User login
The Evolution of Insulin Therapy in Diabetes Mellitus
Introduction
Basal insulin has been an important treatment option for patients with diabetes mellitus (DM) and, along with prandial insulin, has undergone major improvements in terms of purity and similarity to the action of physiologic human insulin. (see The Evolution of Insulin Therapy in Diabetes Mellitus in this supplement.) Lente and Ultralente formulations were used for decades but are no longer available. The use of neutral protamine Hagedorn (NPH) insulin is also being replaced with the basal insulin analogs detemir and glargine.1 Basal insulin analogs generally cause less severe and nocturnal hypoglycemia compared with NPH insulin owing to their improved pharmacologic profiles.2-4 In comparison to NPH insulin, insulin glargine causes similar weight gain, whereas insulin detemir causes less weight gain.2-4 In addition, insulin detemir has been associated with a glucose-lowering effect that is more predictable than that of NPH insulin.5 Despite the improvements observed with basal insulin analogs, their time-action profiles are not completely flat and are shorter than 24 hours in many patients.5,6 In addition, severe hypoglycemia remains a concern, particularly in patients with type 1 DM (T1DM).7,8 Consequently, the search for a better basal insulin continues.
The ideal basal insulin should possess numerous attributes. While each of the attributes listed in the TABLE is important, an overarching difficulty with basal insulin therapy is the need for administration at the same time each day.9 This dosing limitation may be most difficult for those with busy or erratic schedules or who may forget to administer their insulin dose. This article will review the clinical experience with insulin degludec, an ultra–long-acting insulin under review by the US Food and Drug Administration (FDA).
TABLE
Attributes of the ideal basal insulin9
| Delivers a steady, stable, peakless, continuous insulin concentration for at least 24 hours, in a predictable manner, with low intraindividual and interindividual variability |
| Does not cause side effects such as weight gain or hypoglycemia |
| Does not induce mitogenicity |
| Can be used as monotherapy, as part of basal-bolus therapy, or in combination with oral glucose-lowering therapy |
| Equally efficacious, safe, and well-tolerated in patients with type 1 or type 2 diabetes mellitus |
| Indian Journal of Endocrinology and Metabolism. Copyright 2011 by MEDKNOW PUBLICATIONS AND MEDIA PVT LTD. Reproduced with permission of MEDKNOW PUBLICATIONS AND MEDIA PVT LTD in the format Journal via Copyright Clearance Center. |
Clinical Pharmacology of Insulin Degludec
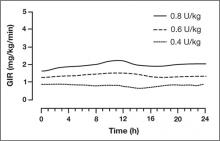
Removal of threonine at position 30 of the B chain of human insulin and the addition of a 16-carbon fatty diacid attached to lysine at position 29 of the B chain of human insulin via a glutamic acid spacer result in the insulin degludec molecule, which has several differences from available basal insulin analogs. Experimental investigations indicated that conditions mimicking subcutaneous injection of insulin degludec resulted in a reorganization of the insulin degludec molecule from dihexamers to multihexamer assemblies that remain in solution at physiologic pH.10 Slow release of zinc ions from the multihexamers leads to the slow release of insulin degludec monomers, which are easily absorbed into the systemic circulation.11 The result is a half-life of insulin degludec that is longer than 24 hours, with a level that is detectable in circulation for at least 96 hours after administration of the dose.10,12 The pharmacodynamic result is a relatively flat and consistent blood glucose–lowering effect with insulin degludec (FIGURE 1) reported to be longer than 24 hours in patients with T1DM or type 2 DM (T2DM).11,12
A randomized, double-blind, two-period, crossover comparison of insulin degludec and insulin glargine in patients with T1DM (N = 66) reported a half-life of 25.4 hours with insulin degludec compared with 12.5 hours with insulin glargine.13 The serum exposure of insulin degludec was similar between the first and second 12-hour period postdose. On the other hand, approximately 60% of the serum exposure to insulin glargine occurred over the first 12 hours following administration. These results highlight that insulin degludec is an ultra–long-acting insulin preparation with improved pharmacodynamic stability.
Analysis of data in 54 patients with T1DM reported that the within-subject pharmacodynamic variability was lower with insulin degludec compared with insulin glargine during a 24-hour euglycemic glucose clamp.14 Over 24 hours, the coefficient of variation (CV) with insulin degludec was lower for the area under the glucose infusion rate curve (AUCGIR) for total AUCGIR,0-24h (CV, 23% vs. 72%; P < .001), for GIRmax (CV, 21% vs. 53%; P < .0001), and for the fluctuation around the mean GIR value over 24 hours (CV, 31% vs. 62%; P < .001).
The findings from these investigations demonstrate that insulin degludec has a long half-life, resulting in a prolonged duration of blood glucose lowering with low within-subject pharmacodynamic variability.
FIGURE 1
Mean 24-hour glucose infusion rates (GIR) of insulin degludec at steady state
Copyright 2011 American Diabetes Association. From Diabetes®, Vol. 60,
Suppl. 1; 2011. Reprinted by permission of the American Diabetes Association.
Efficacy, Safety, and Tolerability of Insulin Degludec
Type 2 Diabetes Mellitus
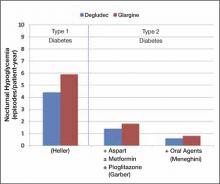
Insulin degludec has been compared with insulin glargine in combination with oral glucose-lowering agents or in combination with a prandial insulin analog; one study investigated insulin degludec and insulin aspart in basal-bolus therapy in T2DM. In the basal-bolus treat-to-target trial, 992 patients with T2DM (mean A1C 8.3%) were randomized to receive insulin degludec or insulin glargine, each in combination with prandial insulin aspart ± metformin ± pioglitazone.15 Basal insulin was titrated to achieve a fasting plasma glucose (FPG) <90 mg/dL. At 1 year, mean A1C values were reduced by 1.1% and 1.2% with insulin degludec and insulin glargine, respectively (estimated treatment difference [ETD], 0.08%; 95% confidence interval (CI), –0.05 to 0.21). FPG was reduced by 41 and 36 mg/dL, respectively (ETD, –5.2 mg/dL; 95% CI, –11.7 to 1.1; P = non significant [NS]). Overall, the rates of confirmed hypoglycemia (plasma glucose <56 mg/dL or severe episodes requiring assistance) were lower in the group treated with insulin degludec than in the group treated with insulin glargine (11.1 vs 13.6 episodes/patient-year; estimated rate ratio [ERR], 0.82; 95% CI, 0.69 to 0.99; P = .0359). Nocturnal confirmed hypoglycemia, defined as episodes occurring between midnight and 6 am, occurred significantly less frequently in the insulin degludec group compared with the insulin glargine group (1.4 vs 1.8 episodes/patient-year, respectively; ERR, 0.75; 95% CI, 0.58 to 0.99; P = .0399) (FIGURE 2). Rates of other adverse events were similar between the 2 groups. At 1 year, the total mean daily insulin doses were 1.46 and 1.42 U/kg in the insulin degludec and insulin glargine groups, respectively, with a ~50:50 basal:bolus ratio for both groups.
