User login
How should you evaluate a patient who has a cytologic diagnosis of atypical glandular cells (AGC)?
Charles J. Dunton, MD (Examining the Evidence; August 2011)
What is optimal surveillance after treatment for high-grade cervical intraepithelial neoplasia (CIN)?
Alan G. Waxman, MD, MPH (Examining the Evidence; June 2011)
2 HPV vaccines, 7 questions that you need answered
Neal M. Lonky, MD, MPH, and an expert panel (August 2010)
Dr. Massad reports no financial relationships relevant to this article.
The societal shifts of the 1960s generated many changes—among them, permanently altered sexual mores. That may be a primary reason why the incidence of vulvar intraepithelial neoplasia (VIN) increased more than 400% between 1973 and 2000, says L. Stewart Massad, Jr, MD, chairman of the Practice Committee of the American Society for Colposcopy and Cervical Pathology (ASCCP) and member of the ACOG Committee on Gynecologic Practice—and one of the authors of a new joint Committee Opinion on the management of VIN.1 This precancer is often associated with carcinogenic types of human papillomavirus (HPV), the most common sexually transmitted disease in the nation.
The 400% statistic caught the attention of OBG Management. The editors invited Dr. Massad to discuss the subject of VIN at length, elaborating on key issues such as its prevention, identification, treatment, and surveillance.
How to identify a VIN lesion
OBG Management: What is VIN? What does it look like?
Dr. Massad: VIN is a premalignant condition of the vulva that may present as unifocal or multifocal lesions. These lesions may be flesh-colored, hypopigmented, or hyperpigmented (FIGURE). They also may be erythematous, flat, or raised. They can be found on any part of the vulva. The dysplastic cells may extend into hair shafts or sweat glands; they don’t penetrate the basement membrane, however, so, by definition, they aren’t invasive.
Usual-type VIN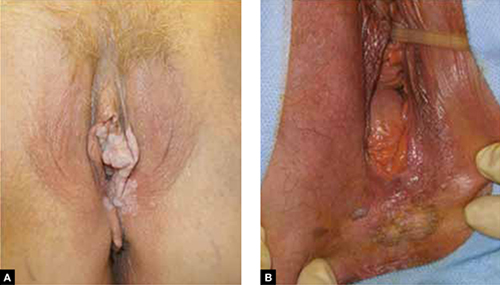
A. This warty lesion is hyperpigmented around the periphery, hypopigmented in the center. B. Another warty lesion. Both images reflect the application of acetic acid.OBG Management: Why has the incidence increased so considerably?
Dr. Massad: The data we have on incidence comes from the Surveillance, Epidemiology and End Results (SEER) program of the National Cancer Institute, as reported by Judson and colleagues.2 Although better reporting of findings of VIN may play a role, the rising incidence seems to be attributable to changes in sexual behavior over the past half century. The incidence of vulvar cancer rose during the same period—about 20%.3 The much slower growth in the incidence of vulvar cancer suggests that treatment of VIN has blunted the risk of cancer.
OBG Management: Is VIN associated with any particular type of HPV?
Dr. Massad: Yes, more than 80% of VIN lesions are associated with HPV 16.
OBG Management: One study from 2005 noted that the mean age of women with VIN decreased from 50 years before 1980 to 39 years in subsequent years.4 Why are more younger women developing VIN?
Dr. Massad: The study that showed that age shift was from New Zealand. The authors speculated that the change was due to earlier sexual activity among women who smoke: HPV, especially HPV 16, and smoking are important risk factors for VIN. The question hasn’t been definitively answered.
OBG Management: What are the risk factors for VIN?
Dr. Massad: Smoking is a big one. More than 50% of women who have VIN are smokers. Thirty percent have concurrent or prior cervical intraepithelial neoplasia (CIN) or vaginal intraepithelial neoplasia (VAIN). The risk of invasion rises with age at the time of the initial diagnosis and with longer follow-up. I can talk about surveillance a little later.
Are some lesions more worrisome than others?
OBG Management: Are VIN lesions categorized similarly to CIN lesions—that is, using three different grades of severity?
Dr. Massad: Until recently, that was the case, but it is no longer so. Broadly, there are now two classes of VIN, according to the International Society for the Study of Vulvovaginal Disease (ISSVD): usual-type VIN and differentiated VIN.
