for adverse events from the mesh. The FDA’s notification recommendations regarding mesh included the following13:
- Obtain specialized training for each mesh implantation technique, and be cognizant of risks.
- Be vigilant for potential adverse events from mesh, including erosion and infection.
- Be observant for complications associated with tools of transvaginal placement (ie, bowel, bladder, and vessel perforation).
- Inform patients that implantation of mesh is permanent and complications may require additional surgery for correction.
- Be aware that complications may affect quality of life—eg, pain with intercourse, scarring, and vaginal wall narrowing (POP repair).
- Provide patients with written copy of patient labeling from the surgical mesh manufacturer.
In 2011, the FDA issued a formal warning to providers that transvaginal mesh posed meaningful risks beyond nonmesh surgery. The FDA’s bulletin draws attention to how the mesh is placed more so than the material per se.19,21 Mesh was a Class II device for sacrocolpopexy or midurethral sling and, similarly, the transvaginal kit was also a Class II device. Overall, use of mesh midurethral slings has been well received as treatment for SUI. The FDA also accepted it for POP, however, but with increasingly strong warnings. The FDA’s 2011 communication stated, “This update is to inform you that serious complications associated with surgical mesh for transvaginal repair of POP are not rare….Furthermore, it is not clear that transvaginal POP repair with mesh is more effective than traditional non-mesh repair in all patients with POP and it may expose patients to greater risk.”7,13
In 2014 the FDA proposed reclassifying mesh to a Class III device, which would require that manufacturers obtain approval, based on safety and effectiveness, before selling mesh. Not until 2016 did the FDA actually reclassify the mess as Class III. Of course, during this time, mesh manufacturers were well aware of the substantial problems the products were causing.13
After serious problems with mesh became well known, and especially after FDA warnings, the use of mesh other than as indicated by the FDA was increasingly risky from a legal (as well as a health) standpoint. As long as mesh was still on the market, of course, it was available for use. But use of mesh for POP procedures without good indications in a way that was contrary to the FDA warnings might well be negligent.
Changes to informed consent
The FDA warnings also should have changed the informed consent for the use of mesh.22 Informed consent commonly consists of the following:
- informing the patient of the proposed procedure
- describing risks (and benefits) of the proposed process
- explaining reasonable alternatives
- noting the risks of taking no action.
Information that is material to a decision should be disclosed. If mesh were going to be used, after the problems of mesh were known and identified by the FDA (other than midurethral slings as treatment of SUI), the risks should have been clearly identified for patients, with alternatives outlined. The American College of Obstetricians and Gynecologists Committee on Ethics has 8 fundamental concepts with regard to informed consent that are worth keeping in mind23:
- Obtaining informed consent for medical treatment and research is an ethical requirement.
- The process expresses respect for the patient as a person.
- It protects patients against unwanted treatment and allows patients’ active involvement in medical planning and care.
- Communication is of paramount importance.
- Informed consent is a process and not a signature on a form.
- A commitment to informed consent and to provision of medical benefit to the patient are linked to provision of care.
- If obtaining informed consent is impossible, a designated surrogate should be identified representing the patient’s best interests.
- Knowledge on the part of the provider regarding state and federal requirements is necessary.
Continue to: Lawsuits line up...
Lawsuits line up
The widespread use of a product with a significant percentage of injuries and eventually with warnings about injuries from use sounds like the formula for a lot of lawsuits. This certainly has happened. A large number of suits—both class actions and individual actions—were filed as a result of mesh injuries.24 These suits were overwhelmingly against the manufacturer, although some included physicians.7 Device makers are more attractive defendants for several reasons. First, they have very deep pockets. In addition, jurors are generally much less sympathetic to large companies than to doctors. Large class actions meant that there were many different patients among the plaintiffs, and medical malpractice claims in most states have a number of trial difficulties not present in other product liability cases. Common defendants have included Johnson & Johnson, Boston Scientific, and Medtronic.
Some of the cases resulted in very large damage awards against manufacturers based on various kinds of product(s) liability. Many other cases were settled or tried with relatively small damages. There were, in addition, a number of instances in which the manufacturers were not liable. Of the 32 plaintiffs who have gone to trial thus far, 24 have obtained verdicts totaling $345 million ($14 million average). The cases that have settled have been for much less—perhaps $60,000 on average. A number of cases remain unresolved. To date, the estimate is that 100,000 women have received almost $8 billion from 7 device manufacturers to resolve claims.25
Some state attorneys general have gotten into the process as well. Attorneys general from California, Kentucky, Mississippi, and Washington have filed lawsuits against Johnson & Johnson, claiming that they deceived doctors and patients about the risks of their pelvic mesh. The states claim that marketing and instructional literature should have contained more information about the risks. Some physicians in these states have expressed concern that these lawsuit risks may do more harm than good because the suits conflate mesh used to treat incontinence with the more risky mesh for POP.26
The “ugly” of class action lawsuits
We have discussed both the sad (the injuries to patients) and the bad (the slow regulatory response and continuing injuries). (The ethics of the marketing by the manufacturers might also be raised as the bad.27) Next, let’s look briefly at the ugly.
Some of the patients affected by mesh injuries have been victimized a second time by medical “lenders” and some of their attorneys. Press reports describe patients with modest awards paying 40% in attorney fees (on the high side for personal injury settlements) plus extravagant costs—leaving modest amounts of actual recovery.25
Worse still, a process of “medical lending” has arisen in mesh cases.28 Medical lenders may contact mesh victims offering to pay up front for surgery to remove mesh, and then place a lien against the settlement for repayment at a much higher rate. They might pay the surgeon $2,500 for the surgery, but place a lien on the settlement amount for $60,000.29,30 In addition, there are allegations that lawyers may recruit the doctors to overstate the injuries or do unnecessary removal surgery because that will likely up the award.31 A quick Google search indicates dozens of offers of cash now for your mesh lawsuit (transvaginal and hernia repair).
The patient in our hypothetical case at the beginning had a fairly typical experience. She was a member of a class filing and received a modest settlement. The attorneys representing the class were allowed by the court to charge substantial attorneys’ fees and costs. The patient had the good sense to avoid medical lenders, although other members of the class did use medical lenders and are now filing complaints about the way they were treated by these lenders.
- Maintain surgical skills and be open to new technology. Medical practice requires constant updating and use of new and improved technology as it comes along. By definition, new technology often requires new skills and understanding. A significant portion of surgeons using mesh indicated that they had not read the instructions for use, or had done so only once.1 CME programs that include surgical education remain of particular value.
- Whether new technology or old, it is essential to keep up to date on all FDA bulletins pertinent to devices and pharmaceuticals that you use and prescribe. For example, in 2016 and 2018 the FDA warned that the use of a very old class of drugs (fluoroquinolones) should be limited. It advised "that the serious side effects associated with fluoroquinolones generally outweigh the benefits for patients with acute sinusitis, acute bronchitis, and uncomplicated urinary tract infections who have other treatment options. For patients with these conditions, fluoroquinolones should be reserved for those who do not have alternative treatment options."2 Continued, unnecessary prescriptions for fluoroquinolones would put a physician at some legal risk whether or not the physician had paid any attention to the warning.
- Informed consent is a very important legal and medical process. Take it seriously, and make sure the patient has the information necessary to make informed decisions about treatment. Document the process and the information provided. In some cases consider directing patients to appropriate literature or websites of the manufacturers.
- As to the use of mesh, if not following FDA advice, it is important to document the reason for this and to document the informed consent especially carefully.
- Follow patients after mesh placement for a minimum of 1 year and emphasize to patients they should convey signs and symptoms of complications from initial placement.3 High-risk patients should be of particular concern and be monitored very closely.
References
- Kirkpatrick G, Faber KD, Fromer DL. Transvaginal mesh placement and the instructions for use: a survey of North American urologists. J Urol. https://doi.org/10.1016/j.urpr.2018.05.004.
- FDA Drug Safety Communication: FDA advises restricting fluoroquinolone antibiotic use for certain uncomplicated infections; warns about disabling side effects that can occur together. July 26, 2016. https://www.fda.gov/Drugs/DrugSafety/ucm500143.htm. Accessed June 19, 2019.
- Karlovsky ME. How to avoid and deal with pelvic mesh litigation. Curr Urol Rep. 2016;17:55.
- Maral I, Ozkardeş H, Peşkircioğlu L, et al. Prevalence of stress urinary incontinence in both sexes at or after age 15 years: a cross-sectional study. J Urol. 2001;165:408-412.
- Olsen AL, Smith VJ, Bergstrom JO, et al. Epidemiology of surgically managed pelvic organ prolapse and urinary incontinence. Obstet Gynecol. 1997;89:501-506.
- Chang J, Lee D. Midurethral slings in the mesh litigation era. Transl Androl Urol. 2017;6(suppl 2): S68-S75.
- Mattingly R, ed. TeLinde's Operative Gynecology, 5th edition. Lippincott, William, and Wilkins: Philadelphia, PA; 1997.
- Burch J. Urethrovaginal fixation to Cooper's ligament for correction of stress incontinence, cystocele, and prolapse. Am J Obstet Gynecol. 1961;81:281-290.
- Ulmsten U, Falconer C, Johnson P, et al. A multicenter study of tension-free vaginal tape (TVT) for surgical treatment of stress urinary incontinence. Int Urogynecol J Pelvic Floor Dysfunct. 1998;9:210-213.
- Kuhlmann-Capek MJ, Kilic GS, Shah AB, et al. Enmeshed in controversy: use of vaginal mesh in the current medicolegal environment. Female Pelvic Med Reconstr Surg. 2015;21:241-243.
- Powell SF. Changing our minds: reforming the FDA medical device reclassification process. Food Drug Law J. 2018;73:177-209.
- US Food and Drug Administration. Surgical Mesh for Treatment of Women with Pelvic Organ Prolapse and Stress Urinary Incontinence. September 2011. https://www.thesenatorsfirm.com/documents/OBS.pdf. Accessed June 19, 2019.
- Maher C, Feiner B, Baessler K, et al. Surgical management of pelvic organ prolapse in women. Cochrane Database Syst Rev. 2013;(4):CD004014.
- Ganj FA, Ibeanu OA, Bedestani A, Nolan TE, Chesson RR. Complications of transvaginal monofilament polypropylene mesh in pelvic organ prolapse repair. Int Urogynecol J Pelvic Floor Dysfunct. 2009;20:919-925.
- Sung VW, Rogers RG, Schaffer JI, et al. Graft use in transvaginal pelvic organ prolapse repair: a systematic review. Obstet Gynecol. 2008;112:1131-1142.
- FDA public health notification: serious complications associated with transvaginal placement of surgical mesh in repair of pelvic organ prolapse and stress urinary incontinence. October 20, 2008. http://www.fda.gov/MedicalDevices/Safety/AlertsandNotices/PublicHealthNotifications/ucm061976.htm. Accessed February 14, 2019.
- Riegel v. Medtronic, 552 U.S. 312 (2008).
- Whitney DW. Guide to preemption of state-law claims against Class III PMA medical devices. Food Drug Law J. 2010;65:113-139.
- Alam P, Iglesia CB. Informed consent for reconstructive pelvic surgery. Obstet Gynecol Clin North Am. 2016;43:131-139.
- Nosti PA, Iglesia CB. Medicolegal issues surrounding devices and mesh for surgical treatment of prolapse and incontinence. Clin Obstet Gynecol. 2013;56:221-228.
- Shepherd CG. Transvaginal mesh litigation: a new opportunity to resolve mass medical device failure claims. Tennessee Law Rev. 2012;80:3:477-94.
- Karlovsky ME. How to avoid and deal with pelvic mesh litigation. Curr Urol Rep. 2016;17:55.
- Cohn JA, Timbrook Brown E, Kowalik CG, et al. The mesh controversy. F1000Research website. https://f1000research.com/articles/5-2423/v1. Accessed June 17, 2019.
- Obstetrics and Gynecology Devices Panel Meeting, February 12, 2019. US Food and Drug Administration website. https://www.fda.gov/media/122867/download. Accessed June 19, 2019.
- Mucowski SJ, Jurnalov C, Phelps JY. Use of vaginal mesh in the face of recent FDA warnings and litigation. Am J Obstet Gynecol. 2010;203:103.e1-e4.
- American College of Obstetricians and Gynecologists Committee on Ethics. ACOG Committee Opinion No. 439: informed consent. Obstet Gynecol. 2009;114(2 pt 1):401-408.
- Souders CP, Eilber KS, McClelland L, et al. The truth behind transvaginal mesh litigation: devices, timelines, and provider characteristics. Female Pelvic Med Reconstr Surg. 2018;24:21-25.
- Goldstein M. As pelvic mesh settlements near $8 billion, women question lawyers' fees. New York Times. February 1, 2019. https://www.nytimes.com/2019/02/01/business/pelvic-mesh-settlements-lawyers.html. Accessed June 19, 2019.
- Johnson G. Surgeons fear pelvic mesh lawsuits will spook patients. Associated Press News. January 10, 2019. https://www.apnews.com/25777c3c33e3489283b1dc2ebdde6b55. Accessed June 19, 2019.
- Clarke RN. Medical device marketing and the ethics of vaginal mesh kit marketing. In The Innovation and Evolution of Medical Devices. New York, NY: Springer; 2019:103-123.
- Top 5 drug and medical device developments of 2018. Law 360. January 1, 2019. Accessed through LexisNexis.
- Frankel A, Dye J. The Lien Machine. New breed of investor profits by financing surgeries for desperate women patients. Reuters. August 18, 2015. https://www.reuters.com/investigates/special-report/usa-litigation-mesh/. Accessed June 19, 2019.
- Sullivan T. New report looks at intersection of "medical lending" and pelvic mesh lawsuits. Policy & Medicine. May 5, 2018. https://www.policymed.com/2015/08/medical-lending-and-pelvic-mesh-litigation.html. Accessed June 19, 2019.
- Goldstein M, Sliver-Greensberg J. How profiteers lure women into often-unneeded surgery. New York Times. April 14, 2018. https://www.nytimes.com/2018/04/14/business/vaginal-mesh-surgery-lawsuits-financing.html. Accessed June 19, 2019.
CASE Complications with mesh placement for SUI
A 47-year-old woman (G4 P3013) presents 5 months posthysterectomy with evidence of urinary tract infection (UTI). Escherichia coli is isolated, and she responds to antibiotic therapy.
Her surgical history includes a mini-sling procedure using a needleless device and mesh placement in order to correct progressive worsening of loss of urine when coughing and sneezing. She also reported slight pelvic pain, dysuria, and urgency upon urination at that time. After subsequent development of pelvic organ prolapse (POP), she underwent the vaginal hysterectomy.
Following her UTI treatment, a host of problems occur for the patient, including pelvic pain and dyspareunia. Her male partner reports “feeling something during sex,” especially at the anterior vaginal wall. A plain radiograph of the abdomen identifies a 2 cm x 2 cm stone over the vaginal mesh. In consultation with female pelvic medicine and reconstructive surgery subspecialists, lithotripsy is performed, with the stone fragmented. The patient remains symptomatic, however.
The mesh is noted to be eroding through the vaginal wall. An attempt is made to excise the mesh, initially via transuretheral resection, then through a laparoscopic approach. Due to the mesh being embedded in the tissue, however, an open approach is undertaken. Extensive excision of the mesh and stone fragments is performed. Postoperatively, the patient reports “dry vagina,” with no other genitourinary complaints.
The patient sues. She sues the mesh manufacturer. She also seeks to sue the gynecologist who placed the sling and vaginal mesh (as she says she was not informed of “all the risks” of vaginal mesh placement. She is part of a class action lawsuit, along with thousands of other women.
WHAT’S THE VERDICT?
The device manufacturer settled out of court with the class action suit. (The gynecologist was never formally a defendant because the patient/plaintiff was advised to “drop the physician from the suit.”) The attorneys representing the class action received 40% of the award plus presented costs for the representation. The class as a whole received a little more than 50% of the negotiated award. The patient in this case received $60,000.
Medical background
Stress urinary incontinence (SUI) is a prevalent condition; it affects 35% of women.1 Overall, 80% of women aged 80 or younger will undergo some form of surgery for POP during their lifetime.2 The pathophysiology of SUI includes urethral hypermobility and intrinsic sphincter deficiency.3
Surgical correction for urinary incontinence: A timeline
Use of the gracilis muscle flap to surgically correct urinary incontinence was introduced in 1907. This technique has been replaced by today’s more common Burch procedure, which was first described in 1961. Surgical mesh use dates back to the 1950s, when it was primarily used for abdominal hernia repair. Tension-free tape was introduced in 1995.4-6
Continue to: In the late 1990s the US Food and Drug Administration...
In the late 1990s the US Food and Drug Administration (FDA) permitted use of the first transvaginal meshes, which were designed to treat SUI—the midurethral sling. These mesh slings were so successful that similar meshes were developed to treat POP.7 Almost immediately there were problems with the new POP devices, and 3 years later Boston Scientific recalled its device.8 Nonetheless, the FDA cleared more than 150 devices using surgical mesh for urogynecologic indications (FIGURE).9

Mesh complications
Managing complications from intravesical mesh is a clinically challenging problem. Bladder perforation, stone formation, and penetration through the vagina can occur. Bladder-related complications can manifest as recurrent UTIs and obstructive urinary symptoms, especially in association with stone formation. From the gynecologic perspective, the more common complications with mesh utilization are pelvic pain, groin pain, dyspareunia, contracture and scarring of mesh, and narrowing of the vaginal canal.10 Mesh erosion problems will occur in an estimated 10% to 25% of transvaginal mesh POP implants.11
In 2008, a comparison of transvaginal mesh to native tissue repair (suture-based) or other (biologic) grafts was published.12 The bottom line: there is insufficient evidence to suggest that transvaginal mesh significantly improves outcomes for both posterior and apical defects.
Legal background
Mesh used for surgical purposes is a medical device, which legally is a product—a special product to be sure, but a product nonetheless. Products are subject to product liability rules. Mesh is also subject to an FDA regulatory system. We will briefly discuss products liability and the regulation of devices, both of which have played important roles in mesh-related injuries.
Products liability
As a general matter, defective products subject their manufacturer and seller to liability. There are several legal theories regarding product liability: negligence (in which the defect was caused through carelessness), breach of warranty or guarantee (in addition to express warranties, there are a number of implied warranties for products, including that it is fit for its intended purpose), and strict liability (there was a defect in the product, but it may not have been because of negligence). The product may be defective in the way it was designed, manufactured, or packaged, or it may be defective because adequate instructions and warning were not given to consumers.
Of course, not every product involved in an injury is defective—most automobile accidents, for example, are not the result of any defect in the automobile. In medicine, almost no product (device or pharmaceutical) is entirely safe. In some ways they are unavoidably unsafe and bound to cause some injuries. But when injuries are caused by a defect in the product (design or manufacturing defect or failure to warn), then there may be products liability. Most products liability cases arise under state law.
FDA’s device regulations
Both drugs and medical devices are subject to FDA review and ordinarily require some form of FDA clearance before they may be marketed. In the case of devices, the FDA has 3 classes, with an increase in risk to the user from Class I to III. Various levels of FDA review are required before marketing of the device is permitted, again with the intensity of review increasing from I to III as follows:
- Class I devices pose the least risk, have the least regulation, and are subject to general controls (ie, manufacturing and marketing practices).
- Class II devices pose slightly higher risks and are subject to special controls in addition to the criteria for Class I.
- Class III devices pose the most risk to patients and require premarket approval (scientific review and studies are required to ensure efficacy and safety).13
Continue to: There are a number of limits on manufacturer liability for defective devices...
There are a number of limits on manufacturer liability for defective devices. For Class III devices, the thorough FDA review of the safety of a device may limit the ability of an injured patient to sue based on the state product liability laws.14 For the most part, this “preemption” of state law has not played a major role in mesh litigation because they were initially classified as Class II devices which did not require or include a detailed FDA review.15
The duty to warn of the dangers and risk of medical devices means that manufacturers (or sellers) of devices are obligated to inform health care providers and other medical personnel of the risks. Unlike other manufacturers, device manufacturers do not have to directly warn consumers—because physicians deal directly with patients and prescribe the devices. Therefore, the health care providers, rather than the manufacturers, are obligated to inform the patient.16 This is known as the learned intermediary rule. Manufacturers may still be liable for failure to warn if they do not convey to health care providers proper warnings.
Manufacturers and sellers are not the only entities that may be subject to liability caused by medical devices. Hospitals or other entities that stock and care for devices are responsible for maintaining the safety and functionality of devices in their care.
Health care providers also may be responsible for injuries from medical devices. Generally, that liability is based on negligence. Negligence may relate to selecting an improper device, installing or using it incorrectly, or failing to give the patient adequate information (or informed consent) about the device and alternatives to it.17
A look at the mesh mess
There are a lot of distressing problems and professional disappointments in dissecting the “mesh mess,” including a failure of the FDA to regulate effectively, the extended sale and promotion of intrinsic sphincter deficiency mesh products, the improper use of mesh by physicians even after the risks were known, and, in some instances, the taking advantage of injured patients by attorneys and businesses.18 A lot of finger pointing also has occurred.19 We will recount some of the lowlights of this unfortunate tale.
Continue to: The FDA, in the 1990s, classified the first POP and SUI mesh...
The FDA, in the 1990s, classified the first POP and SUI mesh as Class II after deciding these products were “substantially equivalent” to older surgical meshes. This, of course, proved not to be the case.20 The FDA started receiving thousands of reports of adverse events and, in 2008, warned physicians to be vigilant for adverse events from the mesh. The FDA’s notification recommendations regarding mesh included the following13:
- Obtain specialized training for each mesh implantation technique, and be cognizant of risks.
- Be vigilant for potential adverse events from mesh, including erosion and infection.
- Be observant for complications associated with tools of transvaginal placement (ie, bowel, bladder, and vessel perforation).
- Inform patients that implantation of mesh is permanent and complications may require additional surgery for correction.
- Be aware that complications may affect quality of life—eg, pain with intercourse, scarring, and vaginal wall narrowing (POP repair).
- Provide patients with written copy of patient labeling from the surgical mesh manufacturer.
In 2011, the FDA issued a formal warning to providers that transvaginal mesh posed meaningful risks beyond nonmesh surgery. The FDA’s bulletin draws attention to how the mesh is placed more so than the material per se.19,21 Mesh was a Class II device for sacrocolpopexy or midurethral sling and, similarly, the transvaginal kit was also a Class II device. Overall, use of mesh midurethral slings has been well received as treatment for SUI. The FDA also accepted it for POP, however, but with increasingly strong warnings. The FDA’s 2011 communication stated, “This update is to inform you that serious complications associated with surgical mesh for transvaginal repair of POP are not rare….Furthermore, it is not clear that transvaginal POP repair with mesh is more effective than traditional non-mesh repair in all patients with POP and it may expose patients to greater risk.”7,13
In 2014 the FDA proposed reclassifying mesh to a Class III device, which would require that manufacturers obtain approval, based on safety and effectiveness, before selling mesh. Not until 2016 did the FDA actually reclassify the mess as Class III. Of course, during this time, mesh manufacturers were well aware of the substantial problems the products were causing.13
After serious problems with mesh became well known, and especially after FDA warnings, the use of mesh other than as indicated by the FDA was increasingly risky from a legal (as well as a health) standpoint. As long as mesh was still on the market, of course, it was available for use. But use of mesh for POP procedures without good indications in a way that was contrary to the FDA warnings might well be negligent.
Changes to informed consent
The FDA warnings also should have changed the informed consent for the use of mesh.22 Informed consent commonly consists of the following:
- informing the patient of the proposed procedure
- describing risks (and benefits) of the proposed process
- explaining reasonable alternatives
- noting the risks of taking no action.
Information that is material to a decision should be disclosed. If mesh were going to be used, after the problems of mesh were known and identified by the FDA (other than midurethral slings as treatment of SUI), the risks should have been clearly identified for patients, with alternatives outlined. The American College of Obstetricians and Gynecologists Committee on Ethics has 8 fundamental concepts with regard to informed consent that are worth keeping in mind23:
- Obtaining informed consent for medical treatment and research is an ethical requirement.
- The process expresses respect for the patient as a person.
- It protects patients against unwanted treatment and allows patients’ active involvement in medical planning and care.
- Communication is of paramount importance.
- Informed consent is a process and not a signature on a form.
- A commitment to informed consent and to provision of medical benefit to the patient are linked to provision of care.
- If obtaining informed consent is impossible, a designated surrogate should be identified representing the patient’s best interests.
- Knowledge on the part of the provider regarding state and federal requirements is necessary.
Continue to: Lawsuits line up...
Lawsuits line up
The widespread use of a product with a significant percentage of injuries and eventually with warnings about injuries from use sounds like the formula for a lot of lawsuits. This certainly has happened. A large number of suits—both class actions and individual actions—were filed as a result of mesh injuries.24 These suits were overwhelmingly against the manufacturer, although some included physicians.7 Device makers are more attractive defendants for several reasons. First, they have very deep pockets. In addition, jurors are generally much less sympathetic to large companies than to doctors. Large class actions meant that there were many different patients among the plaintiffs, and medical malpractice claims in most states have a number of trial difficulties not present in other product liability cases. Common defendants have included Johnson & Johnson, Boston Scientific, and Medtronic.
Some of the cases resulted in very large damage awards against manufacturers based on various kinds of product(s) liability. Many other cases were settled or tried with relatively small damages. There were, in addition, a number of instances in which the manufacturers were not liable. Of the 32 plaintiffs who have gone to trial thus far, 24 have obtained verdicts totaling $345 million ($14 million average). The cases that have settled have been for much less—perhaps $60,000 on average. A number of cases remain unresolved. To date, the estimate is that 100,000 women have received almost $8 billion from 7 device manufacturers to resolve claims.25
Some state attorneys general have gotten into the process as well. Attorneys general from California, Kentucky, Mississippi, and Washington have filed lawsuits against Johnson & Johnson, claiming that they deceived doctors and patients about the risks of their pelvic mesh. The states claim that marketing and instructional literature should have contained more information about the risks. Some physicians in these states have expressed concern that these lawsuit risks may do more harm than good because the suits conflate mesh used to treat incontinence with the more risky mesh for POP.26
The “ugly” of class action lawsuits
We have discussed both the sad (the injuries to patients) and the bad (the slow regulatory response and continuing injuries). (The ethics of the marketing by the manufacturers might also be raised as the bad.27) Next, let’s look briefly at the ugly.
Some of the patients affected by mesh injuries have been victimized a second time by medical “lenders” and some of their attorneys. Press reports describe patients with modest awards paying 40% in attorney fees (on the high side for personal injury settlements) plus extravagant costs—leaving modest amounts of actual recovery.25
Worse still, a process of “medical lending” has arisen in mesh cases.28 Medical lenders may contact mesh victims offering to pay up front for surgery to remove mesh, and then place a lien against the settlement for repayment at a much higher rate. They might pay the surgeon $2,500 for the surgery, but place a lien on the settlement amount for $60,000.29,30 In addition, there are allegations that lawyers may recruit the doctors to overstate the injuries or do unnecessary removal surgery because that will likely up the award.31 A quick Google search indicates dozens of offers of cash now for your mesh lawsuit (transvaginal and hernia repair).
The patient in our hypothetical case at the beginning had a fairly typical experience. She was a member of a class filing and received a modest settlement. The attorneys representing the class were allowed by the court to charge substantial attorneys’ fees and costs. The patient had the good sense to avoid medical lenders, although other members of the class did use medical lenders and are now filing complaints about the way they were treated by these lenders.
- Maintain surgical skills and be open to new technology. Medical practice requires constant updating and use of new and improved technology as it comes along. By definition, new technology often requires new skills and understanding. A significant portion of surgeons using mesh indicated that they had not read the instructions for use, or had done so only once.1 CME programs that include surgical education remain of particular value.
- Whether new technology or old, it is essential to keep up to date on all FDA bulletins pertinent to devices and pharmaceuticals that you use and prescribe. For example, in 2016 and 2018 the FDA warned that the use of a very old class of drugs (fluoroquinolones) should be limited. It advised "that the serious side effects associated with fluoroquinolones generally outweigh the benefits for patients with acute sinusitis, acute bronchitis, and uncomplicated urinary tract infections who have other treatment options. For patients with these conditions, fluoroquinolones should be reserved for those who do not have alternative treatment options."2 Continued, unnecessary prescriptions for fluoroquinolones would put a physician at some legal risk whether or not the physician had paid any attention to the warning.
- Informed consent is a very important legal and medical process. Take it seriously, and make sure the patient has the information necessary to make informed decisions about treatment. Document the process and the information provided. In some cases consider directing patients to appropriate literature or websites of the manufacturers.
- As to the use of mesh, if not following FDA advice, it is important to document the reason for this and to document the informed consent especially carefully.
- Follow patients after mesh placement for a minimum of 1 year and emphasize to patients they should convey signs and symptoms of complications from initial placement.3 High-risk patients should be of particular concern and be monitored very closely.
References
- Kirkpatrick G, Faber KD, Fromer DL. Transvaginal mesh placement and the instructions for use: a survey of North American urologists. J Urol. https://doi.org/10.1016/j.urpr.2018.05.004.
- FDA Drug Safety Communication: FDA advises restricting fluoroquinolone antibiotic use for certain uncomplicated infections; warns about disabling side effects that can occur together. July 26, 2016. https://www.fda.gov/Drugs/DrugSafety/ucm500143.htm. Accessed June 19, 2019.
- Karlovsky ME. How to avoid and deal with pelvic mesh litigation. Curr Urol Rep. 2016;17:55.
CASE Complications with mesh placement for SUI
A 47-year-old woman (G4 P3013) presents 5 months posthysterectomy with evidence of urinary tract infection (UTI). Escherichia coli is isolated, and she responds to antibiotic therapy.
Her surgical history includes a mini-sling procedure using a needleless device and mesh placement in order to correct progressive worsening of loss of urine when coughing and sneezing. She also reported slight pelvic pain, dysuria, and urgency upon urination at that time. After subsequent development of pelvic organ prolapse (POP), she underwent the vaginal hysterectomy.
Following her UTI treatment, a host of problems occur for the patient, including pelvic pain and dyspareunia. Her male partner reports “feeling something during sex,” especially at the anterior vaginal wall. A plain radiograph of the abdomen identifies a 2 cm x 2 cm stone over the vaginal mesh. In consultation with female pelvic medicine and reconstructive surgery subspecialists, lithotripsy is performed, with the stone fragmented. The patient remains symptomatic, however.
The mesh is noted to be eroding through the vaginal wall. An attempt is made to excise the mesh, initially via transuretheral resection, then through a laparoscopic approach. Due to the mesh being embedded in the tissue, however, an open approach is undertaken. Extensive excision of the mesh and stone fragments is performed. Postoperatively, the patient reports “dry vagina,” with no other genitourinary complaints.
The patient sues. She sues the mesh manufacturer. She also seeks to sue the gynecologist who placed the sling and vaginal mesh (as she says she was not informed of “all the risks” of vaginal mesh placement. She is part of a class action lawsuit, along with thousands of other women.
WHAT’S THE VERDICT?
The device manufacturer settled out of court with the class action suit. (The gynecologist was never formally a defendant because the patient/plaintiff was advised to “drop the physician from the suit.”) The attorneys representing the class action received 40% of the award plus presented costs for the representation. The class as a whole received a little more than 50% of the negotiated award. The patient in this case received $60,000.
Medical background
Stress urinary incontinence (SUI) is a prevalent condition; it affects 35% of women.1 Overall, 80% of women aged 80 or younger will undergo some form of surgery for POP during their lifetime.2 The pathophysiology of SUI includes urethral hypermobility and intrinsic sphincter deficiency.3
Surgical correction for urinary incontinence: A timeline
Use of the gracilis muscle flap to surgically correct urinary incontinence was introduced in 1907. This technique has been replaced by today’s more common Burch procedure, which was first described in 1961. Surgical mesh use dates back to the 1950s, when it was primarily used for abdominal hernia repair. Tension-free tape was introduced in 1995.4-6
Continue to: In the late 1990s the US Food and Drug Administration...
In the late 1990s the US Food and Drug Administration (FDA) permitted use of the first transvaginal meshes, which were designed to treat SUI—the midurethral sling. These mesh slings were so successful that similar meshes were developed to treat POP.7 Almost immediately there were problems with the new POP devices, and 3 years later Boston Scientific recalled its device.8 Nonetheless, the FDA cleared more than 150 devices using surgical mesh for urogynecologic indications (FIGURE).9

Mesh complications
Managing complications from intravesical mesh is a clinically challenging problem. Bladder perforation, stone formation, and penetration through the vagina can occur. Bladder-related complications can manifest as recurrent UTIs and obstructive urinary symptoms, especially in association with stone formation. From the gynecologic perspective, the more common complications with mesh utilization are pelvic pain, groin pain, dyspareunia, contracture and scarring of mesh, and narrowing of the vaginal canal.10 Mesh erosion problems will occur in an estimated 10% to 25% of transvaginal mesh POP implants.11
In 2008, a comparison of transvaginal mesh to native tissue repair (suture-based) or other (biologic) grafts was published.12 The bottom line: there is insufficient evidence to suggest that transvaginal mesh significantly improves outcomes for both posterior and apical defects.
Legal background
Mesh used for surgical purposes is a medical device, which legally is a product—a special product to be sure, but a product nonetheless. Products are subject to product liability rules. Mesh is also subject to an FDA regulatory system. We will briefly discuss products liability and the regulation of devices, both of which have played important roles in mesh-related injuries.
Products liability
As a general matter, defective products subject their manufacturer and seller to liability. There are several legal theories regarding product liability: negligence (in which the defect was caused through carelessness), breach of warranty or guarantee (in addition to express warranties, there are a number of implied warranties for products, including that it is fit for its intended purpose), and strict liability (there was a defect in the product, but it may not have been because of negligence). The product may be defective in the way it was designed, manufactured, or packaged, or it may be defective because adequate instructions and warning were not given to consumers.
Of course, not every product involved in an injury is defective—most automobile accidents, for example, are not the result of any defect in the automobile. In medicine, almost no product (device or pharmaceutical) is entirely safe. In some ways they are unavoidably unsafe and bound to cause some injuries. But when injuries are caused by a defect in the product (design or manufacturing defect or failure to warn), then there may be products liability. Most products liability cases arise under state law.
FDA’s device regulations
Both drugs and medical devices are subject to FDA review and ordinarily require some form of FDA clearance before they may be marketed. In the case of devices, the FDA has 3 classes, with an increase in risk to the user from Class I to III. Various levels of FDA review are required before marketing of the device is permitted, again with the intensity of review increasing from I to III as follows:
- Class I devices pose the least risk, have the least regulation, and are subject to general controls (ie, manufacturing and marketing practices).
- Class II devices pose slightly higher risks and are subject to special controls in addition to the criteria for Class I.
- Class III devices pose the most risk to patients and require premarket approval (scientific review and studies are required to ensure efficacy and safety).13
Continue to: There are a number of limits on manufacturer liability for defective devices...
There are a number of limits on manufacturer liability for defective devices. For Class III devices, the thorough FDA review of the safety of a device may limit the ability of an injured patient to sue based on the state product liability laws.14 For the most part, this “preemption” of state law has not played a major role in mesh litigation because they were initially classified as Class II devices which did not require or include a detailed FDA review.15
The duty to warn of the dangers and risk of medical devices means that manufacturers (or sellers) of devices are obligated to inform health care providers and other medical personnel of the risks. Unlike other manufacturers, device manufacturers do not have to directly warn consumers—because physicians deal directly with patients and prescribe the devices. Therefore, the health care providers, rather than the manufacturers, are obligated to inform the patient.16 This is known as the learned intermediary rule. Manufacturers may still be liable for failure to warn if they do not convey to health care providers proper warnings.
Manufacturers and sellers are not the only entities that may be subject to liability caused by medical devices. Hospitals or other entities that stock and care for devices are responsible for maintaining the safety and functionality of devices in their care.
Health care providers also may be responsible for injuries from medical devices. Generally, that liability is based on negligence. Negligence may relate to selecting an improper device, installing or using it incorrectly, or failing to give the patient adequate information (or informed consent) about the device and alternatives to it.17
A look at the mesh mess
There are a lot of distressing problems and professional disappointments in dissecting the “mesh mess,” including a failure of the FDA to regulate effectively, the extended sale and promotion of intrinsic sphincter deficiency mesh products, the improper use of mesh by physicians even after the risks were known, and, in some instances, the taking advantage of injured patients by attorneys and businesses.18 A lot of finger pointing also has occurred.19 We will recount some of the lowlights of this unfortunate tale.
Continue to: The FDA, in the 1990s, classified the first POP and SUI mesh...
The FDA, in the 1990s, classified the first POP and SUI mesh as Class II after deciding these products were “substantially equivalent” to older surgical meshes. This, of course, proved not to be the case.20 The FDA started receiving thousands of reports of adverse events and, in 2008, warned physicians to be vigilant for adverse events from the mesh. The FDA’s notification recommendations regarding mesh included the following13:
- Obtain specialized training for each mesh implantation technique, and be cognizant of risks.
- Be vigilant for potential adverse events from mesh, including erosion and infection.
- Be observant for complications associated with tools of transvaginal placement (ie, bowel, bladder, and vessel perforation).
- Inform patients that implantation of mesh is permanent and complications may require additional surgery for correction.
- Be aware that complications may affect quality of life—eg, pain with intercourse, scarring, and vaginal wall narrowing (POP repair).
- Provide patients with written copy of patient labeling from the surgical mesh manufacturer.
In 2011, the FDA issued a formal warning to providers that transvaginal mesh posed meaningful risks beyond nonmesh surgery. The FDA’s bulletin draws attention to how the mesh is placed more so than the material per se.19,21 Mesh was a Class II device for sacrocolpopexy or midurethral sling and, similarly, the transvaginal kit was also a Class II device. Overall, use of mesh midurethral slings has been well received as treatment for SUI. The FDA also accepted it for POP, however, but with increasingly strong warnings. The FDA’s 2011 communication stated, “This update is to inform you that serious complications associated with surgical mesh for transvaginal repair of POP are not rare….Furthermore, it is not clear that transvaginal POP repair with mesh is more effective than traditional non-mesh repair in all patients with POP and it may expose patients to greater risk.”7,13
In 2014 the FDA proposed reclassifying mesh to a Class III device, which would require that manufacturers obtain approval, based on safety and effectiveness, before selling mesh. Not until 2016 did the FDA actually reclassify the mess as Class III. Of course, during this time, mesh manufacturers were well aware of the substantial problems the products were causing.13
After serious problems with mesh became well known, and especially after FDA warnings, the use of mesh other than as indicated by the FDA was increasingly risky from a legal (as well as a health) standpoint. As long as mesh was still on the market, of course, it was available for use. But use of mesh for POP procedures without good indications in a way that was contrary to the FDA warnings might well be negligent.
Changes to informed consent
The FDA warnings also should have changed the informed consent for the use of mesh.22 Informed consent commonly consists of the following:
- informing the patient of the proposed procedure
- describing risks (and benefits) of the proposed process
- explaining reasonable alternatives
- noting the risks of taking no action.
Information that is material to a decision should be disclosed. If mesh were going to be used, after the problems of mesh were known and identified by the FDA (other than midurethral slings as treatment of SUI), the risks should have been clearly identified for patients, with alternatives outlined. The American College of Obstetricians and Gynecologists Committee on Ethics has 8 fundamental concepts with regard to informed consent that are worth keeping in mind23:
- Obtaining informed consent for medical treatment and research is an ethical requirement.
- The process expresses respect for the patient as a person.
- It protects patients against unwanted treatment and allows patients’ active involvement in medical planning and care.
- Communication is of paramount importance.
- Informed consent is a process and not a signature on a form.
- A commitment to informed consent and to provision of medical benefit to the patient are linked to provision of care.
- If obtaining informed consent is impossible, a designated surrogate should be identified representing the patient’s best interests.
- Knowledge on the part of the provider regarding state and federal requirements is necessary.
Continue to: Lawsuits line up...
Lawsuits line up
The widespread use of a product with a significant percentage of injuries and eventually with warnings about injuries from use sounds like the formula for a lot of lawsuits. This certainly has happened. A large number of suits—both class actions and individual actions—were filed as a result of mesh injuries.24 These suits were overwhelmingly against the manufacturer, although some included physicians.7 Device makers are more attractive defendants for several reasons. First, they have very deep pockets. In addition, jurors are generally much less sympathetic to large companies than to doctors. Large class actions meant that there were many different patients among the plaintiffs, and medical malpractice claims in most states have a number of trial difficulties not present in other product liability cases. Common defendants have included Johnson & Johnson, Boston Scientific, and Medtronic.
Some of the cases resulted in very large damage awards against manufacturers based on various kinds of product(s) liability. Many other cases were settled or tried with relatively small damages. There were, in addition, a number of instances in which the manufacturers were not liable. Of the 32 plaintiffs who have gone to trial thus far, 24 have obtained verdicts totaling $345 million ($14 million average). The cases that have settled have been for much less—perhaps $60,000 on average. A number of cases remain unresolved. To date, the estimate is that 100,000 women have received almost $8 billion from 7 device manufacturers to resolve claims.25
Some state attorneys general have gotten into the process as well. Attorneys general from California, Kentucky, Mississippi, and Washington have filed lawsuits against Johnson & Johnson, claiming that they deceived doctors and patients about the risks of their pelvic mesh. The states claim that marketing and instructional literature should have contained more information about the risks. Some physicians in these states have expressed concern that these lawsuit risks may do more harm than good because the suits conflate mesh used to treat incontinence with the more risky mesh for POP.26
The “ugly” of class action lawsuits
We have discussed both the sad (the injuries to patients) and the bad (the slow regulatory response and continuing injuries). (The ethics of the marketing by the manufacturers might also be raised as the bad.27) Next, let’s look briefly at the ugly.
Some of the patients affected by mesh injuries have been victimized a second time by medical “lenders” and some of their attorneys. Press reports describe patients with modest awards paying 40% in attorney fees (on the high side for personal injury settlements) plus extravagant costs—leaving modest amounts of actual recovery.25
Worse still, a process of “medical lending” has arisen in mesh cases.28 Medical lenders may contact mesh victims offering to pay up front for surgery to remove mesh, and then place a lien against the settlement for repayment at a much higher rate. They might pay the surgeon $2,500 for the surgery, but place a lien on the settlement amount for $60,000.29,30 In addition, there are allegations that lawyers may recruit the doctors to overstate the injuries or do unnecessary removal surgery because that will likely up the award.31 A quick Google search indicates dozens of offers of cash now for your mesh lawsuit (transvaginal and hernia repair).
The patient in our hypothetical case at the beginning had a fairly typical experience. She was a member of a class filing and received a modest settlement. The attorneys representing the class were allowed by the court to charge substantial attorneys’ fees and costs. The patient had the good sense to avoid medical lenders, although other members of the class did use medical lenders and are now filing complaints about the way they were treated by these lenders.
- Maintain surgical skills and be open to new technology. Medical practice requires constant updating and use of new and improved technology as it comes along. By definition, new technology often requires new skills and understanding. A significant portion of surgeons using mesh indicated that they had not read the instructions for use, or had done so only once.1 CME programs that include surgical education remain of particular value.
- Whether new technology or old, it is essential to keep up to date on all FDA bulletins pertinent to devices and pharmaceuticals that you use and prescribe. For example, in 2016 and 2018 the FDA warned that the use of a very old class of drugs (fluoroquinolones) should be limited. It advised "that the serious side effects associated with fluoroquinolones generally outweigh the benefits for patients with acute sinusitis, acute bronchitis, and uncomplicated urinary tract infections who have other treatment options. For patients with these conditions, fluoroquinolones should be reserved for those who do not have alternative treatment options."2 Continued, unnecessary prescriptions for fluoroquinolones would put a physician at some legal risk whether or not the physician had paid any attention to the warning.
- Informed consent is a very important legal and medical process. Take it seriously, and make sure the patient has the information necessary to make informed decisions about treatment. Document the process and the information provided. In some cases consider directing patients to appropriate literature or websites of the manufacturers.
- As to the use of mesh, if not following FDA advice, it is important to document the reason for this and to document the informed consent especially carefully.
- Follow patients after mesh placement for a minimum of 1 year and emphasize to patients they should convey signs and symptoms of complications from initial placement.3 High-risk patients should be of particular concern and be monitored very closely.
References
- Kirkpatrick G, Faber KD, Fromer DL. Transvaginal mesh placement and the instructions for use: a survey of North American urologists. J Urol. https://doi.org/10.1016/j.urpr.2018.05.004.
- FDA Drug Safety Communication: FDA advises restricting fluoroquinolone antibiotic use for certain uncomplicated infections; warns about disabling side effects that can occur together. July 26, 2016. https://www.fda.gov/Drugs/DrugSafety/ucm500143.htm. Accessed June 19, 2019.
- Karlovsky ME. How to avoid and deal with pelvic mesh litigation. Curr Urol Rep. 2016;17:55.
- Maral I, Ozkardeş H, Peşkircioğlu L, et al. Prevalence of stress urinary incontinence in both sexes at or after age 15 years: a cross-sectional study. J Urol. 2001;165:408-412.
- Olsen AL, Smith VJ, Bergstrom JO, et al. Epidemiology of surgically managed pelvic organ prolapse and urinary incontinence. Obstet Gynecol. 1997;89:501-506.
- Chang J, Lee D. Midurethral slings in the mesh litigation era. Transl Androl Urol. 2017;6(suppl 2): S68-S75.
- Mattingly R, ed. TeLinde's Operative Gynecology, 5th edition. Lippincott, William, and Wilkins: Philadelphia, PA; 1997.
- Burch J. Urethrovaginal fixation to Cooper's ligament for correction of stress incontinence, cystocele, and prolapse. Am J Obstet Gynecol. 1961;81:281-290.
- Ulmsten U, Falconer C, Johnson P, et al. A multicenter study of tension-free vaginal tape (TVT) for surgical treatment of stress urinary incontinence. Int Urogynecol J Pelvic Floor Dysfunct. 1998;9:210-213.
- Kuhlmann-Capek MJ, Kilic GS, Shah AB, et al. Enmeshed in controversy: use of vaginal mesh in the current medicolegal environment. Female Pelvic Med Reconstr Surg. 2015;21:241-243.
- Powell SF. Changing our minds: reforming the FDA medical device reclassification process. Food Drug Law J. 2018;73:177-209.
- US Food and Drug Administration. Surgical Mesh for Treatment of Women with Pelvic Organ Prolapse and Stress Urinary Incontinence. September 2011. https://www.thesenatorsfirm.com/documents/OBS.pdf. Accessed June 19, 2019.
- Maher C, Feiner B, Baessler K, et al. Surgical management of pelvic organ prolapse in women. Cochrane Database Syst Rev. 2013;(4):CD004014.
- Ganj FA, Ibeanu OA, Bedestani A, Nolan TE, Chesson RR. Complications of transvaginal monofilament polypropylene mesh in pelvic organ prolapse repair. Int Urogynecol J Pelvic Floor Dysfunct. 2009;20:919-925.
- Sung VW, Rogers RG, Schaffer JI, et al. Graft use in transvaginal pelvic organ prolapse repair: a systematic review. Obstet Gynecol. 2008;112:1131-1142.
- FDA public health notification: serious complications associated with transvaginal placement of surgical mesh in repair of pelvic organ prolapse and stress urinary incontinence. October 20, 2008. http://www.fda.gov/MedicalDevices/Safety/AlertsandNotices/PublicHealthNotifications/ucm061976.htm. Accessed February 14, 2019.
- Riegel v. Medtronic, 552 U.S. 312 (2008).
- Whitney DW. Guide to preemption of state-law claims against Class III PMA medical devices. Food Drug Law J. 2010;65:113-139.
- Alam P, Iglesia CB. Informed consent for reconstructive pelvic surgery. Obstet Gynecol Clin North Am. 2016;43:131-139.
- Nosti PA, Iglesia CB. Medicolegal issues surrounding devices and mesh for surgical treatment of prolapse and incontinence. Clin Obstet Gynecol. 2013;56:221-228.
- Shepherd CG. Transvaginal mesh litigation: a new opportunity to resolve mass medical device failure claims. Tennessee Law Rev. 2012;80:3:477-94.
- Karlovsky ME. How to avoid and deal with pelvic mesh litigation. Curr Urol Rep. 2016;17:55.
- Cohn JA, Timbrook Brown E, Kowalik CG, et al. The mesh controversy. F1000Research website. https://f1000research.com/articles/5-2423/v1. Accessed June 17, 2019.
- Obstetrics and Gynecology Devices Panel Meeting, February 12, 2019. US Food and Drug Administration website. https://www.fda.gov/media/122867/download. Accessed June 19, 2019.
- Mucowski SJ, Jurnalov C, Phelps JY. Use of vaginal mesh in the face of recent FDA warnings and litigation. Am J Obstet Gynecol. 2010;203:103.e1-e4.
- American College of Obstetricians and Gynecologists Committee on Ethics. ACOG Committee Opinion No. 439: informed consent. Obstet Gynecol. 2009;114(2 pt 1):401-408.
- Souders CP, Eilber KS, McClelland L, et al. The truth behind transvaginal mesh litigation: devices, timelines, and provider characteristics. Female Pelvic Med Reconstr Surg. 2018;24:21-25.
- Goldstein M. As pelvic mesh settlements near $8 billion, women question lawyers' fees. New York Times. February 1, 2019. https://www.nytimes.com/2019/02/01/business/pelvic-mesh-settlements-lawyers.html. Accessed June 19, 2019.
- Johnson G. Surgeons fear pelvic mesh lawsuits will spook patients. Associated Press News. January 10, 2019. https://www.apnews.com/25777c3c33e3489283b1dc2ebdde6b55. Accessed June 19, 2019.
- Clarke RN. Medical device marketing and the ethics of vaginal mesh kit marketing. In The Innovation and Evolution of Medical Devices. New York, NY: Springer; 2019:103-123.
- Top 5 drug and medical device developments of 2018. Law 360. January 1, 2019. Accessed through LexisNexis.
- Frankel A, Dye J. The Lien Machine. New breed of investor profits by financing surgeries for desperate women patients. Reuters. August 18, 2015. https://www.reuters.com/investigates/special-report/usa-litigation-mesh/. Accessed June 19, 2019.
- Sullivan T. New report looks at intersection of "medical lending" and pelvic mesh lawsuits. Policy & Medicine. May 5, 2018. https://www.policymed.com/2015/08/medical-lending-and-pelvic-mesh-litigation.html. Accessed June 19, 2019.
- Goldstein M, Sliver-Greensberg J. How profiteers lure women into often-unneeded surgery. New York Times. April 14, 2018. https://www.nytimes.com/2018/04/14/business/vaginal-mesh-surgery-lawsuits-financing.html. Accessed June 19, 2019.
- Maral I, Ozkardeş H, Peşkircioğlu L, et al. Prevalence of stress urinary incontinence in both sexes at or after age 15 years: a cross-sectional study. J Urol. 2001;165:408-412.
- Olsen AL, Smith VJ, Bergstrom JO, et al. Epidemiology of surgically managed pelvic organ prolapse and urinary incontinence. Obstet Gynecol. 1997;89:501-506.
- Chang J, Lee D. Midurethral slings in the mesh litigation era. Transl Androl Urol. 2017;6(suppl 2): S68-S75.
- Mattingly R, ed. TeLinde's Operative Gynecology, 5th edition. Lippincott, William, and Wilkins: Philadelphia, PA; 1997.
- Burch J. Urethrovaginal fixation to Cooper's ligament for correction of stress incontinence, cystocele, and prolapse. Am J Obstet Gynecol. 1961;81:281-290.
- Ulmsten U, Falconer C, Johnson P, et al. A multicenter study of tension-free vaginal tape (TVT) for surgical treatment of stress urinary incontinence. Int Urogynecol J Pelvic Floor Dysfunct. 1998;9:210-213.
- Kuhlmann-Capek MJ, Kilic GS, Shah AB, et al. Enmeshed in controversy: use of vaginal mesh in the current medicolegal environment. Female Pelvic Med Reconstr Surg. 2015;21:241-243.
- Powell SF. Changing our minds: reforming the FDA medical device reclassification process. Food Drug Law J. 2018;73:177-209.
- US Food and Drug Administration. Surgical Mesh for Treatment of Women with Pelvic Organ Prolapse and Stress Urinary Incontinence. September 2011. https://www.thesenatorsfirm.com/documents/OBS.pdf. Accessed June 19, 2019.
- Maher C, Feiner B, Baessler K, et al. Surgical management of pelvic organ prolapse in women. Cochrane Database Syst Rev. 2013;(4):CD004014.
