User login
Clostridium difficile infection (CDI) is the result of a Gram-positive bacterium, whose exotoxins are commonly associated with infectious, watery diarrhea.1Clostridium difficile infection is associated with a significant cost burden, and over the past several years, the incidence and severity of CDI have been on the rise.2,3
There are several known risk factors for CDI. The most well-elucidated risk factor is the use of antibiotics, especially fluoroquinolones, clindamycin, broad-spectrum penicillins, and broad-spectrum cephalosporins.4,5 Other risk factors include advancing age, immunosuppression, a high burden of comorbidities, hospitalization, and antineoplastic agent use.6-8 Over the past decade, gastric acid suppression has come under increased scrutiny as a possible risk factor for CDI; specifically, exposure to proton pump inhibitors (PPIs) and histamine 2 receptor antagonists (H2RAs).8-14 With the reported overuse of PPIs, the importance of understanding safety risks associated with these agents is becoming increasingly necessary.15
In 2012, the FDA issued a public safety announcement reporting a possible association between CDI and patients undergoing treatment with PPIs.16 A large meta-analysis by Janarthanan and colleagues in 2012 evaluated 23 studies with nearly 300,000 patients, showing a 1.6-fold increase in CDI in patients exposed to a PPI.8 Another large meta-analysis noted that 39 studies showed a statistically significant association between PPI use and the risk of developing CDI (odds ratio [OR] 1.74) compared with nonusers.17 A recent study by McDonald and colleagues demonstrated patients with continuous PPI use had an elevated risk of CDI recurrence compared with patients not on continuous PPI therapy.18 These large studies did not focus analysis on elderly, hospitalized patients with significant comorbidities. There are several proposed mechanisms for the reported association between PPI use and CDI. The most widely accepted mechanism is that gastric acid suppression disrupts normal gastrointestinal flora and allows for bacterial overgrowth.19-21There are few studies that have evaluated the association between PPI use and CDI in elderly, hospitalized patients. Studies conducted in a similar patient population have demonstrated no association between PPI use and CDI.22,23 Shah and colleagues reported that treatment with gastric acid antisecretory agents does not increase the risk of developing CDI among elderly, hospitalized patients who also had severe disability.23 Lowe and colleagues demonstrated no association between PPI therapy and hospitalization for elderly outpatients with CDI.22 A study was needed to determine the association between PPI use and CDI in hospitalized, elderly patients with a high burden of comorbidities.
Related: Cleaning Up? Microfiber May Be Better
Objectives
The primary objective of this study was to determine whether there is an association between PPI exposure and CDI in elderly, hospitalized patients. The secondary objective was to determine the risk factors for the development of CDI in elderly, hospitalized patients.
Methods
Approval for this study was obtained from the Emory University Institutional Review Board and the VA Research and Development Committee. The study was a single-center, retrospective, medical record review of patients with a CDI polymerase chain reaction (PCR) assay, conducted at the Atlanta VAMC between August 20, 2011, and August 20, 2013.
Two reports for the study period were generated from TheraDoc (Premier Inc., Salt Lake City, UT) medical record software: all patients with a positive CDI PCR assay and all patients with a negative CDI PCR assay. All adult inpatients aged ≥ 18 years with a positive CDI PCR assay and diarrhea were included. Patients with CDI were randomly matched 1:1, based on age, with a control patient from a large sample of eligible CDI negative assays. Any duplicate positive CDI PCR assays were deleted, and only the first positive test was analyzed. Confirmation that PCR assay with liquid stool was being performed per manufacturer recommendations was obtained from microbiology laboratory staff.
Patient-specific data were collected from the VA Computerized Patient Record System (CPRS). Potential covariates for analyses were selected based on previous literature regarding possible associations between PPI and CDI. Data were collected on patient age, gender, PPI exposure, PPI agent, PPI dose, concomitant medications, high-risk antibiotic use, comorbidities (including diabetes, chronic renal failure, liver disease, anemia, coagulopathy, myocardial infarction, chronic heart failure, peripheral vascular disease, chronic obstructive pulmonary disease, hypertension, hypothyroidism, and any alcohol or drug abuse), length of hospital stay, bed location, and first vs recurrent CDI. Proton pump inhibitor exposure was defined as use of any PPI during hospitalization or within 2 months prior to hospitalization. High-risk antibiotics were defined as fluoroquinolones, broad-spectrum penicillins, broad-spectrum cephalosporins, and clindamycin.
Statistical Analysis
Two-sided Wilcoxon rank sum and chi-square tests were used to compare the selected variables between CDI cases and non-CDI controls. A multivariate logistic regression model was fitted to the data using CDI as the outcome and PPI use as the main exposure of interest. The large number of covariates of interest relative to the sample size suggests conditional maximum likelihood methods of estimation.24
Separate models were run using each case-control pair as a separate stratum in the model (125 pairs) as well as pooling similar-age strata to reduce the 125 pairs to 46 pooled sets. However, when comparing the Akaike information criterion (AIC; an objective measure to determine relative quality of multivariate models where a lower AIC value is preferred) between these individual and pooled strata models, the model that controlled for 125 individual case-control strata was overwhelmingly suggested as the better model (AIC, 175 vs 255, respectively).25 Analyses were conducted with SAS 9.2 (SAS Institute Inc., Cary, NC).
Results
A total of 128 patients were positive for CDI during the 2-year study period. Three of these patients were excluded from the study due to outpatient status. The remaining 125 patients were matched 1:1 with patients negative for CDI to yield a total study population of 250 patients.
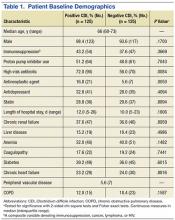
Baseline demographics are shown in Table 1. The majority of patients included were males with a median age of 66 years. Nearly half of all patients in both groups had chronic renal failure, diabetes, or anemia. Comorbidities were numerous but were not significantly different between the positive and negative CDI groups. No significant difference in immunosuppression or PPI use was detected between the 2 groups. However, there were significantly more patients taking a high-risk antibiotic or an antineoplastic agent in the positive CDI group compared with the negative CDI group. The average length of hospital stay averaged 10 to 12 days and did not statistically differ between the 2 groups.