Based on these findings, insulin degludec was associated with glycemic control similar to insulin glargine when given as basal-bolus therapy. Overall, confirmed and nocturnal hypoglycemia occurred less frequently with insulin degludec than with insulin glargine.
FIGURE 2
Incidences of nocturnal hypoglycemia with insulin degludec and insulin glargine15,16,18
Type 1 Diabetes Mellitus
Insulin degludec has been investigated in the treatment of patients with T1DM. Two randomized trials involved basal-bolus therapy in combination with insulin aspart. A 1-year treat-to-target trial in 629 adults with T1DM (mean A1C 7.7%) compared insulin degludec with insulin glargine, each given once daily in a basal-bolus regimen with mealtime insulin aspart.16 Both groups were reported to have improved glycemic control, with overall A1C decreased by 0.4%. Similar proportions of patients achieved A1C <7.0% with insulin degludec and insulin glargine (40% vs 43%; P = NS). Mean FPG values were reduced similarly (ETD, 5.9 mg/dL; P = .35). Compared with insulin glargine, rates of confirmed nocturnal hypoglycemia were 25% lower with insulin degludec (4.4 vs 5.9 episodes/patient-year; ERR, 0.75; 95% CI, 0.59 to 0.96; P = .021), whereas rates of overall confirmed hypoglycemia were similar between treatment groups (42.5 vs 40.2 episodes/patient-year; ERR, 1.07; 95% CI, 0.89 to 1.28; P = .48). Overall rates of other adverse events were similar between groups.
Insulin degludec in a fixed-ratio combined formulation with insulin aspart (IDegAsp) was compared with insulin detemir and insulin aspart in basal-bolus therapy in a 26-week, open-label, treat-to-target trial involving 548 patients with T1DM (mean A1C, 8.3%; mean FPG, 189 mg/dL at baseline).17 IDegAsp was given once daily at any meal, with insulin aspart at the remaining meals, whereas insulin detemir was administered according to approved labeling with mealtime insulin aspart at all meals. The mean decrease in A1C was similar for IDegAsp and insulin detemir/insulin aspart (0.73% vs 0.68%, respectively). The decrease in mean FPG was also similar between groups (P = .52). The mean total daily insulin doses were 69 U (0.86 U/kg) for IDegAsp and 79 U (1.00 U/kg) for insulin detemir and insulin aspart. Rates of severe hypoglycemia were 0.33 and 0.42 episodes/patient-year with IDegAsp and insulin detemir, respectively. Rates of overall confirmed hypoglycemia were similar (39 vs 44 episodes/patient-year; P = .27), whereas confirmed nocturnal hypoglycemia was reported significantly less frequently with IDegAsp (3.7 vs 5.7 episodes/patient-year, respectively; P = .0003). Weight increase was significantly greater (by 1.04 kg) with IDegAsp compared with insulin detemir (P = .0021). Overall rates of other adverse events were similar between treatment groups.
Results from trials in patients with T1DM and T2DM are consistent and suggest comparable glycemic lowering between insulin degludec and the basal insulin analogs detemir and glargine, with less frequent nocturnal hypoglycemia in those treated with insulin degludec compared with insulins glargine and detemir (FIGURE 2).
Flexibility of Dosing Time
Optimal glycemic benefits are achieved with the injection of basal insulin at a consistent time each day. However, consistent timing may be difficult owing to patients’ busy or erratic schedules and/or in patients who may at times forget to administer their medications. These patient factors can lead to wide variability in the dosing interval and suboptimal results in fasting glucose control. These challenges may be improved upon with the investigational agent insulin degludec due to the stable and prolonged time-action profile of insulin degludec coupled with low within-subject pharmacodynamic variability, allowing for a more flexible once-daily dosing time. A 26-week, randomized, open-label trial in patients with T2DM (N = 459) aimed to compare insulin degludec in the setting of variable dosing intervals by administering insulin degludec once daily using a flexible regimen compared with insulin glargine given once daily at the same time each day.18 Both insulins were added to an existing regimen of oral glucose-lowering therapy (if any) and titrated to achieve FPG <90 mg/dL. To ensure variability in the dosing interval, the once-daily regimen of insulin degludec involved a compulsory, rotating morning and evening schedule, creating 8- to 40-hour dosing intervals. From a baseline mean of 8.4%, A1C values were reduced by 1.28% and 1.26% with insulin degludec and insulin glargine, respectively, at 26 weeks, confirming noninferiority of the flexible regimen of once-daily insulin degludec compared with insulin glargine given at the same time each day. The mean FPG at week 26 was significantly lower for insulin degludec than insulin glargine (104 vs 112 mg/dL, respectively; P = .04). The rates of confirmed hypoglycemia (3.6 vs 3.5 episodes/patient-year) and nocturnal hypoglycemia (0.6 vs 0.8 episodes/patient-year) for insulin degludec compared with insulin glargine, respectively, and the numbers of severe hypoglycemia events (2 episodes/group), were similar between treatment groups. This trial demonstrates that when needed to accommodate changes in the patient’s daily schedule, insulin degludec may be administered at differing times from day to day without compromising glycemic control or safety compared with insulin glargine administered at the same time each day.
CONCLUSIONS
Insulin degludec, an ultra–long-acting basal insulin analog, possesses several desirable attributes. Findings from clinical trials have demonstrated that the new-generation once-daily basal insulin degludec provides similar A1C control compared to insulin glargine both administered as basal-oral therapy or in combination with insulin aspart, with the added benefit of lower rates of hypoglycemia, particularly nocturnal hypoglycemia. Insulin degludec has also been shown to offer dosing flexibility, with administration at any time of the day without compromising glycemic control or safety. Insulin degludec, pending FDA approval, will be an additional treatment to help patients with T1DM or T2DM achieve glycemic control.
1. Rodbard HW, Jellinger PS, Davidson JA, et al. Statement by an American Association of Clinical Endocrinologists/American College of Endocrinology consensus panel on type 2 diabetes mellitus: an algorithm for glycemic control [published correction appears in Endocr Pract. 2009;15(7):768-770]. Endocr Pract. 2009;15(6):540-559.
2. Bartley PC, Bogoev M, Larsen J, Philotheou A. Long-term efficacy and safety of insulin detemir compared to Neutral Protamine Hagedorn insulin in patients with type 1 diabetes using a treat-to-target basal-bolus regimen with insulin aspart at meals: a 2-year, randomized, controlled trial. Diabet Med. 2008;25(4):442-449.
3. Riddle MC, Rosenstock J, Gerich J. Insulin Glargine 4002 Study Investigators. The treat-to-target trial: randomized addition of glargine or human NPH insulin to oral therapy of type 2 diabetic patients. Diabetes Care. 2003;26(11):3080—3086.
