More than 5 million patients are admitted to ICUs each year in the United States, and approximately 2% to 10% of these patients develop acute kidney injury requiring renal replacement therapy (AKI-RRT). AKI-RRT carries high morbidity and mortality (Hoste EA, et al. Intensive Care Med. 2015;41:1411) and is associated with renal and systemic complications, such as cardiovascular disease. RRT, frequently provided by nephrologists and/or intensivists, is a supportive therapy that can be life-saving when provided to the right patient at the right time. However, several questions related to the provision of RRT still remain, including the optimal timing of RRT initiation, the development of quality metrics for optimal RRT deliverables and monitoring, and the optimal strategy of RRT de-escalation and risk-stratification of renal recovery. Overall, there is paucity of randomized trials and standardized risk-stratification tools that can guide RRT in the ICU.
Current vexed questions of RRT deliverables in the ICU
There is ongoing research aiming to answer critical questions that can potentially improve current standards of RRT.
What is the optimal time of RRT initiation for critically ill patients with AKI?
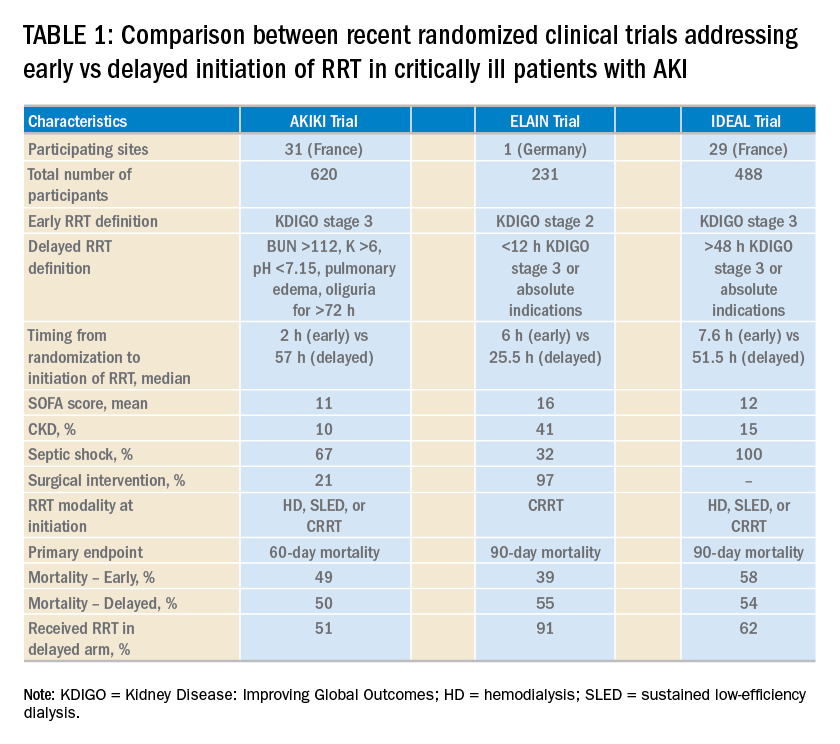
Over the last 2 years, three randomized clinical trials have attempted to address this important question involving heterogeneous ICU populations and distinct research hypotheses and study designs. Two of these studies, AKIKI (Gaudry S, et al. N Engl J Med. 2016;375:122) and IDEAL-ICU (Barbar SD, et al. N Engl J Med. 2018;379:1431) yielded no significant difference in the primary outcome of 60-day and 90-day all-cause mortality between the early vs delayed RRT initiation strategies, respectively (Table 1). Further, AKIKI showed no difference in RRT dependence at 60 days and higher catheter-related infections and hypophosphatemia in the early initiation arm. It is important to note that IDEAL-ICU was stopped early for futility after the second planned interim analysis with only 56% of patients enrolled (main hypothesis was that early RRT initiation reduced 90-day all-cause mortality by 10%). In contrast, the ELAIN trial (Zarbock A, et al. JAMA. 2016;315:2190) showed a significant 90-day mortality reduction (39% vs 55%), reduced RRT need (9 days vs 25 days), and reduced length of stay (51 days vs 82 days) favoring early RRT initiation strategy. A larger study (STARRT-AKI) addressing this question with a more pragmatic approach (incorporating clinical judgment and equipoise among intensivists and nephrologists for patient eligibility) is underway. However, it is possible that STARRT-AKI will not provide a definitive answer for the inevitable search for implementing RRT initiation protocols in the ICU. Therefore, the scientific community may need to redirect the research focus to risk-stratification tools that can assist in the identification of patients who could benefit from early RRT initiation through an individualized approach rather than a standardized protocol.
How can RRT deliverables in the ICU be effectively and systematically monitored?
The provision of RRT to ICU patients with AKI requires an iterative adjustment of the RRT prescription and goals of therapy to accommodate changes in the clinical status with emphasis in hemodynamics, multiorgan failure, and fluid overload (Neyra JA. Clin Nephrol. 2018;90:1). The utilization of static and functional tests or point-of-care ultrasonography to assess hemodynamic variables can be useful. Furthermore, the implementation of customized and automated flowsheets in the electronic health record can facilitate remote monitoring. It is, therefore, essential that the multidisciplinary ICU team develops a process to monitor and ensure RRT deliverables. In this context, the standardization and monitoring of quality metrics (dose, modality, anticoagulation, filter life, downtime, etc) and the development of effective quality management systems are critically important. However, big multicenter data are direly needed to provide insight in this arena.