- Ganj FA, Ibeanu OA, Bedestani A, Nolan TE, Chesson RR. Complications of transvaginal monofilament polypropylene mesh in pelvic organ prolapse repair. Int Urogynecol J Pelvic Floor Dysfunct. 2009;20:919-925.
- Sung VW, Rogers RG, Schaffer JI, et al. Graft use in transvaginal pelvic organ prolapse repair: a systematic review. Obstet Gynecol. 2008;112:1131-1142.
- FDA public health notification: serious complications associated with transvaginal placement of surgical mesh in repair of pelvic organ prolapse and stress urinary incontinence. October 20, 2008. http://www.fda.gov/MedicalDevices/Safety/AlertsandNotices/PublicHealthNotifications/ucm061976.htm. Accessed February 14, 2019.
- Riegel v. Medtronic, 552 U.S. 312 (2008).
- Whitney DW. Guide to preemption of state-law claims against Class III PMA medical devices. Food Drug Law J. 2010;65:113-139.
- Alam P, Iglesia CB. Informed consent for reconstructive pelvic surgery. Obstet Gynecol Clin North Am. 2016;43:131-139.
- Nosti PA, Iglesia CB. Medicolegal issues surrounding devices and mesh for surgical treatment of prolapse and incontinence. Clin Obstet Gynecol. 2013;56:221-228.
- Shepherd CG. Transvaginal mesh litigation: a new opportunity to resolve mass medical device failure claims. Tennessee Law Rev. 2012;80:3:477-94.
- Karlovsky ME. How to avoid and deal with pelvic mesh litigation. Curr Urol Rep. 2016;17:55.
- Cohn JA, Timbrook Brown E, Kowalik CG, et al. The mesh controversy. F1000Research website. https://f1000research.com/articles/5-2423/v1. Accessed June 17, 2019.
- Obstetrics and Gynecology Devices Panel Meeting, February 12, 2019. US Food and Drug Administration website. https://www.fda.gov/media/122867/download. Accessed June 19, 2019.
- Mucowski SJ, Jurnalov C, Phelps JY. Use of vaginal mesh in the face of recent FDA warnings and litigation. Am J Obstet Gynecol. 2010;203:103.e1-e4.
- American College of Obstetricians and Gynecologists Committee on Ethics. ACOG Committee Opinion No. 439: informed consent. Obstet Gynecol. 2009;114(2 pt 1):401-408.
- Souders CP, Eilber KS, McClelland L, et al. The truth behind transvaginal mesh litigation: devices, timelines, and provider characteristics. Female Pelvic Med Reconstr Surg. 2018;24:21-25.
- Goldstein M. As pelvic mesh settlements near $8 billion, women question lawyers' fees. New York Times. February 1, 2019. https://www.nytimes.com/2019/02/01/business/pelvic-mesh-settlements-lawyers.html. Accessed June 19, 2019.
- Johnson G. Surgeons fear pelvic mesh lawsuits will spook patients. Associated Press News. January 10, 2019. https://www.apnews.com/25777c3c33e3489283b1dc2ebdde6b55. Accessed June 19, 2019.
- Clarke RN. Medical device marketing and the ethics of vaginal mesh kit marketing. In The Innovation and Evolution of Medical Devices. New York, NY: Springer; 2019:103-123.
- Top 5 drug and medical device developments of 2018. Law 360. January 1, 2019. Accessed through LexisNexis.
- Frankel A, Dye J. The Lien Machine. New breed of investor profits by financing surgeries for desperate women patients. Reuters. August 18, 2015. https://www.reuters.com/investigates/special-report/usa-litigation-mesh/. Accessed June 19, 2019.
- Sullivan T. New report looks at intersection of "medical lending" and pelvic mesh lawsuits. Policy & Medicine. May 5, 2018. https://www.policymed.com/2015/08/medical-lending-and-pelvic-mesh-litigation.html. Accessed June 19, 2019.
- Goldstein M, Sliver-Greensberg J. How profiteers lure women into often-unneeded surgery. New York Times. April 14, 2018. https://www.nytimes.com/2018/04/14/business/vaginal-mesh-surgery-lawsuits-financing.html. Accessed June 19, 2019.
Pregnancy test missed before IUD placement? Your liability.
CASE: Gynecologist accused of placing an IUD without performing a pregnancy test
A 34-year-old woman (G4 P3013) presents to her gynecologist for planned placement of the Mirena Intrauterine System (Bayer HealthCare). She was divorced 2 months ago and is interested in birth control. She smokes 1.5 packs per day, and her history includes irregular menses, an earlier Pap smear result of atypical squamous cells of undetermined significance (ASCUS) with negative colposcopy results, polycystic ovary syndrome, obesity, migraine headaches with aura, bilateral carpel tunnel surgery, and a herniated L4.5 disc treated conservatively. She has no history of any psychiatric problems.
One week before intrauterine device (IUD) placement, she discussed the options with her gynecologist and received a Mirena patient brochure. At the office visit for IUD placement, the patient stated she had a negative home pregnancy test 1 week earlier. She did not tell the gynecologist that she had taken Plan B One-Step (levonorgestrel, 1.5 mg) emergency contraception 2 weeks prior to presenting to her gynecologist after receiving it from a Planned Parenthood office following condom breakage during coitus. IUD placement was uncomplicated.
After noting spotting several weeks later, she contacted her gynecologist’s office. Results of an office urine pregnancy test were positive; the serum human chorionic gonadotropin (hCG) level was reported at 65,000 mIU/mL.The results of a pelvic sonogram showed a 12 5/7-week intrauterine gestation. The gynecologist unsuccessfully tried to remove the IUD. Options for termination or continuation of the pregnancy were discussed. The patient felt the gynecologist strongly encouraged, “almost insisting on,” termination. Termination could not be performed locally as her state laws did not allow second trimester abortion; the gynecologist provided out-of-state clinic options.
The patient aborted the pregnancy in a neighboring state. She was opposed to the termination but decided it was not a good time for her to have a baby. She felt the staff at the facility were “cold” and had a “we got to get this done attitude.” As she left the clinic, she saw people picketing outside and found the whole process “psychologically traumatic.” When bleeding persisted, she sought care from another gynecologist. Pelvic sonography results showed retained products of conception (POC). The new gynecologist performed operative hysteroscopy to remove the POC. The patient became depressed and felt as if she was a victim of pain and suffering.
The patient’s attorney filed a medical malpractice claim against the gynecologist who inserted the IUD, accusing her of negligence for not performing a pregnancy test immediately before IUD insertion.
In a deposition, the patient stated she bought the home pregnancy test in a “dollar store” and was worried about its accuracy, but never told the gynecologist. Conception probably occurred 2 weeks prior to IUD insertion, correlating with the broken condom and taking of Plan B. She did not think the gynecologist needed to know this as it “would not have made any difference in her care.”
The gynecologist confirmed that the patient’s record included “Patient stated ‘pregnancy test negative within 1 week of IUD placement.’” The gynecologist did not feel that obtaining the date of the patient’s last menstrual period (LMP) was required since she asked if the patient had protected coitus since her LMP and the patient answered yes. The gynecologist thought that if a pregnancy were in utero, Mirena placement would prevent implantation. She believed that she had obtained proper informed consent and that the patient acknowledged receiving and reading the Mirena patient information prior to placement. The gynecologist stated she also provided other birth control options.
The patient’s expert witness testified that the gynecologist fell below the standard of care by not obtaining a pregnancy test prior to IUD insertion.
The gynecologist’s expert witness argued that the patient told the gynecologist that she did not have unprotected coitus. The patient herself withheld information from the gynecologist that she had taken Plan B due to condom breakage. The physician’s attorney also noted that the pelvic exam at time of IUD placement was normal.
What’s the verdict?
The patient has a fairly good case. The gynecologist may not have been sufficiently careful, given all of the facts in this case, to ensure that the patient was not pregnant. An expert is testifying that this fell below the acceptable level of care in the profession. At the same time, the failure of the patient to reveal some information may result in reduced damages through “comparative negligence.” Because there will be several questions of fact for a jury to decide, as well as some emotional elements in this case, the outcome of a trial is uncertain. This suggests that a negotiated settlement before trial should be considered.
Read about medical considerations of a pregnancy with an IUD.
Medical considerations
First, some background information on Mirena.
Indications for Mirena
Here are indications for Mirena1:
- intrauterine contraception for up to 5 years
- treatment of heavy menstrual bleeding for women who choose to use intrauterine contraception as their method of contraception.
Prior to insertion, the following are recommended2:
- a complete medical and social history should be obtained to determine conditions that might influence the selection of a levonorgestrel-releasing intrauterine system (LNG IUS) for contraception
- if indicated, perform a physical examination, and appropriate tests for any forms of genital or other sexually transmitted infections
- there is no requirement for prepregnancy test.
Contraindications for Mirena
Contraindications for Mirena include2:
- pregnancy or suspicion of pregnancy; cannot be used for postcoital contraception
- congenital or acquired uterine anomaly including fibroids if they distort the uterine cavity
- acute pelvic inflammatory disease or a history of pelvic inflammatory disease unless there has been a subsequent intrauterine pregnancy
- postpartum endometritis or infected abortion in the past 3 months
- known or suspected uterine or cervical neoplasia
- known or suspected breast cancer or other progestin-sensitive cancer, now or in the past
- uterine bleeding of unknown etiology
- untreated acute cervicitis or vaginitis, including bacterial vaginosis or other lower genital tract infections until infection is controlled
- acute liver disease or liver tumor (benign or malignant)
- conditions associated with increased susceptibility to pelvic infections
- a previously inserted IUD that has not been removed
- hypersensitivity to any component of this product.
Is Mirena a postcoital contraceptive?
The American College of Obstetricians and Gynecologists (ACOG) bulletin on long-acting reversible contraception states “the levonorgestrel intrauterine system has not been studied for emergency contraception.”3 Ongoing studies are comparing the levonor‑gestrel IUD to the copper IUD for emergency contraception.4
Related Article:
Webcast: Emergency contraception: How to choose the right one for your patient
Accuracy of home pregnancy tests
Although the first home pregnancy test was introduced in 1976,5 there are now several home pregnancy tests available over the counter, most designed to detect urinary levels of hCG at ≥25 mIU/mL. The tests identify hCG, hyperglycosylated hCG, and free Betasubunit hCG in urine. When Cole and colleagues evaluated the validity of urinary tests including assessment of 18 brands, results noted that sensitivity of 12.4 mIU/mL of hCG detected 95% of pregnancies at time of missed menses.6 Some brands required 100 mIU/mL levels of hCG for positive results. The authors concluded “the utility of home pregnancy tests is questioned.”6 For urinary levels of hCG, see TABLE.
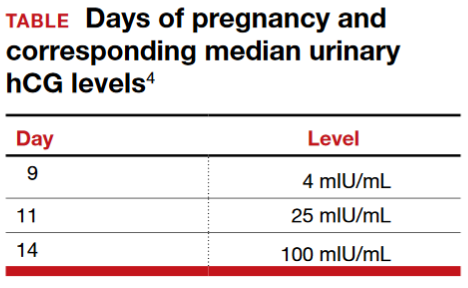
Pregnancy with an IUD
The gynecologist’s concern about pregnancy when an IUD is inserted was valid.
With regard to pregnancy with Mirena in place, the full prescribing information states2:
Intrauterine Pregnancy: If pregnancy occurs while using Mirena, remove Mirena because leaving it in place may increase the risk of spontaneous abortion and preterm labor. Removal of Mirena or probing of the uterus may also result in spontaneous abortion. In the event of an intrauterine pregnancy with Mirena, consider the following:
Septic abortion
In patients becoming pregnant with an IUD in place, septic abortion - with septicemia, septic shock, and death may occur.
Continuation of pregnancy
If a woman becomes pregnant with Mirena in place and if Mirena cannot be removed or the woman chooses not to have it removed, warn her that failure to remove Mirena increases the risk of miscarriage, sepsis, premature labor and premature delivery. Follow her pregnancy closely and advise her to report immediately any symptom that suggests complications of the pregnancy.
Concern for microbial invasion of the amniotic cavity must be considered. Kim and colleagues addressed pregnancy prognosis with an IUD in situ in a retrospective study of 12,297 pregnancies; 196 had an IUD with singleton gestation.7 The study revealed a higher incidence of histologic chorioamnionitis and/or funisitis when compared with those without an IUD (54.2% vs 14.7%, respectively; P<.001). The authors concluded that pregnant women with an IUD in utero are at very high risk for adverse pregnancy outcomes. Brahmi and colleagues8 reported similar risks with higher incidence of spontaneous abortion, preterm delivery, and septic abortion.
Related Article:
Overcoming LARC complications: 7 case challenges
Efficacy and safety concerns with emergency contraception
The efficacy and safety of emergency contraception using levonorgestrel oral tablets (Plan B One-Step; Duramed Pharmaceuticals) is another concern. Plan B One-Step should be taken orally as soon as possible within 72 hours after unprotected intercourse or a known or suspected contraceptive failure. Efficacy is better if Plan B is taken as soon as possible after unprotected intercourse. There are 2 dosages: 1 tablet of levonorgestrel 1.5 mg or 2 tablets of levonorgestrel 0.75 mg. The second 0.75-mg tablet should be taken 12 hours after the first dose.9
Plan B can be used at any time during the menstrual cycle. In a series of 2,445 women aged 15 to 48 years who took levonorgestrel tablets for emergency contraception (Phase IV clinical trial), 5 pregnancies occurred (0.2%).10
ACOG advises that emergency contraception using a pill or the copper IUD should be initiated as soon as possible (up to 5 days) after unprotected coitus or inadequately protected coitus.9
Retained products of contraception
ACOG Practice Bulletin No. 135 on complications associated with second trimesterabortion discusses retained POC.11 The approach to second trimester abortion includes dilation and evacuation (D&E) as well as medical therapy with mifepristone and misoprostol. D&E, a safe and effective approach with advantages over medical abortion, is associated with fewer complications (up to 4%) versus medical abortion (29%); the primary complication is retained POC (placenta).11
Read about the legal considerations of this case.
Legal considerations
The malpractice lawsuit filed in this case claims that the gynecologist failed to exercise the level of care of a reasonably prudent practitioner under the circumstances and was therefore negligent or in breach of a duty to the patient.
First, a lawyer would look for a medical error that was related to some harm. Keep in mind that not all medical errors are negligent or subject to liability. Many medical errors occur even though the physician has exercised all reasonable care and engaged in sound practice, given today’s medical knowledge and facilities. When harm is caused through medical error that was careless or otherwise does not meet the standard of care, financial recovery is possible for the patient through a malpractice claim.12
In this case, the expert witnesses’ statements focus on the issue of conducting a pregnancy test prior to IUD insertion. The patient’s expert testified that failure to perform a pregnancy test was below an acceptable standard of care. That opinion may have been based on the typical practice of gynecologists, widely accepted medical text books, and formal practice standards of professional organizations.13
Cost-benefit analysis. Additional support for the claim that not performing the pregnancy test is negligent comes from applying a cost-benefit analysis. In this analysis, the risks and costs of performing a pregnancy test are compared with the benefits of doing the test.
In this case, the cost of conducting the pregnancy test is very low: essentially risk-freeand relatively inexpensive. On the other hand, the harm that could be avoided would be significant. Kim and colleagues suggest that pregnant women with an IUD in utero are at very high risk for adverse pregnancy outcomes.7 Given that women receiving IUDs are candidates for pregnancy (and perhaps do not know they are pregnant), a simple, risk-free pregnancy test would seem to be an efficient way to avoid a nontrivial harm.14
Did she have unprotected sex? The gynecologist’s expert notes that the patient told the gynecologist that she did not have unprotected coitus. Furthermore, the patient withheld from the gynecologist the information that she had taken Plan B because of a broken condom. Is this a defense against the malpractice claim? The answer is “possibly no,” or “possibly somewhat.”
As for unprotected coitus, the patient could easily have misunderstood the question. Technically, the answer “no” was correct. She had not had unprotected sex—it is just that the protection (condom) failed. It does not appear from the facts that she disclosed or was asked about Plan B or other information related to possible failed contraception. As to whether the patient’s failure to provide that information could be a defense for the physician, the best answer is “possibly” and “somewhat.” (See below.)15
Withholding information. Patients, of course, have a responsibility to inform their physicians of information they know is relevant. Many patients, however, will not know what is relevant (or why), or will not be fully disclosing.
Professionals cannot ignore the fact that their patients and clients are often confused, do not understand what is important and relevant, and cannot always be relied upon. For that very reason, professionals generally are obliged to start with the proposition that they may not have all of the relevant information. In this case, this lack of information makes the cost-calculation of performing a pregnancy test that much more important. The risk of not knowing whether a patient is pregnant includes the fact that many patients just will not know or cannot say with assurance.16
A “somewhat” defense and comparative negligence
Earlier we referred to a “somewhat” defense. Almost all states now have some form of “comparative negligence,” meaning that the patient’s recovery is reduced by the proportion of the blame (negligence) that is attributed to the patient. The most common form of comparative negligence works this way: If there are damages of $100,000, and the jury finds that the fault is 20% the patient’s and 80% the physician’s, the patient would receive $80,000 recovery. (In the past, the concept of “contributory negligence” could result in the plaintiff being precluded from any recovery if the plaintiff was partially negligent—those days are mostly gone.)
Related Article:
Informed consent: The more you know, the more you and your patient are protected
Statement of risks, informed consent, and liability
The gynecologist must provide an adequate description of the IUD risks. The case facts indicate that appropriate risks were discussed and literature provided, so it appears there was probably appropriate informed consent in this case. If not true, this would provide another basis for recovery.
Two other aspects of this case could be the basis for liability. We can assume that the attempted removal of the IUD was performed competently.16 In addition, if the IUD was defective in terms of design, manufacture, or warnings, the manufacturer of the device could be subject to liability.17
Final verdict: Out of court settlement
Why would the gynecologist and the insurance company settle this case? After all, they have some arguments on their side, and physicians win the majority of malpractice cases that go to trial.18 On the other hand, the patient’s expert witness’ testimony and the cost-benefit analysis of the pregnancy test are strong, contrary claims.
Cases are settled for a variety of reasons. Litigation is inherently risky. In this case, we assume that the court denied a motion to dismiss the case before trial because there is a legitimate question of fact concerning what a reasonably prudent gynecologist would have done under the circumstances. That means a jury would probably decide the issue of medical judgment, which is generally disconcerting. Furthermore, the comparative negligence defense that the patient did not tell the gynecologist about the failed condom/Plan B would most likely reduce the amount of damages, but not eliminate liability. The questions regarding the pressure to terminate a second trimester pregnancy might well complicate a jury’s view.
Other considerations include the high costs in time, money, uncertainty, and disruption associated with litigation. The settlement amount was not stated, but the process of negotiating a settlement would allow factoring in the comparative negligence aspect of the case. It would be reasonable for this case to settle before trial.
Should the physician have apologized before trial? The gynecologist could have sent a statement of regret or apology to the patient before a lawsuit was filed. Most states now have statutes that preclude such statements of regret or apology from being used against the physician. Many experts now favor apology statements as a way to reduce the risk of malpractice suits being filed.19
Related Article:
Medical errors: Meeting ethical obligations and reducing liability with proper communication
Defensive medicine. There has been much discussion of “defensive medicine” in recent years.20 It is appropriately criticized when additional testing is solely used to protect the physician from liability. However, much of defensive medicine is not only to protect the physician but also to protect the patient from potential physical and mental harm. In this case, it would have been “careful medicine” in addition to “defensive medicine.”
Share your thoughts! Send your Letter to the Editor to rbarbieri@frontlinemedcom.com. Please include your name and the city and state in which you practice.
- Heikinheimo O, Gemell-Danielsson K. Emerging indications for the levonorgestrel-releasing intrauterine system. ACTA Obstet Gynecol Scand. 2012;91(1):3–9.
- Mirena [prescribing information]. Whippany, NJ: Bayer HealthCare Pharmaceuticals Inc; 2000.
- American College of Obstetricians and Gynecologists. Practice Bulletin No. 121: Long-acting reversible contraception: Implants and intrauterine devices. Obstet Gynecol. 2011;118(1):184–196.
- Rapid EC–Random clinical trial assessing pregnancy with intrauterine devices for emergency contraception. Clinical Trials Identifier: NCT02175030. https://www.clinicaltrials.gov/ct2/show/NCT02175030?term=NCT02175030&rank=1. Updated May 1, 2017. Accessed May 11, 2017.
- Gnoth C, Johnson S. Strips of hope: Accuracy of home pregnancy tests and new developments. Gerburtshilfe Frauenheilkd. 2014;74(7):661–669.
- Cole LA, Khanlian SA, Sutton JM, Davies S, Rayburn WF. Accuracy of home pregnancy tests at the time of missed menses. Am J Obstet Gynecol. 2004;190(1):100–105.
- Kim S, Romero R, Kusanovic J, et al. The prognosis of pregnancy conceived despite the presence of an intrauterine device (IUD). J Perinatal Med. 2010;38(1):45–53.
- Brahmi D, Steenland M, Renner R, Gaffield M, Curtis K. Pregnancy outcomes with an IUD in situ: a systematic review. Contraception. 2012;85(2):131–139.
- American College of Obstetricians and Gynecologists. Practice Bulletin No. 152: Emergency contraception. Obstet Gynecol. 2015;126(3):685–686.
- Chen Q, Xiang W, Zhang D, et al. Efficacy and safety of a levonorgestrel enteric-coated tablet as an over-the-counter drug for emergency contraception: a Phase IV clinical trial. Hum Reprod. 2011;26(9):2316–2321.
- American College of Obstetricians and Gynecologists. Practice Bulletin No. 135: Second-trimester abortion. Obstet Gynecol. 2013;121(6):1395–1406.
- White A, Pichert J, Bledsoe S, Irwin C, Entman S. Cause and effect analysis of closed claims in obstetrics and gynecology. Obstet Gynecol. 2005;105(5 pt1):1031–1038.
- Mehlman M. Professional power and the standard of care in medicine. Case Western Reserve University Scholarly Commons. 2012: Paper 574. http://scholarlycommons.law.case.edu/cgi/viewcontent.cgi?article=1576&context=faculty_publications. Accessed May 11, 2017.
- Klein D, Arnold J, Reese E. Provision of contraception: key recommendations from the CDC. Am Fam Physician. 2015;91(9);625–633.
- Reyes J, Reyes R. The effects of malpractice liability on obstetrics and gynecology: taking the measure of a crisis. N England Law Rev. 2012;47;315–348. https://www.scribd.com/document/136514285/Reyes-Reyes-The-Effect-of-Malpractice-Liability-on-Obstetrics-and -Gynecology#fullscreen&from_embed. Accessed May 11, 2017.
- Peckham C. Medscape Malpractice Report 2015: Why Ob/Gyns get sued. http://www.medscape.com/features/slideshow/malpractice-report-2015/obgyn#page=1. Published January 22, 2016. Accessed May 11. 2017.
- Rheingold P, Paris D. Contraceptives. In: Vargo JJ, ed. Products Liability Practice Guide New York, New York: Matthew Bender & Company; 2017;C:62.
- Jena AB, Chandra A, Lakdawalla D, Seabury S. Outcomes of medical malpractice litigation against US physicians. Arch Intern Med. 2012;172(11):892–894.
- Helmreich JS. Does sorry incriminate? Evidence, harm and the protection of apology. Cornell J Law Public Policy. 2012;21(3);567–609. http://scholarship.law.cornell.edu/cgi/viewcontent.cgi?article=1363&context=cjlpp.
- Baicker K, Wright B, Olson N. Reevaluating reports of defensive medicine. J Health Polit Policy Law. 2015;40(6);1157–1177.
CASE: Gynecologist accused of placing an IUD without performing a pregnancy test
A 34-year-old woman (G4 P3013) presents to her gynecologist for planned placement of the Mirena Intrauterine System (Bayer HealthCare). She was divorced 2 months ago and is interested in birth control. She smokes 1.5 packs per day, and her history includes irregular menses, an earlier Pap smear result of atypical squamous cells of undetermined significance (ASCUS) with negative colposcopy results, polycystic ovary syndrome, obesity, migraine headaches with aura, bilateral carpel tunnel surgery, and a herniated L4.5 disc treated conservatively. She has no history of any psychiatric problems.
One week before intrauterine device (IUD) placement, she discussed the options with her gynecologist and received a Mirena patient brochure. At the office visit for IUD placement, the patient stated she had a negative home pregnancy test 1 week earlier. She did not tell the gynecologist that she had taken Plan B One-Step (levonorgestrel, 1.5 mg) emergency contraception 2 weeks prior to presenting to her gynecologist after receiving it from a Planned Parenthood office following condom breakage during coitus. IUD placement was uncomplicated.
After noting spotting several weeks later, she contacted her gynecologist’s office. Results of an office urine pregnancy test were positive; the serum human chorionic gonadotropin (hCG) level was reported at 65,000 mIU/mL.The results of a pelvic sonogram showed a 12 5/7-week intrauterine gestation. The gynecologist unsuccessfully tried to remove the IUD. Options for termination or continuation of the pregnancy were discussed. The patient felt the gynecologist strongly encouraged, “almost insisting on,” termination. Termination could not be performed locally as her state laws did not allow second trimester abortion; the gynecologist provided out-of-state clinic options.
The patient aborted the pregnancy in a neighboring state. She was opposed to the termination but decided it was not a good time for her to have a baby. She felt the staff at the facility were “cold” and had a “we got to get this done attitude.” As she left the clinic, she saw people picketing outside and found the whole process “psychologically traumatic.” When bleeding persisted, she sought care from another gynecologist. Pelvic sonography results showed retained products of conception (POC). The new gynecologist performed operative hysteroscopy to remove the POC. The patient became depressed and felt as if she was a victim of pain and suffering.
The patient’s attorney filed a medical malpractice claim against the gynecologist who inserted the IUD, accusing her of negligence for not performing a pregnancy test immediately before IUD insertion.
In a deposition, the patient stated she bought the home pregnancy test in a “dollar store” and was worried about its accuracy, but never told the gynecologist. Conception probably occurred 2 weeks prior to IUD insertion, correlating with the broken condom and taking of Plan B. She did not think the gynecologist needed to know this as it “would not have made any difference in her care.”
The gynecologist confirmed that the patient’s record included “Patient stated ‘pregnancy test negative within 1 week of IUD placement.’” The gynecologist did not feel that obtaining the date of the patient’s last menstrual period (LMP) was required since she asked if the patient had protected coitus since her LMP and the patient answered yes. The gynecologist thought that if a pregnancy were in utero, Mirena placement would prevent implantation. She believed that she had obtained proper informed consent and that the patient acknowledged receiving and reading the Mirena patient information prior to placement. The gynecologist stated she also provided other birth control options.
The patient’s expert witness testified that the gynecologist fell below the standard of care by not obtaining a pregnancy test prior to IUD insertion.
The gynecologist’s expert witness argued that the patient told the gynecologist that she did not have unprotected coitus. The patient herself withheld information from the gynecologist that she had taken Plan B due to condom breakage. The physician’s attorney also noted that the pelvic exam at time of IUD placement was normal.
What’s the verdict?
The patient has a fairly good case. The gynecologist may not have been sufficiently careful, given all of the facts in this case, to ensure that the patient was not pregnant. An expert is testifying that this fell below the acceptable level of care in the profession. At the same time, the failure of the patient to reveal some information may result in reduced damages through “comparative negligence.” Because there will be several questions of fact for a jury to decide, as well as some emotional elements in this case, the outcome of a trial is uncertain. This suggests that a negotiated settlement before trial should be considered.
Read about medical considerations of a pregnancy with an IUD.
Medical considerations
First, some background information on Mirena.
Indications for Mirena
Here are indications for Mirena1:
- intrauterine contraception for up to 5 years
- treatment of heavy menstrual bleeding for women who choose to use intrauterine contraception as their method of contraception.
Prior to insertion, the following are recommended2:
- a complete medical and social history should be obtained to determine conditions that might influence the selection of a levonorgestrel-releasing intrauterine system (LNG IUS) for contraception
- if indicated, perform a physical examination, and appropriate tests for any forms of genital or other sexually transmitted infections
- there is no requirement for prepregnancy test.
Contraindications for Mirena
Contraindications for Mirena include2:
- pregnancy or suspicion of pregnancy; cannot be used for postcoital contraception
- congenital or acquired uterine anomaly including fibroids if they distort the uterine cavity
- acute pelvic inflammatory disease or a history of pelvic inflammatory disease unless there has been a subsequent intrauterine pregnancy
- postpartum endometritis or infected abortion in the past 3 months
- known or suspected uterine or cervical neoplasia
- known or suspected breast cancer or other progestin-sensitive cancer, now or in the past
- uterine bleeding of unknown etiology
- untreated acute cervicitis or vaginitis, including bacterial vaginosis or other lower genital tract infections until infection is controlled
- acute liver disease or liver tumor (benign or malignant)
- conditions associated with increased susceptibility to pelvic infections
- a previously inserted IUD that has not been removed
- hypersensitivity to any component of this product.
Is Mirena a postcoital contraceptive?
The American College of Obstetricians and Gynecologists (ACOG) bulletin on long-acting reversible contraception states “the levonorgestrel intrauterine system has not been studied for emergency contraception.”3 Ongoing studies are comparing the levonor‑gestrel IUD to the copper IUD for emergency contraception.4
Related Article:
Webcast: Emergency contraception: How to choose the right one for your patient
Accuracy of home pregnancy tests
Although the first home pregnancy test was introduced in 1976,5 there are now several home pregnancy tests available over the counter, most designed to detect urinary levels of hCG at ≥25 mIU/mL. The tests identify hCG, hyperglycosylated hCG, and free Betasubunit hCG in urine. When Cole and colleagues evaluated the validity of urinary tests including assessment of 18 brands, results noted that sensitivity of 12.4 mIU/mL of hCG detected 95% of pregnancies at time of missed menses.6 Some brands required 100 mIU/mL levels of hCG for positive results. The authors concluded “the utility of home pregnancy tests is questioned.”6 For urinary levels of hCG, see TABLE.

Pregnancy with an IUD
The gynecologist’s concern about pregnancy when an IUD is inserted was valid.
With regard to pregnancy with Mirena in place, the full prescribing information states2:
Intrauterine Pregnancy: If pregnancy occurs while using Mirena, remove Mirena because leaving it in place may increase the risk of spontaneous abortion and preterm labor. Removal of Mirena or probing of the uterus may also result in spontaneous abortion. In the event of an intrauterine pregnancy with Mirena, consider the following:
Septic abortion
In patients becoming pregnant with an IUD in place, septic abortion - with septicemia, septic shock, and death may occur.
Continuation of pregnancy
If a woman becomes pregnant with Mirena in place and if Mirena cannot be removed or the woman chooses not to have it removed, warn her that failure to remove Mirena increases the risk of miscarriage, sepsis, premature labor and premature delivery. Follow her pregnancy closely and advise her to report immediately any symptom that suggests complications of the pregnancy.
Concern for microbial invasion of the amniotic cavity must be considered. Kim and colleagues addressed pregnancy prognosis with an IUD in situ in a retrospective study of 12,297 pregnancies; 196 had an IUD with singleton gestation.7 The study revealed a higher incidence of histologic chorioamnionitis and/or funisitis when compared with those without an IUD (54.2% vs 14.7%, respectively; P<.001). The authors concluded that pregnant women with an IUD in utero are at very high risk for adverse pregnancy outcomes. Brahmi and colleagues8 reported similar risks with higher incidence of spontaneous abortion, preterm delivery, and septic abortion.
Related Article:
Overcoming LARC complications: 7 case challenges
Efficacy and safety concerns with emergency contraception
The efficacy and safety of emergency contraception using levonorgestrel oral tablets (Plan B One-Step; Duramed Pharmaceuticals) is another concern. Plan B One-Step should be taken orally as soon as possible within 72 hours after unprotected intercourse or a known or suspected contraceptive failure. Efficacy is better if Plan B is taken as soon as possible after unprotected intercourse. There are 2 dosages: 1 tablet of levonorgestrel 1.5 mg or 2 tablets of levonorgestrel 0.75 mg. The second 0.75-mg tablet should be taken 12 hours after the first dose.9
Plan B can be used at any time during the menstrual cycle. In a series of 2,445 women aged 15 to 48 years who took levonorgestrel tablets for emergency contraception (Phase IV clinical trial), 5 pregnancies occurred (0.2%).10
ACOG advises that emergency contraception using a pill or the copper IUD should be initiated as soon as possible (up to 5 days) after unprotected coitus or inadequately protected coitus.9
Retained products of contraception
ACOG Practice Bulletin No. 135 on complications associated with second trimesterabortion discusses retained POC.11 The approach to second trimester abortion includes dilation and evacuation (D&E) as well as medical therapy with mifepristone and misoprostol. D&E, a safe and effective approach with advantages over medical abortion, is associated with fewer complications (up to 4%) versus medical abortion (29%); the primary complication is retained POC (placenta).11
Read about the legal considerations of this case.
Legal considerations
The malpractice lawsuit filed in this case claims that the gynecologist failed to exercise the level of care of a reasonably prudent practitioner under the circumstances and was therefore negligent or in breach of a duty to the patient.
First, a lawyer would look for a medical error that was related to some harm. Keep in mind that not all medical errors are negligent or subject to liability. Many medical errors occur even though the physician has exercised all reasonable care and engaged in sound practice, given today’s medical knowledge and facilities. When harm is caused through medical error that was careless or otherwise does not meet the standard of care, financial recovery is possible for the patient through a malpractice claim.12
In this case, the expert witnesses’ statements focus on the issue of conducting a pregnancy test prior to IUD insertion. The patient’s expert testified that failure to perform a pregnancy test was below an acceptable standard of care. That opinion may have been based on the typical practice of gynecologists, widely accepted medical text books, and formal practice standards of professional organizations.13
Cost-benefit analysis. Additional support for the claim that not performing the pregnancy test is negligent comes from applying a cost-benefit analysis. In this analysis, the risks and costs of performing a pregnancy test are compared with the benefits of doing the test.
In this case, the cost of conducting the pregnancy test is very low: essentially risk-freeand relatively inexpensive. On the other hand, the harm that could be avoided would be significant. Kim and colleagues suggest that pregnant women with an IUD in utero are at very high risk for adverse pregnancy outcomes.7 Given that women receiving IUDs are candidates for pregnancy (and perhaps do not know they are pregnant), a simple, risk-free pregnancy test would seem to be an efficient way to avoid a nontrivial harm.14
Did she have unprotected sex? The gynecologist’s expert notes that the patient told the gynecologist that she did not have unprotected coitus. Furthermore, the patient withheld from the gynecologist the information that she had taken Plan B because of a broken condom. Is this a defense against the malpractice claim? The answer is “possibly no,” or “possibly somewhat.”
As for unprotected coitus, the patient could easily have misunderstood the question. Technically, the answer “no” was correct. She had not had unprotected sex—it is just that the protection (condom) failed. It does not appear from the facts that she disclosed or was asked about Plan B or other information related to possible failed contraception. As to whether the patient’s failure to provide that information could be a defense for the physician, the best answer is “possibly” and “somewhat.” (See below.)15
Withholding information. Patients, of course, have a responsibility to inform their physicians of information they know is relevant. Many patients, however, will not know what is relevant (or why), or will not be fully disclosing.
Professionals cannot ignore the fact that their patients and clients are often confused, do not understand what is important and relevant, and cannot always be relied upon. For that very reason, professionals generally are obliged to start with the proposition that they may not have all of the relevant information. In this case, this lack of information makes the cost-calculation of performing a pregnancy test that much more important. The risk of not knowing whether a patient is pregnant includes the fact that many patients just will not know or cannot say with assurance.16
A “somewhat” defense and comparative negligence
Earlier we referred to a “somewhat” defense. Almost all states now have some form of “comparative negligence,” meaning that the patient’s recovery is reduced by the proportion of the blame (negligence) that is attributed to the patient. The most common form of comparative negligence works this way: If there are damages of $100,000, and the jury finds that the fault is 20% the patient’s and 80% the physician’s, the patient would receive $80,000 recovery. (In the past, the concept of “contributory negligence” could result in the plaintiff being precluded from any recovery if the plaintiff was partially negligent—those days are mostly gone.)
Related Article:
Informed consent: The more you know, the more you and your patient are protected
Statement of risks, informed consent, and liability
The gynecologist must provide an adequate description of the IUD risks. The case facts indicate that appropriate risks were discussed and literature provided, so it appears there was probably appropriate informed consent in this case. If not true, this would provide another basis for recovery.
Two other aspects of this case could be the basis for liability. We can assume that the attempted removal of the IUD was performed competently.16 In addition, if the IUD was defective in terms of design, manufacture, or warnings, the manufacturer of the device could be subject to liability.17
Final verdict: Out of court settlement
Why would the gynecologist and the insurance company settle this case? After all, they have some arguments on their side, and physicians win the majority of malpractice cases that go to trial.18 On the other hand, the patient’s expert witness’ testimony and the cost-benefit analysis of the pregnancy test are strong, contrary claims.
Cases are settled for a variety of reasons. Litigation is inherently risky. In this case, we assume that the court denied a motion to dismiss the case before trial because there is a legitimate question of fact concerning what a reasonably prudent gynecologist would have done under the circumstances. That means a jury would probably decide the issue of medical judgment, which is generally disconcerting. Furthermore, the comparative negligence defense that the patient did not tell the gynecologist about the failed condom/Plan B would most likely reduce the amount of damages, but not eliminate liability. The questions regarding the pressure to terminate a second trimester pregnancy might well complicate a jury’s view.
Other considerations include the high costs in time, money, uncertainty, and disruption associated with litigation. The settlement amount was not stated, but the process of negotiating a settlement would allow factoring in the comparative negligence aspect of the case. It would be reasonable for this case to settle before trial.
Should the physician have apologized before trial? The gynecologist could have sent a statement of regret or apology to the patient before a lawsuit was filed. Most states now have statutes that preclude such statements of regret or apology from being used against the physician. Many experts now favor apology statements as a way to reduce the risk of malpractice suits being filed.19
Related Article:
Medical errors: Meeting ethical obligations and reducing liability with proper communication
Defensive medicine. There has been much discussion of “defensive medicine” in recent years.20 It is appropriately criticized when additional testing is solely used to protect the physician from liability. However, much of defensive medicine is not only to protect the physician but also to protect the patient from potential physical and mental harm. In this case, it would have been “careful medicine” in addition to “defensive medicine.”
Share your thoughts! Send your Letter to the Editor to rbarbieri@frontlinemedcom.com. Please include your name and the city and state in which you practice.
CASE: Gynecologist accused of placing an IUD without performing a pregnancy test
A 34-year-old woman (G4 P3013) presents to her gynecologist for planned placement of the Mirena Intrauterine System (Bayer HealthCare). She was divorced 2 months ago and is interested in birth control. She smokes 1.5 packs per day, and her history includes irregular menses, an earlier Pap smear result of atypical squamous cells of undetermined significance (ASCUS) with negative colposcopy results, polycystic ovary syndrome, obesity, migraine headaches with aura, bilateral carpel tunnel surgery, and a herniated L4.5 disc treated conservatively. She has no history of any psychiatric problems.
One week before intrauterine device (IUD) placement, she discussed the options with her gynecologist and received a Mirena patient brochure. At the office visit for IUD placement, the patient stated she had a negative home pregnancy test 1 week earlier. She did not tell the gynecologist that she had taken Plan B One-Step (levonorgestrel, 1.5 mg) emergency contraception 2 weeks prior to presenting to her gynecologist after receiving it from a Planned Parenthood office following condom breakage during coitus. IUD placement was uncomplicated.
After noting spotting several weeks later, she contacted her gynecologist’s office. Results of an office urine pregnancy test were positive; the serum human chorionic gonadotropin (hCG) level was reported at 65,000 mIU/mL.The results of a pelvic sonogram showed a 12 5/7-week intrauterine gestation. The gynecologist unsuccessfully tried to remove the IUD. Options for termination or continuation of the pregnancy were discussed. The patient felt the gynecologist strongly encouraged, “almost insisting on,” termination. Termination could not be performed locally as her state laws did not allow second trimester abortion; the gynecologist provided out-of-state clinic options.
The patient aborted the pregnancy in a neighboring state. She was opposed to the termination but decided it was not a good time for her to have a baby. She felt the staff at the facility were “cold” and had a “we got to get this done attitude.” As she left the clinic, she saw people picketing outside and found the whole process “psychologically traumatic.” When bleeding persisted, she sought care from another gynecologist. Pelvic sonography results showed retained products of conception (POC). The new gynecologist performed operative hysteroscopy to remove the POC. The patient became depressed and felt as if she was a victim of pain and suffering.
The patient’s attorney filed a medical malpractice claim against the gynecologist who inserted the IUD, accusing her of negligence for not performing a pregnancy test immediately before IUD insertion.
In a deposition, the patient stated she bought the home pregnancy test in a “dollar store” and was worried about its accuracy, but never told the gynecologist. Conception probably occurred 2 weeks prior to IUD insertion, correlating with the broken condom and taking of Plan B. She did not think the gynecologist needed to know this as it “would not have made any difference in her care.”
The gynecologist confirmed that the patient’s record included “Patient stated ‘pregnancy test negative within 1 week of IUD placement.’” The gynecologist did not feel that obtaining the date of the patient’s last menstrual period (LMP) was required since she asked if the patient had protected coitus since her LMP and the patient answered yes. The gynecologist thought that if a pregnancy were in utero, Mirena placement would prevent implantation. She believed that she had obtained proper informed consent and that the patient acknowledged receiving and reading the Mirena patient information prior to placement. The gynecologist stated she also provided other birth control options.
The patient’s expert witness testified that the gynecologist fell below the standard of care by not obtaining a pregnancy test prior to IUD insertion.
The gynecologist’s expert witness argued that the patient told the gynecologist that she did not have unprotected coitus. The patient herself withheld information from the gynecologist that she had taken Plan B due to condom breakage. The physician’s attorney also noted that the pelvic exam at time of IUD placement was normal.
What’s the verdict?
The patient has a fairly good case. The gynecologist may not have been sufficiently careful, given all of the facts in this case, to ensure that the patient was not pregnant. An expert is testifying that this fell below the acceptable level of care in the profession. At the same time, the failure of the patient to reveal some information may result in reduced damages through “comparative negligence.” Because there will be several questions of fact for a jury to decide, as well as some emotional elements in this case, the outcome of a trial is uncertain. This suggests that a negotiated settlement before trial should be considered.
Read about medical considerations of a pregnancy with an IUD.
Medical considerations
First, some background information on Mirena.
Indications for Mirena
Here are indications for Mirena1:
- intrauterine contraception for up to 5 years
- treatment of heavy menstrual bleeding for women who choose to use intrauterine contraception as their method of contraception.
Prior to insertion, the following are recommended2:
- a complete medical and social history should be obtained to determine conditions that might influence the selection of a levonorgestrel-releasing intrauterine system (LNG IUS) for contraception
- if indicated, perform a physical examination, and appropriate tests for any forms of genital or other sexually transmitted infections
- there is no requirement for prepregnancy test.
Contraindications for Mirena
Contraindications for Mirena include2:
- pregnancy or suspicion of pregnancy; cannot be used for postcoital contraception
- congenital or acquired uterine anomaly including fibroids if they distort the uterine cavity
- acute pelvic inflammatory disease or a history of pelvic inflammatory disease unless there has been a subsequent intrauterine pregnancy
- postpartum endometritis or infected abortion in the past 3 months
- known or suspected uterine or cervical neoplasia
- known or suspected breast cancer or other progestin-sensitive cancer, now or in the past
- uterine bleeding of unknown etiology
- untreated acute cervicitis or vaginitis, including bacterial vaginosis or other lower genital tract infections until infection is controlled
- acute liver disease or liver tumor (benign or malignant)
- conditions associated with increased susceptibility to pelvic infections
- a previously inserted IUD that has not been removed
- hypersensitivity to any component of this product.
Is Mirena a postcoital contraceptive?
The American College of Obstetricians and Gynecologists (ACOG) bulletin on long-acting reversible contraception states “the levonorgestrel intrauterine system has not been studied for emergency contraception.”3 Ongoing studies are comparing the levonor‑gestrel IUD to the copper IUD for emergency contraception.4
Related Article:
Webcast: Emergency contraception: How to choose the right one for your patient
Accuracy of home pregnancy tests
Although the first home pregnancy test was introduced in 1976,5 there are now several home pregnancy tests available over the counter, most designed to detect urinary levels of hCG at ≥25 mIU/mL. The tests identify hCG, hyperglycosylated hCG, and free Betasubunit hCG in urine. When Cole and colleagues evaluated the validity of urinary tests including assessment of 18 brands, results noted that sensitivity of 12.4 mIU/mL of hCG detected 95% of pregnancies at time of missed menses.6 Some brands required 100 mIU/mL levels of hCG for positive results. The authors concluded “the utility of home pregnancy tests is questioned.”6 For urinary levels of hCG, see TABLE.

Pregnancy with an IUD
The gynecologist’s concern about pregnancy when an IUD is inserted was valid.
With regard to pregnancy with Mirena in place, the full prescribing information states2:
Intrauterine Pregnancy: If pregnancy occurs while using Mirena, remove Mirena because leaving it in place may increase the risk of spontaneous abortion and preterm labor. Removal of Mirena or probing of the uterus may also result in spontaneous abortion. In the event of an intrauterine pregnancy with Mirena, consider the following:
Septic abortion
In patients becoming pregnant with an IUD in place, septic abortion - with septicemia, septic shock, and death may occur.
Continuation of pregnancy
If a woman becomes pregnant with Mirena in place and if Mirena cannot be removed or the woman chooses not to have it removed, warn her that failure to remove Mirena increases the risk of miscarriage, sepsis, premature labor and premature delivery. Follow her pregnancy closely and advise her to report immediately any symptom that suggests complications of the pregnancy.
Concern for microbial invasion of the amniotic cavity must be considered. Kim and colleagues addressed pregnancy prognosis with an IUD in situ in a retrospective study of 12,297 pregnancies; 196 had an IUD with singleton gestation.7 The study revealed a higher incidence of histologic chorioamnionitis and/or funisitis when compared with those without an IUD (54.2% vs 14.7%, respectively; P<.001). The authors concluded that pregnant women with an IUD in utero are at very high risk for adverse pregnancy outcomes. Brahmi and colleagues8 reported similar risks with higher incidence of spontaneous abortion, preterm delivery, and septic abortion.
Related Article:
Overcoming LARC complications: 7 case challenges
Efficacy and safety concerns with emergency contraception
The efficacy and safety of emergency contraception using levonorgestrel oral tablets (Plan B One-Step; Duramed Pharmaceuticals) is another concern. Plan B One-Step should be taken orally as soon as possible within 72 hours after unprotected intercourse or a known or suspected contraceptive failure. Efficacy is better if Plan B is taken as soon as possible after unprotected intercourse. There are 2 dosages: 1 tablet of levonorgestrel 1.5 mg or 2 tablets of levonorgestrel 0.75 mg. The second 0.75-mg tablet should be taken 12 hours after the first dose.9
Plan B can be used at any time during the menstrual cycle. In a series of 2,445 women aged 15 to 48 years who took levonorgestrel tablets for emergency contraception (Phase IV clinical trial), 5 pregnancies occurred (0.2%).10
ACOG advises that emergency contraception using a pill or the copper IUD should be initiated as soon as possible (up to 5 days) after unprotected coitus or inadequately protected coitus.9
Retained products of contraception
ACOG Practice Bulletin No. 135 on complications associated with second trimesterabortion discusses retained POC.11 The approach to second trimester abortion includes dilation and evacuation (D&E) as well as medical therapy with mifepristone and misoprostol. D&E, a safe and effective approach with advantages over medical abortion, is associated with fewer complications (up to 4%) versus medical abortion (29%); the primary complication is retained POC (placenta).11
Read about the legal considerations of this case.
Legal considerations
The malpractice lawsuit filed in this case claims that the gynecologist failed to exercise the level of care of a reasonably prudent practitioner under the circumstances and was therefore negligent or in breach of a duty to the patient.
First, a lawyer would look for a medical error that was related to some harm. Keep in mind that not all medical errors are negligent or subject to liability. Many medical errors occur even though the physician has exercised all reasonable care and engaged in sound practice, given today’s medical knowledge and facilities. When harm is caused through medical error that was careless or otherwise does not meet the standard of care, financial recovery is possible for the patient through a malpractice claim.12
In this case, the expert witnesses’ statements focus on the issue of conducting a pregnancy test prior to IUD insertion. The patient’s expert testified that failure to perform a pregnancy test was below an acceptable standard of care. That opinion may have been based on the typical practice of gynecologists, widely accepted medical text books, and formal practice standards of professional organizations.13
Cost-benefit analysis. Additional support for the claim that not performing the pregnancy test is negligent comes from applying a cost-benefit analysis. In this analysis, the risks and costs of performing a pregnancy test are compared with the benefits of doing the test.
In this case, the cost of conducting the pregnancy test is very low: essentially risk-freeand relatively inexpensive. On the other hand, the harm that could be avoided would be significant. Kim and colleagues suggest that pregnant women with an IUD in utero are at very high risk for adverse pregnancy outcomes.7 Given that women receiving IUDs are candidates for pregnancy (and perhaps do not know they are pregnant), a simple, risk-free pregnancy test would seem to be an efficient way to avoid a nontrivial harm.14
Did she have unprotected sex? The gynecologist’s expert notes that the patient told the gynecologist that she did not have unprotected coitus. Furthermore, the patient withheld from the gynecologist the information that she had taken Plan B because of a broken condom. Is this a defense against the malpractice claim? The answer is “possibly no,” or “possibly somewhat.”
As for unprotected coitus, the patient could easily have misunderstood the question. Technically, the answer “no” was correct. She had not had unprotected sex—it is just that the protection (condom) failed. It does not appear from the facts that she disclosed or was asked about Plan B or other information related to possible failed contraception. As to whether the patient’s failure to provide that information could be a defense for the physician, the best answer is “possibly” and “somewhat.” (See below.)15
Withholding information. Patients, of course, have a responsibility to inform their physicians of information they know is relevant. Many patients, however, will not know what is relevant (or why), or will not be fully disclosing.
Professionals cannot ignore the fact that their patients and clients are often confused, do not understand what is important and relevant, and cannot always be relied upon. For that very reason, professionals generally are obliged to start with the proposition that they may not have all of the relevant information. In this case, this lack of information makes the cost-calculation of performing a pregnancy test that much more important. The risk of not knowing whether a patient is pregnant includes the fact that many patients just will not know or cannot say with assurance.16
A “somewhat” defense and comparative negligence
Earlier we referred to a “somewhat” defense. Almost all states now have some form of “comparative negligence,” meaning that the patient’s recovery is reduced by the proportion of the blame (negligence) that is attributed to the patient. The most common form of comparative negligence works this way: If there are damages of $100,000, and the jury finds that the fault is 20% the patient’s and 80% the physician’s, the patient would receive $80,000 recovery. (In the past, the concept of “contributory negligence” could result in the plaintiff being precluded from any recovery if the plaintiff was partially negligent—those days are mostly gone.)
Related Article:
Informed consent: The more you know, the more you and your patient are protected
Statement of risks, informed consent, and liability
The gynecologist must provide an adequate description of the IUD risks. The case facts indicate that appropriate risks were discussed and literature provided, so it appears there was probably appropriate informed consent in this case. If not true, this would provide another basis for recovery.
Two other aspects of this case could be the basis for liability. We can assume that the attempted removal of the IUD was performed competently.16 In addition, if the IUD was defective in terms of design, manufacture, or warnings, the manufacturer of the device could be subject to liability.17
Final verdict: Out of court settlement
Why would the gynecologist and the insurance company settle this case? After all, they have some arguments on their side, and physicians win the majority of malpractice cases that go to trial.18 On the other hand, the patient’s expert witness’ testimony and the cost-benefit analysis of the pregnancy test are strong, contrary claims.
Cases are settled for a variety of reasons. Litigation is inherently risky. In this case, we assume that the court denied a motion to dismiss the case before trial because there is a legitimate question of fact concerning what a reasonably prudent gynecologist would have done under the circumstances. That means a jury would probably decide the issue of medical judgment, which is generally disconcerting. Furthermore, the comparative negligence defense that the patient did not tell the gynecologist about the failed condom/Plan B would most likely reduce the amount of damages, but not eliminate liability. The questions regarding the pressure to terminate a second trimester pregnancy might well complicate a jury’s view.
Other considerations include the high costs in time, money, uncertainty, and disruption associated with litigation. The settlement amount was not stated, but the process of negotiating a settlement would allow factoring in the comparative negligence aspect of the case. It would be reasonable for this case to settle before trial.