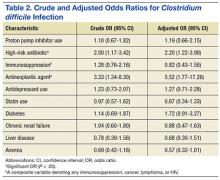
Crude ORs (cORs) and adjusted ORs (aORs) were calculated for the primary and secondary outcome measures (Table 2). There was not a statistically significant association between PPI use and CDI (cOR 1.10, 95% confidence interval [CI] 0.67-1.82; aOR 1.19, 95% CI 0.66-2.15). Other known risk factors were also evaluated for association. A statistically significant association did not exist between CDI and immunosuppression, antidepressant use, statin use, diabetes, chronic renal failure, liver disease, or anemia. However, the statistical analysis did suggest an association between CDI and high-risk antibiotic use (aOR 2.20, 95% CI 1.22-3.99) and antineoplastic agent use (aOR 5.52, 95% CI 1.77-17.26).
A sensitivity analysis was conducted to determine whether there were differing associations with CDI by PPI dose or specific agent. In both sensitivity analyses, there were no statistically significant differences in CDI between patients who took once-daily vs twice-daily PPI dosing or those who took pantoprazole vs omeprazole.
Discussion
The objective of this study was to evaluate the association between PPI use and CDI in an aging, hospitalized population. When adjusted for known risk factors, there was no association between CDI and patients exposed to PPI therapy.
Previous studies evaluating PPI use and CDI have shown conflicting results. Large meta-analyses have shown an increase in CDI in patients exposed to a PPI, whereas other studies have shown no association. In the studies that did not link PPI use and CDI, patients were elderly, hospitalized, and had other CDI risk factors. The patients in this study were hospitalized, with a median age of 66 years. They were significantly immunosuppressed and had a very high burden of comorbidities. A possible explanation for the lack of association between PPI use and CDI is that, in patients with several existing risk factors for CDI, adding a PPI confers no additional effect on CDI risk.
Well-known risk factors, including high-risk antibiotic use and antineoplastic chemotherapy use, were confirmed by this study. Other known risk factors, including immunosuppression and diabetes, were not observed to have an association with CDI in this study. This is perhaps for the same reason that PPI exposure did not show a significant association. In a study published in 2010, Howell and colleagues showed that the risk of CDI increased as acid suppression increased in a dose-dependent fashion.9 There was no association between PPI dose and PPI agent on the primary outcome measure.
About half of all patients in the current study were exposed to PPI therapy, which was a surprisingly high number. Although this study did not evaluate appropriate use of PPI therapy, it exposes the high rate of PPI use at the study site. It is known that PPI use has associated risks, and it is important that physicians continue to be vigilant in their prescribing habits.
Related: The Importance of an Antimicrobial Stewardship Program
Limitations and Future Directions
Several limitations of this study should be noted. A relatively narrow patient population was examined, which limits the generalizability of these findings. However, health care providers treating older, hospitalized patients with a high burden of comorbidities may find the results meaningful. This study was retrospective and included a relatively small sample size, which may limit the ability to detect a statistically significant difference.
Data were not collected on the duration of PPI therapy. A longer duration of therapy has been shown in previous studies to be significantly associated with CDI.26 It is unclear in this patient population whether there would have been an association between PPI duration of treatment and CDI.
Outpatient PPI exposure was determined using CPRS refill history. Patients were considered to have PPI exposure if they filled ≥ 1 prescription for a PPI within 2 months of hospitalization. Using this methodology to determine PPI exposure may not have identified patients who took over-the-counter PPIs or did not report filling a prescription for a PPI from an outside pharmacy, which would have resulted in an underestimation of PPI use in this sample. Furthermore, it is difficult to determine adherence to a prescribed regimen for outpatients.
Pantoprazole and omeprazole are the formulary PPIs at the study site. Conducting research at an institution with a formulary prevents evaluation of other PPIs, including esomeprazole, rabeprazole, dexlansoprazole, and lansoprazole. This is not seen as a significant limitation, as there have not been significant differences in the PPI agent and CDI widely reported in the literature.
Data on H2RA exposure were not collected. Any possible effect of H2RA exposure and CDI cannot be accounted for in this study. It is not likely that H2RA exposure would be associated with an increased risk of CDI in this patient population, as several studies have shown less of an association between CDI and H2RA compared with CDI and PPI use.
Further investigation to evaluate the association between CDI and PPI exposure in an elderly, hospitalized population is needed. Larger studies in these patients that evaluate duration of PPI therapy would be beneficial.
Related: Antidepressants Plus NSAIDs and the Risk of Intracranial Hemorrhage
Conclusion
In an elderly, hospitalized patient population with a high comorbidity burden, this study did not detect a statistically significant association between PPI exposure and CDI. Despite this, providers should continue to consider discontinuation of unnecessary PPI therapy.
Acknowledgements
The authors wish to thank Mehran Salles, PhD, PharmD, for her assistance. Study findings were presented at the 2014 Southeastern Residency Conference in Athens, Georgia, on May 1, 2014.
Author disclosures
The authors report no actual or potential conflicts of interest with regard to this article.
Disclaimer
The opinions expressed herein are those of the authors and do not necessarily reflect those of Federal Practitioner Frontline Medical Communications Inc., the U.S. Government, or any of its agencies. This article may discuss unlabeled or investigational use of certain drugs. Please review complete prescribing information for specific drugs or drug combinations—including indications, contraindications, warnings, and adverse effects—before administering pharmacologic therapy to patients.
1. Poutanen SM, Simor AE. Clostridium difficile-associated diarrhea in adults. CMAJ. 2004;171(1):51-58.
2. Clostridium difficile infection. Centers for Disease Control and Prevention Website. http://www.cdc.gov/HAI/organisms/cdiff/Cdiff_infect.html. Updated February 25, 2015. Accessed October 5, 2015.
3. Song X, Bartlett JG, Speck K, Naegeli A, Carroll K, Perl TM. Rising economic impact of Clostridium difficile-associated disease in adult hospitalized patient population. Infect Control Hosp Epidemiol. 2008;29(9):823-828.
4. Bartlett JG. Narrative review: the new epidemic of Clostridium difficile-associated enteric disease. Ann Intern Med. 2006;145(10):758-764.
5. Baxter R, Ray GT, Fireman BH. Case-control study of antibiotic use and subsequent Clostridium difficile-associated diarrhea in hospitalized patients. Infect Control Hosp Epidemiol. 2008;29(1):44-50.
6. Anand A, Glatt AE. Clostridium difficile infection associated with antineoplastic chemotherapy: a review. Clin Infect Dis. 1993;17(1):109-113.