4. Hermansen K, Davies M, Derezinski T, Martinez Ravn G, Clauson P, Home P. A 26-week, randomized, parallel, treat-to-target trial comparing insulin detemir with NPH insulin as add-on therapy to oral glucose-lowering drugs in insulin-naive people with type 2 diabetes [published correction appears in Diabetes Care. 2007;30(4):1035] Diabetes Care. 2006;29(6):1269-1274.
5. Heise T, Nosek L, Rønn BB, et al. Lower within-subject variability of insulin detemir in comparison to NPH insulin and insulin glargine in people with type 1 diabetes. Diabetes. 2004;53(6):1614-1620.
6. Ashwell SG, Gebbie J, Home PD. Twice-daily compared with once-daily insulin glargine in people with type 1 diabetes using meal-time insulin aspart. Diabet Med. 2006;23(8):879-886.
7. Donnelly LA, Morris AD, Frier BM, et al. DARTS/MEMO Collaboration. Frequency and predictors of hypoglycaemia in type 1 and insulin-treated type 2 diabetes: a population-based study. Diabet Med. 2005;22(6):749-755.
8. Hermansen K, Dornhorst A, Sreenan S. Observational, open-label study of type 1 and type 2 diabetes patients switching from human insulin to insulin analogue basal-bolus regimens: insights from the PREDICTIVE study. Curr Med Res Opin. 2009;25(11):2601-2608.
9. Kalra S, Unnikrishnan AG, Baruah M, Kalra B. Degludec insulin: a novel basal insulin. Indian J Endocrinol Metab. 2011;15(suppl 1):S12-S16.
10. Jonassen I, Havelund S, Ribel U, et al. Insulin degludec: Multi-hexamer formation is the underlying basis for this new generation ultra-long acting basal insulin. Paper presented at: European Association for the Study of Diabetes Annual Meeting; September 20-24, 2010; Stockholm, Sweden.
11. Kurtzhals P, Heise T, Strauss HM, et al. Multi-hexamer formation is the underlying mechanism behind the ultra-long glucose-lowering effect of insulin degludec. Paper presented at: American Diabetes Association 71st Scientific Sessions; June 24-28, 2011; San Diego, CA.
12. Nosek L, Heise T, Bøttcher SG, Hastrup H, Haahr H. Ultra-long-acting insulin degludec has a flat and stable glucose-lowering effect. Paper presented at: American Diabetes Association 71st Scientific Sessions; June 24-28, 2011; San Diego, CA.
13. Heise T, Hövelmann U, Nosek L, Bøttcher SG, Granhall C, Haahr H. Insulin degludec has a two-fold longer half-life and a more consistent pharmacokinetic profile than insulin glargine. Paper presented at: American Diabetes Association 71st Scientific Sessions; June 24-28, 2011; San Diego, CA.
14. Heise T, Hermanski L, Nosek L, Feldmann A, Rasmussen S, Haahr H. The pharmacodynamic variability of insulin degludec is consistently lower than insulin glargine over 24 hours at steady state. Diabetes. 2011;60(suppl 1):A263.-Poster 960-P.
Garber AJ, King AB, Del Prato S, et al. 15.NN1250-3582 (BEGIN BB T2D) Trial Investigators. Insulin degludec, an ultra-longacting basal insulin, versus insulin glargine in basal-bolus treatment with mealtime insulin aspart in type 2 diabetes (BEGIN Basal-Bolus Type 2): a phase 3, randomised, open-label, treat-to-target non-inferiority trial. Lancet. 2012;379(9825):1498-1507.
16. Heller S, Buse J, Fisher M, et al. BEGIN Basal-Bolus Type 1 Trial Investigators. Insulin degludec, an ultra-longacting basal insulin, versus insulin glargine in basal-bolus treatment with mealtime insulin aspart in type 1 diabetes (BEGIN Basal-Blus Type 1): a phase 3, randomised, open-label, treat-to-target non-inferiority trial. Lancet. 2012;379(9825):1489-1497.
17. Hirsch IB, Franek E, Courreges JP, Mersebach H, Dykiel P, Bode BW. Efficacy and safety of a new basal insulin with a bolus boost (IDegAsp) used once daily in combination wtih insulin apart (IAsp) in people wth type 1 diabetes. Diabetes. 2011;60(suppl 1):A292.-Poster 1064-P.
18. Meneghini L, Atkin SL, Bain S, et al. Flexible once-daily dosing of insulin degludec does not compromise glycemic control or safety compared to insulin glargine given once daily at the same time each day in people with type 2 diabetes. Paper presented at: American Diabetes Association 71st Scientific Sessions; June 24-28, 2011; San Diego, CA.
The Evolution of Insulin Therapy in Diabetes Mellitus
Introduction
Basal insulin has been an important treatment option for patients with diabetes mellitus (DM) and, along with prandial insulin, has undergone major improvements in terms of purity and similarity to the action of physiologic human insulin. (see The Evolution of Insulin Therapy in Diabetes Mellitus in this supplement.) Lente and Ultralente formulations were used for decades but are no longer available. The use of neutral protamine Hagedorn (NPH) insulin is also being replaced with the basal insulin analogs detemir and glargine.1 Basal insulin analogs generally cause less severe and nocturnal hypoglycemia compared with NPH insulin owing to their improved pharmacologic profiles.2-4 In comparison to NPH insulin, insulin glargine causes similar weight gain, whereas insulin detemir causes less weight gain.2-4 In addition, insulin detemir has been associated with a glucose-lowering effect that is more predictable than that of NPH insulin.5 Despite the improvements observed with basal insulin analogs, their time-action profiles are not completely flat and are shorter than 24 hours in many patients.5,6 In addition, severe hypoglycemia remains a concern, particularly in patients with type 1 DM (T1DM).7,8 Consequently, the search for a better basal insulin continues.
The ideal basal insulin should possess numerous attributes. While each of the attributes listed in the TABLE is important, an overarching difficulty with basal insulin therapy is the need for administration at the same time each day.9 This dosing limitation may be most difficult for those with busy or erratic schedules or who may forget to administer their insulin dose. This article will review the clinical experience with insulin degludec, an ultra–long-acting insulin under review by the US Food and Drug Administration (FDA).
