User login
Adopting NQF Practices
In November 1999, the Institute of Medicine released its landmark report entitled To Err Is Human: Building A Safer Health System.1 The report claimed that more than 1 million people in the United States suffer from preventable medical injuries each year and that as many as 98,000 people die annually in hospitals from medical errors. Although evidence‐based methods are available to prevent adverse events, there is concern that the current lack of standardization among hospitals implementing such safe practices has the potential to both diffuse and dilute efforts to improve patient safety.
To address this issue, the National Quality Forum (NQF) in 2003 released an evidence‐based consensus report that presented 30 safe practices for better health care with a recommendation that all be universally adopted.2 The purpose of this study is to use information collected from a voluntary patient safety program in Georgia3 and an Agency for Healthcare Research and Quality (AHRQ) reporting demonstration study4 to (1) describe the current statewide adoption rates for NQF medication safe practices and safety culture (Table 1), and (2) examine if hospital adoption varies by hospital size, ownership, and rural or urban location.
| NQF Safe Practice No. | Key Word | Full Description of Safe Practice |
|---|---|---|
| ||
| 1 | Culture of safety | Create a health care culture of safety. |
| 5 | Consultant pharmacists | Pharmacists should actively participate in the medication‐use process, including, at a minimum, being available for consultation with prescribers and reviewing medication orders. |
| 6 | Verbal orders | Verbal orders should be recorded whenever possible and immediately read back to the prescriber. |
| 7 | Abbreviations | Use of standardized abbreviations and dosage designations. |
| 9 | Information transfer | Ensure that care information, especially changes in orders and new diagnostic information, is transmitted to all providers. |
| 12 | CPOE adoption | Implement a computerized prescriber order entry system |
| 27 | Clean workspaces | Keep workspaces where medications are prepared clean, orderly, well lighted, and free of clutter, distraction, and noise. |
| 28 | Labeling and storage | Standardize the methods for labeling, packaging, and storing medications. |
| 29 | High‐alert medications | Identify all high alert drugs. |
| 30 | Unit dosing | Dispense medications in unit‐dose or, when appropriate, unit‐of‐use form whenever possible. |
METHODS
Setting and Exclusions
The Partnership for Health and Accountability (PHA), a voluntary and peer‐review‐protected statewide hospital patient safety program, was established in Georgia in 2001 under the administration of the Georgia Hospital Association. All 148 nonfederal adult acute care hospitals in the state of Georgia participate in some aspect of the initiative. This represents a broad cross section of hospital types nationwide, with 55% of the hospitals having fewer than 100 beds, 25% having 100‐299 beds, and 20% having more than 300 beds. Hospitals are almost evenly divided between urban (54%) and rural (46%) locations.
Survey Instruments
One component of the PHA program focuses on safe medication use (SMU) with a goal of reducing the frequency of medication‐related errors in acute care hospitals. In 2004 all active acute care hospital members of GHA were eligible to participate in the SMU self‐assessment, and all but 1 hospital (147 of 148 hospitals, 97.3%) completed the self‐assessment survey.
The SMU self‐assessment is a 99‐item survey that addresses error reporting and event capture, the prescribing process, order processing and dispensing, medication administration and monitoring, patient involvement, policy and administration, and practitioner education and development. For each item, hospitals report on a 1‐5 scale the current status of adoption, ranging from no discussion to full implementation.
A second component of the PHA program identifies critical organizational tactics and strategies required for a culture of safety. Once every 2 years, top and midlevel managers complete a Strategies for Leadership self‐assessment. Results from this survey are disseminated to member hospitals to promote a culture of safety. Regular audioconferences are held to network and share successful intervention strategies aimed at establishing free and open communication, improving organizational learning, and promoting nonpunitive reporting of adverse events. A total of 147 hospitals (97.3%) completed the 2003 Strategies for Leadership survey.
The Strategies for Leadership self‐assessment is a 75‐item survey that addresses 7 broad categories: top leadership priorities, strategic planning, nonpunitive environment, patient and community focus, information analysis, human resources, and work environment. Hospital managers describe current status using a scale ranging from 1 (no discussion) to 5 (> 90% implementation).
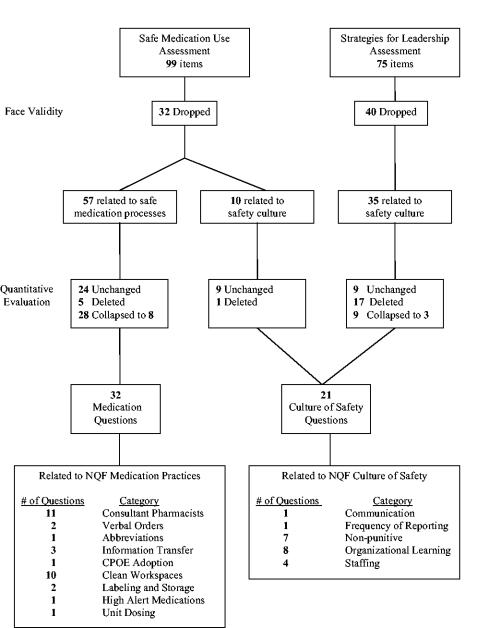
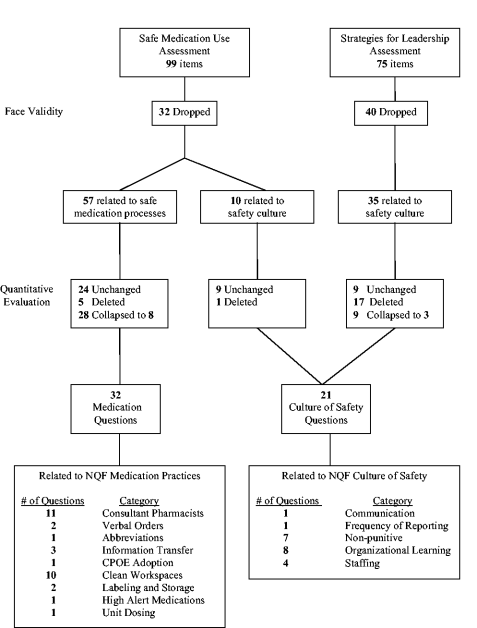
Several steps were used to create the final study measures. First, the SMU and Leadership survey questions were reviewed to see if they addressed 1 of the 10 NQF indicators under study (Table 1). Quantitative analysis was then used to eliminate, collapse, and/or confirm the grouping arrangement. Given the broad and nonspecific nature of create a culture of safety, domains from the Hospital Survey on Patient Safety Culture5 were used to classify specific aspects of safety culture. For the purposes of this study, 5 of the 12 domains were used to categorize hospital responses. The domains used were (1) feedback and communication about error, (2) frequency of reporting, (3) promoting a nonpunitive environment, (4) encouraging organizational learning and continuous improvement, and (5) maintaining safe staffing.
Mapping Survey Questions to Safe Practices
A subset (n = 57) of the SMU survey questions directly related to safe medication processes (ie, prescribing, transcribing, dispensing, administration, and monitoring) were selected for inclusion in the study (Fig. 1). A nonoverlapping subset of Leadership (n = 35) and SMU (n = 10) survey questions related to safety culture were also identified. Clinical members of the project team independently reviewed and mapped medication process survey questions to 1 of 9 NQF indicators of safe medication practices. Assignment was based on face validity and best fit with the intent of the NQF indicator. Social science team members mapped culture‐related survey questions to the NQF indicator create a health care culture of safety using the 5 domains of safety culture.5
Grouping Similar Questions
A Pearson correlation matrix was used to confirm the factor analysis and determine if multiple questions related to a single safe practice could be reduced to 1 composite measure. If analysis supported the use of a composite score, responses to similar questions at the hospital level were averaged, and the hospital's final average was the measure used for analyses. Finally, the project team reviewed the a priori mapping along with the results of the correlation and factor analyses and reached consensus on the final number and mapping scheme of survey questions to NQF safe practices. Of the original 45 culture‐of‐safety questions, 21 were used for this analysis, and of the original 57 safe medication process questions, 32 were used.
