User login
CLICK HERE to access several articles on treating fertility issues.
Dr. Abusief reports no financial relationships relevant to this article. Dr. Adamson reports that he receives research grants from LabCorp and Auxogyn, and is the founder and CEO of Advanced Reproductive Care.
Infertility is not just a woman’s issue; it is a couple’s issue. According to the Centers for Disease Control and Prevention, one-third of cases of infertility are caused by a reproductive problem for the woman, one-third are caused by a problem for the man, and one-third are due to problems for both partners or to unknown causes.1
Here, we discuss three developments within the past 12 months related to the treatment of infertility:
- The International Federation of Gynecology and Obstetrics (FIGO) Committee on Reproductive Medicine—charged with developing evidence-based, cost-effective guidelines that would be accepted as standards for increasing access to quality reproductive medical care in all countries of the world—has developed The FIGO Fertility Tool Box™.
- Smoking cigarettes negatively affects a man’s and woman’s fertility, yet smoking’s contribution to infertility is under-recognized. The Practice Committee of the American Society for Reproductive Medicine culled the evidence, and published its review on the effects of smoking on fertility.
- Results of a large, population-wide cohort study shed light on the association of in vitro fertilization (IVF) and intracytoplasmic sperm injection (ICSI) with birth defects and whether underlying factors present in patients with infertility also may play a role.
FIGO offers Tools for managing infertility
Adamson GD. A quick guide to the FIGO Fertility Tool Box. The FIGO Fertility Tool Box. The International Federation of Gynecology and Obstetrics Web site. http://www.arcfertility.com/figo. Published 2013. Accessed January 21, 2013.
FIGO has as members 125 national ObGyn societies. The FIGO Committee on Reproductive Medicine’s mission is to create access to quality reproductive medical care and is focused on helping infertile women become pregnant and/or on alleviating the burden of infertility. The Committee has just released The FIGO Fertility Tool Box™ to further this goal.
Who should use the Tool Box?
Anybody who wants to help infertile people! It is designed for health-care workers and others who want to make a difference in the lives of infertile people. The Tool Box can be accessed electronically on both computers and cell phones at http://www.figo.org/news/resources/FIGO_Fertility_Tool_Box.
What’s in the Tool Box?
Seven Tools help you tackle the disease/ disability of infertility. Each Tool provides information on how to manage a particular aspect of infertility:
- Tool 1: The FIGO Fertility Daisy—why we should care about infertility
- Tool 2: Overcome personal barriers
- Tool 3: Overcome societal barriers
- Tool 4: Diagnose infertility
- Tool 5: Treat infertility
- Tool 6: Refer/resolve infertility
- Tool 7: Prevent infertility.
The Tools Pyramid ( FIGURE 1 ) contains these seven Tools.
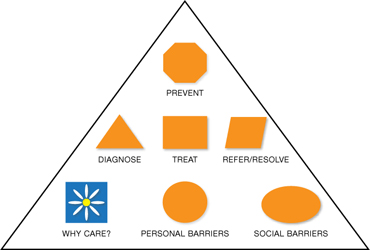
FIGURE 1 The Tools Pyramid™
How do the Tools work?
Each of the seven Tools consists of three levels:
Level 1: Basic Tools. The first level consists of 7 Basic Tools™, which contain information that is brief and succinct—just a simple statement or summary of the Daisy and each of the six Pyramids of Action. The Basic Tools are colored orange.
Level 2: Support Tools. The second level is Support Tools™, which provide more information and detail—enough so that you know what to do to take action. Support Tools are colored green.
Level 3: Reference Tools. The third level is Reference Tools™, which are lists of references that provide evidence for the information and recommended actions in the Basic and Support Tools. Reference Tools are colored white.
The Glossary provides definitions and explanations of abbreviations and acronyms and is colored white like the References. By coloring the levels icons this way, you can always tell whether you are using a Basic Tool, Support Tool, or Reference Tool.
The Levels Pyramid (FIGURE 2 ) shows how the Basic Tools, Support Tools, and Reference Tools relate to each other. You can choose your Tool and level by clicking on the icons from your computer or cellphone.
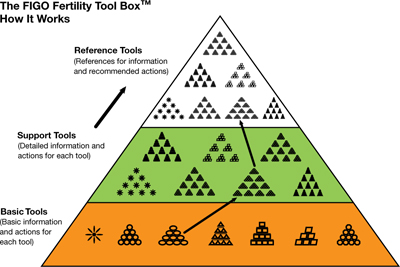
FIGURE 2 The Levels Pyramid™
How do I know what to do?—The Actions Pyramids™
With the exception of the Daisy (Tool 1), all Tools have the shape of a pyramid ( FIGURE 3 ). At the base of each pyramid are actions that can be taken in low-resource settings; that is, they are often simpler, elementary, involve fewer people and are low-cost interventions or opportunities. Overall, there are 64 total actions described in the seven Tools.
As you move higher in the pyramid, generally more resources are required to take actions that are usually more complex. You can think of it as a kind of ladder—as you climb higher it usually gets a bit more complex or complicated. Sometimes, however, it might also be easier higher up on the ladder, and the elementary aspects might be those most challenging.
If you are interested in helping a patient with infertility, you are encouraged to do whatever you can do at any level in any of the Tools in The Actions Pyramid. In the online version, you can click on the actions arrow or icons to click immediately to the action you wish to learn about and do.
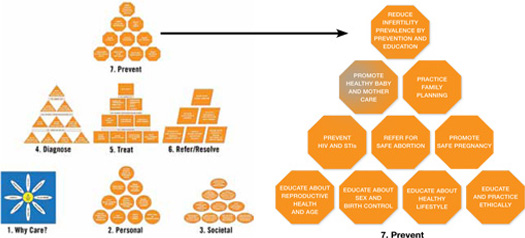
FIGURE 3 The Actions Pyramid™
Which Tool should I use?
The one you think will work best for you and will give you some results quickly. Doing something is better than doing nothing. There is no right or wrong way to make a start. Then, if you want to do more you can choose other Tools or individual aspects of other Tools to build on what you have already achieved. Or, if you want to be very systematic and are very committed you can start with Tool 1 and work your way through the entire Tool Box.
What if I can’t implement some of the recommendations?
Then drop it and move onto something that you can do or implement. No single component of the Tool Box is so essential to helping infertile couples that your efforts will fail if you can’t apply it. Using even one or two actions in one or two Tools will empower you to help many infertile individuals.
What if I want to change a Tool?
Just do it. The Tool Box is made to be changed so that it can be adapted to work in any type of health-care setting anywhere in the world. You know what works best in your situation. Just never stop caring and trying to help infertile people.
Does the Tool Box have a compliments and complaints section?
Yes, it is called the FIGO Committee on Reproductive Medicine. E-mail us at fertilitytool box@figo.org. We would love to hear from you about what you like and what works in the Tool Box and what doesn’t. We hope to constantly improve The FIGO Fertility Tool Box to make it a better Tool to help you tackle the disease/disability of infertility.
This Tool Box now gives providers at any level of women’s health care anywhere in the world easy electronic access to comprehensive evidence-based actions that can be used to help those with infertility
Smoking, by either partner, active or passive, negatively affects reproductive health
Pfeifer S, Fritz M, Goldberg J, et al; the Practice Committee of the American Society for Reproductive Medicine. Smoking and infertility: a committee opinion. Fertil Steril. 2012;98(6):1400–1406.
Approximately 30% of reproductive-age women and 35% of reproductive-age men smoke cigarettes. Although smoking has been linked to many adverse health effects, the substantial detrimental effects of cigarette smoking on fecundity and reproduction are under-recognized. In a recent publication, the Practice Committee of the American Society for Reproductive Medicine reviewed the effects of smoking on fertility.
Smoking’s ill effects on fertility
Conception delay
Smokers are at an increased risk for infertility and conception delay. Independent of other factors, smoking has a negative impact on fecundity, with a trend toward increased time to conception with increased number of cigarettes smoked.2,3 The percentage of women experiencing conception delay for more than 12 months was shown to be 54% higher in smoking versus nonsmoking women in one study.3 These authors found that active smoking by either partner had an adverse effect on conception. Furthermore, the impact of passive smoking by either partner was found to be only slightly less than the impact found for active smoking by either partner.3
Ovarian follicular depletion
Basal levels of follicle-stimulating hormone (FSH) are significantly higher in smokers, with one study demonstrating a 66% increase in smoking versus nonsmoking women and a 39% increase in passive versus nonsmoking women.4 Chemicals in cigarette smoke appear to accelerate follicular depletion and loss of reproductive function, and menopause has been found to occur 1 to 4 years earlier in smoking versus nonsmoking women.2
Effects on sperm parameters
Poor function. Smoking has been found to reduce sperm density, motility, and possibly morphology. Sperm function tests appear to be 22% poorer in smokers versus nonsmokers, and the effects are dose-dependent.