7. Bignardi GE. Risk factors for Clostridium difficile infection. J Hosp Infect. 1998;40(1):1-15.
8. Janarthanan S, Ditah I, Adler DG, Ehrinpreis MN. Clostridium difficile-associated diarrhea and proton pump inhibitor therapy: a meta-analysis. Am J Gastroenterol. 2012;107(7):1001-1010.
9. Howell MD, Novack V, Grgurich P, et al. Iatrogenic gastric acid suppression and the risk of nosocomial Clostridium difficile infection. Arch Intern Med. 2010;170(9):784-790.
10. Aseeri M, Schroeder T, Kramer J, Zackula R. Gastric acid suppression by proton pump inhibitors as a risk factor for Clostridium difficile-associated diarrhea in hospitalized patients. Am J Gastroenterol. 2008;103(9):2308-2313.
11. Dalton BR, Lye-Maccannell T, Henderson EA, Maccannell DR, Louie TJ. Proton pump inhibitors increase significantly the risk of Clostridium difficile infection in a low-endemicity, non-outbreak hospital setting. Aliment Pharmacol Ther. 2009;29(6):626-634.
12. Dial S, Alrasadi K, Manoukian C, Huang A, Menzies D. Risk of Clostridium difficile diarrhea among hospital inpatients prescribed proton pump inhibitors: cohort and case-control studies. CMAJ. 2004;171(1):33-38.
13. Linsky A, Gupta K, Lawler EV, Fonda JR, Hermos JA. Proton pump inhibitors and risk for recurrent Clostridium difficile infection. Arch Intern Med. 2010;170(9):772-778.
14. Yearsley KA, Gilby LJ, Ramadas AV, Kubiak EM, Fone DL, Allison MC. Proton pump inhibitor therapy is a risk factor for Clostridium difficile-associated diarrhoea. Aliment Pharmacol Ther. 2006;24(4):613-619.
15. Nardino RJ, Vender RJ, Herbert PN. Overuse of acid-suppressive therapy in hospitalized patients. Am J Gastroenterol. 2000;95(11):3118-3122.
16. U.S. Food and Drug Administration. FDA drug safety communication: Clostridium difficile-associated diarrhea can be associated with stomach acid drugs known as proton pump inhibitors (PPIs). http://www.fda.gov/Drugs/DrugSafety/ucm290510.htm. Updated February 15, 2013. Accessed October 5, 2015.
17. Kwok CS, Arthur AK, Anibueze CI, Singh S, Cavallazzi R, Loke YK. Risk of Clostridium difficile infection with acid suppressing drugs and antibiotics: meta-analysis. Am J Gastroenterol. 2012;107(7):1011-1019.
18. McDonald EG, Milligan J, Frenette C, Lee TC. Continuous proton pump inhibitor therapy and the associated risk of recurrent Clostridium difficile infection. JAMA Intern Med. 2015;175(5):784-791.
19. Lewis SJ, Franco S, Young G, O'Keefe SJ. Altered bowel function and duodenal bacterial overgrowth in patients treated with omeprazole. Aliment Pharmacol Ther. 1996;10(4):557-561.
20. Theisen J, Nehra D, Citron D, et al. Suppression of gastric acid secretion in patients with gastroesophageal reflux disease results in gastric bacterial overgrowth and deconjugation of bile acids. J Gastrointest Surg. 2000;4(1):50-54.
21. Williams C, McColl KE. Review article: proton pump inhibitors and bacterial overgrowth. Aliment Pharmacol Ther. 2006;23(1):3-10.
22. Lowe DO, Mamdani MM, Kopp A, Low DE, Juurlink DN. Proton pump inhibitors and hospitalization for Clostridium difficile-associated disease: a population-based study. Clin Infect Dis. 2006;43(10):1272-1276.
23. Shah S, Lewis A, Leopold D, Dunstan F, Woodhouse K. Gastric acid suppression does not promote clostridial diarrhoea in the elderly. QJM. 2000;93(3):175-181.
24. Kleinbaum DG, Klein M. Logistic Regression: A Self-Learning Text. 3rd ed. New York, NY: Springer; 2010.
25. Akaike H. A new look at the statistical model identification. IEEE Transact Autom Contr. 1974;19(6):716-723.
26. Barletta JF, El-Ibiary SY, Davis LE, Nguyen B, Raney CR. Proton pump inhibitors and the risk for hospital-acquired Clostridium difficile infection. Mayo Clin Proc. 2013;88(10):1085-1090.
Clostridium difficile infection (CDI) is the result of a Gram-positive bacterium, whose exotoxins are commonly associated with infectious, watery diarrhea.1Clostridium difficile infection is associated with a significant cost burden, and over the past several years, the incidence and severity of CDI have been on the rise.2,3
There are several known risk factors for CDI. The most well-elucidated risk factor is the use of antibiotics, especially fluoroquinolones, clindamycin, broad-spectrum penicillins, and broad-spectrum cephalosporins.4,5 Other risk factors include advancing age, immunosuppression, a high burden of comorbidities, hospitalization, and antineoplastic agent use.6-8 Over the past decade, gastric acid suppression has come under increased scrutiny as a possible risk factor for CDI; specifically, exposure to proton pump inhibitors (PPIs) and histamine 2 receptor antagonists (H2RAs).8-14 With the reported overuse of PPIs, the importance of understanding safety risks associated with these agents is becoming increasingly necessary.15
In 2012, the FDA issued a public safety announcement reporting a possible association between CDI and patients undergoing treatment with PPIs.16 A large meta-analysis by Janarthanan and colleagues in 2012 evaluated 23 studies with nearly 300,000 patients, showing a 1.6-fold increase in CDI in patients exposed to a PPI.8 Another large meta-analysis noted that 39 studies showed a statistically significant association between PPI use and the risk of developing CDI (odds ratio [OR] 1.74) compared with nonusers.17 A recent study by McDonald and colleagues demonstrated patients with continuous PPI use had an elevated risk of CDI recurrence compared with patients not on continuous PPI therapy.18 These large studies did not focus analysis on elderly, hospitalized patients with significant comorbidities. There are several proposed mechanisms for the reported association between PPI use and CDI. The most widely accepted mechanism is that gastric acid suppression disrupts normal gastrointestinal flora and allows for bacterial overgrowth.19-21There are few studies that have evaluated the association between PPI use and CDI in elderly, hospitalized patients. Studies conducted in a similar patient population have demonstrated no association between PPI use and CDI.22,23 Shah and colleagues reported that treatment with gastric acid antisecretory agents does not increase the risk of developing CDI among elderly, hospitalized patients who also had severe disability.23 Lowe and colleagues demonstrated no association between PPI therapy and hospitalization for elderly outpatients with CDI.22 A study was needed to determine the association between PPI use and CDI in hospitalized, elderly patients with a high burden of comorbidities.