Should the physician have apologized before trial? The gynecologist could have sent a statement of regret or apology to the patient before a lawsuit was filed. Most states now have statutes that preclude such statements of regret or apology from being used against the physician. Many experts now favor apology statements as a way to reduce the risk of malpractice suits being filed.19
Related Article:
Medical errors: Meeting ethical obligations and reducing liability with proper communication
Defensive medicine. There has been much discussion of “defensive medicine” in recent years.20 It is appropriately criticized when additional testing is solely used to protect the physician from liability. However, much of defensive medicine is not only to protect the physician but also to protect the patient from potential physical and mental harm. In this case, it would have been “careful medicine” in addition to “defensive medicine.”
Share your thoughts! Send your Letter to the Editor to rbarbieri@frontlinemedcom.com. Please include your name and the city and state in which you practice.
- Heikinheimo O, Gemell-Danielsson K. Emerging indications for the levonorgestrel-releasing intrauterine system. ACTA Obstet Gynecol Scand. 2012;91(1):3–9.
- Mirena [prescribing information]. Whippany, NJ: Bayer HealthCare Pharmaceuticals Inc; 2000.
- American College of Obstetricians and Gynecologists. Practice Bulletin No. 121: Long-acting reversible contraception: Implants and intrauterine devices. Obstet Gynecol. 2011;118(1):184–196.
- Rapid EC–Random clinical trial assessing pregnancy with intrauterine devices for emergency contraception. Clinical Trials Identifier: NCT02175030. https://www.clinicaltrials.gov/ct2/show/NCT02175030?term=NCT02175030&rank=1. Updated May 1, 2017. Accessed May 11, 2017.
- Gnoth C, Johnson S. Strips of hope: Accuracy of home pregnancy tests and new developments. Gerburtshilfe Frauenheilkd. 2014;74(7):661–669.
- Cole LA, Khanlian SA, Sutton JM, Davies S, Rayburn WF. Accuracy of home pregnancy tests at the time of missed menses. Am J Obstet Gynecol. 2004;190(1):100–105.
- Kim S, Romero R, Kusanovic J, et al. The prognosis of pregnancy conceived despite the presence of an intrauterine device (IUD). J Perinatal Med. 2010;38(1):45–53.
- Brahmi D, Steenland M, Renner R, Gaffield M, Curtis K. Pregnancy outcomes with an IUD in situ: a systematic review. Contraception. 2012;85(2):131–139.
- American College of Obstetricians and Gynecologists. Practice Bulletin No. 152: Emergency contraception. Obstet Gynecol. 2015;126(3):685–686.
- Chen Q, Xiang W, Zhang D, et al. Efficacy and safety of a levonorgestrel enteric-coated tablet as an over-the-counter drug for emergency contraception: a Phase IV clinical trial. Hum Reprod. 2011;26(9):2316–2321.
- American College of Obstetricians and Gynecologists. Practice Bulletin No. 135: Second-trimester abortion. Obstet Gynecol. 2013;121(6):1395–1406.
- White A, Pichert J, Bledsoe S, Irwin C, Entman S. Cause and effect analysis of closed claims in obstetrics and gynecology. Obstet Gynecol. 2005;105(5 pt1):1031–1038.
- Mehlman M. Professional power and the standard of care in medicine. Case Western Reserve University Scholarly Commons. 2012: Paper 574. http://scholarlycommons.law.case.edu/cgi/viewcontent.cgi?article=1576&context=faculty_publications. Accessed May 11, 2017.
- Klein D, Arnold J, Reese E. Provision of contraception: key recommendations from the CDC. Am Fam Physician. 2015;91(9);625–633.
- Reyes J, Reyes R. The effects of malpractice liability on obstetrics and gynecology: taking the measure of a crisis. N England Law Rev. 2012;47;315–348. https://www.scribd.com/document/136514285/Reyes-Reyes-The-Effect-of-Malpractice-Liability-on-Obstetrics-and -Gynecology#fullscreen&from_embed. Accessed May 11, 2017.
- Peckham C. Medscape Malpractice Report 2015: Why Ob/Gyns get sued. http://www.medscape.com/features/slideshow/malpractice-report-2015/obgyn#page=1. Published January 22, 2016. Accessed May 11. 2017.
- Rheingold P, Paris D. Contraceptives. In: Vargo JJ, ed. Products Liability Practice Guide New York, New York: Matthew Bender & Company; 2017;C:62.
- Jena AB, Chandra A, Lakdawalla D, Seabury S. Outcomes of medical malpractice litigation against US physicians. Arch Intern Med. 2012;172(11):892–894.
- Helmreich JS. Does sorry incriminate? Evidence, harm and the protection of apology. Cornell J Law Public Policy. 2012;21(3);567–609. http://scholarship.law.cornell.edu/cgi/viewcontent.cgi?article=1363&context=cjlpp.
- Baicker K, Wright B, Olson N. Reevaluating reports of defensive medicine. J Health Polit Policy Law. 2015;40(6);1157–1177.
- Heikinheimo O, Gemell-Danielsson K. Emerging indications for the levonorgestrel-releasing intrauterine system. ACTA Obstet Gynecol Scand. 2012;91(1):3–9.
- Mirena [prescribing information]. Whippany, NJ: Bayer HealthCare Pharmaceuticals Inc; 2000.
- American College of Obstetricians and Gynecologists. Practice Bulletin No. 121: Long-acting reversible contraception: Implants and intrauterine devices. Obstet Gynecol. 2011;118(1):184–196.
- Rapid EC–Random clinical trial assessing pregnancy with intrauterine devices for emergency contraception. Clinical Trials Identifier: NCT02175030. https://www.clinicaltrials.gov/ct2/show/NCT02175030?term=NCT02175030&rank=1. Updated May 1, 2017. Accessed May 11, 2017.
- Gnoth C, Johnson S. Strips of hope: Accuracy of home pregnancy tests and new developments. Gerburtshilfe Frauenheilkd. 2014;74(7):661–669.
- Cole LA, Khanlian SA, Sutton JM, Davies S, Rayburn WF. Accuracy of home pregnancy tests at the time of missed menses. Am J Obstet Gynecol. 2004;190(1):100–105.
- Kim S, Romero R, Kusanovic J, et al. The prognosis of pregnancy conceived despite the presence of an intrauterine device (IUD). J Perinatal Med. 2010;38(1):45–53.
- Brahmi D, Steenland M, Renner R, Gaffield M, Curtis K. Pregnancy outcomes with an IUD in situ: a systematic review. Contraception. 2012;85(2):131–139.
- American College of Obstetricians and Gynecologists. Practice Bulletin No. 152: Emergency contraception. Obstet Gynecol. 2015;126(3):685–686.
- Chen Q, Xiang W, Zhang D, et al. Efficacy and safety of a levonorgestrel enteric-coated tablet as an over-the-counter drug for emergency contraception: a Phase IV clinical trial. Hum Reprod. 2011;26(9):2316–2321.
- American College of Obstetricians and Gynecologists. Practice Bulletin No. 135: Second-trimester abortion. Obstet Gynecol. 2013;121(6):1395–1406.
- White A, Pichert J, Bledsoe S, Irwin C, Entman S. Cause and effect analysis of closed claims in obstetrics and gynecology. Obstet Gynecol. 2005;105(5 pt1):1031–1038.
- Mehlman M. Professional power and the standard of care in medicine. Case Western Reserve University Scholarly Commons. 2012: Paper 574. http://scholarlycommons.law.case.edu/cgi/viewcontent.cgi?article=1576&context=faculty_publications. Accessed May 11, 2017.
- Klein D, Arnold J, Reese E. Provision of contraception: key recommendations from the CDC. Am Fam Physician. 2015;91(9);625–633.
- Reyes J, Reyes R. The effects of malpractice liability on obstetrics and gynecology: taking the measure of a crisis. N England Law Rev. 2012;47;315–348. https://www.scribd.com/document/136514285/Reyes-Reyes-The-Effect-of-Malpractice-Liability-on-Obstetrics-and -Gynecology#fullscreen&from_embed. Accessed May 11, 2017.
- Peckham C. Medscape Malpractice Report 2015: Why Ob/Gyns get sued. http://www.medscape.com/features/slideshow/malpractice-report-2015/obgyn#page=1. Published January 22, 2016. Accessed May 11. 2017.
- Rheingold P, Paris D. Contraceptives. In: Vargo JJ, ed. Products Liability Practice Guide New York, New York: Matthew Bender & Company; 2017;C:62.
- Jena AB, Chandra A, Lakdawalla D, Seabury S. Outcomes of medical malpractice litigation against US physicians. Arch Intern Med. 2012;172(11):892–894.
- Helmreich JS. Does sorry incriminate? Evidence, harm and the protection of apology. Cornell J Law Public Policy. 2012;21(3);567–609. http://scholarship.law.cornell.edu/cgi/viewcontent.cgi?article=1363&context=cjlpp.
- Baicker K, Wright B, Olson N. Reevaluating reports of defensive medicine. J Health Polit Policy Law. 2015;40(6);1157–1177.
Informed consent: The more you know, the more you and your patient are protected
CASE: Surgeon accused of performing tubal ligation without consent
A patient was scheduled for an elective cesarean delivery to be performed by her ObGyn (Dr. Surgeon) at the nearby medical center. The patient was asked to sign an electronic signature pad in her ObGyn’s office, which transposed her signature onto an electronic form that she could not see at the time. She signed it. The consent was not printed out in the office but was added to her electronic medical record, and a copy was sent to her via email. Among other things, the consent included, “[Name] hereby agrees that all appropriate medical and surgical procedures as determined by the physicians and others in this hospital are in my best interest. No further consent is required to any of the treatment in this hospital.”
In the hospital, Dr. Surgeon spoke preoper- atively with the patient about cesarean delivery, the various risks and benefits, and the possibility and risks of an alternative trial of labor. Dr. Surgeon noted the conversation in the patient’s chart.
A nurse brought a standard hard copy “Zee Hospital Surgical Consent Form” to the patient. In a relevant part it provided, “I hereby consent to the surgical procedure Dr. Surgeon has discussed with me: _______” (the blank was filled in with “cesarean delivery”). The form continued: “He/She has explained the risks and benefits. I also authorize Dr. Surgeon, and such assistants as he/she may select, to perform this procedure. In his/her medical judgment, if additional procedures are appropriate, I hereby consent to their performance, in addition to the procedures listed in this form.” The patient signed the form.
While Dr. Surgeon was scrubbing for the delivery, the patient’s husband (also a surgeon at the hospital; Dr. Husband), stopped by, thanked Dr. Surgeon, and said, “Oh, by the way, my wife would like you to do a tubal ligation as well—she really wants it for health reasons. Her chronic hypertension skyrocketed during this pregnancy, and we don’t want any more children.”
“She didn’t mention that a little earlier while I was talking with her,” replied Dr. Surgeon, “but I can see how it would have slipped her mind.”
Dr. Surgeon performed the cesarean delivery and tubal ligation. All went well, with a healthy baby and mother. Several months later, the patient and Dr. Husband separated and sought divorce.
The patient, surprised by the cost of the hospital bill (Dr. Surgeon did not bill for his surgical services as a professional courtesy), was astonished to see a charge related to tubal ligation. Knowing how common billing mistakes were at Zee Hospital, she called to have the bill corrected. The clerk informed her that her medical record showed that a tubal ligation had been performed and that the bill was correct.
The patient sued Dr. Surgeon, Zee Hospital, and her (now former) husband, Dr. Husband, both for the cesarean delivery and the tubal ligation. Her claims are primarily based on the lack of informed consent.
What’s the verdict?
The patient likely has a strong case regarding the tubal ligation claim, but a weak case related to the cesarean delivery claim.
Read the ethical, medical, and legal implications of this case
Ethical and medical considerations
Although this case seems too strange for fiction, the basic facts are taken from events that did occur at a major institution. The puzzling features of this case are meant to be a cautionary tale: it is easy in the rush with the pressure of clinical practice to view informed consent as a bothersome technical detail. Yet as the following discussion suggests, adhering closely to the tenets of informed consent protects not only the fundamental interests of the patient but also the physicians and medical institutions.
Informed consent serves as protective communication
Informed consent at its core is a “process of communication” that involves you as the health care provider and the patient. It provides authority for an activity based upon an understanding of what that activity entails.1,2 Aspects of informed consent, from the physician−patient perspective, include the following:
- disclosure
- comprehension
- voluntary choice
- authorization.
In one other sense, informed consent is based on a fiduciary relationship between the ObGyn and patient.3 Overall, the process consists of an educational communication by the physician to the patient. Ideally, providers perceive the process from an ethical point of view that has been formalized by cases and statutes.4
Informed consent protects one of the most basic values of medicine and society: autonomy. From the perspective of moral philosophers, the principle of autonomy establishes the moral right to choose and follow one’s own plan for life and action.5,6 For ObGyns, the patient’s autonomy and her ability to participate in the medical decision-making process is of paramount importance. Informed consent is also a reflection of trust inherent to the physician−patient relationship.4
Informed consent is too often viewed as a mere legal formality. In truth, it melds legal and ethical values and concerns. The President’s Commission reflected this, noting that informed consent is rooted in “the fundamental recognition that adults are entitled to accept or reject health care intervention(s) on the basis of their own personal values and in furtherance of their own personal goals.”7
The historic perspective of informed consent dates back to Egyptian, Greek, and Roman eras. Dhar and Dhar suggest that the concept of “physicians’ love for humanity—philantropia” dates back to Plato and is complemented by the term “philotechnia” (love of medicine), all of which have evolved into today’s use of the terms “risks, benefits, and alternatives.”8
We emphasize that informed consent is much more than a legal concept. It has strong clinical roots because it provides an opportunity for physicians to improve communication with their patients. Informed consent is not a form; it is a process to be taken seriously.
Legal principles of informed consent
The famous New York case of Mary Schloendorff v. Society of New York Hospital, in 1914, heralded a principle that remains central in American law. Justice Benjamin Cardozo, writing for the majority, held that, “Every human being of adult years and sound mind has a right to determine what shall be done with his own body; and a surgeon who performs an operation without his patient’s consent commits an assault for which he is liable in damages.”9 The surgeon in the Schloendorff case had undertaken a gynecologic procedure—removal of a fibroid tumor—without patient consent. (In that case the hospital rather than the physician was sued, but the principle clearly applied to the physician.)
Over the last century, the American law of informed consent has developed in a number of ways.10 Lack of informed consent is now almost always considered a form of negligence rather than an intentional tort of battery. The details of the legal requirements vary from state to state as a result of statutory changes and court decisions. But in one way or another, to be “informed,” consent generally must include 4 things:
- a description of the procedure or intervention that is proposed
- the risks and benefits of the proposal—the focus here is generally on the risks of the treatment
- alternatives, if there are any (eg, pharmacologic vs surgical treatment)
- the consequences of not undertaking the proposed treatment (eg, the refusal to have a Papanicolaou smear).
A fifth point might be added—the offer to answer any questions or provide additional information.
These 4 or 5 basic items and the expanded list are efforts to simply describe the information that a reasonable person would need in order to make a decision that represents the patient’s values, personality, and preferences. (Informed consent is in some ways an ongoing process—since a patient may withdraw his/her consent.)
Exceptions to the informed consent requirement
Before turning to the facts in the hypothetical case, it is worth noting that there are 2 common exceptions to the informed consent requirement. The first is an emergency exception. When someone requires immediate attention and the patient is not conscious or capable of consent (nor is a “next of kin” available), treatment may proceed.
The second is therapeutic exception. Its designation is narrow, and it is risky to rely on it except in extreme circumstances. But when the very process of informing the patient of all the risks of a proposed treatment would create significant additional risks for the patient, the consent process may be modified. For example, for an extremely suggestable patient, describing certain risks might, in a psychosomatic way, cause the risk to be realized. In such cases, the record must be clearly documented. It is generally best to discuss the matter with a family member or other surrogate decision maker.
Read what went wrong in this case
What went wrong with consent in this case?
Our case illustrates a number of problems that occur when informed consent is not properly completed.
The electronic signature on the broadly stated consent form the patient initially signed in the office was nearly useless. She did not know what she was signing, did not have any chance to read it before signing, and was provided no help with any of the information factors of informed consent.
The surgical consent form is among the most interesting elements of this case. The form itself was seriously flawed because it contained no real evidence that the patient received information about the risks and alternatives. If the form is all there was, it would be a problem. But the conversation that Dr. Surgeon documented with the patient seemed to provide the basic elements of informed consent, including discussion of risks and benefits.
Oral informed consent is recognized in most states. To his credit, Dr. Surgeon appropriately recorded the conversation in the record. The risk of oral informed consent not backed up by text signature is that, if a dispute arises about the consent, it is difficult to prove details of what was said. (There was, of course, no such dispute about the cesarean delivery as it turned out in this case.)
Technological add-ons to consent: Pros and cons
Video and computer software are increasingly becoming an integral part of the informed consent process, and may improve comprehension by patients.11 Electronic consent may be helpful in proving what the patient was told during the consent process. A difficulty can result from overreliance on the electronic aspect and forgetting the human part of the informed consent equation. The health care team often can be productive parts of the informed consent process, but the surgeon must take ultimate responsibility for the informed consent.12
Was there informed consent for the tubal ligation?
The major problem in this case, of course, was the tubal ligation. It does not take much of an understanding of the legal niceties of informed consent to know that there was no real consent to this procedure. Dr. Husband did not have authority to consent, and his comment to Dr. Surgeon did not qualify as consent.
The hospital consent form may appear to provide some legal protection (“In his medical judgment, if additional procedures are appropriate, I hereby consent to doing those….”). Such language was once common in informed consent forms, but it offered little real consent except for trivial incidental processes (removal of an appendix) or where there was a real medical necessity for doing an expanded procedure (removal of a previously unknown cancerous growth).
Thus, Dr. Surgeon performed the sterilization without consent and may well be liable for that part of the surgery even though it did not turn out badly in a medical sense. If not for the tubal ligation, the damages would probably have been trivial. The real loss here is not a medical injury; it is the loss of reproductive capacity.
Protecting reproductive capacity. Modern law has been especially sensitive to protecting decisions regarding reproductive capacity. Therefore, the absence of clear consent to permanent sterilization is legally problematic. Dr. Surgeon may claim that he reasonably believed that the husband could give surrogate consent and it was too late to check with the patient herself. This situation does not fit well with the emergency exception, and it appears from the facts that Dr. Surgeon acted without informed consent to the sterilization.
Was it negligence or battery?
Dr. Surgeon. The most likely basis for liability for Dr. Surgeon is negligence. There is some argument that the tort of battery is a possibility because there was no consent at all for the sterilization. The claim would be that it was not the “information” that was lacking; it was the consent itself. The fact that Dr. Surgeon did not charge for his services would not absolve him of liability.
Dr. Husband. The potential liability of Dr. Husband is complicated by questions of whether he was acting in the capacity of a physician (which would likely involve the question of whether his malpractice insurance would be available), the degree to which he was acting in good faith, and facts we do not have in this case. If Dr. Husband gave consent (and thereby “caused”) the sterilization knowing that his wife did not want to have it or because of animus toward her (they were about to be separated and divorced, after all), there is the possibility of liability. (In some states a form of interspousal liability might complicate some of these claims—but that is a topic for another day.) He essentially took action for the purpose of wrongly causing the sterilization—which may be a battery (an intentional offensive or harmful touching). The legal rules around battery allow punitive damages as well as compensatory damages. In addition, many malpractice insurance policies provide limited coverage for intentional torts. To complicate matters further, it is not clear that Dr. Husband’s actions were related to his practice of medicine in any event (although Dr. Surgeon might claim that Dr. Husband’s expressed concern about his wife’s hypertension was enough to create a malpractice issue if Dr. Surgeon did not perform the verbally requested tubal ligation).
If, as we have speculated, Dr. Husband’s actions were motivated by improper personal considerations at the expense of a patient, he may also face medical board complaints from the patient. It is plausible that a state law makes it a criminal offense to wrongfully (or fraudulently) consent to medical treatment, particularly if related to reproductive capacity.
The hospital may face liability on several grounds, depending in part on the relationships between the hospital, Dr. Surgeon, and Dr. Husband. If Dr. Surgeon is an employee or agent of the hospital, he would be liable for his negligence. Even if Dr. Surgeon is technically an independent contractor, the failure of the hospital to offer more oversight concerning the surgical procedures in its facilities could give rise to a claim of negligence.
As to Dr. Husband, many of the same considerations are present. In addition, even if he is an agent of the hospital, the hospital may claim that his actions (especially if motivated by personal considerations) were a “lark of his own” and not in the course of his employment by the hospital.
Read about the clinical opportunity of informed consent
The clinical opportunity of informed consent
More than a technical legal doctrine, informed consent provides ObGyns an important opportunity for better communications with patients, and is a chance to create reasonable expectations and a more therapeutic relationship that involves the patient in care and decision making. It is likely that engaging the patient in good informed consent processes can set the stage for improved outcomes.
Interactive dialogue with the patient is one advised approach.10 This undertaking in part reflects that, as patients have more ready access to information in the digital age, they are positioned to play a more active role in health care decision making.
The benefits of informed consent are likely to be greatest if you view the process not as a technical legal requirement but as an excellent opportunity to engage the patient in her own care and treatment. Planning, intervention, and evaluation of care options as well as education regarding the medical problem at hand are integral to the informed consent process. And, of course, the right to consent is a “basic patient right” that in a sense guarantees that he/she has the right to make informed decisions regarding one’s care.6
Special considerations
Informed consent most often is associated with but not limited to surgical procedures (often performed with the use of surgical instruments and/or devices). It applies to diagnostic interventions as well as treatment. The more invasive or risky an intervention, the more important it is that the information is thorough.14,15
Pharmaceuticals have informed consent issues. The theory has been that pharmaceutical companies inform physicians of the risks and instructions for the use of pharmaceuticals and the physicians inform the patients. Indeed, traditionally pharmaceutical companies have gained immunity for “failure to warn” patients because the physicians were the “learned intermediaries” providing information to the public. Patient package inserts and pharmacists have taken over the informational role, but informed consent does apply to pharmaceuticals.
It is also worth noting that informed consent in any formal research study or the trial of new techniques, compounds, or devices requires a special process of approval by an institutional review board.
Set the stage for best outcomes
The main objective of informed consent is promoting the autonomy of your patient. That requires that she understand the risks, benefits, and alternatives associated with the procedure and the risks associated with a refusal of treatment. Done properly, this can result in your patient gaining confidence and trust in you as her provider.
Informed consent is a process that reflects our interactions with our patients. It is part of the broader commitment by the medical profession to “first do no harm.”
Share your thoughts! Send your Letter to the Editor to rbarbieri@frontlinemedcom.com. Please include your name and the city and state in which you practice.
- Grady C. Enduring and emerging challenges of informed consent. N Engl J Med. 2015;372(22):855–862.
- Faden RR, Beauchamp TL. History and theory of informed consent. New York, New York: Oxford University Press; 1986.
- Paterick TJ, Carson G, Allen M, Paterick TE. Medical informed consent: general considerations for physicians. Mayo Clin Proc. 2008;83(3):313–319.
- American College of Obstetricians and Gynecologists Committee on Ethics. ACOG Committee Opinion No. 439: Informed consent (reaffirmed 2015). Obstet Gynecol. 2009;114(2 pt 1):401–408.
- Jonsen A, Siegler M, Winslade W. Clinical Ethics: A Practical Approach to Ethical Decisions in Clinical Medicine. New York, New York: McGraw Hill; 2010.
- Menendez J. Informed consent: essential legal and ethical principles for nurses. JONAS Healthc Law Ethics Regul. 2013;15(4):140–144.
- Abram MB, Ballantine HT, Dunlop GR, et al; President’s Commission for the Study of Ethical Problems in Medicine and Biomedical and Behavioral Research. Making Health Care Decisions: The Ethical and Legal Implications of Informed Consent in the Patient-Practitioner Relationship. Volume One: Report. https://repository.library.georgetown.edu/bitstream/handle/10822/559354/making_health_care_decisions.pdf?sequence=1. Published October 1982. Accessed January 9, 2017.
- Dhar H, Dhar D. Informed consent in clinical practice and literature overview. Arch Gynecol Obstet. 2012;286(3):649–651.
- NE Schloendorff v Society of New York Hospital, 211 NY 125, 106 NE 93 1914.
- Avery D. Summary of informed consent and refusal. Am J Clinical Med. 2009;6(3):28–29.
- Pallett A, Phippen N, Miller C, Barnett J. Informed consent for hysterectomy: Does a video presentation improve patient comprehension? [poster 17F]. Obstet Gynecol. 2016;127(suppl):55S.
- Cainzos M, Gonzalez-Vinagre S. Informed consent in surgery. World J Surg. 2014;38(7):1587–1593.
- Weaver J. A problem with informed consent. J Am Coll Surg. 2009;209(2):286–287.
- Bunch W. Informed consent. Clin Orthop Relat Res. 2000;378:71–77.
- Peltier LF. Orthopedics: a history and iconography (Norman Orthopedic, No. 3). San Francisco, California: Jeremy Norman Co; 1993.
CASE: Surgeon accused of performing tubal ligation without consent
A patient was scheduled for an elective cesarean delivery to be performed by her ObGyn (Dr. Surgeon) at the nearby medical center. The patient was asked to sign an electronic signature pad in her ObGyn’s office, which transposed her signature onto an electronic form that she could not see at the time. She signed it. The consent was not printed out in the office but was added to her electronic medical record, and a copy was sent to her via email. Among other things, the consent included, “[Name] hereby agrees that all appropriate medical and surgical procedures as determined by the physicians and others in this hospital are in my best interest. No further consent is required to any of the treatment in this hospital.”
In the hospital, Dr. Surgeon spoke preoper- atively with the patient about cesarean delivery, the various risks and benefits, and the possibility and risks of an alternative trial of labor. Dr. Surgeon noted the conversation in the patient’s chart.
A nurse brought a standard hard copy “Zee Hospital Surgical Consent Form” to the patient. In a relevant part it provided, “I hereby consent to the surgical procedure Dr. Surgeon has discussed with me: _______” (the blank was filled in with “cesarean delivery”). The form continued: “He/She has explained the risks and benefits. I also authorize Dr. Surgeon, and such assistants as he/she may select, to perform this procedure. In his/her medical judgment, if additional procedures are appropriate, I hereby consent to their performance, in addition to the procedures listed in this form.” The patient signed the form.
While Dr. Surgeon was scrubbing for the delivery, the patient’s husband (also a surgeon at the hospital; Dr. Husband), stopped by, thanked Dr. Surgeon, and said, “Oh, by the way, my wife would like you to do a tubal ligation as well—she really wants it for health reasons. Her chronic hypertension skyrocketed during this pregnancy, and we don’t want any more children.”
“She didn’t mention that a little earlier while I was talking with her,” replied Dr. Surgeon, “but I can see how it would have slipped her mind.”
Dr. Surgeon performed the cesarean delivery and tubal ligation. All went well, with a healthy baby and mother. Several months later, the patient and Dr. Husband separated and sought divorce.
The patient, surprised by the cost of the hospital bill (Dr. Surgeon did not bill for his surgical services as a professional courtesy), was astonished to see a charge related to tubal ligation. Knowing how common billing mistakes were at Zee Hospital, she called to have the bill corrected. The clerk informed her that her medical record showed that a tubal ligation had been performed and that the bill was correct.
The patient sued Dr. Surgeon, Zee Hospital, and her (now former) husband, Dr. Husband, both for the cesarean delivery and the tubal ligation. Her claims are primarily based on the lack of informed consent.
What’s the verdict?
The patient likely has a strong case regarding the tubal ligation claim, but a weak case related to the cesarean delivery claim.
Read the ethical, medical, and legal implications of this case
Ethical and medical considerations
Although this case seems too strange for fiction, the basic facts are taken from events that did occur at a major institution. The puzzling features of this case are meant to be a cautionary tale: it is easy in the rush with the pressure of clinical practice to view informed consent as a bothersome technical detail. Yet as the following discussion suggests, adhering closely to the tenets of informed consent protects not only the fundamental interests of the patient but also the physicians and medical institutions.
Informed consent serves as protective communication
Informed consent at its core is a “process of communication” that involves you as the health care provider and the patient. It provides authority for an activity based upon an understanding of what that activity entails.1,2 Aspects of informed consent, from the physician−patient perspective, include the following:
- disclosure
- comprehension
- voluntary choice
- authorization.
In one other sense, informed consent is based on a fiduciary relationship between the ObGyn and patient.3 Overall, the process consists of an educational communication by the physician to the patient. Ideally, providers perceive the process from an ethical point of view that has been formalized by cases and statutes.4
Informed consent protects one of the most basic values of medicine and society: autonomy. From the perspective of moral philosophers, the principle of autonomy establishes the moral right to choose and follow one’s own plan for life and action.5,6 For ObGyns, the patient’s autonomy and her ability to participate in the medical decision-making process is of paramount importance. Informed consent is also a reflection of trust inherent to the physician−patient relationship.4
Informed consent is too often viewed as a mere legal formality. In truth, it melds legal and ethical values and concerns. The President’s Commission reflected this, noting that informed consent is rooted in “the fundamental recognition that adults are entitled to accept or reject health care intervention(s) on the basis of their own personal values and in furtherance of their own personal goals.”7
The historic perspective of informed consent dates back to Egyptian, Greek, and Roman eras. Dhar and Dhar suggest that the concept of “physicians’ love for humanity—philantropia” dates back to Plato and is complemented by the term “philotechnia” (love of medicine), all of which have evolved into today’s use of the terms “risks, benefits, and alternatives.”8
We emphasize that informed consent is much more than a legal concept. It has strong clinical roots because it provides an opportunity for physicians to improve communication with their patients. Informed consent is not a form; it is a process to be taken seriously.
Legal principles of informed consent
The famous New York case of Mary Schloendorff v. Society of New York Hospital, in 1914, heralded a principle that remains central in American law. Justice Benjamin Cardozo, writing for the majority, held that, “Every human being of adult years and sound mind has a right to determine what shall be done with his own body; and a surgeon who performs an operation without his patient’s consent commits an assault for which he is liable in damages.”9 The surgeon in the Schloendorff case had undertaken a gynecologic procedure—removal of a fibroid tumor—without patient consent. (In that case the hospital rather than the physician was sued, but the principle clearly applied to the physician.)
Over the last century, the American law of informed consent has developed in a number of ways.10 Lack of informed consent is now almost always considered a form of negligence rather than an intentional tort of battery. The details of the legal requirements vary from state to state as a result of statutory changes and court decisions. But in one way or another, to be “informed,” consent generally must include 4 things:
- a description of the procedure or intervention that is proposed
- the risks and benefits of the proposal—the focus here is generally on the risks of the treatment
- alternatives, if there are any (eg, pharmacologic vs surgical treatment)
- the consequences of not undertaking the proposed treatment (eg, the refusal to have a Papanicolaou smear).
A fifth point might be added—the offer to answer any questions or provide additional information.
These 4 or 5 basic items and the expanded list are efforts to simply describe the information that a reasonable person would need in order to make a decision that represents the patient’s values, personality, and preferences. (Informed consent is in some ways an ongoing process—since a patient may withdraw his/her consent.)
Exceptions to the informed consent requirement
Before turning to the facts in the hypothetical case, it is worth noting that there are 2 common exceptions to the informed consent requirement. The first is an emergency exception. When someone requires immediate attention and the patient is not conscious or capable of consent (nor is a “next of kin” available), treatment may proceed.
The second is therapeutic exception. Its designation is narrow, and it is risky to rely on it except in extreme circumstances. But when the very process of informing the patient of all the risks of a proposed treatment would create significant additional risks for the patient, the consent process may be modified. For example, for an extremely suggestable patient, describing certain risks might, in a psychosomatic way, cause the risk to be realized. In such cases, the record must be clearly documented. It is generally best to discuss the matter with a family member or other surrogate decision maker.
Read what went wrong in this case
What went wrong with consent in this case?
Our case illustrates a number of problems that occur when informed consent is not properly completed.
The electronic signature on the broadly stated consent form the patient initially signed in the office was nearly useless. She did not know what she was signing, did not have any chance to read it before signing, and was provided no help with any of the information factors of informed consent.
The surgical consent form is among the most interesting elements of this case. The form itself was seriously flawed because it contained no real evidence that the patient received information about the risks and alternatives. If the form is all there was, it would be a problem. But the conversation that Dr. Surgeon documented with the patient seemed to provide the basic elements of informed consent, including discussion of risks and benefits.
Oral informed consent is recognized in most states. To his credit, Dr. Surgeon appropriately recorded the conversation in the record. The risk of oral informed consent not backed up by text signature is that, if a dispute arises about the consent, it is difficult to prove details of what was said. (There was, of course, no such dispute about the cesarean delivery as it turned out in this case.)
Technological add-ons to consent: Pros and cons
Video and computer software are increasingly becoming an integral part of the informed consent process, and may improve comprehension by patients.11 Electronic consent may be helpful in proving what the patient was told during the consent process. A difficulty can result from overreliance on the electronic aspect and forgetting the human part of the informed consent equation. The health care team often can be productive parts of the informed consent process, but the surgeon must take ultimate responsibility for the informed consent.12
Was there informed consent for the tubal ligation?
The major problem in this case, of course, was the tubal ligation. It does not take much of an understanding of the legal niceties of informed consent to know that there was no real consent to this procedure. Dr. Husband did not have authority to consent, and his comment to Dr. Surgeon did not qualify as consent.
The hospital consent form may appear to provide some legal protection (“In his medical judgment, if additional procedures are appropriate, I hereby consent to doing those….”). Such language was once common in informed consent forms, but it offered little real consent except for trivial incidental processes (removal of an appendix) or where there was a real medical necessity for doing an expanded procedure (removal of a previously unknown cancerous growth).
Thus, Dr. Surgeon performed the sterilization without consent and may well be liable for that part of the surgery even though it did not turn out badly in a medical sense. If not for the tubal ligation, the damages would probably have been trivial. The real loss here is not a medical injury; it is the loss of reproductive capacity.
Protecting reproductive capacity. Modern law has been especially sensitive to protecting decisions regarding reproductive capacity. Therefore, the absence of clear consent to permanent sterilization is legally problematic. Dr. Surgeon may claim that he reasonably believed that the husband could give surrogate consent and it was too late to check with the patient herself. This situation does not fit well with the emergency exception, and it appears from the facts that Dr. Surgeon acted without informed consent to the sterilization.
Was it negligence or battery?
Dr. Surgeon. The most likely basis for liability for Dr. Surgeon is negligence. There is some argument that the tort of battery is a possibility because there was no consent at all for the sterilization. The claim would be that it was not the “information” that was lacking; it was the consent itself. The fact that Dr. Surgeon did not charge for his services would not absolve him of liability.
Dr. Husband. The potential liability of Dr. Husband is complicated by questions of whether he was acting in the capacity of a physician (which would likely involve the question of whether his malpractice insurance would be available), the degree to which he was acting in good faith, and facts we do not have in this case. If Dr. Husband gave consent (and thereby “caused”) the sterilization knowing that his wife did not want to have it or because of animus toward her (they were about to be separated and divorced, after all), there is the possibility of liability. (In some states a form of interspousal liability might complicate some of these claims—but that is a topic for another day.) He essentially took action for the purpose of wrongly causing the sterilization—which may be a battery (an intentional offensive or harmful touching). The legal rules around battery allow punitive damages as well as compensatory damages. In addition, many malpractice insurance policies provide limited coverage for intentional torts. To complicate matters further, it is not clear that Dr. Husband’s actions were related to his practice of medicine in any event (although Dr. Surgeon might claim that Dr. Husband’s expressed concern about his wife’s hypertension was enough to create a malpractice issue if Dr. Surgeon did not perform the verbally requested tubal ligation).
If, as we have speculated, Dr. Husband’s actions were motivated by improper personal considerations at the expense of a patient, he may also face medical board complaints from the patient. It is plausible that a state law makes it a criminal offense to wrongfully (or fraudulently) consent to medical treatment, particularly if related to reproductive capacity.
The hospital may face liability on several grounds, depending in part on the relationships between the hospital, Dr. Surgeon, and Dr. Husband. If Dr. Surgeon is an employee or agent of the hospital, he would be liable for his negligence. Even if Dr. Surgeon is technically an independent contractor, the failure of the hospital to offer more oversight concerning the surgical procedures in its facilities could give rise to a claim of negligence.
As to Dr. Husband, many of the same considerations are present. In addition, even if he is an agent of the hospital, the hospital may claim that his actions (especially if motivated by personal considerations) were a “lark of his own” and not in the course of his employment by the hospital.
Read about the clinical opportunity of informed consent
The clinical opportunity of informed consent
More than a technical legal doctrine, informed consent provides ObGyns an important opportunity for better communications with patients, and is a chance to create reasonable expectations and a more therapeutic relationship that involves the patient in care and decision making. It is likely that engaging the patient in good informed consent processes can set the stage for improved outcomes.
Interactive dialogue with the patient is one advised approach.10 This undertaking in part reflects that, as patients have more ready access to information in the digital age, they are positioned to play a more active role in health care decision making.
The benefits of informed consent are likely to be greatest if you view the process not as a technical legal requirement but as an excellent opportunity to engage the patient in her own care and treatment. Planning, intervention, and evaluation of care options as well as education regarding the medical problem at hand are integral to the informed consent process. And, of course, the right to consent is a “basic patient right” that in a sense guarantees that he/she has the right to make informed decisions regarding one’s care.6
Special considerations
Informed consent most often is associated with but not limited to surgical procedures (often performed with the use of surgical instruments and/or devices). It applies to diagnostic interventions as well as treatment. The more invasive or risky an intervention, the more important it is that the information is thorough.14,15
Pharmaceuticals have informed consent issues. The theory has been that pharmaceutical companies inform physicians of the risks and instructions for the use of pharmaceuticals and the physicians inform the patients. Indeed, traditionally pharmaceutical companies have gained immunity for “failure to warn” patients because the physicians were the “learned intermediaries” providing information to the public. Patient package inserts and pharmacists have taken over the informational role, but informed consent does apply to pharmaceuticals.
It is also worth noting that informed consent in any formal research study or the trial of new techniques, compounds, or devices requires a special process of approval by an institutional review board.
Set the stage for best outcomes
The main objective of informed consent is promoting the autonomy of your patient. That requires that she understand the risks, benefits, and alternatives associated with the procedure and the risks associated with a refusal of treatment. Done properly, this can result in your patient gaining confidence and trust in you as her provider.
Informed consent is a process that reflects our interactions with our patients. It is part of the broader commitment by the medical profession to “first do no harm.”
Share your thoughts! Send your Letter to the Editor to rbarbieri@frontlinemedcom.com. Please include your name and the city and state in which you practice.
CASE: Surgeon accused of performing tubal ligation without consent
A patient was scheduled for an elective cesarean delivery to be performed by her ObGyn (Dr. Surgeon) at the nearby medical center. The patient was asked to sign an electronic signature pad in her ObGyn’s office, which transposed her signature onto an electronic form that she could not see at the time. She signed it. The consent was not printed out in the office but was added to her electronic medical record, and a copy was sent to her via email. Among other things, the consent included, “[Name] hereby agrees that all appropriate medical and surgical procedures as determined by the physicians and others in this hospital are in my best interest. No further consent is required to any of the treatment in this hospital.”
In the hospital, Dr. Surgeon spoke preoper- atively with the patient about cesarean delivery, the various risks and benefits, and the possibility and risks of an alternative trial of labor. Dr. Surgeon noted the conversation in the patient’s chart.
A nurse brought a standard hard copy “Zee Hospital Surgical Consent Form” to the patient. In a relevant part it provided, “I hereby consent to the surgical procedure Dr. Surgeon has discussed with me: _______” (the blank was filled in with “cesarean delivery”). The form continued: “He/She has explained the risks and benefits. I also authorize Dr. Surgeon, and such assistants as he/she may select, to perform this procedure. In his/her medical judgment, if additional procedures are appropriate, I hereby consent to their performance, in addition to the procedures listed in this form.” The patient signed the form.
While Dr. Surgeon was scrubbing for the delivery, the patient’s husband (also a surgeon at the hospital; Dr. Husband), stopped by, thanked Dr. Surgeon, and said, “Oh, by the way, my wife would like you to do a tubal ligation as well—she really wants it for health reasons. Her chronic hypertension skyrocketed during this pregnancy, and we don’t want any more children.”
“She didn’t mention that a little earlier while I was talking with her,” replied Dr. Surgeon, “but I can see how it would have slipped her mind.”
Dr. Surgeon performed the cesarean delivery and tubal ligation. All went well, with a healthy baby and mother. Several months later, the patient and Dr. Husband separated and sought divorce.
The patient, surprised by the cost of the hospital bill (Dr. Surgeon did not bill for his surgical services as a professional courtesy), was astonished to see a charge related to tubal ligation. Knowing how common billing mistakes were at Zee Hospital, she called to have the bill corrected. The clerk informed her that her medical record showed that a tubal ligation had been performed and that the bill was correct.
The patient sued Dr. Surgeon, Zee Hospital, and her (now former) husband, Dr. Husband, both for the cesarean delivery and the tubal ligation. Her claims are primarily based on the lack of informed consent.
What’s the verdict?
The patient likely has a strong case regarding the tubal ligation claim, but a weak case related to the cesarean delivery claim.
Read the ethical, medical, and legal implications of this case
Ethical and medical considerations
Although this case seems too strange for fiction, the basic facts are taken from events that did occur at a major institution. The puzzling features of this case are meant to be a cautionary tale: it is easy in the rush with the pressure of clinical practice to view informed consent as a bothersome technical detail. Yet as the following discussion suggests, adhering closely to the tenets of informed consent protects not only the fundamental interests of the patient but also the physicians and medical institutions.
Informed consent serves as protective communication
Informed consent at its core is a “process of communication” that involves you as the health care provider and the patient. It provides authority for an activity based upon an understanding of what that activity entails.1,2 Aspects of informed consent, from the physician−patient perspective, include the following:
- disclosure
- comprehension
- voluntary choice
- authorization.
In one other sense, informed consent is based on a fiduciary relationship between the ObGyn and patient.3 Overall, the process consists of an educational communication by the physician to the patient. Ideally, providers perceive the process from an ethical point of view that has been formalized by cases and statutes.4
Informed consent protects one of the most basic values of medicine and society: autonomy. From the perspective of moral philosophers, the principle of autonomy establishes the moral right to choose and follow one’s own plan for life and action.5,6 For ObGyns, the patient’s autonomy and her ability to participate in the medical decision-making process is of paramount importance. Informed consent is also a reflection of trust inherent to the physician−patient relationship.4
Informed consent is too often viewed as a mere legal formality. In truth, it melds legal and ethical values and concerns. The President’s Commission reflected this, noting that informed consent is rooted in “the fundamental recognition that adults are entitled to accept or reject health care intervention(s) on the basis of their own personal values and in furtherance of their own personal goals.”7
The historic perspective of informed consent dates back to Egyptian, Greek, and Roman eras. Dhar and Dhar suggest that the concept of “physicians’ love for humanity—philantropia” dates back to Plato and is complemented by the term “philotechnia” (love of medicine), all of which have evolved into today’s use of the terms “risks, benefits, and alternatives.”8
We emphasize that informed consent is much more than a legal concept. It has strong clinical roots because it provides an opportunity for physicians to improve communication with their patients. Informed consent is not a form; it is a process to be taken seriously.
Legal principles of informed consent
The famous New York case of Mary Schloendorff v. Society of New York Hospital, in 1914, heralded a principle that remains central in American law. Justice Benjamin Cardozo, writing for the majority, held that, “Every human being of adult years and sound mind has a right to determine what shall be done with his own body; and a surgeon who performs an operation without his patient’s consent commits an assault for which he is liable in damages.”9 The surgeon in the Schloendorff case had undertaken a gynecologic procedure—removal of a fibroid tumor—without patient consent. (In that case the hospital rather than the physician was sued, but the principle clearly applied to the physician.)
Over the last century, the American law of informed consent has developed in a number of ways.10 Lack of informed consent is now almost always considered a form of negligence rather than an intentional tort of battery. The details of the legal requirements vary from state to state as a result of statutory changes and court decisions. But in one way or another, to be “informed,” consent generally must include 4 things:
- a description of the procedure or intervention that is proposed
- the risks and benefits of the proposal—the focus here is generally on the risks of the treatment
- alternatives, if there are any (eg, pharmacologic vs surgical treatment)
- the consequences of not undertaking the proposed treatment (eg, the refusal to have a Papanicolaou smear).
A fifth point might be added—the offer to answer any questions or provide additional information.
These 4 or 5 basic items and the expanded list are efforts to simply describe the information that a reasonable person would need in order to make a decision that represents the patient’s values, personality, and preferences. (Informed consent is in some ways an ongoing process—since a patient may withdraw his/her consent.)
Exceptions to the informed consent requirement
Before turning to the facts in the hypothetical case, it is worth noting that there are 2 common exceptions to the informed consent requirement. The first is an emergency exception. When someone requires immediate attention and the patient is not conscious or capable of consent (nor is a “next of kin” available), treatment may proceed.
The second is therapeutic exception. Its designation is narrow, and it is risky to rely on it except in extreme circumstances. But when the very process of informing the patient of all the risks of a proposed treatment would create significant additional risks for the patient, the consent process may be modified. For example, for an extremely suggestable patient, describing certain risks might, in a psychosomatic way, cause the risk to be realized. In such cases, the record must be clearly documented. It is generally best to discuss the matter with a family member or other surrogate decision maker.
Read what went wrong in this case
What went wrong with consent in this case?
Our case illustrates a number of problems that occur when informed consent is not properly completed.
The electronic signature on the broadly stated consent form the patient initially signed in the office was nearly useless. She did not know what she was signing, did not have any chance to read it before signing, and was provided no help with any of the information factors of informed consent.
The surgical consent form is among the most interesting elements of this case. The form itself was seriously flawed because it contained no real evidence that the patient received information about the risks and alternatives. If the form is all there was, it would be a problem. But the conversation that Dr. Surgeon documented with the patient seemed to provide the basic elements of informed consent, including discussion of risks and benefits.
Oral informed consent is recognized in most states. To his credit, Dr. Surgeon appropriately recorded the conversation in the record. The risk of oral informed consent not backed up by text signature is that, if a dispute arises about the consent, it is difficult to prove details of what was said. (There was, of course, no such dispute about the cesarean delivery as it turned out in this case.)
Technological add-ons to consent: Pros and cons
Video and computer software are increasingly becoming an integral part of the informed consent process, and may improve comprehension by patients.11 Electronic consent may be helpful in proving what the patient was told during the consent process. A difficulty can result from overreliance on the electronic aspect and forgetting the human part of the informed consent equation. The health care team often can be productive parts of the informed consent process, but the surgeon must take ultimate responsibility for the informed consent.12
Was there informed consent for the tubal ligation?
The major problem in this case, of course, was the tubal ligation. It does not take much of an understanding of the legal niceties of informed consent to know that there was no real consent to this procedure. Dr. Husband did not have authority to consent, and his comment to Dr. Surgeon did not qualify as consent.
The hospital consent form may appear to provide some legal protection (“In his medical judgment, if additional procedures are appropriate, I hereby consent to doing those….”). Such language was once common in informed consent forms, but it offered little real consent except for trivial incidental processes (removal of an appendix) or where there was a real medical necessity for doing an expanded procedure (removal of a previously unknown cancerous growth).
Thus, Dr. Surgeon performed the sterilization without consent and may well be liable for that part of the surgery even though it did not turn out badly in a medical sense. If not for the tubal ligation, the damages would probably have been trivial. The real loss here is not a medical injury; it is the loss of reproductive capacity.
Protecting reproductive capacity. Modern law has been especially sensitive to protecting decisions regarding reproductive capacity. Therefore, the absence of clear consent to permanent sterilization is legally problematic. Dr. Surgeon may claim that he reasonably believed that the husband could give surrogate consent and it was too late to check with the patient herself. This situation does not fit well with the emergency exception, and it appears from the facts that Dr. Surgeon acted without informed consent to the sterilization.
Was it negligence or battery?
Dr. Surgeon. The most likely basis for liability for Dr. Surgeon is negligence. There is some argument that the tort of battery is a possibility because there was no consent at all for the sterilization. The claim would be that it was not the “information” that was lacking; it was the consent itself. The fact that Dr. Surgeon did not charge for his services would not absolve him of liability.
Dr. Husband. The potential liability of Dr. Husband is complicated by questions of whether he was acting in the capacity of a physician (which would likely involve the question of whether his malpractice insurance would be available), the degree to which he was acting in good faith, and facts we do not have in this case. If Dr. Husband gave consent (and thereby “caused”) the sterilization knowing that his wife did not want to have it or because of animus toward her (they were about to be separated and divorced, after all), there is the possibility of liability. (In some states a form of interspousal liability might complicate some of these claims—but that is a topic for another day.) He essentially took action for the purpose of wrongly causing the sterilization—which may be a battery (an intentional offensive or harmful touching). The legal rules around battery allow punitive damages as well as compensatory damages. In addition, many malpractice insurance policies provide limited coverage for intentional torts. To complicate matters further, it is not clear that Dr. Husband’s actions were related to his practice of medicine in any event (although Dr. Surgeon might claim that Dr. Husband’s expressed concern about his wife’s hypertension was enough to create a malpractice issue if Dr. Surgeon did not perform the verbally requested tubal ligation).
If, as we have speculated, Dr. Husband’s actions were motivated by improper personal considerations at the expense of a patient, he may also face medical board complaints from the patient. It is plausible that a state law makes it a criminal offense to wrongfully (or fraudulently) consent to medical treatment, particularly if related to reproductive capacity.
The hospital may face liability on several grounds, depending in part on the relationships between the hospital, Dr. Surgeon, and Dr. Husband. If Dr. Surgeon is an employee or agent of the hospital, he would be liable for his negligence. Even if Dr. Surgeon is technically an independent contractor, the failure of the hospital to offer more oversight concerning the surgical procedures in its facilities could give rise to a claim of negligence.
As to Dr. Husband, many of the same considerations are present. In addition, even if he is an agent of the hospital, the hospital may claim that his actions (especially if motivated by personal considerations) were a “lark of his own” and not in the course of his employment by the hospital.
Read about the clinical opportunity of informed consent
The clinical opportunity of informed consent
More than a technical legal doctrine, informed consent provides ObGyns an important opportunity for better communications with patients, and is a chance to create reasonable expectations and a more therapeutic relationship that involves the patient in care and decision making. It is likely that engaging the patient in good informed consent processes can set the stage for improved outcomes.
Interactive dialogue with the patient is one advised approach.10 This undertaking in part reflects that, as patients have more ready access to information in the digital age, they are positioned to play a more active role in health care decision making.
The benefits of informed consent are likely to be greatest if you view the process not as a technical legal requirement but as an excellent opportunity to engage the patient in her own care and treatment. Planning, intervention, and evaluation of care options as well as education regarding the medical problem at hand are integral to the informed consent process. And, of course, the right to consent is a “basic patient right” that in a sense guarantees that he/she has the right to make informed decisions regarding one’s care.6
Special considerations
Informed consent most often is associated with but not limited to surgical procedures (often performed with the use of surgical instruments and/or devices). It applies to diagnostic interventions as well as treatment. The more invasive or risky an intervention, the more important it is that the information is thorough.14,15
Pharmaceuticals have informed consent issues. The theory has been that pharmaceutical companies inform physicians of the risks and instructions for the use of pharmaceuticals and the physicians inform the patients. Indeed, traditionally pharmaceutical companies have gained immunity for “failure to warn” patients because the physicians were the “learned intermediaries” providing information to the public. Patient package inserts and pharmacists have taken over the informational role, but informed consent does apply to pharmaceuticals.
It is also worth noting that informed consent in any formal research study or the trial of new techniques, compounds, or devices requires a special process of approval by an institutional review board.
Set the stage for best outcomes
The main objective of informed consent is promoting the autonomy of your patient. That requires that she understand the risks, benefits, and alternatives associated with the procedure and the risks associated with a refusal of treatment. Done properly, this can result in your patient gaining confidence and trust in you as her provider.
Informed consent is a process that reflects our interactions with our patients. It is part of the broader commitment by the medical profession to “first do no harm.”
Share your thoughts! Send your Letter to the Editor to rbarbieri@frontlinemedcom.com. Please include your name and the city and state in which you practice.
- Grady C. Enduring and emerging challenges of informed consent. N Engl J Med. 2015;372(22):855–862.
- Faden RR, Beauchamp TL. History and theory of informed consent. New York, New York: Oxford University Press; 1986.
- Paterick TJ, Carson G, Allen M, Paterick TE. Medical informed consent: general considerations for physicians. Mayo Clin Proc. 2008;83(3):313–319.
- American College of Obstetricians and Gynecologists Committee on Ethics. ACOG Committee Opinion No. 439: Informed consent (reaffirmed 2015). Obstet Gynecol. 2009;114(2 pt 1):401–408.