ACOG and ASCCP have embraced this classification system, although not all pathologists have done so, and clinicians may still see reports using the old three-tier system.
Usual-type VIN is associated with infection with high-risk types of HPV—most notably, HPV 16, as I mentioned. Histologically, usual-type VIN can mimic common genital warts, and the warty subtype shows keratosis at the surface, a spiky or undulating surface, and vertical maturation of cells in the lesion but with pleomorphic cells filling half or more of the epithelial thickness. The basaloid subtype of usual VIN shows little maturation.
Differentiated VIN exhibits more subtle atypia, with keratin pearls and an eosinophilic cytoplasm.
Biologically, usual-type VIN is associated with HPV and linked to smoking and sexual activity, as we discussed. As its name suggests, it is found more frequently than differentiated VIN. It is most common in women in their late 30s to early 50s.
In contrast, differentiated VIN is not associated with HPV and is more common in postmenopausal women; it is frequently seen with lichen sclerosus.
OBG Management: When did this new way of classifying VIN—as usual-type and differentiated—originate?
Dr. Massad: The ISVVD classification system changed in 2004. Before then, it paralleled the CIN classification system, with three grades of intraepithelial neoplasia corresponding to the thickness of the epithelium filled by dysplastic cells: VIN 1, 2, and 3. However, VIN 1 was not really neoplastic. It reflected infection with HPV, and although it might progress to higher-grade dysplasia or cancer, the risk was minimal. So the ISVVD revised the classification system to include only high-grade VIN—the old VIN 2 and VIN 3. HPV-associated lesions with dysplastic cells confined to the lower third of the epithelium are managed like genital warts, with observation for spontaneous regression or treatment with topical therapy or surgery.
How to screen for VIN
OBG Management: What screening strategy is recommended for VIN?
Dr. Massad: There is no such recommendation. Screening for VIN hasn’t been implemented for several reasons. Most important, other than inspection of the vulva by a clinician, there is no good screening test. VIN isn’t very common, so mass inspection for lesions is unlikely to be cost-effective. The sensitivity and specificity of inspection by a clinician aren’t known. Most lesions are found by women, their partners, or clinicians before cancer develops. And most disease is treated before cancer arises.
OBG Management: Isn’t there a need for heightened scrutiny of the vulva?
Dr. Massad: Yes. Women should examine their genitalia several times a year and seek attention if anything changes. That’s especially true for women who have risk factors, such as smoking, immunosuppression, and a history of being treated for cervical dysplasia. It’s the same concept we employ when teaching women to identify early breast lesions through self-examination.
The biggest challenges in detecting VIN are educating women to report vulvar skin changes to their clinicians for assessment and educating clinicians to examine the vulva before inserting the speculum for cervical screening and vaginal inspection.
OBG Management: Is another challenge distinguishing some forms of VIN from genital warts?
Dr. Massad: It can be a challenge, but clinicians should recall that warts are most common among women around the time of the onset of sexual activity. Older women sometimes develop warts with a new sexual partner. However, when women in their 40s and older develop new warty lesions, always suspect VIN. A woman in her 60s or older who has a new, warty-appearing vulvar lesion should be assumed to have VIN or cancer.
OBG Management: Does VIN ever regress spontaneously?
Dr. Massad: Yes. There have been reports of spontaneous regression of VIN, especially in young women. Regrettably, there are also reports of progression to cancer during observation. There are no characteristics that allow us to distinguish lesions that are going to progress from those that will regress. The ACOG-ASCCP Committee Opinion recommends treatment of all VIN.1
Can VIN be prevented?
OBG Management: The Committee Opinion recommends that the quadrivalent HPV vaccine be offered to women “in target populations” because it can decrease the risk of VIN. What are those target populations?
Dr. Massad: The target population for HPV vaccination is 11- and 12-year-old girls, but catch-up vaccination is acceptable in patients as old as 26 years.
OBG Management: Why isn’t the bivalent vaccine recommended?
Dr. Massad: Only the quadrivalent vaccine has been approved by the US Food and Drug Administration (FDA) for prevention of VIN, although, in theory, the bivalent vaccine ought to be effective as well.
When to biopsy
OBG Management: Do you recommend that any suspect lesion on the vulva be biopsied?