Data Analysis
Bivariate analyses using SPSS software were conducted to examine the association between hospital structural characteristics (urban or rural location, network affiliation, academic affiliation, bed size) and adoption of each NQF safe practice.
RESULTS
Medication Safety
Table 2 shows the overall rate of adoption by all hospitals of the safe practices related to medication use. Full implementation was defined as implementation in greater than 90% of the organization. There has been almost universal adoption of 3 safe practices: processes to standardize labeling and storage of medications (133 of 147, 90.5%), identification of high‐alert medications (119 of 147, 81.0%), and use of unit doses when appropriate (119 of 147, 81.0%). CPOE systems, on the other hand, had been implemented in fewer than 3% (4 of 147) of the hospitals by early 2004. The remaining 5 medication practices showed intermediate adoption (between 48.3% and 69.7%): ensuring information transfer, minimizing verbal orders, providing clean workspaces with minimal distractions, availability of consultant pharmacists, and minimizing abbreviations.
| NQF Safe Practice | Proportion of Hospitals Reporting > 90% Implementation | Association with Hospital Structural Characteristics* |
|---|---|---|
| ||
| #5 Consultant pharmacists | 52.0% | More likely in mid‐size hospitals |
| #6 Verbal orders | 63.3% | None |
| #7 Abbreviations | 48.3% | None |
| #9 Information transfer | 69.7% | None |
| #12 CPOE adoption | 2.7% | None |
| #27 Clean workspaces | 53.7% | Less likely in large hospitals |
| #28 Labeling and storage | 90.5% | None |
| #29 High‐alert medications | 81.0% | None |
| #30 Unit dosing | 81.0% | More likely in for‐profit hospitals |
Variation in Adoption by Hospital Characteristics
There was only limited variation in adoption by hospital characteristics as summarized in Table 2 and discussed in more detail below. For‐profit hospitals were most likely to have a unit dose medication distribution system in place (93.1% vs. 78.2%, P = .037). For‐profit hospitals were also more likely (83.1% vs. 58.4%, P = .004) to have fully implemented a policy to read back verbal orders. The likelihood of adopting a policy to eliminate verbal orders did not vary significantly by hospital characteristics. The prevalence of distractions was also seen as a problem for writing orders and medication administration, with the largest hospitals more frequently reporting this challenge (59.2% vs. 29.6%, P = .005). Midsize hospitals (100299 beds) were more likely than larger or smaller hospitals to report that a pharmacist reviewed and approved all nonemergency orders prior to dispensing. (76.3% vs. 45.0%, P = .001).
Barriers to Adoption of Medication Safe Practices
Ensuring that new prescribers had access to all currently prescribed medications, including both dose and frequency was a challenge for many hospitals. More than 30% of hospitals (45 of 147) did not have this capability consistently throughout the institution, and that capability did not vary by hospital size or geographic location. Although most hospitals (93 of 147, 63.3%) had a read‐back policy for verbal orders, only 36.1% of hospitals (53 of 147) had fully implemented a policy to eliminate or minimize the use of verbal orders. Two aspects of the medication preparation environment also appeared to be problematic for the surveyed hospitals: appropriate space for medication preparation and a distraction‐free environment. Only half the hospitals (74 of 147) reported that medications were prepared in an environment that minimized distractions, and 53.7% (79 of 147) reported that pharmacists were provided with sufficient space. Although more than 90% of hospitals reported that pharmacists were available for consultation even when the pharmacy was closed, fewer than half the hospitals (71 of 147, 48.3%) reported that pharmacists were involved on patient care units as a resource for clinical decision support. There also were gaps in the patient information available when preparing medications, in particular, pregnancy status (82 of 147, 55.8%) and medications prescribed before hospitalization (85 of 147, 57.8%). Fewer than half the hospitals (67 of 147, 45.5%) had fully implemented a policy to minimize use of dangerous abbreviations. Most hospitals (91 of 147, 61.9%), however, did report that they had methods in place to proactively review processes for communicating medication orders and then redesign if appropriate.
Safety Culture
Table 3 shows the self‐reported adoption of safety culture as defined by the Hospital Patient Safety Culture Survey5 domains. Hospital safety culture was highest in several areas related to nonpunitive policies. For example, the vast majority of hospitals reported that no disciplinary actions were taken against employees for nonmalicious errors, that a formal hospital‐wide nonpunitive policy for staff and employees was in place, and that the hospital had a user‐friendly and confidential error‐reporting system in place. A smaller proportion of hospitals (75 of 147, 51.0%) provided specific resources to support employees involved in error or sponsor unit visits by senior management to promote blame‐free discussion and reporting of errors (64 of 147, 43.5%). For‐profit hospitals (63.3% vs. 38.5%, P = .014) and small hospitals (49.2% vs. 18.5%, P = .004) were more likely to have unit visits by senior management. An even smaller minority of hospitals reported having used dedicated observers to catch errors as they occur (32 of 147, 21.8%) or that they provided direct incentives to caregivers for reporting errors (31 of 147, 21.1%).
| Safety of Culture Category | Specific Attribute | Overall Adoption | Association with Hospital Structural Characteristics* |
|---|---|---|---|
| |||
| Communication | Safety alert process | 59.9% | None |
| Frequency of reporting | Confidential error reporting system | 70.1% | None |
| Non‐punitive environment | Nonpunitive policies | 76.2% | None |
| Employee resources | 51.0% | None | |
| Unit visits | 43.5% | Unit visits more likely in small hospitals and for‐profit hospitals | |
| Organizational learning | Annual safety plans | 76.7% | None |
| Teams analyze errors | 72.1% | None | |
| Data analysis guides QI | 69.4% | Using data analysis to guide QI initiatives less likely in large hospitals | |
| Proactive evaluations before implementation | 44.9% | None | |
| Piloting processes | 42.9% | None | |
| Staffing | Adequate staffing ratios | 72.8% | None |
| Limited work hours | 57.6% | Limiting staff work hours less likely in large hospitals | |
In regard to organizational policies, three‐fourths of hospitals did have a patient safety plan that was reviewed annually by senior leadership. Most hospitals (106 of 147, 72.1%) used multidisciplinary teams to regularly analyze errors after they occurred and to identify possible system changes with no significant differences in adoption rates across hospital types. Most hospitals (102 of 147, 69.4%) also used data analysis to drive patient safety quality improvement efforts. Surprisingly, this was least common in the largest hospitals (48.1% vs. 74.2%, P = .008). Overall, hospitals were much less likely to have adopted the use of proactive techniques such as failure modes and effects analysis (FMEA) before implementation of major system changes or the piloting of new processes prior to implementation. Adoption rates for these activities were below 50% for all hospital demographic groups.
In terms of strategies for maintaining safe staffing levels, most hospitals reported they maintained safe staffing through adequate staffing ratios (107 of 147, 72.8%), whereas a smaller number (84 of 147, 57.1%) reported maintaining safe staffing by limiting work hours. Large hospitals were the least likely to limit work hours (33.3% vs. 63.2%, P = .005).
DISCUSSION
This is the first study to use existing data sources to characterize the current progress and barriers to further adoption of NQF safe practices and safety culture related to medication use in a statewide sample of hospitals. Several findings are notable. First, most of the hospitals surveyed had adopted 7 of 9 medication‐related NQF safe practices by 2004. Similar to findings from the earlier ISMP Safety Self‐Assessment for Hospitals, hospitals scored most highly on practices related to drug storage, packaging, and labeling and lowest on CPOE implementation.12 Results from the 2003 Leapfrog Group Quality and Safety Survey also found that only 3.7% of participating hospitals had fully implemented a CPOE system.13 Medication safe practices that directly affect physicians, such as verbal orders, standardized abbreviations, and access to relevant clinical information when prescribing had only intermediate adoption rates.