TABLE
Attributes of the ideal basal insulin9
| Delivers a steady, stable, peakless, continuous insulin concentration for at least 24 hours, in a predictable manner, with low intraindividual and interindividual variability |
| Does not cause side effects such as weight gain or hypoglycemia |
| Does not induce mitogenicity |
| Can be used as monotherapy, as part of basal-bolus therapy, or in combination with oral glucose-lowering therapy |
| Equally efficacious, safe, and well-tolerated in patients with type 1 or type 2 diabetes mellitus |
| Indian Journal of Endocrinology and Metabolism. Copyright 2011 by MEDKNOW PUBLICATIONS AND MEDIA PVT LTD. Reproduced with permission of MEDKNOW PUBLICATIONS AND MEDIA PVT LTD in the format Journal via Copyright Clearance Center. |
Clinical Pharmacology of Insulin Degludec
Removal of threonine at position 30 of the B chain of human insulin and the addition of a 16-carbon fatty diacid attached to lysine at position 29 of the B chain of human insulin via a glutamic acid spacer result in the insulin degludec molecule, which has several differences from available basal insulin analogs. Experimental investigations indicated that conditions mimicking subcutaneous injection of insulin degludec resulted in a reorganization of the insulin degludec molecule from dihexamers to multihexamer assemblies that remain in solution at physiologic pH.10 Slow release of zinc ions from the multihexamers leads to the slow release of insulin degludec monomers, which are easily absorbed into the systemic circulation.11 The result is a half-life of insulin degludec that is longer than 24 hours, with a level that is detectable in circulation for at least 96 hours after administration of the dose.10,12 The pharmacodynamic result is a relatively flat and consistent blood glucose–lowering effect with insulin degludec (FIGURE 1) reported to be longer than 24 hours in patients with T1DM or type 2 DM (T2DM).11,12
A randomized, double-blind, two-period, crossover comparison of insulin degludec and insulin glargine in patients with T1DM (N = 66) reported a half-life of 25.4 hours with insulin degludec compared with 12.5 hours with insulin glargine.13 The serum exposure of insulin degludec was similar between the first and second 12-hour period postdose. On the other hand, approximately 60% of the serum exposure to insulin glargine occurred over the first 12 hours following administration. These results highlight that insulin degludec is an ultra–long-acting insulin preparation with improved pharmacodynamic stability.
Analysis of data in 54 patients with T1DM reported that the within-subject pharmacodynamic variability was lower with insulin degludec compared with insulin glargine during a 24-hour euglycemic glucose clamp.14 Over 24 hours, the coefficient of variation (CV) with insulin degludec was lower for the area under the glucose infusion rate curve (AUCGIR) for total AUCGIR,0-24h (CV, 23% vs. 72%; P < .001), for GIRmax (CV, 21% vs. 53%; P < .0001), and for the fluctuation around the mean GIR value over 24 hours (CV, 31% vs. 62%; P < .001).
The findings from these investigations demonstrate that insulin degludec has a long half-life, resulting in a prolonged duration of blood glucose lowering with low within-subject pharmacodynamic variability.
FIGURE 1
Mean 24-hour glucose infusion rates (GIR) of insulin degludec at steady state
Copyright 2011 American Diabetes Association. From Diabetes®, Vol. 60,
Suppl. 1; 2011. Reprinted by permission of the American Diabetes Association.
Efficacy, Safety, and Tolerability of Insulin Degludec
Type 2 Diabetes Mellitus
Insulin degludec has been compared with insulin glargine in combination with oral glucose-lowering agents or in combination with a prandial insulin analog; one study investigated insulin degludec and insulin aspart in basal-bolus therapy in T2DM. In the basal-bolus treat-to-target trial, 992 patients with T2DM (mean A1C 8.3%) were randomized to receive insulin degludec or insulin glargine, each in combination with prandial insulin aspart ± metformin ± pioglitazone.15 Basal insulin was titrated to achieve a fasting plasma glucose (FPG) <90 mg/dL. At 1 year, mean A1C values were reduced by 1.1% and 1.2% with insulin degludec and insulin glargine, respectively (estimated treatment difference [ETD], 0.08%; 95% confidence interval (CI), –0.05 to 0.21). FPG was reduced by 41 and 36 mg/dL, respectively (ETD, –5.2 mg/dL; 95% CI, –11.7 to 1.1; P = non significant [NS]). Overall, the rates of confirmed hypoglycemia (plasma glucose <56 mg/dL or severe episodes requiring assistance) were lower in the group treated with insulin degludec than in the group treated with insulin glargine (11.1 vs 13.6 episodes/patient-year; estimated rate ratio [ERR], 0.82; 95% CI, 0.69 to 0.99; P = .0359). Nocturnal confirmed hypoglycemia, defined as episodes occurring between midnight and 6 am, occurred significantly less frequently in the insulin degludec group compared with the insulin glargine group (1.4 vs 1.8 episodes/patient-year, respectively; ERR, 0.75; 95% CI, 0.58 to 0.99; P = .0399) (FIGURE 2). Rates of other adverse events were similar between the 2 groups. At 1 year, the total mean daily insulin doses were 1.46 and 1.42 U/kg in the insulin degludec and insulin glargine groups, respectively, with a ~50:50 basal:bolus ratio for both groups.
Based on these findings, insulin degludec was associated with glycemic control similar to insulin glargine when given as basal-bolus therapy. Overall, confirmed and nocturnal hypoglycemia occurred less frequently with insulin degludec than with insulin glargine.
FIGURE 2
Incidences of nocturnal hypoglycemia with insulin degludec and insulin glargine15,16,18
Type 1 Diabetes Mellitus
Insulin degludec has been investigated in the treatment of patients with T1DM. Two randomized trials involved basal-bolus therapy in combination with insulin aspart. A 1-year treat-to-target trial in 629 adults with T1DM (mean A1C 7.7%) compared insulin degludec with insulin glargine, each given once daily in a basal-bolus regimen with mealtime insulin aspart.16 Both groups were reported to have improved glycemic control, with overall A1C decreased by 0.4%. Similar proportions of patients achieved A1C <7.0% with insulin degludec and insulin glargine (40% vs 43%; P = NS). Mean FPG values were reduced similarly (ETD, 5.9 mg/dL; P = .35). Compared with insulin glargine, rates of confirmed nocturnal hypoglycemia were 25% lower with insulin degludec (4.4 vs 5.9 episodes/patient-year; ERR, 0.75; 95% CI, 0.59 to 0.96; P = .021), whereas rates of overall confirmed hypoglycemia were similar between treatment groups (42.5 vs 40.2 episodes/patient-year; ERR, 1.07; 95% CI, 0.89 to 1.28; P = .48). Overall rates of other adverse events were similar between groups.
