User login
Pyogenic liver abscess
A 58-year-old woman presents with fever, chills, vomiting, and right-upperquadrant abdominal pain. She has had diarrhea for several days and has lost 15 lb over the last 6 weeks. Six months ago, she took a cruise through the Panama Canal to Nicaragua, Costa Rica, Mexico, and El Salvador.
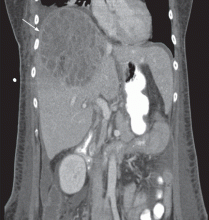
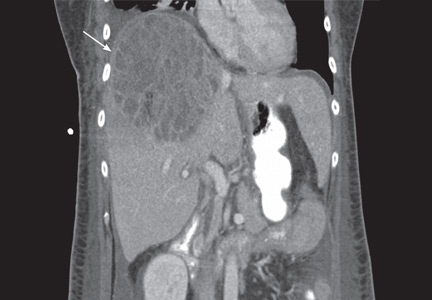
The patient undergoes CT-guided liver biopsy with drain placement. Cultures grow Klebsiella pneumoniae, and she is started on intravenous piperacillin-tazobactam for 4 weeks, followed by 4 weeks of oral ciprofloxacin.
Follow-up evaluation at 4 weeks and at 8 weeks shows improvement in the patient’s condition, with CT of the abdomen and pelvis showing a gradual decrease in the size of the abscess.
INCREASING PREVALENCE
K pneumoniae has emerged as the most common organism seen in pyogenic liver abscess.1 Initially seen in Taiwan in the 1980s, K pneumoniae liver abscess is becoming more common in the United States.2 Diabetes and impaired fasting glucose have both been implicated as potential risk factors, but the condition is also seen in nondiabetic patients.3 Although pyogenic liver abscess is commonly a sequela of biliary disease, K pneumoniae liver abscess is more often cryptogenic. 1 Clinical manifestations usually include fever, abdominal pain in the right upper quadrant, nausea, and vomiting.
In this patient, no clear relationship was established between her travel and her illness.
GREATER RISK OF SPREAD
K pneumoniae liver abscess is more likely to spread than polymicrobial liver abscess.3 It is associated with endophthalmitis, meningitis, brain abscess, and septic pulmonary embolism.3 Diabetic patients are particularly susceptible to metastatic foci.3 The reason is not well understood, but poor glycemic control leading to impaired neutrophil phagocytosis is thought to play a role.4
DIAGNOSIS AND TREATMENT
Liver abscesses are associated with elevated alkaline phosphatase levels, hyperbilirubinemia, leukocytosis, hypoalbuminemia, and anemia. As bacteremia is often seen with K pneumoniae liver abscess, blood cultures should be obtained.1 Imaging studies should include right-upper-quadrant ultrasonography if suspicion is high for concomitant biliary disease, and also CT with intravenous contrast to better quantify the dimensions of the abscess.5
Treatment includes empiric parenteral antibiotics and percutaneous drainage. In addition, culture of purulent material for aerobic and anaerobic organisms helps guide antibiotic treatment.
The antibiotic regimen should consist of a first- or third-generation beta-lactamase inhibitor, with or without an aminoglycoside.3 Patients unable to tolerate beta-lactam antibiotics can be given a fluoroquinolone.
The results of cultures and determination of antibiotic sensitivities help to further modifiy antibiotic therapy. Antibiotic therapy may be needed for 4 to 6 weeks. Parenteral antibiotics are recommended initially, and if a patient responds to therapy, treatment can be switched to oral antibiotics to complete the course of treatment.
Although antibiotics with percutaneous drainage are the recommended course of therapy, surgical drainage is sometimes necessary and is best done with the input of a hepatobiliary surgeon. Patients with abscesses larger than 5 cm who had surgical drainage had better clinical outcomes than those who had percutaneous drainage,6 but monitoring the response to antibiotics and the patient’s clinical course is very important when determining the need for emergency surgical intervention vs percutaneous drainage.6
Follow-up imaging is necessary to evaluate the response to therapy, to determine the continued need for antibiotics, and to assess for any further need for drainage.
- Pope JV, Teich DL, Clardy P, McGillicuddy DC. Klebsiella pneumoniae liver abscess: an emerging problem in North America. J Emerg Med 2008; Epub ahead of print.
- Frazee BW, Hansen S, Lambert L. Invasive infection with hypermucoviscous Klebsiella pneumoniae: multiple cases presenting to a single emergency department in the United States. Ann Emerg Med 2009; 53:639–642.
- Lee SS, Chen YS, Tsai HC, et al. Predictors of septic metastatic infection and mortality among patients with Klebsiella pneumoniae liver abscess. Clin Infect Dis 2008; 47:642–650.
- Lin JC, Siu LK, Fung CP, et al. Impaired phagocytosis of capsular serotypes K1 or K2 Klebsiella pneumoniae in type 2 diabetes mellitus patients with poor glycemic control. J Clin Endocrinol Metab 2006; 91:3084–3087.
- Golia P, Sadler M. Pyogenic liver abscess: Klebsiella as an emerging pathogen. Emerg Radiol 2006; 13:87–88.
- Tan YM, Chung AY, Chow PK, et al. An appraisal of surgical and percutaneous drainage for pyogenic liver abscesses larger than 5 cm. Ann Surg 2005; 241:485–490.
A 58-year-old woman presents with fever, chills, vomiting, and right-upperquadrant abdominal pain. She has had diarrhea for several days and has lost 15 lb over the last 6 weeks. Six months ago, she took a cruise through the Panama Canal to Nicaragua, Costa Rica, Mexico, and El Salvador.
The patient undergoes CT-guided liver biopsy with drain placement. Cultures grow Klebsiella pneumoniae, and she is started on intravenous piperacillin-tazobactam for 4 weeks, followed by 4 weeks of oral ciprofloxacin.
Follow-up evaluation at 4 weeks and at 8 weeks shows improvement in the patient’s condition, with CT of the abdomen and pelvis showing a gradual decrease in the size of the abscess.
INCREASING PREVALENCE
K pneumoniae has emerged as the most common organism seen in pyogenic liver abscess.1 Initially seen in Taiwan in the 1980s, K pneumoniae liver abscess is becoming more common in the United States.2 Diabetes and impaired fasting glucose have both been implicated as potential risk factors, but the condition is also seen in nondiabetic patients.3 Although pyogenic liver abscess is commonly a sequela of biliary disease, K pneumoniae liver abscess is more often cryptogenic. 1 Clinical manifestations usually include fever, abdominal pain in the right upper quadrant, nausea, and vomiting.
In this patient, no clear relationship was established between her travel and her illness.
GREATER RISK OF SPREAD
K pneumoniae liver abscess is more likely to spread than polymicrobial liver abscess.3 It is associated with endophthalmitis, meningitis, brain abscess, and septic pulmonary embolism.3 Diabetic patients are particularly susceptible to metastatic foci.3 The reason is not well understood, but poor glycemic control leading to impaired neutrophil phagocytosis is thought to play a role.4
DIAGNOSIS AND TREATMENT
Liver abscesses are associated with elevated alkaline phosphatase levels, hyperbilirubinemia, leukocytosis, hypoalbuminemia, and anemia. As bacteremia is often seen with K pneumoniae liver abscess, blood cultures should be obtained.1 Imaging studies should include right-upper-quadrant ultrasonography if suspicion is high for concomitant biliary disease, and also CT with intravenous contrast to better quantify the dimensions of the abscess.5
Treatment includes empiric parenteral antibiotics and percutaneous drainage. In addition, culture of purulent material for aerobic and anaerobic organisms helps guide antibiotic treatment.
The antibiotic regimen should consist of a first- or third-generation beta-lactamase inhibitor, with or without an aminoglycoside.3 Patients unable to tolerate beta-lactam antibiotics can be given a fluoroquinolone.
The results of cultures and determination of antibiotic sensitivities help to further modifiy antibiotic therapy. Antibiotic therapy may be needed for 4 to 6 weeks. Parenteral antibiotics are recommended initially, and if a patient responds to therapy, treatment can be switched to oral antibiotics to complete the course of treatment.
Although antibiotics with percutaneous drainage are the recommended course of therapy, surgical drainage is sometimes necessary and is best done with the input of a hepatobiliary surgeon. Patients with abscesses larger than 5 cm who had surgical drainage had better clinical outcomes than those who had percutaneous drainage,6 but monitoring the response to antibiotics and the patient’s clinical course is very important when determining the need for emergency surgical intervention vs percutaneous drainage.6
Follow-up imaging is necessary to evaluate the response to therapy, to determine the continued need for antibiotics, and to assess for any further need for drainage.
A 58-year-old woman presents with fever, chills, vomiting, and right-upperquadrant abdominal pain. She has had diarrhea for several days and has lost 15 lb over the last 6 weeks. Six months ago, she took a cruise through the Panama Canal to Nicaragua, Costa Rica, Mexico, and El Salvador.
The patient undergoes CT-guided liver biopsy with drain placement. Cultures grow Klebsiella pneumoniae, and she is started on intravenous piperacillin-tazobactam for 4 weeks, followed by 4 weeks of oral ciprofloxacin.
Follow-up evaluation at 4 weeks and at 8 weeks shows improvement in the patient’s condition, with CT of the abdomen and pelvis showing a gradual decrease in the size of the abscess.
INCREASING PREVALENCE
K pneumoniae has emerged as the most common organism seen in pyogenic liver abscess.1 Initially seen in Taiwan in the 1980s, K pneumoniae liver abscess is becoming more common in the United States.2 Diabetes and impaired fasting glucose have both been implicated as potential risk factors, but the condition is also seen in nondiabetic patients.3 Although pyogenic liver abscess is commonly a sequela of biliary disease, K pneumoniae liver abscess is more often cryptogenic. 1 Clinical manifestations usually include fever, abdominal pain in the right upper quadrant, nausea, and vomiting.
