User login
To the Editor:
Nevus sebaceous of Jadassohn (or nevus sebaceous [NS]) is a congenital hamartomatous disorder initially described by Jadassohn1 in 1895. Nevus sebaceous occurs in 0.3% of newborns2 and is most commonly identified on the face and scalp.3,4 Mehregan and Pinkus5 characterized NS as an organoid tumor containing multiple skin components with 3 life stages. The first stage—occurring during infancy—consists of immature hair follicles and sebaceous glands. The second stage—beginning at puberty—shows development of sebaceous glands, epidermal hyperplasia, and maturation of apocrine glands. The final stage involves formation of secondary benign and malignant neoplasms.
Historically, basal cell carcinoma (BCC) was thought to be the most common neoplasm arising in NS.5-8 In 1993, Ackerman et al9 introduced a new definition of trichoblastoma (TB), expanding the definition to encompass previously excluded benign follicular neoplasms. Large studies conducted after this new definition was proposed suggested that syringocystadenoma papilliferum and TB develop more frequently than does BCC.3,4,10-15 Furthermore, Cribier et al4 and Merrot et al15 reviewed prior cases of NS using the new definition and asserted that the majority of previously diagnosed cases of BCC were considered to be TB under the new criteria. With the advent of modern diagnostic testing, the rate of secondary benign neoplasm growth is now thought to be between 7% and 19%, with syringocystadenoma papilliferum arising in 2% to 13% of cases and TB in 1.5% to 7%.3,4,10-14 Malignant neoplasms are observed much less frequently, with BCC arising in 0% to 1% of NS cases.
Nevus sebaceous lesions typically enlarge during puberty, while malignant neoplasms occur almost exclusively in adulthood,4,10-12 suggesting that hormones contribute to NS stage progression. We present the case of a woman who developed BCC in a previously asymptomatic NS during pregnancy.
A 32-year-old woman who was otherwise healthy presented to our dermatology clinic with a pink-yellow verrucous plaque on the right temporal hairline extending to the preauricular area of the face. The patient had no personal or family history of skin cancer and no history of tanning bed use. She reported that the lesion had been present since birth. A diagnosis of NS was made.
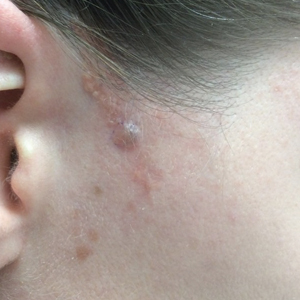
Two years later, she presented with a new bleeding growth atop the previously diagnosed NS that had been present for approximately 4 months (Figure). At this visit she was pregnant (30 weeks’ gestation). Physical examination revealed a 4-mm, brown, pearly papule at the inferior margin of the previously noted pink verrucous plaque on the right temporal hairline. A biopsy was performed and histopathology displayed aggregates of basaloid cells with a high nuclear to cytoplasmic ratio, peripheral palisading, and abundant melanin, consistent with pigmented BCC. The patient was referred for Mohs micrographic surgery; the lesion was removed with clear margins. The patient had no recurrence of BCC at 36-month follow-up.
Few studies have looked at the signal transduction pathways leading to malignant neoplasm formation in NS. Nevus sebaceous lesions are theorized to result from postzygotic genetic mutations in HRAS and KRAS oncogenes,16,17 which also are altered in squamous cell carcinoma and BCC.18 Similarly, Xin et al19 detected loss of heterozygosity of the human patched gene, PTCH, a tumor suppressor in the hedgehog pathway that has been implicated in sporadic BCC formation, suggesting that this loss of heterozygosity may predispose to secondary BCC formation.20,21 However, loss of PTCH heterozygosity could not be replicated by Takata et al22 and Levinsohn et al.16
Increased numbers of androgen receptors have been demonstrated in NS basal keratinocytes and sebaceous glands.23 Nevus sebaceous lesions enlarge during puberty,5 and malignant neoplasms arise almost exclusively in adulthood.3,4,10-13 The androgen surge during puberty and increased androgen levels in adulthood may promote sebaceous gland development and epidermal hyperplasia that result in progression of NS lesions from the first stage to the second stage. Basal cell carcinomas also express androgen receptors and have abnormal androgen hormone metabolism,24,25 though they do not display a notable number of estrogen or progesterone receptors.26 Therefore, increased androgen levels in adulthood also may contribute to progression to secondary neoplasm formation in the third stage.