- Jonsen A, Siegler M, Winslade W. Clinical Ethics: A Practical Approach to Ethical Decisions in Clinical Medicine. New York, New York: McGraw Hill; 2010.
- Menendez J. Informed consent: essential legal and ethical principles for nurses. JONAS Healthc Law Ethics Regul. 2013;15(4):140–144.
- Abram MB, Ballantine HT, Dunlop GR, et al; President’s Commission for the Study of Ethical Problems in Medicine and Biomedical and Behavioral Research. Making Health Care Decisions: The Ethical and Legal Implications of Informed Consent in the Patient-Practitioner Relationship. Volume One: Report. https://repository.library.georgetown.edu/bitstream/handle/10822/559354/making_health_care_decisions.pdf?sequence=1. Published October 1982. Accessed January 9, 2017.
- Dhar H, Dhar D. Informed consent in clinical practice and literature overview. Arch Gynecol Obstet. 2012;286(3):649–651.
- NE Schloendorff v Society of New York Hospital, 211 NY 125, 106 NE 93 1914.
- Avery D. Summary of informed consent and refusal. Am J Clinical Med. 2009;6(3):28–29.
- Pallett A, Phippen N, Miller C, Barnett J. Informed consent for hysterectomy: Does a video presentation improve patient comprehension? [poster 17F]. Obstet Gynecol. 2016;127(suppl):55S.
- Cainzos M, Gonzalez-Vinagre S. Informed consent in surgery. World J Surg. 2014;38(7):1587–1593.
- Weaver J. A problem with informed consent. J Am Coll Surg. 2009;209(2):286–287.
- Bunch W. Informed consent. Clin Orthop Relat Res. 2000;378:71–77.
- Peltier LF. Orthopedics: a history and iconography (Norman Orthopedic, No. 3). San Francisco, California: Jeremy Norman Co; 1993.
- Grady C. Enduring and emerging challenges of informed consent. N Engl J Med. 2015;372(22):855–862.
- Faden RR, Beauchamp TL. History and theory of informed consent. New York, New York: Oxford University Press; 1986.
- Paterick TJ, Carson G, Allen M, Paterick TE. Medical informed consent: general considerations for physicians. Mayo Clin Proc. 2008;83(3):313–319.
- American College of Obstetricians and Gynecologists Committee on Ethics. ACOG Committee Opinion No. 439: Informed consent (reaffirmed 2015). Obstet Gynecol. 2009;114(2 pt 1):401–408.
- Jonsen A, Siegler M, Winslade W. Clinical Ethics: A Practical Approach to Ethical Decisions in Clinical Medicine. New York, New York: McGraw Hill; 2010.
- Menendez J. Informed consent: essential legal and ethical principles for nurses. JONAS Healthc Law Ethics Regul. 2013;15(4):140–144.
- Abram MB, Ballantine HT, Dunlop GR, et al; President’s Commission for the Study of Ethical Problems in Medicine and Biomedical and Behavioral Research. Making Health Care Decisions: The Ethical and Legal Implications of Informed Consent in the Patient-Practitioner Relationship. Volume One: Report. https://repository.library.georgetown.edu/bitstream/handle/10822/559354/making_health_care_decisions.pdf?sequence=1. Published October 1982. Accessed January 9, 2017.
- Dhar H, Dhar D. Informed consent in clinical practice and literature overview. Arch Gynecol Obstet. 2012;286(3):649–651.
- NE Schloendorff v Society of New York Hospital, 211 NY 125, 106 NE 93 1914.
- Avery D. Summary of informed consent and refusal. Am J Clinical Med. 2009;6(3):28–29.
- Pallett A, Phippen N, Miller C, Barnett J. Informed consent for hysterectomy: Does a video presentation improve patient comprehension? [poster 17F]. Obstet Gynecol. 2016;127(suppl):55S.
- Cainzos M, Gonzalez-Vinagre S. Informed consent in surgery. World J Surg. 2014;38(7):1587–1593.
- Weaver J. A problem with informed consent. J Am Coll Surg. 2009;209(2):286–287.
- Bunch W. Informed consent. Clin Orthop Relat Res. 2000;378:71–77.
- Peltier LF. Orthopedics: a history and iconography (Norman Orthopedic, No. 3). San Francisco, California: Jeremy Norman Co; 1993.
Patient with a breast mass: Why did she pursue litigation?
CASE: After routine mammography results, DCIS found
A 49-year-old woman (G2 P2002) with a history of fibrocystic breast disease presented with a left breast mass that she found a month ago on self-examination. The patient faithfully had obtained routine mammograms since age 40. This year, after reporting the mass and with spot films obtained as recommended by the radiologist, a new cluster of microcalcifications was identified on the report: “spot compression” assessment identified a 3-cm mass and noted “s/p breast augmentation.”
The radiologist interpreted the spot films to be benign. His report stated that “15% of breast cancers are not detected by mammogram and breast self-exam is recommended monthly from 40 years of age.”
The gynecologist recommended a 6-month follow up. When the patient complied, the radiologist’s report again noted calcifications believed to be nonmalignant. Six months later, the patient presented with bloody nipple discharge from her left breast with apparent “eczema-like” lesions on the areola. The patient noted that her “left implant felt different.”
The patient’s surgical history included breast augmentation “years ago.” Her family history was negative for breast cancer. Her medications included hormone therapy (conjugated estrogens 0.625 mg with medroxyprogesterone acetate 2.5 mg daily) for vaginal atrophy. Other medical conditions included irritable bowel syndrome (managed with diet), anxiety and mood swings (for which she was taking sertraline), decreased libido, and irregular vaginal bleeding (after the patient refused endometrial sampling, she was switched to oral contraceptives to address the problem). In addition, her hypertension was being treated with hydrochlorothiazide.
At the gynecologist’s suggestion, a dermatology consultation was obtained.
The dermatologist gave a diagnosis of Paget disease with high-grade ductal carcinoma-in-situ (DCIS). The interval from screening mammogram to DCIS diagnosis had been 8 months. The dermatologist referred the patient to a breast surgeon. A discussion ensued between the breast surgeon and the dermatologist concerning the difficulty of making a diagnosis of breast cancer in a woman with breast augmentation.
The patient underwent modified radical mastectomy, and histopathology revealed DCIS with clear margins; lymph nodes were negative.
The patient filed a malpractice suit against the gynecologist related to the delayed breast mass evaluation and management. She remained upset that when she called the gynecologist’s office to convey her concerns regarding the left nipple discharge and implant concerns, “she was blown off.” She felt there was a clear “failure to communicate on critical matters of her health.” She alleged that the gynecologist, not the dermatologist, should have referred her to a breast surgeon.
WHAT 'S THE VERDICT?
In the end, the patient decided not to pursue the lawsuit.
Related article:
Who is liable when a surgical error occurs?
Medical considerations
Breast cancer is the most common female malignancy, with 232,340 cases occurring annually in the United States. It is the second leading cause of cancer-related death in US women.1 For this case discussion, we review the role of breast cancer screening, including breast self-examination and mammography.
Is breast self-examination recommended?
Recently, medical care has evolved from “breast self-examination” (BSE) to “breast self-awareness.”2 The concept of BSE and concerns about it stem in part from “the Shanghai study.”3 In this prospective randomized trial, 266,064 female textile workers were randomly assigned to “rigorous and repetitive training in BSE” versus no instruction and no BSE performance. The former group had twice as many breast biopsies than the latter group (2,761 biopsies in the BSE group vs 1,505 in the control group). There was no difference in the number of breast cancers diagnosed among the groups—864 in the BSE group and 896 in the control group (relative risk [RR], 0.97; 95% confidence interval [CI], 0.88–1.06; P = .47). Other studies also support lack of efficacy regarding BSE.4
The potential for psychological harm, unnecessary biopsy, and additional imaging in association with false-positive findings is a concern2 (TABLE). The American College of Obstetricians and Gynecologists (ACOG) states, “breast self-examination may be appropriate for certain high-risk populations and for other women who chose to follow this approach.”2,5,6 The US Preventive Services Task Force (USPSTF) guidelines say that there is “insufficient evidence to assess risks vs benefits, including harms,” and recommends against teaching BSE.7 The American Cancer Society puts BSE in the “optional” category.2,8
Is there a middle of the road strategy? Perhaps. The concept of breast-awareness was developed so that women understand how their breasts look and feel.2,5 The concept does not advocate monthly BSE. The Mayo Clinic reported that, of 592 breast cancers, 57% were detected following abnormal screening mammography, 30% by BSE and 14% by clinical examination by a clinician. Furthermore, 38% of women with a palpable abnormality had a normal mammogram within the preceding 13 months.9 McBride and colleagues aptly addressed this: “Healthcare providers can educate their patients that breast awareness, in essence, is a two-step process. First, it requires that women be familiar with their breasts and aware of new changes and, second, have an understanding of the implications of these changes which includes informing their health care provider promptly.”10 The concept of “know what is normal for you” as conveyed by The Susan G. Komen Foundation succinctly encourages communication with patients.2,11
Mammography
The latest technique in mammography is digital or 3D mammography, also known as tomosynthesis. The technique is similar to 2D mammography with the addition of digital cameras. A study published in the radiology literature noted that the 2 methods were equivalent.12 One possible advantage of 3D mammography is that the 3D images are stored in computer files and are more easily incorporated into the electronic medical record.
What about mammography after breast augmentation?
While breast augmentation is not associated with an increase in breast cancer,13 mammography following breast augmentation can be more difficult to interpret and may result in a delay in diagnosis. In a prospective study of asymptomatic women who were diagnosed with breast cancer, 137 had augmentation and 685 did not. Miglioretti and colleagues noted that the sensitivity of screening mammography was lower in the augmentation cohort.14 To enhance accuracy, breast implant displacement views (in which the breast tissue is pulled forward and the implant is displaced posteriorly to improve visualization) have been recommended.15 A retrospective review provides data reporting no effect on interpretation of mammograms following augmentation.16 The American Cancer Society recommends the same screening for women with implants as without implants, starting at age 40 years.17
Paget disease of the breast
Paget disease of the breast was first described by Sir James Paget in 1874. He also defined Paget disease of extramammary tissue, bone, vulva, and penis. Paget disease of the breast is a rare type of cancer in which the skin and nipple are involved frequently in association with DCIS or invasive breast cancer. The skin has an eczema-like appearance. Characteristic Paget (malignant) cells are large with clear cytoplasm (clear halo) and eccentric, hyperchromatic nuclei throughout the dermis. Assessment includes mammography and biopsy with immunohistochemical staining. Treatment varies by case and can include lumpectomy or mastectomy and chemotherapy and/or radiation therapy. Medications, including tamoxifen and anastrazole, have been recommended. Prognosis depends on nodal involvement. The disease is more common in women older than age 50.18
Related article:
The medicolegal considerations of interacting with your patients online
Legal issues: What was the gynecologist’s obligation?
The question remains, did the gynecologist have an obligation to obtain diagnostic mammography and ultrasound of the breast? Would it have been prudent for immediate referral to a breast surgeon? These are critical questions.
Negligence and the standard of care
The malpractice lawsuit against this gynecologist is based on negligence. In essence, it is a claim that the management provided fell below a standard of care that would be given by a reasonably careful and prudent gynecologist under the circumstances. This generally means that the care was less than the profession itself would find acceptable. Here the claim is essentially a diagnostic error claim, a common basis of malpractice.19 The delay in diagnosing breast cancer is a leading cause of malpractice claims against gynecologists.20,21
It is axiomatic that not all bad outcomes are the result of error, and not all errors are a result of an unacceptable standard of care. It is only bad outcomes resulting from careless or negligent errors that give rise to malpractice. But malpractice claims are often filed without knowing whether or not there was negligence—and, as we will see, many of those claims are without merit.
Was there negligence?
Ordinarily the plaintiff/patient must demonstrate that the care by the physician fell below the standard of care and, as a result, the patient suffered an injury. Stated another way, the plaintiff must show that the physician’s actions were unreasonable given all of the circumstances. In this case, the plaintiff must demonstrate that the gynecologist’s care was not appropriate. Failure to refer to another physician or provide additional testing is likely to be the major claim for negligence or inappropriate care.
Did that negligence cause injury?
Even if the plaintiff can demonstrate that the care was negligent, to prevail the plaintiff must also demonstrate that that negligence caused the injury—and that might be difficult.
What injury was caused by the delay in discovering the cancer? It did not apparently lead to the patient’s death. Can it be proven that the delay clearly shortened the patient’s life expectancy or required additional expensive and painful treatment? That may be difficult to demonstrate.
Causation. The causation factor could appear in many cancer or similar medical cases. On one hand, causation is a critical matter, but on the other hand, delay in treating cancer might have a very adverse effect on patients.
In recent years, about half the states have solved this dilemma by recognizing the concept of “loss of chance.”22 Essentially, this means that the health care provider, by delaying treatment, diminished the possibility that the patient would survive or recover fully.
There are significant variations among states in the loss-of-chance concept, many being quite technical.22 Thus, it is possible that a delay that reduced the chance of recovery or survival could be the injury and causal connection between the injury and the negligence. Even in states that recognize the loss-of-chance concept, the patient still must prove loss of chance.
Lessons learned from this case study
- Breast self-awareness has replaced (substituted) breast self-examination
- ACOG recommends breast mammography beginning at age 40 years
- Breast augmentation affects mammographic interpretation
- Prehaps if better communication had been provided initially, the patient would not have sought legal counsel or filed a weak suit
Related article:
Is the smartphone recording while the patient is under anesthesia?
Is this a strong malpractice case?
In the case presented here, it is not clear that the patient could show a meaningful loss of chance. If there is a delay in the breast cancer diagnosis, tumor doubling time would be an issue. While it is impossible to assess growth rate when a breast cancer is in its preclinical microscopic stage, doubling time can be 100 to 200 days. Therefore, it would take 20 years for the tumor to reach a 1- to 2-cm diameter. A log-normal distribution has been suggested for determining tumor growth.23
Although the facts in this case are sketchy, this does not look like a strong malpractice case. Given the expense, difficulty, and length of time it takes to pursue a malpractice case (especially for someone battling cancer), an obvious question is: Why would a patient file a lawsuit in these circumstances? There is no single answer to that, but the hope of getting rich is unlikely a primary motivation. Ironically, many malpractice cases are filed in which there was no error (or at least no negligent error).24
The search for what really happened, or why the bad event happened, is key. In other circumstances, it may be a desire for revenge or to protect other patients from similar bad results. Studies repeatedly have shown a somewhat limited correlation between negligent error and the decision to file a malpractice claim.24–26 In this case, the patient’s sense of being “blown off” during a particularly difficult time may represent the reason why she filed a malpractice lawsuit. Communication gaffes and poor physician-patient relationships undoubtedly contribute to medical malpractice claims.27,28 Improving communication with patients probably improves care, but it also almost certainly reduces the risk of a malpractice claim.29
Why a lawyer would accept this case is also unclear, but that is an issue for another day. Also for another day is the issue of product liability concerning breast implants. Those legal issues and related liability, primarily directed to the manufacturers of the implants, are interesting topics. They are also complex and will be the subject of a future article.
Finally, the patient’s decision to not pursue her lawsuit does not come as a surprise. A relatively small percentage of malpractice claims result in any meaningful financial recovery for the plaintiff. Few cases go to trial, and of those that do result in a verdict, about 75% of the verdicts are in favor of the physician.25,30 Many cases just fade away, either because the plaintiff never pursues them or because they are dismissed by a court at an early stage. Nonetheless, for the physician, even winning a malpractice case is disruptive and difficult. So in addition to ensuring careful, quality, and up-to-date care, a physician should seek to maintain good relationships and communication with patients to reduce the probability of even weak lawsuits being filed.
Share your thoughts! Send your Letter to the Editor to rbarbieri@frontlinemedcom.com. Please include your name and the city and state in which you practice.
- Siegel R, Naishadham D, Jemal A. Cancer statistics, 2013. CA Cancer J Clin. 2013;63(1):11–30.
- Mark K, Temkin S, Terplan M. Breast self-awareness: the evidence behind the euphemism. Obstet Gynecol. 2014;123(4):734–745.
- Thomas D, Gao D, Self S, et al. Randomized trial of breast self-examination in Shanghai: methodology and preliminary results. J Natl Cancer Inst. 1997;89(5):355–365.
- Jones S. Regular self-examination or clinical examination for early detection of breast cancer. Int J Epidemiol. 2008;37(6):1219.
- ACOG Committee on Practice Bulletins–Gynecology. ACOG Practice Bulletin No. 122. American College of Obstetricians and Gynecologists website. http://www.acog.org/Resources-And-Publications/Practice-Bulletins/Committee-on-Practice-Bulletins-Gynecology/Breast-Cancer-Screening. Published August 2011; reaffirmed 2014. Accessed November 10, 2016.
- ACOG Statement on breast cancer screening guidelines. American College of Obstetricians and Gynecologists website. http://www.acog.org/About-ACOG/News-Room/Statements/2016/ACOG-Statement-on-Breast-Cancer-Screening-Guidelines. Published January 11, 2016. Accessed November 10, 2016.
- Final Recommendation Statement: Breast Cancer: Screening. U.S. Preventive Services Task Force web site. https://www.uspreventiveservicestaskforce.org/Page/Document/RecommendationStatementFinal/breast-cancer-screening1. Published September 2016. Accessed November 10, 2016.
- American Cancer Society recommendations for early breast cancer detection in women without breast symptoms. American Cancer Society website. http://www.cancer.org/cancer/breastcancer/moreinformation/breastcancerearlydetection/breast-cancer-early-detection-acs-recs. Updated October 20, 2015. Accessed November 10, 2016.
- Mathis K, Hoskin T, Boughey J, et al. Palpable presentation of breast cancer persists in the era of screening mammography. J Am Coll Surg. 2010;210(3):314–318.
- MacBride M, Pruthi S, Bevers T. The evolution of breast self-examination to breast awareness. Breast J. 2012;18(6):641–643.
- Susan G. Komen Foundation. Breast self-awareness messages. http://ww5.komen.org/Breastcancer/Breastselfawareness.html. Accessed November 10, 2016.
- DelTurco MR, Mantellini P, Ciatto S, et al. Full-field digital versus screen-film mammography: comparative accuracy in concurrent screening cohorts. AJR. 2007;189(4):860–866.
- Susan G. Koman Foundation. Factors that do not increase breast cancer risk: Breast implants. https://ww5.komen.org/BreastCancer/FactorsThatDoNotIncreaseRisk.html. Updated October 28, 2016. Accessed November 10, 2016.
- Miglioretti D, Rutter, C, Geller B, et al. Effect of breast augmentation on the accuracy of mammography and cancer characteristics. JAMA. 2004;291(4):442–450.
- Eklund GW, Busby RC, Miller SH, Job JS. Improved imaging of the augmented breast. Am J Roentgenol. 1988;151(3):469–473.
- Kam K, Lee E, Pairwan S, et al. The effect of breast implants on mammogram outcomes. Am Surg. 2015;81(10):1053–1056.
- American Cancer Society. Mammograms and other imaging tests. http://www.cancer.org/acs/groups/cid/documents/webcontent/003178-pdf.pdf. Revised April 25, 2016. Accessed November 10, 2016.
- Dalberg K, Hellborg H, Warnberg F. Paget’s disease of the nipple in a population based cohort. Breast Cancer Res Treat. 2008;111(2):313–319.
- Saber Tehrani A, Lee H, Mathews S, et al. 25-year summary of US malpractice claims for diagnostic errors 1986–2010: an analysis from the National Practitioner Data Bank. BMJ Qual Saf. 2013;22(8):672–680.
- White AA, Pichert JW, Bledsoe SH, Irwin C, Entman SS. Cause and effect analysis of closed claims in obstetrics and gynecology. Obstet Gynecol. 2005;105(5 pt 1):1031–1038.
- Ward CJ, Green VL. Risk management and medico-legal issues in breast cancer. Clin Obstet Gynecol. 2016;59(2):439–446.
- Guest L, Schap D, Tran T. The “Loss of Chance” rule as a special category of damages in medical malpractice: a state-by-state analysis. J Legal Econ. 2015;21(2):53–107.
- Kuroishi T, Tominaga S, Morimoto T, et al. Tumor growth rate and prognosis of breast cancer mainly detected by mass screening. Jpn J Cancer Res. 1990;81(5):454–462.
- Localio AR, Lawthers AG, Brennan TA, et al. Relation between malpractice claims and adverse events due to negligence: results of the Harvard Medical Practice Study III. N Engl J Medicine. 1991;325(4):245–251.
- Gandhi TK, Kachalia A, Thomas EJ, et al. Missed and delayed diagnoses in the ambulatory setting: a study of closed malpractice claims. Ann Intern Med. 2006;145(7):488–496.
- Studdert DM, Mello MM, Gawande AA, et al. Claims, errors, and compensation payments in medical malpractice litigation. N Engl J Med. 2006;354(19):2024–2033.
- Huntington B, Kuhn N. Communication gaffes: a root cause of malpractice claims. Proc (Bayl Univ Med Cent). 2003;16(2):157–161.
- Moore PJ, Adler NE, Robertson PA. Medical malpractice: the effect of doctor-patient relations on medical patient perceptions and malpractice intentions. West J Med. 2000;173(4):244–250.
- Hickson GB, Jenkins AD. Identifying and addressing communication failures as a means of reducing unnecessary malpractice claims. NC Med J. 2007;68(5):362–364.
- Jena AB, Chandra A, Lakdawalla D, Seabury S. Outcomes of medical malpractice litigation against US physicians. Arch Intern Med. 2012;172(11):892–894.
CASE: After routine mammography results, DCIS found
A 49-year-old woman (G2 P2002) with a history of fibrocystic breast disease presented with a left breast mass that she found a month ago on self-examination. The patient faithfully had obtained routine mammograms since age 40. This year, after reporting the mass and with spot films obtained as recommended by the radiologist, a new cluster of microcalcifications was identified on the report: “spot compression” assessment identified a 3-cm mass and noted “s/p breast augmentation.”
The radiologist interpreted the spot films to be benign. His report stated that “15% of breast cancers are not detected by mammogram and breast self-exam is recommended monthly from 40 years of age.”
The gynecologist recommended a 6-month follow up. When the patient complied, the radiologist’s report again noted calcifications believed to be nonmalignant. Six months later, the patient presented with bloody nipple discharge from her left breast with apparent “eczema-like” lesions on the areola. The patient noted that her “left implant felt different.”
The patient’s surgical history included breast augmentation “years ago.” Her family history was negative for breast cancer. Her medications included hormone therapy (conjugated estrogens 0.625 mg with medroxyprogesterone acetate 2.5 mg daily) for vaginal atrophy. Other medical conditions included irritable bowel syndrome (managed with diet), anxiety and mood swings (for which she was taking sertraline), decreased libido, and irregular vaginal bleeding (after the patient refused endometrial sampling, she was switched to oral contraceptives to address the problem). In addition, her hypertension was being treated with hydrochlorothiazide.
At the gynecologist’s suggestion, a dermatology consultation was obtained.
The dermatologist gave a diagnosis of Paget disease with high-grade ductal carcinoma-in-situ (DCIS). The interval from screening mammogram to DCIS diagnosis had been 8 months. The dermatologist referred the patient to a breast surgeon. A discussion ensued between the breast surgeon and the dermatologist concerning the difficulty of making a diagnosis of breast cancer in a woman with breast augmentation.
The patient underwent modified radical mastectomy, and histopathology revealed DCIS with clear margins; lymph nodes were negative.
The patient filed a malpractice suit against the gynecologist related to the delayed breast mass evaluation and management. She remained upset that when she called the gynecologist’s office to convey her concerns regarding the left nipple discharge and implant concerns, “she was blown off.” She felt there was a clear “failure to communicate on critical matters of her health.” She alleged that the gynecologist, not the dermatologist, should have referred her to a breast surgeon.
WHAT 'S THE VERDICT?
In the end, the patient decided not to pursue the lawsuit.
Related article:
Who is liable when a surgical error occurs?
Medical considerations
Breast cancer is the most common female malignancy, with 232,340 cases occurring annually in the United States. It is the second leading cause of cancer-related death in US women.1 For this case discussion, we review the role of breast cancer screening, including breast self-examination and mammography.
Is breast self-examination recommended?
Recently, medical care has evolved from “breast self-examination” (BSE) to “breast self-awareness.”2 The concept of BSE and concerns about it stem in part from “the Shanghai study.”3 In this prospective randomized trial, 266,064 female textile workers were randomly assigned to “rigorous and repetitive training in BSE” versus no instruction and no BSE performance. The former group had twice as many breast biopsies than the latter group (2,761 biopsies in the BSE group vs 1,505 in the control group). There was no difference in the number of breast cancers diagnosed among the groups—864 in the BSE group and 896 in the control group (relative risk [RR], 0.97; 95% confidence interval [CI], 0.88–1.06; P = .47). Other studies also support lack of efficacy regarding BSE.4
The potential for psychological harm, unnecessary biopsy, and additional imaging in association with false-positive findings is a concern2 (TABLE). The American College of Obstetricians and Gynecologists (ACOG) states, “breast self-examination may be appropriate for certain high-risk populations and for other women who chose to follow this approach.”2,5,6 The US Preventive Services Task Force (USPSTF) guidelines say that there is “insufficient evidence to assess risks vs benefits, including harms,” and recommends against teaching BSE.7 The American Cancer Society puts BSE in the “optional” category.2,8
Is there a middle of the road strategy? Perhaps. The concept of breast-awareness was developed so that women understand how their breasts look and feel.2,5 The concept does not advocate monthly BSE. The Mayo Clinic reported that, of 592 breast cancers, 57% were detected following abnormal screening mammography, 30% by BSE and 14% by clinical examination by a clinician. Furthermore, 38% of women with a palpable abnormality had a normal mammogram within the preceding 13 months.9 McBride and colleagues aptly addressed this: “Healthcare providers can educate their patients that breast awareness, in essence, is a two-step process. First, it requires that women be familiar with their breasts and aware of new changes and, second, have an understanding of the implications of these changes which includes informing their health care provider promptly.”10 The concept of “know what is normal for you” as conveyed by The Susan G. Komen Foundation succinctly encourages communication with patients.2,11
Mammography
The latest technique in mammography is digital or 3D mammography, also known as tomosynthesis. The technique is similar to 2D mammography with the addition of digital cameras. A study published in the radiology literature noted that the 2 methods were equivalent.12 One possible advantage of 3D mammography is that the 3D images are stored in computer files and are more easily incorporated into the electronic medical record.
What about mammography after breast augmentation?
While breast augmentation is not associated with an increase in breast cancer,13 mammography following breast augmentation can be more difficult to interpret and may result in a delay in diagnosis. In a prospective study of asymptomatic women who were diagnosed with breast cancer, 137 had augmentation and 685 did not. Miglioretti and colleagues noted that the sensitivity of screening mammography was lower in the augmentation cohort.14 To enhance accuracy, breast implant displacement views (in which the breast tissue is pulled forward and the implant is displaced posteriorly to improve visualization) have been recommended.15 A retrospective review provides data reporting no effect on interpretation of mammograms following augmentation.16 The American Cancer Society recommends the same screening for women with implants as without implants, starting at age 40 years.17
Paget disease of the breast
Paget disease of the breast was first described by Sir James Paget in 1874. He also defined Paget disease of extramammary tissue, bone, vulva, and penis. Paget disease of the breast is a rare type of cancer in which the skin and nipple are involved frequently in association with DCIS or invasive breast cancer. The skin has an eczema-like appearance. Characteristic Paget (malignant) cells are large with clear cytoplasm (clear halo) and eccentric, hyperchromatic nuclei throughout the dermis. Assessment includes mammography and biopsy with immunohistochemical staining. Treatment varies by case and can include lumpectomy or mastectomy and chemotherapy and/or radiation therapy. Medications, including tamoxifen and anastrazole, have been recommended. Prognosis depends on nodal involvement. The disease is more common in women older than age 50.18
Related article:
The medicolegal considerations of interacting with your patients online
Legal issues: What was the gynecologist’s obligation?
The question remains, did the gynecologist have an obligation to obtain diagnostic mammography and ultrasound of the breast? Would it have been prudent for immediate referral to a breast surgeon? These are critical questions.
Negligence and the standard of care
The malpractice lawsuit against this gynecologist is based on negligence. In essence, it is a claim that the management provided fell below a standard of care that would be given by a reasonably careful and prudent gynecologist under the circumstances. This generally means that the care was less than the profession itself would find acceptable. Here the claim is essentially a diagnostic error claim, a common basis of malpractice.19 The delay in diagnosing breast cancer is a leading cause of malpractice claims against gynecologists.20,21
It is axiomatic that not all bad outcomes are the result of error, and not all errors are a result of an unacceptable standard of care. It is only bad outcomes resulting from careless or negligent errors that give rise to malpractice. But malpractice claims are often filed without knowing whether or not there was negligence—and, as we will see, many of those claims are without merit.
Was there negligence?
Ordinarily the plaintiff/patient must demonstrate that the care by the physician fell below the standard of care and, as a result, the patient suffered an injury. Stated another way, the plaintiff must show that the physician’s actions were unreasonable given all of the circumstances. In this case, the plaintiff must demonstrate that the gynecologist’s care was not appropriate. Failure to refer to another physician or provide additional testing is likely to be the major claim for negligence or inappropriate care.
Did that negligence cause injury?
Even if the plaintiff can demonstrate that the care was negligent, to prevail the plaintiff must also demonstrate that that negligence caused the injury—and that might be difficult.
What injury was caused by the delay in discovering the cancer? It did not apparently lead to the patient’s death. Can it be proven that the delay clearly shortened the patient’s life expectancy or required additional expensive and painful treatment? That may be difficult to demonstrate.
Causation. The causation factor could appear in many cancer or similar medical cases. On one hand, causation is a critical matter, but on the other hand, delay in treating cancer might have a very adverse effect on patients.
In recent years, about half the states have solved this dilemma by recognizing the concept of “loss of chance.”22 Essentially, this means that the health care provider, by delaying treatment, diminished the possibility that the patient would survive or recover fully.
There are significant variations among states in the loss-of-chance concept, many being quite technical.22 Thus, it is possible that a delay that reduced the chance of recovery or survival could be the injury and causal connection between the injury and the negligence. Even in states that recognize the loss-of-chance concept, the patient still must prove loss of chance.
Lessons learned from this case study
- Breast self-awareness has replaced (substituted) breast self-examination
- ACOG recommends breast mammography beginning at age 40 years
- Breast augmentation affects mammographic interpretation
- Prehaps if better communication had been provided initially, the patient would not have sought legal counsel or filed a weak suit
Related article:
Is the smartphone recording while the patient is under anesthesia?
Is this a strong malpractice case?
In the case presented here, it is not clear that the patient could show a meaningful loss of chance. If there is a delay in the breast cancer diagnosis, tumor doubling time would be an issue. While it is impossible to assess growth rate when a breast cancer is in its preclinical microscopic stage, doubling time can be 100 to 200 days. Therefore, it would take 20 years for the tumor to reach a 1- to 2-cm diameter. A log-normal distribution has been suggested for determining tumor growth.23
Although the facts in this case are sketchy, this does not look like a strong malpractice case. Given the expense, difficulty, and length of time it takes to pursue a malpractice case (especially for someone battling cancer), an obvious question is: Why would a patient file a lawsuit in these circumstances? There is no single answer to that, but the hope of getting rich is unlikely a primary motivation. Ironically, many malpractice cases are filed in which there was no error (or at least no negligent error).24
The search for what really happened, or why the bad event happened, is key. In other circumstances, it may be a desire for revenge or to protect other patients from similar bad results. Studies repeatedly have shown a somewhat limited correlation between negligent error and the decision to file a malpractice claim.24–26 In this case, the patient’s sense of being “blown off” during a particularly difficult time may represent the reason why she filed a malpractice lawsuit. Communication gaffes and poor physician-patient relationships undoubtedly contribute to medical malpractice claims.27,28 Improving communication with patients probably improves care, but it also almost certainly reduces the risk of a malpractice claim.29
Why a lawyer would accept this case is also unclear, but that is an issue for another day. Also for another day is the issue of product liability concerning breast implants. Those legal issues and related liability, primarily directed to the manufacturers of the implants, are interesting topics. They are also complex and will be the subject of a future article.
Finally, the patient’s decision to not pursue her lawsuit does not come as a surprise. A relatively small percentage of malpractice claims result in any meaningful financial recovery for the plaintiff. Few cases go to trial, and of those that do result in a verdict, about 75% of the verdicts are in favor of the physician.25,30 Many cases just fade away, either because the plaintiff never pursues them or because they are dismissed by a court at an early stage. Nonetheless, for the physician, even winning a malpractice case is disruptive and difficult. So in addition to ensuring careful, quality, and up-to-date care, a physician should seek to maintain good relationships and communication with patients to reduce the probability of even weak lawsuits being filed.
Share your thoughts! Send your Letter to the Editor to rbarbieri@frontlinemedcom.com. Please include your name and the city and state in which you practice.
CASE: After routine mammography results, DCIS found
A 49-year-old woman (G2 P2002) with a history of fibrocystic breast disease presented with a left breast mass that she found a month ago on self-examination. The patient faithfully had obtained routine mammograms since age 40. This year, after reporting the mass and with spot films obtained as recommended by the radiologist, a new cluster of microcalcifications was identified on the report: “spot compression” assessment identified a 3-cm mass and noted “s/p breast augmentation.”
The radiologist interpreted the spot films to be benign. His report stated that “15% of breast cancers are not detected by mammogram and breast self-exam is recommended monthly from 40 years of age.”
The gynecologist recommended a 6-month follow up. When the patient complied, the radiologist’s report again noted calcifications believed to be nonmalignant. Six months later, the patient presented with bloody nipple discharge from her left breast with apparent “eczema-like” lesions on the areola. The patient noted that her “left implant felt different.”
The patient’s surgical history included breast augmentation “years ago.” Her family history was negative for breast cancer. Her medications included hormone therapy (conjugated estrogens 0.625 mg with medroxyprogesterone acetate 2.5 mg daily) for vaginal atrophy. Other medical conditions included irritable bowel syndrome (managed with diet), anxiety and mood swings (for which she was taking sertraline), decreased libido, and irregular vaginal bleeding (after the patient refused endometrial sampling, she was switched to oral contraceptives to address the problem). In addition, her hypertension was being treated with hydrochlorothiazide.
At the gynecologist’s suggestion, a dermatology consultation was obtained.
The dermatologist gave a diagnosis of Paget disease with high-grade ductal carcinoma-in-situ (DCIS). The interval from screening mammogram to DCIS diagnosis had been 8 months. The dermatologist referred the patient to a breast surgeon. A discussion ensued between the breast surgeon and the dermatologist concerning the difficulty of making a diagnosis of breast cancer in a woman with breast augmentation.
The patient underwent modified radical mastectomy, and histopathology revealed DCIS with clear margins; lymph nodes were negative.
The patient filed a malpractice suit against the gynecologist related to the delayed breast mass evaluation and management. She remained upset that when she called the gynecologist’s office to convey her concerns regarding the left nipple discharge and implant concerns, “she was blown off.” She felt there was a clear “failure to communicate on critical matters of her health.” She alleged that the gynecologist, not the dermatologist, should have referred her to a breast surgeon.
WHAT 'S THE VERDICT?
In the end, the patient decided not to pursue the lawsuit.
Related article:
Who is liable when a surgical error occurs?
Medical considerations
Breast cancer is the most common female malignancy, with 232,340 cases occurring annually in the United States. It is the second leading cause of cancer-related death in US women.1 For this case discussion, we review the role of breast cancer screening, including breast self-examination and mammography.
Is breast self-examination recommended?
Recently, medical care has evolved from “breast self-examination” (BSE) to “breast self-awareness.”2 The concept of BSE and concerns about it stem in part from “the Shanghai study.”3 In this prospective randomized trial, 266,064 female textile workers were randomly assigned to “rigorous and repetitive training in BSE” versus no instruction and no BSE performance. The former group had twice as many breast biopsies than the latter group (2,761 biopsies in the BSE group vs 1,505 in the control group). There was no difference in the number of breast cancers diagnosed among the groups—864 in the BSE group and 896 in the control group (relative risk [RR], 0.97; 95% confidence interval [CI], 0.88–1.06; P = .47). Other studies also support lack of efficacy regarding BSE.4
The potential for psychological harm, unnecessary biopsy, and additional imaging in association with false-positive findings is a concern2 (TABLE). The American College of Obstetricians and Gynecologists (ACOG) states, “breast self-examination may be appropriate for certain high-risk populations and for other women who chose to follow this approach.”2,5,6 The US Preventive Services Task Force (USPSTF) guidelines say that there is “insufficient evidence to assess risks vs benefits, including harms,” and recommends against teaching BSE.7 The American Cancer Society puts BSE in the “optional” category.2,8
Is there a middle of the road strategy? Perhaps. The concept of breast-awareness was developed so that women understand how their breasts look and feel.2,5 The concept does not advocate monthly BSE. The Mayo Clinic reported that, of 592 breast cancers, 57% were detected following abnormal screening mammography, 30% by BSE and 14% by clinical examination by a clinician. Furthermore, 38% of women with a palpable abnormality had a normal mammogram within the preceding 13 months.9 McBride and colleagues aptly addressed this: “Healthcare providers can educate their patients that breast awareness, in essence, is a two-step process. First, it requires that women be familiar with their breasts and aware of new changes and, second, have an understanding of the implications of these changes which includes informing their health care provider promptly.”10 The concept of “know what is normal for you” as conveyed by The Susan G. Komen Foundation succinctly encourages communication with patients.2,11
Mammography
The latest technique in mammography is digital or 3D mammography, also known as tomosynthesis. The technique is similar to 2D mammography with the addition of digital cameras. A study published in the radiology literature noted that the 2 methods were equivalent.12 One possible advantage of 3D mammography is that the 3D images are stored in computer files and are more easily incorporated into the electronic medical record.
What about mammography after breast augmentation?
While breast augmentation is not associated with an increase in breast cancer,13 mammography following breast augmentation can be more difficult to interpret and may result in a delay in diagnosis. In a prospective study of asymptomatic women who were diagnosed with breast cancer, 137 had augmentation and 685 did not. Miglioretti and colleagues noted that the sensitivity of screening mammography was lower in the augmentation cohort.14 To enhance accuracy, breast implant displacement views (in which the breast tissue is pulled forward and the implant is displaced posteriorly to improve visualization) have been recommended.15 A retrospective review provides data reporting no effect on interpretation of mammograms following augmentation.16 The American Cancer Society recommends the same screening for women with implants as without implants, starting at age 40 years.17
Paget disease of the breast
Paget disease of the breast was first described by Sir James Paget in 1874. He also defined Paget disease of extramammary tissue, bone, vulva, and penis. Paget disease of the breast is a rare type of cancer in which the skin and nipple are involved frequently in association with DCIS or invasive breast cancer. The skin has an eczema-like appearance. Characteristic Paget (malignant) cells are large with clear cytoplasm (clear halo) and eccentric, hyperchromatic nuclei throughout the dermis. Assessment includes mammography and biopsy with immunohistochemical staining. Treatment varies by case and can include lumpectomy or mastectomy and chemotherapy and/or radiation therapy. Medications, including tamoxifen and anastrazole, have been recommended. Prognosis depends on nodal involvement. The disease is more common in women older than age 50.18
Related article:
The medicolegal considerations of interacting with your patients online
Legal issues: What was the gynecologist’s obligation?
The question remains, did the gynecologist have an obligation to obtain diagnostic mammography and ultrasound of the breast? Would it have been prudent for immediate referral to a breast surgeon? These are critical questions.
Negligence and the standard of care
The malpractice lawsuit against this gynecologist is based on negligence. In essence, it is a claim that the management provided fell below a standard of care that would be given by a reasonably careful and prudent gynecologist under the circumstances. This generally means that the care was less than the profession itself would find acceptable. Here the claim is essentially a diagnostic error claim, a common basis of malpractice.19 The delay in diagnosing breast cancer is a leading cause of malpractice claims against gynecologists.20,21
It is axiomatic that not all bad outcomes are the result of error, and not all errors are a result of an unacceptable standard of care. It is only bad outcomes resulting from careless or negligent errors that give rise to malpractice. But malpractice claims are often filed without knowing whether or not there was negligence—and, as we will see, many of those claims are without merit.
Was there negligence?
Ordinarily the plaintiff/patient must demonstrate that the care by the physician fell below the standard of care and, as a result, the patient suffered an injury. Stated another way, the plaintiff must show that the physician’s actions were unreasonable given all of the circumstances. In this case, the plaintiff must demonstrate that the gynecologist’s care was not appropriate. Failure to refer to another physician or provide additional testing is likely to be the major claim for negligence or inappropriate care.
Did that negligence cause injury?
Even if the plaintiff can demonstrate that the care was negligent, to prevail the plaintiff must also demonstrate that that negligence caused the injury—and that might be difficult.
What injury was caused by the delay in discovering the cancer? It did not apparently lead to the patient’s death. Can it be proven that the delay clearly shortened the patient’s life expectancy or required additional expensive and painful treatment? That may be difficult to demonstrate.
Causation. The causation factor could appear in many cancer or similar medical cases. On one hand, causation is a critical matter, but on the other hand, delay in treating cancer might have a very adverse effect on patients.
In recent years, about half the states have solved this dilemma by recognizing the concept of “loss of chance.”22 Essentially, this means that the health care provider, by delaying treatment, diminished the possibility that the patient would survive or recover fully.
There are significant variations among states in the loss-of-chance concept, many being quite technical.22 Thus, it is possible that a delay that reduced the chance of recovery or survival could be the injury and causal connection between the injury and the negligence. Even in states that recognize the loss-of-chance concept, the patient still must prove loss of chance.
Lessons learned from this case study
- Breast self-awareness has replaced (substituted) breast self-examination
- ACOG recommends breast mammography beginning at age 40 years
- Breast augmentation affects mammographic interpretation
- Prehaps if better communication had been provided initially, the patient would not have sought legal counsel or filed a weak suit
Related article:
Is the smartphone recording while the patient is under anesthesia?
Is this a strong malpractice case?
In the case presented here, it is not clear that the patient could show a meaningful loss of chance. If there is a delay in the breast cancer diagnosis, tumor doubling time would be an issue. While it is impossible to assess growth rate when a breast cancer is in its preclinical microscopic stage, doubling time can be 100 to 200 days. Therefore, it would take 20 years for the tumor to reach a 1- to 2-cm diameter. A log-normal distribution has been suggested for determining tumor growth.23
Although the facts in this case are sketchy, this does not look like a strong malpractice case. Given the expense, difficulty, and length of time it takes to pursue a malpractice case (especially for someone battling cancer), an obvious question is: Why would a patient file a lawsuit in these circumstances? There is no single answer to that, but the hope of getting rich is unlikely a primary motivation. Ironically, many malpractice cases are filed in which there was no error (or at least no negligent error).24
The search for what really happened, or why the bad event happened, is key. In other circumstances, it may be a desire for revenge or to protect other patients from similar bad results. Studies repeatedly have shown a somewhat limited correlation between negligent error and the decision to file a malpractice claim.24–26 In this case, the patient’s sense of being “blown off” during a particularly difficult time may represent the reason why she filed a malpractice lawsuit. Communication gaffes and poor physician-patient relationships undoubtedly contribute to medical malpractice claims.27,28 Improving communication with patients probably improves care, but it also almost certainly reduces the risk of a malpractice claim.29
Why a lawyer would accept this case is also unclear, but that is an issue for another day. Also for another day is the issue of product liability concerning breast implants. Those legal issues and related liability, primarily directed to the manufacturers of the implants, are interesting topics. They are also complex and will be the subject of a future article.
Finally, the patient’s decision to not pursue her lawsuit does not come as a surprise. A relatively small percentage of malpractice claims result in any meaningful financial recovery for the plaintiff. Few cases go to trial, and of those that do result in a verdict, about 75% of the verdicts are in favor of the physician.25,30 Many cases just fade away, either because the plaintiff never pursues them or because they are dismissed by a court at an early stage. Nonetheless, for the physician, even winning a malpractice case is disruptive and difficult. So in addition to ensuring careful, quality, and up-to-date care, a physician should seek to maintain good relationships and communication with patients to reduce the probability of even weak lawsuits being filed.
Share your thoughts! Send your Letter to the Editor to rbarbieri@frontlinemedcom.com. Please include your name and the city and state in which you practice.
- Siegel R, Naishadham D, Jemal A. Cancer statistics, 2013. CA Cancer J Clin. 2013;63(1):11–30.
- Mark K, Temkin S, Terplan M. Breast self-awareness: the evidence behind the euphemism. Obstet Gynecol. 2014;123(4):734–745.
- Thomas D, Gao D, Self S, et al. Randomized trial of breast self-examination in Shanghai: methodology and preliminary results. J Natl Cancer Inst. 1997;89(5):355–365.
- Jones S. Regular self-examination or clinical examination for early detection of breast cancer. Int J Epidemiol. 2008;37(6):1219.
- ACOG Committee on Practice Bulletins–Gynecology. ACOG Practice Bulletin No. 122. American College of Obstetricians and Gynecologists website. http://www.acog.org/Resources-And-Publications/Practice-Bulletins/Committee-on-Practice-Bulletins-Gynecology/Breast-Cancer-Screening. Published August 2011; reaffirmed 2014. Accessed November 10, 2016.
- ACOG Statement on breast cancer screening guidelines. American College of Obstetricians and Gynecologists website. http://www.acog.org/About-ACOG/News-Room/Statements/2016/ACOG-Statement-on-Breast-Cancer-Screening-Guidelines. Published January 11, 2016. Accessed November 10, 2016.
- Final Recommendation Statement: Breast Cancer: Screening. U.S. Preventive Services Task Force web site. https://www.uspreventiveservicestaskforce.org/Page/Document/RecommendationStatementFinal/breast-cancer-screening1. Published September 2016. Accessed November 10, 2016.
- American Cancer Society recommendations for early breast cancer detection in women without breast symptoms. American Cancer Society website. http://www.cancer.org/cancer/breastcancer/moreinformation/breastcancerearlydetection/breast-cancer-early-detection-acs-recs. Updated October 20, 2015. Accessed November 10, 2016.
- Mathis K, Hoskin T, Boughey J, et al. Palpable presentation of breast cancer persists in the era of screening mammography. J Am Coll Surg. 2010;210(3):314–318.
- MacBride M, Pruthi S, Bevers T. The evolution of breast self-examination to breast awareness. Breast J. 2012;18(6):641–643.
- Susan G. Komen Foundation. Breast self-awareness messages. http://ww5.komen.org/Breastcancer/Breastselfawareness.html. Accessed November 10, 2016.
- DelTurco MR, Mantellini P, Ciatto S, et al. Full-field digital versus screen-film mammography: comparative accuracy in concurrent screening cohorts. AJR. 2007;189(4):860–866.
- Susan G. Koman Foundation. Factors that do not increase breast cancer risk: Breast implants. https://ww5.komen.org/BreastCancer/FactorsThatDoNotIncreaseRisk.html. Updated October 28, 2016. Accessed November 10, 2016.
- Miglioretti D, Rutter, C, Geller B, et al. Effect of breast augmentation on the accuracy of mammography and cancer characteristics. JAMA. 2004;291(4):442–450.
- Eklund GW, Busby RC, Miller SH, Job JS. Improved imaging of the augmented breast. Am J Roentgenol. 1988;151(3):469–473.
- Kam K, Lee E, Pairwan S, et al. The effect of breast implants on mammogram outcomes. Am Surg. 2015;81(10):1053–1056.
- American Cancer Society. Mammograms and other imaging tests. http://www.cancer.org/acs/groups/cid/documents/webcontent/003178-pdf.pdf. Revised April 25, 2016. Accessed November 10, 2016.
- Dalberg K, Hellborg H, Warnberg F. Paget’s disease of the nipple in a population based cohort. Breast Cancer Res Treat. 2008;111(2):313–319.
- Saber Tehrani A, Lee H, Mathews S, et al. 25-year summary of US malpractice claims for diagnostic errors 1986–2010: an analysis from the National Practitioner Data Bank. BMJ Qual Saf. 2013;22(8):672–680.
- White AA, Pichert JW, Bledsoe SH, Irwin C, Entman SS. Cause and effect analysis of closed claims in obstetrics and gynecology. Obstet Gynecol. 2005;105(5 pt 1):1031–1038.
- Ward CJ, Green VL. Risk management and medico-legal issues in breast cancer. Clin Obstet Gynecol. 2016;59(2):439–446.
- Guest L, Schap D, Tran T. The “Loss of Chance” rule as a special category of damages in medical malpractice: a state-by-state analysis. J Legal Econ. 2015;21(2):53–107.
- Kuroishi T, Tominaga S, Morimoto T, et al. Tumor growth rate and prognosis of breast cancer mainly detected by mass screening. Jpn J Cancer Res. 1990;81(5):454–462.
- Localio AR, Lawthers AG, Brennan TA, et al. Relation between malpractice claims and adverse events due to negligence: results of the Harvard Medical Practice Study III. N Engl J Medicine. 1991;325(4):245–251.
- Gandhi TK, Kachalia A, Thomas EJ, et al. Missed and delayed diagnoses in the ambulatory setting: a study of closed malpractice claims. Ann Intern Med. 2006;145(7):488–496.
- Studdert DM, Mello MM, Gawande AA, et al. Claims, errors, and compensation payments in medical malpractice litigation. N Engl J Med. 2006;354(19):2024–2033.
- Huntington B, Kuhn N. Communication gaffes: a root cause of malpractice claims. Proc (Bayl Univ Med Cent). 2003;16(2):157–161.
- Moore PJ, Adler NE, Robertson PA. Medical malpractice: the effect of doctor-patient relations on medical patient perceptions and malpractice intentions. West J Med. 2000;173(4):244–250.
- Hickson GB, Jenkins AD. Identifying and addressing communication failures as a means of reducing unnecessary malpractice claims. NC Med J. 2007;68(5):362–364.
- Jena AB, Chandra A, Lakdawalla D, Seabury S. Outcomes of medical malpractice litigation against US physicians. Arch Intern Med. 2012;172(11):892–894.
- Siegel R, Naishadham D, Jemal A. Cancer statistics, 2013. CA Cancer J Clin. 2013;63(1):11–30.
- Mark K, Temkin S, Terplan M. Breast self-awareness: the evidence behind the euphemism. Obstet Gynecol. 2014;123(4):734–745.
- Thomas D, Gao D, Self S, et al. Randomized trial of breast self-examination in Shanghai: methodology and preliminary results. J Natl Cancer Inst. 1997;89(5):355–365.
- Jones S. Regular self-examination or clinical examination for early detection of breast cancer. Int J Epidemiol. 2008;37(6):1219.
- ACOG Committee on Practice Bulletins–Gynecology. ACOG Practice Bulletin No. 122. American College of Obstetricians and Gynecologists website. http://www.acog.org/Resources-And-Publications/Practice-Bulletins/Committee-on-Practice-Bulletins-Gynecology/Breast-Cancer-Screening. Published August 2011; reaffirmed 2014. Accessed November 10, 2016.
- ACOG Statement on breast cancer screening guidelines. American College of Obstetricians and Gynecologists website. http://www.acog.org/About-ACOG/News-Room/Statements/2016/ACOG-Statement-on-Breast-Cancer-Screening-Guidelines. Published January 11, 2016. Accessed November 10, 2016.
- Final Recommendation Statement: Breast Cancer: Screening. U.S. Preventive Services Task Force web site. https://www.uspreventiveservicestaskforce.org/Page/Document/RecommendationStatementFinal/breast-cancer-screening1. Published September 2016. Accessed November 10, 2016.
- American Cancer Society recommendations for early breast cancer detection in women without breast symptoms. American Cancer Society website. http://www.cancer.org/cancer/breastcancer/moreinformation/breastcancerearlydetection/breast-cancer-early-detection-acs-recs. Updated October 20, 2015. Accessed November 10, 2016.
- Mathis K, Hoskin T, Boughey J, et al. Palpable presentation of breast cancer persists in the era of screening mammography. J Am Coll Surg. 2010;210(3):314–318.
- MacBride M, Pruthi S, Bevers T. The evolution of breast self-examination to breast awareness. Breast J. 2012;18(6):641–643.
- Susan G. Komen Foundation. Breast self-awareness messages. http://ww5.komen.org/Breastcancer/Breastselfawareness.html. Accessed November 10, 2016.
- DelTurco MR, Mantellini P, Ciatto S, et al. Full-field digital versus screen-film mammography: comparative accuracy in concurrent screening cohorts. AJR. 2007;189(4):860–866.