Dr. Massad: The decision to biopsy should be individualized. However, women who have apparent warts that fail to respond to topical therapy should undergo biopsy, as should older women with warty lesions. Keep in mind that older women may develop verrucous carcinomas and may benefit from excision of enlarging warty lesions even if a biopsy is reported as only condylomata. Clinicians should not biopsy varicosities or obvious flat nevi.
OBG Management: Is colposcopy ever helpful in assessing vulvar lesions?
Dr. Massad: Most vulvar lesions can be identified without colposcopy, but colposcopy is useful in determining the extent of lesions. It often reveals subclinical disease not evident at the time of vulvar inspection.
OBG Management: When colposcopy is used, is the procedure the same as for cervical examination?
Dr. Massad: Not exactly. The clinician should apply 5% acetic acid for 5 minutes using a gauze sponge, but the magnification should be 63 to 103—not 153, as it is for cervical examination. It’s important to distinguish hyperplasia from VIN. In general, hyperplastic lesions are faint, gray, diffuse, and flat, whereas VIN lesions are raised and irregular in shape, with sharp borders.
OBG Management: What about toluidine blue? Is it useful in inspection of lesions?
Dr. Massad: Toluidine blue stains skin that is irritated. It isn’t very specific for VIN or vulvar cancer, and it can make colposcopy difficult, so experts no longer recommend it.
OBG Management: What are the treatment options for VIN?
Dr. Massad: They include surgical excision, laser ablation, and topical therapy with 5% imiquimod. All are potentially effective. The Committee Opinion doesn’t specify a preference, except to say that excision is advised when there is any suspicion of cancer to preserve a sample for pathologic analysis. Ablation destroys the lesion, making assessment of possible invasion impossible, and imiquimod may allow disease to progress during observation.
OBG Management: The Committee Opinion recommends wide local excision when cancer is suspected. What size of margin is optimal?
Dr. Massad: In general, a margin of 5 to 10 mm around the lesion is recommended. Vulvectomy isn’t needed because close follow-up usually identifies recurrence before invasion occurs.
OBG Management: When is laser ablation a good choice?
Dr. Massad: Whenever a biopsy shows VIN and cancer is not suspected. Laser ablation is ideal when lesions are multifocal or extensive, although repeated treatments may be required to resolve small foci of residual disease. Done with careful attention to power density and depth of ablation, laser therapy can be less disfiguring than excision.
OBG Management: You mentioned 5% imiquimod. Is there evidence that it’s effective in the treatment of VIN?
Dr. Massad: Multiple randomized, controlled trials have shown 5% imiquimod to be effective against VIN, although the agent does not have approval from the FDA for that indication.5,6 Lower concentrations of imiquimod have not been studied in the treatment of VIN. Women treated with this topical therapy should be followed every 4 weeks with colposcopy because progression to cancer has been reported during imiquimod therapy. Lesions that fail to respond completely after a full course of imiquimod should be treated with excision or laser ablation.
Surveillance is critical
OBG Management: According to the Committee Opinion, the recurrence rate of VIN can reach 30% to 50%.7 Why so high?
Dr. Massad: Usual-type VIN reflects exposure to carcinogenic HPV, and differentiated VIN arises from a vulvar dystrophy. In both situations, treatments destroy VIN and arrest progress to cancer, but the entire vulvar skin remains subject to the inciting condition.
OBG Management: Would skinning vulvectomy eliminate the risk of recurrence?
Dr. Massad: Full vulvectomy is crippling and usually unnecessary. Most patients and clinicians accept the risk of recurrence of VIN to avoid the side effects of radical treatment.
OBG Management: What kind of surveillance is recommended after treatment?
Dr. Massad: Patients should perform vulvar self-examination every few months. They should also be examined 6 and 12 months after initial treatment and annually thereafter because the risk of recurrence may persist for years.
Because VIN is associated with carcinogenic HPV, women with VIN should undergo an annual Pap test.
OBG Management: Thank you, Dr. Massad. Let’s hope the incidence of this precancer begins to decline.
- Recommend the quadrivalent HPV vaccine for girls in the target age range (11 and 12 years old) to reduce the risk of VIN.
- Encourage smoking cessation.
- Make it a practice to inspect the vulva before inserting the speculum for cervical examination.
- Biopsy most pigmented lesions on the vulva. Biopsy all warty lesions in postmenopausal women and in women who fail topical treatment for genital warts.
- Treat all VIN lesions. When cancer is suspected, use wide local excision with a margin of 5 to 10 mm.