Similarly, cases of rapid growth of NS lesions during pregnancy, a state of increased testosterone production,27 have
- Jadassohn J. Bemerkugen zur Histologie der systematisirten Naevi und uber “Talgdru˝sen-Naevi”. Arch Dermatol Syph. 1895;33:355-372.
- Alper J, Holmes LB, Mihm MC Jr. Birthmarks with serious medical significance: nevocullular nevi, sebaceous nevi, and multiple café au lait spots. J Pediatr. 1979;95:696-700.
- Muñoz-Pérez MA, García-Hernandez MJ, Ríos JJ, et al. Sebaceus naevi: a clinicopathologic study. J Eur Acad Dermatol Venereol. 2002;16:319-324.
- Cribier B, Scrivener Y, Grosshans E. Tumors arising in nevus sebaceus: a study of 596 cases. J Am Acad Dermatol. 2000;42:263-268.
- Mehregan AH, Pinkus H. Life history of organoid nevi. Special reference to nevus sebaceus of Jadassohn. Arch Dermatol. 1965;91:574-588.
- Jones EW, Heyl T. Naevus sebaceus. a report of 140 cases with special regard to the development of secondary malignant tumours. Br J Dermatol. 1970;82:99-117.
- Serpas de López RM, Hernández-Pérez E. Jadassohn’s sebaceous nevus. J Dermatol Surg Oncol. 1985;11:68-72.
- Smolin T, Hundeiker M. Squamous epithelial and basal cell carcinomas in naevus sebaceus (Jadassohn). Z Hautkr. 1986;61:267-282.
- Ackerman B, Reddy VB, Soyer HP. Neoplasms with Follicular Differentiation. New York, NY: Ardor Scribendi; 1993.
- Kaddu S, Schäppi H, Kerl H, et al. Trichoblastoma and sebaceoma in nevus sebaceus. Am J Dermatopathol. 1999;21:552-556.
- Idriss MH, Elston DM. Secondary neoplasms associated with nevus sebaceus of Jadassohn: a study of 707 cases. J Am Acad Dermatol. 2014;70:332-337.
- Hsu MC, Liau JY, Hong JL, et al. Secondary neoplasms arising from nevus sebaceus: a retrospective study of 450 cases in Taiwan. J Dermatol. 2016;43:175-180.
- Santibanez-Gallerani A, Marshall D, Duarte AM, et al. Should nevus sebaceus of Jadassohn in children be excised? a study of 757 cases, and literature review. J Craniofac Surg. 2003;14:658-660.
- Jaqueti G, Requena L, Sánchez Yus E. Trichoblastoma is the most common neoplasm developed in nevus sebaceus of Jadassohn: a clinicopathologic study of a series of 155 cases. Am J Dermatopathol. 2000;22:108-118.
- Merrot O, Cotten H, Patenotre P, et al. Sebaceous hamartoma of Jadassohn: trichoblastoma mimicking basal cell carcinoma? Ann Chir Plast Esthet. 2002;47:210-213.
- Levinsohn JL, Tian LC, Boyden LM, et al. Whole-exome sequencing reveals somatic mutations in HRAS and KRAS, which cause nevus sebaceus. J Invest Dermatol. 2013;133:827-830.
- Groesser L, Herschberger E, Ruetten A, et al. Postzygotic HRAS and KRAS mutations cause nevus sebaceous and Schimmelpenning syndrome. Nat Genet. 2012;44:783-787.
- Pierceall WE, Goldberg LH, Tainsky MA, et al. Ras gene mutation and amplification in human nonmelanoma skin cancers. Mol Carcinog. 1991;4:196-202.
- Xin H, Matt D, Qin JZ, et al. The sebaceous nevus: a nevus with deletions of the PTCH gene. Cancer Res. 1999;59:1834-1836.
- Johnson RL, Rothman AL, Xie J, et al. Human homolog of patched, a candidate gene for the basal cell nevus syndrome. Science. 1996;272:1668-1671.
- Gailani MR, Ståhle-Bäckdahl M, Leffell DJ, et al. The role of the human homologue of Drosophila patched in sporadic basal cell carcinomas. Nat Genet. 1996;14:78-81.
- Takata M, Tojo M, Hatta N, et al. No evidence of deregulated patched-hedgehog signaling pathway in trichoblastomas and other tumors arising within nevus sebaceous. J Invest Dermatol. 2001;117:1666-1670.