Most hospitals have developed policies around nonpunitive safety cultures, but fewer have adopted proactive error reduction systems. Safety culture is more difficult to measure than safe medication processes. A previous survey of Iowa hospitals assessed only whether hospitals reported progress toward creating a culture of safety.14 In this study we attempted to break down the broad concept of safety culture into specific actionable components. Three widely recognized components of a safe hospital culture are creating a nonpunitive environment for staff, using data to identify and analyze errors and system causes, and safe staffing levels.8 Most but not all surveyed hospitals had adopted these safety culture strategies. Other more resource‐intensive practices, such as unit visits by senior management and FMEA, were less likely to have been adopted. The adoption rates reported here for 20032004 are in most cases higher than those found in the 2000 ISMP survey, which may be explained by the more recent survey reported here and variations in question wording as well as response scoring.
Variations in adoption rates of NQF‐recommended safe practices generally were not explained by hospital characteristics such as ownership, size, or geographic location. Instead, barriers appear to be related to resource constraints as well as the ability of hospitals to directly control the specific safe practice. The ISMP survey found that hospital demographic factors explained only 3% of the variation in adoption, which is similar to our finding of few differences in adoption of safe practices by hospital type. Cost and health care culture may explain why certain safe practices remain less than fully adopted.12 Resource constraints may explain the lower adoption rate of several practices: CPOE, pharmacist consultation, and physical environment improvements. Other safe practices with lower adoption rates require active physician participation, for example, minimizing verbal orders, standardizing abbreviations, and ensuring accurate information transfers. Hospital‐based physicians can play a key role in advocating for effective processes to promote these practices.
Another general factor that distinguished highly adopted practices from less adopted practices was the extent to which reactive as opposed to proactive actions were required. Hospitals were more likely to report reactive policies such as reading back verbal orders than proactive policies to minimize verbal orders. Pharmacists were generally available for telephone consultation but in only half the hospitals were they available on the hospital units for consultation. A similar pattern was seen for culture of safety practices; systems were generally in place for nonpunitive error reporting, but a minority of hospitals had senior leadership making unit rounds or multidisciplinary teams proactively testing new systems to identify potential errors before they occur. Again, there is a leadership role that hospital‐based physicians can play as effective team builders for safety culture and as clinical leaders for improvement of medication processes. Much research has demonstrated the impact that a culture of safety can have on error reduction.811 As physicians who spend most of their clinical time directly on patient care units, hospital‐based physicians are uniquely positioned to promote positive changes in culture. Research on the impact of hospitalists on hospital costs and patient outcomes should be broadened to include an assessment of their impact on safety culture and error reduction.
This study had several limitations, the first being that it was based on voluntarily provided self‐assessment data. The surveys used in this project have been refined and administered over 3 years in a nonpunitive process improvement program with a consistently high participation rate. The hospital‐reported survey results have not been independently verified for accuracy, similar to most of the prior research in this area. The surveys measure management's perception of safety culture and do not assess actual employee perceptions of the safety culture on their particular units. Thus, although management may believe they are implementing policies to create a nonpunitive environment, actual assessments of employees' views are needed to confirm this. Because the study was based on previously collected data, several steps were used to map the existing questions to NQF safe practices. Given the broad nature of the NQF topics, at least 1 relevant survey question was identified for each of the medication‐related safe practices. When more than 1 question was judged to be relevant, the responses were averaged. The survey was limited to adult acute care hospitals in Georgia, which may not be nationally representative, and federal and Veterans Administration hospitals were not included. However, Georgia has a relatively high proportion of smaller rural hospitals and offers interesting baseline data on similar rates of adoption of safe practices in rural and smaller hospitals compared with that in urban hospitals. Because we were using previously collected surveys, we could only look at the adoption of selected safe practices. Further work is needed to look at the adoption of other safe practices.
In summary, it is encouraging that the most studied NQF‐recommended safe practices have already been adopted by a wide range of hospitals, including rural and small hospitals. Resource constraints as well as health care culture and structure remain barriers to broader diffusion. Some barriers may be addressed by technology and improvements in physical environments, but others relate to culture and may be more challenging to address. Active physician participation in medication‐related patient safety initiatives will be key to promoting further adoption of safe practices.
- Kohn LT,Corrigan JM, andDonaldson MS, eds.To Err Is Human: Building a Safer Health System: A Report from the Committee on Quality of Healthcare in America.Institute of Medicine,National Academy of Sciences.Washington, DC:National Academy Press,1999.
- The National Quality Forum.Safe practices for better healthcare: a consensus report. NQF publication no. NQFCR‐05‐03;2003.
- Georgia Hospital Association. Available at: http://www.gha.org.
- ,,.Voluntary hospital coalitions to promote patient safety: why, how and can they work? In:Advances in Patient Safety: From Research to Implementation.Rockville, MD:AHRQ;2005.
- Agency for Healthcare Research and Quality (AHRQ).The Hospital Survey on Patient Safety Toolkit 2004. Sponsored by the Medical Errors Workgroup of the Quality Interagency Coordination Task Force (QuIC), developed by Westat.Rockville, MD:AHRQ;2004.
- Vaughan, Diane.1996.The Challenger launch decision: risky technology, culture, and deviance at NASA.Chicago:University of Chicago Press.
- ,,,,,.Overcoming barriers to adopting and implementing computerized physician order entry systems in U.S. hospitals.Health Aff.2004;23(4):184–190.
- ,.Safety culture assessment: a tool for improving patient safety in healthcare organizations.Qual Saf in Health Care.2003;12(suppl. 2):17–23.
- ,,.Error, stress, and teamwork in medicine and aviation: cross‐sectional surveys.Hum Perf Extrem Environ.2001;6:6–11.
- ,,,.Using a multihospital survey to examine the safety culture.Jt Comm J Qual Saf.2004;30:125–32.
- ,,,.A review of the literature examining linkages between organizational factors, medical errors, and patient safety.Med Care Res Rev.2004;61:3–37.
- ,,,,,.Findings from the ISMP medication safety self‐assessment for hospitals.Jt Comm J Qual Saf.2003;29:586–597.
- ,.Hospital implementation of computerized provider order entry systems: results from the 2003 Leapfrog Group Quality and Safety Survey.J Healthc Inf Manag.2005;19(4):55–65.
- ,,,,.National Quality Forum 30 safe practices: priority and progress in Iowa hospitals.Am J Med Qual.2006;21:101–108.
In November 1999, the Institute of Medicine released its landmark report entitled To Err Is Human: Building A Safer Health System.1 The report claimed that more than 1 million people in the United States suffer from preventable medical injuries each year and that as many as 98,000 people die annually in hospitals from medical errors. Although evidence‐based methods are available to prevent adverse events, there is concern that the current lack of standardization among hospitals implementing such safe practices has the potential to both diffuse and dilute efforts to improve patient safety.