Insulin degludec in a fixed-ratio combined formulation with insulin aspart (IDegAsp) was compared with insulin detemir and insulin aspart in basal-bolus therapy in a 26-week, open-label, treat-to-target trial involving 548 patients with T1DM (mean A1C, 8.3%; mean FPG, 189 mg/dL at baseline).17 IDegAsp was given once daily at any meal, with insulin aspart at the remaining meals, whereas insulin detemir was administered according to approved labeling with mealtime insulin aspart at all meals. The mean decrease in A1C was similar for IDegAsp and insulin detemir/insulin aspart (0.73% vs 0.68%, respectively). The decrease in mean FPG was also similar between groups (P = .52). The mean total daily insulin doses were 69 U (0.86 U/kg) for IDegAsp and 79 U (1.00 U/kg) for insulin detemir and insulin aspart. Rates of severe hypoglycemia were 0.33 and 0.42 episodes/patient-year with IDegAsp and insulin detemir, respectively. Rates of overall confirmed hypoglycemia were similar (39 vs 44 episodes/patient-year; P = .27), whereas confirmed nocturnal hypoglycemia was reported significantly less frequently with IDegAsp (3.7 vs 5.7 episodes/patient-year, respectively; P = .0003). Weight increase was significantly greater (by 1.04 kg) with IDegAsp compared with insulin detemir (P = .0021). Overall rates of other adverse events were similar between treatment groups.
Results from trials in patients with T1DM and T2DM are consistent and suggest comparable glycemic lowering between insulin degludec and the basal insulin analogs detemir and glargine, with less frequent nocturnal hypoglycemia in those treated with insulin degludec compared with insulins glargine and detemir (FIGURE 2).
Flexibility of Dosing Time
Optimal glycemic benefits are achieved with the injection of basal insulin at a consistent time each day. However, consistent timing may be difficult owing to patients’ busy or erratic schedules and/or in patients who may at times forget to administer their medications. These patient factors can lead to wide variability in the dosing interval and suboptimal results in fasting glucose control. These challenges may be improved upon with the investigational agent insulin degludec due to the stable and prolonged time-action profile of insulin degludec coupled with low within-subject pharmacodynamic variability, allowing for a more flexible once-daily dosing time. A 26-week, randomized, open-label trial in patients with T2DM (N = 459) aimed to compare insulin degludec in the setting of variable dosing intervals by administering insulin degludec once daily using a flexible regimen compared with insulin glargine given once daily at the same time each day.18 Both insulins were added to an existing regimen of oral glucose-lowering therapy (if any) and titrated to achieve FPG <90 mg/dL. To ensure variability in the dosing interval, the once-daily regimen of insulin degludec involved a compulsory, rotating morning and evening schedule, creating 8- to 40-hour dosing intervals. From a baseline mean of 8.4%, A1C values were reduced by 1.28% and 1.26% with insulin degludec and insulin glargine, respectively, at 26 weeks, confirming noninferiority of the flexible regimen of once-daily insulin degludec compared with insulin glargine given at the same time each day. The mean FPG at week 26 was significantly lower for insulin degludec than insulin glargine (104 vs 112 mg/dL, respectively; P = .04). The rates of confirmed hypoglycemia (3.6 vs 3.5 episodes/patient-year) and nocturnal hypoglycemia (0.6 vs 0.8 episodes/patient-year) for insulin degludec compared with insulin glargine, respectively, and the numbers of severe hypoglycemia events (2 episodes/group), were similar between treatment groups. This trial demonstrates that when needed to accommodate changes in the patient’s daily schedule, insulin degludec may be administered at differing times from day to day without compromising glycemic control or safety compared with insulin glargine administered at the same time each day.
CONCLUSIONS
Insulin degludec, an ultra–long-acting basal insulin analog, possesses several desirable attributes. Findings from clinical trials have demonstrated that the new-generation once-daily basal insulin degludec provides similar A1C control compared to insulin glargine both administered as basal-oral therapy or in combination with insulin aspart, with the added benefit of lower rates of hypoglycemia, particularly nocturnal hypoglycemia. Insulin degludec has also been shown to offer dosing flexibility, with administration at any time of the day without compromising glycemic control or safety. Insulin degludec, pending FDA approval, will be an additional treatment to help patients with T1DM or T2DM achieve glycemic control.
The Evolution of Insulin Therapy in Diabetes Mellitus
Introduction
Basal insulin has been an important treatment option for patients with diabetes mellitus (DM) and, along with prandial insulin, has undergone major improvements in terms of purity and similarity to the action of physiologic human insulin. (see The Evolution of Insulin Therapy in Diabetes Mellitus in this supplement.) Lente and Ultralente formulations were used for decades but are no longer available. The use of neutral protamine Hagedorn (NPH) insulin is also being replaced with the basal insulin analogs detemir and glargine.1 Basal insulin analogs generally cause less severe and nocturnal hypoglycemia compared with NPH insulin owing to their improved pharmacologic profiles.2-4 In comparison to NPH insulin, insulin glargine causes similar weight gain, whereas insulin detemir causes less weight gain.2-4 In addition, insulin detemir has been associated with a glucose-lowering effect that is more predictable than that of NPH insulin.5 Despite the improvements observed with basal insulin analogs, their time-action profiles are not completely flat and are shorter than 24 hours in many patients.5,6 In addition, severe hypoglycemia remains a concern, particularly in patients with type 1 DM (T1DM).7,8 Consequently, the search for a better basal insulin continues.
The ideal basal insulin should possess numerous attributes. While each of the attributes listed in the TABLE is important, an overarching difficulty with basal insulin therapy is the need for administration at the same time each day.9 This dosing limitation may be most difficult for those with busy or erratic schedules or who may forget to administer their insulin dose. This article will review the clinical experience with insulin degludec, an ultra–long-acting insulin under review by the US Food and Drug Administration (FDA).
TABLE
Attributes of the ideal basal insulin9
| Delivers a steady, stable, peakless, continuous insulin concentration for at least 24 hours, in a predictable manner, with low intraindividual and interindividual variability |
| Does not cause side effects such as weight gain or hypoglycemia |
| Does not induce mitogenicity |
| Can be used as monotherapy, as part of basal-bolus therapy, or in combination with oral glucose-lowering therapy |
| Equally efficacious, safe, and well-tolerated in patients with type 1 or type 2 diabetes mellitus |
| Indian Journal of Endocrinology and Metabolism. Copyright 2011 by MEDKNOW PUBLICATIONS AND MEDIA PVT LTD. Reproduced with permission of MEDKNOW PUBLICATIONS AND MEDIA PVT LTD in the format Journal via Copyright Clearance Center. |
Clinical Pharmacology of Insulin Degludec
Removal of threonine at position 30 of the B chain of human insulin and the addition of a 16-carbon fatty diacid attached to lysine at position 29 of the B chain of human insulin via a glutamic acid spacer result in the insulin degludec molecule, which has several differences from available basal insulin analogs. Experimental investigations indicated that conditions mimicking subcutaneous injection of insulin degludec resulted in a reorganization of the insulin degludec molecule from dihexamers to multihexamer assemblies that remain in solution at physiologic pH.10 Slow release of zinc ions from the multihexamers leads to the slow release of insulin degludec monomers, which are easily absorbed into the systemic circulation.11 The result is a half-life of insulin degludec that is longer than 24 hours, with a level that is detectable in circulation for at least 96 hours after administration of the dose.10,12 The pharmacodynamic result is a relatively flat and consistent blood glucose–lowering effect with insulin degludec (FIGURE 1) reported to be longer than 24 hours in patients with T1DM or type 2 DM (T2DM).11,12
A randomized, double-blind, two-period, crossover comparison of insulin degludec and insulin glargine in patients with T1DM (N = 66) reported a half-life of 25.4 hours with insulin degludec compared with 12.5 hours with insulin glargine.13 The serum exposure of insulin degludec was similar between the first and second 12-hour period postdose. On the other hand, approximately 60% of the serum exposure to insulin glargine occurred over the first 12 hours following administration. These results highlight that insulin degludec is an ultra–long-acting insulin preparation with improved pharmacodynamic stability.