Related: Cleaning Up? Microfiber May Be Better
Objectives
The primary objective of this study was to determine whether there is an association between PPI exposure and CDI in elderly, hospitalized patients. The secondary objective was to determine the risk factors for the development of CDI in elderly, hospitalized patients.
Methods
Approval for this study was obtained from the Emory University Institutional Review Board and the VA Research and Development Committee. The study was a single-center, retrospective, medical record review of patients with a CDI polymerase chain reaction (PCR) assay, conducted at the Atlanta VAMC between August 20, 2011, and August 20, 2013.
Two reports for the study period were generated from TheraDoc (Premier Inc., Salt Lake City, UT) medical record software: all patients with a positive CDI PCR assay and all patients with a negative CDI PCR assay. All adult inpatients aged ≥ 18 years with a positive CDI PCR assay and diarrhea were included. Patients with CDI were randomly matched 1:1, based on age, with a control patient from a large sample of eligible CDI negative assays. Any duplicate positive CDI PCR assays were deleted, and only the first positive test was analyzed. Confirmation that PCR assay with liquid stool was being performed per manufacturer recommendations was obtained from microbiology laboratory staff.
Patient-specific data were collected from the VA Computerized Patient Record System (CPRS). Potential covariates for analyses were selected based on previous literature regarding possible associations between PPI and CDI. Data were collected on patient age, gender, PPI exposure, PPI agent, PPI dose, concomitant medications, high-risk antibiotic use, comorbidities (including diabetes, chronic renal failure, liver disease, anemia, coagulopathy, myocardial infarction, chronic heart failure, peripheral vascular disease, chronic obstructive pulmonary disease, hypertension, hypothyroidism, and any alcohol or drug abuse), length of hospital stay, bed location, and first vs recurrent CDI. Proton pump inhibitor exposure was defined as use of any PPI during hospitalization or within 2 months prior to hospitalization. High-risk antibiotics were defined as fluoroquinolones, broad-spectrum penicillins, broad-spectrum cephalosporins, and clindamycin.
Statistical Analysis
Two-sided Wilcoxon rank sum and chi-square tests were used to compare the selected variables between CDI cases and non-CDI controls. A multivariate logistic regression model was fitted to the data using CDI as the outcome and PPI use as the main exposure of interest. The large number of covariates of interest relative to the sample size suggests conditional maximum likelihood methods of estimation.24
Separate models were run using each case-control pair as a separate stratum in the model (125 pairs) as well as pooling similar-age strata to reduce the 125 pairs to 46 pooled sets. However, when comparing the Akaike information criterion (AIC; an objective measure to determine relative quality of multivariate models where a lower AIC value is preferred) between these individual and pooled strata models, the model that controlled for 125 individual case-control strata was overwhelmingly suggested as the better model (AIC, 175 vs 255, respectively).25 Analyses were conducted with SAS 9.2 (SAS Institute Inc., Cary, NC).
Results
A total of 128 patients were positive for CDI during the 2-year study period. Three of these patients were excluded from the study due to outpatient status. The remaining 125 patients were matched 1:1 with patients negative for CDI to yield a total study population of 250 patients.
Baseline demographics are shown in Table 1. The majority of patients included were males with a median age of 66 years. Nearly half of all patients in both groups had chronic renal failure, diabetes, or anemia. Comorbidities were numerous but were not significantly different between the positive and negative CDI groups. No significant difference in immunosuppression or PPI use was detected between the 2 groups. However, there were significantly more patients taking a high-risk antibiotic or an antineoplastic agent in the positive CDI group compared with the negative CDI group. The average length of hospital stay averaged 10 to 12 days and did not statistically differ between the 2 groups.
Crude ORs (cORs) and adjusted ORs (aORs) were calculated for the primary and secondary outcome measures (Table 2). There was not a statistically significant association between PPI use and CDI (cOR 1.10, 95% confidence interval [CI] 0.67-1.82; aOR 1.19, 95% CI 0.66-2.15). Other known risk factors were also evaluated for association. A statistically significant association did not exist between CDI and immunosuppression, antidepressant use, statin use, diabetes, chronic renal failure, liver disease, or anemia. However, the statistical analysis did suggest an association between CDI and high-risk antibiotic use (aOR 2.20, 95% CI 1.22-3.99) and antineoplastic agent use (aOR 5.52, 95% CI 1.77-17.26).
A sensitivity analysis was conducted to determine whether there were differing associations with CDI by PPI dose or specific agent. In both sensitivity analyses, there were no statistically significant differences in CDI between patients who took once-daily vs twice-daily PPI dosing or those who took pantoprazole vs omeprazole.
Discussion
The objective of this study was to evaluate the association between PPI use and CDI in an aging, hospitalized population. When adjusted for known risk factors, there was no association between CDI and patients exposed to PPI therapy.
Previous studies evaluating PPI use and CDI have shown conflicting results. Large meta-analyses have shown an increase in CDI in patients exposed to a PPI, whereas other studies have shown no association. In the studies that did not link PPI use and CDI, patients were elderly, hospitalized, and had other CDI risk factors. The patients in this study were hospitalized, with a median age of 66 years. They were significantly immunosuppressed and had a very high burden of comorbidities. A possible explanation for the lack of association between PPI use and CDI is that, in patients with several existing risk factors for CDI, adding a PPI confers no additional effect on CDI risk.
Well-known risk factors, including high-risk antibiotic use and antineoplastic chemotherapy use, were confirmed by this study. Other known risk factors, including immunosuppression and diabetes, were not observed to have an association with CDI in this study. This is perhaps for the same reason that PPI exposure did not show a significant association. In a study published in 2010, Howell and colleagues showed that the risk of CDI increased as acid suppression increased in a dose-dependent fashion.9 There was no association between PPI dose and PPI agent on the primary outcome measure.