In this patient, no clear relationship was established between her travel and her illness.
GREATER RISK OF SPREAD
K pneumoniae liver abscess is more likely to spread than polymicrobial liver abscess.3 It is associated with endophthalmitis, meningitis, brain abscess, and septic pulmonary embolism.3 Diabetic patients are particularly susceptible to metastatic foci.3 The reason is not well understood, but poor glycemic control leading to impaired neutrophil phagocytosis is thought to play a role.4
DIAGNOSIS AND TREATMENT
Liver abscesses are associated with elevated alkaline phosphatase levels, hyperbilirubinemia, leukocytosis, hypoalbuminemia, and anemia. As bacteremia is often seen with K pneumoniae liver abscess, blood cultures should be obtained.1 Imaging studies should include right-upper-quadrant ultrasonography if suspicion is high for concomitant biliary disease, and also CT with intravenous contrast to better quantify the dimensions of the abscess.5
Treatment includes empiric parenteral antibiotics and percutaneous drainage. In addition, culture of purulent material for aerobic and anaerobic organisms helps guide antibiotic treatment.
The antibiotic regimen should consist of a first- or third-generation beta-lactamase inhibitor, with or without an aminoglycoside.3 Patients unable to tolerate beta-lactam antibiotics can be given a fluoroquinolone.
The results of cultures and determination of antibiotic sensitivities help to further modifiy antibiotic therapy. Antibiotic therapy may be needed for 4 to 6 weeks. Parenteral antibiotics are recommended initially, and if a patient responds to therapy, treatment can be switched to oral antibiotics to complete the course of treatment.
Although antibiotics with percutaneous drainage are the recommended course of therapy, surgical drainage is sometimes necessary and is best done with the input of a hepatobiliary surgeon. Patients with abscesses larger than 5 cm who had surgical drainage had better clinical outcomes than those who had percutaneous drainage,6 but monitoring the response to antibiotics and the patient’s clinical course is very important when determining the need for emergency surgical intervention vs percutaneous drainage.6
Follow-up imaging is necessary to evaluate the response to therapy, to determine the continued need for antibiotics, and to assess for any further need for drainage.
- Pope JV, Teich DL, Clardy P, McGillicuddy DC. Klebsiella pneumoniae liver abscess: an emerging problem in North America. J Emerg Med 2008; Epub ahead of print.
- Frazee BW, Hansen S, Lambert L. Invasive infection with hypermucoviscous Klebsiella pneumoniae: multiple cases presenting to a single emergency department in the United States. Ann Emerg Med 2009; 53:639–642.
- Lee SS, Chen YS, Tsai HC, et al. Predictors of septic metastatic infection and mortality among patients with Klebsiella pneumoniae liver abscess. Clin Infect Dis 2008; 47:642–650.
- Lin JC, Siu LK, Fung CP, et al. Impaired phagocytosis of capsular serotypes K1 or K2 Klebsiella pneumoniae in type 2 diabetes mellitus patients with poor glycemic control. J Clin Endocrinol Metab 2006; 91:3084–3087.
- Golia P, Sadler M. Pyogenic liver abscess: Klebsiella as an emerging pathogen. Emerg Radiol 2006; 13:87–88.
- Tan YM, Chung AY, Chow PK, et al. An appraisal of surgical and percutaneous drainage for pyogenic liver abscesses larger than 5 cm. Ann Surg 2005; 241:485–490.
- Pope JV, Teich DL, Clardy P, McGillicuddy DC. Klebsiella pneumoniae liver abscess: an emerging problem in North America. J Emerg Med 2008; Epub ahead of print.
- Frazee BW, Hansen S, Lambert L. Invasive infection with hypermucoviscous Klebsiella pneumoniae: multiple cases presenting to a single emergency department in the United States. Ann Emerg Med 2009; 53:639–642.
- Lee SS, Chen YS, Tsai HC, et al. Predictors of septic metastatic infection and mortality among patients with Klebsiella pneumoniae liver abscess. Clin Infect Dis 2008; 47:642–650.
- Lin JC, Siu LK, Fung CP, et al. Impaired phagocytosis of capsular serotypes K1 or K2 Klebsiella pneumoniae in type 2 diabetes mellitus patients with poor glycemic control. J Clin Endocrinol Metab 2006; 91:3084–3087.
- Golia P, Sadler M. Pyogenic liver abscess: Klebsiella as an emerging pathogen. Emerg Radiol 2006; 13:87–88.
- Tan YM, Chung AY, Chow PK, et al. An appraisal of surgical and percutaneous drainage for pyogenic liver abscesses larger than 5 cm. Ann Surg 2005; 241:485–490.
Does vitamin D deficiency play a role in the pathogenesis of chronic heart failure? Do supplements improve survival?
Vitamin D deficiency may play a role in the pathogenesis of chronic heart failure, but whether giving patients supplements to raise their vitamin D levels into the normal range improves their survival is not clear.
ASSOCIATION BETWEEN VITAMIN D DEFICIENCY AND OTHER DISORDERS
In the mid-17th century, Whistler and Glisson independently described rickets as a severe bone-deforming disease characterized by growth retardation, bending of the spine, deformities of the legs, and weak and toneless muscles. Histologically, rickets is characterized by impaired mineralization of the cartilage in the epiphyseal growth plates in children. In 1919, Sir Edward Mellanby identified vitamin D deficiency as the cause.
Osteomalacia, another disease caused by vitamin D deficiency, is a disorder of mineralization of newly formed bone matrix in adults. Vitamin D, therefore, has well-known roles in maintaining bone health and calcium and phosphorus homeostasis.
In addition, vitamin D deficiency has been shown in recent years to be associated with myocardial dysfunction.1,2
VITAMIN D METABOLISM IS COMPLEX
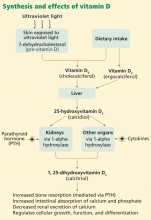
In skin exposed to ultraviolet B light, the provitamin 7-dehydrocholesterol is converted to vitamin D3 (cholecalciferol). Vitamin D3 is also obtained from dietary sources. However, many scientists consider vitamin D more a hormone than a classic vitamin, as adequate exposure to sunlight may negate the need for dietary supplements.
The active form of vitamin D is synthesized by hydroxylation in the liver and kidney. In the liver, hepatic enzymes add a hydroxyl (OH) group to vitamin D3, changing it to 25-hydroxyvitamin D3. In the kidney, 25-hydroxyvitamin D3 receives another hydroxyl group, converting it to the biologically active metabolite 1,25-dihydroxyvitamin D3 (calcitriol). This renal hydroxylation is via 1-alpha-hydroxylase activity and is directly under control of parathyroid hormone (PTH), and indirectly under control of the serum concentrations of calcium.
Interestingly, a number of different organ cells, including cardiomyocytes, also express 1-alpha-hydroxylase and therefore also convert 25-hydroxyvitamin D3 to 1,25-dihydroxyvitamin D3. Unlike the renal hydroxylation, this extrarenal process depends on cytokine activation and on serum levels of 25-hydroxyvitamin D3.3 Low levels of 25-hydroxyvitamin D3 lead to alterations in cellular control over growth, differentiation, and function.
The active form of vitamin D is transported protein-bound in the blood to various target organs, where it is delivered in free form to cells. Specific nuclear receptor proteins are found in many organs not classically considered target organs for vitamin D, including the skin, brain, skeletal muscles, cardiomyocytes, vascular endothelial cells, circulating monocytes, and activated B and T lymphocytes. Vitamin D plays a significant role in the autocrine and paracrine regulation of cellular function, growth, and differentiation in various organs.3
MOST HEART FAILURE PATIENTS HAVE LOW VITAMIN D LEVELS
More than 40% of men and 50% of women in the United States have low vitamin D levels (< 30 ng/mL [75 nmol/L])—and low levels in adults are associated with both coronary artery disease and heart failure.4 Most patients with heart failure have low levels.5,6 Therefore, screening for vitamin D deficiency in patients with heart failure is appropriate and encouraged.
Low vitamin D levels carry a poor prognosis. Pilz et al5 measured baseline 25-hydroxyvitamin D3 levels in 3,299 patients referred for elective coronary angiography and followed them prospectively for a median of 7.7 years. Even after adjustment for cardiac risk factors, patients who had low 25-hydroxyvitamin D3 levels were more likely to die of heart failure or sudden cardiac death than patients with normal levels.
Boxer et al7 found an association between low 25-hydroxyvitamin D3 levels and low exercise capacity and frailty in patients with systolic heart failure.
LOW VITAMIN D CONTRIBUTES TO THE PATHOGENESIS OF HEART FAILURE
In recent years, ideas about the pathophysiology of heart failure have expanded from a purely hemodynamic view to a more complex concept involving inflammatory cytokines and neurohormonal overactivation.8
Animal studies first showed vitamin D to inhibit the renin-angiotensin-aldosterone system, activation of which contributes to the salt and water retention seen in heart failure.4,9
In addition, vitamin D has a number of effects that should help prevent hypertension, an important risk factor for heart failure. It protects the kidney by suppressing the reninangiotensin-aldosterone system, prevents secondary hyperparathyroidism and its effects on vascular stiffness, prevents insulin resistance, and suppresses inflammation, which protects vascular endothelial cells.10
The first studies to show a connection between cardiovascular homeostasis and vitamin D status were in animal models more than 20 years ago. These studies showed that 1,25-dihydroxyvitamin D3 acts directly on cardiomyocyte vitamin D receptors, which are widely distributed throughout the body in several tissue types.11
Excess PTH levels associated with low vitamin D levels may play a role in cardiovascular disease by leading to cardiomyocyte hypertrophy and interstitial fibrosis of the heart.12 Animal studies have found that vitamin D suppresses cardiac hypertrophy.13 Vitamin D also plays a role in cardiomyocyte relaxation and may abrogate the hypercontractility associated with diastolic heart failure.2,14
Currently, it is unclear whether vitamin D deficiency is a causative risk factor for heart failure or simply a reflection of the poor functional status of patients with heart failure that leads to decreased exposure to sunlight. This debate will continue until further randomized clinical trials address this association.