- Susan G. Koman Foundation. Factors that do not increase breast cancer risk: Breast implants. https://ww5.komen.org/BreastCancer/FactorsThatDoNotIncreaseRisk.html. Updated October 28, 2016. Accessed November 10, 2016.
- Miglioretti D, Rutter, C, Geller B, et al. Effect of breast augmentation on the accuracy of mammography and cancer characteristics. JAMA. 2004;291(4):442–450.
- Eklund GW, Busby RC, Miller SH, Job JS. Improved imaging of the augmented breast. Am J Roentgenol. 1988;151(3):469–473.
- Kam K, Lee E, Pairwan S, et al. The effect of breast implants on mammogram outcomes. Am Surg. 2015;81(10):1053–1056.
- American Cancer Society. Mammograms and other imaging tests. http://www.cancer.org/acs/groups/cid/documents/webcontent/003178-pdf.pdf. Revised April 25, 2016. Accessed November 10, 2016.
- Dalberg K, Hellborg H, Warnberg F. Paget’s disease of the nipple in a population based cohort. Breast Cancer Res Treat. 2008;111(2):313–319.
- Saber Tehrani A, Lee H, Mathews S, et al. 25-year summary of US malpractice claims for diagnostic errors 1986–2010: an analysis from the National Practitioner Data Bank. BMJ Qual Saf. 2013;22(8):672–680.
- White AA, Pichert JW, Bledsoe SH, Irwin C, Entman SS. Cause and effect analysis of closed claims in obstetrics and gynecology. Obstet Gynecol. 2005;105(5 pt 1):1031–1038.
- Ward CJ, Green VL. Risk management and medico-legal issues in breast cancer. Clin Obstet Gynecol. 2016;59(2):439–446.
- Guest L, Schap D, Tran T. The “Loss of Chance” rule as a special category of damages in medical malpractice: a state-by-state analysis. J Legal Econ. 2015;21(2):53–107.
- Kuroishi T, Tominaga S, Morimoto T, et al. Tumor growth rate and prognosis of breast cancer mainly detected by mass screening. Jpn J Cancer Res. 1990;81(5):454–462.
- Localio AR, Lawthers AG, Brennan TA, et al. Relation between malpractice claims and adverse events due to negligence: results of the Harvard Medical Practice Study III. N Engl J Medicine. 1991;325(4):245–251.
- Gandhi TK, Kachalia A, Thomas EJ, et al. Missed and delayed diagnoses in the ambulatory setting: a study of closed malpractice claims. Ann Intern Med. 2006;145(7):488–496.
- Studdert DM, Mello MM, Gawande AA, et al. Claims, errors, and compensation payments in medical malpractice litigation. N Engl J Med. 2006;354(19):2024–2033.
- Huntington B, Kuhn N. Communication gaffes: a root cause of malpractice claims. Proc (Bayl Univ Med Cent). 2003;16(2):157–161.
- Moore PJ, Adler NE, Robertson PA. Medical malpractice: the effect of doctor-patient relations on medical patient perceptions and malpractice intentions. West J Med. 2000;173(4):244–250.
- Hickson GB, Jenkins AD. Identifying and addressing communication failures as a means of reducing unnecessary malpractice claims. NC Med J. 2007;68(5):362–364.
- Jena AB, Chandra A, Lakdawalla D, Seabury S. Outcomes of medical malpractice litigation against US physicians. Arch Intern Med. 2012;172(11):892–894.
4 Supreme Court decisions important to ObGyns from the 2015−2016 term
Each year, the decisions of the Supreme Court have a significant impact on ObGyn practice. During the 2015–2016 term, which ended in June, the Court issued important rulings on abortion facilities, Affordable Care Act (ACA) contraception coverage, health care False Claims Act (FCA) liability, and state health care data collection. The American Medical Association (AMA), the Association of American Medical Colleges (AAMC), the American College of Obstetricians and Gynecologists (ACOG), and other organizations that represent health care professionals play an important role in health-related Supreme Court cases. For example, amicus curiae (“friend of the Court”) briefs are filed not by parties to a case but by organizations that have a special insight into or interest in a case. Although the extent to which amicus briefs influence cases is often unclear, organization representatives think their briefs make a difference, and briefs undoubtedly do in some cases.
The 2016 presidential election will determine the Supreme Court make-up for the next term, but in this article we consider recent cases that affect ObGyns’ practice in particular. We start with the cases in which professional organizations filed amicus briefs and then turn to other notable cases.
1. Abortion access in Texas and other states
The most important ObGyn case of the 2015–2016 term was Whole Woman’s Health v Hellerstedt.1
At stake. Texas adopted a statute requiring 1) that physicians who perform abortions have admitting privileges at a hospital within 30 miles of the clinic and 2) that abortion clinics meet the state’s standards for ambulatory surgical centers. The current law, upheld by the Court some years ago, is that state laws affecting abortion are unconstitutional if they “unduly burden” the right to abortion. By undue burden, the Court meant, “Regulations that have the purpose or effect of presenting a substantial obstacle to a woman seeking an abortion impose an undue burden on the right.” The question in the Texas case was whether the statute’s 2 requirements were undue.
ACOG, AMA, and other groups filed a brief stating that the Texas law did not promote the welfare of women but instead was unnecessary and not “supported by accepted medical practice or scientific evidence.”2 In another brief the Society of Hospital Medicine and the Society of ObGyn Hospitalists also indicated that having admitting privileges is appropriate only for physicians who regularly admit patients to a hospital.3
A brief filed by the American Association of Pro-Life Obstetricians and Gynecologists and several other organizations argued the other side: “The surgical center and admitting privileges requirements imposed by the Act reflect the professional standard of practice for outpatient gynecological and similar surgery.”4
Final ruling. In a 5−3 decision, the Court struck down the Texas law for providing little or no health benefits while significantly burdening abortion facility access. Many clinics had closed or were in plans to because of the difficulty and expense of complying with the law. This case has national implications. Similar laws, either in place or being considered in other states, will almost certainly be ruled unconstitutional.
2. Contraceptive coverage
The case of Zubik v Burwell was closely watched this past year.
At stake. Under the ACA, a nonprofit religious organization may certify its objection to its insurance plan’s contraception coverage, at which point other arrangements are made to provide contraceptive coverage through the same plan. Religious organizations objected to the certification requirement.
A brief filed by ACOG, Physicians for Reproductive Health, and other groups emphasized the importance of providing contraceptives and contraceptive counseling as part of regular health care and suggested that the current accommodation for religious organizations is appropriate.5
After hearing the formal oral arguments, the Court asked for additional briefs on “whether contraceptive coverage could be provided to petitioners’ employees, through petitioners’ insurance companies, without any such notice from petitioners.”6
Final ruling. The parties agreed such a system would resolve the issue, so the Court sent the case back to the lower court to work out the details. In effect, the case was mediated—an unusual if not unique action for the Court. The resolution probably will achieve what the briefs sought—access to contraceptives and continuity of care.
3. Fraud and abuse litigation
The FCA, which provides for triple damages (3 times actual damages) and stiff civil penalties for anyone who presents the federal government (Medicare, Medicaid) with false claims for goods or services, is a major means of uncovering and punishing health care fraud and abuse. In health care, this law has been used to prosecute cases involving services paid for but not provided, unnecessary services, and off-label pharmaceutical promotion.
An important part of the FCA is that it allows a private intervenor (whistleblower) to initiate an action against a health care provider. The government may then take up the case. If not, the intervenor may pursue it; the incentive is 15% to 30% of the damages the government is awarded.
At stake. The Court was asked if “implied certification” applies to FCA cases.7 Implied certification means that requesting a payment from Medicare or Medicaid implies that the provider is not knowingly withholding information material to the government’s decision to pay the claim. In separately filed briefs, AMA et al8 and American Hospital Association (AHA) et al9 argued that applying implied certification to FCA cases would expand FCA litigation (particularly by intervenors), which is already expensive for health care institutions.
Final ruling. The Court unanimously adopted implied certification but noted that nondisclosure of information must be shown to be a material misrepresentation rather than a trivial regulatory or contractual violation. Furthermore, the Court emphasized that the basis for a claim must be an allegation of fraud, not of malpractice. These findings, which certainly are not what the health care organizations had hoped for, likely will lead to an increase in FCA cases.
4. Collection of state health care data
In Vermont, and about 20 other states that collect data on health care utilization and costs, health insurers and other entities are required to submit detailed reports about health care claims.10 Some insurers objected to this requirement.
At stake. An AHA–AAMC brief noted the importance of health care data and of Vermont’s collecting these data as contributing to better, more efficient health care delivery.11 Another brief, filed by AMA and the Vermont Medical Society, presented more legal or statutory arguments.12
Final ruling. The Court held that the Vermont plan and similar plans violate the federal Employee Retirement Income Security Act of 1974. As health insurance companies and other entities already provide detailed utilization and cost data to the federal government, producing up to 50 additional reports for state governments would be burdensome. Any state that wants the information, the Court said, should obtain it from the federal government.
More notable 2015-2016 Supreme Court decisions.
The Court:
- permitted limited consideration of race in university admissions. ACOG, AAMC, and AMA with many other groups filed an amicus brief supporting medical school and university affirmative action programs.1
- held that a state must give full faith and credit to the adoption orders of the courts of other states (this case involved an LGBT couple).2
- held that states may require (without a search warrant) a breathalyzer test, but not a blood test, for a driver suspected of drinking.3
- narrowed the ability of the federal government to seize or restrain (before trial) the assets of a person charged with criminal health care offenses.4
- temporarily stayed the August 2016 US Department of Education order to schools to allow transgender students to use the facilities in which they feel "most comfortable." The Court likely will take up this case very soon.5
- Fisher v University of Texas at Austin et al, No. 14-981 (2016). https://www.supremecourt.gov/opinions/15pdf/14-981_4g15.pdf. Accessed August 30, 2016.
- V.L. v E.L. et al, No. 15-648 (2016). https://www.supremecourt.gov/opinions/15pdf/15-648_d18e.pdf. Accessed August 30, 2016.
- Birchfield v North Dakota, No. 14-1468 (2016). https://www.supremecourt.gov/opinions/15pdf/14-1468_8n59.pdf. Accessed August 30, 2016.
- Luis v United States, No. 14-419 (2016). https://www.supremecourt.gov/opinions/15pdf/14-419_nmip.pdf. Accessed August 30, 2016.
- Gloucester County School Board v G.G., by his next friend and mother, Deidre Grimm, No. 16A52 (2016). https://www.supremecourt.gov/opinions/15pdf/16a52_8759.pdf. Accessed August 30, 2016.
What’s to come
The Court’s recent decisions on access to abortion services and contraceptives were good for patients of ObGyns, but its decisions on health care FCA liability and state health care data collection were, arguably, not as good for ObGyn business practices.
The Court itself had an unusual year. Justice Scalia died in February, and Congress’s inaction on seating a replacement meant that most of the term’s cases were decided by an 8-member Court. Nevertheless, the Court was deadlocked 4−4 on only 4 of the 80 cases it heard. In addition, it was relatively agreed on outcomes; in only about one-third of cases were there more than 2 justices disagreeing with the outcome.
It is unlikely that a replacement for Justice Scalia will be confirmed before the Court begins its new term in October. The need to replace Justice Scalia and the potential turnover of other Court members—Justice Ginsburg is 83, Justice Kennedy is 80, and Justice Breyer is 78—are reminders of the importance of this year’s presidential election. In the meantime, the Court is accepting the cases that will make up the coming term’s docket, and ObGyns undoubtedly will play a role in cases that involve health care.
Share your thoughts! Send your Letter to the Editor to rbarbieri@frontlinemedcom.com. Please include your name and the city and state in which you practice.
- Whole Woman's Health et al v Hellerstedt, Commissioner, Texas Department of State Health Services, et al, No. 15-274 (2016). https://www.supremecourt.gov/opinions/15pdf/15-274_new_e18f.pdf. Accessed August 30, 2016.
- Amici curiae brief filed by American College of Obstetricians and Gynecologists, American Medical Association, American Academy of Family Physicians, American Osteopathic Association, and American Academy of Pediatrics in support of petitioners. Whole Woman's Health et al v Cole, Commissioner, Texas Department of State Health Services, et al, No. 15-274 (2016). http://www.scotusblog.com/wp-content/uploads/2016/01/ACOG-WilmerHale.pdf. Accessed August 30, 2016.
- Amici curiae brief filed by Society of Hospital Medicine and Society of Ob/Gyn Hospitalists in support of petitioners. Whole Woman's Health et al v Cole, Commissioner, Texas Department of State Health Services, et al, No. 15-274 (2016). http://www.scotusblog.com/wp-content/uploads/2016/01/Society-of-Hospital-Medicine-Crowell.pdf. Accessed August 30, 2016.
- Amici curiae brief filed by American Association of Pro-Life Obstetricians and Gynecologists, American College of Pediatricians, Christian Medical & Dental Association, Catholic Medical Association, and Physicians for Life in support of respondents. Whole Woman's Health et al v Hellerstedt, Commissioner, Texas Department of State Health Services, et al. http://www.scotusblog.com/wp-content/uploads/2016/02/15-274-bsac-American-Association-of-Pro-Life-Obstetricians-and-Gynecolog....pdf. Accessed August 30, 2016.
- Amici curiae brief filed by American College of Obstetricians and Gynecologists, Physicians for Reproductive Health, American Academy of Family Physicians, American Nurses Association, et al in support of the government and affirmance. Zubik et al v Burwell, Secretary of Health and Human Services, et al, Nos. 14-1418, 14-1458, 14-1505, 15-35, 15-105, 15-119, and 15-191. http://www.scotusblog.com/wp-content/uploads/2016/02/Docfoc.com-Amicus-Brief-Zubik-v.-Burwell.pdf. Accessed August 30, 2016.
- Zubik et al v Burwell, Secretary of Health and Human Services, et al, No. 14-1418 (2016). https://www.supremecourt.gov/opinions/15pdf/14-1418_8758.pdf. Accessed August 30, 2016.
- Universal Health Services, Inc v United States et al ex rel. Escobar et al, No. 15-7 (2016). https://www.supremecourt.gov/opinions/15pdf/15-7_a074.pdf. Accessed August 30, 2016.
- Amici curiae brief filed by American Medical Association, National Association of Chain Drug Stores, National Association of Manufacturers, American Tort Reform Association and NFIB Small Business Legal Center in support of petitioner. Universal Health Services, Inc v United States and Commonwealth of Massachusetts ex rel. Escobar and Correa, No. 15-7 (2016). http://www.scotusblog.com/wp-content/uploads/2016/01/15-7-tsac-American-Medical-Association.pdf. Accessed August 30, 2016.
- Amici curiae brief filed by American Hospital Association, Federation of American Hospitals, and Association of American Medical Colleges in support of petitioner. Universal Health Services, Inc v United States and Commonwealth of Massachusetts ex rel. Escobar and Correa, No. 15-7 (2016). http://www.scotusblog.com/wp-content/uploads/2016/01/15-7tsacAHAFAHAAMC.pdf. Accessed August 30, 2016.
- Gobeille, Chair, Vermont Green Mountain Care Board, v Liberty Mutual Insurance Co, No. 14-181 (2016). https://www.supremecourt.gov/opinions/15pdf/14-181_5426.pdf. Accessed August 30, 2016.
- Amici curiae brief filed by American Hospital Association and Association of American Medical Colleges in support of petitioner. Gobeille, Chair, Vermont Green Mountain Care Board, v Liberty Mutual Insurance Co, No. 14-181 (2016). http://www.scotusblog.com/wp-content/uploads/2015/09/150904-amicus-gobeille-liberty.pdf. Accessed August 30, 2016.
- Amici curiae brief filed by American Medical Association and Vermont Medical Society in support of petitioner. Gobeille, Chair, Vermont Green Mountain Care Board, v Liberty Mutual Insurance Co, No. 14-181 (2016). http://www.scotusblog.com/wp-content/uploads/2015/09/VHCURES-Amicus-Brief-of-American-Medical-Association.pdf. Accessed August 30, 2016.
Each year, the decisions of the Supreme Court have a significant impact on ObGyn practice. During the 2015–2016 term, which ended in June, the Court issued important rulings on abortion facilities, Affordable Care Act (ACA) contraception coverage, health care False Claims Act (FCA) liability, and state health care data collection. The American Medical Association (AMA), the Association of American Medical Colleges (AAMC), the American College of Obstetricians and Gynecologists (ACOG), and other organizations that represent health care professionals play an important role in health-related Supreme Court cases. For example, amicus curiae (“friend of the Court”) briefs are filed not by parties to a case but by organizations that have a special insight into or interest in a case. Although the extent to which amicus briefs influence cases is often unclear, organization representatives think their briefs make a difference, and briefs undoubtedly do in some cases.
The 2016 presidential election will determine the Supreme Court make-up for the next term, but in this article we consider recent cases that affect ObGyns’ practice in particular. We start with the cases in which professional organizations filed amicus briefs and then turn to other notable cases.
1. Abortion access in Texas and other states
The most important ObGyn case of the 2015–2016 term was Whole Woman’s Health v Hellerstedt.1
At stake. Texas adopted a statute requiring 1) that physicians who perform abortions have admitting privileges at a hospital within 30 miles of the clinic and 2) that abortion clinics meet the state’s standards for ambulatory surgical centers. The current law, upheld by the Court some years ago, is that state laws affecting abortion are unconstitutional if they “unduly burden” the right to abortion. By undue burden, the Court meant, “Regulations that have the purpose or effect of presenting a substantial obstacle to a woman seeking an abortion impose an undue burden on the right.” The question in the Texas case was whether the statute’s 2 requirements were undue.
ACOG, AMA, and other groups filed a brief stating that the Texas law did not promote the welfare of women but instead was unnecessary and not “supported by accepted medical practice or scientific evidence.”2 In another brief the Society of Hospital Medicine and the Society of ObGyn Hospitalists also indicated that having admitting privileges is appropriate only for physicians who regularly admit patients to a hospital.3
A brief filed by the American Association of Pro-Life Obstetricians and Gynecologists and several other organizations argued the other side: “The surgical center and admitting privileges requirements imposed by the Act reflect the professional standard of practice for outpatient gynecological and similar surgery.”4
Final ruling. In a 5−3 decision, the Court struck down the Texas law for providing little or no health benefits while significantly burdening abortion facility access. Many clinics had closed or were in plans to because of the difficulty and expense of complying with the law. This case has national implications. Similar laws, either in place or being considered in other states, will almost certainly be ruled unconstitutional.
2. Contraceptive coverage
The case of Zubik v Burwell was closely watched this past year.
At stake. Under the ACA, a nonprofit religious organization may certify its objection to its insurance plan’s contraception coverage, at which point other arrangements are made to provide contraceptive coverage through the same plan. Religious organizations objected to the certification requirement.
A brief filed by ACOG, Physicians for Reproductive Health, and other groups emphasized the importance of providing contraceptives and contraceptive counseling as part of regular health care and suggested that the current accommodation for religious organizations is appropriate.5
After hearing the formal oral arguments, the Court asked for additional briefs on “whether contraceptive coverage could be provided to petitioners’ employees, through petitioners’ insurance companies, without any such notice from petitioners.”6
Final ruling. The parties agreed such a system would resolve the issue, so the Court sent the case back to the lower court to work out the details. In effect, the case was mediated—an unusual if not unique action for the Court. The resolution probably will achieve what the briefs sought—access to contraceptives and continuity of care.
3. Fraud and abuse litigation
The FCA, which provides for triple damages (3 times actual damages) and stiff civil penalties for anyone who presents the federal government (Medicare, Medicaid) with false claims for goods or services, is a major means of uncovering and punishing health care fraud and abuse. In health care, this law has been used to prosecute cases involving services paid for but not provided, unnecessary services, and off-label pharmaceutical promotion.
An important part of the FCA is that it allows a private intervenor (whistleblower) to initiate an action against a health care provider. The government may then take up the case. If not, the intervenor may pursue it; the incentive is 15% to 30% of the damages the government is awarded.
At stake. The Court was asked if “implied certification” applies to FCA cases.7 Implied certification means that requesting a payment from Medicare or Medicaid implies that the provider is not knowingly withholding information material to the government’s decision to pay the claim. In separately filed briefs, AMA et al8 and American Hospital Association (AHA) et al9 argued that applying implied certification to FCA cases would expand FCA litigation (particularly by intervenors), which is already expensive for health care institutions.
Final ruling. The Court unanimously adopted implied certification but noted that nondisclosure of information must be shown to be a material misrepresentation rather than a trivial regulatory or contractual violation. Furthermore, the Court emphasized that the basis for a claim must be an allegation of fraud, not of malpractice. These findings, which certainly are not what the health care organizations had hoped for, likely will lead to an increase in FCA cases.
4. Collection of state health care data
In Vermont, and about 20 other states that collect data on health care utilization and costs, health insurers and other entities are required to submit detailed reports about health care claims.10 Some insurers objected to this requirement.
At stake. An AHA–AAMC brief noted the importance of health care data and of Vermont’s collecting these data as contributing to better, more efficient health care delivery.11 Another brief, filed by AMA and the Vermont Medical Society, presented more legal or statutory arguments.12
Final ruling. The Court held that the Vermont plan and similar plans violate the federal Employee Retirement Income Security Act of 1974. As health insurance companies and other entities already provide detailed utilization and cost data to the federal government, producing up to 50 additional reports for state governments would be burdensome. Any state that wants the information, the Court said, should obtain it from the federal government.
More notable 2015-2016 Supreme Court decisions.
The Court:
- permitted limited consideration of race in university admissions. ACOG, AAMC, and AMA with many other groups filed an amicus brief supporting medical school and university affirmative action programs.1
- held that a state must give full faith and credit to the adoption orders of the courts of other states (this case involved an LGBT couple).2
- held that states may require (without a search warrant) a breathalyzer test, but not a blood test, for a driver suspected of drinking.3
- narrowed the ability of the federal government to seize or restrain (before trial) the assets of a person charged with criminal health care offenses.4
- temporarily stayed the August 2016 US Department of Education order to schools to allow transgender students to use the facilities in which they feel "most comfortable." The Court likely will take up this case very soon.5
- Fisher v University of Texas at Austin et al, No. 14-981 (2016). https://www.supremecourt.gov/opinions/15pdf/14-981_4g15.pdf. Accessed August 30, 2016.
- V.L. v E.L. et al, No. 15-648 (2016). https://www.supremecourt.gov/opinions/15pdf/15-648_d18e.pdf. Accessed August 30, 2016.
- Birchfield v North Dakota, No. 14-1468 (2016). https://www.supremecourt.gov/opinions/15pdf/14-1468_8n59.pdf. Accessed August 30, 2016.
- Luis v United States, No. 14-419 (2016). https://www.supremecourt.gov/opinions/15pdf/14-419_nmip.pdf. Accessed August 30, 2016.
- Gloucester County School Board v G.G., by his next friend and mother, Deidre Grimm, No. 16A52 (2016). https://www.supremecourt.gov/opinions/15pdf/16a52_8759.pdf. Accessed August 30, 2016.
What’s to come
The Court’s recent decisions on access to abortion services and contraceptives were good for patients of ObGyns, but its decisions on health care FCA liability and state health care data collection were, arguably, not as good for ObGyn business practices.
The Court itself had an unusual year. Justice Scalia died in February, and Congress’s inaction on seating a replacement meant that most of the term’s cases were decided by an 8-member Court. Nevertheless, the Court was deadlocked 4−4 on only 4 of the 80 cases it heard. In addition, it was relatively agreed on outcomes; in only about one-third of cases were there more than 2 justices disagreeing with the outcome.
It is unlikely that a replacement for Justice Scalia will be confirmed before the Court begins its new term in October. The need to replace Justice Scalia and the potential turnover of other Court members—Justice Ginsburg is 83, Justice Kennedy is 80, and Justice Breyer is 78—are reminders of the importance of this year’s presidential election. In the meantime, the Court is accepting the cases that will make up the coming term’s docket, and ObGyns undoubtedly will play a role in cases that involve health care.
Share your thoughts! Send your Letter to the Editor to rbarbieri@frontlinemedcom.com. Please include your name and the city and state in which you practice.
Each year, the decisions of the Supreme Court have a significant impact on ObGyn practice. During the 2015–2016 term, which ended in June, the Court issued important rulings on abortion facilities, Affordable Care Act (ACA) contraception coverage, health care False Claims Act (FCA) liability, and state health care data collection. The American Medical Association (AMA), the Association of American Medical Colleges (AAMC), the American College of Obstetricians and Gynecologists (ACOG), and other organizations that represent health care professionals play an important role in health-related Supreme Court cases. For example, amicus curiae (“friend of the Court”) briefs are filed not by parties to a case but by organizations that have a special insight into or interest in a case. Although the extent to which amicus briefs influence cases is often unclear, organization representatives think their briefs make a difference, and briefs undoubtedly do in some cases.
The 2016 presidential election will determine the Supreme Court make-up for the next term, but in this article we consider recent cases that affect ObGyns’ practice in particular. We start with the cases in which professional organizations filed amicus briefs and then turn to other notable cases.
1. Abortion access in Texas and other states
The most important ObGyn case of the 2015–2016 term was Whole Woman’s Health v Hellerstedt.1
At stake. Texas adopted a statute requiring 1) that physicians who perform abortions have admitting privileges at a hospital within 30 miles of the clinic and 2) that abortion clinics meet the state’s standards for ambulatory surgical centers. The current law, upheld by the Court some years ago, is that state laws affecting abortion are unconstitutional if they “unduly burden” the right to abortion. By undue burden, the Court meant, “Regulations that have the purpose or effect of presenting a substantial obstacle to a woman seeking an abortion impose an undue burden on the right.” The question in the Texas case was whether the statute’s 2 requirements were undue.
ACOG, AMA, and other groups filed a brief stating that the Texas law did not promote the welfare of women but instead was unnecessary and not “supported by accepted medical practice or scientific evidence.”2 In another brief the Society of Hospital Medicine and the Society of ObGyn Hospitalists also indicated that having admitting privileges is appropriate only for physicians who regularly admit patients to a hospital.3
A brief filed by the American Association of Pro-Life Obstetricians and Gynecologists and several other organizations argued the other side: “The surgical center and admitting privileges requirements imposed by the Act reflect the professional standard of practice for outpatient gynecological and similar surgery.”4
Final ruling. In a 5−3 decision, the Court struck down the Texas law for providing little or no health benefits while significantly burdening abortion facility access. Many clinics had closed or were in plans to because of the difficulty and expense of complying with the law. This case has national implications. Similar laws, either in place or being considered in other states, will almost certainly be ruled unconstitutional.
2. Contraceptive coverage
The case of Zubik v Burwell was closely watched this past year.
At stake. Under the ACA, a nonprofit religious organization may certify its objection to its insurance plan’s contraception coverage, at which point other arrangements are made to provide contraceptive coverage through the same plan. Religious organizations objected to the certification requirement.
A brief filed by ACOG, Physicians for Reproductive Health, and other groups emphasized the importance of providing contraceptives and contraceptive counseling as part of regular health care and suggested that the current accommodation for religious organizations is appropriate.5
After hearing the formal oral arguments, the Court asked for additional briefs on “whether contraceptive coverage could be provided to petitioners’ employees, through petitioners’ insurance companies, without any such notice from petitioners.”6
Final ruling. The parties agreed such a system would resolve the issue, so the Court sent the case back to the lower court to work out the details. In effect, the case was mediated—an unusual if not unique action for the Court. The resolution probably will achieve what the briefs sought—access to contraceptives and continuity of care.
3. Fraud and abuse litigation
The FCA, which provides for triple damages (3 times actual damages) and stiff civil penalties for anyone who presents the federal government (Medicare, Medicaid) with false claims for goods or services, is a major means of uncovering and punishing health care fraud and abuse. In health care, this law has been used to prosecute cases involving services paid for but not provided, unnecessary services, and off-label pharmaceutical promotion.
An important part of the FCA is that it allows a private intervenor (whistleblower) to initiate an action against a health care provider. The government may then take up the case. If not, the intervenor may pursue it; the incentive is 15% to 30% of the damages the government is awarded.
At stake. The Court was asked if “implied certification” applies to FCA cases.7 Implied certification means that requesting a payment from Medicare or Medicaid implies that the provider is not knowingly withholding information material to the government’s decision to pay the claim. In separately filed briefs, AMA et al8 and American Hospital Association (AHA) et al9 argued that applying implied certification to FCA cases would expand FCA litigation (particularly by intervenors), which is already expensive for health care institutions.
Final ruling. The Court unanimously adopted implied certification but noted that nondisclosure of information must be shown to be a material misrepresentation rather than a trivial regulatory or contractual violation. Furthermore, the Court emphasized that the basis for a claim must be an allegation of fraud, not of malpractice. These findings, which certainly are not what the health care organizations had hoped for, likely will lead to an increase in FCA cases.
4. Collection of state health care data
In Vermont, and about 20 other states that collect data on health care utilization and costs, health insurers and other entities are required to submit detailed reports about health care claims.10 Some insurers objected to this requirement.
At stake. An AHA–AAMC brief noted the importance of health care data and of Vermont’s collecting these data as contributing to better, more efficient health care delivery.11 Another brief, filed by AMA and the Vermont Medical Society, presented more legal or statutory arguments.12
Final ruling. The Court held that the Vermont plan and similar plans violate the federal Employee Retirement Income Security Act of 1974. As health insurance companies and other entities already provide detailed utilization and cost data to the federal government, producing up to 50 additional reports for state governments would be burdensome. Any state that wants the information, the Court said, should obtain it from the federal government.
More notable 2015-2016 Supreme Court decisions.
The Court:
- permitted limited consideration of race in university admissions. ACOG, AAMC, and AMA with many other groups filed an amicus brief supporting medical school and university affirmative action programs.1
- held that a state must give full faith and credit to the adoption orders of the courts of other states (this case involved an LGBT couple).2
- held that states may require (without a search warrant) a breathalyzer test, but not a blood test, for a driver suspected of drinking.3
- narrowed the ability of the federal government to seize or restrain (before trial) the assets of a person charged with criminal health care offenses.4
- temporarily stayed the August 2016 US Department of Education order to schools to allow transgender students to use the facilities in which they feel "most comfortable." The Court likely will take up this case very soon.5
- Fisher v University of Texas at Austin et al, No. 14-981 (2016). https://www.supremecourt.gov/opinions/15pdf/14-981_4g15.pdf. Accessed August 30, 2016.
- V.L. v E.L. et al, No. 15-648 (2016). https://www.supremecourt.gov/opinions/15pdf/15-648_d18e.pdf. Accessed August 30, 2016.
- Birchfield v North Dakota, No. 14-1468 (2016). https://www.supremecourt.gov/opinions/15pdf/14-1468_8n59.pdf. Accessed August 30, 2016.
- Luis v United States, No. 14-419 (2016). https://www.supremecourt.gov/opinions/15pdf/14-419_nmip.pdf. Accessed August 30, 2016.
- Gloucester County School Board v G.G., by his next friend and mother, Deidre Grimm, No. 16A52 (2016). https://www.supremecourt.gov/opinions/15pdf/16a52_8759.pdf. Accessed August 30, 2016.
What’s to come
The Court’s recent decisions on access to abortion services and contraceptives were good for patients of ObGyns, but its decisions on health care FCA liability and state health care data collection were, arguably, not as good for ObGyn business practices.
The Court itself had an unusual year. Justice Scalia died in February, and Congress’s inaction on seating a replacement meant that most of the term’s cases were decided by an 8-member Court. Nevertheless, the Court was deadlocked 4−4 on only 4 of the 80 cases it heard. In addition, it was relatively agreed on outcomes; in only about one-third of cases were there more than 2 justices disagreeing with the outcome.
It is unlikely that a replacement for Justice Scalia will be confirmed before the Court begins its new term in October. The need to replace Justice Scalia and the potential turnover of other Court members—Justice Ginsburg is 83, Justice Kennedy is 80, and Justice Breyer is 78—are reminders of the importance of this year’s presidential election. In the meantime, the Court is accepting the cases that will make up the coming term’s docket, and ObGyns undoubtedly will play a role in cases that involve health care.
Share your thoughts! Send your Letter to the Editor to rbarbieri@frontlinemedcom.com. Please include your name and the city and state in which you practice.
- Whole Woman's Health et al v Hellerstedt, Commissioner, Texas Department of State Health Services, et al, No. 15-274 (2016). https://www.supremecourt.gov/opinions/15pdf/15-274_new_e18f.pdf. Accessed August 30, 2016.
- Amici curiae brief filed by American College of Obstetricians and Gynecologists, American Medical Association, American Academy of Family Physicians, American Osteopathic Association, and American Academy of Pediatrics in support of petitioners. Whole Woman's Health et al v Cole, Commissioner, Texas Department of State Health Services, et al, No. 15-274 (2016). http://www.scotusblog.com/wp-content/uploads/2016/01/ACOG-WilmerHale.pdf. Accessed August 30, 2016.
- Amici curiae brief filed by Society of Hospital Medicine and Society of Ob/Gyn Hospitalists in support of petitioners. Whole Woman's Health et al v Cole, Commissioner, Texas Department of State Health Services, et al, No. 15-274 (2016). http://www.scotusblog.com/wp-content/uploads/2016/01/Society-of-Hospital-Medicine-Crowell.pdf. Accessed August 30, 2016.
- Amici curiae brief filed by American Association of Pro-Life Obstetricians and Gynecologists, American College of Pediatricians, Christian Medical & Dental Association, Catholic Medical Association, and Physicians for Life in support of respondents. Whole Woman's Health et al v Hellerstedt, Commissioner, Texas Department of State Health Services, et al. http://www.scotusblog.com/wp-content/uploads/2016/02/15-274-bsac-American-Association-of-Pro-Life-Obstetricians-and-Gynecolog....pdf. Accessed August 30, 2016.
- Amici curiae brief filed by American College of Obstetricians and Gynecologists, Physicians for Reproductive Health, American Academy of Family Physicians, American Nurses Association, et al in support of the government and affirmance. Zubik et al v Burwell, Secretary of Health and Human Services, et al, Nos. 14-1418, 14-1458, 14-1505, 15-35, 15-105, 15-119, and 15-191. http://www.scotusblog.com/wp-content/uploads/2016/02/Docfoc.com-Amicus-Brief-Zubik-v.-Burwell.pdf. Accessed August 30, 2016.
- Zubik et al v Burwell, Secretary of Health and Human Services, et al, No. 14-1418 (2016). https://www.supremecourt.gov/opinions/15pdf/14-1418_8758.pdf. Accessed August 30, 2016.
- Universal Health Services, Inc v United States et al ex rel. Escobar et al, No. 15-7 (2016). https://www.supremecourt.gov/opinions/15pdf/15-7_a074.pdf. Accessed August 30, 2016.
- Amici curiae brief filed by American Medical Association, National Association of Chain Drug Stores, National Association of Manufacturers, American Tort Reform Association and NFIB Small Business Legal Center in support of petitioner. Universal Health Services, Inc v United States and Commonwealth of Massachusetts ex rel. Escobar and Correa, No. 15-7 (2016). http://www.scotusblog.com/wp-content/uploads/2016/01/15-7-tsac-American-Medical-Association.pdf. Accessed August 30, 2016.
- Amici curiae brief filed by American Hospital Association, Federation of American Hospitals, and Association of American Medical Colleges in support of petitioner. Universal Health Services, Inc v United States and Commonwealth of Massachusetts ex rel. Escobar and Correa, No. 15-7 (2016). http://www.scotusblog.com/wp-content/uploads/2016/01/15-7tsacAHAFAHAAMC.pdf. Accessed August 30, 2016.
- Gobeille, Chair, Vermont Green Mountain Care Board, v Liberty Mutual Insurance Co, No. 14-181 (2016). https://www.supremecourt.gov/opinions/15pdf/14-181_5426.pdf. Accessed August 30, 2016.
- Amici curiae brief filed by American Hospital Association and Association of American Medical Colleges in support of petitioner. Gobeille, Chair, Vermont Green Mountain Care Board, v Liberty Mutual Insurance Co, No. 14-181 (2016). http://www.scotusblog.com/wp-content/uploads/2015/09/150904-amicus-gobeille-liberty.pdf. Accessed August 30, 2016.
- Amici curiae brief filed by American Medical Association and Vermont Medical Society in support of petitioner. Gobeille, Chair, Vermont Green Mountain Care Board, v Liberty Mutual Insurance Co, No. 14-181 (2016). http://www.scotusblog.com/wp-content/uploads/2015/09/VHCURES-Amicus-Brief-of-American-Medical-Association.pdf. Accessed August 30, 2016.
- Whole Woman's Health et al v Hellerstedt, Commissioner, Texas Department of State Health Services, et al, No. 15-274 (2016). https://www.supremecourt.gov/opinions/15pdf/15-274_new_e18f.pdf. Accessed August 30, 2016.
- Amici curiae brief filed by American College of Obstetricians and Gynecologists, American Medical Association, American Academy of Family Physicians, American Osteopathic Association, and American Academy of Pediatrics in support of petitioners. Whole Woman's Health et al v Cole, Commissioner, Texas Department of State Health Services, et al, No. 15-274 (2016). http://www.scotusblog.com/wp-content/uploads/2016/01/ACOG-WilmerHale.pdf. Accessed August 30, 2016.
- Amici curiae brief filed by Society of Hospital Medicine and Society of Ob/Gyn Hospitalists in support of petitioners. Whole Woman's Health et al v Cole, Commissioner, Texas Department of State Health Services, et al, No. 15-274 (2016). http://www.scotusblog.com/wp-content/uploads/2016/01/Society-of-Hospital-Medicine-Crowell.pdf. Accessed August 30, 2016.
- Amici curiae brief filed by American Association of Pro-Life Obstetricians and Gynecologists, American College of Pediatricians, Christian Medical & Dental Association, Catholic Medical Association, and Physicians for Life in support of respondents. Whole Woman's Health et al v Hellerstedt, Commissioner, Texas Department of State Health Services, et al. http://www.scotusblog.com/wp-content/uploads/2016/02/15-274-bsac-American-Association-of-Pro-Life-Obstetricians-and-Gynecolog....pdf. Accessed August 30, 2016.
- Amici curiae brief filed by American College of Obstetricians and Gynecologists, Physicians for Reproductive Health, American Academy of Family Physicians, American Nurses Association, et al in support of the government and affirmance. Zubik et al v Burwell, Secretary of Health and Human Services, et al, Nos. 14-1418, 14-1458, 14-1505, 15-35, 15-105, 15-119, and 15-191. http://www.scotusblog.com/wp-content/uploads/2016/02/Docfoc.com-Amicus-Brief-Zubik-v.-Burwell.pdf. Accessed August 30, 2016.
- Zubik et al v Burwell, Secretary of Health and Human Services, et al, No. 14-1418 (2016). https://www.supremecourt.gov/opinions/15pdf/14-1418_8758.pdf. Accessed August 30, 2016.
- Universal Health Services, Inc v United States et al ex rel. Escobar et al, No. 15-7 (2016). https://www.supremecourt.gov/opinions/15pdf/15-7_a074.pdf. Accessed August 30, 2016.
- Amici curiae brief filed by American Medical Association, National Association of Chain Drug Stores, National Association of Manufacturers, American Tort Reform Association and NFIB Small Business Legal Center in support of petitioner. Universal Health Services, Inc v United States and Commonwealth of Massachusetts ex rel. Escobar and Correa, No. 15-7 (2016). http://www.scotusblog.com/wp-content/uploads/2016/01/15-7-tsac-American-Medical-Association.pdf. Accessed August 30, 2016.
- Amici curiae brief filed by American Hospital Association, Federation of American Hospitals, and Association of American Medical Colleges in support of petitioner. Universal Health Services, Inc v United States and Commonwealth of Massachusetts ex rel. Escobar and Correa, No. 15-7 (2016). http://www.scotusblog.com/wp-content/uploads/2016/01/15-7tsacAHAFAHAAMC.pdf. Accessed August 30, 2016.
- Gobeille, Chair, Vermont Green Mountain Care Board, v Liberty Mutual Insurance Co, No. 14-181 (2016). https://www.supremecourt.gov/opinions/15pdf/14-181_5426.pdf. Accessed August 30, 2016.
- Amici curiae brief filed by American Hospital Association and Association of American Medical Colleges in support of petitioner. Gobeille, Chair, Vermont Green Mountain Care Board, v Liberty Mutual Insurance Co, No. 14-181 (2016). http://www.scotusblog.com/wp-content/uploads/2015/09/150904-amicus-gobeille-liberty.pdf. Accessed August 30, 2016.
- Amici curiae brief filed by American Medical Association and Vermont Medical Society in support of petitioner. Gobeille, Chair, Vermont Green Mountain Care Board, v Liberty Mutual Insurance Co, No. 14-181 (2016). http://www.scotusblog.com/wp-content/uploads/2015/09/VHCURES-Amicus-Brief-of-American-Medical-Association.pdf. Accessed August 30, 2016.
In this Article
- Whole Woman’s Health v Hellerstedt
- Fraud and abuse litigation
- What’s to come
Who is liable when a surgical error occurs?
CASE Surgeon accused of operating outside her scope of expertise
A 38-year-old woman (G2 P2002) presented to the emergency department (ED) with acute pelvic pain involving the right lower quadrant (RLQ). The patient had a history of stage IV endometriosis and chronic pelvic pain, primarily affecting the RLQ, that was treated by total laparoscopic hysterectomy with bilateral salpingo-oophorectomy 6 months earlier. Pertinent findings on physical examination included hypoactive bowel sounds and rebound tenderness. The ED physician ordered a computed tomography (CT) scan of the abdomen, which showed no evidence of ureteral injury or other abnormality. The gynecologist who performed the surgery 6 months ago evaluated the patient in the ED.
The gynecologist decided to perform operative laparoscopy because of the severity of the patient’s pain and duration of symptoms. Informed consent obtained in the ED before the patient received analgesics included a handwritten note that said “and other indicated procedures.” The patient signed the document prior to being taken to the operating room (OR). Time out occurred in the OR before anesthesia induction. The gynecologist proceeded with laparoscopic adhesiolysis with planned appendectomy, as she was trained. A normal appendix was noted and left intact. RLQ adhesions involving the colon and abdominal wall were treated with electrosurgical cautery. When the gynecologist found adhesions between the liver and diaphragm in the right upper quadrant (RUQ), she continued adhesiolysis. However, the diaphragm was inadvertently punctured.
As the gynecologist attempted to suture the defect laparoscopically, she encountered difficulty and converted to laparotomy. Adhesions were dense and initially precluded adequate closure of the diaphragmatic defect. The gynecologist persisted and ultimately the closure was adequate; laparotomy concluded. Postoperatively, the patient was given a diagnosis of atelectasis, primarily on the right side; a chest tube was placed by the general surgery team. The patient had an uneventful postoperative period and was discharged on postoperative day 5. One month later she returned to the ED with evidence of pneumonia; she was given a diagnosis of empyema, and antibiotics were administered. She responded well and was discharged after 6 days.
The patient filed a malpractice lawsuit against the gynecologist, the hospital, and associated practitioners. The suit made 3 negligence claims: 1) the surgery was improperly performed, as evidenced by the diaphragmatic perforation; 2) the gynecologist was not adequately trained for RUQ surgery, and 3) the hospital should not have permitted RUQ surgery to proceed. The liability claim cited the lack of qualification of a gynecologic surgeon to proceed with surgical intervention near the diaphragm and the associated consequences of practicing outside the scope of expertise.
Fitz-Hugh Curtis syndrome, a complication of pelvic inflammatory disease that may cause adhesions, was raised as the initial finding at the second surgical procedure and documented as such in the operative report. The plaintiff’s counsel questioned whether surgical correction of this syndrome was within the realm of a gynecologic surgeon. The plaintiff’s counsel argued that the laparoscopic surgical procedure involved bowel and liver; diaphragmatic adhesiolysis was not indicated, especially with normal abdominal CT scan results and the absence of RUQ symptoms. The claim specified that the surgery and care, as a consequence of the RUQ adhesiolysis, resulted in atelectasis, pneumonia, and empyema, with pain and suffering. The plaintiff sought unspecified monetary damages for these results.
What’s the verdict?
The case is in negotiation prior to trial.
Legal and medical considerations
“To err is not just human but intrinsically biological and no profession is exempt from fallibility.”1
Error and liability
To err may be human, but human error is not necessarily the cause of every suboptimal medical outcome. In fact, the overall surgical complication rate has been reported at 3.4%.2 Even when there is an error, it may not have been the kind of error that gives rise to medical malpractice liability. When it comes to surgical errors, the most common are those that actually relate to medications given at surgery that appear to be more common—one recent study found that 1 in 20 perioperative medication administrations resulted in a medication error or an adverse drug event.3
Medical error vs medical malpractice
The fact is that medical error and medical malpractice (or professional negligence) are not the same thing. It is critical to understand the difference.
Medical error is the third leading cause of death in the United States.4 It is defined as “the failure of a planned action to be completed as intended or the use of a wrong plan to achieve an aim,”5 or, in the Canadian literature, “an act of omission or commission in planning or execution that contributes or could contribute to an unintended result.”6 The gamut of medical errors spans (among others) problems with technique, judgment, medication administration, diagnostic and surgical errors, and incomplete record keeping.5
Negligent error, on the other hand, is generally a subset of medical error recognized by the law. It is error that occurs because of carelessness. Technically, to give rise to liability for negligence (or malpractice) there must be duty, breach, causation, and injury. That is, the physician must owe a duty to the patient, the duty must have been breached, and that breach must have caused an injury.7
Usually the duty in medical practice is that the physician must have acted as a reasonable and prudent professional would have performed under the circumstances. For the most part, malpractice is a level of practice that the profession itself would not view as reasonable practice.8 Specialists usually are held to the higher standards of the specialty. It also can be negligent to undertake practice or a procedure for which the physician is not adequately trained, or for failing to refer the patient to another more qualified physician.
The duty in medicine usually arises from the physician-patient relationship (clearly present here). It is reasonably clear in this case that there was an injury, but, in fact, the question is whether the physician acted carelessly in a way that caused that injury. Our facts leave some ambiguity—unfortunately,a common problem in the real world.
It is possible that the gynecologist was negligent in puncturing the diaphragm. It may have been carelessness, for example, in the way the procedure was performed, or in the decision to proceed despite the difficulties encountered. It is also possible that the gynecologist was not appropriately trained and experienced in the surgery that was undertaken, in which case the decision to do the surgery (rather than to refer to another physician) could well have been negligent. In either of those cases, negligence liability (malpractice) is a possibility.
Proving negligence. It is the plaintiff (the patient) who must prove the elements of negligence (including causation).8 The plaintiff will have to demonstrate not only carelessness, but that carelessness is what caused the injuries for which she is seeking compensation. In this case, the injuries are the consequence of puncturing the diaphragm. The potential damages would be the money to cover the additional medical costs and other expenses, lost wages, and noneconomic damages such as pain and suffering.
The hospital’s role in negligence
The issue of informed consent is also raised in this case, with a handwritten note prior to surgery (but the focus of this article is on medical errors). In addition to the gynecologist, the hospital and other medical personnelwere sued. The hospital is responsible for the acts of its agents, notably its employees. Even if the physicians are not technically hospital employees, the hospital may in some cases be responsible. Among other things, the hospital likely has an obligation to prevent physicians from undertaking inappropriate procedures, including those for which the physician is not appropriately trained. If the gynecologist in this case did not have privileges to perform surgery in this category, the hospital may have an obligation to not schedule the surgery or to intraoperatively question her credentials for such a procedure. In any event, the hospital will have a major role in this case and its interests may, in some instances, be inconsistent with the interests of the physician.
Why settlement discussions?
The case description ends with a note that settlement discussions were underway. If the plaintiff must prove all of the elements of negligence, why have these discussions? First, such discussions are common in almost all negligence cases. This does not mean that the case actually will be settled by the insurance company representing the physician or hospital; many malpractice cases simply fade away because the patient drops the action. Second, there are ambiguities in the facts, and it is sometimes impossible to determine whether or not a jury would find negligence. The hospital may be inclined to settle if there is any realistic chance of a jury ruling against it. Paying a small settlement may be worth avoiding high legal expenses and the risk of an adverse outcome at trial.9
Reducing medical/surgical error through a team approach
Recognizing that “human performance can be affected by many factors that include circadian rhythms, state of mind, physical health, attitude, emotions, propensity for certain common mistakes and errors, and cognitive biases,”10 health care professionals have a commitment to reduce the errors in the interest of patient safety and best practice.
The surgical environment is an opportunity to provide a team approach to patient safety. Surgical risk is a reflection of operative performance, the main factor in the development of postoperative complications.11 We wish to broaden the perspective that gynecologic surgeons, like all surgeons, must keep in mind a number of concerns that can be associated with problems related to surgical procedures, including12:
- visual perception difficulties
- stress
- loss of haptic perception (feedback using touch), as with robot-assisted procedures
- lack of situational awareness (a term we borrow from the aviation industry)
- long-term (and short-term) memory problems.
Analysis of surgical errors shows that they are related to, in order of frequency 1:
- surgical technique
- judgment
- inattention to detail
- incomplete understanding of the problem or surgical situation.
Medical errors: Caring for the second victim (you)

Patrice M. Weiss, MD
We use the term “victim” to refer to the patient and her family following a medical error. The phrase “the second victim” was coined by Dr. Albert Wu in an article in the British Medical Journal1 and describes how a clinician and team of health care professionals also can be affected by medical errors.
What signs and symptoms identify a second victim?Those suffering as a second victim may show signs of depression, loss of joy in work, and difficulty sleeping. They also may replay the events, question their own ability, and feel fearful about making another error. These reactions can lead to burnout—a serious issue that 46% of physicians report.2
As colleagues of those involved in a medical error, we should be cognizant of changes in behavior such as excessive irritability, showing up late for work, or agitation. It may be easier to recognize these symptoms in others rather than in ourselves because we often do not take time to examine how our experiences may affect us personally. Heightening awareness can help us recognize those suffering as second victims and identify the second victim symptoms in ourselves.
How can we help second victims?One challenge second victims face is not being allowed to discuss a medical error. Certainly, due to confidentiality requirements during professional liability cases, we should not talk freely about the event. However, silence creates a barrier that prevents a second victim from processing the incident.
Some hospitals offer forums to discuss medical errors, with the goal of preventing reoccurrence: morbidity and mortality conferences, morning report, Quality Assurance and Performance Improvement meetings, and root cause analyses. These forums often are not perceived by institutions’ employees in a positive way. Are they really meant to improve patient care or do they single out an individual or group in a “name/blame/shame game”? An intimidating process will only worsen a second victim’s symptoms. It is not necessary, however, to create a whole new process; it is possible to restructure, reframe, and change the culture of an existing practice.
Some institutions have developed a formalized program to help second victims. The University of Missouri has a “forYOU team,” an internal, rapid response group that provides emotional first aid to the entire team involved in a medical error case. These responders are not from human resources and do not need to be sought out; they are peers who have been educated about the struggles of the second victim. They will not discuss the case or how care was rendered; they naturally and instinctively provide emotional support to their colleagues.
At my institution, the Carilion Clinic at the Virginia Tech Carilion School of Medicine, “The Trust Program” encourages truth, respectfulness, understanding, support, and transparency. All health care clinicians receive basic training, but many have volunteered for additional instruction to become mentors because they have experienced second-victim symptoms themselves.
Clinicians want assistance when dealing with a medical error. One poll reports that 90% of physicians felt that health care organizations did not adequately help them cope with the stresses associated with a medical error.3 The goal is to have all institutions recognize that clinicians can be affected by a medical error and offer support.
To hear an expanded audiocast from Dr. Weiss on “the second victim” click here.
Dr. Weiss is Professor, Department of Obstetrics & Gynecology, Virginia Tech Carilion School of Medicine, and Chief Medical Officer and Executive Vice President, Carilion Clinic, Roanoke, Virginia.
The author reports no financial relationships relevant to this article.
References
- Wu AW. Medical error: the second victim. BMJ. 2000;320(7237):726–727.
- Peckham C. Medscape Physician Lifestyle Report 2015. Medscape website. http://www.medscape.com/features/slideshow/lifestyle/2015/public/overview#1. Published January 26, 2015. Accessed May 24, 2016.
- White AA, Waterman AD, McCotter P, Boyle DJ, Gallagher TH. Supporting health care workers after medical error: considerations for health care leaders. JCOM. 2008;15(5):240–247.