Analysis of data in 54 patients with T1DM reported that the within-subject pharmacodynamic variability was lower with insulin degludec compared with insulin glargine during a 24-hour euglycemic glucose clamp.14 Over 24 hours, the coefficient of variation (CV) with insulin degludec was lower for the area under the glucose infusion rate curve (AUCGIR) for total AUCGIR,0-24h (CV, 23% vs. 72%; P < .001), for GIRmax (CV, 21% vs. 53%; P < .0001), and for the fluctuation around the mean GIR value over 24 hours (CV, 31% vs. 62%; P < .001).
The findings from these investigations demonstrate that insulin degludec has a long half-life, resulting in a prolonged duration of blood glucose lowering with low within-subject pharmacodynamic variability.
FIGURE 1
Mean 24-hour glucose infusion rates (GIR) of insulin degludec at steady state
Copyright 2011 American Diabetes Association. From Diabetes®, Vol. 60,
Suppl. 1; 2011. Reprinted by permission of the American Diabetes Association.
Efficacy, Safety, and Tolerability of Insulin Degludec
Type 2 Diabetes Mellitus
Insulin degludec has been compared with insulin glargine in combination with oral glucose-lowering agents or in combination with a prandial insulin analog; one study investigated insulin degludec and insulin aspart in basal-bolus therapy in T2DM. In the basal-bolus treat-to-target trial, 992 patients with T2DM (mean A1C 8.3%) were randomized to receive insulin degludec or insulin glargine, each in combination with prandial insulin aspart ± metformin ± pioglitazone.15 Basal insulin was titrated to achieve a fasting plasma glucose (FPG) <90 mg/dL. At 1 year, mean A1C values were reduced by 1.1% and 1.2% with insulin degludec and insulin glargine, respectively (estimated treatment difference [ETD], 0.08%; 95% confidence interval (CI), –0.05 to 0.21). FPG was reduced by 41 and 36 mg/dL, respectively (ETD, –5.2 mg/dL; 95% CI, –11.7 to 1.1; P = non significant [NS]). Overall, the rates of confirmed hypoglycemia (plasma glucose <56 mg/dL or severe episodes requiring assistance) were lower in the group treated with insulin degludec than in the group treated with insulin glargine (11.1 vs 13.6 episodes/patient-year; estimated rate ratio [ERR], 0.82; 95% CI, 0.69 to 0.99; P = .0359). Nocturnal confirmed hypoglycemia, defined as episodes occurring between midnight and 6 am, occurred significantly less frequently in the insulin degludec group compared with the insulin glargine group (1.4 vs 1.8 episodes/patient-year, respectively; ERR, 0.75; 95% CI, 0.58 to 0.99; P = .0399) (FIGURE 2). Rates of other adverse events were similar between the 2 groups. At 1 year, the total mean daily insulin doses were 1.46 and 1.42 U/kg in the insulin degludec and insulin glargine groups, respectively, with a ~50:50 basal:bolus ratio for both groups.
Based on these findings, insulin degludec was associated with glycemic control similar to insulin glargine when given as basal-bolus therapy. Overall, confirmed and nocturnal hypoglycemia occurred less frequently with insulin degludec than with insulin glargine.
FIGURE 2
Incidences of nocturnal hypoglycemia with insulin degludec and insulin glargine15,16,18
Type 1 Diabetes Mellitus
Insulin degludec has been investigated in the treatment of patients with T1DM. Two randomized trials involved basal-bolus therapy in combination with insulin aspart. A 1-year treat-to-target trial in 629 adults with T1DM (mean A1C 7.7%) compared insulin degludec with insulin glargine, each given once daily in a basal-bolus regimen with mealtime insulin aspart.16 Both groups were reported to have improved glycemic control, with overall A1C decreased by 0.4%. Similar proportions of patients achieved A1C <7.0% with insulin degludec and insulin glargine (40% vs 43%; P = NS). Mean FPG values were reduced similarly (ETD, 5.9 mg/dL; P = .35). Compared with insulin glargine, rates of confirmed nocturnal hypoglycemia were 25% lower with insulin degludec (4.4 vs 5.9 episodes/patient-year; ERR, 0.75; 95% CI, 0.59 to 0.96; P = .021), whereas rates of overall confirmed hypoglycemia were similar between treatment groups (42.5 vs 40.2 episodes/patient-year; ERR, 1.07; 95% CI, 0.89 to 1.28; P = .48). Overall rates of other adverse events were similar between groups.
Insulin degludec in a fixed-ratio combined formulation with insulin aspart (IDegAsp) was compared with insulin detemir and insulin aspart in basal-bolus therapy in a 26-week, open-label, treat-to-target trial involving 548 patients with T1DM (mean A1C, 8.3%; mean FPG, 189 mg/dL at baseline).17 IDegAsp was given once daily at any meal, with insulin aspart at the remaining meals, whereas insulin detemir was administered according to approved labeling with mealtime insulin aspart at all meals. The mean decrease in A1C was similar for IDegAsp and insulin detemir/insulin aspart (0.73% vs 0.68%, respectively). The decrease in mean FPG was also similar between groups (P = .52). The mean total daily insulin doses were 69 U (0.86 U/kg) for IDegAsp and 79 U (1.00 U/kg) for insulin detemir and insulin aspart. Rates of severe hypoglycemia were 0.33 and 0.42 episodes/patient-year with IDegAsp and insulin detemir, respectively. Rates of overall confirmed hypoglycemia were similar (39 vs 44 episodes/patient-year; P = .27), whereas confirmed nocturnal hypoglycemia was reported significantly less frequently with IDegAsp (3.7 vs 5.7 episodes/patient-year, respectively; P = .0003). Weight increase was significantly greater (by 1.04 kg) with IDegAsp compared with insulin detemir (P = .0021). Overall rates of other adverse events were similar between treatment groups.
Results from trials in patients with T1DM and T2DM are consistent and suggest comparable glycemic lowering between insulin degludec and the basal insulin analogs detemir and glargine, with less frequent nocturnal hypoglycemia in those treated with insulin degludec compared with insulins glargine and detemir (FIGURE 2).
