Intravenous albumin is a human-derived blood product studied widely in a variety of patient populations. Despite its frequent use in critical care, few high-quality studies have demonstrated improvements in patient-important outcomes. Compared with crystalloids, albumin increases the risk of fluid overload and bleeding and infections in patients undergoing cardiac surgery.1,2 In addition, albumin is costly, and its production is fraught with donor supply chain ethical concerns (the majority of albumin is derived from paid plasma donors).
Albumin use is highly variable between countries, hospitals, and even clinicians within the same specialty due to several factors, including the perception of minimal risk with albumin, concerns regarding insufficient short-term hemodynamic response to crystalloid, and lack of high-quality evidence to inform clinical practice. We will discuss when intensivists should consider albumin use (with prescription personalized to patient context) and when it should be avoided due to the concerns for patient harm.
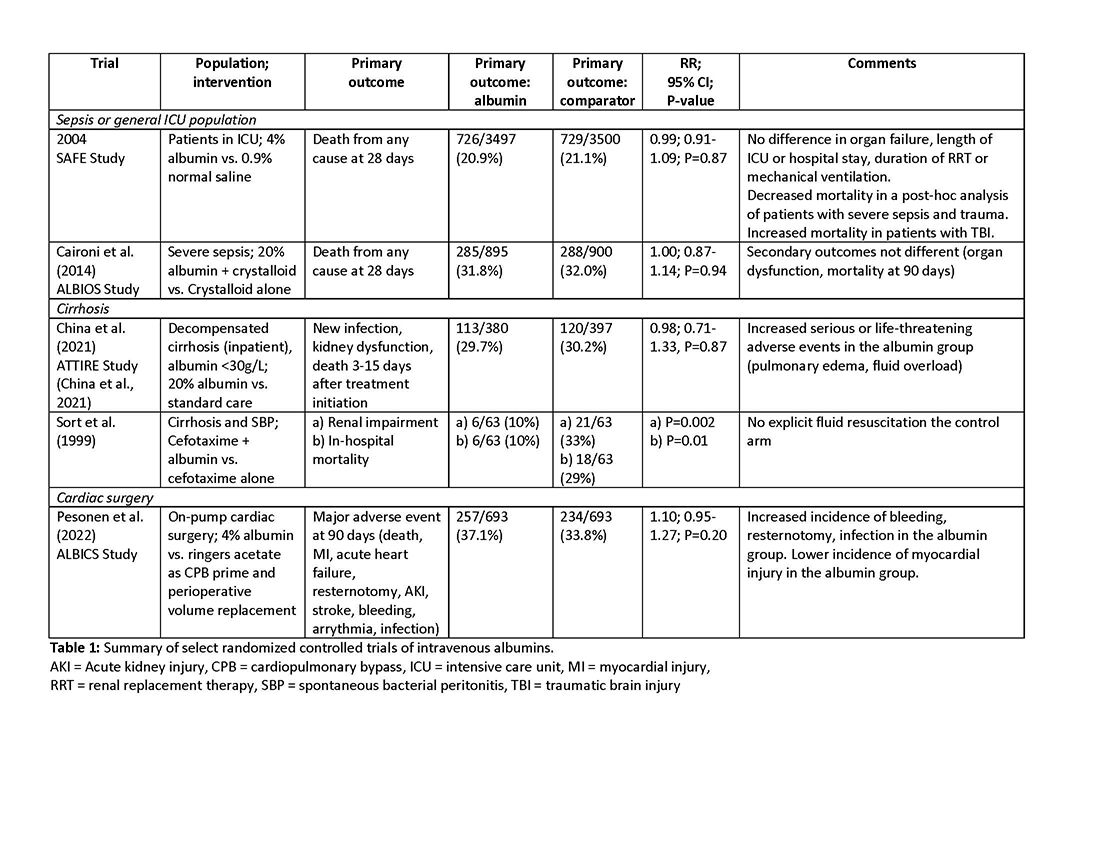
An intensivist might consider albumin as a reasonable treatment option in patients with cirrhosis undergoing large volume paracentesis to prevent paracentesis-induced circulatory dysfunction, and in patients with cirrhosis and spontaneous bacterial peritonitis (SBP), as data suggests use in this setting leads to a reduction in mortality.3 Clinicians should be aware that even for these widely accepted albumin indications, which are supported by published guidelines, the certainty of evidence is low, recommendations are weak (conditional), and, therefore, albumin should always be personalized to the patient based on volume of paracentesis fluid removed, prior history of hypotension after procedures, and degree of renal dysfunction.4
There are also several conditions for which an intensivist might consider albumin and for which albumin is commonly administered but lacks high-quality studies to support its use either as a frontline or rescue fluid therapy. One such condition is type 1 hepatorenal syndrome (HRS), for which albumin is widely used; however, there are no randomized controlled trials that have compared albumin with placebo. Instead, all studies examining this indication have included albumin in both treatment and control arms, while evaluating a variety of drug therapies that included terlipressin, midodrine, and octreotide. This does not allow for evaluation of albumin and its effect on outcomes in HRS. Intensivists should be aware of the concerns that the combination of terlipressin, an agent commonly used for HRS, and albumin may increase the risk of respiratory failure, fluid overload, and mortality.5 Albumin could also be considered in patients with sepsis after a trial of crystalloid, another indication lacking randomized controlled trial data. There are studies examining albumin as a frontline volume replacement in patients with sepsis, and this is recommended against in clinical practice guidelines, given no evidence of benefit.4 Lastly, albumin could be considered in patients with, or at high risk for, intradialytic hypotension, although there are no clinical studies demonstrating superiority of this approach over other fluid alternatives, and consideration should first be given to less costly alternative strategies (for example, oral midodrine, high dialysate sodium, lower dialysate temperature, isolated ultrafiltration).