VITAMIN D AND HEART TRANSPLANTATION
One would expect that patients with endstage organ failure would be at high risk of vitamin D deficiency because of limited sunlight exposure. However, few studies have evaluated the role of this vitamin in heart transplant recipients.
Stein and colleagues15 measured serum 25-hydroxyvitamin D3 immediately after transplantation in 46 heart and 23 liver transplant recipients. Levels were low in both types of transplant recipients, but liver transplant recipients had significantly lower levels than heart transplant patients. This could be explained by malabsorption and impaired synthesis of 25-hydroxyvitamin D3 in end-stage liver disease.
Also, an important point is that osteoporosis is prevalent in postcardiac transplant patients and likely related to the immunosuppressive agents these patients must take.16 In theory, increasing the body’s stores of vitamin D during the pretransplant period could lower the rate of bone loss and osteoporosis after cardiac transplantation.
Further investigation is needed to determine whether restoring adequate levels of vitamin D at the time of or after transplantation prevents graft rejection or improves survival.
VITAMIN D SUPPLEMENTATION AND SURVIVAL IN HEART FAILURE
The best laboratory test to assess vitamin D levels is the serum 25-hydroxyvitamin D3 concentration. A level between 20 and 30 ng/mL (50–75 nmol/L) is considered insufficient, and a level below 20 ng/mL (50 nmol/L) represents vitamin D deficiency.4,5,11
Vitamin D insufficiency is typically treated with 800 to 1,000 IU of vitamin D3 daily, whereas deficiency requires 50,000 IU of vitamin D3 weekly for 6 to 8 weeks, followed by 800 to 1,000 IU daily.19 The goal of therapy is to increase the serum 25-hydroxyvitamin D3 level above 30 ng/mL.19
Currently, it is unknown if vitamin D supplementation improves survival in heart failure. We recommend testing for vitamin D deficiency in all patients with heart failure and treating them as described above. For heart failure patients that are not deficient, daily intake of 800 to 1,000 IU of vitamin D is reasonable. Our review underscores the need for more studies to evaluate the efficacy of vitamin D replacement in improving survival in patients with heart failure.
KEY POINTS
- Screening for vitamin D deficiency in patients with heart failure is appropriate and encouraged.
- Vitamin D deficiency is common in patients with heart failure and in heart transplant recipients.
- In theory, achieving adequate levels of vitamin D would have a beneficial effect on patients with heart failure.
- Randomized controlled trials are needed to determine if vitamin D supplementation confers a survival benefit in patients with heart failure who have deficient vitamin D levels.
- Nibbelink KA, Tishkoff DX, Hershey SD, Rahman A, Simpson RU. 1,25(OH)2-vitamin D3 actions on cell proliferation, size, gene expression, and receptor localization, in the HL-1 cardiac myocyte. J Steroid Biochem Mol Biol 2007; 103:533–537.
- Tishkoff DX, Nibbelink KA, Holmberg KH, Dandu L, Simpson RU. Functional vitamin D receptor (VDR) in the t-tubules of cardiac myocytes: VDR knockout cardiomyocyte contractility. Endocrinology 2008; 149:558–564.
- Peterlik M, Cross HS. Vitamin D and calcium deficits predispose for multiple chronic diseases. Eur J Clin Invest 2005; 35:290–304.
- Kim DH, Sabour S, Sagar UN, Adams S, Whellan DJ. Prevalence of hypovitaminosis D in cardiovascular diseases (from the National Health and Nutrition Examination Survey 2001 to 2004). Am J Cardiol 2008; 102:1540–1544.
- Pilz S, März W, Wellnitz B, et al. Association of vitamin D deficiency with heart failure and sudden cardiac death in a large cross-sectional study of patients referred for coronary angiography. J Clin Endocrinol Metab 2008; 93:3927–3935.
- Zittermann A, Schleithoff SS, Koerfer R. Vitamin D insufficiency in congestive heart failure: why and what to do about it? Heart Fail Rev 2006; 11:25–33.
- Boxer RS, Dauser DA, Walsh SJ, Hager WD, Kenny AM. The association between vitamin D and inflammation with the 6-minute walk and frailty in patients with heart failure. J Am Geriatr Soc 2008; 56:454–461.
- Schleithoff SS, Zittermann A, Tenderich G, Berthold HK, Stehle P, Koerfer R. Vitamin D supplementation improves cytokine profiles in patients with congestive heart failure: a double-blind, randomized, placebo-controlled trial. Am J Clin Nutr 2006; 83:754–759.
- Li YC, Kong J, Wei M, Chen ZF, Liu SQ, Cao LP. 1,25-Dihydroxyvitamin D(3) is a negative endocrine regulator of the renin-angiotensin system. J Clin Invest 2002; 110:229–238.
- Pilz S, Tomaschitz A, Ritz E, Pieber TR; Medscape. Vitamin D status and arterial hypertension: a systematic review. Nat Rev Cardiol 2009; 6:621–630.
- Nemerovski CW, Dorsch MP, Simpson RU, Bone HG, Aaronson KD, Bleske BE. Vitamin D and cardiovascular disease. Pharmacotherapy 2009; 29:691–708.
- Rostand SG, Drüeke TB. Parathyroid hormone, vitamin D, and cardiovascular disease in chronic renal failure. Kidney Int 1999; 56:383–392.
- Wu J, Garami M, Cheng T, Gardner DG. 1,25(OH)2 vitamin D3, and retinoic acid antagonize endothelin-stimulated hypertrophy of neonatal rat cardiac myocytes. J Clin Invest 1996; 97:1577–1588.
- Green JJ, Robinson DA, Wilson GE, Simpson RU, Westfall MV. Calcitriol modulation of cardiac contractile performance via protein kinase C. J Mol Cell Cardiol 2006; 41:350–359.
- Stein EM, Cohen A, Freeby M, et al. Severe vitamin D deficiency among heart and liver transplant recipients. Clin Transplant 2009; (Epub ahead of print)
- Shane E, Rivas M, McMahon DJ, et al. Bone loss and turnover after cardiac transplantation. J Clin Endocrinol Metab 1997; 82:1497–1506.
- Norman AW, Bouillon R, Whiting SJ, Vieth R, Lips P. 13th Workshop consensus for vitamin D nutritional guidelines. J Steroid Biochem Mol Biol 2007; 103:204–205.
- Vieth R, Bischoff-Ferrari H, Boucher BJ, et al. The urgent need to recommend an intake of vitamin D that is effective. Am J Clin Nutr 2007; 85:649–650.
- Dawson-Hughes B, Heaney RP, Holick MF, Lips P, Meunier PJ, Vieth R. Estimates of optimal vitamin D status. Osteoporos Int 2005; 16:713–716.
Vitamin D deficiency may play a role in the pathogenesis of chronic heart failure, but whether giving patients supplements to raise their vitamin D levels into the normal range improves their survival is not clear.
ASSOCIATION BETWEEN VITAMIN D DEFICIENCY AND OTHER DISORDERS
In the mid-17th century, Whistler and Glisson independently described rickets as a severe bone-deforming disease characterized by growth retardation, bending of the spine, deformities of the legs, and weak and toneless muscles. Histologically, rickets is characterized by impaired mineralization of the cartilage in the epiphyseal growth plates in children. In 1919, Sir Edward Mellanby identified vitamin D deficiency as the cause.
Osteomalacia, another disease caused by vitamin D deficiency, is a disorder of mineralization of newly formed bone matrix in adults. Vitamin D, therefore, has well-known roles in maintaining bone health and calcium and phosphorus homeostasis.
In addition, vitamin D deficiency has been shown in recent years to be associated with myocardial dysfunction.1,2
VITAMIN D METABOLISM IS COMPLEX
In skin exposed to ultraviolet B light, the provitamin 7-dehydrocholesterol is converted to vitamin D3 (cholecalciferol). Vitamin D3 is also obtained from dietary sources. However, many scientists consider vitamin D more a hormone than a classic vitamin, as adequate exposure to sunlight may negate the need for dietary supplements.
The active form of vitamin D is synthesized by hydroxylation in the liver and kidney. In the liver, hepatic enzymes add a hydroxyl (OH) group to vitamin D3, changing it to 25-hydroxyvitamin D3. In the kidney, 25-hydroxyvitamin D3 receives another hydroxyl group, converting it to the biologically active metabolite 1,25-dihydroxyvitamin D3 (calcitriol). This renal hydroxylation is via 1-alpha-hydroxylase activity and is directly under control of parathyroid hormone (PTH), and indirectly under control of the serum concentrations of calcium.
Interestingly, a number of different organ cells, including cardiomyocytes, also express 1-alpha-hydroxylase and therefore also convert 25-hydroxyvitamin D3 to 1,25-dihydroxyvitamin D3. Unlike the renal hydroxylation, this extrarenal process depends on cytokine activation and on serum levels of 25-hydroxyvitamin D3.3 Low levels of 25-hydroxyvitamin D3 lead to alterations in cellular control over growth, differentiation, and function.
The active form of vitamin D is transported protein-bound in the blood to various target organs, where it is delivered in free form to cells. Specific nuclear receptor proteins are found in many organs not classically considered target organs for vitamin D, including the skin, brain, skeletal muscles, cardiomyocytes, vascular endothelial cells, circulating monocytes, and activated B and T lymphocytes. Vitamin D plays a significant role in the autocrine and paracrine regulation of cellular function, growth, and differentiation in various organs.3
MOST HEART FAILURE PATIENTS HAVE LOW VITAMIN D LEVELS
More than 40% of men and 50% of women in the United States have low vitamin D levels (< 30 ng/mL [75 nmol/L])—and low levels in adults are associated with both coronary artery disease and heart failure.4 Most patients with heart failure have low levels.5,6 Therefore, screening for vitamin D deficiency in patients with heart failure is appropriate and encouraged.