“Inadequacy” with regard to surgical proceduresIndication for surgery is intrinsic to provision of appropriate care. Surgery inherently poses the possibility of unexpected problems. Adequate training and skill, therefore, must include the ability to deal with a range of problems that arise in the course of surgery. The spectrum related to inadequacy as related to surgical problems includes “failed surgery,” defined as “if despite the utmost care of everyone involved and with the responsible consideration of all knowledge, the designed aim is not achieved, surgery by itself has failed.”5 Of paramount importance is the surgeon’s knowledge of technology and the ability to troubleshoot, as well as the OR team’s responsibility for proper maintenance of equipment to ensure optimal functionality.1
Aviation industry studies indicate that “high performing cockpit crews have been shown to devote one third of their communications to discuss threats and mistakes in their environment, while poor performing teams devoted much less, about 5%, of their time to such.”1,13 A well-trained and well-motivated OR nursing team has been equated with reduction in operative time and rate of conversion to laparotomy.14 Outdated instruments may also contribute to surgical errors.1
Moving the “learning curve” out of the OR and into the simulation lab remains valuable, which is also confirmed by the aviation industry.15 The significance of loss of haptic perception continues to be debated between laparoscopic (straight-stick) surgeons and those performing robotic approaches. Does haptic perception play a major role in surgical intervention? Most surgeons do not view loss of haptic perception, as with minimally invasive procedures, as a major impediment to successful surgery. From the legal perspective, loss of haptic perception has not been well addressed.
The American College of Obstetricians and Gynecologists has focused on patient safety in the surgical environment including concerns for wrong-patient surgery, wrong-side surgery, wrong-level surgery, and wrong-part surgery.16 The Joint Commission has identified factors that may enhance the risk of wrong-site surgery: multiple surgeons involved in the case, multiple procedures during a single surgical visit, unusual time pressures to start or complete the surgery, and unusual physical characteristics including morbid obesity or physical deformity.16
10 starting points for medical error preventionSo what are we to do? Consider:
- Using a preprocedure verification checklist.
- Marking the operative site.
- Completing a time out process prior to starting the procedure, according to the Joint Commission protocol. [For more information on Joint Commission-recommended time out protocols and ways to prevent medical errors, click here.]
- Involving the patient in the identification and procedure definition process. (This is an important part of informed consent.)
- Providing appropriate proctoring and sign-off for new procedures and technology.
- Avoiding sleep deprivation situations, especially with regard to emergency procedures.
- Using only radiopaque-labeled materials placed into the operating cavity.
- Considering medication effect on a fetus, if applicable.
- Reducing distractions from pagers, telephone calls, etc.
- Maintaining a “sterile cockpit” (or distraction free) environment for everyone in the OR.
Set the stage for best outcomesA true team approach is an excellent modus operandi before, during, and after surgery,setting the stage for best outcomes for patients.
“As human beings, surgeons will commit errors and for this reason they have to adopt and utilize stringent defense systems to minimize the incidence of these adverse events … Transparency is the first step on the way to a new safety culture with the acknowledgement of errors when they occur with adoption of systems destined to establish their cause and future prevention.”1
Share your thoughts! Send your Letter to the Editor to rbarbieri@frontlinemedcom.com. Please include your name and the city and state in which you practice.
- Galleano R, Franceschi A, Ciciliot M, Falchero F, Cuschieri A. Errors in laparoscopic surgery: what surgeons should know. Mineva Chir. 2011;66(2):107−117.
- Fabri P, Zyas-Castro J. Human error, not communication and systems, underlies surgical complications. Surgery. 2008;144(4):557−565.
- Nanji KC, Patel A, Shaikh S, Seger DL, Bates DW. Evaluation of perioperative medication errors and adverse drug events. Anesthesiology. 2016;124(1):25−34.
- Makary MA, Daniel M. Medical error−the third leading cause of death in the US. BMJ. 2016;353:i2139. doi:10.1136/bmj.i2139. Balogun J, Bramall A, Berstein M. How surgical trainees handle catastrophic errors: a qualitative study. J Surg Educ. 2015;72(6):1179−1184.
- Grober E, Bohnen J. Defining medical error. Can J Surg. 2005;48(1):39−44.
- Anderson RE, ed. Medical Malpractice: A Physician's Sourcebook. Totowa, NJ: Humana Press, Inc; 2004.
- Mehlman MJ. Professional power and the standard of care in medicine. 44 Arizona State Law J. 2012;44:1165−1777. http://papers.ssrn.com/sol3/papers.cfm?abstract_id=2205485. Revised February 13, 2013.
- Hyman DA, Silver C. On the table: an examination of medical malpractice, litigation, and methods of reform: healthcare quality, patient safety, and the culture of medicine: "Denial Ain't Just a River in Egypt." New Eng Law Rev. 2012;46:417−931.
- Landers R. Reducing surgical errors: implementing a three-hinge approach to success. AORN J. 2015;101(6):657−665.
- Pettigrew R, Burns H, Carter D. Evaluating surgical risk: the importance of technical factors in determining outcome. Br J Surg. 1987;74(9):791−794.
- Parker W. Understanding errors during laparoscopic surgery. Obstet Gynecol Clin North Am. 2010;37(3):437−449.
- Sexton JB, Helmreich RL. Analyzing cockpit communications: the links between language, performance, error, and workload. Hum Perf Extrem Environ. 2000;5(1):63−68.
- Kenyon T, Lenker M, Bax R, Swanstrom L. Cost and benefit of the trained laparoscopic team: a comparative study of a designated nursing team vs. a non-trained team. Surg Endosc. 1997;11(8):812−814.
- Woodman R. Surgeons should train like pilots. BMJ. 1999;319:1321.
- American College of Obstetrics and Gynecology. ACOG Committee Opinion No. 464: Patient safety in the surgical environment. Obstet Gynecol. 2010;116(3):786−790.
CASE Surgeon accused of operating outside her scope of expertise
A 38-year-old woman (G2 P2002) presented to the emergency department (ED) with acute pelvic pain involving the right lower quadrant (RLQ). The patient had a history of stage IV endometriosis and chronic pelvic pain, primarily affecting the RLQ, that was treated by total laparoscopic hysterectomy with bilateral salpingo-oophorectomy 6 months earlier. Pertinent findings on physical examination included hypoactive bowel sounds and rebound tenderness. The ED physician ordered a computed tomography (CT) scan of the abdomen, which showed no evidence of ureteral injury or other abnormality. The gynecologist who performed the surgery 6 months ago evaluated the patient in the ED.
The gynecologist decided to perform operative laparoscopy because of the severity of the patient’s pain and duration of symptoms. Informed consent obtained in the ED before the patient received analgesics included a handwritten note that said “and other indicated procedures.” The patient signed the document prior to being taken to the operating room (OR). Time out occurred in the OR before anesthesia induction. The gynecologist proceeded with laparoscopic adhesiolysis with planned appendectomy, as she was trained. A normal appendix was noted and left intact. RLQ adhesions involving the colon and abdominal wall were treated with electrosurgical cautery. When the gynecologist found adhesions between the liver and diaphragm in the right upper quadrant (RUQ), she continued adhesiolysis. However, the diaphragm was inadvertently punctured.
As the gynecologist attempted to suture the defect laparoscopically, she encountered difficulty and converted to laparotomy. Adhesions were dense and initially precluded adequate closure of the diaphragmatic defect. The gynecologist persisted and ultimately the closure was adequate; laparotomy concluded. Postoperatively, the patient was given a diagnosis of atelectasis, primarily on the right side; a chest tube was placed by the general surgery team. The patient had an uneventful postoperative period and was discharged on postoperative day 5. One month later she returned to the ED with evidence of pneumonia; she was given a diagnosis of empyema, and antibiotics were administered. She responded well and was discharged after 6 days.
The patient filed a malpractice lawsuit against the gynecologist, the hospital, and associated practitioners. The suit made 3 negligence claims: 1) the surgery was improperly performed, as evidenced by the diaphragmatic perforation; 2) the gynecologist was not adequately trained for RUQ surgery, and 3) the hospital should not have permitted RUQ surgery to proceed. The liability claim cited the lack of qualification of a gynecologic surgeon to proceed with surgical intervention near the diaphragm and the associated consequences of practicing outside the scope of expertise.
Fitz-Hugh Curtis syndrome, a complication of pelvic inflammatory disease that may cause adhesions, was raised as the initial finding at the second surgical procedure and documented as such in the operative report. The plaintiff’s counsel questioned whether surgical correction of this syndrome was within the realm of a gynecologic surgeon. The plaintiff’s counsel argued that the laparoscopic surgical procedure involved bowel and liver; diaphragmatic adhesiolysis was not indicated, especially with normal abdominal CT scan results and the absence of RUQ symptoms. The claim specified that the surgery and care, as a consequence of the RUQ adhesiolysis, resulted in atelectasis, pneumonia, and empyema, with pain and suffering. The plaintiff sought unspecified monetary damages for these results.
What’s the verdict?
The case is in negotiation prior to trial.
Legal and medical considerations
“To err is not just human but intrinsically biological and no profession is exempt from fallibility.”1
Error and liability
To err may be human, but human error is not necessarily the cause of every suboptimal medical outcome. In fact, the overall surgical complication rate has been reported at 3.4%.2 Even when there is an error, it may not have been the kind of error that gives rise to medical malpractice liability. When it comes to surgical errors, the most common are those that actually relate to medications given at surgery that appear to be more common—one recent study found that 1 in 20 perioperative medication administrations resulted in a medication error or an adverse drug event.3
Medical error vs medical malpractice
The fact is that medical error and medical malpractice (or professional negligence) are not the same thing. It is critical to understand the difference.
Medical error is the third leading cause of death in the United States.4 It is defined as “the failure of a planned action to be completed as intended or the use of a wrong plan to achieve an aim,”5 or, in the Canadian literature, “an act of omission or commission in planning or execution that contributes or could contribute to an unintended result.”6 The gamut of medical errors spans (among others) problems with technique, judgment, medication administration, diagnostic and surgical errors, and incomplete record keeping.5
Negligent error, on the other hand, is generally a subset of medical error recognized by the law. It is error that occurs because of carelessness. Technically, to give rise to liability for negligence (or malpractice) there must be duty, breach, causation, and injury. That is, the physician must owe a duty to the patient, the duty must have been breached, and that breach must have caused an injury.7
Usually the duty in medical practice is that the physician must have acted as a reasonable and prudent professional would have performed under the circumstances. For the most part, malpractice is a level of practice that the profession itself would not view as reasonable practice.8 Specialists usually are held to the higher standards of the specialty. It also can be negligent to undertake practice or a procedure for which the physician is not adequately trained, or for failing to refer the patient to another more qualified physician.
The duty in medicine usually arises from the physician-patient relationship (clearly present here). It is reasonably clear in this case that there was an injury, but, in fact, the question is whether the physician acted carelessly in a way that caused that injury. Our facts leave some ambiguity—unfortunately,a common problem in the real world.
It is possible that the gynecologist was negligent in puncturing the diaphragm. It may have been carelessness, for example, in the way the procedure was performed, or in the decision to proceed despite the difficulties encountered. It is also possible that the gynecologist was not appropriately trained and experienced in the surgery that was undertaken, in which case the decision to do the surgery (rather than to refer to another physician) could well have been negligent. In either of those cases, negligence liability (malpractice) is a possibility.
Proving negligence. It is the plaintiff (the patient) who must prove the elements of negligence (including causation).8 The plaintiff will have to demonstrate not only carelessness, but that carelessness is what caused the injuries for which she is seeking compensation. In this case, the injuries are the consequence of puncturing the diaphragm. The potential damages would be the money to cover the additional medical costs and other expenses, lost wages, and noneconomic damages such as pain and suffering.
The hospital’s role in negligence
The issue of informed consent is also raised in this case, with a handwritten note prior to surgery (but the focus of this article is on medical errors). In addition to the gynecologist, the hospital and other medical personnelwere sued. The hospital is responsible for the acts of its agents, notably its employees. Even if the physicians are not technically hospital employees, the hospital may in some cases be responsible. Among other things, the hospital likely has an obligation to prevent physicians from undertaking inappropriate procedures, including those for which the physician is not appropriately trained. If the gynecologist in this case did not have privileges to perform surgery in this category, the hospital may have an obligation to not schedule the surgery or to intraoperatively question her credentials for such a procedure. In any event, the hospital will have a major role in this case and its interests may, in some instances, be inconsistent with the interests of the physician.
Why settlement discussions?
The case description ends with a note that settlement discussions were underway. If the plaintiff must prove all of the elements of negligence, why have these discussions? First, such discussions are common in almost all negligence cases. This does not mean that the case actually will be settled by the insurance company representing the physician or hospital; many malpractice cases simply fade away because the patient drops the action. Second, there are ambiguities in the facts, and it is sometimes impossible to determine whether or not a jury would find negligence. The hospital may be inclined to settle if there is any realistic chance of a jury ruling against it. Paying a small settlement may be worth avoiding high legal expenses and the risk of an adverse outcome at trial.9
Reducing medical/surgical error through a team approach
Recognizing that “human performance can be affected by many factors that include circadian rhythms, state of mind, physical health, attitude, emotions, propensity for certain common mistakes and errors, and cognitive biases,”10 health care professionals have a commitment to reduce the errors in the interest of patient safety and best practice.
The surgical environment is an opportunity to provide a team approach to patient safety. Surgical risk is a reflection of operative performance, the main factor in the development of postoperative complications.11 We wish to broaden the perspective that gynecologic surgeons, like all surgeons, must keep in mind a number of concerns that can be associated with problems related to surgical procedures, including12:
- visual perception difficulties
- stress
- loss of haptic perception (feedback using touch), as with robot-assisted procedures
- lack of situational awareness (a term we borrow from the aviation industry)
- long-term (and short-term) memory problems.
Analysis of surgical errors shows that they are related to, in order of frequency 1:
- surgical technique
- judgment
- inattention to detail
- incomplete understanding of the problem or surgical situation.
Medical errors: Caring for the second victim (you)

Patrice M. Weiss, MD
We use the term “victim” to refer to the patient and her family following a medical error. The phrase “the second victim” was coined by Dr. Albert Wu in an article in the British Medical Journal1 and describes how a clinician and team of health care professionals also can be affected by medical errors.
What signs and symptoms identify a second victim?Those suffering as a second victim may show signs of depression, loss of joy in work, and difficulty sleeping. They also may replay the events, question their own ability, and feel fearful about making another error. These reactions can lead to burnout—a serious issue that 46% of physicians report.2
As colleagues of those involved in a medical error, we should be cognizant of changes in behavior such as excessive irritability, showing up late for work, or agitation. It may be easier to recognize these symptoms in others rather than in ourselves because we often do not take time to examine how our experiences may affect us personally. Heightening awareness can help us recognize those suffering as second victims and identify the second victim symptoms in ourselves.
How can we help second victims?One challenge second victims face is not being allowed to discuss a medical error. Certainly, due to confidentiality requirements during professional liability cases, we should not talk freely about the event. However, silence creates a barrier that prevents a second victim from processing the incident.
Some hospitals offer forums to discuss medical errors, with the goal of preventing reoccurrence: morbidity and mortality conferences, morning report, Quality Assurance and Performance Improvement meetings, and root cause analyses. These forums often are not perceived by institutions’ employees in a positive way. Are they really meant to improve patient care or do they single out an individual or group in a “name/blame/shame game”? An intimidating process will only worsen a second victim’s symptoms. It is not necessary, however, to create a whole new process; it is possible to restructure, reframe, and change the culture of an existing practice.
Some institutions have developed a formalized program to help second victims. The University of Missouri has a “forYOU team,” an internal, rapid response group that provides emotional first aid to the entire team involved in a medical error case. These responders are not from human resources and do not need to be sought out; they are peers who have been educated about the struggles of the second victim. They will not discuss the case or how care was rendered; they naturally and instinctively provide emotional support to their colleagues.
At my institution, the Carilion Clinic at the Virginia Tech Carilion School of Medicine, “The Trust Program” encourages truth, respectfulness, understanding, support, and transparency. All health care clinicians receive basic training, but many have volunteered for additional instruction to become mentors because they have experienced second-victim symptoms themselves.
Clinicians want assistance when dealing with a medical error. One poll reports that 90% of physicians felt that health care organizations did not adequately help them cope with the stresses associated with a medical error.3 The goal is to have all institutions recognize that clinicians can be affected by a medical error and offer support.
To hear an expanded audiocast from Dr. Weiss on “the second victim” click here.
Dr. Weiss is Professor, Department of Obstetrics & Gynecology, Virginia Tech Carilion School of Medicine, and Chief Medical Officer and Executive Vice President, Carilion Clinic, Roanoke, Virginia.
The author reports no financial relationships relevant to this article.
References
- Wu AW. Medical error: the second victim. BMJ. 2000;320(7237):726–727.
- Peckham C. Medscape Physician Lifestyle Report 2015. Medscape website. http://www.medscape.com/features/slideshow/lifestyle/2015/public/overview#1. Published January 26, 2015. Accessed May 24, 2016.
- White AA, Waterman AD, McCotter P, Boyle DJ, Gallagher TH. Supporting health care workers after medical error: considerations for health care leaders. JCOM. 2008;15(5):240–247.
“Inadequacy” with regard to surgical proceduresIndication for surgery is intrinsic to provision of appropriate care. Surgery inherently poses the possibility of unexpected problems. Adequate training and skill, therefore, must include the ability to deal with a range of problems that arise in the course of surgery. The spectrum related to inadequacy as related to surgical problems includes “failed surgery,” defined as “if despite the utmost care of everyone involved and with the responsible consideration of all knowledge, the designed aim is not achieved, surgery by itself has failed.”5 Of paramount importance is the surgeon’s knowledge of technology and the ability to troubleshoot, as well as the OR team’s responsibility for proper maintenance of equipment to ensure optimal functionality.1
Aviation industry studies indicate that “high performing cockpit crews have been shown to devote one third of their communications to discuss threats and mistakes in their environment, while poor performing teams devoted much less, about 5%, of their time to such.”1,13 A well-trained and well-motivated OR nursing team has been equated with reduction in operative time and rate of conversion to laparotomy.14 Outdated instruments may also contribute to surgical errors.1
Moving the “learning curve” out of the OR and into the simulation lab remains valuable, which is also confirmed by the aviation industry.15 The significance of loss of haptic perception continues to be debated between laparoscopic (straight-stick) surgeons and those performing robotic approaches. Does haptic perception play a major role in surgical intervention? Most surgeons do not view loss of haptic perception, as with minimally invasive procedures, as a major impediment to successful surgery. From the legal perspective, loss of haptic perception has not been well addressed.
The American College of Obstetricians and Gynecologists has focused on patient safety in the surgical environment including concerns for wrong-patient surgery, wrong-side surgery, wrong-level surgery, and wrong-part surgery.16 The Joint Commission has identified factors that may enhance the risk of wrong-site surgery: multiple surgeons involved in the case, multiple procedures during a single surgical visit, unusual time pressures to start or complete the surgery, and unusual physical characteristics including morbid obesity or physical deformity.16
10 starting points for medical error preventionSo what are we to do? Consider:
- Using a preprocedure verification checklist.
- Marking the operative site.
- Completing a time out process prior to starting the procedure, according to the Joint Commission protocol. [For more information on Joint Commission-recommended time out protocols and ways to prevent medical errors, click here.]
- Involving the patient in the identification and procedure definition process. (This is an important part of informed consent.)
- Providing appropriate proctoring and sign-off for new procedures and technology.
- Avoiding sleep deprivation situations, especially with regard to emergency procedures.
- Using only radiopaque-labeled materials placed into the operating cavity.
- Considering medication effect on a fetus, if applicable.
- Reducing distractions from pagers, telephone calls, etc.
- Maintaining a “sterile cockpit” (or distraction free) environment for everyone in the OR.
Set the stage for best outcomesA true team approach is an excellent modus operandi before, during, and after surgery,setting the stage for best outcomes for patients.
“As human beings, surgeons will commit errors and for this reason they have to adopt and utilize stringent defense systems to minimize the incidence of these adverse events … Transparency is the first step on the way to a new safety culture with the acknowledgement of errors when they occur with adoption of systems destined to establish their cause and future prevention.”1
Share your thoughts! Send your Letter to the Editor to rbarbieri@frontlinemedcom.com. Please include your name and the city and state in which you practice.
CASE Surgeon accused of operating outside her scope of expertise
A 38-year-old woman (G2 P2002) presented to the emergency department (ED) with acute pelvic pain involving the right lower quadrant (RLQ). The patient had a history of stage IV endometriosis and chronic pelvic pain, primarily affecting the RLQ, that was treated by total laparoscopic hysterectomy with bilateral salpingo-oophorectomy 6 months earlier. Pertinent findings on physical examination included hypoactive bowel sounds and rebound tenderness. The ED physician ordered a computed tomography (CT) scan of the abdomen, which showed no evidence of ureteral injury or other abnormality. The gynecologist who performed the surgery 6 months ago evaluated the patient in the ED.
The gynecologist decided to perform operative laparoscopy because of the severity of the patient’s pain and duration of symptoms. Informed consent obtained in the ED before the patient received analgesics included a handwritten note that said “and other indicated procedures.” The patient signed the document prior to being taken to the operating room (OR). Time out occurred in the OR before anesthesia induction. The gynecologist proceeded with laparoscopic adhesiolysis with planned appendectomy, as she was trained. A normal appendix was noted and left intact. RLQ adhesions involving the colon and abdominal wall were treated with electrosurgical cautery. When the gynecologist found adhesions between the liver and diaphragm in the right upper quadrant (RUQ), she continued adhesiolysis. However, the diaphragm was inadvertently punctured.
As the gynecologist attempted to suture the defect laparoscopically, she encountered difficulty and converted to laparotomy. Adhesions were dense and initially precluded adequate closure of the diaphragmatic defect. The gynecologist persisted and ultimately the closure was adequate; laparotomy concluded. Postoperatively, the patient was given a diagnosis of atelectasis, primarily on the right side; a chest tube was placed by the general surgery team. The patient had an uneventful postoperative period and was discharged on postoperative day 5. One month later she returned to the ED with evidence of pneumonia; she was given a diagnosis of empyema, and antibiotics were administered. She responded well and was discharged after 6 days.
The patient filed a malpractice lawsuit against the gynecologist, the hospital, and associated practitioners. The suit made 3 negligence claims: 1) the surgery was improperly performed, as evidenced by the diaphragmatic perforation; 2) the gynecologist was not adequately trained for RUQ surgery, and 3) the hospital should not have permitted RUQ surgery to proceed. The liability claim cited the lack of qualification of a gynecologic surgeon to proceed with surgical intervention near the diaphragm and the associated consequences of practicing outside the scope of expertise.
Fitz-Hugh Curtis syndrome, a complication of pelvic inflammatory disease that may cause adhesions, was raised as the initial finding at the second surgical procedure and documented as such in the operative report. The plaintiff’s counsel questioned whether surgical correction of this syndrome was within the realm of a gynecologic surgeon. The plaintiff’s counsel argued that the laparoscopic surgical procedure involved bowel and liver; diaphragmatic adhesiolysis was not indicated, especially with normal abdominal CT scan results and the absence of RUQ symptoms. The claim specified that the surgery and care, as a consequence of the RUQ adhesiolysis, resulted in atelectasis, pneumonia, and empyema, with pain and suffering. The plaintiff sought unspecified monetary damages for these results.
What’s the verdict?
The case is in negotiation prior to trial.
Legal and medical considerations
“To err is not just human but intrinsically biological and no profession is exempt from fallibility.”1
Error and liability
To err may be human, but human error is not necessarily the cause of every suboptimal medical outcome. In fact, the overall surgical complication rate has been reported at 3.4%.2 Even when there is an error, it may not have been the kind of error that gives rise to medical malpractice liability. When it comes to surgical errors, the most common are those that actually relate to medications given at surgery that appear to be more common—one recent study found that 1 in 20 perioperative medication administrations resulted in a medication error or an adverse drug event.3
Medical error vs medical malpractice
The fact is that medical error and medical malpractice (or professional negligence) are not the same thing. It is critical to understand the difference.
Medical error is the third leading cause of death in the United States.4 It is defined as “the failure of a planned action to be completed as intended or the use of a wrong plan to achieve an aim,”5 or, in the Canadian literature, “an act of omission or commission in planning or execution that contributes or could contribute to an unintended result.”6 The gamut of medical errors spans (among others) problems with technique, judgment, medication administration, diagnostic and surgical errors, and incomplete record keeping.5
Negligent error, on the other hand, is generally a subset of medical error recognized by the law. It is error that occurs because of carelessness. Technically, to give rise to liability for negligence (or malpractice) there must be duty, breach, causation, and injury. That is, the physician must owe a duty to the patient, the duty must have been breached, and that breach must have caused an injury.7
Usually the duty in medical practice is that the physician must have acted as a reasonable and prudent professional would have performed under the circumstances. For the most part, malpractice is a level of practice that the profession itself would not view as reasonable practice.8 Specialists usually are held to the higher standards of the specialty. It also can be negligent to undertake practice or a procedure for which the physician is not adequately trained, or for failing to refer the patient to another more qualified physician.
The duty in medicine usually arises from the physician-patient relationship (clearly present here). It is reasonably clear in this case that there was an injury, but, in fact, the question is whether the physician acted carelessly in a way that caused that injury. Our facts leave some ambiguity—unfortunately,a common problem in the real world.
It is possible that the gynecologist was negligent in puncturing the diaphragm. It may have been carelessness, for example, in the way the procedure was performed, or in the decision to proceed despite the difficulties encountered. It is also possible that the gynecologist was not appropriately trained and experienced in the surgery that was undertaken, in which case the decision to do the surgery (rather than to refer to another physician) could well have been negligent. In either of those cases, negligence liability (malpractice) is a possibility.
Proving negligence. It is the plaintiff (the patient) who must prove the elements of negligence (including causation).8 The plaintiff will have to demonstrate not only carelessness, but that carelessness is what caused the injuries for which she is seeking compensation. In this case, the injuries are the consequence of puncturing the diaphragm. The potential damages would be the money to cover the additional medical costs and other expenses, lost wages, and noneconomic damages such as pain and suffering.
The hospital’s role in negligence
The issue of informed consent is also raised in this case, with a handwritten note prior to surgery (but the focus of this article is on medical errors). In addition to the gynecologist, the hospital and other medical personnelwere sued. The hospital is responsible for the acts of its agents, notably its employees. Even if the physicians are not technically hospital employees, the hospital may in some cases be responsible. Among other things, the hospital likely has an obligation to prevent physicians from undertaking inappropriate procedures, including those for which the physician is not appropriately trained. If the gynecologist in this case did not have privileges to perform surgery in this category, the hospital may have an obligation to not schedule the surgery or to intraoperatively question her credentials for such a procedure. In any event, the hospital will have a major role in this case and its interests may, in some instances, be inconsistent with the interests of the physician.
Why settlement discussions?
The case description ends with a note that settlement discussions were underway. If the plaintiff must prove all of the elements of negligence, why have these discussions? First, such discussions are common in almost all negligence cases. This does not mean that the case actually will be settled by the insurance company representing the physician or hospital; many malpractice cases simply fade away because the patient drops the action. Second, there are ambiguities in the facts, and it is sometimes impossible to determine whether or not a jury would find negligence. The hospital may be inclined to settle if there is any realistic chance of a jury ruling against it. Paying a small settlement may be worth avoiding high legal expenses and the risk of an adverse outcome at trial.9
Reducing medical/surgical error through a team approach
Recognizing that “human performance can be affected by many factors that include circadian rhythms, state of mind, physical health, attitude, emotions, propensity for certain common mistakes and errors, and cognitive biases,”10 health care professionals have a commitment to reduce the errors in the interest of patient safety and best practice.
The surgical environment is an opportunity to provide a team approach to patient safety. Surgical risk is a reflection of operative performance, the main factor in the development of postoperative complications.11 We wish to broaden the perspective that gynecologic surgeons, like all surgeons, must keep in mind a number of concerns that can be associated with problems related to surgical procedures, including12:
- visual perception difficulties
- stress
- loss of haptic perception (feedback using touch), as with robot-assisted procedures
- lack of situational awareness (a term we borrow from the aviation industry)
- long-term (and short-term) memory problems.
Analysis of surgical errors shows that they are related to, in order of frequency 1:
- surgical technique
- judgment
- inattention to detail
- incomplete understanding of the problem or surgical situation.
Medical errors: Caring for the second victim (you)

Patrice M. Weiss, MD
We use the term “victim” to refer to the patient and her family following a medical error. The phrase “the second victim” was coined by Dr. Albert Wu in an article in the British Medical Journal1 and describes how a clinician and team of health care professionals also can be affected by medical errors.
What signs and symptoms identify a second victim?Those suffering as a second victim may show signs of depression, loss of joy in work, and difficulty sleeping. They also may replay the events, question their own ability, and feel fearful about making another error. These reactions can lead to burnout—a serious issue that 46% of physicians report.2
As colleagues of those involved in a medical error, we should be cognizant of changes in behavior such as excessive irritability, showing up late for work, or agitation. It may be easier to recognize these symptoms in others rather than in ourselves because we often do not take time to examine how our experiences may affect us personally. Heightening awareness can help us recognize those suffering as second victims and identify the second victim symptoms in ourselves.
How can we help second victims?One challenge second victims face is not being allowed to discuss a medical error. Certainly, due to confidentiality requirements during professional liability cases, we should not talk freely about the event. However, silence creates a barrier that prevents a second victim from processing the incident.
Some hospitals offer forums to discuss medical errors, with the goal of preventing reoccurrence: morbidity and mortality conferences, morning report, Quality Assurance and Performance Improvement meetings, and root cause analyses. These forums often are not perceived by institutions’ employees in a positive way. Are they really meant to improve patient care or do they single out an individual or group in a “name/blame/shame game”? An intimidating process will only worsen a second victim’s symptoms. It is not necessary, however, to create a whole new process; it is possible to restructure, reframe, and change the culture of an existing practice.
Some institutions have developed a formalized program to help second victims. The University of Missouri has a “forYOU team,” an internal, rapid response group that provides emotional first aid to the entire team involved in a medical error case. These responders are not from human resources and do not need to be sought out; they are peers who have been educated about the struggles of the second victim. They will not discuss the case or how care was rendered; they naturally and instinctively provide emotional support to their colleagues.
At my institution, the Carilion Clinic at the Virginia Tech Carilion School of Medicine, “The Trust Program” encourages truth, respectfulness, understanding, support, and transparency. All health care clinicians receive basic training, but many have volunteered for additional instruction to become mentors because they have experienced second-victim symptoms themselves.
Clinicians want assistance when dealing with a medical error. One poll reports that 90% of physicians felt that health care organizations did not adequately help them cope with the stresses associated with a medical error.3 The goal is to have all institutions recognize that clinicians can be affected by a medical error and offer support.
To hear an expanded audiocast from Dr. Weiss on “the second victim” click here.
Dr. Weiss is Professor, Department of Obstetrics & Gynecology, Virginia Tech Carilion School of Medicine, and Chief Medical Officer and Executive Vice President, Carilion Clinic, Roanoke, Virginia.
The author reports no financial relationships relevant to this article.
References
- Wu AW. Medical error: the second victim. BMJ. 2000;320(7237):726–727.
- Peckham C. Medscape Physician Lifestyle Report 2015. Medscape website. http://www.medscape.com/features/slideshow/lifestyle/2015/public/overview#1. Published January 26, 2015. Accessed May 24, 2016.
- White AA, Waterman AD, McCotter P, Boyle DJ, Gallagher TH. Supporting health care workers after medical error: considerations for health care leaders. JCOM. 2008;15(5):240–247.
“Inadequacy” with regard to surgical proceduresIndication for surgery is intrinsic to provision of appropriate care. Surgery inherently poses the possibility of unexpected problems. Adequate training and skill, therefore, must include the ability to deal with a range of problems that arise in the course of surgery. The spectrum related to inadequacy as related to surgical problems includes “failed surgery,” defined as “if despite the utmost care of everyone involved and with the responsible consideration of all knowledge, the designed aim is not achieved, surgery by itself has failed.”5 Of paramount importance is the surgeon’s knowledge of technology and the ability to troubleshoot, as well as the OR team’s responsibility for proper maintenance of equipment to ensure optimal functionality.1
Aviation industry studies indicate that “high performing cockpit crews have been shown to devote one third of their communications to discuss threats and mistakes in their environment, while poor performing teams devoted much less, about 5%, of their time to such.”1,13 A well-trained and well-motivated OR nursing team has been equated with reduction in operative time and rate of conversion to laparotomy.14 Outdated instruments may also contribute to surgical errors.1
Moving the “learning curve” out of the OR and into the simulation lab remains valuable, which is also confirmed by the aviation industry.15 The significance of loss of haptic perception continues to be debated between laparoscopic (straight-stick) surgeons and those performing robotic approaches. Does haptic perception play a major role in surgical intervention? Most surgeons do not view loss of haptic perception, as with minimally invasive procedures, as a major impediment to successful surgery. From the legal perspective, loss of haptic perception has not been well addressed.
The American College of Obstetricians and Gynecologists has focused on patient safety in the surgical environment including concerns for wrong-patient surgery, wrong-side surgery, wrong-level surgery, and wrong-part surgery.16 The Joint Commission has identified factors that may enhance the risk of wrong-site surgery: multiple surgeons involved in the case, multiple procedures during a single surgical visit, unusual time pressures to start or complete the surgery, and unusual physical characteristics including morbid obesity or physical deformity.16
10 starting points for medical error preventionSo what are we to do? Consider:
- Using a preprocedure verification checklist.
- Marking the operative site.
- Completing a time out process prior to starting the procedure, according to the Joint Commission protocol. [For more information on Joint Commission-recommended time out protocols and ways to prevent medical errors, click here.]
- Involving the patient in the identification and procedure definition process. (This is an important part of informed consent.)
- Providing appropriate proctoring and sign-off for new procedures and technology.
- Avoiding sleep deprivation situations, especially with regard to emergency procedures.
- Using only radiopaque-labeled materials placed into the operating cavity.
- Considering medication effect on a fetus, if applicable.
- Reducing distractions from pagers, telephone calls, etc.
- Maintaining a “sterile cockpit” (or distraction free) environment for everyone in the OR.
Set the stage for best outcomesA true team approach is an excellent modus operandi before, during, and after surgery,setting the stage for best outcomes for patients.
“As human beings, surgeons will commit errors and for this reason they have to adopt and utilize stringent defense systems to minimize the incidence of these adverse events … Transparency is the first step on the way to a new safety culture with the acknowledgement of errors when they occur with adoption of systems destined to establish their cause and future prevention.”1
Share your thoughts! Send your Letter to the Editor to rbarbieri@frontlinemedcom.com. Please include your name and the city and state in which you practice.
- Galleano R, Franceschi A, Ciciliot M, Falchero F, Cuschieri A. Errors in laparoscopic surgery: what surgeons should know. Mineva Chir. 2011;66(2):107−117.
- Fabri P, Zyas-Castro J. Human error, not communication and systems, underlies surgical complications. Surgery. 2008;144(4):557−565.
- Nanji KC, Patel A, Shaikh S, Seger DL, Bates DW. Evaluation of perioperative medication errors and adverse drug events. Anesthesiology. 2016;124(1):25−34.
- Makary MA, Daniel M. Medical error−the third leading cause of death in the US. BMJ. 2016;353:i2139. doi:10.1136/bmj.i2139. Balogun J, Bramall A, Berstein M. How surgical trainees handle catastrophic errors: a qualitative study. J Surg Educ. 2015;72(6):1179−1184.
- Grober E, Bohnen J. Defining medical error. Can J Surg. 2005;48(1):39−44.
- Anderson RE, ed. Medical Malpractice: A Physician's Sourcebook. Totowa, NJ: Humana Press, Inc; 2004.
- Mehlman MJ. Professional power and the standard of care in medicine. 44 Arizona State Law J. 2012;44:1165−1777. http://papers.ssrn.com/sol3/papers.cfm?abstract_id=2205485. Revised February 13, 2013.
- Hyman DA, Silver C. On the table: an examination of medical malpractice, litigation, and methods of reform: healthcare quality, patient safety, and the culture of medicine: "Denial Ain't Just a River in Egypt." New Eng Law Rev. 2012;46:417−931.
- Landers R. Reducing surgical errors: implementing a three-hinge approach to success. AORN J. 2015;101(6):657−665.
- Pettigrew R, Burns H, Carter D. Evaluating surgical risk: the importance of technical factors in determining outcome. Br J Surg. 1987;74(9):791−794.
- Parker W. Understanding errors during laparoscopic surgery. Obstet Gynecol Clin North Am. 2010;37(3):437−449.
- Sexton JB, Helmreich RL. Analyzing cockpit communications: the links between language, performance, error, and workload. Hum Perf Extrem Environ. 2000;5(1):63−68.
- Kenyon T, Lenker M, Bax R, Swanstrom L. Cost and benefit of the trained laparoscopic team: a comparative study of a designated nursing team vs. a non-trained team. Surg Endosc. 1997;11(8):812−814.
- Woodman R. Surgeons should train like pilots. BMJ. 1999;319:1321.
- American College of Obstetrics and Gynecology. ACOG Committee Opinion No. 464: Patient safety in the surgical environment. Obstet Gynecol. 2010;116(3):786−790.
- Galleano R, Franceschi A, Ciciliot M, Falchero F, Cuschieri A. Errors in laparoscopic surgery: what surgeons should know. Mineva Chir. 2011;66(2):107−117.
- Fabri P, Zyas-Castro J. Human error, not communication and systems, underlies surgical complications. Surgery. 2008;144(4):557−565.
- Nanji KC, Patel A, Shaikh S, Seger DL, Bates DW. Evaluation of perioperative medication errors and adverse drug events. Anesthesiology. 2016;124(1):25−34.
- Makary MA, Daniel M. Medical error−the third leading cause of death in the US. BMJ. 2016;353:i2139. doi:10.1136/bmj.i2139. Balogun J, Bramall A, Berstein M. How surgical trainees handle catastrophic errors: a qualitative study. J Surg Educ. 2015;72(6):1179−1184.
- Grober E, Bohnen J. Defining medical error. Can J Surg. 2005;48(1):39−44.
- Anderson RE, ed. Medical Malpractice: A Physician's Sourcebook. Totowa, NJ: Humana Press, Inc; 2004.
- Mehlman MJ. Professional power and the standard of care in medicine. 44 Arizona State Law J. 2012;44:1165−1777. http://papers.ssrn.com/sol3/papers.cfm?abstract_id=2205485. Revised February 13, 2013.
- Hyman DA, Silver C. On the table: an examination of medical malpractice, litigation, and methods of reform: healthcare quality, patient safety, and the culture of medicine: "Denial Ain't Just a River in Egypt." New Eng Law Rev. 2012;46:417−931.
- Landers R. Reducing surgical errors: implementing a three-hinge approach to success. AORN J. 2015;101(6):657−665.
- Pettigrew R, Burns H, Carter D. Evaluating surgical risk: the importance of technical factors in determining outcome. Br J Surg. 1987;74(9):791−794.
- Parker W. Understanding errors during laparoscopic surgery. Obstet Gynecol Clin North Am. 2010;37(3):437−449.
- Sexton JB, Helmreich RL. Analyzing cockpit communications: the links between language, performance, error, and workload. Hum Perf Extrem Environ. 2000;5(1):63−68.
- Kenyon T, Lenker M, Bax R, Swanstrom L. Cost and benefit of the trained laparoscopic team: a comparative study of a designated nursing team vs. a non-trained team. Surg Endosc. 1997;11(8):812−814.
- Woodman R. Surgeons should train like pilots. BMJ. 1999;319:1321.
- American College of Obstetrics and Gynecology. ACOG Committee Opinion No. 464: Patient safety in the surgical environment. Obstet Gynecol. 2010;116(3):786−790.
In this Article
- Medical error vs negligence
- Caring for the second victim
- 10 starting points for reducing medical errors
The medicolegal considerations of interacting with your patients online
CASE: Patient discloses personal information in electronic communication. How to respond and what’s at stake?
Your nurse comes to you with a dilemma. Last Friday she received an email from a patient, sent to the nurse’s personal email account (G-mail) that conveyed information regarding the patient’s recent treatment for a herpetic vulvar lesion. The text details presumed exposure, date and time, number of sexual partners, concernfor “spread of disease,” and the patient’s desire to have a comprehensive sexually transmitted infection screening as soon as possible.
Your nurse has years of professional experience, but she is perhaps not the most savvy with regard to current information technology and social media. Nonetheless, she knows it is best not to immediately respond to the patient’s email without checking with you. She tracks you down on Monday morning to review the email and the dilemma she feels she has been placed in. What’s the best next step?
While discussing the general question with the staff, another nurse notes that there have been some reviews of the office on social media. It seems that this second nurse tweets and texts with patients all the time. The office manager strongly suggests that the office “join the 21st Century” by setting up a Facebook page and using their webpage to attract new patients and communicate with current patients.
How do you prepare for this? Is your staff knowledgeable about the dos and don’ts of social media?
The use of social media by health care providers has been growing for several years. Back in 2011 a large survey by QuantiaMD revealed that 87% of physicians used social media for personal reasons, and 67% of them used it professionally.1 How they used it for professional purposes also was explored in 2011, with almost 3 of 4 physicians using it for social networking and more than half engaging with their own institution’s social media (FIGURE).2 In 2013, 53% of physicians indicated that their practice had a Facebook platform, 28% had a presence on LinkedIn, and 21% were on Twitter.3 Not surprisingly, social media use is higher among younger physicians4; the 2016 equivalents to these percentages most likely are higher.
| Health providers’ use of social media for professional reasons2 |
 |
In 2011, a survey found that most health providers used social networking, their institutions’ own social media, and Internet forums, boards, and communities for professional reasons. |
Patients’ outreach through social media regarding health care information continues to grow, with 33.8% asking for health advice using social media.5 While email and other social media open the possibility of improved communication with patients, they also present a number of important professional and legal issues that deserve special consideration.6 Each medium presents its own challenges, but there are 4 categories of concern related to basic values and rights that we consider important to review:
- confidentiality
- dual relationships and conflicts of interest
- quality of care and advice
- general professionalism (including advertising).
Confidentiality
Few values of the medical profession are of longer standing than the commitment to maintain patient privacy. Fifth Century BC obligations continue to apply to the technology of the 21st Century AD. And the challenges are significant.
Email is not secure
In the opening case, the choice to email her clinician was apparently the patient’s. She probably does not realize that email is not very confidential, although it is undoubtedly in the Terms of Service Agreement she clicked through. Her email was likely scanned by her email service provider—Google, in this case—as well as the nurse. If, however, the physician’s office responds by email, it may well compound the confidentiality problem by further distributing the information through yet another email provider.
If, as a physician, you encourage email communication by your patients, a smart approach is to emphasize that such communications are not very confidential. At a minimum, until a secure email system can be established, it is best not to transmit medical information via email and to inform patients of the risk of such communication. In the case above, the nurse who received the email should respond to the patient by telephone (much more secure). Or she can respond to the patient by email (not including the patient’s message in the return), writing that, because email communications are inherently not confidential, she suggests a phone call or personal visit.
This case also notes that the patient sent the email to the nurse’s personal account, not to an office email account. Sending medical emails to an employee’s personal account raises additional problems of confidentiality and appropriate controls. It should be made clear that employees should not be discussing private medical matters via their own email accounts.
Other forms of social media are also not secure
Similar concerns arise about texting and using Twitter by the second nurse. These activities apparently had been unknown to the physician, but the practice still may be responsible for her actions. These are insecure forms of communication and raise serious ethical and legal concerns.
Other social media pose confidentiality risks as well. For example, a physician was dismissed from a position and reprimanded by the medical board for posting patient information on Facebook,7 and an ObGyn caused problems by posting a nasty note about a patient who showed up late for an appointment.8 Too many patients may not understand that posting on social media is the equivalent of standing on a street corner yelling private information. Social media sites that invite the discussion of personal matters are an invitation to trouble.
Physicians are ethically obliged to protect confidentiality
Professional standards place significant ethical obligations on physicians to protect patient confidentiality. The American Medical Association (AMA) has an ethics opinion on professionalism with social media,9 as does the American College of Obstetricians and Gynecologists (ACOG).10 Another excellent discussion of ethical and practical issues is a joint position paper by the American College of Physicians and the Federation of State Medical Boards.11 Both documents focus attention on issues of confidentiality.
Physicians are legally obliged to protect confidentiality
There are many legal protections for confidentiality that can be implicated by electronic communications and social media. All states provide protection for unwarranted disclosure of private patient information. Such disclosures made electronically are included.12 Indeed, because electronic disclosures may be broadcast more widely, they may be especially dangerous. The misuse of social media may result in license discipline by the state board, regulatory sanctions, or civil liability (rare, but criminal sanctions are a possibility in extreme circumstances).
In addition to state laws regarding confidentiality, there are a number of federal laws that cover confidential medical information. None is more important than the Health Insurance Portability and Accountability Act (HIPAA) and the more recent HITECH amendments (Health Information Technology for Economic and Clinical Health).13 These laws have both privacy provisions and security (including “encryption”) requirements. These are complicated laws but at their core are the notions that health care providers and some others:
- are responsible for maintaining the security and privacy of health information
- may not transmit (even unintentionally) such information to others without patient permission or legal authority.14
- may not transmit (even unintentionally) such information to others without patient permission or legal authority.
A good source of step-by-step information about these laws is “Health information privacy: Covered entities and business associates,” on the US Health and Human Services website.14
HITECH also provides for notice to patients when health information is inappropriately transmitted. Thus, a missing USB flash drive with patient information may require notification to thousands of patients.15 Any consideration of the use of email or social media in medical practice must take into account the HIPAA/HITECH obligations to protect the security of patient health information. There can be serious professional consequences for failing to follow the HIPAA requirements.16
Dual relationships and conflicts of interest
In our hypothetical case, the office manager’s suggestion that the office use Facebook and their website to attract new patients also may raise confidentiality problems. The Facebook suggestion especially needs to be considered carefully. Facebook use is estimated to be 63% to 96% among students and 13% to 47% among health care professionals.17 Facebook is most often seen as an interactive social site; it risks blurring the lines between personal and professional relationships.9 There is a consensus that a physician should not “friend” patients on Facebook. The AMA ethics opinion notes that “physicians must maintain appropriate boundaries of the patient-physician relationship in accordance with professional ethical guidelines, just as they would in any other context.”9
Separate personal and professional contacts
Difficulties with interactive social media are not limited to the physicians in a practice. The problems increase with the number of staff members who post or respond on social media. Control of social media is essential. The practice must ensure that staff members do not slip into inappropriate personal comments and relationships. Staff should understand (and be reminded of) the necessity of separating personal and professional contacts.
Avoid misunderstandings
In addition, whatever the intent of the physician and staff may be, it is essentially impossible to know how patients will interpret interactions on these social media. The very informal, off-the-cuff, chatty way in which Facebook and similar sites are used invites misunderstandings, and maintaining professional boundaries is necessary.
Ground rules
All of this is not to say that professionals should never use Facebook or similar sites. Rather, if used, ground rules need to be established.
Social media communications must:
- be professional and not related to personal matters
- not be used to give medical advice
- be controlled by high level staff
- be reviewed periodically.
Staff training
Particularly for interactive social media (email, texts, Twitter, Facebook, etc), it is essential that there be both clear policies and good staff training (TABLE).9–11,18 There really should be no “making it up as we go along.” Staff on a social media lark of their own can be disastrous for the practice. Policies need to be updated frequently, and staff training reinforced and repeated periodically.
Quality of care and advice
Start with your website
Institutions’ websites are major sources of health care information: Nearly 32% of US adults would be very likely to prefer a hospital based on its website.5 Your website can be an important face of your practice to the community—for good or for bad. On one hand, the practice can control what is on a website and, unlike some social media, it will not be directed to individual patients. Done well, it “provides golden opportunities for marketing physician services, as well as for contributing to public health by providing high-quality online content that is both accurate and understandable to laypeople.”19 Done badly, it can convey incorrect and harmful information and discredit the medical practice that established it.
Your website introduces the practice and settings, but it will serve another purpose to thousands of people who likely will see it over time as a source of credible health information. The importance of ensuring that your website is carefully constructed to provide, or link to, good medical advice that contributes to quality of care cannot be overstated.
A good website begins with a clear statement of the reasons and goals for having the site. Professional design assistance generally is used to create the site, but that design process needs to be overseen by a medical professional to ensure that it conveys the sense of the practice and provides completely accurate information. A homepage of dancing clowns with stethoscopes may seem good to a 20-something-year-old designer, but it is not appropriate for a physician. It will be the practice, not the designer, who is held accountable for the site content. Links to other sites need to be vetted and used with care. Patients and other members of the public may well take the links as carrying the endorsement of the practice and its physicians.
Perhaps the greatest risk of a website is that it will not be kept current. Unfortunately, they do not update themselves. Some knowledgeable staff member must frequently review it to update everything from office hours and personnel to links to other sites. In addition, the physicians periodically must review it to ensure that all medical information is up to date and accurate. Old, outdated information about the office can put off potential patients. Outdated medical information may be harmful to patients who rely on it.
Any professional website should include disclaimers informing users that the site is not intended to establish a professional relationship or to give professional advice. The nature and extent of the disclaimer will depend on the type of information on the site. An example of a particularly thorough disclaimer is the Mayo Clinic disclaimer and terms of use (http://www.mayoclinic.org/about-this-site/terms-conditions-use-policy).
General professionalism
At the end of the day, social media are an outreach from a medical practice and from the profession to the public.20 Failure to treat these platforms with appropriate professional standards may result in professional discipline, damages, or civil penalties. Almost all of the reviews of social media use in health care practice note that the risks of inappropriate use are not only to the individual physician but also to the general medical profession, which may be undermined. Consider posting policies of the relevent state medical boards, the AMA, and ACOG in your office after you have had a discussion with your staff about them.21
The AMA statement includes a provision that a physician seeing unprofessional social media conduct by a colleague has the responsibility to bring that to the attention of the colleague. If the colleague does not correct a significant problem, “the physician should report the matter to appropriate authorities.”9
Bottom line
Any practitioner considering the use of social media must view it as a major step that requires caution, expert assistance, and constant attention to potential privacy, quality, and professionalism issues. If you are considering it, ensure that all staff associated with the practice understand and agree to the established limits on social media use.
Share your thoughts on this article! Send your Letter to the Editor to rbarbieri@frontlinemedcom.com. Please include your name and the city and state in which you practice.
- Modahl M, Tompsett L, Moorhead T. Doctors, patients, & social media. Quantia MD website. http://www.quantiamd.com/q-qcp/DoctorsPatientSocialMedia.pdf. Published September 2011. Accessed February 18, 2016.
- Kuberacka A, Wengrojj J, Fabozzi N. Social media use in U.S. healthcare provider institutions: Insights from Frost & Sullivan and iHT2 survey. Frost and Sullivan website. http://ihealthtran.com/pdf/frostiht2survey.pdf. Published August 30, 2011. Accessed February 18, 2016.
- O’Connor ME. How do tech savvy physicians use health technology and social media? Health Care Social Media website. http://hcsmmonitor.com/2014/01/08/how-do-tech-savvy-physicians-use-health-technology-and-social-media/. Published January 8, 2014. Accessed February 18, 2016.
- American Medical Association (AMA) Insurance. 2014 work/life profiles of today’s U.S. physician. AMA Insurance website. https://www.amainsure.com/work-life-profiles-of-todays-us-physician.html. Published April 2014. Accessed February 18, 2016.
- National Research Corporation. 2013 National Market Insights Survey: Health care social media website. https://healthcaresocialmedia.files.wordpress.com/2014/04/nrc-infographiclong.jpg. Accessed February 18, 2016.
- Suby C. Social media in health care: benefits, concerns and guidelines for use. Creat Nurs. 2013;19(3):140–147.
- Conaboy C. For doctors, social media a tricky case. Boston Globe. http://www.boston.com/lifestyle/health/articles/2011/04/20/for_doctors_social_media_a_tricky_case/?page=full. Published April 20, 2011. Accessed February 18, 2016.
- Matyszczyk C. Outcry as ob-gyn uses Facebook to complain about patient. CNET. http://www.cnet.com/news/outcry-as-ob-gyn-uses-facebook-to-complain-about-patient/Minion Pro. Published February 9, 2013. Accessed February 18, 2016.
- American Medical Association (AMA). Opinion 9.124: Professionalism in the use of social media. AMA website. http://www.ama-assn.org/ama/pub/physician-resources/medical-ethics/code-medical-ethics/opinion9124.page? Published June 2011. Accessed February 18, 2016.
- American College of Obstetricians and Gynecologists (ACOG) Committee on Professional Liability. ACOG Committee Opinion No. 622: professional use of digital and social media. Obstet Gynecol. 2015;125(2):516-520.
- Farnan JM, Sulmasy LS, Worster BK, et al. Online medical professionalism: patient and public relationships: Policy Statement From the American College of Physicians and the Federation of State Medical Boards. Ann Intern Med. 2013;158(8):620–627.
- Hader A, Drown E. Patient privacy and social media. AANA J. 2010;78(4):270–274.