No link to male infertility, yet. While evidence suggests an adverse effect on sperm function from smoking, available data do not conclusively demonstrate a reduction in male fertility due to smoking. This could be due to secondary confounding effects of partner status.2
Maternal smoking may decrease sperm counts in offspring, according to Storgaard and colleagues, who found that men whose mothers had smoked more than 10 cigarettes per day had lower sperm densities than men with nonsmoking mothers.5
Mutagenic potential
Tobacco smoke exposure may harm gametogenesis by adversely affecting chromosomes and damaging the meiotic spindle and has been associated with an increased risk of trisomy 21 offspring resulting from maternal nondisjunction.6,7 Gene damage in sperm may be secondary to direct binding of tobacco smoke constituents or chemical byproducts to DNA, creating premutational lesions or “adducts.” These mutagenic adducts have been found in greater numbers in embryos from smokers versus nonsmokers, suggesting a mechanism for the transmission of adversely modified DNA from parental smoking.2
Early pregnancy effects
Smoking increases the risk of spontaneous miscarriage in both natural and assisted conceptions and has been linked to an increased risk for bacterial vaginosis, which in turn increases the risk for second trimester miscarriage and preterm labor.2 Studies also have identified an increased risk of ectopic pregnancy in smokers, including one study demonstrating an odds ratio (OR) for ectopic pregnancy of 3.5 (95% confidence interval [CI], 1.4–8.6) in women who smoked more than 20 cigarettes per day versus nonsmokers.6
Assisted reproductive therapies rendered less effective
Studies of IVF have demonstrated that smokers versus nonsmokers have an increased gonadotropin requirement for ovarian stimulation, lower peak estradiol levels, elevated testosterone levels, fewer oocytes retrieved, higher numbers of cancelled cycles, thicker zone pellucida, lower implantation rates, and an increased rate of failed fertilization.2 In order to achieve conception, smokers require nearly twice the number of IVF cycles versus nonsmokers.2 Authors of a 5-year, prospective study controlling for potential confounders found that if a woman ever smoked in her lifetime, her risk of failing to conceive with assisted reproductive technologies (ART) more than doubled (relative risk, 2.5; 95% CI, 1.38–4.55). Each year of smoking was associated with a 9% increase in the risk of unsuccessful ART cycles (95% CI, 1.02–1.15;
P <.01).8
We have an important role in helping patients quit
A study involving smoking cessation in infertile women found that simple interventions, such as counseling, education, and encouragement during each clinic visit, were more successful than merely providing educational materials and Web site addresses. The rates of smoking cessation increased from 4% at baseline to 24% after 12 months.9
The Public Health Service and National Cancer Institute offer validated, office-based intervention guidelines for smoking cessation, including a five-step approach2 :
- Ask about smoking at every opportunity
- Advise all smokers to stop
- Assist willingness to stop
- Assist patients in stopping (including through the use of pharmaceuticals and carbon monoxide handheld monitors)
- Arrange follow-up visits.
The use of adjunctive medical therapies, including nicotine replacement therapy and/or buproprion, has resulted in a twofold increase in the proportion of nonpregnant women who quit smoking.2 These medical therapies may be useful if behavioral approaches alone fail—although their use has not been studied in infertile women. Smoking cessation rates appear to be higher in infertile versus pregnant women, yet only 18% of women referred for infertility care have received advice on smoking cessation from their referring provider.9
The detrimental effects of smoking on reproductive health are substantial. Nonsmokers with excessive exposure to tobacco smoke have adverse reproductive effects that may be as great as those observed in smokers.
Studies suggest that much of the reduced fecundity observed in smokers may be reversed within 1 year of smoking cessation.2 Clinicians who care for smokers with infertility have a tremendous opportunity to facilitate smoking cessation in their patients and their partners. Smoking-cessation intervention should be a key component of effective treatment of infertility.
The safety of assisted reproductive technologies
Davies ML, Moore VM, Willson KJ, et al. Reproductive technologies and the risk of birth defects. N Engl J Med. 2012;366(19):1803–1813.
Since the birth of Louise Brown, the first baby born after being conceived with in vitro fertilization (IVF) in 1978, IVF has become a pillar in the treatment of infertility. Although recognized as a highly effective treatment, the safety of IVF and its related technologies, such as intracytoplasmic sperm injection (ICSI), has been questioned. Studies have linked the use of assisted reproduction, including IVF and ICSI, with an increased risk of birth defects.10-15 Findings, however, were limited by small sample sizes and lack of appropriate controls. Furthermore, it has been unclear if this increased risk is due to factors related to treatment or to an underlying factor present in patients with infertility. It also has been unclear whether there is a differential in risk according to the type of ART used. In a large population-wide cohort study, Davies and colleagues linked a census of treatment with ART in South Australia to a registry of births and terminations with a gestation period of at least 20 weeks or a birth weight of 400 g and registries of birth defects.
The authors compared the risk of birth defects in pregnancies among women who had conceived with the use of ART, women with spontaneous pregnancies who had had a previous birth after ART treatment, women with a diagnosis of infertility who had conceived without ART, and pregnancies in women without infertility. Births and pregnancy terminations secondary to birth defects were studied to assess the birth defect risk from pregnancy to a child’s fifth birthday.
Details of the trial
A total of 308,974 births were included in the analysis. Births in women who conceived with the use of ART were associated with a significant increase in risk of birth defects (8.3%) compared with births conceived spontaneously in fertile women (8.3% vs 5.8%, respectively; unadjusted OR, 1.47; 95% CI, 1.33-1.62). This effect remained significant after multivariate adjustment (adjusted OR, 1.28; 95% CI, 1.16-1.41).
While there was no significant association between ART and the risk of specific syndromes such as Down’s, Turner’s, Edward’s, and others, there was a significantly increased adjusted OR for any defect and multiple defects in births conceived with ART versus those conceived spontaneously in fertile women.
The OR for birth defects associated with IVF was 1.26 (95% CI, 1.07-1.48) in unadjusted analyses and 1.07 (95% CI, 0.9-1.26) after multivariate adjustment. The OR for birth defects associated with IVF with ICSI were 1.77 (95% CI, 1.47-2.12) in unadjusted and 1.57 (95% CI, 1.30-1.90) after multivariate analysis. Compared with ICSI, IVF was associated with a reduced risk of any birth defect (OR, 0.68; 95% CI, 0.53-0.87).
Births after gamete intrafallopian transfer, intrauterine insemination, or the use of clomiphene citrate at home were associated with significantly increased risks of any birth defect in adjusted analyses. Births after conception with donor insemination and clinically supervised ovulation induction were not associated with an increased risk of birth defects. Births occurring after spontaneous conception in women with a history of a previous birth with ART were also associated with an increased risk of birth defects, even after adjustment for confounders (adjusted OR, 1.25; 95% CI, 1.01-1.56). Births occurring after spontaneous conception in women with a history of infertility without previous ART treatment were also significantly associated with a small increased risk in birth defects (OR, 1.29; 95% CI, 0.99-1.68).
ICSI and birth-defect association persisted
In this large observational study, the authors confirmed findings from previous studies11,12,16-18 that the number of birth defects found in pregnancies conceived with ART are higher than the number found in pregnancies conceived spontaneously. In this study, after multivariate adjustment, the association between IVF and an increased risk of birth defects was found to be no longer significant, but the risk remained elevated after ART with ICSI. These findings are similar to results in previous studies.18,19 The increased risk may be secondary to the ICSI procedure itself19,20 or to underlying male infertility factors leading to the use of ICSI.14
Birth defects appeared to be highest in fresh embryo cycles of ICSI versus IVF and lowest in frozen-embryo cycles. A reduction in birth defects with cryopreservation may be secondary to a reduced likelihood that cryopreserved embryos would survive the thawing process as well as the temporal separation of the developing embryo from a hormonally stimulated cycle.21-23 Treatment with ART was associated with an increased risk of cardiovascular, musculoskeletal, urogenital, and gastrointestinal defects, as well as cerebral palsy. The observation of an increased risk of cerebral palsy with ART treatment is consistent with findings from a previous study. Strömberg and colleagues found that the risk of cerebral palsy was increased by a factor of 3.7 among multiples conceived with IVF and 2.8 among singletons conceived with IVF.24
Davies and colleagues also observed that the risk of a birth defect was increased among women with a history of infertility who were able to conceive without ART,25 a finding observed in a previous large Danish registry.15
Although the vast majority of births resulting from assisted reproduction were free of birth defects, treatment with ART was associated with an increased risk of birth defects, compared with spontaneous conception. After adjustment for potential confounders, including maternal age, the risk persisted for conceptions associated with ICSI but not IVF.