Flexibility of Dosing Time
Optimal glycemic benefits are achieved with the injection of basal insulin at a consistent time each day. However, consistent timing may be difficult owing to patients’ busy or erratic schedules and/or in patients who may at times forget to administer their medications. These patient factors can lead to wide variability in the dosing interval and suboptimal results in fasting glucose control. These challenges may be improved upon with the investigational agent insulin degludec due to the stable and prolonged time-action profile of insulin degludec coupled with low within-subject pharmacodynamic variability, allowing for a more flexible once-daily dosing time. A 26-week, randomized, open-label trial in patients with T2DM (N = 459) aimed to compare insulin degludec in the setting of variable dosing intervals by administering insulin degludec once daily using a flexible regimen compared with insulin glargine given once daily at the same time each day.18 Both insulins were added to an existing regimen of oral glucose-lowering therapy (if any) and titrated to achieve FPG <90 mg/dL. To ensure variability in the dosing interval, the once-daily regimen of insulin degludec involved a compulsory, rotating morning and evening schedule, creating 8- to 40-hour dosing intervals. From a baseline mean of 8.4%, A1C values were reduced by 1.28% and 1.26% with insulin degludec and insulin glargine, respectively, at 26 weeks, confirming noninferiority of the flexible regimen of once-daily insulin degludec compared with insulin glargine given at the same time each day. The mean FPG at week 26 was significantly lower for insulin degludec than insulin glargine (104 vs 112 mg/dL, respectively; P = .04). The rates of confirmed hypoglycemia (3.6 vs 3.5 episodes/patient-year) and nocturnal hypoglycemia (0.6 vs 0.8 episodes/patient-year) for insulin degludec compared with insulin glargine, respectively, and the numbers of severe hypoglycemia events (2 episodes/group), were similar between treatment groups. This trial demonstrates that when needed to accommodate changes in the patient’s daily schedule, insulin degludec may be administered at differing times from day to day without compromising glycemic control or safety compared with insulin glargine administered at the same time each day.
CONCLUSIONS
Insulin degludec, an ultra–long-acting basal insulin analog, possesses several desirable attributes. Findings from clinical trials have demonstrated that the new-generation once-daily basal insulin degludec provides similar A1C control compared to insulin glargine both administered as basal-oral therapy or in combination with insulin aspart, with the added benefit of lower rates of hypoglycemia, particularly nocturnal hypoglycemia. Insulin degludec has also been shown to offer dosing flexibility, with administration at any time of the day without compromising glycemic control or safety. Insulin degludec, pending FDA approval, will be an additional treatment to help patients with T1DM or T2DM achieve glycemic control.
1. Rodbard HW, Jellinger PS, Davidson JA, et al. Statement by an American Association of Clinical Endocrinologists/American College of Endocrinology consensus panel on type 2 diabetes mellitus: an algorithm for glycemic control [published correction appears in Endocr Pract. 2009;15(7):768-770]. Endocr Pract. 2009;15(6):540-559.
2. Bartley PC, Bogoev M, Larsen J, Philotheou A. Long-term efficacy and safety of insulin detemir compared to Neutral Protamine Hagedorn insulin in patients with type 1 diabetes using a treat-to-target basal-bolus regimen with insulin aspart at meals: a 2-year, randomized, controlled trial. Diabet Med. 2008;25(4):442-449.
3. Riddle MC, Rosenstock J, Gerich J. Insulin Glargine 4002 Study Investigators. The treat-to-target trial: randomized addition of glargine or human NPH insulin to oral therapy of type 2 diabetic patients. Diabetes Care. 2003;26(11):3080—3086.
4. Hermansen K, Davies M, Derezinski T, Martinez Ravn G, Clauson P, Home P. A 26-week, randomized, parallel, treat-to-target trial comparing insulin detemir with NPH insulin as add-on therapy to oral glucose-lowering drugs in insulin-naive people with type 2 diabetes [published correction appears in Diabetes Care. 2007;30(4):1035] Diabetes Care. 2006;29(6):1269-1274.
5. Heise T, Nosek L, Rønn BB, et al. Lower within-subject variability of insulin detemir in comparison to NPH insulin and insulin glargine in people with type 1 diabetes. Diabetes. 2004;53(6):1614-1620.
6. Ashwell SG, Gebbie J, Home PD. Twice-daily compared with once-daily insulin glargine in people with type 1 diabetes using meal-time insulin aspart. Diabet Med. 2006;23(8):879-886.
7. Donnelly LA, Morris AD, Frier BM, et al. DARTS/MEMO Collaboration. Frequency and predictors of hypoglycaemia in type 1 and insulin-treated type 2 diabetes: a population-based study. Diabet Med. 2005;22(6):749-755.
8. Hermansen K, Dornhorst A, Sreenan S. Observational, open-label study of type 1 and type 2 diabetes patients switching from human insulin to insulin analogue basal-bolus regimens: insights from the PREDICTIVE study. Curr Med Res Opin. 2009;25(11):2601-2608.
9. Kalra S, Unnikrishnan AG, Baruah M, Kalra B. Degludec insulin: a novel basal insulin. Indian J Endocrinol Metab. 2011;15(suppl 1):S12-S16.
10. Jonassen I, Havelund S, Ribel U, et al. Insulin degludec: Multi-hexamer formation is the underlying basis for this new generation ultra-long acting basal insulin. Paper presented at: European Association for the Study of Diabetes Annual Meeting; September 20-24, 2010; Stockholm, Sweden.
11. Kurtzhals P, Heise T, Strauss HM, et al. Multi-hexamer formation is the underlying mechanism behind the ultra-long glucose-lowering effect of insulin degludec. Paper presented at: American Diabetes Association 71st Scientific Sessions; June 24-28, 2011; San Diego, CA.
12. Nosek L, Heise T, Bøttcher SG, Hastrup H, Haahr H. Ultra-long-acting insulin degludec has a flat and stable glucose-lowering effect. Paper presented at: American Diabetes Association 71st Scientific Sessions; June 24-28, 2011; San Diego, CA.
13. Heise T, Hövelmann U, Nosek L, Bøttcher SG, Granhall C, Haahr H. Insulin degludec has a two-fold longer half-life and a more consistent pharmacokinetic profile than insulin glargine. Paper presented at: American Diabetes Association 71st Scientific Sessions; June 24-28, 2011; San Diego, CA.
14. Heise T, Hermanski L, Nosek L, Feldmann A, Rasmussen S, Haahr H. The pharmacodynamic variability of insulin degludec is consistently lower than insulin glargine over 24 hours at steady state. Diabetes. 2011;60(suppl 1):A263.-Poster 960-P.
Garber AJ, King AB, Del Prato S, et al. 15.NN1250-3582 (BEGIN BB T2D) Trial Investigators. Insulin degludec, an ultra-longacting basal insulin, versus insulin glargine in basal-bolus treatment with mealtime insulin aspart in type 2 diabetes (BEGIN Basal-Bolus Type 2): a phase 3, randomised, open-label, treat-to-target non-inferiority trial. Lancet. 2012;379(9825):1498-1507.