To address this issue, the National Quality Forum (NQF) in 2003 released an evidence‐based consensus report that presented 30 safe practices for better health care with a recommendation that all be universally adopted.2 The purpose of this study is to use information collected from a voluntary patient safety program in Georgia3 and an Agency for Healthcare Research and Quality (AHRQ) reporting demonstration study4 to (1) describe the current statewide adoption rates for NQF medication safe practices and safety culture (Table 1), and (2) examine if hospital adoption varies by hospital size, ownership, and rural or urban location.
| NQF Safe Practice No. | Key Word | Full Description of Safe Practice |
|---|---|---|
| ||
| 1 | Culture of safety | Create a health care culture of safety. |
| 5 | Consultant pharmacists | Pharmacists should actively participate in the medication‐use process, including, at a minimum, being available for consultation with prescribers and reviewing medication orders. |
| 6 | Verbal orders | Verbal orders should be recorded whenever possible and immediately read back to the prescriber. |
| 7 | Abbreviations | Use of standardized abbreviations and dosage designations. |
| 9 | Information transfer | Ensure that care information, especially changes in orders and new diagnostic information, is transmitted to all providers. |
| 12 | CPOE adoption | Implement a computerized prescriber order entry system |
| 27 | Clean workspaces | Keep workspaces where medications are prepared clean, orderly, well lighted, and free of clutter, distraction, and noise. |
| 28 | Labeling and storage | Standardize the methods for labeling, packaging, and storing medications. |
| 29 | High‐alert medications | Identify all high alert drugs. |
| 30 | Unit dosing | Dispense medications in unit‐dose or, when appropriate, unit‐of‐use form whenever possible. |
METHODS
Setting and Exclusions
The Partnership for Health and Accountability (PHA), a voluntary and peer‐review‐protected statewide hospital patient safety program, was established in Georgia in 2001 under the administration of the Georgia Hospital Association. All 148 nonfederal adult acute care hospitals in the state of Georgia participate in some aspect of the initiative. This represents a broad cross section of hospital types nationwide, with 55% of the hospitals having fewer than 100 beds, 25% having 100‐299 beds, and 20% having more than 300 beds. Hospitals are almost evenly divided between urban (54%) and rural (46%) locations.
Survey Instruments
One component of the PHA program focuses on safe medication use (SMU) with a goal of reducing the frequency of medication‐related errors in acute care hospitals. In 2004 all active acute care hospital members of GHA were eligible to participate in the SMU self‐assessment, and all but 1 hospital (147 of 148 hospitals, 97.3%) completed the self‐assessment survey.
The SMU self‐assessment is a 99‐item survey that addresses error reporting and event capture, the prescribing process, order processing and dispensing, medication administration and monitoring, patient involvement, policy and administration, and practitioner education and development. For each item, hospitals report on a 1‐5 scale the current status of adoption, ranging from no discussion to full implementation.
A second component of the PHA program identifies critical organizational tactics and strategies required for a culture of safety. Once every 2 years, top and midlevel managers complete a Strategies for Leadership self‐assessment. Results from this survey are disseminated to member hospitals to promote a culture of safety. Regular audioconferences are held to network and share successful intervention strategies aimed at establishing free and open communication, improving organizational learning, and promoting nonpunitive reporting of adverse events. A total of 147 hospitals (97.3%) completed the 2003 Strategies for Leadership survey.
The Strategies for Leadership self‐assessment is a 75‐item survey that addresses 7 broad categories: top leadership priorities, strategic planning, nonpunitive environment, patient and community focus, information analysis, human resources, and work environment. Hospital managers describe current status using a scale ranging from 1 (no discussion) to 5 (> 90% implementation).
Several steps were used to create the final study measures. First, the SMU and Leadership survey questions were reviewed to see if they addressed 1 of the 10 NQF indicators under study (Table 1). Quantitative analysis was then used to eliminate, collapse, and/or confirm the grouping arrangement. Given the broad and nonspecific nature of create a culture of safety, domains from the Hospital Survey on Patient Safety Culture5 were used to classify specific aspects of safety culture. For the purposes of this study, 5 of the 12 domains were used to categorize hospital responses. The domains used were (1) feedback and communication about error, (2) frequency of reporting, (3) promoting a nonpunitive environment, (4) encouraging organizational learning and continuous improvement, and (5) maintaining safe staffing.
Mapping Survey Questions to Safe Practices
A subset (n = 57) of the SMU survey questions directly related to safe medication processes (ie, prescribing, transcribing, dispensing, administration, and monitoring) were selected for inclusion in the study (Fig. 1). A nonoverlapping subset of Leadership (n = 35) and SMU (n = 10) survey questions related to safety culture were also identified. Clinical members of the project team independently reviewed and mapped medication process survey questions to 1 of 9 NQF indicators of safe medication practices. Assignment was based on face validity and best fit with the intent of the NQF indicator. Social science team members mapped culture‐related survey questions to the NQF indicator create a health care culture of safety using the 5 domains of safety culture.5

Grouping Similar Questions
A Pearson correlation matrix was used to confirm the factor analysis and determine if multiple questions related to a single safe practice could be reduced to 1 composite measure. If analysis supported the use of a composite score, responses to similar questions at the hospital level were averaged, and the hospital's final average was the measure used for analyses. Finally, the project team reviewed the a priori mapping along with the results of the correlation and factor analyses and reached consensus on the final number and mapping scheme of survey questions to NQF safe practices. Of the original 45 culture‐of‐safety questions, 21 were used for this analysis, and of the original 57 safe medication process questions, 32 were used.
Data Analysis
Bivariate analyses using SPSS software were conducted to examine the association between hospital structural characteristics (urban or rural location, network affiliation, academic affiliation, bed size) and adoption of each NQF safe practice.
RESULTS
Medication Safety
Table 2 shows the overall rate of adoption by all hospitals of the safe practices related to medication use. Full implementation was defined as implementation in greater than 90% of the organization. There has been almost universal adoption of 3 safe practices: processes to standardize labeling and storage of medications (133 of 147, 90.5%), identification of high‐alert medications (119 of 147, 81.0%), and use of unit doses when appropriate (119 of 147, 81.0%). CPOE systems, on the other hand, had been implemented in fewer than 3% (4 of 147) of the hospitals by early 2004. The remaining 5 medication practices showed intermediate adoption (between 48.3% and 69.7%): ensuring information transfer, minimizing verbal orders, providing clean workspaces with minimal distractions, availability of consultant pharmacists, and minimizing abbreviations.
| NQF Safe Practice | Proportion of Hospitals Reporting > 90% Implementation | Association with Hospital Structural Characteristics* |
|---|---|---|
| ||
| #5 Consultant pharmacists | 52.0% | More likely in mid‐size hospitals |
| #6 Verbal orders | 63.3% | None |
| #7 Abbreviations | 48.3% | None |
| #9 Information transfer | 69.7% | None |
| #12 CPOE adoption | 2.7% | None |
| #27 Clean workspaces | 53.7% | Less likely in large hospitals |
| #28 Labeling and storage | 90.5% | None |
| #29 High‐alert medications | 81.0% | None |
| #30 Unit dosing | 81.0% | More likely in for‐profit hospitals |
Variation in Adoption by Hospital Characteristics
There was only limited variation in adoption by hospital characteristics as summarized in Table 2 and discussed in more detail below. For‐profit hospitals were most likely to have a unit dose medication distribution system in place (93.1% vs. 78.2%, P = .037). For‐profit hospitals were also more likely (83.1% vs. 58.4%, P = .004) to have fully implemented a policy to read back verbal orders. The likelihood of adopting a policy to eliminate verbal orders did not vary significantly by hospital characteristics. The prevalence of distractions was also seen as a problem for writing orders and medication administration, with the largest hospitals more frequently reporting this challenge (59.2% vs. 29.6%, P = .005). Midsize hospitals (100299 beds) were more likely than larger or smaller hospitals to report that a pharmacist reviewed and approved all nonemergency orders prior to dispensing. (76.3% vs. 45.0%, P = .001).