- Hamilton KS, Johnson S, Smoller BR. The role of androgen receptors in the clinical course of nevus sebaceus of Jadassohn. Mod Pathol. 2001;14:539-542.
- Moretti G, Cardo P, Rampini E, et al. Testosterone metabolism in basal cell epitheliomas. J Invest Dermatol. 1978;71:361-362.
- Bayer-Garner IB, Givens V, Smoller B. Immunohistochemical staining for androgen receptors: a sensitive marker of sebaceous differentiation. Am J Dermatopathol. 1999;21:426-431.
- Rogers GS, Flowers JL, Pollack SV, et al. Determination of sex steroid receptor in human basal cell carcinoma. J Am Acad Dermatol. 1988;18:1039-1043.
- Bammann BL, Coulam CB, Jiang NS. Total and free testosterone during pregnancy. Am J Obstet Gynecol. 1980;137:293-298.
- Terenzi V, Indrizzi E, Buonaccorsi S, et al. Nevus sebaceus of Jadassohn. J Craniofac Surg. 2006;17:1234-1239.
- Moody MN, Landau JM, Goldberg LH. Nevus sebaceous revisited. Pediatr Dermatol. 2012;29:15-23.
- Lillis PJ, Ceilley RI. Multiple tumors arising in nevus sebaceus. Cutis. 1979;23:310-314.
- Chun K, Vázquez M, Sánchez JL. Nevus sebaceus: clinical outcomeand considerations for prophylactic excision. Int J Dermatol. 1995;34:538-541.
To the Editor:
Nevus sebaceous of Jadassohn (or nevus sebaceous [NS]) is a congenital hamartomatous disorder initially described by Jadassohn1 in 1895. Nevus sebaceous occurs in 0.3% of newborns2 and is most commonly identified on the face and scalp.3,4 Mehregan and Pinkus5 characterized NS as an organoid tumor containing multiple skin components with 3 life stages. The first stage—occurring during infancy—consists of immature hair follicles and sebaceous glands. The second stage—beginning at puberty—shows development of sebaceous glands, epidermal hyperplasia, and maturation of apocrine glands. The final stage involves formation of secondary benign and malignant neoplasms.
Historically, basal cell carcinoma (BCC) was thought to be the most common neoplasm arising in NS.5-8 In 1993, Ackerman et al9 introduced a new definition of trichoblastoma (TB), expanding the definition to encompass previously excluded benign follicular neoplasms. Large studies conducted after this new definition was proposed suggested that syringocystadenoma papilliferum and TB develop more frequently than does BCC.3,4,10-15 Furthermore, Cribier et al4 and Merrot et al15 reviewed prior cases of NS using the new definition and asserted that the majority of previously diagnosed cases of BCC were considered to be TB under the new criteria. With the advent of modern diagnostic testing, the rate of secondary benign neoplasm growth is now thought to be between 7% and 19%, with syringocystadenoma papilliferum arising in 2% to 13% of cases and TB in 1.5% to 7%.3,4,10-14 Malignant neoplasms are observed much less frequently, with BCC arising in 0% to 1% of NS cases.
Nevus sebaceous lesions typically enlarge during puberty, while malignant neoplasms occur almost exclusively in adulthood,4,10-12 suggesting that hormones contribute to NS stage progression. We present the case of a woman who developed BCC in a previously asymptomatic NS during pregnancy.
A 32-year-old woman who was otherwise healthy presented to our dermatology clinic with a pink-yellow verrucous plaque on the right temporal hairline extending to the preauricular area of the face. The patient had no personal or family history of skin cancer and no history of tanning bed use. She reported that the lesion had been present since birth. A diagnosis of NS was made.
Two years later, she presented with a new bleeding growth atop the previously diagnosed NS that had been present for approximately 4 months (Figure). At this visit she was pregnant (30 weeks’ gestation). Physical examination revealed a 4-mm, brown, pearly papule at the inferior margin of the previously noted pink verrucous plaque on the right temporal hairline. A biopsy was performed and histopathology displayed aggregates of basaloid cells with a high nuclear to cytoplasmic ratio, peripheral palisading, and abundant melanin, consistent with pigmented BCC. The patient was referred for Mohs micrographic surgery; the lesion was removed with clear margins. The patient had no recurrence of BCC at 36-month follow-up.