About half of all patients in the current study were exposed to PPI therapy, which was a surprisingly high number. Although this study did not evaluate appropriate use of PPI therapy, it exposes the high rate of PPI use at the study site. It is known that PPI use has associated risks, and it is important that physicians continue to be vigilant in their prescribing habits.
Related: The Importance of an Antimicrobial Stewardship Program
Limitations and Future Directions
Several limitations of this study should be noted. A relatively narrow patient population was examined, which limits the generalizability of these findings. However, health care providers treating older, hospitalized patients with a high burden of comorbidities may find the results meaningful. This study was retrospective and included a relatively small sample size, which may limit the ability to detect a statistically significant difference.
Data were not collected on the duration of PPI therapy. A longer duration of therapy has been shown in previous studies to be significantly associated with CDI.26 It is unclear in this patient population whether there would have been an association between PPI duration of treatment and CDI.
Outpatient PPI exposure was determined using CPRS refill history. Patients were considered to have PPI exposure if they filled ≥ 1 prescription for a PPI within 2 months of hospitalization. Using this methodology to determine PPI exposure may not have identified patients who took over-the-counter PPIs or did not report filling a prescription for a PPI from an outside pharmacy, which would have resulted in an underestimation of PPI use in this sample. Furthermore, it is difficult to determine adherence to a prescribed regimen for outpatients.
Pantoprazole and omeprazole are the formulary PPIs at the study site. Conducting research at an institution with a formulary prevents evaluation of other PPIs, including esomeprazole, rabeprazole, dexlansoprazole, and lansoprazole. This is not seen as a significant limitation, as there have not been significant differences in the PPI agent and CDI widely reported in the literature.
Data on H2RA exposure were not collected. Any possible effect of H2RA exposure and CDI cannot be accounted for in this study. It is not likely that H2RA exposure would be associated with an increased risk of CDI in this patient population, as several studies have shown less of an association between CDI and H2RA compared with CDI and PPI use.
Further investigation to evaluate the association between CDI and PPI exposure in an elderly, hospitalized population is needed. Larger studies in these patients that evaluate duration of PPI therapy would be beneficial.
Related: Antidepressants Plus NSAIDs and the Risk of Intracranial Hemorrhage
Conclusion
In an elderly, hospitalized patient population with a high comorbidity burden, this study did not detect a statistically significant association between PPI exposure and CDI. Despite this, providers should continue to consider discontinuation of unnecessary PPI therapy.
Acknowledgements
The authors wish to thank Mehran Salles, PhD, PharmD, for her assistance. Study findings were presented at the 2014 Southeastern Residency Conference in Athens, Georgia, on May 1, 2014.
Author disclosures
The authors report no actual or potential conflicts of interest with regard to this article.
Disclaimer
The opinions expressed herein are those of the authors and do not necessarily reflect those of Federal Practitioner Frontline Medical Communications Inc., the U.S. Government, or any of its agencies. This article may discuss unlabeled or investigational use of certain drugs. Please review complete prescribing information for specific drugs or drug combinations—including indications, contraindications, warnings, and adverse effects—before administering pharmacologic therapy to patients.
Clostridium difficile infection (CDI) is the result of a Gram-positive bacterium, whose exotoxins are commonly associated with infectious, watery diarrhea.1Clostridium difficile infection is associated with a significant cost burden, and over the past several years, the incidence and severity of CDI have been on the rise.2,3
There are several known risk factors for CDI. The most well-elucidated risk factor is the use of antibiotics, especially fluoroquinolones, clindamycin, broad-spectrum penicillins, and broad-spectrum cephalosporins.4,5 Other risk factors include advancing age, immunosuppression, a high burden of comorbidities, hospitalization, and antineoplastic agent use.6-8 Over the past decade, gastric acid suppression has come under increased scrutiny as a possible risk factor for CDI; specifically, exposure to proton pump inhibitors (PPIs) and histamine 2 receptor antagonists (H2RAs).8-14 With the reported overuse of PPIs, the importance of understanding safety risks associated with these agents is becoming increasingly necessary.15
In 2012, the FDA issued a public safety announcement reporting a possible association between CDI and patients undergoing treatment with PPIs.16 A large meta-analysis by Janarthanan and colleagues in 2012 evaluated 23 studies with nearly 300,000 patients, showing a 1.6-fold increase in CDI in patients exposed to a PPI.8 Another large meta-analysis noted that 39 studies showed a statistically significant association between PPI use and the risk of developing CDI (odds ratio [OR] 1.74) compared with nonusers.17 A recent study by McDonald and colleagues demonstrated patients with continuous PPI use had an elevated risk of CDI recurrence compared with patients not on continuous PPI therapy.18 These large studies did not focus analysis on elderly, hospitalized patients with significant comorbidities. There are several proposed mechanisms for the reported association between PPI use and CDI. The most widely accepted mechanism is that gastric acid suppression disrupts normal gastrointestinal flora and allows for bacterial overgrowth.19-21There are few studies that have evaluated the association between PPI use and CDI in elderly, hospitalized patients. Studies conducted in a similar patient population have demonstrated no association between PPI use and CDI.22,23 Shah and colleagues reported that treatment with gastric acid antisecretory agents does not increase the risk of developing CDI among elderly, hospitalized patients who also had severe disability.23 Lowe and colleagues demonstrated no association between PPI therapy and hospitalization for elderly outpatients with CDI.22 A study was needed to determine the association between PPI use and CDI in hospitalized, elderly patients with a high burden of comorbidities.
Related: Cleaning Up? Microfiber May Be Better
Objectives
The primary objective of this study was to determine whether there is an association between PPI exposure and CDI in elderly, hospitalized patients. The secondary objective was to determine the risk factors for the development of CDI in elderly, hospitalized patients.
Methods
Approval for this study was obtained from the Emory University Institutional Review Board and the VA Research and Development Committee. The study was a single-center, retrospective, medical record review of patients with a CDI polymerase chain reaction (PCR) assay, conducted at the Atlanta VAMC between August 20, 2011, and August 20, 2013.
Two reports for the study period were generated from TheraDoc (Premier Inc., Salt Lake City, UT) medical record software: all patients with a positive CDI PCR assay and all patients with a negative CDI PCR assay. All adult inpatients aged ≥ 18 years with a positive CDI PCR assay and diarrhea were included. Patients with CDI were randomly matched 1:1, based on age, with a control patient from a large sample of eligible CDI negative assays. Any duplicate positive CDI PCR assays were deleted, and only the first positive test was analyzed. Confirmation that PCR assay with liquid stool was being performed per manufacturer recommendations was obtained from microbiology laboratory staff.