While the exact mechanisms responsible for this increased risk remain unknown, the finding of an increased risk of birth defects among women with infertility who conceived without ART indicates that inherent patient factors, rather than assisted reproductive technologies alone, contribute to the risk. These findings can help to guide couples considering assisted reproduction for the treatment of infertility.
We want to hear from you! Tell us what you think.
1. Infertility FAQs. Centers for Disease Control and Prevention Web site. http://www.cdc.gov/reproductivehealth/infertility. Updated April 19 2012. Accessed January 20, 2013.
2. Pfeifer S, Fritz M, Goldberg J, et al. the Practice Committee of the American Society for Reproductive Medicine. Smoking and infertility: a committee opinion. Fertil Steril. 2012;98(6):1400-1406.
3. Hull MG, North K, Taylor H, Farrow A, Ford WC. Delayed conception and active and passive smoking: The Avon Longitudinal Study of Pregnancy and Childhood Study Team. Fertil Steril. 2000;74(4):725-733.
4. Cooper GS, Baird DD, Hulka BS, Weinberg CR, Savitz DA, Hughes CL, Jr. Follicle-stimulating hormone concentrations in relation to active and passive smoking. Obstet Gynecol. 1995;85(3):407-411.
5. Storgaard L, Bonde JP, Ernst E, et al. Does smoking during pregnancy affect sons’ sperm counts? Epidemiology. 2003;14(3):278-286.
6. Yang Q, Sherman SL, Hassold TJ, et al. Risk factors for trisomy 21: maternal cigarette smoking and oral contraceptive use in a population-based case-control study. Genet Med. 1999;1(3):80-88.
7. Zenzes MT, Wang P, Casper RF. Cigarette smoking may affect meiotic maturation of human oocytes. Hum Reprod. 1995;10(12):3213-3217.
8. Klonoff-Cohen H, Natarajan L, Marrs R, Yee B. Effects of female and male smoking on success rates of IVF and gamete intra-Fallopian transfer. Hum Reprod. 2001;16(7):1382-1390.
9. Hughes EG, Lamont DA, Beecroft ML, Wilson DM, Brennan BG, Rice SC. Randomized trial of a “stage of change” oriented smoking cessation intervention in infertile and pregnant women. Fertil Steril. 2000;74(3):498-503.
10. Rimm AA, Katayama AC, Diaz M, Katayama KP. A meta-analysis of controlled studies comparing major malformation rates in IVF and ICSI infants with naturally conceived children. J Assist Reprod Genet. 2004;21(12):437-443.
11. Hansen M, Kurinczuk JJ, Bower C, Webb S. The risk of major birth defects after intracytoplasmic sperm injection and in vitro fertilization. N Engl J Med. 2002;346(10):725-730.
12. Hansen M, Bower C, Milne E, de Klerk N, Kurinczuk JJ. Assisted reproductive technologies and the risk of birth defects—a systematic review. Hum Reprod. 2005;20(2):328-338.
13. Schieve LA, Rasmussen SA, Reefhuis J. Risk of birth defects among children conceived with assisted reproductive technology: providing an epidemiologic context to the data. Fertil Steril. 2005;84(5):1320-1324.
14. Lie RT, Lyngstadaas A, Ørstavik KH, Bakketeig LS, Jacobsen G, Tanbo T. Birth defects in children conceived by ICSI compared with children conceived by other IVF-methods; a meta-analysis. Int J Epidemiol. 2005;34(3):696-701.
15. Zhu JL, Basso O, Obel C, Bille C, Olsen J. Infertility infertility treatment, and congenital malformations: Danish national birth cohort. BMJ. 2006;333(7570):679.-
16. Reefhuis J, Honein MA, Schieve LA, Correa A, Hobbs CA, Rasmussen SA. National Birth Defects Prevention Study. Assisted reproductive technology and major structural birth defects in the United States. Hum Reprod. 2009;24(2):360-366.
17. El-Chaar D, Yang Q, Gao J, et al. Risk of birth defects increased in pregnancies conceived by assisted human reproduction. Fertil Steril. 2009;92(5):1557-1561.
18. Källén B, Finnström O, Nygren KG, Olausson PO. In vitro fertilization (IVF) in Sweden: infant outcome after different IVF fertilization methods. Fertil Steril. 2005;84(3):611-617.
19. Bonduelle M, Wennerholm U, Loft A, et al. A multi-centre cohort study of the physical health of 5-year-old children conceived after intracytoplasmic sperm injection, in vitro fertilization and natural conception. Hum Reprod. 2005;20(2):413-419.
20. Kurinczuk JJ. Safety issues in assisted reproduction technology: from theory to reality — just what are the data telling us about ICSI offspring health and future fertility and should we be concerned? Hum Reprod. 2003;18(5):925-931.
21. Pinborg A, Loft A, Aaris Henningsen AK, Rasmussen S, Andersen AN. Infant outcome of 957 singletons born after frozen embryo replacement: the Danish National Cohort Study 1995-2006. Fertil Steril. 2010;94(4):1320-1327.
22. Halliday JL, Ukoumunne OC, Baker HW, et al. Increased risk of blastogenesis birth defects, arising in the first 4 weeks of pregnancy, after assisted reproductive technologies. Hum Reprod. 2010;25(1):59-65.
23. Wennerholm U, Söderström-Anttila V, Bergh C, et al. Children born after cryopreservation of embryos or oocytes: a systematic review of outcome data. Hum Reprod. 2009;24(9):2158-2172.
24. Strömberg B, Dahlquist G, Ericson A, Finnström O, Köster M, Stjernqvist K. Neurological sequelae in children born after in-vitro fertilisation: a population based study. Lancet. 2002;359(9305):461-465.
25. Davies MJ, Moore VM, Willson KJ, et al. Reproductive technologies and the risk of birth defects. N Engl J Med. 2012;366(19):1803-1813.
CLICK HERE to access several articles on treating fertility issues.
Dr. Abusief reports no financial relationships relevant to this article. Dr. Adamson reports that he receives research grants from LabCorp and Auxogyn, and is the founder and CEO of Advanced Reproductive Care.
Infertility is not just a woman’s issue; it is a couple’s issue. According to the Centers for Disease Control and Prevention, one-third of cases of infertility are caused by a reproductive problem for the woman, one-third are caused by a problem for the man, and one-third are due to problems for both partners or to unknown causes.1
Here, we discuss three developments within the past 12 months related to the treatment of infertility:
- The International Federation of Gynecology and Obstetrics (FIGO) Committee on Reproductive Medicine—charged with developing evidence-based, cost-effective guidelines that would be accepted as standards for increasing access to quality reproductive medical care in all countries of the world—has developed The FIGO Fertility Tool Box™.
- Smoking cigarettes negatively affects a man’s and woman’s fertility, yet smoking’s contribution to infertility is under-recognized. The Practice Committee of the American Society for Reproductive Medicine culled the evidence, and published its review on the effects of smoking on fertility.
- Results of a large, population-wide cohort study shed light on the association of in vitro fertilization (IVF) and intracytoplasmic sperm injection (ICSI) with birth defects and whether underlying factors present in patients with infertility also may play a role.
FIGO offers Tools for managing infertility
Adamson GD. A quick guide to the FIGO Fertility Tool Box. The FIGO Fertility Tool Box. The International Federation of Gynecology and Obstetrics Web site. http://www.arcfertility.com/figo. Published 2013. Accessed January 21, 2013.
FIGO has as members 125 national ObGyn societies. The FIGO Committee on Reproductive Medicine’s mission is to create access to quality reproductive medical care and is focused on helping infertile women become pregnant and/or on alleviating the burden of infertility. The Committee has just released The FIGO Fertility Tool Box™ to further this goal.
Who should use the Tool Box?
Anybody who wants to help infertile people! It is designed for health-care workers and others who want to make a difference in the lives of infertile people. The Tool Box can be accessed electronically on both computers and cell phones at http://www.figo.org/news/resources/FIGO_Fertility_Tool_Box.