Few studies have looked at the signal transduction pathways leading to malignant neoplasm formation in NS. Nevus sebaceous lesions are theorized to result from postzygotic genetic mutations in HRAS and KRAS oncogenes,16,17 which also are altered in squamous cell carcinoma and BCC.18 Similarly, Xin et al19 detected loss of heterozygosity of the human patched gene, PTCH, a tumor suppressor in the hedgehog pathway that has been implicated in sporadic BCC formation, suggesting that this loss of heterozygosity may predispose to secondary BCC formation.20,21 However, loss of PTCH heterozygosity could not be replicated by Takata et al22 and Levinsohn et al.16
Increased numbers of androgen receptors have been demonstrated in NS basal keratinocytes and sebaceous glands.23 Nevus sebaceous lesions enlarge during puberty,5 and malignant neoplasms arise almost exclusively in adulthood.3,4,10-13 The androgen surge during puberty and increased androgen levels in adulthood may promote sebaceous gland development and epidermal hyperplasia that result in progression of NS lesions from the first stage to the second stage. Basal cell carcinomas also express androgen receptors and have abnormal androgen hormone metabolism,24,25 though they do not display a notable number of estrogen or progesterone receptors.26 Therefore, increased androgen levels in adulthood also may contribute to progression to secondary neoplasm formation in the third stage.
Similarly, cases of rapid growth of NS lesions during pregnancy, a state of increased testosterone production,27 have
To the Editor:
Nevus sebaceous of Jadassohn (or nevus sebaceous [NS]) is a congenital hamartomatous disorder initially described by Jadassohn1 in 1895. Nevus sebaceous occurs in 0.3% of newborns2 and is most commonly identified on the face and scalp.3,4 Mehregan and Pinkus5 characterized NS as an organoid tumor containing multiple skin components with 3 life stages. The first stage—occurring during infancy—consists of immature hair follicles and sebaceous glands. The second stage—beginning at puberty—shows development of sebaceous glands, epidermal hyperplasia, and maturation of apocrine glands. The final stage involves formation of secondary benign and malignant neoplasms.
Historically, basal cell carcinoma (BCC) was thought to be the most common neoplasm arising in NS.5-8 In 1993, Ackerman et al9 introduced a new definition of trichoblastoma (TB), expanding the definition to encompass previously excluded benign follicular neoplasms. Large studies conducted after this new definition was proposed suggested that syringocystadenoma papilliferum and TB develop more frequently than does BCC.3,4,10-15 Furthermore, Cribier et al4 and Merrot et al15 reviewed prior cases of NS using the new definition and asserted that the majority of previously diagnosed cases of BCC were considered to be TB under the new criteria. With the advent of modern diagnostic testing, the rate of secondary benign neoplasm growth is now thought to be between 7% and 19%, with syringocystadenoma papilliferum arising in 2% to 13% of cases and TB in 1.5% to 7%.3,4,10-14 Malignant neoplasms are observed much less frequently, with BCC arising in 0% to 1% of NS cases.
Nevus sebaceous lesions typically enlarge during puberty, while malignant neoplasms occur almost exclusively in adulthood,4,10-12 suggesting that hormones contribute to NS stage progression. We present the case of a woman who developed BCC in a previously asymptomatic NS during pregnancy.
A 32-year-old woman who was otherwise healthy presented to our dermatology clinic with a pink-yellow verrucous plaque on the right temporal hairline extending to the preauricular area of the face. The patient had no personal or family history of skin cancer and no history of tanning bed use. She reported that the lesion had been present since birth. A diagnosis of NS was made.
Two years later, she presented with a new bleeding growth atop the previously diagnosed NS that had been present for approximately 4 months (Figure). At this visit she was pregnant (30 weeks’ gestation). Physical examination revealed a 4-mm, brown, pearly papule at the inferior margin of the previously noted pink verrucous plaque on the right temporal hairline. A biopsy was performed and histopathology displayed aggregates of basaloid cells with a high nuclear to cytoplasmic ratio, peripheral palisading, and abundant melanin, consistent with pigmented BCC. The patient was referred for Mohs micrographic surgery; the lesion was removed with clear margins. The patient had no recurrence of BCC at 36-month follow-up.