Low vitamin D levels carry a poor prognosis. Pilz et al5 measured baseline 25-hydroxyvitamin D3 levels in 3,299 patients referred for elective coronary angiography and followed them prospectively for a median of 7.7 years. Even after adjustment for cardiac risk factors, patients who had low 25-hydroxyvitamin D3 levels were more likely to die of heart failure or sudden cardiac death than patients with normal levels.
Boxer et al7 found an association between low 25-hydroxyvitamin D3 levels and low exercise capacity and frailty in patients with systolic heart failure.
LOW VITAMIN D CONTRIBUTES TO THE PATHOGENESIS OF HEART FAILURE
In recent years, ideas about the pathophysiology of heart failure have expanded from a purely hemodynamic view to a more complex concept involving inflammatory cytokines and neurohormonal overactivation.8
Animal studies first showed vitamin D to inhibit the renin-angiotensin-aldosterone system, activation of which contributes to the salt and water retention seen in heart failure.4,9
In addition, vitamin D has a number of effects that should help prevent hypertension, an important risk factor for heart failure. It protects the kidney by suppressing the reninangiotensin-aldosterone system, prevents secondary hyperparathyroidism and its effects on vascular stiffness, prevents insulin resistance, and suppresses inflammation, which protects vascular endothelial cells.10
The first studies to show a connection between cardiovascular homeostasis and vitamin D status were in animal models more than 20 years ago. These studies showed that 1,25-dihydroxyvitamin D3 acts directly on cardiomyocyte vitamin D receptors, which are widely distributed throughout the body in several tissue types.11
Excess PTH levels associated with low vitamin D levels may play a role in cardiovascular disease by leading to cardiomyocyte hypertrophy and interstitial fibrosis of the heart.12 Animal studies have found that vitamin D suppresses cardiac hypertrophy.13 Vitamin D also plays a role in cardiomyocyte relaxation and may abrogate the hypercontractility associated with diastolic heart failure.2,14
Currently, it is unclear whether vitamin D deficiency is a causative risk factor for heart failure or simply a reflection of the poor functional status of patients with heart failure that leads to decreased exposure to sunlight. This debate will continue until further randomized clinical trials address this association.
VITAMIN D AND HEART TRANSPLANTATION
One would expect that patients with endstage organ failure would be at high risk of vitamin D deficiency because of limited sunlight exposure. However, few studies have evaluated the role of this vitamin in heart transplant recipients.
Stein and colleagues15 measured serum 25-hydroxyvitamin D3 immediately after transplantation in 46 heart and 23 liver transplant recipients. Levels were low in both types of transplant recipients, but liver transplant recipients had significantly lower levels than heart transplant patients. This could be explained by malabsorption and impaired synthesis of 25-hydroxyvitamin D3 in end-stage liver disease.
Also, an important point is that osteoporosis is prevalent in postcardiac transplant patients and likely related to the immunosuppressive agents these patients must take.16 In theory, increasing the body’s stores of vitamin D during the pretransplant period could lower the rate of bone loss and osteoporosis after cardiac transplantation.
Further investigation is needed to determine whether restoring adequate levels of vitamin D at the time of or after transplantation prevents graft rejection or improves survival.
VITAMIN D SUPPLEMENTATION AND SURVIVAL IN HEART FAILURE
The best laboratory test to assess vitamin D levels is the serum 25-hydroxyvitamin D3 concentration. A level between 20 and 30 ng/mL (50–75 nmol/L) is considered insufficient, and a level below 20 ng/mL (50 nmol/L) represents vitamin D deficiency.4,5,11
Vitamin D insufficiency is typically treated with 800 to 1,000 IU of vitamin D3 daily, whereas deficiency requires 50,000 IU of vitamin D3 weekly for 6 to 8 weeks, followed by 800 to 1,000 IU daily.19 The goal of therapy is to increase the serum 25-hydroxyvitamin D3 level above 30 ng/mL.19
Currently, it is unknown if vitamin D supplementation improves survival in heart failure. We recommend testing for vitamin D deficiency in all patients with heart failure and treating them as described above. For heart failure patients that are not deficient, daily intake of 800 to 1,000 IU of vitamin D is reasonable. Our review underscores the need for more studies to evaluate the efficacy of vitamin D replacement in improving survival in patients with heart failure.
KEY POINTS
- Screening for vitamin D deficiency in patients with heart failure is appropriate and encouraged.
- Vitamin D deficiency is common in patients with heart failure and in heart transplant recipients.
- In theory, achieving adequate levels of vitamin D would have a beneficial effect on patients with heart failure.
- Randomized controlled trials are needed to determine if vitamin D supplementation confers a survival benefit in patients with heart failure who have deficient vitamin D levels.
Vitamin D deficiency may play a role in the pathogenesis of chronic heart failure, but whether giving patients supplements to raise their vitamin D levels into the normal range improves their survival is not clear.
ASSOCIATION BETWEEN VITAMIN D DEFICIENCY AND OTHER DISORDERS
In the mid-17th century, Whistler and Glisson independently described rickets as a severe bone-deforming disease characterized by growth retardation, bending of the spine, deformities of the legs, and weak and toneless muscles. Histologically, rickets is characterized by impaired mineralization of the cartilage in the epiphyseal growth plates in children. In 1919, Sir Edward Mellanby identified vitamin D deficiency as the cause.
Osteomalacia, another disease caused by vitamin D deficiency, is a disorder of mineralization of newly formed bone matrix in adults. Vitamin D, therefore, has well-known roles in maintaining bone health and calcium and phosphorus homeostasis.
In addition, vitamin D deficiency has been shown in recent years to be associated with myocardial dysfunction.1,2
VITAMIN D METABOLISM IS COMPLEX
In skin exposed to ultraviolet B light, the provitamin 7-dehydrocholesterol is converted to vitamin D3 (cholecalciferol). Vitamin D3 is also obtained from dietary sources. However, many scientists consider vitamin D more a hormone than a classic vitamin, as adequate exposure to sunlight may negate the need for dietary supplements.
The active form of vitamin D is synthesized by hydroxylation in the liver and kidney. In the liver, hepatic enzymes add a hydroxyl (OH) group to vitamin D3, changing it to 25-hydroxyvitamin D3. In the kidney, 25-hydroxyvitamin D3 receives another hydroxyl group, converting it to the biologically active metabolite 1,25-dihydroxyvitamin D3 (calcitriol). This renal hydroxylation is via 1-alpha-hydroxylase activity and is directly under control of parathyroid hormone (PTH), and indirectly under control of the serum concentrations of calcium.
Interestingly, a number of different organ cells, including cardiomyocytes, also express 1-alpha-hydroxylase and therefore also convert 25-hydroxyvitamin D3 to 1,25-dihydroxyvitamin D3. Unlike the renal hydroxylation, this extrarenal process depends on cytokine activation and on serum levels of 25-hydroxyvitamin D3.3 Low levels of 25-hydroxyvitamin D3 lead to alterations in cellular control over growth, differentiation, and function.
The active form of vitamin D is transported protein-bound in the blood to various target organs, where it is delivered in free form to cells. Specific nuclear receptor proteins are found in many organs not classically considered target organs for vitamin D, including the skin, brain, skeletal muscles, cardiomyocytes, vascular endothelial cells, circulating monocytes, and activated B and T lymphocytes. Vitamin D plays a significant role in the autocrine and paracrine regulation of cellular function, growth, and differentiation in various organs.3
MOST HEART FAILURE PATIENTS HAVE LOW VITAMIN D LEVELS
More than 40% of men and 50% of women in the United States have low vitamin D levels (< 30 ng/mL [75 nmol/L])—and low levels in adults are associated with both coronary artery disease and heart failure.4 Most patients with heart failure have low levels.5,6 Therefore, screening for vitamin D deficiency in patients with heart failure is appropriate and encouraged.
Low vitamin D levels carry a poor prognosis. Pilz et al5 measured baseline 25-hydroxyvitamin D3 levels in 3,299 patients referred for elective coronary angiography and followed them prospectively for a median of 7.7 years. Even after adjustment for cardiac risk factors, patients who had low 25-hydroxyvitamin D3 levels were more likely to die of heart failure or sudden cardiac death than patients with normal levels.
Boxer et al7 found an association between low 25-hydroxyvitamin D3 levels and low exercise capacity and frailty in patients with systolic heart failure.
LOW VITAMIN D CONTRIBUTES TO THE PATHOGENESIS OF HEART FAILURE
In recent years, ideas about the pathophysiology of heart failure have expanded from a purely hemodynamic view to a more complex concept involving inflammatory cytokines and neurohormonal overactivation.8
Animal studies first showed vitamin D to inhibit the renin-angiotensin-aldosterone system, activation of which contributes to the salt and water retention seen in heart failure.4,9
In addition, vitamin D has a number of effects that should help prevent hypertension, an important risk factor for heart failure. It protects the kidney by suppressing the reninangiotensin-aldosterone system, prevents secondary hyperparathyroidism and its effects on vascular stiffness, prevents insulin resistance, and suppresses inflammation, which protects vascular endothelial cells.10
The first studies to show a connection between cardiovascular homeostasis and vitamin D status were in animal models more than 20 years ago. These studies showed that 1,25-dihydroxyvitamin D3 acts directly on cardiomyocyte vitamin D receptors, which are widely distributed throughout the body in several tissue types.11
Excess PTH levels associated with low vitamin D levels may play a role in cardiovascular disease by leading to cardiomyocyte hypertrophy and interstitial fibrosis of the heart.12 Animal studies have found that vitamin D suppresses cardiac hypertrophy.13 Vitamin D also plays a role in cardiomyocyte relaxation and may abrogate the hypercontractility associated with diastolic heart failure.2,14
Currently, it is unclear whether vitamin D deficiency is a causative risk factor for heart failure or simply a reflection of the poor functional status of patients with heart failure that leads to decreased exposure to sunlight. This debate will continue until further randomized clinical trials address this association.