What’s in the Tool Box?
Seven Tools help you tackle the disease/ disability of infertility. Each Tool provides information on how to manage a particular aspect of infertility:
- Tool 1: The FIGO Fertility Daisy—why we should care about infertility
- Tool 2: Overcome personal barriers
- Tool 3: Overcome societal barriers
- Tool 4: Diagnose infertility
- Tool 5: Treat infertility
- Tool 6: Refer/resolve infertility
- Tool 7: Prevent infertility.
The Tools Pyramid ( FIGURE 1 ) contains these seven Tools.

FIGURE 1 The Tools Pyramid™
How do the Tools work?
Each of the seven Tools consists of three levels:
Level 1: Basic Tools. The first level consists of 7 Basic Tools™, which contain information that is brief and succinct—just a simple statement or summary of the Daisy and each of the six Pyramids of Action. The Basic Tools are colored orange.
Level 2: Support Tools. The second level is Support Tools™, which provide more information and detail—enough so that you know what to do to take action. Support Tools are colored green.
Level 3: Reference Tools. The third level is Reference Tools™, which are lists of references that provide evidence for the information and recommended actions in the Basic and Support Tools. Reference Tools are colored white.
The Glossary provides definitions and explanations of abbreviations and acronyms and is colored white like the References. By coloring the levels icons this way, you can always tell whether you are using a Basic Tool, Support Tool, or Reference Tool.
The Levels Pyramid (FIGURE 2 ) shows how the Basic Tools, Support Tools, and Reference Tools relate to each other. You can choose your Tool and level by clicking on the icons from your computer or cellphone.

FIGURE 2 The Levels Pyramid™
How do I know what to do?—The Actions Pyramids™
With the exception of the Daisy (Tool 1), all Tools have the shape of a pyramid ( FIGURE 3 ). At the base of each pyramid are actions that can be taken in low-resource settings; that is, they are often simpler, elementary, involve fewer people and are low-cost interventions or opportunities. Overall, there are 64 total actions described in the seven Tools.
As you move higher in the pyramid, generally more resources are required to take actions that are usually more complex. You can think of it as a kind of ladder—as you climb higher it usually gets a bit more complex or complicated. Sometimes, however, it might also be easier higher up on the ladder, and the elementary aspects might be those most challenging.
If you are interested in helping a patient with infertility, you are encouraged to do whatever you can do at any level in any of the Tools in The Actions Pyramid. In the online version, you can click on the actions arrow or icons to click immediately to the action you wish to learn about and do.

FIGURE 3 The Actions Pyramid™
Which Tool should I use?
The one you think will work best for you and will give you some results quickly. Doing something is better than doing nothing. There is no right or wrong way to make a start. Then, if you want to do more you can choose other Tools or individual aspects of other Tools to build on what you have already achieved. Or, if you want to be very systematic and are very committed you can start with Tool 1 and work your way through the entire Tool Box.
What if I can’t implement some of the recommendations?
Then drop it and move onto something that you can do or implement. No single component of the Tool Box is so essential to helping infertile couples that your efforts will fail if you can’t apply it. Using even one or two actions in one or two Tools will empower you to help many infertile individuals.
What if I want to change a Tool?
Just do it. The Tool Box is made to be changed so that it can be adapted to work in any type of health-care setting anywhere in the world. You know what works best in your situation. Just never stop caring and trying to help infertile people.
Does the Tool Box have a compliments and complaints section?
Yes, it is called the FIGO Committee on Reproductive Medicine. E-mail us at fertilitytool box@figo.org. We would love to hear from you about what you like and what works in the Tool Box and what doesn’t. We hope to constantly improve The FIGO Fertility Tool Box to make it a better Tool to help you tackle the disease/disability of infertility.
This Tool Box now gives providers at any level of women’s health care anywhere in the world easy electronic access to comprehensive evidence-based actions that can be used to help those with infertility
Smoking, by either partner, active or passive, negatively affects reproductive health
Pfeifer S, Fritz M, Goldberg J, et al; the Practice Committee of the American Society for Reproductive Medicine. Smoking and infertility: a committee opinion. Fertil Steril. 2012;98(6):1400–1406.
Approximately 30% of reproductive-age women and 35% of reproductive-age men smoke cigarettes. Although smoking has been linked to many adverse health effects, the substantial detrimental effects of cigarette smoking on fecundity and reproduction are under-recognized. In a recent publication, the Practice Committee of the American Society for Reproductive Medicine reviewed the effects of smoking on fertility.
Smoking’s ill effects on fertility
Conception delay
Smokers are at an increased risk for infertility and conception delay. Independent of other factors, smoking has a negative impact on fecundity, with a trend toward increased time to conception with increased number of cigarettes smoked.2,3 The percentage of women experiencing conception delay for more than 12 months was shown to be 54% higher in smoking versus nonsmoking women in one study.3 These authors found that active smoking by either partner had an adverse effect on conception. Furthermore, the impact of passive smoking by either partner was found to be only slightly less than the impact found for active smoking by either partner.3
Ovarian follicular depletion
Basal levels of follicle-stimulating hormone (FSH) are significantly higher in smokers, with one study demonstrating a 66% increase in smoking versus nonsmoking women and a 39% increase in passive versus nonsmoking women.4 Chemicals in cigarette smoke appear to accelerate follicular depletion and loss of reproductive function, and menopause has been found to occur 1 to 4 years earlier in smoking versus nonsmoking women.2
Effects on sperm parameters
Poor function. Smoking has been found to reduce sperm density, motility, and possibly morphology. Sperm function tests appear to be 22% poorer in smokers versus nonsmokers, and the effects are dose-dependent.
No link to male infertility, yet. While evidence suggests an adverse effect on sperm function from smoking, available data do not conclusively demonstrate a reduction in male fertility due to smoking. This could be due to secondary confounding effects of partner status.2
Maternal smoking may decrease sperm counts in offspring, according to Storgaard and colleagues, who found that men whose mothers had smoked more than 10 cigarettes per day had lower sperm densities than men with nonsmoking mothers.5
Mutagenic potential
Tobacco smoke exposure may harm gametogenesis by adversely affecting chromosomes and damaging the meiotic spindle and has been associated with an increased risk of trisomy 21 offspring resulting from maternal nondisjunction.6,7 Gene damage in sperm may be secondary to direct binding of tobacco smoke constituents or chemical byproducts to DNA, creating premutational lesions or “adducts.” These mutagenic adducts have been found in greater numbers in embryos from smokers versus nonsmokers, suggesting a mechanism for the transmission of adversely modified DNA from parental smoking.2
Early pregnancy effects
Smoking increases the risk of spontaneous miscarriage in both natural and assisted conceptions and has been linked to an increased risk for bacterial vaginosis, which in turn increases the risk for second trimester miscarriage and preterm labor.2 Studies also have identified an increased risk of ectopic pregnancy in smokers, including one study demonstrating an odds ratio (OR) for ectopic pregnancy of 3.5 (95% confidence interval [CI], 1.4–8.6) in women who smoked more than 20 cigarettes per day versus nonsmokers.6
Assisted reproductive therapies rendered less effective
Studies of IVF have demonstrated that smokers versus nonsmokers have an increased gonadotropin requirement for ovarian stimulation, lower peak estradiol levels, elevated testosterone levels, fewer oocytes retrieved, higher numbers of cancelled cycles, thicker zone pellucida, lower implantation rates, and an increased rate of failed fertilization.2 In order to achieve conception, smokers require nearly twice the number of IVF cycles versus nonsmokers.2 Authors of a 5-year, prospective study controlling for potential confounders found that if a woman ever smoked in her lifetime, her risk of failing to conceive with assisted reproductive technologies (ART) more than doubled (relative risk, 2.5; 95% CI, 1.38–4.55). Each year of smoking was associated with a 9% increase in the risk of unsuccessful ART cycles (95% CI, 1.02–1.15;
P <.01).8
We have an important role in helping patients quit
A study involving smoking cessation in infertile women found that simple interventions, such as counseling, education, and encouragement during each clinic visit, were more successful than merely providing educational materials and Web site addresses. The rates of smoking cessation increased from 4% at baseline to 24% after 12 months.9
The Public Health Service and National Cancer Institute offer validated, office-based intervention guidelines for smoking cessation, including a five-step approach2 :
- Ask about smoking at every opportunity
- Advise all smokers to stop
- Assist willingness to stop
- Assist patients in stopping (including through the use of pharmaceuticals and carbon monoxide handheld monitors)
- Arrange follow-up visits.