Few studies have looked at the signal transduction pathways leading to malignant neoplasm formation in NS. Nevus sebaceous lesions are theorized to result from postzygotic genetic mutations in HRAS and KRAS oncogenes,16,17 which also are altered in squamous cell carcinoma and BCC.18 Similarly, Xin et al19 detected loss of heterozygosity of the human patched gene, PTCH, a tumor suppressor in the hedgehog pathway that has been implicated in sporadic BCC formation, suggesting that this loss of heterozygosity may predispose to secondary BCC formation.20,21 However, loss of PTCH heterozygosity could not be replicated by Takata et al22 and Levinsohn et al.16
Increased numbers of androgen receptors have been demonstrated in NS basal keratinocytes and sebaceous glands.23 Nevus sebaceous lesions enlarge during puberty,5 and malignant neoplasms arise almost exclusively in adulthood.3,4,10-13 The androgen surge during puberty and increased androgen levels in adulthood may promote sebaceous gland development and epidermal hyperplasia that result in progression of NS lesions from the first stage to the second stage. Basal cell carcinomas also express androgen receptors and have abnormal androgen hormone metabolism,24,25 though they do not display a notable number of estrogen or progesterone receptors.26 Therefore, increased androgen levels in adulthood also may contribute to progression to secondary neoplasm formation in the third stage.
Similarly, cases of rapid growth of NS lesions during pregnancy, a state of increased testosterone production,27 have
- Jadassohn J. Bemerkugen zur Histologie der systematisirten Naevi und uber “Talgdru˝sen-Naevi”. Arch Dermatol Syph. 1895;33:355-372.
- Alper J, Holmes LB, Mihm MC Jr. Birthmarks with serious medical significance: nevocullular nevi, sebaceous nevi, and multiple café au lait spots. J Pediatr. 1979;95:696-700.
- Muñoz-Pérez MA, García-Hernandez MJ, Ríos JJ, et al. Sebaceus naevi: a clinicopathologic study. J Eur Acad Dermatol Venereol. 2002;16:319-324.
- Cribier B, Scrivener Y, Grosshans E. Tumors arising in nevus sebaceus: a study of 596 cases. J Am Acad Dermatol. 2000;42:263-268.
- Mehregan AH, Pinkus H. Life history of organoid nevi. Special reference to nevus sebaceus of Jadassohn. Arch Dermatol. 1965;91:574-588.
- Jones EW, Heyl T. Naevus sebaceus. a report of 140 cases with special regard to the development of secondary malignant tumours. Br J Dermatol. 1970;82:99-117.
- Serpas de López RM, Hernández-Pérez E. Jadassohn’s sebaceous nevus. J Dermatol Surg Oncol. 1985;11:68-72.
- Smolin T, Hundeiker M. Squamous epithelial and basal cell carcinomas in naevus sebaceus (Jadassohn). Z Hautkr. 1986;61:267-282.
- Ackerman B, Reddy VB, Soyer HP. Neoplasms with Follicular Differentiation. New York, NY: Ardor Scribendi; 1993.
- Kaddu S, Schäppi H, Kerl H, et al. Trichoblastoma and sebaceoma in nevus sebaceus. Am J Dermatopathol. 1999;21:552-556.
- Idriss MH, Elston DM. Secondary neoplasms associated with nevus sebaceus of Jadassohn: a study of 707 cases. J Am Acad Dermatol. 2014;70:332-337.
- Hsu MC, Liau JY, Hong JL, et al. Secondary neoplasms arising from nevus sebaceus: a retrospective study of 450 cases in Taiwan. J Dermatol. 2016;43:175-180.
- Santibanez-Gallerani A, Marshall D, Duarte AM, et al. Should nevus sebaceus of Jadassohn in children be excised? a study of 757 cases, and literature review. J Craniofac Surg. 2003;14:658-660.
- Jaqueti G, Requena L, Sánchez Yus E. Trichoblastoma is the most common neoplasm developed in nevus sebaceus of Jadassohn: a clinicopathologic study of a series of 155 cases. Am J Dermatopathol. 2000;22:108-118.
- Merrot O, Cotten H, Patenotre P, et al. Sebaceous hamartoma of Jadassohn: trichoblastoma mimicking basal cell carcinoma? Ann Chir Plast Esthet. 2002;47:210-213.
- Levinsohn JL, Tian LC, Boyden LM, et al. Whole-exome sequencing reveals somatic mutations in HRAS and KRAS, which cause nevus sebaceus. J Invest Dermatol. 2013;133:827-830.