- Kavoussi SC, Huang JJ, Tsai JC, Kempton JE. HIPAA for physicians in the information age. Conn Med. 2014;78(7):425–427.
- U.S. Department of Health & Human Services (HHS). Health information privacy: Covered entities and business associates. HHS website. http://www.hhs.gov/ocr/privacy/hipaa/understanding/coveredentities/. Published March 14, 2012. Accessed February 18, 2016.
- Perna G. Breach report: lost flash drive at Kaiser Permanente affects 49,000 patients. Healthcare Informatics website. http://www.healthcare-informatics.com/news-item/breach-report-lost-flash-drive-kaiser-permanente-affects-49000-patients. Published December 11, 2013. Accessed February 18, 2016.
- McBride M. How to ensure your social media efforts are HIPAA-compliant. Med Econ. 2012;89:70–74.
- Von Muhlen M, Ohno-Machado L. Reviewing social media use by clinicians. J Am Med Inform Assoc. 2012;19(5):777–781.
- Omurtag K, Turek P. Incorporating social media into practice: a blueprint for reproductive health providers. Clin Obstet Gynecol. 2013;56(3):463–470.
- Radmanesh A, Duszak R, Fitzgerald R. Social media and public outreach: a physician primer. Am J Neuroradiol. 2015;36(7):1223–1224.
- Grajales FJ 3rd, Sheps S, Ho K, Novak-Lauscher H, Eysenbach G. Social media: a review and tutorial of applications in medicine and health care. J Med Internet Res. 2014;16(2):e13.
- ACOG Today. Social media guide: how to comment with patients and spread women’s health messages. American Congress of Obstetricians and Gynecologists website. http://www.acog.org/-/media/ACOG-Today/acogToday201211.pdf. Published November 2012. Accessed February 18, 2016.
CASE: Patient discloses personal information in electronic communication. How to respond and what’s at stake?
Your nurse comes to you with a dilemma. Last Friday she received an email from a patient, sent to the nurse’s personal email account (G-mail) that conveyed information regarding the patient’s recent treatment for a herpetic vulvar lesion. The text details presumed exposure, date and time, number of sexual partners, concernfor “spread of disease,” and the patient’s desire to have a comprehensive sexually transmitted infection screening as soon as possible.
Your nurse has years of professional experience, but she is perhaps not the most savvy with regard to current information technology and social media. Nonetheless, she knows it is best not to immediately respond to the patient’s email without checking with you. She tracks you down on Monday morning to review the email and the dilemma she feels she has been placed in. What’s the best next step?
While discussing the general question with the staff, another nurse notes that there have been some reviews of the office on social media. It seems that this second nurse tweets and texts with patients all the time. The office manager strongly suggests that the office “join the 21st Century” by setting up a Facebook page and using their webpage to attract new patients and communicate with current patients.
How do you prepare for this? Is your staff knowledgeable about the dos and don’ts of social media?
The use of social media by health care providers has been growing for several years. Back in 2011 a large survey by QuantiaMD revealed that 87% of physicians used social media for personal reasons, and 67% of them used it professionally.1 How they used it for professional purposes also was explored in 2011, with almost 3 of 4 physicians using it for social networking and more than half engaging with their own institution’s social media (FIGURE).2 In 2013, 53% of physicians indicated that their practice had a Facebook platform, 28% had a presence on LinkedIn, and 21% were on Twitter.3 Not surprisingly, social media use is higher among younger physicians4; the 2016 equivalents to these percentages most likely are higher.
| Health providers’ use of social media for professional reasons2 |
 |
In 2011, a survey found that most health providers used social networking, their institutions’ own social media, and Internet forums, boards, and communities for professional reasons. |
Patients’ outreach through social media regarding health care information continues to grow, with 33.8% asking for health advice using social media.5 While email and other social media open the possibility of improved communication with patients, they also present a number of important professional and legal issues that deserve special consideration.6 Each medium presents its own challenges, but there are 4 categories of concern related to basic values and rights that we consider important to review:
- confidentiality
- dual relationships and conflicts of interest
- quality of care and advice
- general professionalism (including advertising).
Confidentiality
Few values of the medical profession are of longer standing than the commitment to maintain patient privacy. Fifth Century BC obligations continue to apply to the technology of the 21st Century AD. And the challenges are significant.
Email is not secure
In the opening case, the choice to email her clinician was apparently the patient’s. She probably does not realize that email is not very confidential, although it is undoubtedly in the Terms of Service Agreement she clicked through. Her email was likely scanned by her email service provider—Google, in this case—as well as the nurse. If, however, the physician’s office responds by email, it may well compound the confidentiality problem by further distributing the information through yet another email provider.
If, as a physician, you encourage email communication by your patients, a smart approach is to emphasize that such communications are not very confidential. At a minimum, until a secure email system can be established, it is best not to transmit medical information via email and to inform patients of the risk of such communication. In the case above, the nurse who received the email should respond to the patient by telephone (much more secure). Or she can respond to the patient by email (not including the patient’s message in the return), writing that, because email communications are inherently not confidential, she suggests a phone call or personal visit.
This case also notes that the patient sent the email to the nurse’s personal account, not to an office email account. Sending medical emails to an employee’s personal account raises additional problems of confidentiality and appropriate controls. It should be made clear that employees should not be discussing private medical matters via their own email accounts.
Other forms of social media are also not secure
Similar concerns arise about texting and using Twitter by the second nurse. These activities apparently had been unknown to the physician, but the practice still may be responsible for her actions. These are insecure forms of communication and raise serious ethical and legal concerns.
Other social media pose confidentiality risks as well. For example, a physician was dismissed from a position and reprimanded by the medical board for posting patient information on Facebook,7 and an ObGyn caused problems by posting a nasty note about a patient who showed up late for an appointment.8 Too many patients may not understand that posting on social media is the equivalent of standing on a street corner yelling private information. Social media sites that invite the discussion of personal matters are an invitation to trouble.
Physicians are ethically obliged to protect confidentiality
Professional standards place significant ethical obligations on physicians to protect patient confidentiality. The American Medical Association (AMA) has an ethics opinion on professionalism with social media,9 as does the American College of Obstetricians and Gynecologists (ACOG).10 Another excellent discussion of ethical and practical issues is a joint position paper by the American College of Physicians and the Federation of State Medical Boards.11 Both documents focus attention on issues of confidentiality.
Physicians are legally obliged to protect confidentiality
There are many legal protections for confidentiality that can be implicated by electronic communications and social media. All states provide protection for unwarranted disclosure of private patient information. Such disclosures made electronically are included.12 Indeed, because electronic disclosures may be broadcast more widely, they may be especially dangerous. The misuse of social media may result in license discipline by the state board, regulatory sanctions, or civil liability (rare, but criminal sanctions are a possibility in extreme circumstances).
In addition to state laws regarding confidentiality, there are a number of federal laws that cover confidential medical information. None is more important than the Health Insurance Portability and Accountability Act (HIPAA) and the more recent HITECH amendments (Health Information Technology for Economic and Clinical Health).13 These laws have both privacy provisions and security (including “encryption”) requirements. These are complicated laws but at their core are the notions that health care providers and some others:
- are responsible for maintaining the security and privacy of health information
- may not transmit (even unintentionally) such information to others without patient permission or legal authority.14
- may not transmit (even unintentionally) such information to others without patient permission or legal authority.
A good source of step-by-step information about these laws is “Health information privacy: Covered entities and business associates,” on the US Health and Human Services website.14
HITECH also provides for notice to patients when health information is inappropriately transmitted. Thus, a missing USB flash drive with patient information may require notification to thousands of patients.15 Any consideration of the use of email or social media in medical practice must take into account the HIPAA/HITECH obligations to protect the security of patient health information. There can be serious professional consequences for failing to follow the HIPAA requirements.16
Dual relationships and conflicts of interest
In our hypothetical case, the office manager’s suggestion that the office use Facebook and their website to attract new patients also may raise confidentiality problems. The Facebook suggestion especially needs to be considered carefully. Facebook use is estimated to be 63% to 96% among students and 13% to 47% among health care professionals.17 Facebook is most often seen as an interactive social site; it risks blurring the lines between personal and professional relationships.9 There is a consensus that a physician should not “friend” patients on Facebook. The AMA ethics opinion notes that “physicians must maintain appropriate boundaries of the patient-physician relationship in accordance with professional ethical guidelines, just as they would in any other context.”9
Separate personal and professional contacts
Difficulties with interactive social media are not limited to the physicians in a practice. The problems increase with the number of staff members who post or respond on social media. Control of social media is essential. The practice must ensure that staff members do not slip into inappropriate personal comments and relationships. Staff should understand (and be reminded of) the necessity of separating personal and professional contacts.
Avoid misunderstandings
In addition, whatever the intent of the physician and staff may be, it is essentially impossible to know how patients will interpret interactions on these social media. The very informal, off-the-cuff, chatty way in which Facebook and similar sites are used invites misunderstandings, and maintaining professional boundaries is necessary.
Ground rules
All of this is not to say that professionals should never use Facebook or similar sites. Rather, if used, ground rules need to be established.
Social media communications must:
- be professional and not related to personal matters
- not be used to give medical advice
- be controlled by high level staff
- be reviewed periodically.
Staff training
Particularly for interactive social media (email, texts, Twitter, Facebook, etc), it is essential that there be both clear policies and good staff training (TABLE).9–11,18 There really should be no “making it up as we go along.” Staff on a social media lark of their own can be disastrous for the practice. Policies need to be updated frequently, and staff training reinforced and repeated periodically.
Quality of care and advice
Start with your website
Institutions’ websites are major sources of health care information: Nearly 32% of US adults would be very likely to prefer a hospital based on its website.5 Your website can be an important face of your practice to the community—for good or for bad. On one hand, the practice can control what is on a website and, unlike some social media, it will not be directed to individual patients. Done well, it “provides golden opportunities for marketing physician services, as well as for contributing to public health by providing high-quality online content that is both accurate and understandable to laypeople.”19 Done badly, it can convey incorrect and harmful information and discredit the medical practice that established it.
Your website introduces the practice and settings, but it will serve another purpose to thousands of people who likely will see it over time as a source of credible health information. The importance of ensuring that your website is carefully constructed to provide, or link to, good medical advice that contributes to quality of care cannot be overstated.
A good website begins with a clear statement of the reasons and goals for having the site. Professional design assistance generally is used to create the site, but that design process needs to be overseen by a medical professional to ensure that it conveys the sense of the practice and provides completely accurate information. A homepage of dancing clowns with stethoscopes may seem good to a 20-something-year-old designer, but it is not appropriate for a physician. It will be the practice, not the designer, who is held accountable for the site content. Links to other sites need to be vetted and used with care. Patients and other members of the public may well take the links as carrying the endorsement of the practice and its physicians.
Perhaps the greatest risk of a website is that it will not be kept current. Unfortunately, they do not update themselves. Some knowledgeable staff member must frequently review it to update everything from office hours and personnel to links to other sites. In addition, the physicians periodically must review it to ensure that all medical information is up to date and accurate. Old, outdated information about the office can put off potential patients. Outdated medical information may be harmful to patients who rely on it.
Any professional website should include disclaimers informing users that the site is not intended to establish a professional relationship or to give professional advice. The nature and extent of the disclaimer will depend on the type of information on the site. An example of a particularly thorough disclaimer is the Mayo Clinic disclaimer and terms of use (http://www.mayoclinic.org/about-this-site/terms-conditions-use-policy).
General professionalism
At the end of the day, social media are an outreach from a medical practice and from the profession to the public.20 Failure to treat these platforms with appropriate professional standards may result in professional discipline, damages, or civil penalties. Almost all of the reviews of social media use in health care practice note that the risks of inappropriate use are not only to the individual physician but also to the general medical profession, which may be undermined. Consider posting policies of the relevent state medical boards, the AMA, and ACOG in your office after you have had a discussion with your staff about them.21
The AMA statement includes a provision that a physician seeing unprofessional social media conduct by a colleague has the responsibility to bring that to the attention of the colleague. If the colleague does not correct a significant problem, “the physician should report the matter to appropriate authorities.”9
Bottom line
Any practitioner considering the use of social media must view it as a major step that requires caution, expert assistance, and constant attention to potential privacy, quality, and professionalism issues. If you are considering it, ensure that all staff associated with the practice understand and agree to the established limits on social media use.
Share your thoughts on this article! Send your Letter to the Editor to rbarbieri@frontlinemedcom.com. Please include your name and the city and state in which you practice.
CASE: Patient discloses personal information in electronic communication. How to respond and what’s at stake?
Your nurse comes to you with a dilemma. Last Friday she received an email from a patient, sent to the nurse’s personal email account (G-mail) that conveyed information regarding the patient’s recent treatment for a herpetic vulvar lesion. The text details presumed exposure, date and time, number of sexual partners, concernfor “spread of disease,” and the patient’s desire to have a comprehensive sexually transmitted infection screening as soon as possible.
Your nurse has years of professional experience, but she is perhaps not the most savvy with regard to current information technology and social media. Nonetheless, she knows it is best not to immediately respond to the patient’s email without checking with you. She tracks you down on Monday morning to review the email and the dilemma she feels she has been placed in. What’s the best next step?
While discussing the general question with the staff, another nurse notes that there have been some reviews of the office on social media. It seems that this second nurse tweets and texts with patients all the time. The office manager strongly suggests that the office “join the 21st Century” by setting up a Facebook page and using their webpage to attract new patients and communicate with current patients.
How do you prepare for this? Is your staff knowledgeable about the dos and don’ts of social media?
The use of social media by health care providers has been growing for several years. Back in 2011 a large survey by QuantiaMD revealed that 87% of physicians used social media for personal reasons, and 67% of them used it professionally.1 How they used it for professional purposes also was explored in 2011, with almost 3 of 4 physicians using it for social networking and more than half engaging with their own institution’s social media (FIGURE).2 In 2013, 53% of physicians indicated that their practice had a Facebook platform, 28% had a presence on LinkedIn, and 21% were on Twitter.3 Not surprisingly, social media use is higher among younger physicians4; the 2016 equivalents to these percentages most likely are higher.
| Health providers’ use of social media for professional reasons2 |
 |
In 2011, a survey found that most health providers used social networking, their institutions’ own social media, and Internet forums, boards, and communities for professional reasons. |
Patients’ outreach through social media regarding health care information continues to grow, with 33.8% asking for health advice using social media.5 While email and other social media open the possibility of improved communication with patients, they also present a number of important professional and legal issues that deserve special consideration.6 Each medium presents its own challenges, but there are 4 categories of concern related to basic values and rights that we consider important to review:
- confidentiality
- dual relationships and conflicts of interest
- quality of care and advice
- general professionalism (including advertising).
Confidentiality
Few values of the medical profession are of longer standing than the commitment to maintain patient privacy. Fifth Century BC obligations continue to apply to the technology of the 21st Century AD. And the challenges are significant.
Email is not secure
In the opening case, the choice to email her clinician was apparently the patient’s. She probably does not realize that email is not very confidential, although it is undoubtedly in the Terms of Service Agreement she clicked through. Her email was likely scanned by her email service provider—Google, in this case—as well as the nurse. If, however, the physician’s office responds by email, it may well compound the confidentiality problem by further distributing the information through yet another email provider.
If, as a physician, you encourage email communication by your patients, a smart approach is to emphasize that such communications are not very confidential. At a minimum, until a secure email system can be established, it is best not to transmit medical information via email and to inform patients of the risk of such communication. In the case above, the nurse who received the email should respond to the patient by telephone (much more secure). Or she can respond to the patient by email (not including the patient’s message in the return), writing that, because email communications are inherently not confidential, she suggests a phone call or personal visit.
This case also notes that the patient sent the email to the nurse’s personal account, not to an office email account. Sending medical emails to an employee’s personal account raises additional problems of confidentiality and appropriate controls. It should be made clear that employees should not be discussing private medical matters via their own email accounts.
Other forms of social media are also not secure
Similar concerns arise about texting and using Twitter by the second nurse. These activities apparently had been unknown to the physician, but the practice still may be responsible for her actions. These are insecure forms of communication and raise serious ethical and legal concerns.
Other social media pose confidentiality risks as well. For example, a physician was dismissed from a position and reprimanded by the medical board for posting patient information on Facebook,7 and an ObGyn caused problems by posting a nasty note about a patient who showed up late for an appointment.8 Too many patients may not understand that posting on social media is the equivalent of standing on a street corner yelling private information. Social media sites that invite the discussion of personal matters are an invitation to trouble.
Physicians are ethically obliged to protect confidentiality
Professional standards place significant ethical obligations on physicians to protect patient confidentiality. The American Medical Association (AMA) has an ethics opinion on professionalism with social media,9 as does the American College of Obstetricians and Gynecologists (ACOG).10 Another excellent discussion of ethical and practical issues is a joint position paper by the American College of Physicians and the Federation of State Medical Boards.11 Both documents focus attention on issues of confidentiality.
Physicians are legally obliged to protect confidentiality
There are many legal protections for confidentiality that can be implicated by electronic communications and social media. All states provide protection for unwarranted disclosure of private patient information. Such disclosures made electronically are included.12 Indeed, because electronic disclosures may be broadcast more widely, they may be especially dangerous. The misuse of social media may result in license discipline by the state board, regulatory sanctions, or civil liability (rare, but criminal sanctions are a possibility in extreme circumstances).
In addition to state laws regarding confidentiality, there are a number of federal laws that cover confidential medical information. None is more important than the Health Insurance Portability and Accountability Act (HIPAA) and the more recent HITECH amendments (Health Information Technology for Economic and Clinical Health).13 These laws have both privacy provisions and security (including “encryption”) requirements. These are complicated laws but at their core are the notions that health care providers and some others:
- are responsible for maintaining the security and privacy of health information
- may not transmit (even unintentionally) such information to others without patient permission or legal authority.14
- may not transmit (even unintentionally) such information to others without patient permission or legal authority.
A good source of step-by-step information about these laws is “Health information privacy: Covered entities and business associates,” on the US Health and Human Services website.14
HITECH also provides for notice to patients when health information is inappropriately transmitted. Thus, a missing USB flash drive with patient information may require notification to thousands of patients.15 Any consideration of the use of email or social media in medical practice must take into account the HIPAA/HITECH obligations to protect the security of patient health information. There can be serious professional consequences for failing to follow the HIPAA requirements.16
Dual relationships and conflicts of interest
In our hypothetical case, the office manager’s suggestion that the office use Facebook and their website to attract new patients also may raise confidentiality problems. The Facebook suggestion especially needs to be considered carefully. Facebook use is estimated to be 63% to 96% among students and 13% to 47% among health care professionals.17 Facebook is most often seen as an interactive social site; it risks blurring the lines between personal and professional relationships.9 There is a consensus that a physician should not “friend” patients on Facebook. The AMA ethics opinion notes that “physicians must maintain appropriate boundaries of the patient-physician relationship in accordance with professional ethical guidelines, just as they would in any other context.”9
Separate personal and professional contacts
Difficulties with interactive social media are not limited to the physicians in a practice. The problems increase with the number of staff members who post or respond on social media. Control of social media is essential. The practice must ensure that staff members do not slip into inappropriate personal comments and relationships. Staff should understand (and be reminded of) the necessity of separating personal and professional contacts.
Avoid misunderstandings
In addition, whatever the intent of the physician and staff may be, it is essentially impossible to know how patients will interpret interactions on these social media. The very informal, off-the-cuff, chatty way in which Facebook and similar sites are used invites misunderstandings, and maintaining professional boundaries is necessary.
Ground rules
All of this is not to say that professionals should never use Facebook or similar sites. Rather, if used, ground rules need to be established.
Social media communications must:
- be professional and not related to personal matters
- not be used to give medical advice
- be controlled by high level staff
- be reviewed periodically.
Staff training
Particularly for interactive social media (email, texts, Twitter, Facebook, etc), it is essential that there be both clear policies and good staff training (TABLE).9–11,18 There really should be no “making it up as we go along.” Staff on a social media lark of their own can be disastrous for the practice. Policies need to be updated frequently, and staff training reinforced and repeated periodically.
Quality of care and advice
Start with your website
Institutions’ websites are major sources of health care information: Nearly 32% of US adults would be very likely to prefer a hospital based on its website.5 Your website can be an important face of your practice to the community—for good or for bad. On one hand, the practice can control what is on a website and, unlike some social media, it will not be directed to individual patients. Done well, it “provides golden opportunities for marketing physician services, as well as for contributing to public health by providing high-quality online content that is both accurate and understandable to laypeople.”19 Done badly, it can convey incorrect and harmful information and discredit the medical practice that established it.
Your website introduces the practice and settings, but it will serve another purpose to thousands of people who likely will see it over time as a source of credible health information. The importance of ensuring that your website is carefully constructed to provide, or link to, good medical advice that contributes to quality of care cannot be overstated.
A good website begins with a clear statement of the reasons and goals for having the site. Professional design assistance generally is used to create the site, but that design process needs to be overseen by a medical professional to ensure that it conveys the sense of the practice and provides completely accurate information. A homepage of dancing clowns with stethoscopes may seem good to a 20-something-year-old designer, but it is not appropriate for a physician. It will be the practice, not the designer, who is held accountable for the site content. Links to other sites need to be vetted and used with care. Patients and other members of the public may well take the links as carrying the endorsement of the practice and its physicians.
Perhaps the greatest risk of a website is that it will not be kept current. Unfortunately, they do not update themselves. Some knowledgeable staff member must frequently review it to update everything from office hours and personnel to links to other sites. In addition, the physicians periodically must review it to ensure that all medical information is up to date and accurate. Old, outdated information about the office can put off potential patients. Outdated medical information may be harmful to patients who rely on it.
Any professional website should include disclaimers informing users that the site is not intended to establish a professional relationship or to give professional advice. The nature and extent of the disclaimer will depend on the type of information on the site. An example of a particularly thorough disclaimer is the Mayo Clinic disclaimer and terms of use (http://www.mayoclinic.org/about-this-site/terms-conditions-use-policy).
General professionalism
At the end of the day, social media are an outreach from a medical practice and from the profession to the public.20 Failure to treat these platforms with appropriate professional standards may result in professional discipline, damages, or civil penalties. Almost all of the reviews of social media use in health care practice note that the risks of inappropriate use are not only to the individual physician but also to the general medical profession, which may be undermined. Consider posting policies of the relevent state medical boards, the AMA, and ACOG in your office after you have had a discussion with your staff about them.21
The AMA statement includes a provision that a physician seeing unprofessional social media conduct by a colleague has the responsibility to bring that to the attention of the colleague. If the colleague does not correct a significant problem, “the physician should report the matter to appropriate authorities.”9
Bottom line
Any practitioner considering the use of social media must view it as a major step that requires caution, expert assistance, and constant attention to potential privacy, quality, and professionalism issues. If you are considering it, ensure that all staff associated with the practice understand and agree to the established limits on social media use.
Share your thoughts on this article! Send your Letter to the Editor to rbarbieri@frontlinemedcom.com. Please include your name and the city and state in which you practice.
- Modahl M, Tompsett L, Moorhead T. Doctors, patients, & social media. Quantia MD website. http://www.quantiamd.com/q-qcp/DoctorsPatientSocialMedia.pdf. Published September 2011. Accessed February 18, 2016.
- Kuberacka A, Wengrojj J, Fabozzi N. Social media use in U.S. healthcare provider institutions: Insights from Frost & Sullivan and iHT2 survey. Frost and Sullivan website. http://ihealthtran.com/pdf/frostiht2survey.pdf. Published August 30, 2011. Accessed February 18, 2016.
- O’Connor ME. How do tech savvy physicians use health technology and social media? Health Care Social Media website. http://hcsmmonitor.com/2014/01/08/how-do-tech-savvy-physicians-use-health-technology-and-social-media/. Published January 8, 2014. Accessed February 18, 2016.
- American Medical Association (AMA) Insurance. 2014 work/life profiles of today’s U.S. physician. AMA Insurance website. https://www.amainsure.com/work-life-profiles-of-todays-us-physician.html. Published April 2014. Accessed February 18, 2016.
- National Research Corporation. 2013 National Market Insights Survey: Health care social media website. https://healthcaresocialmedia.files.wordpress.com/2014/04/nrc-infographiclong.jpg. Accessed February 18, 2016.
- Suby C. Social media in health care: benefits, concerns and guidelines for use. Creat Nurs. 2013;19(3):140–147.
- Conaboy C. For doctors, social media a tricky case. Boston Globe. http://www.boston.com/lifestyle/health/articles/2011/04/20/for_doctors_social_media_a_tricky_case/?page=full. Published April 20, 2011. Accessed February 18, 2016.
- Matyszczyk C. Outcry as ob-gyn uses Facebook to complain about patient. CNET. http://www.cnet.com/news/outcry-as-ob-gyn-uses-facebook-to-complain-about-patient/Minion Pro. Published February 9, 2013. Accessed February 18, 2016.
- American Medical Association (AMA). Opinion 9.124: Professionalism in the use of social media. AMA website. http://www.ama-assn.org/ama/pub/physician-resources/medical-ethics/code-medical-ethics/opinion9124.page? Published June 2011. Accessed February 18, 2016.
- American College of Obstetricians and Gynecologists (ACOG) Committee on Professional Liability. ACOG Committee Opinion No. 622: professional use of digital and social media. Obstet Gynecol. 2015;125(2):516-520.
- Farnan JM, Sulmasy LS, Worster BK, et al. Online medical professionalism: patient and public relationships: Policy Statement From the American College of Physicians and the Federation of State Medical Boards. Ann Intern Med. 2013;158(8):620–627.
- Hader A, Drown E. Patient privacy and social media. AANA J. 2010;78(4):270–274.
- Kavoussi SC, Huang JJ, Tsai JC, Kempton JE. HIPAA for physicians in the information age. Conn Med. 2014;78(7):425–427.
- U.S. Department of Health & Human Services (HHS). Health information privacy: Covered entities and business associates. HHS website. http://www.hhs.gov/ocr/privacy/hipaa/understanding/coveredentities/. Published March 14, 2012. Accessed February 18, 2016.
- Perna G. Breach report: lost flash drive at Kaiser Permanente affects 49,000 patients. Healthcare Informatics website. http://www.healthcare-informatics.com/news-item/breach-report-lost-flash-drive-kaiser-permanente-affects-49000-patients. Published December 11, 2013. Accessed February 18, 2016.
- McBride M. How to ensure your social media efforts are HIPAA-compliant. Med Econ. 2012;89:70–74.
- Von Muhlen M, Ohno-Machado L. Reviewing social media use by clinicians. J Am Med Inform Assoc. 2012;19(5):777–781.
- Omurtag K, Turek P. Incorporating social media into practice: a blueprint for reproductive health providers. Clin Obstet Gynecol. 2013;56(3):463–470.
- Radmanesh A, Duszak R, Fitzgerald R. Social media and public outreach: a physician primer. Am J Neuroradiol. 2015;36(7):1223–1224.
- Grajales FJ 3rd, Sheps S, Ho K, Novak-Lauscher H, Eysenbach G. Social media: a review and tutorial of applications in medicine and health care. J Med Internet Res. 2014;16(2):e13.
- ACOG Today. Social media guide: how to comment with patients and spread women’s health messages. American Congress of Obstetricians and Gynecologists website. http://www.acog.org/-/media/ACOG-Today/acogToday201211.pdf. Published November 2012. Accessed February 18, 2016.
- Modahl M, Tompsett L, Moorhead T. Doctors, patients, & social media. Quantia MD website. http://www.quantiamd.com/q-qcp/DoctorsPatientSocialMedia.pdf. Published September 2011. Accessed February 18, 2016.
- Kuberacka A, Wengrojj J, Fabozzi N. Social media use in U.S. healthcare provider institutions: Insights from Frost & Sullivan and iHT2 survey. Frost and Sullivan website. http://ihealthtran.com/pdf/frostiht2survey.pdf. Published August 30, 2011. Accessed February 18, 2016.
- O’Connor ME. How do tech savvy physicians use health technology and social media? Health Care Social Media website. http://hcsmmonitor.com/2014/01/08/how-do-tech-savvy-physicians-use-health-technology-and-social-media/. Published January 8, 2014. Accessed February 18, 2016.
- American Medical Association (AMA) Insurance. 2014 work/life profiles of today’s U.S. physician. AMA Insurance website. https://www.amainsure.com/work-life-profiles-of-todays-us-physician.html. Published April 2014. Accessed February 18, 2016.
- National Research Corporation. 2013 National Market Insights Survey: Health care social media website. https://healthcaresocialmedia.files.wordpress.com/2014/04/nrc-infographiclong.jpg. Accessed February 18, 2016.
- Suby C. Social media in health care: benefits, concerns and guidelines for use. Creat Nurs. 2013;19(3):140–147.
- Conaboy C. For doctors, social media a tricky case. Boston Globe. http://www.boston.com/lifestyle/health/articles/2011/04/20/for_doctors_social_media_a_tricky_case/?page=full. Published April 20, 2011. Accessed February 18, 2016.
- Matyszczyk C. Outcry as ob-gyn uses Facebook to complain about patient. CNET. http://www.cnet.com/news/outcry-as-ob-gyn-uses-facebook-to-complain-about-patient/Minion Pro. Published February 9, 2013. Accessed February 18, 2016.
- American Medical Association (AMA). Opinion 9.124: Professionalism in the use of social media. AMA website. http://www.ama-assn.org/ama/pub/physician-resources/medical-ethics/code-medical-ethics/opinion9124.page? Published June 2011. Accessed February 18, 2016.
- American College of Obstetricians and Gynecologists (ACOG) Committee on Professional Liability. ACOG Committee Opinion No. 622: professional use of digital and social media. Obstet Gynecol. 2015;125(2):516-520.
- Farnan JM, Sulmasy LS, Worster BK, et al. Online medical professionalism: patient and public relationships: Policy Statement From the American College of Physicians and the Federation of State Medical Boards. Ann Intern Med. 2013;158(8):620–627.
- Hader A, Drown E. Patient privacy and social media. AANA J. 2010;78(4):270–274.
- Kavoussi SC, Huang JJ, Tsai JC, Kempton JE. HIPAA for physicians in the information age. Conn Med. 2014;78(7):425–427.
- U.S. Department of Health & Human Services (HHS). Health information privacy: Covered entities and business associates. HHS website. http://www.hhs.gov/ocr/privacy/hipaa/understanding/coveredentities/. Published March 14, 2012. Accessed February 18, 2016.
- Perna G. Breach report: lost flash drive at Kaiser Permanente affects 49,000 patients. Healthcare Informatics website. http://www.healthcare-informatics.com/news-item/breach-report-lost-flash-drive-kaiser-permanente-affects-49000-patients. Published December 11, 2013. Accessed February 18, 2016.
- McBride M. How to ensure your social media efforts are HIPAA-compliant. Med Econ. 2012;89:70–74.
- Von Muhlen M, Ohno-Machado L. Reviewing social media use by clinicians. J Am Med Inform Assoc. 2012;19(5):777–781.
- Omurtag K, Turek P. Incorporating social media into practice: a blueprint for reproductive health providers. Clin Obstet Gynecol. 2013;56(3):463–470.
- Radmanesh A, Duszak R, Fitzgerald R. Social media and public outreach: a physician primer. Am J Neuroradiol. 2015;36(7):1223–1224.
- Grajales FJ 3rd, Sheps S, Ho K, Novak-Lauscher H, Eysenbach G. Social media: a review and tutorial of applications in medicine and health care. J Med Internet Res. 2014;16(2):e13.
- ACOG Today. Social media guide: how to comment with patients and spread women’s health messages. American Congress of Obstetricians and Gynecologists website. http://www.acog.org/-/media/ACOG-Today/acogToday201211.pdf. Published November 2012. Accessed February 18, 2016.
In this Article
- Health providers’ use of social media
- Protecting confidentiality
- Creating a social media policy
6 Supreme Court decisions that affected ObGyns in 2015
You might ask, why do I need to know what the Supreme Court does, it will not affect me! Well, we all know that is not exactly true. We chose these 6 cases because of their importance to ObGyns. In a number of them, the American Medical Association (AMA) or specific specialty board filed amicus curiae briefs, which suggested that the profession felt these were especially critical cases. (An amicus brief is a “friend of the Court” brief filed not by one of the affected parties, but by an organization or person with an interest or special expertise in the case.) You can find additional analysis of the 2015 term of the Supreme Court at the website of the National Register (http://www.nationalregister.org/pub/the-national-registerreport-pub/the-register-report-fall-2015/the-aca-survives-and-same-sex-marriage-thrives-the-2014-2015-supreme-court/).
1. Affordable Care Act upheld
King v Burwell was likely the Court’s most important case for physicians and their patients during the 2015 term.
At stake. The question was whether or not people who use the federal Affordable Care Act (ACA) Exchange could receive the same subsidy as those who use the state established Exchanges.1
Final ruling. The Court said “yes,” they could receive the same subsidy, ruling in favor of King.
Key points of the case
The ACA provides for state Exchanges (an electronic marketplace in which people can compare and purchase health insurance policies), but most states did not establish Exchanges. As a result, in many states the federal Exchange became the default. Because the ACA subsidies (which help many people afford mandated insurance coverage) are processed through exchanges, the question of whether those who used the federal Exchange received the subsidy was enormously important. Subsidies for millions of individuals depended on it.
The language of the ACA provides that the insurance subsidy is available only if the person(s) enroll in “an Exchange established by the State” (emphasis added).1 This case was about what “Exchange established by the State” means. A 6-justice majority held that the best interpretation of the statute was that it permitted subsidies through the federal Exchange. This was a difficult decision because, as the majority noted, the ACA is sloppily written. Nonetheless, the Court had to do its best to read the language in the context of the whole statute. Six justices held that the statute meant that the subsidies would cover people who signed up through federal as well as state Exchanges.
The dissenting justices essentially took the position that the language of the statute is clear. Among other things, the dissent said, it means that the words “established by the state” have no meaning at all in the statute, and it is unclear why Congress did not say “Exchange” instead of “Exchange established by the state.”
The results of the case were that the subsidies granted through the federal Exchange will continue. It will not expand the subsidies. Had the decision gone the other way, there would have been a real challenge to the future of the ACA. For that reason a number of medical and health care organizations filed amicus briefs with the Court in this case.
2. State licensing boards and antitrust
At stake. Antitrust laws prohibit combinations and conspiracies in restraint of trade. Competitors cannot come together to seek to set prices, divide the market, or prevent new competitors from entering the market. Since the 1940s, however, the Supreme Court has recognized a “state action” exception to the antitrust laws. The question before the Court this term was whether or not a state licensing board is included in the state-action immunity.2
The AMA and others filed an amicus brief in this case, noting threat to the public health if the Court disrupted state medical boards’ regulation of professional licensing and unauthorized practice.
Final ruling. The Court rejected this argument. It held that, where a state board is “controlled by active market participants” (as most state professional boards are), antitrust immunity is not automatic. For the immunity to protect boards, 2 conditions must exist, the Court held:
1. The state must have articulated a clear policy to allow the regulation that is an anticompetitive conduct (eg, licensing)
2. The state must have provided active supervision of the anticompetitive conduct. This requires that the state appoint someone or some group to approve policies of the board.
The first of these requirements often would be met by the statute setting up the board. The Court focused some attention on the second requirement. It concluded that “the adequacy of supervision otherwise will depend on all the circumstances of a case.”2
Key points of the case
In most states, this decision will require some kind of restructuring so that the professional boards are not the final decision makers but, in effect, only make recommendations to a “supervisor.” The Court gave short shrift to the AMA’s concerns that the decision might make it more difficult to attract really good professionals to the boards. The possibility of personal liability probably can be dealt with, but it deserves attention. The problems with litigation and antitrust claims, and reviews of decisions of a board (potentially by nonprofessionals) hardly can be a plus in attracting the right professionals to the boards.
This case will give rise to considerable litigation for many years. Medical boards should endeavor to get ahead of the issue by immediately studying ways in which the concerns of the Federal Trade Commissioncan be accommodated without significantly reducing the public protection that is part of well-administered professional licensing.
3. State reimbursement for Medicaid services
Medicaid is a federal-state program, and federal law requires, in part, that states must “assure that payments are … sufficient to enlist enough providers so that care and services are available under the plan at least to the extent that such care and services are available to the general population in the geographic area.”3 Providers claimed that the state had failed to establish such a payment system. A number of medical groups, including the AMA, filed amicus briefs in support of the providers.
At stake. State funding for various Medicaid services has been a problem for many health care professionals for some time. But the Medicaid law does not clearly give providers the right to file lawsuits claiming inadequate reimbursement,4 so the question in this case was whether or not there was an implied right to do so.
Final ruling. The Court, in a 5-4 decision, held that Medicaid providers do not have the authority to sue states in federal courts torequire that the states provide higher Medicaid rates for services.4 As a practical matter, this decision leaves with the states broad authority to set Medicaid reimbursement rates. It is possible, of course, that in the future Congress would change the law to grant such rights or more clearly set reimbursement rates.
4. False Claims Act cases
Unfortunately, False Claims Act cases occur in health care. False claims transpire when someone (or an organization) presents to the government a claim for payment that is not legitimate or is for inadequate or low quality services. False claims include everything from fraudulent billing for services never performed to a pharmaceutical company’s promotion of a drug for off-label use.
The federal False Claims Act incentivizes private whistleblowers (often disgruntled employees) to initiate false claims lawsuits. (The government may then choose to take over the false claims or allow the private whistleblower to pursue them.)
At stake. Because Medicare and Medicaid are federally financed programs, it is common for providers who participate in those programs to be subject to false claims.5 This term the Court heard an important False Claims Act case involving the statute of limitations and multiple claims based on the same activity. The AMA joined an amicus brief urging the Court to prohibit both kinds of expansion.
Final ruling. The Court agreed that the statute of limitations for these claims should not be extended, but it did determine that the “first-to-file” limitation in the statute “keeps new claims out of court only while related claims are still alive, not in perpetuity.”5 The result of this second holding is that the “firstto-file” rule does not preclude another false claims suit that is duplicative to be filed as soon as the prior suit is no longer pending.
It is not clear that the practical effect of the decision will be great, but it may in some cases open up clinicians to multiple, serial lawsuits over the same claims.
5. Pregnancy and employment discrimination
The federal Pregnancy Discrimination Act (PDA) prohibits discrimination “because of or on the basis of pregnancy, childbirth, or related medical conditions.” It also requires that employers treat “women affected by pregnancy . . . the same for all employment-related purposes . . . as other persons not so affected but similar in their ability or inability to work.”
At stake. United Parcel Service (UPS) declined to give a pregnant employee a “light duty” accommodation during pregnancy so that she could avoid having to lift heavy packages. But it had given other employees just that kind of accommodation when they were injured or lost certification to drive a delivery truck. The suit claimed violation of the PDA.6
Final ruling. The Court held that UPS violated the PDA by giving some employees this accommodation but refusing it to pregnant workers who requested it.6 Once an organization voluntarily grants a particular accommodation to, for example, a temporarily injured worker, it may be required to provide similar accommodation to pregnant workers. The case probably will increase employment protection for pregnant women.
6. Children “testifying” in abuse cases
All states require physicians and teachers, among others, to report suspected child abuse. An ongoing question has been whether those reporters may be called to testify about what the child said at the time of abuse discovery/suspicion. This case involved a teacher, but it could as easily have been a physician.7
Key points of the case. Teachers found suspicious injuries on 3-year-old L.P. The child gave conflicting statements about what happened, but claimed that Clark had hurt him. At Clark’s criminal trial, L.P. did not testify, but the state wanted to introduce L.P.’s statements to the teachers as evidence of Clark’s guilt.
At stake. The question was whether or not this introduction would violate the right of Clark to “confront his accuser” as guaranteed by the Constitution.
Final ruling. All 9 justices said it was proper to allow L.P.’s statements to be introduced at trial.7 Essentially this was permitted because the primary purpose of L.P.’s statements was not to gather testimony to be presented at trial.
The final opinion creates incentives for some prosecutors to make abuse victims unavailable in order to allow their testimony by hearsay. Aside from the legal technicalities, the likelihood of wrongful convictions under such a system must be considered for the long run.
Other major 2015 Supreme Court decisions
- The Constitution requires states to recognize same-sex marriages and also accept such marriages performed in other states.1 (The American Medical Association joined an amicus brief in this case.)
- The Court turned down the appeal of several inmates who had received lethal injection death sentences. They challenged the mix of drugs that Oklahoma planned to use to execute them.2
- In 2 cases justices went out of the way to raise questions about the constitutionality of the death penalty. This may suggest that the Court will take up this issue in the near future.2,3
- Federal housing discrimination laws were expanded to cases in which there is “disparate impact” discrimination.4
- The Court narrowed prosecution for “threatening” statements in social network/Internet communications by holding that negligence in the communication is not sufficient for conviction under federal law.5
- The Court held that the Environmental Protection Agency violated the Clean Air Act with its power plant emission regulations because it failed to do a proper cost-benefit analysis.6
References
1. Obergefell et al v Hodges, Director, Ohio Department of Health et al, No. 14–556 (2015). http://www.supremecourt.gov/opinions/14pdf/14-556_3204.pdf. Accessed December 14, 2015.
2. Glossip et al v Gross et al, No. 14–7955 (2015). http://www.supremecourt.gov/opinions/14pdf/14-7955_aplc.pdf. Accessed December 14, 2015.
3. Davis, Acting Worden v Ayala, No. 13–1428 (2015). http://www.supremecourt.gov/opinions/14pdf/13-1428_1a7d.pdf. Accessed December 14, 2015.
4. Texas Department of Housing and Community Affairs et al v Inclusive Communities Project, Inc et al, No. 13–1371 (2015). http://www.supremecourt.gov/opinions/14pdf/13-1371_8m58.pdf. Accessed December 14, 2015.
5. Elonis v United States, No. 13–983 (2015). http://www.supremecourt.gov/opinions/14pdf/13-983_7148.pdf. Accessed December 14, 2015.
6. Michigan et al v Environmental Protection Agency et al, No. 14–46 (2015). http://www.supremecourt.gov/opinions/14pdf/14-46_bqmc.pdf. Accessed December 14, 2015.
What’s coming in 2016
This term of the Supreme Court is a reminder of how important the decisions of the Court are to the work of physicians and ObGyns in particular. This trend is continuing—the Court already has, for the next term, accepted cases regarding abortion, contraception, and health care delivery. It will, therefore, be worthwhile to follow the developments at the nation’s highest court.
Share your thoughts on this article! Send your Letter to the Editor to rbarbieri@frontlinemedcom.com. Please include your name and the city and state in which you practice.
1. King et al v Burwell, Secretary of Health and Human Services et al, No. 14–114 (2015). http://www.supremecourt.gov/opinions/14pdf/14-114_qol1.pdf. Accessed December 14, 2015.
2. North Carolina State Board of Dental Examiners v Federal Trade Commission, No. 13–534 (2015). http://www.supremecourt.gov/opinions/14pd/13-534_19m2.pdf. Accessed December 14, 2015.
3. Federal Policy Guidance. Medicaid.gov website. http://medicaid.gov/federal-policy-guidance/federal-policyguidance.html. Accessed December 14, 2015.
4. Armstrong et al v Exceptional Child Center, Inc et al, No. 14–15 (2015). http://www.supremecourt.gov/opinions/14pdf/14-15_d1oe.pdf. Accessed December 14, 2015.
5. Kellogg Brown & Root Services, Inc et al v United States ex rel. Carter, No 12–1497 (2015). http://www.supremecourt.gov/opinions/14pdf/12-1497_2d8f.pdf. Accessed December 14, 2015.
6. Young v United Parcel Service, Inc., No. 12–1226 (2015). http://www.supremecourt.gov/opinions/14pdf/12-1226_k5fl.pdf. Accessed December 14, 2015.
7. Ohio v Clark, No. 13–1352 (2015). http://www.supremecourt.gov/opinion/14pdf/13-1352_ed9l.pdf. Accessed December 14, 2015.
You might ask, why do I need to know what the Supreme Court does, it will not affect me! Well, we all know that is not exactly true. We chose these 6 cases because of their importance to ObGyns. In a number of them, the American Medical Association (AMA) or specific specialty board filed amicus curiae briefs, which suggested that the profession felt these were especially critical cases. (An amicus brief is a “friend of the Court” brief filed not by one of the affected parties, but by an organization or person with an interest or special expertise in the case.) You can find additional analysis of the 2015 term of the Supreme Court at the website of the National Register (http://www.nationalregister.org/pub/the-national-registerreport-pub/the-register-report-fall-2015/the-aca-survives-and-same-sex-marriage-thrives-the-2014-2015-supreme-court/).
1. Affordable Care Act upheld
King v Burwell was likely the Court’s most important case for physicians and their patients during the 2015 term.
At stake. The question was whether or not people who use the federal Affordable Care Act (ACA) Exchange could receive the same subsidy as those who use the state established Exchanges.1
Final ruling. The Court said “yes,” they could receive the same subsidy, ruling in favor of King.
Key points of the case
The ACA provides for state Exchanges (an electronic marketplace in which people can compare and purchase health insurance policies), but most states did not establish Exchanges. As a result, in many states the federal Exchange became the default. Because the ACA subsidies (which help many people afford mandated insurance coverage) are processed through exchanges, the question of whether those who used the federal Exchange received the subsidy was enormously important. Subsidies for millions of individuals depended on it.
The language of the ACA provides that the insurance subsidy is available only if the person(s) enroll in “an Exchange established by the State” (emphasis added).1 This case was about what “Exchange established by the State” means. A 6-justice majority held that the best interpretation of the statute was that it permitted subsidies through the federal Exchange. This was a difficult decision because, as the majority noted, the ACA is sloppily written. Nonetheless, the Court had to do its best to read the language in the context of the whole statute. Six justices held that the statute meant that the subsidies would cover people who signed up through federal as well as state Exchanges.
The dissenting justices essentially took the position that the language of the statute is clear. Among other things, the dissent said, it means that the words “established by the state” have no meaning at all in the statute, and it is unclear why Congress did not say “Exchange” instead of “Exchange established by the state.”
The results of the case were that the subsidies granted through the federal Exchange will continue. It will not expand the subsidies. Had the decision gone the other way, there would have been a real challenge to the future of the ACA. For that reason a number of medical and health care organizations filed amicus briefs with the Court in this case.
2. State licensing boards and antitrust
At stake. Antitrust laws prohibit combinations and conspiracies in restraint of trade. Competitors cannot come together to seek to set prices, divide the market, or prevent new competitors from entering the market. Since the 1940s, however, the Supreme Court has recognized a “state action” exception to the antitrust laws. The question before the Court this term was whether or not a state licensing board is included in the state-action immunity.2
The AMA and others filed an amicus brief in this case, noting threat to the public health if the Court disrupted state medical boards’ regulation of professional licensing and unauthorized practice.
Final ruling. The Court rejected this argument. It held that, where a state board is “controlled by active market participants” (as most state professional boards are), antitrust immunity is not automatic. For the immunity to protect boards, 2 conditions must exist, the Court held:
1. The state must have articulated a clear policy to allow the regulation that is an anticompetitive conduct (eg, licensing)
2. The state must have provided active supervision of the anticompetitive conduct. This requires that the state appoint someone or some group to approve policies of the board.
The first of these requirements often would be met by the statute setting up the board. The Court focused some attention on the second requirement. It concluded that “the adequacy of supervision otherwise will depend on all the circumstances of a case.”2
Key points of the case
In most states, this decision will require some kind of restructuring so that the professional boards are not the final decision makers but, in effect, only make recommendations to a “supervisor.” The Court gave short shrift to the AMA’s concerns that the decision might make it more difficult to attract really good professionals to the boards. The possibility of personal liability probably can be dealt with, but it deserves attention. The problems with litigation and antitrust claims, and reviews of decisions of a board (potentially by nonprofessionals) hardly can be a plus in attracting the right professionals to the boards.
This case will give rise to considerable litigation for many years. Medical boards should endeavor to get ahead of the issue by immediately studying ways in which the concerns of the Federal Trade Commissioncan be accommodated without significantly reducing the public protection that is part of well-administered professional licensing.
3. State reimbursement for Medicaid services
Medicaid is a federal-state program, and federal law requires, in part, that states must “assure that payments are … sufficient to enlist enough providers so that care and services are available under the plan at least to the extent that such care and services are available to the general population in the geographic area.”3 Providers claimed that the state had failed to establish such a payment system. A number of medical groups, including the AMA, filed amicus briefs in support of the providers.
At stake. State funding for various Medicaid services has been a problem for many health care professionals for some time. But the Medicaid law does not clearly give providers the right to file lawsuits claiming inadequate reimbursement,4 so the question in this case was whether or not there was an implied right to do so.
Final ruling. The Court, in a 5-4 decision, held that Medicaid providers do not have the authority to sue states in federal courts torequire that the states provide higher Medicaid rates for services.4 As a practical matter, this decision leaves with the states broad authority to set Medicaid reimbursement rates. It is possible, of course, that in the future Congress would change the law to grant such rights or more clearly set reimbursement rates.
4. False Claims Act cases
Unfortunately, False Claims Act cases occur in health care. False claims transpire when someone (or an organization) presents to the government a claim for payment that is not legitimate or is for inadequate or low quality services. False claims include everything from fraudulent billing for services never performed to a pharmaceutical company’s promotion of a drug for off-label use.
The federal False Claims Act incentivizes private whistleblowers (often disgruntled employees) to initiate false claims lawsuits. (The government may then choose to take over the false claims or allow the private whistleblower to pursue them.)
At stake. Because Medicare and Medicaid are federally financed programs, it is common for providers who participate in those programs to be subject to false claims.5 This term the Court heard an important False Claims Act case involving the statute of limitations and multiple claims based on the same activity. The AMA joined an amicus brief urging the Court to prohibit both kinds of expansion.
Final ruling. The Court agreed that the statute of limitations for these claims should not be extended, but it did determine that the “first-to-file” limitation in the statute “keeps new claims out of court only while related claims are still alive, not in perpetuity.”5 The result of this second holding is that the “firstto-file” rule does not preclude another false claims suit that is duplicative to be filed as soon as the prior suit is no longer pending.
It is not clear that the practical effect of the decision will be great, but it may in some cases open up clinicians to multiple, serial lawsuits over the same claims.
5. Pregnancy and employment discrimination
The federal Pregnancy Discrimination Act (PDA) prohibits discrimination “because of or on the basis of pregnancy, childbirth, or related medical conditions.” It also requires that employers treat “women affected by pregnancy . . . the same for all employment-related purposes . . . as other persons not so affected but similar in their ability or inability to work.”
At stake. United Parcel Service (UPS) declined to give a pregnant employee a “light duty” accommodation during pregnancy so that she could avoid having to lift heavy packages. But it had given other employees just that kind of accommodation when they were injured or lost certification to drive a delivery truck. The suit claimed violation of the PDA.6
Final ruling. The Court held that UPS violated the PDA by giving some employees this accommodation but refusing it to pregnant workers who requested it.6 Once an organization voluntarily grants a particular accommodation to, for example, a temporarily injured worker, it may be required to provide similar accommodation to pregnant workers. The case probably will increase employment protection for pregnant women.
6. Children “testifying” in abuse cases
All states require physicians and teachers, among others, to report suspected child abuse. An ongoing question has been whether those reporters may be called to testify about what the child said at the time of abuse discovery/suspicion. This case involved a teacher, but it could as easily have been a physician.7
Key points of the case. Teachers found suspicious injuries on 3-year-old L.P. The child gave conflicting statements about what happened, but claimed that Clark had hurt him. At Clark’s criminal trial, L.P. did not testify, but the state wanted to introduce L.P.’s statements to the teachers as evidence of Clark’s guilt.
At stake. The question was whether or not this introduction would violate the right of Clark to “confront his accuser” as guaranteed by the Constitution.
Final ruling. All 9 justices said it was proper to allow L.P.’s statements to be introduced at trial.7 Essentially this was permitted because the primary purpose of L.P.’s statements was not to gather testimony to be presented at trial.
The final opinion creates incentives for some prosecutors to make abuse victims unavailable in order to allow their testimony by hearsay. Aside from the legal technicalities, the likelihood of wrongful convictions under such a system must be considered for the long run.
Other major 2015 Supreme Court decisions
- The Constitution requires states to recognize same-sex marriages and also accept such marriages performed in other states.1 (The American Medical Association joined an amicus brief in this case.)
- The Court turned down the appeal of several inmates who had received lethal injection death sentences. They challenged the mix of drugs that Oklahoma planned to use to execute them.2
- In 2 cases justices went out of the way to raise questions about the constitutionality of the death penalty. This may suggest that the Court will take up this issue in the near future.2,3
- Federal housing discrimination laws were expanded to cases in which there is “disparate impact” discrimination.4
- The Court narrowed prosecution for “threatening” statements in social network/Internet communications by holding that negligence in the communication is not sufficient for conviction under federal law.5
- The Court held that the Environmental Protection Agency violated the Clean Air Act with its power plant emission regulations because it failed to do a proper cost-benefit analysis.6
References
1. Obergefell et al v Hodges, Director, Ohio Department of Health et al, No. 14–556 (2015). http://www.supremecourt.gov/opinions/14pdf/14-556_3204.pdf. Accessed December 14, 2015.