The use of adjunctive medical therapies, including nicotine replacement therapy and/or buproprion, has resulted in a twofold increase in the proportion of nonpregnant women who quit smoking.2 These medical therapies may be useful if behavioral approaches alone fail—although their use has not been studied in infertile women. Smoking cessation rates appear to be higher in infertile versus pregnant women, yet only 18% of women referred for infertility care have received advice on smoking cessation from their referring provider.9
The detrimental effects of smoking on reproductive health are substantial. Nonsmokers with excessive exposure to tobacco smoke have adverse reproductive effects that may be as great as those observed in smokers.
Studies suggest that much of the reduced fecundity observed in smokers may be reversed within 1 year of smoking cessation.2 Clinicians who care for smokers with infertility have a tremendous opportunity to facilitate smoking cessation in their patients and their partners. Smoking-cessation intervention should be a key component of effective treatment of infertility.
The safety of assisted reproductive technologies
Davies ML, Moore VM, Willson KJ, et al. Reproductive technologies and the risk of birth defects. N Engl J Med. 2012;366(19):1803–1813.
Since the birth of Louise Brown, the first baby born after being conceived with in vitro fertilization (IVF) in 1978, IVF has become a pillar in the treatment of infertility. Although recognized as a highly effective treatment, the safety of IVF and its related technologies, such as intracytoplasmic sperm injection (ICSI), has been questioned. Studies have linked the use of assisted reproduction, including IVF and ICSI, with an increased risk of birth defects.10-15 Findings, however, were limited by small sample sizes and lack of appropriate controls. Furthermore, it has been unclear if this increased risk is due to factors related to treatment or to an underlying factor present in patients with infertility. It also has been unclear whether there is a differential in risk according to the type of ART used. In a large population-wide cohort study, Davies and colleagues linked a census of treatment with ART in South Australia to a registry of births and terminations with a gestation period of at least 20 weeks or a birth weight of 400 g and registries of birth defects.
The authors compared the risk of birth defects in pregnancies among women who had conceived with the use of ART, women with spontaneous pregnancies who had had a previous birth after ART treatment, women with a diagnosis of infertility who had conceived without ART, and pregnancies in women without infertility. Births and pregnancy terminations secondary to birth defects were studied to assess the birth defect risk from pregnancy to a child’s fifth birthday.
Details of the trial
A total of 308,974 births were included in the analysis. Births in women who conceived with the use of ART were associated with a significant increase in risk of birth defects (8.3%) compared with births conceived spontaneously in fertile women (8.3% vs 5.8%, respectively; unadjusted OR, 1.47; 95% CI, 1.33-1.62). This effect remained significant after multivariate adjustment (adjusted OR, 1.28; 95% CI, 1.16-1.41).
While there was no significant association between ART and the risk of specific syndromes such as Down’s, Turner’s, Edward’s, and others, there was a significantly increased adjusted OR for any defect and multiple defects in births conceived with ART versus those conceived spontaneously in fertile women.
The OR for birth defects associated with IVF was 1.26 (95% CI, 1.07-1.48) in unadjusted analyses and 1.07 (95% CI, 0.9-1.26) after multivariate adjustment. The OR for birth defects associated with IVF with ICSI were 1.77 (95% CI, 1.47-2.12) in unadjusted and 1.57 (95% CI, 1.30-1.90) after multivariate analysis. Compared with ICSI, IVF was associated with a reduced risk of any birth defect (OR, 0.68; 95% CI, 0.53-0.87).
Births after gamete intrafallopian transfer, intrauterine insemination, or the use of clomiphene citrate at home were associated with significantly increased risks of any birth defect in adjusted analyses. Births after conception with donor insemination and clinically supervised ovulation induction were not associated with an increased risk of birth defects. Births occurring after spontaneous conception in women with a history of a previous birth with ART were also associated with an increased risk of birth defects, even after adjustment for confounders (adjusted OR, 1.25; 95% CI, 1.01-1.56). Births occurring after spontaneous conception in women with a history of infertility without previous ART treatment were also significantly associated with a small increased risk in birth defects (OR, 1.29; 95% CI, 0.99-1.68).
ICSI and birth-defect association persisted
In this large observational study, the authors confirmed findings from previous studies11,12,16-18 that the number of birth defects found in pregnancies conceived with ART are higher than the number found in pregnancies conceived spontaneously. In this study, after multivariate adjustment, the association between IVF and an increased risk of birth defects was found to be no longer significant, but the risk remained elevated after ART with ICSI. These findings are similar to results in previous studies.18,19 The increased risk may be secondary to the ICSI procedure itself19,20 or to underlying male infertility factors leading to the use of ICSI.14
Birth defects appeared to be highest in fresh embryo cycles of ICSI versus IVF and lowest in frozen-embryo cycles. A reduction in birth defects with cryopreservation may be secondary to a reduced likelihood that cryopreserved embryos would survive the thawing process as well as the temporal separation of the developing embryo from a hormonally stimulated cycle.21-23 Treatment with ART was associated with an increased risk of cardiovascular, musculoskeletal, urogenital, and gastrointestinal defects, as well as cerebral palsy. The observation of an increased risk of cerebral palsy with ART treatment is consistent with findings from a previous study. Strömberg and colleagues found that the risk of cerebral palsy was increased by a factor of 3.7 among multiples conceived with IVF and 2.8 among singletons conceived with IVF.24
Davies and colleagues also observed that the risk of a birth defect was increased among women with a history of infertility who were able to conceive without ART,25 a finding observed in a previous large Danish registry.15
Although the vast majority of births resulting from assisted reproduction were free of birth defects, treatment with ART was associated with an increased risk of birth defects, compared with spontaneous conception. After adjustment for potential confounders, including maternal age, the risk persisted for conceptions associated with ICSI but not IVF.
While the exact mechanisms responsible for this increased risk remain unknown, the finding of an increased risk of birth defects among women with infertility who conceived without ART indicates that inherent patient factors, rather than assisted reproductive technologies alone, contribute to the risk. These findings can help to guide couples considering assisted reproduction for the treatment of infertility.
We want to hear from you! Tell us what you think.
CLICK HERE to access several articles on treating fertility issues.
Dr. Abusief reports no financial relationships relevant to this article. Dr. Adamson reports that he receives research grants from LabCorp and Auxogyn, and is the founder and CEO of Advanced Reproductive Care.
Infertility is not just a woman’s issue; it is a couple’s issue. According to the Centers for Disease Control and Prevention, one-third of cases of infertility are caused by a reproductive problem for the woman, one-third are caused by a problem for the man, and one-third are due to problems for both partners or to unknown causes.1
Here, we discuss three developments within the past 12 months related to the treatment of infertility:
- The International Federation of Gynecology and Obstetrics (FIGO) Committee on Reproductive Medicine—charged with developing evidence-based, cost-effective guidelines that would be accepted as standards for increasing access to quality reproductive medical care in all countries of the world—has developed The FIGO Fertility Tool Box™.
- Smoking cigarettes negatively affects a man’s and woman’s fertility, yet smoking’s contribution to infertility is under-recognized. The Practice Committee of the American Society for Reproductive Medicine culled the evidence, and published its review on the effects of smoking on fertility.
- Results of a large, population-wide cohort study shed light on the association of in vitro fertilization (IVF) and intracytoplasmic sperm injection (ICSI) with birth defects and whether underlying factors present in patients with infertility also may play a role.
FIGO offers Tools for managing infertility
Adamson GD. A quick guide to the FIGO Fertility Tool Box. The FIGO Fertility Tool Box. The International Federation of Gynecology and Obstetrics Web site. http://www.arcfertility.com/figo. Published 2013. Accessed January 21, 2013.
FIGO has as members 125 national ObGyn societies. The FIGO Committee on Reproductive Medicine’s mission is to create access to quality reproductive medical care and is focused on helping infertile women become pregnant and/or on alleviating the burden of infertility. The Committee has just released The FIGO Fertility Tool Box™ to further this goal.
Who should use the Tool Box?
Anybody who wants to help infertile people! It is designed for health-care workers and others who want to make a difference in the lives of infertile people. The Tool Box can be accessed electronically on both computers and cell phones at http://www.figo.org/news/resources/FIGO_Fertility_Tool_Box.
What’s in the Tool Box?