- Groesser L, Herschberger E, Ruetten A, et al. Postzygotic HRAS and KRAS mutations cause nevus sebaceous and Schimmelpenning syndrome. Nat Genet. 2012;44:783-787.
- Pierceall WE, Goldberg LH, Tainsky MA, et al. Ras gene mutation and amplification in human nonmelanoma skin cancers. Mol Carcinog. 1991;4:196-202.
- Xin H, Matt D, Qin JZ, et al. The sebaceous nevus: a nevus with deletions of the PTCH gene. Cancer Res. 1999;59:1834-1836.
- Johnson RL, Rothman AL, Xie J, et al. Human homolog of patched, a candidate gene for the basal cell nevus syndrome. Science. 1996;272:1668-1671.
- Gailani MR, Ståhle-Bäckdahl M, Leffell DJ, et al. The role of the human homologue of Drosophila patched in sporadic basal cell carcinomas. Nat Genet. 1996;14:78-81.
- Takata M, Tojo M, Hatta N, et al. No evidence of deregulated patched-hedgehog signaling pathway in trichoblastomas and other tumors arising within nevus sebaceous. J Invest Dermatol. 2001;117:1666-1670.
- Hamilton KS, Johnson S, Smoller BR. The role of androgen receptors in the clinical course of nevus sebaceus of Jadassohn. Mod Pathol. 2001;14:539-542.
- Moretti G, Cardo P, Rampini E, et al. Testosterone metabolism in basal cell epitheliomas. J Invest Dermatol. 1978;71:361-362.
- Bayer-Garner IB, Givens V, Smoller B. Immunohistochemical staining for androgen receptors: a sensitive marker of sebaceous differentiation. Am J Dermatopathol. 1999;21:426-431.
- Rogers GS, Flowers JL, Pollack SV, et al. Determination of sex steroid receptor in human basal cell carcinoma. J Am Acad Dermatol. 1988;18:1039-1043.
- Bammann BL, Coulam CB, Jiang NS. Total and free testosterone during pregnancy. Am J Obstet Gynecol. 1980;137:293-298.
- Terenzi V, Indrizzi E, Buonaccorsi S, et al. Nevus sebaceus of Jadassohn. J Craniofac Surg. 2006;17:1234-1239.
- Moody MN, Landau JM, Goldberg LH. Nevus sebaceous revisited. Pediatr Dermatol. 2012;29:15-23.
- Lillis PJ, Ceilley RI. Multiple tumors arising in nevus sebaceus. Cutis. 1979;23:310-314.
- Chun K, Vázquez M, Sánchez JL. Nevus sebaceus: clinical outcomeand considerations for prophylactic excision. Int J Dermatol. 1995;34:538-541.
- Jadassohn J. Bemerkugen zur Histologie der systematisirten Naevi und uber “Talgdru˝sen-Naevi”. Arch Dermatol Syph. 1895;33:355-372.
- Alper J, Holmes LB, Mihm MC Jr. Birthmarks with serious medical significance: nevocullular nevi, sebaceous nevi, and multiple café au lait spots. J Pediatr. 1979;95:696-700.
- Muñoz-Pérez MA, García-Hernandez MJ, Ríos JJ, et al. Sebaceus naevi: a clinicopathologic study. J Eur Acad Dermatol Venereol. 2002;16:319-324.
- Cribier B, Scrivener Y, Grosshans E. Tumors arising in nevus sebaceus: a study of 596 cases. J Am Acad Dermatol. 2000;42:263-268.
- Mehregan AH, Pinkus H. Life history of organoid nevi. Special reference to nevus sebaceus of Jadassohn. Arch Dermatol. 1965;91:574-588.
- Jones EW, Heyl T. Naevus sebaceus. a report of 140 cases with special regard to the development of secondary malignant tumours. Br J Dermatol. 1970;82:99-117.
- Serpas de López RM, Hernández-Pérez E. Jadassohn’s sebaceous nevus. J Dermatol Surg Oncol. 1985;11:68-72.
- Smolin T, Hundeiker M. Squamous epithelial and basal cell carcinomas in naevus sebaceus (Jadassohn). Z Hautkr. 1986;61:267-282.
- Ackerman B, Reddy VB, Soyer HP. Neoplasms with Follicular Differentiation. New York, NY: Ardor Scribendi; 1993.
- Kaddu S, Schäppi H, Kerl H, et al. Trichoblastoma and sebaceoma in nevus sebaceus. Am J Dermatopathol. 1999;21:552-556.