VITAMIN D AND HEART TRANSPLANTATION
One would expect that patients with endstage organ failure would be at high risk of vitamin D deficiency because of limited sunlight exposure. However, few studies have evaluated the role of this vitamin in heart transplant recipients.
Stein and colleagues15 measured serum 25-hydroxyvitamin D3 immediately after transplantation in 46 heart and 23 liver transplant recipients. Levels were low in both types of transplant recipients, but liver transplant recipients had significantly lower levels than heart transplant patients. This could be explained by malabsorption and impaired synthesis of 25-hydroxyvitamin D3 in end-stage liver disease.
Also, an important point is that osteoporosis is prevalent in postcardiac transplant patients and likely related to the immunosuppressive agents these patients must take.16 In theory, increasing the body’s stores of vitamin D during the pretransplant period could lower the rate of bone loss and osteoporosis after cardiac transplantation.
Further investigation is needed to determine whether restoring adequate levels of vitamin D at the time of or after transplantation prevents graft rejection or improves survival.
VITAMIN D SUPPLEMENTATION AND SURVIVAL IN HEART FAILURE
The best laboratory test to assess vitamin D levels is the serum 25-hydroxyvitamin D3 concentration. A level between 20 and 30 ng/mL (50–75 nmol/L) is considered insufficient, and a level below 20 ng/mL (50 nmol/L) represents vitamin D deficiency.4,5,11
Vitamin D insufficiency is typically treated with 800 to 1,000 IU of vitamin D3 daily, whereas deficiency requires 50,000 IU of vitamin D3 weekly for 6 to 8 weeks, followed by 800 to 1,000 IU daily.19 The goal of therapy is to increase the serum 25-hydroxyvitamin D3 level above 30 ng/mL.19
Currently, it is unknown if vitamin D supplementation improves survival in heart failure. We recommend testing for vitamin D deficiency in all patients with heart failure and treating them as described above. For heart failure patients that are not deficient, daily intake of 800 to 1,000 IU of vitamin D is reasonable. Our review underscores the need for more studies to evaluate the efficacy of vitamin D replacement in improving survival in patients with heart failure.
KEY POINTS
- Screening for vitamin D deficiency in patients with heart failure is appropriate and encouraged.
- Vitamin D deficiency is common in patients with heart failure and in heart transplant recipients.
- In theory, achieving adequate levels of vitamin D would have a beneficial effect on patients with heart failure.
- Randomized controlled trials are needed to determine if vitamin D supplementation confers a survival benefit in patients with heart failure who have deficient vitamin D levels.
- Nibbelink KA, Tishkoff DX, Hershey SD, Rahman A, Simpson RU. 1,25(OH)2-vitamin D3 actions on cell proliferation, size, gene expression, and receptor localization, in the HL-1 cardiac myocyte. J Steroid Biochem Mol Biol 2007; 103:533–537.
- Tishkoff DX, Nibbelink KA, Holmberg KH, Dandu L, Simpson RU. Functional vitamin D receptor (VDR) in the t-tubules of cardiac myocytes: VDR knockout cardiomyocyte contractility. Endocrinology 2008; 149:558–564.
- Peterlik M, Cross HS. Vitamin D and calcium deficits predispose for multiple chronic diseases. Eur J Clin Invest 2005; 35:290–304.
- Kim DH, Sabour S, Sagar UN, Adams S, Whellan DJ. Prevalence of hypovitaminosis D in cardiovascular diseases (from the National Health and Nutrition Examination Survey 2001 to 2004). Am J Cardiol 2008; 102:1540–1544.
- Pilz S, März W, Wellnitz B, et al. Association of vitamin D deficiency with heart failure and sudden cardiac death in a large cross-sectional study of patients referred for coronary angiography. J Clin Endocrinol Metab 2008; 93:3927–3935.
- Zittermann A, Schleithoff SS, Koerfer R. Vitamin D insufficiency in congestive heart failure: why and what to do about it? Heart Fail Rev 2006; 11:25–33.
- Boxer RS, Dauser DA, Walsh SJ, Hager WD, Kenny AM. The association between vitamin D and inflammation with the 6-minute walk and frailty in patients with heart failure. J Am Geriatr Soc 2008; 56:454–461.
- Schleithoff SS, Zittermann A, Tenderich G, Berthold HK, Stehle P, Koerfer R. Vitamin D supplementation improves cytokine profiles in patients with congestive heart failure: a double-blind, randomized, placebo-controlled trial. Am J Clin Nutr 2006; 83:754–759.
- Li YC, Kong J, Wei M, Chen ZF, Liu SQ, Cao LP. 1,25-Dihydroxyvitamin D(3) is a negative endocrine regulator of the renin-angiotensin system. J Clin Invest 2002; 110:229–238.
- Pilz S, Tomaschitz A, Ritz E, Pieber TR; Medscape. Vitamin D status and arterial hypertension: a systematic review. Nat Rev Cardiol 2009; 6:621–630.
- Nemerovski CW, Dorsch MP, Simpson RU, Bone HG, Aaronson KD, Bleske BE. Vitamin D and cardiovascular disease. Pharmacotherapy 2009; 29:691–708.
- Rostand SG, Drüeke TB. Parathyroid hormone, vitamin D, and cardiovascular disease in chronic renal failure. Kidney Int 1999; 56:383–392.
- Wu J, Garami M, Cheng T, Gardner DG. 1,25(OH)2 vitamin D3, and retinoic acid antagonize endothelin-stimulated hypertrophy of neonatal rat cardiac myocytes. J Clin Invest 1996; 97:1577–1588.
- Green JJ, Robinson DA, Wilson GE, Simpson RU, Westfall MV. Calcitriol modulation of cardiac contractile performance via protein kinase C. J Mol Cell Cardiol 2006; 41:350–359.
- Stein EM, Cohen A, Freeby M, et al. Severe vitamin D deficiency among heart and liver transplant recipients. Clin Transplant 2009; (Epub ahead of print)
- Shane E, Rivas M, McMahon DJ, et al. Bone loss and turnover after cardiac transplantation. J Clin Endocrinol Metab 1997; 82:1497–1506.
- Norman AW, Bouillon R, Whiting SJ, Vieth R, Lips P. 13th Workshop consensus for vitamin D nutritional guidelines. J Steroid Biochem Mol Biol 2007; 103:204–205.
- Vieth R, Bischoff-Ferrari H, Boucher BJ, et al. The urgent need to recommend an intake of vitamin D that is effective. Am J Clin Nutr 2007; 85:649–650.
- Dawson-Hughes B, Heaney RP, Holick MF, Lips P, Meunier PJ, Vieth R. Estimates of optimal vitamin D status. Osteoporos Int 2005; 16:713–716.
- Nibbelink KA, Tishkoff DX, Hershey SD, Rahman A, Simpson RU. 1,25(OH)2-vitamin D3 actions on cell proliferation, size, gene expression, and receptor localization, in the HL-1 cardiac myocyte. J Steroid Biochem Mol Biol 2007; 103:533–537.
- Tishkoff DX, Nibbelink KA, Holmberg KH, Dandu L, Simpson RU. Functional vitamin D receptor (VDR) in the t-tubules of cardiac myocytes: VDR knockout cardiomyocyte contractility. Endocrinology 2008; 149:558–564.
- Peterlik M, Cross HS. Vitamin D and calcium deficits predispose for multiple chronic diseases. Eur J Clin Invest 2005; 35:290–304.
- Kim DH, Sabour S, Sagar UN, Adams S, Whellan DJ. Prevalence of hypovitaminosis D in cardiovascular diseases (from the National Health and Nutrition Examination Survey 2001 to 2004). Am J Cardiol 2008; 102:1540–1544.
- Pilz S, März W, Wellnitz B, et al. Association of vitamin D deficiency with heart failure and sudden cardiac death in a large cross-sectional study of patients referred for coronary angiography. J Clin Endocrinol Metab 2008; 93:3927–3935.
- Zittermann A, Schleithoff SS, Koerfer R. Vitamin D insufficiency in congestive heart failure: why and what to do about it? Heart Fail Rev 2006; 11:25–33.
- Boxer RS, Dauser DA, Walsh SJ, Hager WD, Kenny AM. The association between vitamin D and inflammation with the 6-minute walk and frailty in patients with heart failure. J Am Geriatr Soc 2008; 56:454–461.
- Schleithoff SS, Zittermann A, Tenderich G, Berthold HK, Stehle P, Koerfer R. Vitamin D supplementation improves cytokine profiles in patients with congestive heart failure: a double-blind, randomized, placebo-controlled trial. Am J Clin Nutr 2006; 83:754–759.
- Li YC, Kong J, Wei M, Chen ZF, Liu SQ, Cao LP. 1,25-Dihydroxyvitamin D(3) is a negative endocrine regulator of the renin-angiotensin system. J Clin Invest 2002; 110:229–238.
- Pilz S, Tomaschitz A, Ritz E, Pieber TR; Medscape. Vitamin D status and arterial hypertension: a systematic review. Nat Rev Cardiol 2009; 6:621–630.
- Nemerovski CW, Dorsch MP, Simpson RU, Bone HG, Aaronson KD, Bleske BE. Vitamin D and cardiovascular disease. Pharmacotherapy 2009; 29:691–708.
- Rostand SG, Drüeke TB. Parathyroid hormone, vitamin D, and cardiovascular disease in chronic renal failure. Kidney Int 1999; 56:383–392.
- Wu J, Garami M, Cheng T, Gardner DG. 1,25(OH)2 vitamin D3, and retinoic acid antagonize endothelin-stimulated hypertrophy of neonatal rat cardiac myocytes. J Clin Invest 1996; 97:1577–1588.