Patient-specific data were collected from the VA Computerized Patient Record System (CPRS). Potential covariates for analyses were selected based on previous literature regarding possible associations between PPI and CDI. Data were collected on patient age, gender, PPI exposure, PPI agent, PPI dose, concomitant medications, high-risk antibiotic use, comorbidities (including diabetes, chronic renal failure, liver disease, anemia, coagulopathy, myocardial infarction, chronic heart failure, peripheral vascular disease, chronic obstructive pulmonary disease, hypertension, hypothyroidism, and any alcohol or drug abuse), length of hospital stay, bed location, and first vs recurrent CDI. Proton pump inhibitor exposure was defined as use of any PPI during hospitalization or within 2 months prior to hospitalization. High-risk antibiotics were defined as fluoroquinolones, broad-spectrum penicillins, broad-spectrum cephalosporins, and clindamycin.
Statistical Analysis
Two-sided Wilcoxon rank sum and chi-square tests were used to compare the selected variables between CDI cases and non-CDI controls. A multivariate logistic regression model was fitted to the data using CDI as the outcome and PPI use as the main exposure of interest. The large number of covariates of interest relative to the sample size suggests conditional maximum likelihood methods of estimation.24
Separate models were run using each case-control pair as a separate stratum in the model (125 pairs) as well as pooling similar-age strata to reduce the 125 pairs to 46 pooled sets. However, when comparing the Akaike information criterion (AIC; an objective measure to determine relative quality of multivariate models where a lower AIC value is preferred) between these individual and pooled strata models, the model that controlled for 125 individual case-control strata was overwhelmingly suggested as the better model (AIC, 175 vs 255, respectively).25 Analyses were conducted with SAS 9.2 (SAS Institute Inc., Cary, NC).
Results
A total of 128 patients were positive for CDI during the 2-year study period. Three of these patients were excluded from the study due to outpatient status. The remaining 125 patients were matched 1:1 with patients negative for CDI to yield a total study population of 250 patients.
Baseline demographics are shown in Table 1. The majority of patients included were males with a median age of 66 years. Nearly half of all patients in both groups had chronic renal failure, diabetes, or anemia. Comorbidities were numerous but were not significantly different between the positive and negative CDI groups. No significant difference in immunosuppression or PPI use was detected between the 2 groups. However, there were significantly more patients taking a high-risk antibiotic or an antineoplastic agent in the positive CDI group compared with the negative CDI group. The average length of hospital stay averaged 10 to 12 days and did not statistically differ between the 2 groups.
Crude ORs (cORs) and adjusted ORs (aORs) were calculated for the primary and secondary outcome measures (Table 2). There was not a statistically significant association between PPI use and CDI (cOR 1.10, 95% confidence interval [CI] 0.67-1.82; aOR 1.19, 95% CI 0.66-2.15). Other known risk factors were also evaluated for association. A statistically significant association did not exist between CDI and immunosuppression, antidepressant use, statin use, diabetes, chronic renal failure, liver disease, or anemia. However, the statistical analysis did suggest an association between CDI and high-risk antibiotic use (aOR 2.20, 95% CI 1.22-3.99) and antineoplastic agent use (aOR 5.52, 95% CI 1.77-17.26).
A sensitivity analysis was conducted to determine whether there were differing associations with CDI by PPI dose or specific agent. In both sensitivity analyses, there were no statistically significant differences in CDI between patients who took once-daily vs twice-daily PPI dosing or those who took pantoprazole vs omeprazole.
Discussion
The objective of this study was to evaluate the association between PPI use and CDI in an aging, hospitalized population. When adjusted for known risk factors, there was no association between CDI and patients exposed to PPI therapy.
Previous studies evaluating PPI use and CDI have shown conflicting results. Large meta-analyses have shown an increase in CDI in patients exposed to a PPI, whereas other studies have shown no association. In the studies that did not link PPI use and CDI, patients were elderly, hospitalized, and had other CDI risk factors. The patients in this study were hospitalized, with a median age of 66 years. They were significantly immunosuppressed and had a very high burden of comorbidities. A possible explanation for the lack of association between PPI use and CDI is that, in patients with several existing risk factors for CDI, adding a PPI confers no additional effect on CDI risk.
Well-known risk factors, including high-risk antibiotic use and antineoplastic chemotherapy use, were confirmed by this study. Other known risk factors, including immunosuppression and diabetes, were not observed to have an association with CDI in this study. This is perhaps for the same reason that PPI exposure did not show a significant association. In a study published in 2010, Howell and colleagues showed that the risk of CDI increased as acid suppression increased in a dose-dependent fashion.9 There was no association between PPI dose and PPI agent on the primary outcome measure.
About half of all patients in the current study were exposed to PPI therapy, which was a surprisingly high number. Although this study did not evaluate appropriate use of PPI therapy, it exposes the high rate of PPI use at the study site. It is known that PPI use has associated risks, and it is important that physicians continue to be vigilant in their prescribing habits.
Related: The Importance of an Antimicrobial Stewardship Program
Limitations and Future Directions
Several limitations of this study should be noted. A relatively narrow patient population was examined, which limits the generalizability of these findings. However, health care providers treating older, hospitalized patients with a high burden of comorbidities may find the results meaningful. This study was retrospective and included a relatively small sample size, which may limit the ability to detect a statistically significant difference.
Data were not collected on the duration of PPI therapy. A longer duration of therapy has been shown in previous studies to be significantly associated with CDI.26 It is unclear in this patient population whether there would have been an association between PPI duration of treatment and CDI.
Outpatient PPI exposure was determined using CPRS refill history. Patients were considered to have PPI exposure if they filled ≥ 1 prescription for a PPI within 2 months of hospitalization. Using this methodology to determine PPI exposure may not have identified patients who took over-the-counter PPIs or did not report filling a prescription for a PPI from an outside pharmacy, which would have resulted in an underestimation of PPI use in this sample. Furthermore, it is difficult to determine adherence to a prescribed regimen for outpatients.
Pantoprazole and omeprazole are the formulary PPIs at the study site. Conducting research at an institution with a formulary prevents evaluation of other PPIs, including esomeprazole, rabeprazole, dexlansoprazole, and lansoprazole. This is not seen as a significant limitation, as there have not been significant differences in the PPI agent and CDI widely reported in the literature.