Seven Tools help you tackle the disease/ disability of infertility. Each Tool provides information on how to manage a particular aspect of infertility:
- Tool 1: The FIGO Fertility Daisy—why we should care about infertility
- Tool 2: Overcome personal barriers
- Tool 3: Overcome societal barriers
- Tool 4: Diagnose infertility
- Tool 5: Treat infertility
- Tool 6: Refer/resolve infertility
- Tool 7: Prevent infertility.
The Tools Pyramid ( FIGURE 1 ) contains these seven Tools.

FIGURE 1 The Tools Pyramid™
How do the Tools work?
Each of the seven Tools consists of three levels:
Level 1: Basic Tools. The first level consists of 7 Basic Tools™, which contain information that is brief and succinct—just a simple statement or summary of the Daisy and each of the six Pyramids of Action. The Basic Tools are colored orange.
Level 2: Support Tools. The second level is Support Tools™, which provide more information and detail—enough so that you know what to do to take action. Support Tools are colored green.
Level 3: Reference Tools. The third level is Reference Tools™, which are lists of references that provide evidence for the information and recommended actions in the Basic and Support Tools. Reference Tools are colored white.
The Glossary provides definitions and explanations of abbreviations and acronyms and is colored white like the References. By coloring the levels icons this way, you can always tell whether you are using a Basic Tool, Support Tool, or Reference Tool.
The Levels Pyramid (FIGURE 2 ) shows how the Basic Tools, Support Tools, and Reference Tools relate to each other. You can choose your Tool and level by clicking on the icons from your computer or cellphone.

FIGURE 2 The Levels Pyramid™
How do I know what to do?—The Actions Pyramids™
With the exception of the Daisy (Tool 1), all Tools have the shape of a pyramid ( FIGURE 3 ). At the base of each pyramid are actions that can be taken in low-resource settings; that is, they are often simpler, elementary, involve fewer people and are low-cost interventions or opportunities. Overall, there are 64 total actions described in the seven Tools.
As you move higher in the pyramid, generally more resources are required to take actions that are usually more complex. You can think of it as a kind of ladder—as you climb higher it usually gets a bit more complex or complicated. Sometimes, however, it might also be easier higher up on the ladder, and the elementary aspects might be those most challenging.
If you are interested in helping a patient with infertility, you are encouraged to do whatever you can do at any level in any of the Tools in The Actions Pyramid. In the online version, you can click on the actions arrow or icons to click immediately to the action you wish to learn about and do.

FIGURE 3 The Actions Pyramid™
Which Tool should I use?
The one you think will work best for you and will give you some results quickly. Doing something is better than doing nothing. There is no right or wrong way to make a start. Then, if you want to do more you can choose other Tools or individual aspects of other Tools to build on what you have already achieved. Or, if you want to be very systematic and are very committed you can start with Tool 1 and work your way through the entire Tool Box.
What if I can’t implement some of the recommendations?
Then drop it and move onto something that you can do or implement. No single component of the Tool Box is so essential to helping infertile couples that your efforts will fail if you can’t apply it. Using even one or two actions in one or two Tools will empower you to help many infertile individuals.
What if I want to change a Tool?
Just do it. The Tool Box is made to be changed so that it can be adapted to work in any type of health-care setting anywhere in the world. You know what works best in your situation. Just never stop caring and trying to help infertile people.
Does the Tool Box have a compliments and complaints section?
Yes, it is called the FIGO Committee on Reproductive Medicine. E-mail us at fertilitytool box@figo.org. We would love to hear from you about what you like and what works in the Tool Box and what doesn’t. We hope to constantly improve The FIGO Fertility Tool Box to make it a better Tool to help you tackle the disease/disability of infertility.
This Tool Box now gives providers at any level of women’s health care anywhere in the world easy electronic access to comprehensive evidence-based actions that can be used to help those with infertility
Smoking, by either partner, active or passive, negatively affects reproductive health
Pfeifer S, Fritz M, Goldberg J, et al; the Practice Committee of the American Society for Reproductive Medicine. Smoking and infertility: a committee opinion. Fertil Steril. 2012;98(6):1400–1406.
Approximately 30% of reproductive-age women and 35% of reproductive-age men smoke cigarettes. Although smoking has been linked to many adverse health effects, the substantial detrimental effects of cigarette smoking on fecundity and reproduction are under-recognized. In a recent publication, the Practice Committee of the American Society for Reproductive Medicine reviewed the effects of smoking on fertility.
Smoking’s ill effects on fertility
Conception delay
Smokers are at an increased risk for infertility and conception delay. Independent of other factors, smoking has a negative impact on fecundity, with a trend toward increased time to conception with increased number of cigarettes smoked.2,3 The percentage of women experiencing conception delay for more than 12 months was shown to be 54% higher in smoking versus nonsmoking women in one study.3 These authors found that active smoking by either partner had an adverse effect on conception. Furthermore, the impact of passive smoking by either partner was found to be only slightly less than the impact found for active smoking by either partner.3
Ovarian follicular depletion
Basal levels of follicle-stimulating hormone (FSH) are significantly higher in smokers, with one study demonstrating a 66% increase in smoking versus nonsmoking women and a 39% increase in passive versus nonsmoking women.4 Chemicals in cigarette smoke appear to accelerate follicular depletion and loss of reproductive function, and menopause has been found to occur 1 to 4 years earlier in smoking versus nonsmoking women.2
Effects on sperm parameters
Poor function. Smoking has been found to reduce sperm density, motility, and possibly morphology. Sperm function tests appear to be 22% poorer in smokers versus nonsmokers, and the effects are dose-dependent.
No link to male infertility, yet. While evidence suggests an adverse effect on sperm function from smoking, available data do not conclusively demonstrate a reduction in male fertility due to smoking. This could be due to secondary confounding effects of partner status.2
Maternal smoking may decrease sperm counts in offspring, according to Storgaard and colleagues, who found that men whose mothers had smoked more than 10 cigarettes per day had lower sperm densities than men with nonsmoking mothers.5
Mutagenic potential
Tobacco smoke exposure may harm gametogenesis by adversely affecting chromosomes and damaging the meiotic spindle and has been associated with an increased risk of trisomy 21 offspring resulting from maternal nondisjunction.6,7 Gene damage in sperm may be secondary to direct binding of tobacco smoke constituents or chemical byproducts to DNA, creating premutational lesions or “adducts.” These mutagenic adducts have been found in greater numbers in embryos from smokers versus nonsmokers, suggesting a mechanism for the transmission of adversely modified DNA from parental smoking.2
Early pregnancy effects
Smoking increases the risk of spontaneous miscarriage in both natural and assisted conceptions and has been linked to an increased risk for bacterial vaginosis, which in turn increases the risk for second trimester miscarriage and preterm labor.2 Studies also have identified an increased risk of ectopic pregnancy in smokers, including one study demonstrating an odds ratio (OR) for ectopic pregnancy of 3.5 (95% confidence interval [CI], 1.4–8.6) in women who smoked more than 20 cigarettes per day versus nonsmokers.6
Assisted reproductive therapies rendered less effective
Studies of IVF have demonstrated that smokers versus nonsmokers have an increased gonadotropin requirement for ovarian stimulation, lower peak estradiol levels, elevated testosterone levels, fewer oocytes retrieved, higher numbers of cancelled cycles, thicker zone pellucida, lower implantation rates, and an increased rate of failed fertilization.2 In order to achieve conception, smokers require nearly twice the number of IVF cycles versus nonsmokers.2 Authors of a 5-year, prospective study controlling for potential confounders found that if a woman ever smoked in her lifetime, her risk of failing to conceive with assisted reproductive technologies (ART) more than doubled (relative risk, 2.5; 95% CI, 1.38–4.55). Each year of smoking was associated with a 9% increase in the risk of unsuccessful ART cycles (95% CI, 1.02–1.15;
P <.01).8
We have an important role in helping patients quit
A study involving smoking cessation in infertile women found that simple interventions, such as counseling, education, and encouragement during each clinic visit, were more successful than merely providing educational materials and Web site addresses. The rates of smoking cessation increased from 4% at baseline to 24% after 12 months.9
The Public Health Service and National Cancer Institute offer validated, office-based intervention guidelines for smoking cessation, including a five-step approach2 :
- Ask about smoking at every opportunity
- Advise all smokers to stop
- Assist willingness to stop
- Assist patients in stopping (including through the use of pharmaceuticals and carbon monoxide handheld monitors)
- Arrange follow-up visits.