- Green JJ, Robinson DA, Wilson GE, Simpson RU, Westfall MV. Calcitriol modulation of cardiac contractile performance via protein kinase C. J Mol Cell Cardiol 2006; 41:350–359.
- Stein EM, Cohen A, Freeby M, et al. Severe vitamin D deficiency among heart and liver transplant recipients. Clin Transplant 2009; (Epub ahead of print)
- Shane E, Rivas M, McMahon DJ, et al. Bone loss and turnover after cardiac transplantation. J Clin Endocrinol Metab 1997; 82:1497–1506.
- Norman AW, Bouillon R, Whiting SJ, Vieth R, Lips P. 13th Workshop consensus for vitamin D nutritional guidelines. J Steroid Biochem Mol Biol 2007; 103:204–205.
- Vieth R, Bischoff-Ferrari H, Boucher BJ, et al. The urgent need to recommend an intake of vitamin D that is effective. Am J Clin Nutr 2007; 85:649–650.
- Dawson-Hughes B, Heaney RP, Holick MF, Lips P, Meunier PJ, Vieth R. Estimates of optimal vitamin D status. Osteoporos Int 2005; 16:713–716.
Does measuring natriuretic peptides have a role in patients with chronic kidney disease?
Yes, measuring the levels of certain natriuretic peptides can help diagnose decompensated heart failure and predict the risk of death and cardiac hospitalization in patients across a wide spectrum of renal function.
However, at this time, it is unclear whether routinely measuring natriuretic peptides will result in any change in the management of patients with chronic kidney disease. Additionally, using these peptides to monitor volume status in dialysis patients has not yet been deemed useful, although it may be complementary to echocardiography in evaluating cardiac risk in patients with end-stage renal disease.
A BRIEF REVIEW OF NATRIURETIC PEPTIDES
Natriuretic peptides include atrial natriuretic peptide, brain natriuretic peptide (BNP), C-type natriuretic peptide, and urodilantin.
BNP, which is homologous to atrial natriuretic peptide, is present in the brain and the heart. The circulating concentration of BNP is less than 20% of the atrial natriuretic peptide level in healthy people, but equals or exceeds that of atrial natriuretic peptide in patients with congestive heart failure.
BNP starts as a precursor protein. This is modified within the cell into a prohormone, proBNP, which is secreted from the left ventricle in response to myocardial wall stress. In the circulation, proBNP is cleaved into a biologically active C-terminal fragment—BNP—and a biologically inactive N-terminal fragment (NT-proBNP).1 NT-proBNP is primarily cleared by the kidney. BNP is cleared by receptor-mediated binding and removed by neutral endopeptidase, as well as by the kidney.
Both BNP and NT-proBNP have been investigated as diagnostic markers of suspected heart disease.
PEPTIDE LEVELS ARE HIGH IN CHRONIC KIDNEY DISEASE AND HEART FAILURE
An estimated 8.3 million people in the United States have stage 3, 4, or 5 chronic kidney disease,2 defined as an estimated glomerular filtration rate of less than 60 mL/min/1.73 m2. Approximately 50% of patients with heart failure have chronic kidney disease, and almost 60% of patients with chronic kidney disease have some abnormality in ventricular function.
A few years ago, researchers began investigating the benefits and limitations of using natriuretic peptides to diagnose cardiac dysfunction (left ventricular structural and functional abnormalities) in patients with chronic kidney disease.
One important study3 was conducted in almost 3,000 patients from the Dallas Heart Study who were between the ages of 30 and 65 years—a relatively young, mostly healthy population. The authors found that natriuretic peptide levels did not vary as long as the estimated glomerular filtration rate was within the normal range. However, when the estimated glomerular filtration rate dropped below a threshold of 90 mL/min/1.73 m2, the concentrations of both NT-proBNP and BNP increased exponentially. NT-proBNP levels rose more than BNP levels, as NT-proBNP is primarily cleared by the kidney.
More recent studies found that the high levels of NT-proBNP in patients with chronic kidney disease do not simply reflect the reduced clearance of this peptide; they also reflect compromised ventricular function.2,4 This relationship was supported by studies of the fractional renal excretion of NT-proBNP and BNP in several populations with and without renal impairment.5 Interestingly, fractional excretion of both peptides remained equivalent across a wide spectrum of renal function. Seemingly, cardiac disease drove the increase in values rather than the degree of renal impairment.
HIGH PEPTIDE LEVELS PREDICT DEATH, HOSPITALIZATION
Both BNP and NT-proBNP are strong predictors of death and cardiac hospitalization in kidney patients.1,4,6
In patients with end-stage renal disease, the risk of cardiovascular disease and death is significantly higher than that in the general population, and BNP has been found to be a valuable prognostic indicator of cardiac disease.7
Multiple studies showed that high levels of natriuretic peptides are associated with a higher risk of death in patients with acute coronary syndrome, independent of traditional cardiovascular risk factors such as electrocardiographic changes and levels of other biomarkers. However, these data were derived from patients with mild renal impairment.2
Apple et al8 compared the prognostic value of NT-proBNP with that of cardiac troponin T in hemodialysis patients who had no symptoms and found that NT-proBNP was more strongly associated with left ventricular systolic dysfunction and subsequent cardiovascular death.
PEPTIDE LEVELS ARE HIGHER IN ANEMIA
A significant number of patients with congestive heart failure have renal insufficiency and low hemoglobin levels, which may increase natriuretic peptide levels. It is unclear why anemia is associated with elevated levels of natriuretic peptides, even in the absence of clinical heart failure and independent of other cardiovascular risk factors.9 Nevertheless, anemia should be taken into consideration and treated effectively when evaluating patients with renal impairment and possible congestive heart failure.
PEPTIDES COMPLEMENT CARDIAC ECHO IN END-STAGE RENAL DISEASE
Numerous studies have found a close association between BNP and NT-proBNP levels and left ventricular mass and systolic function in patients with end-stage renal disease.10,11 Data from the Cardiovascular Risk Extended Evaluation in Dialysis Patients study12 suggest that BNP measurement can be reliably applied in end-stage renal disease to rule out systolic dysfunction and to detect left ventricular hypertrophy, but it has a very low negative predictive value for left ventricular hypertrophy in this patient population: someone with a normal BNP level can still have left ventricular hypertrophy.
In addition, volume status is harder to assess with BNP alone than with echocardiography, and an elevated BNP value is not very specific.13
In essence, both BNP and NT-proBNP can be used to complement echocardiography in evaluating cardiac risk in patients with end-stage renal disease. With additional data, it may be possible in the future to use them as substitutes for echocardiography when managing ventricular abnormalities in patients with end-stage renal disease.
USING SPECIFIC CUT POINTS IN RENAL DISEASE
When evaluating a patient with acute dyspnea and either chronic kidney disease or end-stage renal disease who is receiving dialysis, both BNP and NT-proBNP are affected similarly and necessitate a higher level of interpretation to diagnose decompensated heart failure. Currently, researchers disagree about specific cut points for natriuretic peptides. However, deFilippi and colleagues4 suggested the following cut points for NT-proBNP for diagnosing heart failure in patients of different ages with or without renal impairment:
- Younger than 50 years—450 ng/L
- Age 50 to 75 years—900 ng/L
- Older than 75 years—1,800 ng/L.
A BNP cutoff point of 225 pg/mL can be used for patients with an estimated glomerular filtration rate of less than 60 mL/min/1.73 m2, based on data from the Breathing Not Properly multinational study.14
There is no set cut-point for either BNP or NT-proBNP for predicting death and cardiac hospitalization in renal patients, but abnormally high levels should signal the need to optimize medical management and to monitor more closely.
- Austin WJ, Bhalla V, Hernandez-Arce I, et al. Correlation and prognostic utility of B-type natriuretic peptide and its amino-terminal fragment in patients with chronic kidney disease. Am J Clin Pathol 2006; 126:506–512.
- DeFilippi C, van Kimmenade RR, Pinto YM. Amino-terminal pro-B-type natriuretic peptide testing in renal disease. Am J Cardiol 2008; 101:82–88.
- Das SR, Abdullah SM, Leonard D, et al. Association between renal function and circulating levels of natriuretic peptides (from the Dallas Heart Study). Am J Cardiol 2008; 102:1394–1398.
- DeFilippi CR, Seliger SL, Maynard S, Christenson RH. Impact of renal disease on natriuretic peptide testing for diagnosing decompensated heart failure and predicting mortality. Clin Chem 2007; 53:1511–1519.
- Goetze JP, Jensen G, Møller S, Bendtsen F, Rehfeld JF, Henriksen JH. BNP and N-terminal proBNP are both extracted in the normal kidney. Eur J Clin Invest 2006; 36:8–15.
- Zoccali C. Biomarkers in chronic kidney disease: utility and issues towards better understanding. Curr Opin Nephrol Hypertens 2005; 14:532–537.
- Haapio M, Ronco C. BNP and a renal patient: emphasis on the unique characteristics of B-type natriuretic peptide in end-stage kidney disease. Contrib Nephrol 2008; 161:68–75.
- Apple FS, Murakami MM, Pearce LA, Herzog CA. Multibiomarker risk stratification of N-terminal pro-B-type natriuretic peptide, high-sensitivity C-reactive protein, and cardiac troponin T and I in end-stage renal disease for all-cause death. Clin Chem 2004: 50:2279–2285.
- Hogenhuis J, Voors AA, Jaarsma T, et al. Anemia and renal dysfunction are independently associated with BNP and NT-proBNP levels in patients with heart failure. Eur J Heart Fail 2007; 9:787–794.
- Madsen LH, Ladefoged S, Corell P, Schou M, Hildebrandt PR, Atar D. N-terminal pro brain natriuretic peptide predicts mortality in patients with end-stage renal disease on hemodialysis. Kidney Int 2007; 71:548–554.