16. Heller S, Buse J, Fisher M, et al. BEGIN Basal-Bolus Type 1 Trial Investigators. Insulin degludec, an ultra-longacting basal insulin, versus insulin glargine in basal-bolus treatment with mealtime insulin aspart in type 1 diabetes (BEGIN Basal-Blus Type 1): a phase 3, randomised, open-label, treat-to-target non-inferiority trial. Lancet. 2012;379(9825):1489-1497.
17. Hirsch IB, Franek E, Courreges JP, Mersebach H, Dykiel P, Bode BW. Efficacy and safety of a new basal insulin with a bolus boost (IDegAsp) used once daily in combination wtih insulin apart (IAsp) in people wth type 1 diabetes. Diabetes. 2011;60(suppl 1):A292.-Poster 1064-P.
18. Meneghini L, Atkin SL, Bain S, et al. Flexible once-daily dosing of insulin degludec does not compromise glycemic control or safety compared to insulin glargine given once daily at the same time each day in people with type 2 diabetes. Paper presented at: American Diabetes Association 71st Scientific Sessions; June 24-28, 2011; San Diego, CA.
1. Rodbard HW, Jellinger PS, Davidson JA, et al. Statement by an American Association of Clinical Endocrinologists/American College of Endocrinology consensus panel on type 2 diabetes mellitus: an algorithm for glycemic control [published correction appears in Endocr Pract. 2009;15(7):768-770]. Endocr Pract. 2009;15(6):540-559.
2. Bartley PC, Bogoev M, Larsen J, Philotheou A. Long-term efficacy and safety of insulin detemir compared to Neutral Protamine Hagedorn insulin in patients with type 1 diabetes using a treat-to-target basal-bolus regimen with insulin aspart at meals: a 2-year, randomized, controlled trial. Diabet Med. 2008;25(4):442-449.
3. Riddle MC, Rosenstock J, Gerich J. Insulin Glargine 4002 Study Investigators. The treat-to-target trial: randomized addition of glargine or human NPH insulin to oral therapy of type 2 diabetic patients. Diabetes Care. 2003;26(11):3080—3086.
4. Hermansen K, Davies M, Derezinski T, Martinez Ravn G, Clauson P, Home P. A 26-week, randomized, parallel, treat-to-target trial comparing insulin detemir with NPH insulin as add-on therapy to oral glucose-lowering drugs in insulin-naive people with type 2 diabetes [published correction appears in Diabetes Care. 2007;30(4):1035] Diabetes Care. 2006;29(6):1269-1274.
5. Heise T, Nosek L, Rønn BB, et al. Lower within-subject variability of insulin detemir in comparison to NPH insulin and insulin glargine in people with type 1 diabetes. Diabetes. 2004;53(6):1614-1620.
6. Ashwell SG, Gebbie J, Home PD. Twice-daily compared with once-daily insulin glargine in people with type 1 diabetes using meal-time insulin aspart. Diabet Med. 2006;23(8):879-886.
7. Donnelly LA, Morris AD, Frier BM, et al. DARTS/MEMO Collaboration. Frequency and predictors of hypoglycaemia in type 1 and insulin-treated type 2 diabetes: a population-based study. Diabet Med. 2005;22(6):749-755.
8. Hermansen K, Dornhorst A, Sreenan S. Observational, open-label study of type 1 and type 2 diabetes patients switching from human insulin to insulin analogue basal-bolus regimens: insights from the PREDICTIVE study. Curr Med Res Opin. 2009;25(11):2601-2608.
9. Kalra S, Unnikrishnan AG, Baruah M, Kalra B. Degludec insulin: a novel basal insulin. Indian J Endocrinol Metab. 2011;15(suppl 1):S12-S16.
10. Jonassen I, Havelund S, Ribel U, et al. Insulin degludec: Multi-hexamer formation is the underlying basis for this new generation ultra-long acting basal insulin. Paper presented at: European Association for the Study of Diabetes Annual Meeting; September 20-24, 2010; Stockholm, Sweden.
11. Kurtzhals P, Heise T, Strauss HM, et al. Multi-hexamer formation is the underlying mechanism behind the ultra-long glucose-lowering effect of insulin degludec. Paper presented at: American Diabetes Association 71st Scientific Sessions; June 24-28, 2011; San Diego, CA.
12. Nosek L, Heise T, Bøttcher SG, Hastrup H, Haahr H. Ultra-long-acting insulin degludec has a flat and stable glucose-lowering effect. Paper presented at: American Diabetes Association 71st Scientific Sessions; June 24-28, 2011; San Diego, CA.
13. Heise T, Hövelmann U, Nosek L, Bøttcher SG, Granhall C, Haahr H. Insulin degludec has a two-fold longer half-life and a more consistent pharmacokinetic profile than insulin glargine. Paper presented at: American Diabetes Association 71st Scientific Sessions; June 24-28, 2011; San Diego, CA.
14. Heise T, Hermanski L, Nosek L, Feldmann A, Rasmussen S, Haahr H. The pharmacodynamic variability of insulin degludec is consistently lower than insulin glargine over 24 hours at steady state. Diabetes. 2011;60(suppl 1):A263.-Poster 960-P.
Garber AJ, King AB, Del Prato S, et al. 15.NN1250-3582 (BEGIN BB T2D) Trial Investigators. Insulin degludec, an ultra-longacting basal insulin, versus insulin glargine in basal-bolus treatment with mealtime insulin aspart in type 2 diabetes (BEGIN Basal-Bolus Type 2): a phase 3, randomised, open-label, treat-to-target non-inferiority trial. Lancet. 2012;379(9825):1498-1507.
16. Heller S, Buse J, Fisher M, et al. BEGIN Basal-Bolus Type 1 Trial Investigators. Insulin degludec, an ultra-longacting basal insulin, versus insulin glargine in basal-bolus treatment with mealtime insulin aspart in type 1 diabetes (BEGIN Basal-Blus Type 1): a phase 3, randomised, open-label, treat-to-target non-inferiority trial. Lancet. 2012;379(9825):1489-1497.
17. Hirsch IB, Franek E, Courreges JP, Mersebach H, Dykiel P, Bode BW. Efficacy and safety of a new basal insulin with a bolus boost (IDegAsp) used once daily in combination wtih insulin apart (IAsp) in people wth type 1 diabetes. Diabetes. 2011;60(suppl 1):A292.-Poster 1064-P.
18. Meneghini L, Atkin SL, Bain S, et al. Flexible once-daily dosing of insulin degludec does not compromise glycemic control or safety compared to insulin glargine given once daily at the same time each day in people with type 2 diabetes. Paper presented at: American Diabetes Association 71st Scientific Sessions; June 24-28, 2011; San Diego, CA.