The use of adjunctive medical therapies, including nicotine replacement therapy and/or buproprion, has resulted in a twofold increase in the proportion of nonpregnant women who quit smoking.2 These medical therapies may be useful if behavioral approaches alone fail—although their use has not been studied in infertile women. Smoking cessation rates appear to be higher in infertile versus pregnant women, yet only 18% of women referred for infertility care have received advice on smoking cessation from their referring provider.9
The detrimental effects of smoking on reproductive health are substantial. Nonsmokers with excessive exposure to tobacco smoke have adverse reproductive effects that may be as great as those observed in smokers.
Studies suggest that much of the reduced fecundity observed in smokers may be reversed within 1 year of smoking cessation.2 Clinicians who care for smokers with infertility have a tremendous opportunity to facilitate smoking cessation in their patients and their partners. Smoking-cessation intervention should be a key component of effective treatment of infertility.
The safety of assisted reproductive technologies
Davies ML, Moore VM, Willson KJ, et al. Reproductive technologies and the risk of birth defects. N Engl J Med. 2012;366(19):1803–1813.
Since the birth of Louise Brown, the first baby born after being conceived with in vitro fertilization (IVF) in 1978, IVF has become a pillar in the treatment of infertility. Although recognized as a highly effective treatment, the safety of IVF and its related technologies, such as intracytoplasmic sperm injection (ICSI), has been questioned. Studies have linked the use of assisted reproduction, including IVF and ICSI, with an increased risk of birth defects.10-15 Findings, however, were limited by small sample sizes and lack of appropriate controls. Furthermore, it has been unclear if this increased risk is due to factors related to treatment or to an underlying factor present in patients with infertility. It also has been unclear whether there is a differential in risk according to the type of ART used. In a large population-wide cohort study, Davies and colleagues linked a census of treatment with ART in South Australia to a registry of births and terminations with a gestation period of at least 20 weeks or a birth weight of 400 g and registries of birth defects.
The authors compared the risk of birth defects in pregnancies among women who had conceived with the use of ART, women with spontaneous pregnancies who had had a previous birth after ART treatment, women with a diagnosis of infertility who had conceived without ART, and pregnancies in women without infertility. Births and pregnancy terminations secondary to birth defects were studied to assess the birth defect risk from pregnancy to a child’s fifth birthday.
Details of the trial
A total of 308,974 births were included in the analysis. Births in women who conceived with the use of ART were associated with a significant increase in risk of birth defects (8.3%) compared with births conceived spontaneously in fertile women (8.3% vs 5.8%, respectively; unadjusted OR, 1.47; 95% CI, 1.33-1.62). This effect remained significant after multivariate adjustment (adjusted OR, 1.28; 95% CI, 1.16-1.41).
While there was no significant association between ART and the risk of specific syndromes such as Down’s, Turner’s, Edward’s, and others, there was a significantly increased adjusted OR for any defect and multiple defects in births conceived with ART versus those conceived spontaneously in fertile women.
The OR for birth defects associated with IVF was 1.26 (95% CI, 1.07-1.48) in unadjusted analyses and 1.07 (95% CI, 0.9-1.26) after multivariate adjustment. The OR for birth defects associated with IVF with ICSI were 1.77 (95% CI, 1.47-2.12) in unadjusted and 1.57 (95% CI, 1.30-1.90) after multivariate analysis. Compared with ICSI, IVF was associated with a reduced risk of any birth defect (OR, 0.68; 95% CI, 0.53-0.87).
Births after gamete intrafallopian transfer, intrauterine insemination, or the use of clomiphene citrate at home were associated with significantly increased risks of any birth defect in adjusted analyses. Births after conception with donor insemination and clinically supervised ovulation induction were not associated with an increased risk of birth defects. Births occurring after spontaneous conception in women with a history of a previous birth with ART were also associated with an increased risk of birth defects, even after adjustment for confounders (adjusted OR, 1.25; 95% CI, 1.01-1.56). Births occurring after spontaneous conception in women with a history of infertility without previous ART treatment were also significantly associated with a small increased risk in birth defects (OR, 1.29; 95% CI, 0.99-1.68).
ICSI and birth-defect association persisted
In this large observational study, the authors confirmed findings from previous studies11,12,16-18 that the number of birth defects found in pregnancies conceived with ART are higher than the number found in pregnancies conceived spontaneously. In this study, after multivariate adjustment, the association between IVF and an increased risk of birth defects was found to be no longer significant, but the risk remained elevated after ART with ICSI. These findings are similar to results in previous studies.18,19 The increased risk may be secondary to the ICSI procedure itself19,20 or to underlying male infertility factors leading to the use of ICSI.14
Birth defects appeared to be highest in fresh embryo cycles of ICSI versus IVF and lowest in frozen-embryo cycles. A reduction in birth defects with cryopreservation may be secondary to a reduced likelihood that cryopreserved embryos would survive the thawing process as well as the temporal separation of the developing embryo from a hormonally stimulated cycle.21-23 Treatment with ART was associated with an increased risk of cardiovascular, musculoskeletal, urogenital, and gastrointestinal defects, as well as cerebral palsy. The observation of an increased risk of cerebral palsy with ART treatment is consistent with findings from a previous study. Strömberg and colleagues found that the risk of cerebral palsy was increased by a factor of 3.7 among multiples conceived with IVF and 2.8 among singletons conceived with IVF.24
Davies and colleagues also observed that the risk of a birth defect was increased among women with a history of infertility who were able to conceive without ART,25 a finding observed in a previous large Danish registry.15
Although the vast majority of births resulting from assisted reproduction were free of birth defects, treatment with ART was associated with an increased risk of birth defects, compared with spontaneous conception. After adjustment for potential confounders, including maternal age, the risk persisted for conceptions associated with ICSI but not IVF.
While the exact mechanisms responsible for this increased risk remain unknown, the finding of an increased risk of birth defects among women with infertility who conceived without ART indicates that inherent patient factors, rather than assisted reproductive technologies alone, contribute to the risk. These findings can help to guide couples considering assisted reproduction for the treatment of infertility.
We want to hear from you! Tell us what you think.
1. Infertility FAQs. Centers for Disease Control and Prevention Web site. http://www.cdc.gov/reproductivehealth/infertility. Updated April 19 2012. Accessed January 20, 2013.
2. Pfeifer S, Fritz M, Goldberg J, et al. the Practice Committee of the American Society for Reproductive Medicine. Smoking and infertility: a committee opinion. Fertil Steril. 2012;98(6):1400-1406.
3. Hull MG, North K, Taylor H, Farrow A, Ford WC. Delayed conception and active and passive smoking: The Avon Longitudinal Study of Pregnancy and Childhood Study Team. Fertil Steril. 2000;74(4):725-733.
4. Cooper GS, Baird DD, Hulka BS, Weinberg CR, Savitz DA, Hughes CL, Jr. Follicle-stimulating hormone concentrations in relation to active and passive smoking. Obstet Gynecol. 1995;85(3):407-411.
5. Storgaard L, Bonde JP, Ernst E, et al. Does smoking during pregnancy affect sons’ sperm counts? Epidemiology. 2003;14(3):278-286.
6. Yang Q, Sherman SL, Hassold TJ, et al. Risk factors for trisomy 21: maternal cigarette smoking and oral contraceptive use in a population-based case-control study. Genet Med. 1999;1(3):80-88.
7. Zenzes MT, Wang P, Casper RF. Cigarette smoking may affect meiotic maturation of human oocytes. Hum Reprod. 1995;10(12):3213-3217.
8. Klonoff-Cohen H, Natarajan L, Marrs R, Yee B. Effects of female and male smoking on success rates of IVF and gamete intra-Fallopian transfer. Hum Reprod. 2001;16(7):1382-1390.
9. Hughes EG, Lamont DA, Beecroft ML, Wilson DM, Brennan BG, Rice SC. Randomized trial of a “stage of change” oriented smoking cessation intervention in infertile and pregnant women. Fertil Steril. 2000;74(3):498-503.
10. Rimm AA, Katayama AC, Diaz M, Katayama KP. A meta-analysis of controlled studies comparing major malformation rates in IVF and ICSI infants with naturally conceived children. J Assist Reprod Genet. 2004;21(12):437-443.
11. Hansen M, Kurinczuk JJ, Bower C, Webb S. The risk of major birth defects after intracytoplasmic sperm injection and in vitro fertilization. N Engl J Med. 2002;346(10):725-730.
12. Hansen M, Bower C, Milne E, de Klerk N, Kurinczuk JJ. Assisted reproductive technologies and the risk of birth defects—a systematic review. Hum Reprod. 2005;20(2):328-338.