Barriers to Adoption of Medication Safe Practices
Ensuring that new prescribers had access to all currently prescribed medications, including both dose and frequency was a challenge for many hospitals. More than 30% of hospitals (45 of 147) did not have this capability consistently throughout the institution, and that capability did not vary by hospital size or geographic location. Although most hospitals (93 of 147, 63.3%) had a read‐back policy for verbal orders, only 36.1% of hospitals (53 of 147) had fully implemented a policy to eliminate or minimize the use of verbal orders. Two aspects of the medication preparation environment also appeared to be problematic for the surveyed hospitals: appropriate space for medication preparation and a distraction‐free environment. Only half the hospitals (74 of 147) reported that medications were prepared in an environment that minimized distractions, and 53.7% (79 of 147) reported that pharmacists were provided with sufficient space. Although more than 90% of hospitals reported that pharmacists were available for consultation even when the pharmacy was closed, fewer than half the hospitals (71 of 147, 48.3%) reported that pharmacists were involved on patient care units as a resource for clinical decision support. There also were gaps in the patient information available when preparing medications, in particular, pregnancy status (82 of 147, 55.8%) and medications prescribed before hospitalization (85 of 147, 57.8%). Fewer than half the hospitals (67 of 147, 45.5%) had fully implemented a policy to minimize use of dangerous abbreviations. Most hospitals (91 of 147, 61.9%), however, did report that they had methods in place to proactively review processes for communicating medication orders and then redesign if appropriate.
Safety Culture
Table 3 shows the self‐reported adoption of safety culture as defined by the Hospital Patient Safety Culture Survey5 domains. Hospital safety culture was highest in several areas related to nonpunitive policies. For example, the vast majority of hospitals reported that no disciplinary actions were taken against employees for nonmalicious errors, that a formal hospital‐wide nonpunitive policy for staff and employees was in place, and that the hospital had a user‐friendly and confidential error‐reporting system in place. A smaller proportion of hospitals (75 of 147, 51.0%) provided specific resources to support employees involved in error or sponsor unit visits by senior management to promote blame‐free discussion and reporting of errors (64 of 147, 43.5%). For‐profit hospitals (63.3% vs. 38.5%, P = .014) and small hospitals (49.2% vs. 18.5%, P = .004) were more likely to have unit visits by senior management. An even smaller minority of hospitals reported having used dedicated observers to catch errors as they occur (32 of 147, 21.8%) or that they provided direct incentives to caregivers for reporting errors (31 of 147, 21.1%).
| Safety of Culture Category | Specific Attribute | Overall Adoption | Association with Hospital Structural Characteristics* |
|---|---|---|---|
| |||
| Communication | Safety alert process | 59.9% | None |
| Frequency of reporting | Confidential error reporting system | 70.1% | None |
| Non‐punitive environment | Nonpunitive policies | 76.2% | None |
| Employee resources | 51.0% | None | |
| Unit visits | 43.5% | Unit visits more likely in small hospitals and for‐profit hospitals | |
| Organizational learning | Annual safety plans | 76.7% | None |
| Teams analyze errors | 72.1% | None | |
| Data analysis guides QI | 69.4% | Using data analysis to guide QI initiatives less likely in large hospitals | |
| Proactive evaluations before implementation | 44.9% | None | |
| Piloting processes | 42.9% | None | |
| Staffing | Adequate staffing ratios | 72.8% | None |
| Limited work hours | 57.6% | Limiting staff work hours less likely in large hospitals | |
In regard to organizational policies, three‐fourths of hospitals did have a patient safety plan that was reviewed annually by senior leadership. Most hospitals (106 of 147, 72.1%) used multidisciplinary teams to regularly analyze errors after they occurred and to identify possible system changes with no significant differences in adoption rates across hospital types. Most hospitals (102 of 147, 69.4%) also used data analysis to drive patient safety quality improvement efforts. Surprisingly, this was least common in the largest hospitals (48.1% vs. 74.2%, P = .008). Overall, hospitals were much less likely to have adopted the use of proactive techniques such as failure modes and effects analysis (FMEA) before implementation of major system changes or the piloting of new processes prior to implementation. Adoption rates for these activities were below 50% for all hospital demographic groups.
In terms of strategies for maintaining safe staffing levels, most hospitals reported they maintained safe staffing through adequate staffing ratios (107 of 147, 72.8%), whereas a smaller number (84 of 147, 57.1%) reported maintaining safe staffing by limiting work hours. Large hospitals were the least likely to limit work hours (33.3% vs. 63.2%, P = .005).
DISCUSSION
This is the first study to use existing data sources to characterize the current progress and barriers to further adoption of NQF safe practices and safety culture related to medication use in a statewide sample of hospitals. Several findings are notable. First, most of the hospitals surveyed had adopted 7 of 9 medication‐related NQF safe practices by 2004. Similar to findings from the earlier ISMP Safety Self‐Assessment for Hospitals, hospitals scored most highly on practices related to drug storage, packaging, and labeling and lowest on CPOE implementation.12 Results from the 2003 Leapfrog Group Quality and Safety Survey also found that only 3.7% of participating hospitals had fully implemented a CPOE system.13 Medication safe practices that directly affect physicians, such as verbal orders, standardized abbreviations, and access to relevant clinical information when prescribing had only intermediate adoption rates.
Most hospitals have developed policies around nonpunitive safety cultures, but fewer have adopted proactive error reduction systems. Safety culture is more difficult to measure than safe medication processes. A previous survey of Iowa hospitals assessed only whether hospitals reported progress toward creating a culture of safety.14 In this study we attempted to break down the broad concept of safety culture into specific actionable components. Three widely recognized components of a safe hospital culture are creating a nonpunitive environment for staff, using data to identify and analyze errors and system causes, and safe staffing levels.8 Most but not all surveyed hospitals had adopted these safety culture strategies. Other more resource‐intensive practices, such as unit visits by senior management and FMEA, were less likely to have been adopted. The adoption rates reported here for 20032004 are in most cases higher than those found in the 2000 ISMP survey, which may be explained by the more recent survey reported here and variations in question wording as well as response scoring.
Variations in adoption rates of NQF‐recommended safe practices generally were not explained by hospital characteristics such as ownership, size, or geographic location. Instead, barriers appear to be related to resource constraints as well as the ability of hospitals to directly control the specific safe practice. The ISMP survey found that hospital demographic factors explained only 3% of the variation in adoption, which is similar to our finding of few differences in adoption of safe practices by hospital type. Cost and health care culture may explain why certain safe practices remain less than fully adopted.12 Resource constraints may explain the lower adoption rate of several practices: CPOE, pharmacist consultation, and physical environment improvements. Other safe practices with lower adoption rates require active physician participation, for example, minimizing verbal orders, standardizing abbreviations, and ensuring accurate information transfers. Hospital‐based physicians can play a key role in advocating for effective processes to promote these practices.
Another general factor that distinguished highly adopted practices from less adopted practices was the extent to which reactive as opposed to proactive actions were required. Hospitals were more likely to report reactive policies such as reading back verbal orders than proactive policies to minimize verbal orders. Pharmacists were generally available for telephone consultation but in only half the hospitals were they available on the hospital units for consultation. A similar pattern was seen for culture of safety practices; systems were generally in place for nonpunitive error reporting, but a minority of hospitals had senior leadership making unit rounds or multidisciplinary teams proactively testing new systems to identify potential errors before they occur. Again, there is a leadership role that hospital‐based physicians can play as effective team builders for safety culture and as clinical leaders for improvement of medication processes. Much research has demonstrated the impact that a culture of safety can have on error reduction.811 As physicians who spend most of their clinical time directly on patient care units, hospital‐based physicians are uniquely positioned to promote positive changes in culture. Research on the impact of hospitalists on hospital costs and patient outcomes should be broadened to include an assessment of their impact on safety culture and error reduction.