2. Glossip et al v Gross et al, No. 14–7955 (2015). http://www.supremecourt.gov/opinions/14pdf/14-7955_aplc.pdf. Accessed December 14, 2015.
3. Davis, Acting Worden v Ayala, No. 13–1428 (2015). http://www.supremecourt.gov/opinions/14pdf/13-1428_1a7d.pdf. Accessed December 14, 2015.
4. Texas Department of Housing and Community Affairs et al v Inclusive Communities Project, Inc et al, No. 13–1371 (2015). http://www.supremecourt.gov/opinions/14pdf/13-1371_8m58.pdf. Accessed December 14, 2015.
5. Elonis v United States, No. 13–983 (2015). http://www.supremecourt.gov/opinions/14pdf/13-983_7148.pdf. Accessed December 14, 2015.
6. Michigan et al v Environmental Protection Agency et al, No. 14–46 (2015). http://www.supremecourt.gov/opinions/14pdf/14-46_bqmc.pdf. Accessed December 14, 2015.
What’s coming in 2016
This term of the Supreme Court is a reminder of how important the decisions of the Court are to the work of physicians and ObGyns in particular. This trend is continuing—the Court already has, for the next term, accepted cases regarding abortion, contraception, and health care delivery. It will, therefore, be worthwhile to follow the developments at the nation’s highest court.
Share your thoughts on this article! Send your Letter to the Editor to rbarbieri@frontlinemedcom.com. Please include your name and the city and state in which you practice.
You might ask, why do I need to know what the Supreme Court does, it will not affect me! Well, we all know that is not exactly true. We chose these 6 cases because of their importance to ObGyns. In a number of them, the American Medical Association (AMA) or specific specialty board filed amicus curiae briefs, which suggested that the profession felt these were especially critical cases. (An amicus brief is a “friend of the Court” brief filed not by one of the affected parties, but by an organization or person with an interest or special expertise in the case.) You can find additional analysis of the 2015 term of the Supreme Court at the website of the National Register (http://www.nationalregister.org/pub/the-national-registerreport-pub/the-register-report-fall-2015/the-aca-survives-and-same-sex-marriage-thrives-the-2014-2015-supreme-court/).
1. Affordable Care Act upheld
King v Burwell was likely the Court’s most important case for physicians and their patients during the 2015 term.
At stake. The question was whether or not people who use the federal Affordable Care Act (ACA) Exchange could receive the same subsidy as those who use the state established Exchanges.1
Final ruling. The Court said “yes,” they could receive the same subsidy, ruling in favor of King.
Key points of the case
The ACA provides for state Exchanges (an electronic marketplace in which people can compare and purchase health insurance policies), but most states did not establish Exchanges. As a result, in many states the federal Exchange became the default. Because the ACA subsidies (which help many people afford mandated insurance coverage) are processed through exchanges, the question of whether those who used the federal Exchange received the subsidy was enormously important. Subsidies for millions of individuals depended on it.
The language of the ACA provides that the insurance subsidy is available only if the person(s) enroll in “an Exchange established by the State” (emphasis added).1 This case was about what “Exchange established by the State” means. A 6-justice majority held that the best interpretation of the statute was that it permitted subsidies through the federal Exchange. This was a difficult decision because, as the majority noted, the ACA is sloppily written. Nonetheless, the Court had to do its best to read the language in the context of the whole statute. Six justices held that the statute meant that the subsidies would cover people who signed up through federal as well as state Exchanges.
The dissenting justices essentially took the position that the language of the statute is clear. Among other things, the dissent said, it means that the words “established by the state” have no meaning at all in the statute, and it is unclear why Congress did not say “Exchange” instead of “Exchange established by the state.”
The results of the case were that the subsidies granted through the federal Exchange will continue. It will not expand the subsidies. Had the decision gone the other way, there would have been a real challenge to the future of the ACA. For that reason a number of medical and health care organizations filed amicus briefs with the Court in this case.
2. State licensing boards and antitrust
At stake. Antitrust laws prohibit combinations and conspiracies in restraint of trade. Competitors cannot come together to seek to set prices, divide the market, or prevent new competitors from entering the market. Since the 1940s, however, the Supreme Court has recognized a “state action” exception to the antitrust laws. The question before the Court this term was whether or not a state licensing board is included in the state-action immunity.2
The AMA and others filed an amicus brief in this case, noting threat to the public health if the Court disrupted state medical boards’ regulation of professional licensing and unauthorized practice.
Final ruling. The Court rejected this argument. It held that, where a state board is “controlled by active market participants” (as most state professional boards are), antitrust immunity is not automatic. For the immunity to protect boards, 2 conditions must exist, the Court held:
1. The state must have articulated a clear policy to allow the regulation that is an anticompetitive conduct (eg, licensing)
2. The state must have provided active supervision of the anticompetitive conduct. This requires that the state appoint someone or some group to approve policies of the board.
The first of these requirements often would be met by the statute setting up the board. The Court focused some attention on the second requirement. It concluded that “the adequacy of supervision otherwise will depend on all the circumstances of a case.”2
Key points of the case
In most states, this decision will require some kind of restructuring so that the professional boards are not the final decision makers but, in effect, only make recommendations to a “supervisor.” The Court gave short shrift to the AMA’s concerns that the decision might make it more difficult to attract really good professionals to the boards. The possibility of personal liability probably can be dealt with, but it deserves attention. The problems with litigation and antitrust claims, and reviews of decisions of a board (potentially by nonprofessionals) hardly can be a plus in attracting the right professionals to the boards.
This case will give rise to considerable litigation for many years. Medical boards should endeavor to get ahead of the issue by immediately studying ways in which the concerns of the Federal Trade Commissioncan be accommodated without significantly reducing the public protection that is part of well-administered professional licensing.
3. State reimbursement for Medicaid services
Medicaid is a federal-state program, and federal law requires, in part, that states must “assure that payments are … sufficient to enlist enough providers so that care and services are available under the plan at least to the extent that such care and services are available to the general population in the geographic area.”3 Providers claimed that the state had failed to establish such a payment system. A number of medical groups, including the AMA, filed amicus briefs in support of the providers.
At stake. State funding for various Medicaid services has been a problem for many health care professionals for some time. But the Medicaid law does not clearly give providers the right to file lawsuits claiming inadequate reimbursement,4 so the question in this case was whether or not there was an implied right to do so.
Final ruling. The Court, in a 5-4 decision, held that Medicaid providers do not have the authority to sue states in federal courts torequire that the states provide higher Medicaid rates for services.4 As a practical matter, this decision leaves with the states broad authority to set Medicaid reimbursement rates. It is possible, of course, that in the future Congress would change the law to grant such rights or more clearly set reimbursement rates.
4. False Claims Act cases
Unfortunately, False Claims Act cases occur in health care. False claims transpire when someone (or an organization) presents to the government a claim for payment that is not legitimate or is for inadequate or low quality services. False claims include everything from fraudulent billing for services never performed to a pharmaceutical company’s promotion of a drug for off-label use.
The federal False Claims Act incentivizes private whistleblowers (often disgruntled employees) to initiate false claims lawsuits. (The government may then choose to take over the false claims or allow the private whistleblower to pursue them.)
At stake. Because Medicare and Medicaid are federally financed programs, it is common for providers who participate in those programs to be subject to false claims.5 This term the Court heard an important False Claims Act case involving the statute of limitations and multiple claims based on the same activity. The AMA joined an amicus brief urging the Court to prohibit both kinds of expansion.
Final ruling. The Court agreed that the statute of limitations for these claims should not be extended, but it did determine that the “first-to-file” limitation in the statute “keeps new claims out of court only while related claims are still alive, not in perpetuity.”5 The result of this second holding is that the “firstto-file” rule does not preclude another false claims suit that is duplicative to be filed as soon as the prior suit is no longer pending.
It is not clear that the practical effect of the decision will be great, but it may in some cases open up clinicians to multiple, serial lawsuits over the same claims.
5. Pregnancy and employment discrimination
The federal Pregnancy Discrimination Act (PDA) prohibits discrimination “because of or on the basis of pregnancy, childbirth, or related medical conditions.” It also requires that employers treat “women affected by pregnancy . . . the same for all employment-related purposes . . . as other persons not so affected but similar in their ability or inability to work.”
At stake. United Parcel Service (UPS) declined to give a pregnant employee a “light duty” accommodation during pregnancy so that she could avoid having to lift heavy packages. But it had given other employees just that kind of accommodation when they were injured or lost certification to drive a delivery truck. The suit claimed violation of the PDA.6
Final ruling. The Court held that UPS violated the PDA by giving some employees this accommodation but refusing it to pregnant workers who requested it.6 Once an organization voluntarily grants a particular accommodation to, for example, a temporarily injured worker, it may be required to provide similar accommodation to pregnant workers. The case probably will increase employment protection for pregnant women.
6. Children “testifying” in abuse cases
All states require physicians and teachers, among others, to report suspected child abuse. An ongoing question has been whether those reporters may be called to testify about what the child said at the time of abuse discovery/suspicion. This case involved a teacher, but it could as easily have been a physician.7
Key points of the case. Teachers found suspicious injuries on 3-year-old L.P. The child gave conflicting statements about what happened, but claimed that Clark had hurt him. At Clark’s criminal trial, L.P. did not testify, but the state wanted to introduce L.P.’s statements to the teachers as evidence of Clark’s guilt.
At stake. The question was whether or not this introduction would violate the right of Clark to “confront his accuser” as guaranteed by the Constitution.
Final ruling. All 9 justices said it was proper to allow L.P.’s statements to be introduced at trial.7 Essentially this was permitted because the primary purpose of L.P.’s statements was not to gather testimony to be presented at trial.
The final opinion creates incentives for some prosecutors to make abuse victims unavailable in order to allow their testimony by hearsay. Aside from the legal technicalities, the likelihood of wrongful convictions under such a system must be considered for the long run.
Other major 2015 Supreme Court decisions
- The Constitution requires states to recognize same-sex marriages and also accept such marriages performed in other states.1 (The American Medical Association joined an amicus brief in this case.)
- The Court turned down the appeal of several inmates who had received lethal injection death sentences. They challenged the mix of drugs that Oklahoma planned to use to execute them.2
- In 2 cases justices went out of the way to raise questions about the constitutionality of the death penalty. This may suggest that the Court will take up this issue in the near future.2,3
- Federal housing discrimination laws were expanded to cases in which there is “disparate impact” discrimination.4
- The Court narrowed prosecution for “threatening” statements in social network/Internet communications by holding that negligence in the communication is not sufficient for conviction under federal law.5
- The Court held that the Environmental Protection Agency violated the Clean Air Act with its power plant emission regulations because it failed to do a proper cost-benefit analysis.6
References
1. Obergefell et al v Hodges, Director, Ohio Department of Health et al, No. 14–556 (2015). http://www.supremecourt.gov/opinions/14pdf/14-556_3204.pdf. Accessed December 14, 2015.
2. Glossip et al v Gross et al, No. 14–7955 (2015). http://www.supremecourt.gov/opinions/14pdf/14-7955_aplc.pdf. Accessed December 14, 2015.
3. Davis, Acting Worden v Ayala, No. 13–1428 (2015). http://www.supremecourt.gov/opinions/14pdf/13-1428_1a7d.pdf. Accessed December 14, 2015.
4. Texas Department of Housing and Community Affairs et al v Inclusive Communities Project, Inc et al, No. 13–1371 (2015). http://www.supremecourt.gov/opinions/14pdf/13-1371_8m58.pdf. Accessed December 14, 2015.
5. Elonis v United States, No. 13–983 (2015). http://www.supremecourt.gov/opinions/14pdf/13-983_7148.pdf. Accessed December 14, 2015.
6. Michigan et al v Environmental Protection Agency et al, No. 14–46 (2015). http://www.supremecourt.gov/opinions/14pdf/14-46_bqmc.pdf. Accessed December 14, 2015.
What’s coming in 2016
This term of the Supreme Court is a reminder of how important the decisions of the Court are to the work of physicians and ObGyns in particular. This trend is continuing—the Court already has, for the next term, accepted cases regarding abortion, contraception, and health care delivery. It will, therefore, be worthwhile to follow the developments at the nation’s highest court.
Share your thoughts on this article! Send your Letter to the Editor to rbarbieri@frontlinemedcom.com. Please include your name and the city and state in which you practice.
1. King et al v Burwell, Secretary of Health and Human Services et al, No. 14–114 (2015). http://www.supremecourt.gov/opinions/14pdf/14-114_qol1.pdf. Accessed December 14, 2015.
2. North Carolina State Board of Dental Examiners v Federal Trade Commission, No. 13–534 (2015). http://www.supremecourt.gov/opinions/14pd/13-534_19m2.pdf. Accessed December 14, 2015.
3. Federal Policy Guidance. Medicaid.gov website. http://medicaid.gov/federal-policy-guidance/federal-policyguidance.html. Accessed December 14, 2015.
4. Armstrong et al v Exceptional Child Center, Inc et al, No. 14–15 (2015). http://www.supremecourt.gov/opinions/14pdf/14-15_d1oe.pdf. Accessed December 14, 2015.
5. Kellogg Brown & Root Services, Inc et al v United States ex rel. Carter, No 12–1497 (2015). http://www.supremecourt.gov/opinions/14pdf/12-1497_2d8f.pdf. Accessed December 14, 2015.
6. Young v United Parcel Service, Inc., No. 12–1226 (2015). http://www.supremecourt.gov/opinions/14pdf/12-1226_k5fl.pdf. Accessed December 14, 2015.
7. Ohio v Clark, No. 13–1352 (2015). http://www.supremecourt.gov/opinion/14pdf/13-1352_ed9l.pdf. Accessed December 14, 2015.
1. King et al v Burwell, Secretary of Health and Human Services et al, No. 14–114 (2015). http://www.supremecourt.gov/opinions/14pdf/14-114_qol1.pdf. Accessed December 14, 2015.
2. North Carolina State Board of Dental Examiners v Federal Trade Commission, No. 13–534 (2015). http://www.supremecourt.gov/opinions/14pd/13-534_19m2.pdf. Accessed December 14, 2015.
3. Federal Policy Guidance. Medicaid.gov website. http://medicaid.gov/federal-policy-guidance/federal-policyguidance.html. Accessed December 14, 2015.
4. Armstrong et al v Exceptional Child Center, Inc et al, No. 14–15 (2015). http://www.supremecourt.gov/opinions/14pdf/14-15_d1oe.pdf. Accessed December 14, 2015.
5. Kellogg Brown & Root Services, Inc et al v United States ex rel. Carter, No 12–1497 (2015). http://www.supremecourt.gov/opinions/14pdf/12-1497_2d8f.pdf. Accessed December 14, 2015.
6. Young v United Parcel Service, Inc., No. 12–1226 (2015). http://www.supremecourt.gov/opinions/14pdf/12-1226_k5fl.pdf. Accessed December 14, 2015.
7. Ohio v Clark, No. 13–1352 (2015). http://www.supremecourt.gov/opinion/14pdf/13-1352_ed9l.pdf. Accessed December 14, 2015.
Is the smartphone recording while the patient is under anesthesia?
CASE: Physician defames sedated patient
Our case takes us to the Commonwealth of Virginia. A male patient preparing to undergo a colonoscopy was concerned that, because of grogginess brought on by anesthesia, he might misunderstand postprocedure instructions or advice. He, therefore, turned his cell phone’s record function “on” and put it with his clothes. His clothes were put in a plastic bag, which ended up under the table with him in the operating room.
Following the procedure, as his wife drove him home, the patient replayed the instructions on the cell phone and realized that it had recorded the entire procedure. It quickly became apparent that the medical personnel had engaged in a series of inappropriate and insulting comments at the patient’s expense.
The anesthesiologist, talking to the now-unconscious patient, said, “after five minutes of talking to you in pre-op, I wanted to punch you in the face.” The patient had reported he was taking medication for a mild penile rash. The anesthesiologist warned an assistant not to touch it or “you might get syphilis on your arm or something,” but then noted, “it’s probably tuberculosis of the penis, so you’ll be all right.” There was further mocking of the patient, including a question of whether he was homosexual.
The anesthesiologist and gastroenterologist wanted to avoid talking to the patient after the procedure, and the gastroenterologist instructed an assistant to lie to the patient and convince the patient that the gastroenterologist had already spoken to him following the colonoscopy but, “you just don’t remember it.” In addition, the anesthesiologist announced that she was going to mark “hemorrhoids” on the patient’s chart, which she knew was a false diagnosis.
The patient, who is identified only by initials, is an attorney.1 Of course, the smartphone was “good documentation” of what came out of what the health care team said.
The lawsuit
The patient (now plaintiff) claimed that he was verbally brutalized and suffered anxiety, embarrassment, and loss of sleep for several months.
On the first day of trial, the gastroenterologist was dismissed from the case. The trial went on against the anesthesiologist and the anesthesia practice.
What’s the verdict?
The patient was awarded $500,000, as follows:
- $100,000 for defamation, ($50,000 each for the syphilis and tuberculosis comments),
- $200,000 for medical malpractice
- $200,000 in punitive damages (including $50,000 the jury found that the anesthesia practice should pay).
Caveat. The above facts about this case come from the plaintiff’s complaint1 and various professional commentaries and news sources.2–5 Such sources are not always reliable, so they may not describe accurately all of the relevant events and statements.
Neither of the authors of this column attended the trial or heard the testimony presented. For the purposes of discussing the issues below, however, we treat as true the facts stated above. In addition, some of the legal claims in this case are uncertain. It is entirely possible that an appeal will be made and accepted, and some or all of the damages could be reduced by the trial court or an appellate court. The jury award, therefore, is not necessarily the last word.
Medicolegal takeaways from this case
This case raises a number of professional, ethical, and legal issues. Most fundamentally, the health care team is always expected to prioritize the patient’s best interest. Respect for the patient is an essential element of that.
Behaviors such as those reported about these physicians are “absolutely not to engage in any time,” stated President of the American Society of Anesthesiologists John Absentein, MD.6 A former president of the Academy of Anesthesiology, Kathryn McGoldrick, MD, added some common sense advice that such discussions are “not only offensive but frankly stupid.” As she notes, “we can never be certain that our patients are asleep and wouldn’t have recall.”7
The actions of the physicians also may violate ethical obligations. The very first principle of medical ethics is that the physician shall provide care “with compassion and respect for human dignity and rights.”8
The legal claims included defamation, infliction of emotional distress, privacy (related to medical records), and malpractice. We will take a very brief look at each of those causes of action and then say a word about punitive damages (which the jury awarded in this case).
It is important to remember that state law, rather than federal, is providing the legal principles on which these claims were decided. Federal law might provide some relevant principles in such cases (for example, the First Amendment freedom of speech limits the state defamation rules), but that is the exception. State law is the rule.
Patient−physician recordings and the law
State laws differ regarding when it is legal to record in-person conversations. When everyone in the conversations knows about the recording, it is permissible and can be used in a court of law. In most states it is legal to record when only one party to the conversation has agreed to it, even though others in the conversation are not aware of it (which was the situation in the case discussed here).
In theory, physicians (by contract with patients) might try to limit patients’ rights to record medical services. But that practice would be difficult to implement or enforce in many circumstances. The reality is that audio and video recording devices are so ubiquitous that it is not sensible to avoid all recording of patient contact.
Physicians also might consider the potential such recordings have in some circumstances to improve communication with patients. Permitting the patient to record the patient−physician exchange, for instance, allows the patient the ability to review the advice after having left the office. This could be beneficial from a patient care perspective.
Defamation—award of damages
At its core, defamation is publishing (that is, telling someone other than the plaintiff) something untrue that may be harmful to another person. Generally the harm is reputational and the plaintiff may be affected by loss of business, mental suffering, or loss of esteem in the community.9
Defamation claims are not typical in health care cases. However, these claims are not rare: instances of health care professionals defaming other health care professionals, patients giving negative “reviews,” or health care professionals releasing false information to employers certainly do exist.
In this case, in addition to saying that the patient had syphilis and tuberculosis (both untrue), the physicians said he was a “wimp.” One interesting concept of defamation law that has developed over the centuries is “negligence per se.” This means a falsehood has been published about someone and the falsehood is likely to cause serious reputational harm. Claims that someone has a contagious disease traditionally have been considered negligence per se. Syphilis and tuberculosis fall in that category. On the other hand, saying someone needs to “man up” is usually a matter of opinion, so defamation for such comments is unlikely without special circumstances.
From the anesthesiologist’s perspective, the question is whether anyone who heard the publication really believed that the patient had either of the diseases. A joke that nobody believes to be based on fact generally is not defamatory because it has not harmed the plaintiff.10 It is apparent that the jury felt the patient had been defamed, however, given the $100,000 award for defamation.
In the United States there is special sensitivity to defamation awards because they may implicate the First Amendment’s protection of free speech. That being the case, this award may be particularly open to review by the judge and appellate courts.
Emotional distress—no award of damages
There are 2 kinds of “emotional distress” claimed in this case:
- intentional infliction
- negligent infliction.
Intentional infliction usually requires outrageous conduct by the defendant who acts intentionally or recklessly to inflict severe mental pain on the plaintiff.11 In this case, the element of “intentional” or “reckless” is interesting. While the conduct was outrageous, it is doubtful that there was any way the anesthesiologist could have imagined that these outrageous statements would have been transmitted to the patient/plaintiff.
As for negligent infliction of emotional distress, most states have been wary of opening a Pandora’s Box of litigation. Therefore, they generally require significant physical manifestations of great stress to allow recovery.12 It appears that the jury did not find the elements of either intentional or negligent infliction of emotional distress in this case.
As a side-note, this kind of emotional distress is viewed by the law as different from emotional distress that is incidental to a physical injury (pain and suffering). All states recognize that form of emotional distress.
Privacy—no award of damages
The privacy of medical records has, of course, become a major concern in the last few years. Both federal and state law provides significant penalties for the unauthorized release of medical information. However, in this case, it does not appear that medical information was improp- erly revealed.13
The patient’s complaint suggested that the anesthesiologist’s discussion during the colonoscopy of the medication for the penile rash was unnecessary for health care purposes.1 Therefore, it claims, the discussion violated the state health records privacy law. At the same time there was no indication in the public reports that this caused any harm to the patient.
Medical malpractice—award of damages
Malpractice usually involves professional practice that is unacceptable to the profession itself. It most commonly is negligence, or carelessness, that causes injury to the patient. The gross disregard for professional medical standards here was certainly negligence.14 The plaintiff claimed that discussing the medication for the penile rash and falsification of the medical records constituted malpractice.1
Presumably the jury award for medical malpractice means the jury found that the misconduct of the medical staff caused the emotional harm that the plaintiff experienced (described as embarrassment, loss of sleep, mental anguish, and anxiety), and that those injuries warranted a $200,000 award.
Punitive damage—award of damages
The jury also awarded $200,000 in “punitive” or “exemplary” damages. These are unusual damages, given not so much to compensate the victim but rather as a deterrent for the future. Generally the defendant’s conduct must have been egregious and completely unacceptable.15 Those elements were apparent to the jury from the facts of this case.
What about loss of practice privileges?
It is not unlikely that one or more of the medical professionals might, beyond civil liability, be subject to licensure discipline by the Virginia board. In addition, there are other secondary consequences of this lawsuit. The employment of those involved may be interrupted. (The anesthesiologist is said to have moved to another state, for example.) Hospital privileges also may be affected, as may insurance rates. The results of this award likely will have to be reported to the National Practitioner Data Bank.
As physicians, what’s our takeaway?
Conduct unbecoming a physician remains front and center with a recent essay published in the internal medicine literature.16 The anonymous author attests to witnessing a male gynecologist making sexual comments regarding the patient at the time of vaginal surgery preparation and an obstetrician singing and dancing to a Mexican song while treating his Hispanic patient for postpartum bleeding.
The unusual case of the anesthesiologist that we address was made even more unusual by the fact that it was recorded. Recordings, however, are likely to become ever more common. The advice of everyone’s grandmother is well taken: “Always act as though what you do will be published on the front page of the newspaper.” The ubiquitous presence of video and audio cameras and untold other devices means that someone may well be watching.
Aside from the risk of getting caught, respect for patients and clients is the very foundation of respect and professional care. It is distressing that the anesthesiologist was so disrespectful of a patient. It is equally disappointing that nobody put a stop to it.
Share your thoughts! Send your Letter to the Editor to rbarbieri@frontlinemedcom.com. Please include your name and the city and state in which you practice.
- D.B. v Safe Sedation, Complaint, Civil Action 2014-05265, Circuit Court of Fairfax County.
- Abbott R. Unconscious patient says doctors mocked him. Courthouse News Service. http://www.courthousenews.com/2014/04/22/67225.htm. Updated April 22, 2014. Accessed August 19, 2015.
- Jackman T. Anesthesiologist trashes sedated patient—and it ends up costing her. Washington Post. June 23, 2015. http://www.washingtonpost.com/local/anesthesiologist-trashes-sedated-patient-jury-orders-her-to-pay-500000/2015/06/23/cae05c00-18f3-11e5-ab92-c75ae6ab94b5_story.html. Accessed August 19, 2015.
- Waibel E. Patient says Bethesda practitioners mocked him during the colonoscopy. GazetteNet. May 13, 2014. http://www.gazette.net/article/20140513/NEWS/140519703/1070/patient-says-bethesda-practitioners-mocked-him-during-colonoscopy&template=gazette. Accessed July 15, 2015.
- Vieth P. Fairfax County Circuit Court: Doctors allegedly mocked their unconscious patient. Virginia Lawyers Weekly. May 1, 2014. http://valawyersweekly.com/2014/05/01/doctors-allegedly-mocked-their-unconscious-patient. Accessed August 19, 2015.
- Welch A. Patient sues anesthesiologist who mocked him while sedated. CBS News. http://www.cbsnews.com/news/patient-sues-anesthesiloigst-who mocked-him-while sedated. Accessed July 15, 2015.
- Leins C. Anesthesiologist derides subdued patient, loses lawsuit. US News. June 24, 2015. http://www.usnews.com/news/articles/2015/06/24/anesthesiologist-derides-subdued-patient-loses-lawsuit. Accessed August 19, 2015.
- Principles of medical ethics. American Medical Association Web site. http://www.ama-assn.org/ama/pub/physician-resources/medical-ethics/code-medical-ethics/principles-medical-ethics.page. Accessed August 19, 2015.
- Instruction nos. 24 and 25. Virginia Defamation Lawyer Web site. http://www.virginiadefamationlawyer.com/Instr%2024%20and%2025.pdf. Accessed August 19, 2015.
- Berlik LE. The Virginia model jury instructions for defamation lead to bad verdicts. The Virginia Defamation Law Blog. June 27, 2015. http://www.virginiadefamationlawyer.com/2015/06/the-virginia-model-jury-instructions-for-defamation-lead-to-bad-verdicts.html#more. Accessed August 19, 2015.
- Russo v White, 241 Va 23 (1991).
- Hughes v Moore, 214 Va 27 (1973).
- Law on patient health records/privacy. Virginia Department of Health Professions Web site.
- http://webcache.googleusercontent.com/search?q=cache:asc1xQmBefoJ:https://www.dhp.virginia.gov/dhp_laws/Law_Patient%2520Health%2520Records.doc+&cd=2&hl=en&ct=clnk&gl=us. Accessed August 19, 2015.
- Virginia Medical Malpractice Act. Va Code Ann. 8.01-230, 8.01-243(A).
- Ford CR. Pleading and understanding punitive-damages claims in Virginia. Litigation News. 2008;8(10):1-11.
CASE: Physician defames sedated patient
Our case takes us to the Commonwealth of Virginia. A male patient preparing to undergo a colonoscopy was concerned that, because of grogginess brought on by anesthesia, he might misunderstand postprocedure instructions or advice. He, therefore, turned his cell phone’s record function “on” and put it with his clothes. His clothes were put in a plastic bag, which ended up under the table with him in the operating room.
Following the procedure, as his wife drove him home, the patient replayed the instructions on the cell phone and realized that it had recorded the entire procedure. It quickly became apparent that the medical personnel had engaged in a series of inappropriate and insulting comments at the patient’s expense.
The anesthesiologist, talking to the now-unconscious patient, said, “after five minutes of talking to you in pre-op, I wanted to punch you in the face.” The patient had reported he was taking medication for a mild penile rash. The anesthesiologist warned an assistant not to touch it or “you might get syphilis on your arm or something,” but then noted, “it’s probably tuberculosis of the penis, so you’ll be all right.” There was further mocking of the patient, including a question of whether he was homosexual.
The anesthesiologist and gastroenterologist wanted to avoid talking to the patient after the procedure, and the gastroenterologist instructed an assistant to lie to the patient and convince the patient that the gastroenterologist had already spoken to him following the colonoscopy but, “you just don’t remember it.” In addition, the anesthesiologist announced that she was going to mark “hemorrhoids” on the patient’s chart, which she knew was a false diagnosis.
The patient, who is identified only by initials, is an attorney.1 Of course, the smartphone was “good documentation” of what came out of what the health care team said.
The lawsuit
The patient (now plaintiff) claimed that he was verbally brutalized and suffered anxiety, embarrassment, and loss of sleep for several months.
On the first day of trial, the gastroenterologist was dismissed from the case. The trial went on against the anesthesiologist and the anesthesia practice.
What’s the verdict?
The patient was awarded $500,000, as follows:
- $100,000 for defamation, ($50,000 each for the syphilis and tuberculosis comments),
- $200,000 for medical malpractice
- $200,000 in punitive damages (including $50,000 the jury found that the anesthesia practice should pay).
Caveat. The above facts about this case come from the plaintiff’s complaint1 and various professional commentaries and news sources.2–5 Such sources are not always reliable, so they may not describe accurately all of the relevant events and statements.
Neither of the authors of this column attended the trial or heard the testimony presented. For the purposes of discussing the issues below, however, we treat as true the facts stated above. In addition, some of the legal claims in this case are uncertain. It is entirely possible that an appeal will be made and accepted, and some or all of the damages could be reduced by the trial court or an appellate court. The jury award, therefore, is not necessarily the last word.
Medicolegal takeaways from this case
This case raises a number of professional, ethical, and legal issues. Most fundamentally, the health care team is always expected to prioritize the patient’s best interest. Respect for the patient is an essential element of that.
Behaviors such as those reported about these physicians are “absolutely not to engage in any time,” stated President of the American Society of Anesthesiologists John Absentein, MD.6 A former president of the Academy of Anesthesiology, Kathryn McGoldrick, MD, added some common sense advice that such discussions are “not only offensive but frankly stupid.” As she notes, “we can never be certain that our patients are asleep and wouldn’t have recall.”7
The actions of the physicians also may violate ethical obligations. The very first principle of medical ethics is that the physician shall provide care “with compassion and respect for human dignity and rights.”8
The legal claims included defamation, infliction of emotional distress, privacy (related to medical records), and malpractice. We will take a very brief look at each of those causes of action and then say a word about punitive damages (which the jury awarded in this case).
It is important to remember that state law, rather than federal, is providing the legal principles on which these claims were decided. Federal law might provide some relevant principles in such cases (for example, the First Amendment freedom of speech limits the state defamation rules), but that is the exception. State law is the rule.
Patient−physician recordings and the law
State laws differ regarding when it is legal to record in-person conversations. When everyone in the conversations knows about the recording, it is permissible and can be used in a court of law. In most states it is legal to record when only one party to the conversation has agreed to it, even though others in the conversation are not aware of it (which was the situation in the case discussed here).
In theory, physicians (by contract with patients) might try to limit patients’ rights to record medical services. But that practice would be difficult to implement or enforce in many circumstances. The reality is that audio and video recording devices are so ubiquitous that it is not sensible to avoid all recording of patient contact.
Physicians also might consider the potential such recordings have in some circumstances to improve communication with patients. Permitting the patient to record the patient−physician exchange, for instance, allows the patient the ability to review the advice after having left the office. This could be beneficial from a patient care perspective.
Defamation—award of damages
At its core, defamation is publishing (that is, telling someone other than the plaintiff) something untrue that may be harmful to another person. Generally the harm is reputational and the plaintiff may be affected by loss of business, mental suffering, or loss of esteem in the community.9
Defamation claims are not typical in health care cases. However, these claims are not rare: instances of health care professionals defaming other health care professionals, patients giving negative “reviews,” or health care professionals releasing false information to employers certainly do exist.
In this case, in addition to saying that the patient had syphilis and tuberculosis (both untrue), the physicians said he was a “wimp.” One interesting concept of defamation law that has developed over the centuries is “negligence per se.” This means a falsehood has been published about someone and the falsehood is likely to cause serious reputational harm. Claims that someone has a contagious disease traditionally have been considered negligence per se. Syphilis and tuberculosis fall in that category. On the other hand, saying someone needs to “man up” is usually a matter of opinion, so defamation for such comments is unlikely without special circumstances.
From the anesthesiologist’s perspective, the question is whether anyone who heard the publication really believed that the patient had either of the diseases. A joke that nobody believes to be based on fact generally is not defamatory because it has not harmed the plaintiff.10 It is apparent that the jury felt the patient had been defamed, however, given the $100,000 award for defamation.
In the United States there is special sensitivity to defamation awards because they may implicate the First Amendment’s protection of free speech. That being the case, this award may be particularly open to review by the judge and appellate courts.
Emotional distress—no award of damages
There are 2 kinds of “emotional distress” claimed in this case:
- intentional infliction
- negligent infliction.
Intentional infliction usually requires outrageous conduct by the defendant who acts intentionally or recklessly to inflict severe mental pain on the plaintiff.11 In this case, the element of “intentional” or “reckless” is interesting. While the conduct was outrageous, it is doubtful that there was any way the anesthesiologist could have imagined that these outrageous statements would have been transmitted to the patient/plaintiff.
As for negligent infliction of emotional distress, most states have been wary of opening a Pandora’s Box of litigation. Therefore, they generally require significant physical manifestations of great stress to allow recovery.12 It appears that the jury did not find the elements of either intentional or negligent infliction of emotional distress in this case.
As a side-note, this kind of emotional distress is viewed by the law as different from emotional distress that is incidental to a physical injury (pain and suffering). All states recognize that form of emotional distress.
Privacy—no award of damages
The privacy of medical records has, of course, become a major concern in the last few years. Both federal and state law provides significant penalties for the unauthorized release of medical information. However, in this case, it does not appear that medical information was improp- erly revealed.13
The patient’s complaint suggested that the anesthesiologist’s discussion during the colonoscopy of the medication for the penile rash was unnecessary for health care purposes.1 Therefore, it claims, the discussion violated the state health records privacy law. At the same time there was no indication in the public reports that this caused any harm to the patient.
Medical malpractice—award of damages
Malpractice usually involves professional practice that is unacceptable to the profession itself. It most commonly is negligence, or carelessness, that causes injury to the patient. The gross disregard for professional medical standards here was certainly negligence.14 The plaintiff claimed that discussing the medication for the penile rash and falsification of the medical records constituted malpractice.1
Presumably the jury award for medical malpractice means the jury found that the misconduct of the medical staff caused the emotional harm that the plaintiff experienced (described as embarrassment, loss of sleep, mental anguish, and anxiety), and that those injuries warranted a $200,000 award.
Punitive damage—award of damages
The jury also awarded $200,000 in “punitive” or “exemplary” damages. These are unusual damages, given not so much to compensate the victim but rather as a deterrent for the future. Generally the defendant’s conduct must have been egregious and completely unacceptable.15 Those elements were apparent to the jury from the facts of this case.
What about loss of practice privileges?
It is not unlikely that one or more of the medical professionals might, beyond civil liability, be subject to licensure discipline by the Virginia board. In addition, there are other secondary consequences of this lawsuit. The employment of those involved may be interrupted. (The anesthesiologist is said to have moved to another state, for example.) Hospital privileges also may be affected, as may insurance rates. The results of this award likely will have to be reported to the National Practitioner Data Bank.
As physicians, what’s our takeaway?
Conduct unbecoming a physician remains front and center with a recent essay published in the internal medicine literature.16 The anonymous author attests to witnessing a male gynecologist making sexual comments regarding the patient at the time of vaginal surgery preparation and an obstetrician singing and dancing to a Mexican song while treating his Hispanic patient for postpartum bleeding.
The unusual case of the anesthesiologist that we address was made even more unusual by the fact that it was recorded. Recordings, however, are likely to become ever more common. The advice of everyone’s grandmother is well taken: “Always act as though what you do will be published on the front page of the newspaper.” The ubiquitous presence of video and audio cameras and untold other devices means that someone may well be watching.
Aside from the risk of getting caught, respect for patients and clients is the very foundation of respect and professional care. It is distressing that the anesthesiologist was so disrespectful of a patient. It is equally disappointing that nobody put a stop to it.
Share your thoughts! Send your Letter to the Editor to rbarbieri@frontlinemedcom.com. Please include your name and the city and state in which you practice.
CASE: Physician defames sedated patient
Our case takes us to the Commonwealth of Virginia. A male patient preparing to undergo a colonoscopy was concerned that, because of grogginess brought on by anesthesia, he might misunderstand postprocedure instructions or advice. He, therefore, turned his cell phone’s record function “on” and put it with his clothes. His clothes were put in a plastic bag, which ended up under the table with him in the operating room.
Following the procedure, as his wife drove him home, the patient replayed the instructions on the cell phone and realized that it had recorded the entire procedure. It quickly became apparent that the medical personnel had engaged in a series of inappropriate and insulting comments at the patient’s expense.
The anesthesiologist, talking to the now-unconscious patient, said, “after five minutes of talking to you in pre-op, I wanted to punch you in the face.” The patient had reported he was taking medication for a mild penile rash. The anesthesiologist warned an assistant not to touch it or “you might get syphilis on your arm or something,” but then noted, “it’s probably tuberculosis of the penis, so you’ll be all right.” There was further mocking of the patient, including a question of whether he was homosexual.
The anesthesiologist and gastroenterologist wanted to avoid talking to the patient after the procedure, and the gastroenterologist instructed an assistant to lie to the patient and convince the patient that the gastroenterologist had already spoken to him following the colonoscopy but, “you just don’t remember it.” In addition, the anesthesiologist announced that she was going to mark “hemorrhoids” on the patient’s chart, which she knew was a false diagnosis.
The patient, who is identified only by initials, is an attorney.1 Of course, the smartphone was “good documentation” of what came out of what the health care team said.
The lawsuit
The patient (now plaintiff) claimed that he was verbally brutalized and suffered anxiety, embarrassment, and loss of sleep for several months.
On the first day of trial, the gastroenterologist was dismissed from the case. The trial went on against the anesthesiologist and the anesthesia practice.
What’s the verdict?
The patient was awarded $500,000, as follows:
- $100,000 for defamation, ($50,000 each for the syphilis and tuberculosis comments),
- $200,000 for medical malpractice
- $200,000 in punitive damages (including $50,000 the jury found that the anesthesia practice should pay).
Caveat. The above facts about this case come from the plaintiff’s complaint1 and various professional commentaries and news sources.2–5 Such sources are not always reliable, so they may not describe accurately all of the relevant events and statements.
Neither of the authors of this column attended the trial or heard the testimony presented. For the purposes of discussing the issues below, however, we treat as true the facts stated above. In addition, some of the legal claims in this case are uncertain. It is entirely possible that an appeal will be made and accepted, and some or all of the damages could be reduced by the trial court or an appellate court. The jury award, therefore, is not necessarily the last word.
Medicolegal takeaways from this case
This case raises a number of professional, ethical, and legal issues. Most fundamentally, the health care team is always expected to prioritize the patient’s best interest. Respect for the patient is an essential element of that.
Behaviors such as those reported about these physicians are “absolutely not to engage in any time,” stated President of the American Society of Anesthesiologists John Absentein, MD.6 A former president of the Academy of Anesthesiology, Kathryn McGoldrick, MD, added some common sense advice that such discussions are “not only offensive but frankly stupid.” As she notes, “we can never be certain that our patients are asleep and wouldn’t have recall.”7
The actions of the physicians also may violate ethical obligations. The very first principle of medical ethics is that the physician shall provide care “with compassion and respect for human dignity and rights.”8
The legal claims included defamation, infliction of emotional distress, privacy (related to medical records), and malpractice. We will take a very brief look at each of those causes of action and then say a word about punitive damages (which the jury awarded in this case).
It is important to remember that state law, rather than federal, is providing the legal principles on which these claims were decided. Federal law might provide some relevant principles in such cases (for example, the First Amendment freedom of speech limits the state defamation rules), but that is the exception. State law is the rule.
Patient−physician recordings and the law
State laws differ regarding when it is legal to record in-person conversations. When everyone in the conversations knows about the recording, it is permissible and can be used in a court of law. In most states it is legal to record when only one party to the conversation has agreed to it, even though others in the conversation are not aware of it (which was the situation in the case discussed here).
In theory, physicians (by contract with patients) might try to limit patients’ rights to record medical services. But that practice would be difficult to implement or enforce in many circumstances. The reality is that audio and video recording devices are so ubiquitous that it is not sensible to avoid all recording of patient contact.
Physicians also might consider the potential such recordings have in some circumstances to improve communication with patients. Permitting the patient to record the patient−physician exchange, for instance, allows the patient the ability to review the advice after having left the office. This could be beneficial from a patient care perspective.
Defamation—award of damages
At its core, defamation is publishing (that is, telling someone other than the plaintiff) something untrue that may be harmful to another person. Generally the harm is reputational and the plaintiff may be affected by loss of business, mental suffering, or loss of esteem in the community.9
Defamation claims are not typical in health care cases. However, these claims are not rare: instances of health care professionals defaming other health care professionals, patients giving negative “reviews,” or health care professionals releasing false information to employers certainly do exist.
In this case, in addition to saying that the patient had syphilis and tuberculosis (both untrue), the physicians said he was a “wimp.” One interesting concept of defamation law that has developed over the centuries is “negligence per se.” This means a falsehood has been published about someone and the falsehood is likely to cause serious reputational harm. Claims that someone has a contagious disease traditionally have been considered negligence per se. Syphilis and tuberculosis fall in that category. On the other hand, saying someone needs to “man up” is usually a matter of opinion, so defamation for such comments is unlikely without special circumstances.
From the anesthesiologist’s perspective, the question is whether anyone who heard the publication really believed that the patient had either of the diseases. A joke that nobody believes to be based on fact generally is not defamatory because it has not harmed the plaintiff.10 It is apparent that the jury felt the patient had been defamed, however, given the $100,000 award for defamation.
In the United States there is special sensitivity to defamation awards because they may implicate the First Amendment’s protection of free speech. That being the case, this award may be particularly open to review by the judge and appellate courts.
Emotional distress—no award of damages
There are 2 kinds of “emotional distress” claimed in this case:
- intentional infliction
- negligent infliction.
Intentional infliction usually requires outrageous conduct by the defendant who acts intentionally or recklessly to inflict severe mental pain on the plaintiff.11 In this case, the element of “intentional” or “reckless” is interesting. While the conduct was outrageous, it is doubtful that there was any way the anesthesiologist could have imagined that these outrageous statements would have been transmitted to the patient/plaintiff.
As for negligent infliction of emotional distress, most states have been wary of opening a Pandora’s Box of litigation. Therefore, they generally require significant physical manifestations of great stress to allow recovery.12 It appears that the jury did not find the elements of either intentional or negligent infliction of emotional distress in this case.
As a side-note, this kind of emotional distress is viewed by the law as different from emotional distress that is incidental to a physical injury (pain and suffering). All states recognize that form of emotional distress.
Privacy—no award of damages
The privacy of medical records has, of course, become a major concern in the last few years. Both federal and state law provides significant penalties for the unauthorized release of medical information. However, in this case, it does not appear that medical information was improp- erly revealed.13
The patient’s complaint suggested that the anesthesiologist’s discussion during the colonoscopy of the medication for the penile rash was unnecessary for health care purposes.1 Therefore, it claims, the discussion violated the state health records privacy law. At the same time there was no indication in the public reports that this caused any harm to the patient.
Medical malpractice—award of damages
Malpractice usually involves professional practice that is unacceptable to the profession itself. It most commonly is negligence, or carelessness, that causes injury to the patient. The gross disregard for professional medical standards here was certainly negligence.14 The plaintiff claimed that discussing the medication for the penile rash and falsification of the medical records constituted malpractice.1
Presumably the jury award for medical malpractice means the jury found that the misconduct of the medical staff caused the emotional harm that the plaintiff experienced (described as embarrassment, loss of sleep, mental anguish, and anxiety), and that those injuries warranted a $200,000 award.
Punitive damage—award of damages
The jury also awarded $200,000 in “punitive” or “exemplary” damages. These are unusual damages, given not so much to compensate the victim but rather as a deterrent for the future. Generally the defendant’s conduct must have been egregious and completely unacceptable.15 Those elements were apparent to the jury from the facts of this case.
What about loss of practice privileges?
It is not unlikely that one or more of the medical professionals might, beyond civil liability, be subject to licensure discipline by the Virginia board. In addition, there are other secondary consequences of this lawsuit. The employment of those involved may be interrupted. (The anesthesiologist is said to have moved to another state, for example.) Hospital privileges also may be affected, as may insurance rates. The results of this award likely will have to be reported to the National Practitioner Data Bank.
As physicians, what’s our takeaway?
Conduct unbecoming a physician remains front and center with a recent essay published in the internal medicine literature.16 The anonymous author attests to witnessing a male gynecologist making sexual comments regarding the patient at the time of vaginal surgery preparation and an obstetrician singing and dancing to a Mexican song while treating his Hispanic patient for postpartum bleeding.
The unusual case of the anesthesiologist that we address was made even more unusual by the fact that it was recorded. Recordings, however, are likely to become ever more common. The advice of everyone’s grandmother is well taken: “Always act as though what you do will be published on the front page of the newspaper.” The ubiquitous presence of video and audio cameras and untold other devices means that someone may well be watching.
Aside from the risk of getting caught, respect for patients and clients is the very foundation of respect and professional care. It is distressing that the anesthesiologist was so disrespectful of a patient. It is equally disappointing that nobody put a stop to it.
Share your thoughts! Send your Letter to the Editor to rbarbieri@frontlinemedcom.com. Please include your name and the city and state in which you practice.
- D.B. v Safe Sedation, Complaint, Civil Action 2014-05265, Circuit Court of Fairfax County.
- Abbott R. Unconscious patient says doctors mocked him. Courthouse News Service. http://www.courthousenews.com/2014/04/22/67225.htm. Updated April 22, 2014. Accessed August 19, 2015.
- Jackman T. Anesthesiologist trashes sedated patient—and it ends up costing her. Washington Post. June 23, 2015. http://www.washingtonpost.com/local/anesthesiologist-trashes-sedated-patient-jury-orders-her-to-pay-500000/2015/06/23/cae05c00-18f3-11e5-ab92-c75ae6ab94b5_story.html. Accessed August 19, 2015.
- Waibel E. Patient says Bethesda practitioners mocked him during the colonoscopy. GazetteNet. May 13, 2014. http://www.gazette.net/article/20140513/NEWS/140519703/1070/patient-says-bethesda-practitioners-mocked-him-during-colonoscopy&template=gazette. Accessed July 15, 2015.
- Vieth P. Fairfax County Circuit Court: Doctors allegedly mocked their unconscious patient. Virginia Lawyers Weekly. May 1, 2014. http://valawyersweekly.com/2014/05/01/doctors-allegedly-mocked-their-unconscious-patient. Accessed August 19, 2015.
- Welch A. Patient sues anesthesiologist who mocked him while sedated. CBS News. http://www.cbsnews.com/news/patient-sues-anesthesiloigst-who mocked-him-while sedated. Accessed July 15, 2015.
- Leins C. Anesthesiologist derides subdued patient, loses lawsuit. US News. June 24, 2015. http://www.usnews.com/news/articles/2015/06/24/anesthesiologist-derides-subdued-patient-loses-lawsuit. Accessed August 19, 2015.
- Principles of medical ethics. American Medical Association Web site. http://www.ama-assn.org/ama/pub/physician-resources/medical-ethics/code-medical-ethics/principles-medical-ethics.page. Accessed August 19, 2015.
- Instruction nos. 24 and 25. Virginia Defamation Lawyer Web site. http://www.virginiadefamationlawyer.com/Instr%2024%20and%2025.pdf. Accessed August 19, 2015.
- Berlik LE. The Virginia model jury instructions for defamation lead to bad verdicts. The Virginia Defamation Law Blog. June 27, 2015. http://www.virginiadefamationlawyer.com/2015/06/the-virginia-model-jury-instructions-for-defamation-lead-to-bad-verdicts.html#more. Accessed August 19, 2015.
- Russo v White, 241 Va 23 (1991).
- Hughes v Moore, 214 Va 27 (1973).
- Law on patient health records/privacy. Virginia Department of Health Professions Web site.
- http://webcache.googleusercontent.com/search?q=cache:asc1xQmBefoJ:https://www.dhp.virginia.gov/dhp_laws/Law_Patient%2520Health%2520Records.doc+&cd=2&hl=en&ct=clnk&gl=us. Accessed August 19, 2015.
- Virginia Medical Malpractice Act. Va Code Ann. 8.01-230, 8.01-243(A).
- Ford CR. Pleading and understanding punitive-damages claims in Virginia. Litigation News. 2008;8(10):1-11.
- D.B. v Safe Sedation, Complaint, Civil Action 2014-05265, Circuit Court of Fairfax County.
- Abbott R. Unconscious patient says doctors mocked him. Courthouse News Service. http://www.courthousenews.com/2014/04/22/67225.htm. Updated April 22, 2014. Accessed August 19, 2015.
- Jackman T. Anesthesiologist trashes sedated patient—and it ends up costing her. Washington Post. June 23, 2015. http://www.washingtonpost.com/local/anesthesiologist-trashes-sedated-patient-jury-orders-her-to-pay-500000/2015/06/23/cae05c00-18f3-11e5-ab92-c75ae6ab94b5_story.html. Accessed August 19, 2015.
- Waibel E. Patient says Bethesda practitioners mocked him during the colonoscopy. GazetteNet. May 13, 2014. http://www.gazette.net/article/20140513/NEWS/140519703/1070/patient-says-bethesda-practitioners-mocked-him-during-colonoscopy&template=gazette. Accessed July 15, 2015.
- Vieth P. Fairfax County Circuit Court: Doctors allegedly mocked their unconscious patient. Virginia Lawyers Weekly. May 1, 2014. http://valawyersweekly.com/2014/05/01/doctors-allegedly-mocked-their-unconscious-patient. Accessed August 19, 2015.
- Welch A. Patient sues anesthesiologist who mocked him while sedated. CBS News. http://www.cbsnews.com/news/patient-sues-anesthesiloigst-who mocked-him-while sedated. Accessed July 15, 2015.
- Leins C. Anesthesiologist derides subdued patient, loses lawsuit. US News. June 24, 2015. http://www.usnews.com/news/articles/2015/06/24/anesthesiologist-derides-subdued-patient-loses-lawsuit. Accessed August 19, 2015.
- Principles of medical ethics. American Medical Association Web site. http://www.ama-assn.org/ama/pub/physician-resources/medical-ethics/code-medical-ethics/principles-medical-ethics.page. Accessed August 19, 2015.
- Instruction nos. 24 and 25. Virginia Defamation Lawyer Web site. http://www.virginiadefamationlawyer.com/Instr%2024%20and%2025.pdf. Accessed August 19, 2015.
- Berlik LE. The Virginia model jury instructions for defamation lead to bad verdicts. The Virginia Defamation Law Blog. June 27, 2015. http://www.virginiadefamationlawyer.com/2015/06/the-virginia-model-jury-instructions-for-defamation-lead-to-bad-verdicts.html#more. Accessed August 19, 2015.
- Russo v White, 241 Va 23 (1991).
- Hughes v Moore, 214 Va 27 (1973).
- Law on patient health records/privacy. Virginia Department of Health Professions Web site.
- http://webcache.googleusercontent.com/search?q=cache:asc1xQmBefoJ:https://www.dhp.virginia.gov/dhp_laws/Law_Patient%2520Health%2520Records.doc+&cd=2&hl=en&ct=clnk&gl=us. Accessed August 19, 2015.
- Virginia Medical Malpractice Act. Va Code Ann. 8.01-230, 8.01-243(A).
- Ford CR. Pleading and understanding punitive-damages claims in Virginia. Litigation News. 2008;8(10):1-11.
In this Article
- The patient’s claims in this case
- Recordings and the law