- Idriss MH, Elston DM. Secondary neoplasms associated with nevus sebaceus of Jadassohn: a study of 707 cases. J Am Acad Dermatol. 2014;70:332-337.
- Hsu MC, Liau JY, Hong JL, et al. Secondary neoplasms arising from nevus sebaceus: a retrospective study of 450 cases in Taiwan. J Dermatol. 2016;43:175-180.
- Santibanez-Gallerani A, Marshall D, Duarte AM, et al. Should nevus sebaceus of Jadassohn in children be excised? a study of 757 cases, and literature review. J Craniofac Surg. 2003;14:658-660.
- Jaqueti G, Requena L, Sánchez Yus E. Trichoblastoma is the most common neoplasm developed in nevus sebaceus of Jadassohn: a clinicopathologic study of a series of 155 cases. Am J Dermatopathol. 2000;22:108-118.
- Merrot O, Cotten H, Patenotre P, et al. Sebaceous hamartoma of Jadassohn: trichoblastoma mimicking basal cell carcinoma? Ann Chir Plast Esthet. 2002;47:210-213.
- Levinsohn JL, Tian LC, Boyden LM, et al. Whole-exome sequencing reveals somatic mutations in HRAS and KRAS, which cause nevus sebaceus. J Invest Dermatol. 2013;133:827-830.
- Groesser L, Herschberger E, Ruetten A, et al. Postzygotic HRAS and KRAS mutations cause nevus sebaceous and Schimmelpenning syndrome. Nat Genet. 2012;44:783-787.
- Pierceall WE, Goldberg LH, Tainsky MA, et al. Ras gene mutation and amplification in human nonmelanoma skin cancers. Mol Carcinog. 1991;4:196-202.
- Xin H, Matt D, Qin JZ, et al. The sebaceous nevus: a nevus with deletions of the PTCH gene. Cancer Res. 1999;59:1834-1836.
- Johnson RL, Rothman AL, Xie J, et al. Human homolog of patched, a candidate gene for the basal cell nevus syndrome. Science. 1996;272:1668-1671.
- Gailani MR, Ståhle-Bäckdahl M, Leffell DJ, et al. The role of the human homologue of Drosophila patched in sporadic basal cell carcinomas. Nat Genet. 1996;14:78-81.
- Takata M, Tojo M, Hatta N, et al. No evidence of deregulated patched-hedgehog signaling pathway in trichoblastomas and other tumors arising within nevus sebaceous. J Invest Dermatol. 2001;117:1666-1670.
- Hamilton KS, Johnson S, Smoller BR. The role of androgen receptors in the clinical course of nevus sebaceus of Jadassohn. Mod Pathol. 2001;14:539-542.
- Moretti G, Cardo P, Rampini E, et al. Testosterone metabolism in basal cell epitheliomas. J Invest Dermatol. 1978;71:361-362.
- Bayer-Garner IB, Givens V, Smoller B. Immunohistochemical staining for androgen receptors: a sensitive marker of sebaceous differentiation. Am J Dermatopathol. 1999;21:426-431.
- Rogers GS, Flowers JL, Pollack SV, et al. Determination of sex steroid receptor in human basal cell carcinoma. J Am Acad Dermatol. 1988;18:1039-1043.
- Bammann BL, Coulam CB, Jiang NS. Total and free testosterone during pregnancy. Am J Obstet Gynecol. 1980;137:293-298.
- Terenzi V, Indrizzi E, Buonaccorsi S, et al. Nevus sebaceus of Jadassohn. J Craniofac Surg. 2006;17:1234-1239.
- Moody MN, Landau JM, Goldberg LH. Nevus sebaceous revisited. Pediatr Dermatol. 2012;29:15-23.
- Lillis PJ, Ceilley RI. Multiple tumors arising in nevus sebaceus. Cutis. 1979;23:310-314.
- Chun K, Vázquez M, Sánchez JL. Nevus sebaceus: clinical outcomeand considerations for prophylactic excision. Int J Dermatol. 1995;34:538-541.
Practice Points
- Benign neoplasms arise more frequently in nevus sebaceous (NS) lesions than do malignant neoplasms.
- The hormonal changes that occur during pregnancy and puberty appear to play a role in the development of neoplasms in NS lesions.
- Monitoring NS lesions more closely during periods of hormonal change may help diagnose malignant transformations in these patients.