- Wang AY, Lai KN. Use of cardiac biomarkers in end-stage renal disease. J Am Soc Nephrol 2008; 19:1643–1652.
- Mallamaci F, Zoccali C, Tripepi G, et al; on behalf of the CREED Investigators. Diagnostic potential of cardiac natriuretic peptides in dialysis patients. Kidney Int 2001; 59:1559–1566.
- Biasioli S, Zamperetti M, Borin D, Guidi G, De Fanti E, Schiavon R. Significance of plasma B-type natriuretic peptide in hemodialysis patients: blood sample timing and comorbidity burden. ASAIO J 2007; 53:587–591.
- McCullough PA, Duc P, Omland T, et al. B-type natriuretic peptide and renal function in the diagnosis of heart failure: an analysis from the Breathing Not Properly multinational study. Am J Kidney Dis 2003; 41:571–579.
Yes, measuring the levels of certain natriuretic peptides can help diagnose decompensated heart failure and predict the risk of death and cardiac hospitalization in patients across a wide spectrum of renal function.
However, at this time, it is unclear whether routinely measuring natriuretic peptides will result in any change in the management of patients with chronic kidney disease. Additionally, using these peptides to monitor volume status in dialysis patients has not yet been deemed useful, although it may be complementary to echocardiography in evaluating cardiac risk in patients with end-stage renal disease.
A BRIEF REVIEW OF NATRIURETIC PEPTIDES
Natriuretic peptides include atrial natriuretic peptide, brain natriuretic peptide (BNP), C-type natriuretic peptide, and urodilantin.
BNP, which is homologous to atrial natriuretic peptide, is present in the brain and the heart. The circulating concentration of BNP is less than 20% of the atrial natriuretic peptide level in healthy people, but equals or exceeds that of atrial natriuretic peptide in patients with congestive heart failure.
BNP starts as a precursor protein. This is modified within the cell into a prohormone, proBNP, which is secreted from the left ventricle in response to myocardial wall stress. In the circulation, proBNP is cleaved into a biologically active C-terminal fragment—BNP—and a biologically inactive N-terminal fragment (NT-proBNP).1 NT-proBNP is primarily cleared by the kidney. BNP is cleared by receptor-mediated binding and removed by neutral endopeptidase, as well as by the kidney.
Both BNP and NT-proBNP have been investigated as diagnostic markers of suspected heart disease.
PEPTIDE LEVELS ARE HIGH IN CHRONIC KIDNEY DISEASE AND HEART FAILURE
An estimated 8.3 million people in the United States have stage 3, 4, or 5 chronic kidney disease,2 defined as an estimated glomerular filtration rate of less than 60 mL/min/1.73 m2. Approximately 50% of patients with heart failure have chronic kidney disease, and almost 60% of patients with chronic kidney disease have some abnormality in ventricular function.
A few years ago, researchers began investigating the benefits and limitations of using natriuretic peptides to diagnose cardiac dysfunction (left ventricular structural and functional abnormalities) in patients with chronic kidney disease.
One important study3 was conducted in almost 3,000 patients from the Dallas Heart Study who were between the ages of 30 and 65 years—a relatively young, mostly healthy population. The authors found that natriuretic peptide levels did not vary as long as the estimated glomerular filtration rate was within the normal range. However, when the estimated glomerular filtration rate dropped below a threshold of 90 mL/min/1.73 m2, the concentrations of both NT-proBNP and BNP increased exponentially. NT-proBNP levels rose more than BNP levels, as NT-proBNP is primarily cleared by the kidney.
More recent studies found that the high levels of NT-proBNP in patients with chronic kidney disease do not simply reflect the reduced clearance of this peptide; they also reflect compromised ventricular function.2,4 This relationship was supported by studies of the fractional renal excretion of NT-proBNP and BNP in several populations with and without renal impairment.5 Interestingly, fractional excretion of both peptides remained equivalent across a wide spectrum of renal function. Seemingly, cardiac disease drove the increase in values rather than the degree of renal impairment.
HIGH PEPTIDE LEVELS PREDICT DEATH, HOSPITALIZATION
Both BNP and NT-proBNP are strong predictors of death and cardiac hospitalization in kidney patients.1,4,6
In patients with end-stage renal disease, the risk of cardiovascular disease and death is significantly higher than that in the general population, and BNP has been found to be a valuable prognostic indicator of cardiac disease.7
Multiple studies showed that high levels of natriuretic peptides are associated with a higher risk of death in patients with acute coronary syndrome, independent of traditional cardiovascular risk factors such as electrocardiographic changes and levels of other biomarkers. However, these data were derived from patients with mild renal impairment.2
Apple et al8 compared the prognostic value of NT-proBNP with that of cardiac troponin T in hemodialysis patients who had no symptoms and found that NT-proBNP was more strongly associated with left ventricular systolic dysfunction and subsequent cardiovascular death.
PEPTIDE LEVELS ARE HIGHER IN ANEMIA
A significant number of patients with congestive heart failure have renal insufficiency and low hemoglobin levels, which may increase natriuretic peptide levels. It is unclear why anemia is associated with elevated levels of natriuretic peptides, even in the absence of clinical heart failure and independent of other cardiovascular risk factors.9 Nevertheless, anemia should be taken into consideration and treated effectively when evaluating patients with renal impairment and possible congestive heart failure.
PEPTIDES COMPLEMENT CARDIAC ECHO IN END-STAGE RENAL DISEASE
Numerous studies have found a close association between BNP and NT-proBNP levels and left ventricular mass and systolic function in patients with end-stage renal disease.10,11 Data from the Cardiovascular Risk Extended Evaluation in Dialysis Patients study12 suggest that BNP measurement can be reliably applied in end-stage renal disease to rule out systolic dysfunction and to detect left ventricular hypertrophy, but it has a very low negative predictive value for left ventricular hypertrophy in this patient population: someone with a normal BNP level can still have left ventricular hypertrophy.
In addition, volume status is harder to assess with BNP alone than with echocardiography, and an elevated BNP value is not very specific.13
In essence, both BNP and NT-proBNP can be used to complement echocardiography in evaluating cardiac risk in patients with end-stage renal disease. With additional data, it may be possible in the future to use them as substitutes for echocardiography when managing ventricular abnormalities in patients with end-stage renal disease.
USING SPECIFIC CUT POINTS IN RENAL DISEASE
When evaluating a patient with acute dyspnea and either chronic kidney disease or end-stage renal disease who is receiving dialysis, both BNP and NT-proBNP are affected similarly and necessitate a higher level of interpretation to diagnose decompensated heart failure. Currently, researchers disagree about specific cut points for natriuretic peptides. However, deFilippi and colleagues4 suggested the following cut points for NT-proBNP for diagnosing heart failure in patients of different ages with or without renal impairment:
- Younger than 50 years—450 ng/L
- Age 50 to 75 years—900 ng/L
- Older than 75 years—1,800 ng/L.
A BNP cutoff point of 225 pg/mL can be used for patients with an estimated glomerular filtration rate of less than 60 mL/min/1.73 m2, based on data from the Breathing Not Properly multinational study.14
There is no set cut-point for either BNP or NT-proBNP for predicting death and cardiac hospitalization in renal patients, but abnormally high levels should signal the need to optimize medical management and to monitor more closely.
Yes, measuring the levels of certain natriuretic peptides can help diagnose decompensated heart failure and predict the risk of death and cardiac hospitalization in patients across a wide spectrum of renal function.
However, at this time, it is unclear whether routinely measuring natriuretic peptides will result in any change in the management of patients with chronic kidney disease. Additionally, using these peptides to monitor volume status in dialysis patients has not yet been deemed useful, although it may be complementary to echocardiography in evaluating cardiac risk in patients with end-stage renal disease.
A BRIEF REVIEW OF NATRIURETIC PEPTIDES
Natriuretic peptides include atrial natriuretic peptide, brain natriuretic peptide (BNP), C-type natriuretic peptide, and urodilantin.
BNP, which is homologous to atrial natriuretic peptide, is present in the brain and the heart. The circulating concentration of BNP is less than 20% of the atrial natriuretic peptide level in healthy people, but equals or exceeds that of atrial natriuretic peptide in patients with congestive heart failure.
BNP starts as a precursor protein. This is modified within the cell into a prohormone, proBNP, which is secreted from the left ventricle in response to myocardial wall stress. In the circulation, proBNP is cleaved into a biologically active C-terminal fragment—BNP—and a biologically inactive N-terminal fragment (NT-proBNP).1 NT-proBNP is primarily cleared by the kidney. BNP is cleared by receptor-mediated binding and removed by neutral endopeptidase, as well as by the kidney.
Both BNP and NT-proBNP have been investigated as diagnostic markers of suspected heart disease.
PEPTIDE LEVELS ARE HIGH IN CHRONIC KIDNEY DISEASE AND HEART FAILURE
An estimated 8.3 million people in the United States have stage 3, 4, or 5 chronic kidney disease,2 defined as an estimated glomerular filtration rate of less than 60 mL/min/1.73 m2. Approximately 50% of patients with heart failure have chronic kidney disease, and almost 60% of patients with chronic kidney disease have some abnormality in ventricular function.
A few years ago, researchers began investigating the benefits and limitations of using natriuretic peptides to diagnose cardiac dysfunction (left ventricular structural and functional abnormalities) in patients with chronic kidney disease.
One important study3 was conducted in almost 3,000 patients from the Dallas Heart Study who were between the ages of 30 and 65 years—a relatively young, mostly healthy population. The authors found that natriuretic peptide levels did not vary as long as the estimated glomerular filtration rate was within the normal range. However, when the estimated glomerular filtration rate dropped below a threshold of 90 mL/min/1.73 m2, the concentrations of both NT-proBNP and BNP increased exponentially. NT-proBNP levels rose more than BNP levels, as NT-proBNP is primarily cleared by the kidney.