Data on H2RA exposure were not collected. Any possible effect of H2RA exposure and CDI cannot be accounted for in this study. It is not likely that H2RA exposure would be associated with an increased risk of CDI in this patient population, as several studies have shown less of an association between CDI and H2RA compared with CDI and PPI use.
Further investigation to evaluate the association between CDI and PPI exposure in an elderly, hospitalized population is needed. Larger studies in these patients that evaluate duration of PPI therapy would be beneficial.
Related: Antidepressants Plus NSAIDs and the Risk of Intracranial Hemorrhage
Conclusion
In an elderly, hospitalized patient population with a high comorbidity burden, this study did not detect a statistically significant association between PPI exposure and CDI. Despite this, providers should continue to consider discontinuation of unnecessary PPI therapy.
Acknowledgements
The authors wish to thank Mehran Salles, PhD, PharmD, for her assistance. Study findings were presented at the 2014 Southeastern Residency Conference in Athens, Georgia, on May 1, 2014.
Author disclosures
The authors report no actual or potential conflicts of interest with regard to this article.
Disclaimer
The opinions expressed herein are those of the authors and do not necessarily reflect those of Federal Practitioner Frontline Medical Communications Inc., the U.S. Government, or any of its agencies. This article may discuss unlabeled or investigational use of certain drugs. Please review complete prescribing information for specific drugs or drug combinations—including indications, contraindications, warnings, and adverse effects—before administering pharmacologic therapy to patients.
1. Poutanen SM, Simor AE. Clostridium difficile-associated diarrhea in adults. CMAJ. 2004;171(1):51-58.
2. Clostridium difficile infection. Centers for Disease Control and Prevention Website. http://www.cdc.gov/HAI/organisms/cdiff/Cdiff_infect.html. Updated February 25, 2015. Accessed October 5, 2015.
3. Song X, Bartlett JG, Speck K, Naegeli A, Carroll K, Perl TM. Rising economic impact of Clostridium difficile-associated disease in adult hospitalized patient population. Infect Control Hosp Epidemiol. 2008;29(9):823-828.
4. Bartlett JG. Narrative review: the new epidemic of Clostridium difficile-associated enteric disease. Ann Intern Med. 2006;145(10):758-764.
5. Baxter R, Ray GT, Fireman BH. Case-control study of antibiotic use and subsequent Clostridium difficile-associated diarrhea in hospitalized patients. Infect Control Hosp Epidemiol. 2008;29(1):44-50.
6. Anand A, Glatt AE. Clostridium difficile infection associated with antineoplastic chemotherapy: a review. Clin Infect Dis. 1993;17(1):109-113.
7. Bignardi GE. Risk factors for Clostridium difficile infection. J Hosp Infect. 1998;40(1):1-15.
8. Janarthanan S, Ditah I, Adler DG, Ehrinpreis MN. Clostridium difficile-associated diarrhea and proton pump inhibitor therapy: a meta-analysis. Am J Gastroenterol. 2012;107(7):1001-1010.
9. Howell MD, Novack V, Grgurich P, et al. Iatrogenic gastric acid suppression and the risk of nosocomial Clostridium difficile infection. Arch Intern Med. 2010;170(9):784-790.
10. Aseeri M, Schroeder T, Kramer J, Zackula R. Gastric acid suppression by proton pump inhibitors as a risk factor for Clostridium difficile-associated diarrhea in hospitalized patients. Am J Gastroenterol. 2008;103(9):2308-2313.
11. Dalton BR, Lye-Maccannell T, Henderson EA, Maccannell DR, Louie TJ. Proton pump inhibitors increase significantly the risk of Clostridium difficile infection in a low-endemicity, non-outbreak hospital setting. Aliment Pharmacol Ther. 2009;29(6):626-634.
12. Dial S, Alrasadi K, Manoukian C, Huang A, Menzies D. Risk of Clostridium difficile diarrhea among hospital inpatients prescribed proton pump inhibitors: cohort and case-control studies. CMAJ. 2004;171(1):33-38.
13. Linsky A, Gupta K, Lawler EV, Fonda JR, Hermos JA. Proton pump inhibitors and risk for recurrent Clostridium difficile infection. Arch Intern Med. 2010;170(9):772-778.
14. Yearsley KA, Gilby LJ, Ramadas AV, Kubiak EM, Fone DL, Allison MC. Proton pump inhibitor therapy is a risk factor for Clostridium difficile-associated diarrhoea. Aliment Pharmacol Ther. 2006;24(4):613-619.
15. Nardino RJ, Vender RJ, Herbert PN. Overuse of acid-suppressive therapy in hospitalized patients. Am J Gastroenterol. 2000;95(11):3118-3122.
16. U.S. Food and Drug Administration. FDA drug safety communication: Clostridium difficile-associated diarrhea can be associated with stomach acid drugs known as proton pump inhibitors (PPIs). http://www.fda.gov/Drugs/DrugSafety/ucm290510.htm. Updated February 15, 2013. Accessed October 5, 2015.
17. Kwok CS, Arthur AK, Anibueze CI, Singh S, Cavallazzi R, Loke YK. Risk of Clostridium difficile infection with acid suppressing drugs and antibiotics: meta-analysis. Am J Gastroenterol. 2012;107(7):1011-1019.
18. McDonald EG, Milligan J, Frenette C, Lee TC. Continuous proton pump inhibitor therapy and the associated risk of recurrent Clostridium difficile infection. JAMA Intern Med. 2015;175(5):784-791.
19. Lewis SJ, Franco S, Young G, O'Keefe SJ. Altered bowel function and duodenal bacterial overgrowth in patients treated with omeprazole. Aliment Pharmacol Ther. 1996;10(4):557-561.
20. Theisen J, Nehra D, Citron D, et al. Suppression of gastric acid secretion in patients with gastroesophageal reflux disease results in gastric bacterial overgrowth and deconjugation of bile acids. J Gastrointest Surg. 2000;4(1):50-54.
21. Williams C, McColl KE. Review article: proton pump inhibitors and bacterial overgrowth. Aliment Pharmacol Ther. 2006;23(1):3-10.
22. Lowe DO, Mamdani MM, Kopp A, Low DE, Juurlink DN. Proton pump inhibitors and hospitalization for Clostridium difficile-associated disease: a population-based study. Clin Infect Dis. 2006;43(10):1272-1276.