13. Schieve LA, Rasmussen SA, Reefhuis J. Risk of birth defects among children conceived with assisted reproductive technology: providing an epidemiologic context to the data. Fertil Steril. 2005;84(5):1320-1324.
14. Lie RT, Lyngstadaas A, Ørstavik KH, Bakketeig LS, Jacobsen G, Tanbo T. Birth defects in children conceived by ICSI compared with children conceived by other IVF-methods; a meta-analysis. Int J Epidemiol. 2005;34(3):696-701.
15. Zhu JL, Basso O, Obel C, Bille C, Olsen J. Infertility infertility treatment, and congenital malformations: Danish national birth cohort. BMJ. 2006;333(7570):679.-
16. Reefhuis J, Honein MA, Schieve LA, Correa A, Hobbs CA, Rasmussen SA. National Birth Defects Prevention Study. Assisted reproductive technology and major structural birth defects in the United States. Hum Reprod. 2009;24(2):360-366.
17. El-Chaar D, Yang Q, Gao J, et al. Risk of birth defects increased in pregnancies conceived by assisted human reproduction. Fertil Steril. 2009;92(5):1557-1561.
18. Källén B, Finnström O, Nygren KG, Olausson PO. In vitro fertilization (IVF) in Sweden: infant outcome after different IVF fertilization methods. Fertil Steril. 2005;84(3):611-617.
19. Bonduelle M, Wennerholm U, Loft A, et al. A multi-centre cohort study of the physical health of 5-year-old children conceived after intracytoplasmic sperm injection, in vitro fertilization and natural conception. Hum Reprod. 2005;20(2):413-419.
20. Kurinczuk JJ. Safety issues in assisted reproduction technology: from theory to reality — just what are the data telling us about ICSI offspring health and future fertility and should we be concerned? Hum Reprod. 2003;18(5):925-931.
21. Pinborg A, Loft A, Aaris Henningsen AK, Rasmussen S, Andersen AN. Infant outcome of 957 singletons born after frozen embryo replacement: the Danish National Cohort Study 1995-2006. Fertil Steril. 2010;94(4):1320-1327.
22. Halliday JL, Ukoumunne OC, Baker HW, et al. Increased risk of blastogenesis birth defects, arising in the first 4 weeks of pregnancy, after assisted reproductive technologies. Hum Reprod. 2010;25(1):59-65.
23. Wennerholm U, Söderström-Anttila V, Bergh C, et al. Children born after cryopreservation of embryos or oocytes: a systematic review of outcome data. Hum Reprod. 2009;24(9):2158-2172.
24. Strömberg B, Dahlquist G, Ericson A, Finnström O, Köster M, Stjernqvist K. Neurological sequelae in children born after in-vitro fertilisation: a population based study. Lancet. 2002;359(9305):461-465.
25. Davies MJ, Moore VM, Willson KJ, et al. Reproductive technologies and the risk of birth defects. N Engl J Med. 2012;366(19):1803-1813.
1. Infertility FAQs. Centers for Disease Control and Prevention Web site. http://www.cdc.gov/reproductivehealth/infertility. Updated April 19 2012. Accessed January 20, 2013.
2. Pfeifer S, Fritz M, Goldberg J, et al. the Practice Committee of the American Society for Reproductive Medicine. Smoking and infertility: a committee opinion. Fertil Steril. 2012;98(6):1400-1406.
3. Hull MG, North K, Taylor H, Farrow A, Ford WC. Delayed conception and active and passive smoking: The Avon Longitudinal Study of Pregnancy and Childhood Study Team. Fertil Steril. 2000;74(4):725-733.
4. Cooper GS, Baird DD, Hulka BS, Weinberg CR, Savitz DA, Hughes CL, Jr. Follicle-stimulating hormone concentrations in relation to active and passive smoking. Obstet Gynecol. 1995;85(3):407-411.
5. Storgaard L, Bonde JP, Ernst E, et al. Does smoking during pregnancy affect sons’ sperm counts? Epidemiology. 2003;14(3):278-286.
6. Yang Q, Sherman SL, Hassold TJ, et al. Risk factors for trisomy 21: maternal cigarette smoking and oral contraceptive use in a population-based case-control study. Genet Med. 1999;1(3):80-88.
7. Zenzes MT, Wang P, Casper RF. Cigarette smoking may affect meiotic maturation of human oocytes. Hum Reprod. 1995;10(12):3213-3217.
8. Klonoff-Cohen H, Natarajan L, Marrs R, Yee B. Effects of female and male smoking on success rates of IVF and gamete intra-Fallopian transfer. Hum Reprod. 2001;16(7):1382-1390.
9. Hughes EG, Lamont DA, Beecroft ML, Wilson DM, Brennan BG, Rice SC. Randomized trial of a “stage of change” oriented smoking cessation intervention in infertile and pregnant women. Fertil Steril. 2000;74(3):498-503.
10. Rimm AA, Katayama AC, Diaz M, Katayama KP. A meta-analysis of controlled studies comparing major malformation rates in IVF and ICSI infants with naturally conceived children. J Assist Reprod Genet. 2004;21(12):437-443.
11. Hansen M, Kurinczuk JJ, Bower C, Webb S. The risk of major birth defects after intracytoplasmic sperm injection and in vitro fertilization. N Engl J Med. 2002;346(10):725-730.
12. Hansen M, Bower C, Milne E, de Klerk N, Kurinczuk JJ. Assisted reproductive technologies and the risk of birth defects—a systematic review. Hum Reprod. 2005;20(2):328-338.
13. Schieve LA, Rasmussen SA, Reefhuis J. Risk of birth defects among children conceived with assisted reproductive technology: providing an epidemiologic context to the data. Fertil Steril. 2005;84(5):1320-1324.
14. Lie RT, Lyngstadaas A, Ørstavik KH, Bakketeig LS, Jacobsen G, Tanbo T. Birth defects in children conceived by ICSI compared with children conceived by other IVF-methods; a meta-analysis. Int J Epidemiol. 2005;34(3):696-701.
15. Zhu JL, Basso O, Obel C, Bille C, Olsen J. Infertility infertility treatment, and congenital malformations: Danish national birth cohort. BMJ. 2006;333(7570):679.-
16. Reefhuis J, Honein MA, Schieve LA, Correa A, Hobbs CA, Rasmussen SA. National Birth Defects Prevention Study. Assisted reproductive technology and major structural birth defects in the United States. Hum Reprod. 2009;24(2):360-366.
17. El-Chaar D, Yang Q, Gao J, et al. Risk of birth defects increased in pregnancies conceived by assisted human reproduction. Fertil Steril. 2009;92(5):1557-1561.
18. Källén B, Finnström O, Nygren KG, Olausson PO. In vitro fertilization (IVF) in Sweden: infant outcome after different IVF fertilization methods. Fertil Steril. 2005;84(3):611-617.
19. Bonduelle M, Wennerholm U, Loft A, et al. A multi-centre cohort study of the physical health of 5-year-old children conceived after intracytoplasmic sperm injection, in vitro fertilization and natural conception. Hum Reprod. 2005;20(2):413-419.
20. Kurinczuk JJ. Safety issues in assisted reproduction technology: from theory to reality — just what are the data telling us about ICSI offspring health and future fertility and should we be concerned? Hum Reprod. 2003;18(5):925-931.
21. Pinborg A, Loft A, Aaris Henningsen AK, Rasmussen S, Andersen AN. Infant outcome of 957 singletons born after frozen embryo replacement: the Danish National Cohort Study 1995-2006. Fertil Steril. 2010;94(4):1320-1327.
22. Halliday JL, Ukoumunne OC, Baker HW, et al. Increased risk of blastogenesis birth defects, arising in the first 4 weeks of pregnancy, after assisted reproductive technologies. Hum Reprod. 2010;25(1):59-65.
23. Wennerholm U, Söderström-Anttila V, Bergh C, et al. Children born after cryopreservation of embryos or oocytes: a systematic review of outcome data. Hum Reprod. 2009;24(9):2158-2172.
24. Strömberg B, Dahlquist G, Ericson A, Finnström O, Köster M, Stjernqvist K. Neurological sequelae in children born after in-vitro fertilisation: a population based study. Lancet. 2002;359(9305):461-465.
25. Davies MJ, Moore VM, Willson KJ, et al. Reproductive technologies and the risk of birth defects. N Engl J Med. 2012;366(19):1803-1813.