More recent studies found that the high levels of NT-proBNP in patients with chronic kidney disease do not simply reflect the reduced clearance of this peptide; they also reflect compromised ventricular function.2,4 This relationship was supported by studies of the fractional renal excretion of NT-proBNP and BNP in several populations with and without renal impairment.5 Interestingly, fractional excretion of both peptides remained equivalent across a wide spectrum of renal function. Seemingly, cardiac disease drove the increase in values rather than the degree of renal impairment.
HIGH PEPTIDE LEVELS PREDICT DEATH, HOSPITALIZATION
Both BNP and NT-proBNP are strong predictors of death and cardiac hospitalization in kidney patients.1,4,6
In patients with end-stage renal disease, the risk of cardiovascular disease and death is significantly higher than that in the general population, and BNP has been found to be a valuable prognostic indicator of cardiac disease.7
Multiple studies showed that high levels of natriuretic peptides are associated with a higher risk of death in patients with acute coronary syndrome, independent of traditional cardiovascular risk factors such as electrocardiographic changes and levels of other biomarkers. However, these data were derived from patients with mild renal impairment.2
Apple et al8 compared the prognostic value of NT-proBNP with that of cardiac troponin T in hemodialysis patients who had no symptoms and found that NT-proBNP was more strongly associated with left ventricular systolic dysfunction and subsequent cardiovascular death.
PEPTIDE LEVELS ARE HIGHER IN ANEMIA
A significant number of patients with congestive heart failure have renal insufficiency and low hemoglobin levels, which may increase natriuretic peptide levels. It is unclear why anemia is associated with elevated levels of natriuretic peptides, even in the absence of clinical heart failure and independent of other cardiovascular risk factors.9 Nevertheless, anemia should be taken into consideration and treated effectively when evaluating patients with renal impairment and possible congestive heart failure.
PEPTIDES COMPLEMENT CARDIAC ECHO IN END-STAGE RENAL DISEASE
Numerous studies have found a close association between BNP and NT-proBNP levels and left ventricular mass and systolic function in patients with end-stage renal disease.10,11 Data from the Cardiovascular Risk Extended Evaluation in Dialysis Patients study12 suggest that BNP measurement can be reliably applied in end-stage renal disease to rule out systolic dysfunction and to detect left ventricular hypertrophy, but it has a very low negative predictive value for left ventricular hypertrophy in this patient population: someone with a normal BNP level can still have left ventricular hypertrophy.
In addition, volume status is harder to assess with BNP alone than with echocardiography, and an elevated BNP value is not very specific.13
In essence, both BNP and NT-proBNP can be used to complement echocardiography in evaluating cardiac risk in patients with end-stage renal disease. With additional data, it may be possible in the future to use them as substitutes for echocardiography when managing ventricular abnormalities in patients with end-stage renal disease.
USING SPECIFIC CUT POINTS IN RENAL DISEASE
When evaluating a patient with acute dyspnea and either chronic kidney disease or end-stage renal disease who is receiving dialysis, both BNP and NT-proBNP are affected similarly and necessitate a higher level of interpretation to diagnose decompensated heart failure. Currently, researchers disagree about specific cut points for natriuretic peptides. However, deFilippi and colleagues4 suggested the following cut points for NT-proBNP for diagnosing heart failure in patients of different ages with or without renal impairment:
- Younger than 50 years—450 ng/L
- Age 50 to 75 years—900 ng/L
- Older than 75 years—1,800 ng/L.
A BNP cutoff point of 225 pg/mL can be used for patients with an estimated glomerular filtration rate of less than 60 mL/min/1.73 m2, based on data from the Breathing Not Properly multinational study.14
There is no set cut-point for either BNP or NT-proBNP for predicting death and cardiac hospitalization in renal patients, but abnormally high levels should signal the need to optimize medical management and to monitor more closely.
- Austin WJ, Bhalla V, Hernandez-Arce I, et al. Correlation and prognostic utility of B-type natriuretic peptide and its amino-terminal fragment in patients with chronic kidney disease. Am J Clin Pathol 2006; 126:506–512.
- DeFilippi C, van Kimmenade RR, Pinto YM. Amino-terminal pro-B-type natriuretic peptide testing in renal disease. Am J Cardiol 2008; 101:82–88.
- Das SR, Abdullah SM, Leonard D, et al. Association between renal function and circulating levels of natriuretic peptides (from the Dallas Heart Study). Am J Cardiol 2008; 102:1394–1398.
- DeFilippi CR, Seliger SL, Maynard S, Christenson RH. Impact of renal disease on natriuretic peptide testing for diagnosing decompensated heart failure and predicting mortality. Clin Chem 2007; 53:1511–1519.
- Goetze JP, Jensen G, Møller S, Bendtsen F, Rehfeld JF, Henriksen JH. BNP and N-terminal proBNP are both extracted in the normal kidney. Eur J Clin Invest 2006; 36:8–15.
- Zoccali C. Biomarkers in chronic kidney disease: utility and issues towards better understanding. Curr Opin Nephrol Hypertens 2005; 14:532–537.
- Haapio M, Ronco C. BNP and a renal patient: emphasis on the unique characteristics of B-type natriuretic peptide in end-stage kidney disease. Contrib Nephrol 2008; 161:68–75.
- Apple FS, Murakami MM, Pearce LA, Herzog CA. Multibiomarker risk stratification of N-terminal pro-B-type natriuretic peptide, high-sensitivity C-reactive protein, and cardiac troponin T and I in end-stage renal disease for all-cause death. Clin Chem 2004: 50:2279–2285.
- Hogenhuis J, Voors AA, Jaarsma T, et al. Anemia and renal dysfunction are independently associated with BNP and NT-proBNP levels in patients with heart failure. Eur J Heart Fail 2007; 9:787–794.
- Madsen LH, Ladefoged S, Corell P, Schou M, Hildebrandt PR, Atar D. N-terminal pro brain natriuretic peptide predicts mortality in patients with end-stage renal disease on hemodialysis. Kidney Int 2007; 71:548–554.
- Wang AY, Lai KN. Use of cardiac biomarkers in end-stage renal disease. J Am Soc Nephrol 2008; 19:1643–1652.
- Mallamaci F, Zoccali C, Tripepi G, et al; on behalf of the CREED Investigators. Diagnostic potential of cardiac natriuretic peptides in dialysis patients. Kidney Int 2001; 59:1559–1566.
- Biasioli S, Zamperetti M, Borin D, Guidi G, De Fanti E, Schiavon R. Significance of plasma B-type natriuretic peptide in hemodialysis patients: blood sample timing and comorbidity burden. ASAIO J 2007; 53:587–591.
- McCullough PA, Duc P, Omland T, et al. B-type natriuretic peptide and renal function in the diagnosis of heart failure: an analysis from the Breathing Not Properly multinational study. Am J Kidney Dis 2003; 41:571–579.
- Austin WJ, Bhalla V, Hernandez-Arce I, et al. Correlation and prognostic utility of B-type natriuretic peptide and its amino-terminal fragment in patients with chronic kidney disease. Am J Clin Pathol 2006; 126:506–512.
- DeFilippi C, van Kimmenade RR, Pinto YM. Amino-terminal pro-B-type natriuretic peptide testing in renal disease. Am J Cardiol 2008; 101:82–88.
- Das SR, Abdullah SM, Leonard D, et al. Association between renal function and circulating levels of natriuretic peptides (from the Dallas Heart Study). Am J Cardiol 2008; 102:1394–1398.
- DeFilippi CR, Seliger SL, Maynard S, Christenson RH. Impact of renal disease on natriuretic peptide testing for diagnosing decompensated heart failure and predicting mortality. Clin Chem 2007; 53:1511–1519.
- Goetze JP, Jensen G, Møller S, Bendtsen F, Rehfeld JF, Henriksen JH. BNP and N-terminal proBNP are both extracted in the normal kidney. Eur J Clin Invest 2006; 36:8–15.
- Zoccali C. Biomarkers in chronic kidney disease: utility and issues towards better understanding. Curr Opin Nephrol Hypertens 2005; 14:532–537.
- Haapio M, Ronco C. BNP and a renal patient: emphasis on the unique characteristics of B-type natriuretic peptide in end-stage kidney disease. Contrib Nephrol 2008; 161:68–75.
- Apple FS, Murakami MM, Pearce LA, Herzog CA. Multibiomarker risk stratification of N-terminal pro-B-type natriuretic peptide, high-sensitivity C-reactive protein, and cardiac troponin T and I in end-stage renal disease for all-cause death. Clin Chem 2004: 50:2279–2285.
- Hogenhuis J, Voors AA, Jaarsma T, et al. Anemia and renal dysfunction are independently associated with BNP and NT-proBNP levels in patients with heart failure. Eur J Heart Fail 2007; 9:787–794.
- Madsen LH, Ladefoged S, Corell P, Schou M, Hildebrandt PR, Atar D. N-terminal pro brain natriuretic peptide predicts mortality in patients with end-stage renal disease on hemodialysis. Kidney Int 2007; 71:548–554.
- Wang AY, Lai KN. Use of cardiac biomarkers in end-stage renal disease. J Am Soc Nephrol 2008; 19:1643–1652.
- Mallamaci F, Zoccali C, Tripepi G, et al; on behalf of the CREED Investigators. Diagnostic potential of cardiac natriuretic peptides in dialysis patients. Kidney Int 2001; 59:1559–1566.
- Biasioli S, Zamperetti M, Borin D, Guidi G, De Fanti E, Schiavon R. Significance of plasma B-type natriuretic peptide in hemodialysis patients: blood sample timing and comorbidity burden. ASAIO J 2007; 53:587–591.
- McCullough PA, Duc P, Omland T, et al. B-type natriuretic peptide and renal function in the diagnosis of heart failure: an analysis from the Breathing Not Properly multinational study. Am J Kidney Dis 2003; 41:571–579.