This study had several limitations, the first being that it was based on voluntarily provided self‐assessment data. The surveys used in this project have been refined and administered over 3 years in a nonpunitive process improvement program with a consistently high participation rate. The hospital‐reported survey results have not been independently verified for accuracy, similar to most of the prior research in this area. The surveys measure management's perception of safety culture and do not assess actual employee perceptions of the safety culture on their particular units. Thus, although management may believe they are implementing policies to create a nonpunitive environment, actual assessments of employees' views are needed to confirm this. Because the study was based on previously collected data, several steps were used to map the existing questions to NQF safe practices. Given the broad nature of the NQF topics, at least 1 relevant survey question was identified for each of the medication‐related safe practices. When more than 1 question was judged to be relevant, the responses were averaged. The survey was limited to adult acute care hospitals in Georgia, which may not be nationally representative, and federal and Veterans Administration hospitals were not included. However, Georgia has a relatively high proportion of smaller rural hospitals and offers interesting baseline data on similar rates of adoption of safe practices in rural and smaller hospitals compared with that in urban hospitals. Because we were using previously collected surveys, we could only look at the adoption of selected safe practices. Further work is needed to look at the adoption of other safe practices.
In summary, it is encouraging that the most studied NQF‐recommended safe practices have already been adopted by a wide range of hospitals, including rural and small hospitals. Resource constraints as well as health care culture and structure remain barriers to broader diffusion. Some barriers may be addressed by technology and improvements in physical environments, but others relate to culture and may be more challenging to address. Active physician participation in medication‐related patient safety initiatives will be key to promoting further adoption of safe practices.
In November 1999, the Institute of Medicine released its landmark report entitled To Err Is Human: Building A Safer Health System.1 The report claimed that more than 1 million people in the United States suffer from preventable medical injuries each year and that as many as 98,000 people die annually in hospitals from medical errors. Although evidence‐based methods are available to prevent adverse events, there is concern that the current lack of standardization among hospitals implementing such safe practices has the potential to both diffuse and dilute efforts to improve patient safety.
To address this issue, the National Quality Forum (NQF) in 2003 released an evidence‐based consensus report that presented 30 safe practices for better health care with a recommendation that all be universally adopted.2 The purpose of this study is to use information collected from a voluntary patient safety program in Georgia3 and an Agency for Healthcare Research and Quality (AHRQ) reporting demonstration study4 to (1) describe the current statewide adoption rates for NQF medication safe practices and safety culture (Table 1), and (2) examine if hospital adoption varies by hospital size, ownership, and rural or urban location.
| NQF Safe Practice No. | Key Word | Full Description of Safe Practice |
|---|---|---|
| ||
| 1 | Culture of safety | Create a health care culture of safety. |
| 5 | Consultant pharmacists | Pharmacists should actively participate in the medication‐use process, including, at a minimum, being available for consultation with prescribers and reviewing medication orders. |
| 6 | Verbal orders | Verbal orders should be recorded whenever possible and immediately read back to the prescriber. |
| 7 | Abbreviations | Use of standardized abbreviations and dosage designations. |
| 9 | Information transfer | Ensure that care information, especially changes in orders and new diagnostic information, is transmitted to all providers. |
| 12 | CPOE adoption | Implement a computerized prescriber order entry system |
| 27 | Clean workspaces | Keep workspaces where medications are prepared clean, orderly, well lighted, and free of clutter, distraction, and noise. |
| 28 | Labeling and storage | Standardize the methods for labeling, packaging, and storing medications. |
| 29 | High‐alert medications | Identify all high alert drugs. |
| 30 | Unit dosing | Dispense medications in unit‐dose or, when appropriate, unit‐of‐use form whenever possible. |
METHODS
Setting and Exclusions
The Partnership for Health and Accountability (PHA), a voluntary and peer‐review‐protected statewide hospital patient safety program, was established in Georgia in 2001 under the administration of the Georgia Hospital Association. All 148 nonfederal adult acute care hospitals in the state of Georgia participate in some aspect of the initiative. This represents a broad cross section of hospital types nationwide, with 55% of the hospitals having fewer than 100 beds, 25% having 100‐299 beds, and 20% having more than 300 beds. Hospitals are almost evenly divided between urban (54%) and rural (46%) locations.
Survey Instruments
One component of the PHA program focuses on safe medication use (SMU) with a goal of reducing the frequency of medication‐related errors in acute care hospitals. In 2004 all active acute care hospital members of GHA were eligible to participate in the SMU self‐assessment, and all but 1 hospital (147 of 148 hospitals, 97.3%) completed the self‐assessment survey.
The SMU self‐assessment is a 99‐item survey that addresses error reporting and event capture, the prescribing process, order processing and dispensing, medication administration and monitoring, patient involvement, policy and administration, and practitioner education and development. For each item, hospitals report on a 1‐5 scale the current status of adoption, ranging from no discussion to full implementation.
A second component of the PHA program identifies critical organizational tactics and strategies required for a culture of safety. Once every 2 years, top and midlevel managers complete a Strategies for Leadership self‐assessment. Results from this survey are disseminated to member hospitals to promote a culture of safety. Regular audioconferences are held to network and share successful intervention strategies aimed at establishing free and open communication, improving organizational learning, and promoting nonpunitive reporting of adverse events. A total of 147 hospitals (97.3%) completed the 2003 Strategies for Leadership survey.
The Strategies for Leadership self‐assessment is a 75‐item survey that addresses 7 broad categories: top leadership priorities, strategic planning, nonpunitive environment, patient and community focus, information analysis, human resources, and work environment. Hospital managers describe current status using a scale ranging from 1 (no discussion) to 5 (> 90% implementation).
Several steps were used to create the final study measures. First, the SMU and Leadership survey questions were reviewed to see if they addressed 1 of the 10 NQF indicators under study (Table 1). Quantitative analysis was then used to eliminate, collapse, and/or confirm the grouping arrangement. Given the broad and nonspecific nature of create a culture of safety, domains from the Hospital Survey on Patient Safety Culture5 were used to classify specific aspects of safety culture. For the purposes of this study, 5 of the 12 domains were used to categorize hospital responses. The domains used were (1) feedback and communication about error, (2) frequency of reporting, (3) promoting a nonpunitive environment, (4) encouraging organizational learning and continuous improvement, and (5) maintaining safe staffing.
Mapping Survey Questions to Safe Practices
A subset (n = 57) of the SMU survey questions directly related to safe medication processes (ie, prescribing, transcribing, dispensing, administration, and monitoring) were selected for inclusion in the study (Fig. 1). A nonoverlapping subset of Leadership (n = 35) and SMU (n = 10) survey questions related to safety culture were also identified. Clinical members of the project team independently reviewed and mapped medication process survey questions to 1 of 9 NQF indicators of safe medication practices. Assignment was based on face validity and best fit with the intent of the NQF indicator. Social science team members mapped culture‐related survey questions to the NQF indicator create a health care culture of safety using the 5 domains of safety culture.5

Grouping Similar Questions
A Pearson correlation matrix was used to confirm the factor analysis and determine if multiple questions related to a single safe practice could be reduced to 1 composite measure. If analysis supported the use of a composite score, responses to similar questions at the hospital level were averaged, and the hospital's final average was the measure used for analyses. Finally, the project team reviewed the a priori mapping along with the results of the correlation and factor analyses and reached consensus on the final number and mapping scheme of survey questions to NQF safe practices. Of the original 45 culture‐of‐safety questions, 21 were used for this analysis, and of the original 57 safe medication process questions, 32 were used.
Data Analysis
Bivariate analyses using SPSS software were conducted to examine the association between hospital structural characteristics (urban or rural location, network affiliation, academic affiliation, bed size) and adoption of each NQF safe practice.