23. Shah S, Lewis A, Leopold D, Dunstan F, Woodhouse K. Gastric acid suppression does not promote clostridial diarrhoea in the elderly. QJM. 2000;93(3):175-181.
24. Kleinbaum DG, Klein M. Logistic Regression: A Self-Learning Text. 3rd ed. New York, NY: Springer; 2010.
25. Akaike H. A new look at the statistical model identification. IEEE Transact Autom Contr. 1974;19(6):716-723.
26. Barletta JF, El-Ibiary SY, Davis LE, Nguyen B, Raney CR. Proton pump inhibitors and the risk for hospital-acquired Clostridium difficile infection. Mayo Clin Proc. 2013;88(10):1085-1090.
1. Poutanen SM, Simor AE. Clostridium difficile-associated diarrhea in adults. CMAJ. 2004;171(1):51-58.
2. Clostridium difficile infection. Centers for Disease Control and Prevention Website. http://www.cdc.gov/HAI/organisms/cdiff/Cdiff_infect.html. Updated February 25, 2015. Accessed October 5, 2015.
3. Song X, Bartlett JG, Speck K, Naegeli A, Carroll K, Perl TM. Rising economic impact of Clostridium difficile-associated disease in adult hospitalized patient population. Infect Control Hosp Epidemiol. 2008;29(9):823-828.
4. Bartlett JG. Narrative review: the new epidemic of Clostridium difficile-associated enteric disease. Ann Intern Med. 2006;145(10):758-764.
5. Baxter R, Ray GT, Fireman BH. Case-control study of antibiotic use and subsequent Clostridium difficile-associated diarrhea in hospitalized patients. Infect Control Hosp Epidemiol. 2008;29(1):44-50.
6. Anand A, Glatt AE. Clostridium difficile infection associated with antineoplastic chemotherapy: a review. Clin Infect Dis. 1993;17(1):109-113.
7. Bignardi GE. Risk factors for Clostridium difficile infection. J Hosp Infect. 1998;40(1):1-15.
8. Janarthanan S, Ditah I, Adler DG, Ehrinpreis MN. Clostridium difficile-associated diarrhea and proton pump inhibitor therapy: a meta-analysis. Am J Gastroenterol. 2012;107(7):1001-1010.
9. Howell MD, Novack V, Grgurich P, et al. Iatrogenic gastric acid suppression and the risk of nosocomial Clostridium difficile infection. Arch Intern Med. 2010;170(9):784-790.
10. Aseeri M, Schroeder T, Kramer J, Zackula R. Gastric acid suppression by proton pump inhibitors as a risk factor for Clostridium difficile-associated diarrhea in hospitalized patients. Am J Gastroenterol. 2008;103(9):2308-2313.
11. Dalton BR, Lye-Maccannell T, Henderson EA, Maccannell DR, Louie TJ. Proton pump inhibitors increase significantly the risk of Clostridium difficile infection in a low-endemicity, non-outbreak hospital setting. Aliment Pharmacol Ther. 2009;29(6):626-634.
12. Dial S, Alrasadi K, Manoukian C, Huang A, Menzies D. Risk of Clostridium difficile diarrhea among hospital inpatients prescribed proton pump inhibitors: cohort and case-control studies. CMAJ. 2004;171(1):33-38.
13. Linsky A, Gupta K, Lawler EV, Fonda JR, Hermos JA. Proton pump inhibitors and risk for recurrent Clostridium difficile infection. Arch Intern Med. 2010;170(9):772-778.
14. Yearsley KA, Gilby LJ, Ramadas AV, Kubiak EM, Fone DL, Allison MC. Proton pump inhibitor therapy is a risk factor for Clostridium difficile-associated diarrhoea. Aliment Pharmacol Ther. 2006;24(4):613-619.
15. Nardino RJ, Vender RJ, Herbert PN. Overuse of acid-suppressive therapy in hospitalized patients. Am J Gastroenterol. 2000;95(11):3118-3122.
16. U.S. Food and Drug Administration. FDA drug safety communication: Clostridium difficile-associated diarrhea can be associated with stomach acid drugs known as proton pump inhibitors (PPIs). http://www.fda.gov/Drugs/DrugSafety/ucm290510.htm. Updated February 15, 2013. Accessed October 5, 2015.
17. Kwok CS, Arthur AK, Anibueze CI, Singh S, Cavallazzi R, Loke YK. Risk of Clostridium difficile infection with acid suppressing drugs and antibiotics: meta-analysis. Am J Gastroenterol. 2012;107(7):1011-1019.
18. McDonald EG, Milligan J, Frenette C, Lee TC. Continuous proton pump inhibitor therapy and the associated risk of recurrent Clostridium difficile infection. JAMA Intern Med. 2015;175(5):784-791.
19. Lewis SJ, Franco S, Young G, O'Keefe SJ. Altered bowel function and duodenal bacterial overgrowth in patients treated with omeprazole. Aliment Pharmacol Ther. 1996;10(4):557-561.
20. Theisen J, Nehra D, Citron D, et al. Suppression of gastric acid secretion in patients with gastroesophageal reflux disease results in gastric bacterial overgrowth and deconjugation of bile acids. J Gastrointest Surg. 2000;4(1):50-54.
21. Williams C, McColl KE. Review article: proton pump inhibitors and bacterial overgrowth. Aliment Pharmacol Ther. 2006;23(1):3-10.
22. Lowe DO, Mamdani MM, Kopp A, Low DE, Juurlink DN. Proton pump inhibitors and hospitalization for Clostridium difficile-associated disease: a population-based study. Clin Infect Dis. 2006;43(10):1272-1276.
23. Shah S, Lewis A, Leopold D, Dunstan F, Woodhouse K. Gastric acid suppression does not promote clostridial diarrhoea in the elderly. QJM. 2000;93(3):175-181.
24. Kleinbaum DG, Klein M. Logistic Regression: A Self-Learning Text. 3rd ed. New York, NY: Springer; 2010.
25. Akaike H. A new look at the statistical model identification. IEEE Transact Autom Contr. 1974;19(6):716-723.
26. Barletta JF, El-Ibiary SY, Davis LE, Nguyen B, Raney CR. Proton pump inhibitors and the risk for hospital-acquired Clostridium difficile infection. Mayo Clin Proc. 2013;88(10):1085-1090.