RESULTS
Medication Safety
Table 2 shows the overall rate of adoption by all hospitals of the safe practices related to medication use. Full implementation was defined as implementation in greater than 90% of the organization. There has been almost universal adoption of 3 safe practices: processes to standardize labeling and storage of medications (133 of 147, 90.5%), identification of high‐alert medications (119 of 147, 81.0%), and use of unit doses when appropriate (119 of 147, 81.0%). CPOE systems, on the other hand, had been implemented in fewer than 3% (4 of 147) of the hospitals by early 2004. The remaining 5 medication practices showed intermediate adoption (between 48.3% and 69.7%): ensuring information transfer, minimizing verbal orders, providing clean workspaces with minimal distractions, availability of consultant pharmacists, and minimizing abbreviations.
| NQF Safe Practice | Proportion of Hospitals Reporting > 90% Implementation | Association with Hospital Structural Characteristics* |
|---|---|---|
| ||
| #5 Consultant pharmacists | 52.0% | More likely in mid‐size hospitals |
| #6 Verbal orders | 63.3% | None |
| #7 Abbreviations | 48.3% | None |
| #9 Information transfer | 69.7% | None |
| #12 CPOE adoption | 2.7% | None |
| #27 Clean workspaces | 53.7% | Less likely in large hospitals |
| #28 Labeling and storage | 90.5% | None |
| #29 High‐alert medications | 81.0% | None |
| #30 Unit dosing | 81.0% | More likely in for‐profit hospitals |
Variation in Adoption by Hospital Characteristics
There was only limited variation in adoption by hospital characteristics as summarized in Table 2 and discussed in more detail below. For‐profit hospitals were most likely to have a unit dose medication distribution system in place (93.1% vs. 78.2%, P = .037). For‐profit hospitals were also more likely (83.1% vs. 58.4%, P = .004) to have fully implemented a policy to read back verbal orders. The likelihood of adopting a policy to eliminate verbal orders did not vary significantly by hospital characteristics. The prevalence of distractions was also seen as a problem for writing orders and medication administration, with the largest hospitals more frequently reporting this challenge (59.2% vs. 29.6%, P = .005). Midsize hospitals (100299 beds) were more likely than larger or smaller hospitals to report that a pharmacist reviewed and approved all nonemergency orders prior to dispensing. (76.3% vs. 45.0%, P = .001).
Barriers to Adoption of Medication Safe Practices
Ensuring that new prescribers had access to all currently prescribed medications, including both dose and frequency was a challenge for many hospitals. More than 30% of hospitals (45 of 147) did not have this capability consistently throughout the institution, and that capability did not vary by hospital size or geographic location. Although most hospitals (93 of 147, 63.3%) had a read‐back policy for verbal orders, only 36.1% of hospitals (53 of 147) had fully implemented a policy to eliminate or minimize the use of verbal orders. Two aspects of the medication preparation environment also appeared to be problematic for the surveyed hospitals: appropriate space for medication preparation and a distraction‐free environment. Only half the hospitals (74 of 147) reported that medications were prepared in an environment that minimized distractions, and 53.7% (79 of 147) reported that pharmacists were provided with sufficient space. Although more than 90% of hospitals reported that pharmacists were available for consultation even when the pharmacy was closed, fewer than half the hospitals (71 of 147, 48.3%) reported that pharmacists were involved on patient care units as a resource for clinical decision support. There also were gaps in the patient information available when preparing medications, in particular, pregnancy status (82 of 147, 55.8%) and medications prescribed before hospitalization (85 of 147, 57.8%). Fewer than half the hospitals (67 of 147, 45.5%) had fully implemented a policy to minimize use of dangerous abbreviations. Most hospitals (91 of 147, 61.9%), however, did report that they had methods in place to proactively review processes for communicating medication orders and then redesign if appropriate.
Safety Culture
Table 3 shows the self‐reported adoption of safety culture as defined by the Hospital Patient Safety Culture Survey5 domains. Hospital safety culture was highest in several areas related to nonpunitive policies. For example, the vast majority of hospitals reported that no disciplinary actions were taken against employees for nonmalicious errors, that a formal hospital‐wide nonpunitive policy for staff and employees was in place, and that the hospital had a user‐friendly and confidential error‐reporting system in place. A smaller proportion of hospitals (75 of 147, 51.0%) provided specific resources to support employees involved in error or sponsor unit visits by senior management to promote blame‐free discussion and reporting of errors (64 of 147, 43.5%). For‐profit hospitals (63.3% vs. 38.5%, P = .014) and small hospitals (49.2% vs. 18.5%, P = .004) were more likely to have unit visits by senior management. An even smaller minority of hospitals reported having used dedicated observers to catch errors as they occur (32 of 147, 21.8%) or that they provided direct incentives to caregivers for reporting errors (31 of 147, 21.1%).
| Safety of Culture Category | Specific Attribute | Overall Adoption | Association with Hospital Structural Characteristics* |
|---|---|---|---|
| |||
| Communication | Safety alert process | 59.9% | None |
| Frequency of reporting | Confidential error reporting system | 70.1% | None |
| Non‐punitive environment | Nonpunitive policies | 76.2% | None |
| Employee resources | 51.0% | None | |
| Unit visits | 43.5% | Unit visits more likely in small hospitals and for‐profit hospitals | |
| Organizational learning | Annual safety plans | 76.7% | None |
| Teams analyze errors | 72.1% | None | |
| Data analysis guides QI | 69.4% | Using data analysis to guide QI initiatives less likely in large hospitals | |
| Proactive evaluations before implementation | 44.9% | None | |
| Piloting processes | 42.9% | None | |
| Staffing | Adequate staffing ratios | 72.8% | None |
| Limited work hours | 57.6% | Limiting staff work hours less likely in large hospitals | |
In regard to organizational policies, three‐fourths of hospitals did have a patient safety plan that was reviewed annually by senior leadership. Most hospitals (106 of 147, 72.1%) used multidisciplinary teams to regularly analyze errors after they occurred and to identify possible system changes with no significant differences in adoption rates across hospital types. Most hospitals (102 of 147, 69.4%) also used data analysis to drive patient safety quality improvement efforts. Surprisingly, this was least common in the largest hospitals (48.1% vs. 74.2%, P = .008). Overall, hospitals were much less likely to have adopted the use of proactive techniques such as failure modes and effects analysis (FMEA) before implementation of major system changes or the piloting of new processes prior to implementation. Adoption rates for these activities were below 50% for all hospital demographic groups.
In terms of strategies for maintaining safe staffing levels, most hospitals reported they maintained safe staffing through adequate staffing ratios (107 of 147, 72.8%), whereas a smaller number (84 of 147, 57.1%) reported maintaining safe staffing by limiting work hours. Large hospitals were the least likely to limit work hours (33.3% vs. 63.2%, P = .005).
DISCUSSION
This is the first study to use existing data sources to characterize the current progress and barriers to further adoption of NQF safe practices and safety culture related to medication use in a statewide sample of hospitals. Several findings are notable. First, most of the hospitals surveyed had adopted 7 of 9 medication‐related NQF safe practices by 2004. Similar to findings from the earlier ISMP Safety Self‐Assessment for Hospitals, hospitals scored most highly on practices related to drug storage, packaging, and labeling and lowest on CPOE implementation.12 Results from the 2003 Leapfrog Group Quality and Safety Survey also found that only 3.7% of participating hospitals had fully implemented a CPOE system.13 Medication safe practices that directly affect physicians, such as verbal orders, standardized abbreviations, and access to relevant clinical information when prescribing had only intermediate adoption rates.
Most hospitals have developed policies around nonpunitive safety cultures, but fewer have adopted proactive error reduction systems. Safety culture is more difficult to measure than safe medication processes. A previous survey of Iowa hospitals assessed only whether hospitals reported progress toward creating a culture of safety.14 In this study we attempted to break down the broad concept of safety culture into specific actionable components. Three widely recognized components of a safe hospital culture are creating a nonpunitive environment for staff, using data to identify and analyze errors and system causes, and safe staffing levels.8 Most but not all surveyed hospitals had adopted these safety culture strategies. Other more resource‐intensive practices, such as unit visits by senior management and FMEA, were less likely to have been adopted. The adoption rates reported here for 20032004 are in most cases higher than those found in the 2000 ISMP survey, which may be explained by the more recent survey reported here and variations in question wording as well as response scoring.
Variations in adoption rates of NQF‐recommended safe practices generally were not explained by hospital characteristics such as ownership, size, or geographic location. Instead, barriers appear to be related to resource constraints as well as the ability of hospitals to directly control the specific safe practice. The ISMP survey found that hospital demographic factors explained only 3% of the variation in adoption, which is similar to our finding of few differences in adoption of safe practices by hospital type. Cost and health care culture may explain why certain safe practices remain less than fully adopted.12 Resource constraints may explain the lower adoption rate of several practices: CPOE, pharmacist consultation, and physical environment improvements. Other safe practices with lower adoption rates require active physician participation, for example, minimizing verbal orders, standardizing abbreviations, and ensuring accurate information transfers. Hospital‐based physicians can play a key role in advocating for effective processes to promote these practices.
Another general factor that distinguished highly adopted practices from less adopted practices was the extent to which reactive as opposed to proactive actions were required. Hospitals were more likely to report reactive policies such as reading back verbal orders than proactive policies to minimize verbal orders. Pharmacists were generally available for telephone consultation but in only half the hospitals were they available on the hospital units for consultation. A similar pattern was seen for culture of safety practices; systems were generally in place for nonpunitive error reporting, but a minority of hospitals had senior leadership making unit rounds or multidisciplinary teams proactively testing new systems to identify potential errors before they occur. Again, there is a leadership role that hospital‐based physicians can play as effective team builders for safety culture and as clinical leaders for improvement of medication processes. Much research has demonstrated the impact that a culture of safety can have on error reduction.811 As physicians who spend most of their clinical time directly on patient care units, hospital‐based physicians are uniquely positioned to promote positive changes in culture. Research on the impact of hospitalists on hospital costs and patient outcomes should be broadened to include an assessment of their impact on safety culture and error reduction.
This study had several limitations, the first being that it was based on voluntarily provided self‐assessment data. The surveys used in this project have been refined and administered over 3 years in a nonpunitive process improvement program with a consistently high participation rate. The hospital‐reported survey results have not been independently verified for accuracy, similar to most of the prior research in this area. The surveys measure management's perception of safety culture and do not assess actual employee perceptions of the safety culture on their particular units. Thus, although management may believe they are implementing policies to create a nonpunitive environment, actual assessments of employees' views are needed to confirm this. Because the study was based on previously collected data, several steps were used to map the existing questions to NQF safe practices. Given the broad nature of the NQF topics, at least 1 relevant survey question was identified for each of the medication‐related safe practices. When more than 1 question was judged to be relevant, the responses were averaged. The survey was limited to adult acute care hospitals in Georgia, which may not be nationally representative, and federal and Veterans Administration hospitals were not included. However, Georgia has a relatively high proportion of smaller rural hospitals and offers interesting baseline data on similar rates of adoption of safe practices in rural and smaller hospitals compared with that in urban hospitals. Because we were using previously collected surveys, we could only look at the adoption of selected safe practices. Further work is needed to look at the adoption of other safe practices.
In summary, it is encouraging that the most studied NQF‐recommended safe practices have already been adopted by a wide range of hospitals, including rural and small hospitals. Resource constraints as well as health care culture and structure remain barriers to broader diffusion. Some barriers may be addressed by technology and improvements in physical environments, but others relate to culture and may be more challenging to address. Active physician participation in medication‐related patient safety initiatives will be key to promoting further adoption of safe practices.
- Kohn LT,Corrigan JM, andDonaldson MS, eds.To Err Is Human: Building a Safer Health System: A Report from the Committee on Quality of Healthcare in America.Institute of Medicine,National Academy of Sciences.Washington, DC:National Academy Press,1999.
- The National Quality Forum.Safe practices for better healthcare: a consensus report. NQF publication no. NQFCR‐05‐03;2003.
- Georgia Hospital Association. Available at: http://www.gha.org.
- ,,.Voluntary hospital coalitions to promote patient safety: why, how and can they work? In:Advances in Patient Safety: From Research to Implementation.Rockville, MD:AHRQ;2005.
- Agency for Healthcare Research and Quality (AHRQ).The Hospital Survey on Patient Safety Toolkit 2004. Sponsored by the Medical Errors Workgroup of the Quality Interagency Coordination Task Force (QuIC), developed by Westat.Rockville, MD:AHRQ;2004.
- Vaughan, Diane.1996.The Challenger launch decision: risky technology, culture, and deviance at NASA.Chicago:University of Chicago Press.
- ,,,,,.Overcoming barriers to adopting and implementing computerized physician order entry systems in U.S. hospitals.Health Aff.2004;23(4):184–190.
- ,.Safety culture assessment: a tool for improving patient safety in healthcare organizations.Qual Saf in Health Care.2003;12(suppl. 2):17–23.
- ,,.Error, stress, and teamwork in medicine and aviation: cross‐sectional surveys.Hum Perf Extrem Environ.2001;6:6–11.
- ,,,.Using a multihospital survey to examine the safety culture.Jt Comm J Qual Saf.2004;30:125–32.
- ,,,.A review of the literature examining linkages between organizational factors, medical errors, and patient safety.Med Care Res Rev.2004;61:3–37.
- ,,,,,.Findings from the ISMP medication safety self‐assessment for hospitals.Jt Comm J Qual Saf.2003;29:586–597.
- ,.Hospital implementation of computerized provider order entry systems: results from the 2003 Leapfrog Group Quality and Safety Survey.J Healthc Inf Manag.2005;19(4):55–65.
- ,,,,.National Quality Forum 30 safe practices: priority and progress in Iowa hospitals.Am J Med Qual.2006;21:101–108.
- Kohn LT,Corrigan JM, andDonaldson MS, eds.To Err Is Human: Building a Safer Health System: A Report from the Committee on Quality of Healthcare in America.Institute of Medicine,National Academy of Sciences.Washington, DC:National Academy Press,1999.
- The National Quality Forum.Safe practices for better healthcare: a consensus report. NQF publication no. NQFCR‐05‐03;2003.
- Georgia Hospital Association. Available at: http://www.gha.org.
- ,,.Voluntary hospital coalitions to promote patient safety: why, how and can they work? In:Advances in Patient Safety: From Research to Implementation.Rockville, MD:AHRQ;2005.
- Agency for Healthcare Research and Quality (AHRQ).The Hospital Survey on Patient Safety Toolkit 2004. Sponsored by the Medical Errors Workgroup of the Quality Interagency Coordination Task Force (QuIC), developed by Westat.Rockville, MD:AHRQ;2004.
- Vaughan, Diane.1996.The Challenger launch decision: risky technology, culture, and deviance at NASA.Chicago:University of Chicago Press.
- ,,,,,.Overcoming barriers to adopting and implementing computerized physician order entry systems in U.S. hospitals.Health Aff.2004;23(4):184–190.
- ,.Safety culture assessment: a tool for improving patient safety in healthcare organizations.Qual Saf in Health Care.2003;12(suppl. 2):17–23.
- ,,.Error, stress, and teamwork in medicine and aviation: cross‐sectional surveys.Hum Perf Extrem Environ.2001;6:6–11.
- ,,,.Using a multihospital survey to examine the safety culture.Jt Comm J Qual Saf.2004;30:125–32.
- ,,,.A review of the literature examining linkages between organizational factors, medical errors, and patient safety.Med Care Res Rev.2004;61:3–37.
- ,,,,,.Findings from the ISMP medication safety self‐assessment for hospitals.Jt Comm J Qual Saf.2003;29:586–597.
- ,.Hospital implementation of computerized provider order entry systems: results from the 2003 Leapfrog Group Quality and Safety Survey.J Healthc Inf Manag.2005;19(4):55–65.
- ,,,,.National Quality Forum 30 safe practices: priority and progress in Iowa hospitals.Am J Med Qual.2006;21:101–108.
Copyright © 2007 Society of Hospital Medicine