- Keep in mind that dysplastic cells can extend into hair follicles and sweat glands.
- Closely follow up all women treated for VIN (6 and 12 months after treatment and annually thereafter) and encourage them to examine their vulva several times every year. Perform an annual Pap test for any woman found to have VIN.
We want to hear from you! Tell us what you think.
1. Committee on Gynecologic Practice; American College of Obstetricians and Gynecologists. Committee Opinion #509: Management of vulvar intraepithelial neoplasia. Obstet Gynecol. 2011;118(5):1192-1194.
2. Judson PL, Habermann EB, Baxter NN, Durham SB, Virnig BA. Trends in the incidence of invasive and in situ vulvar carcinoma. Obstet Gynecol. 2006;107(5):1018-1022.
3. Markowitz LE, Dunne EF, Saraiya M, Lawson HW, Chesson H, Unger ER. Quadrivalent human papillomavirus vaccine: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep. 2007;56(RR-2):1-24.
4. Jones RW, Rowan DM, Stewart AW. Vulvar intraepithelial neoplasia: aspects of the natural history and outcome in 405 women. Obstet Gynecol. 2005;106(6):1319-1326.
5. Van Seters M, van Beurden M, ten Kate FJW, et al. Treatment of vulvar intraepithelial neoplasia with topical imiquimod. N Engl J Med. 2008;358(14):1465-1473.
6. Terlou A, van Seters M, Ewing PC, et al. Treatment of vaginal intraepithelial neoplasia with topical imiquimod: seven years median follow-up of a randomized clinical trial. Gynecol Oncol. 2011;121(1):157-162.
7. Hillemanns P, Wang X, Staehle S, Michels W, Dannecker C. Evaluation of different treatment modalities for vulvar intraepithelial neoplasia (VIN): CO2 laser vaporization, photodynamic therapy, excision and vulvectomy. Gynecol Oncol. 2006;100(2):271-275.
How should you evaluate a patient who has a cytologic diagnosis of atypical glandular cells (AGC)?
Charles J. Dunton, MD (Examining the Evidence; August 2011)
What is optimal surveillance after treatment for high-grade cervical intraepithelial neoplasia (CIN)?
Alan G. Waxman, MD, MPH (Examining the Evidence; June 2011)
2 HPV vaccines, 7 questions that you need answered
Neal M. Lonky, MD, MPH, and an expert panel (August 2010)
Dr. Massad reports no financial relationships relevant to this article.
The societal shifts of the 1960s generated many changes—among them, permanently altered sexual mores. That may be a primary reason why the incidence of vulvar intraepithelial neoplasia (VIN) increased more than 400% between 1973 and 2000, says L. Stewart Massad, Jr, MD, chairman of the Practice Committee of the American Society for Colposcopy and Cervical Pathology (ASCCP) and member of the ACOG Committee on Gynecologic Practice—and one of the authors of a new joint Committee Opinion on the management of VIN.1 This precancer is often associated with carcinogenic types of human papillomavirus (HPV), the most common sexually transmitted disease in the nation.
The 400% statistic caught the attention of OBG Management. The editors invited Dr. Massad to discuss the subject of VIN at length, elaborating on key issues such as its prevention, identification, treatment, and surveillance.
How to identify a VIN lesion
OBG Management: What is VIN? What does it look like?
Dr. Massad: VIN is a premalignant condition of the vulva that may present as unifocal or multifocal lesions. These lesions may be flesh-colored, hypopigmented, or hyperpigmented (FIGURE). They also may be erythematous, flat, or raised. They can be found on any part of the vulva. The dysplastic cells may extend into hair shafts or sweat glands; they don’t penetrate the basement membrane, however, so, by definition, they aren’t invasive.
Usual-type VIN
A. This warty lesion is hyperpigmented around the periphery, hypopigmented in the center. B. Another warty lesion. Both images reflect the application of acetic acid.OBG Management: Why has the incidence increased so considerably?
Dr. Massad: The data we have on incidence comes from the Surveillance, Epidemiology and End Results (SEER) program of the National Cancer Institute, as reported by Judson and colleagues.2 Although better reporting of findings of VIN may play a role, the rising incidence seems to be attributable to changes in sexual behavior over the past half century. The incidence of vulvar cancer rose during the same period—about 20%.3 The much slower growth in the incidence of vulvar cancer suggests that treatment of VIN has blunted the risk of cancer.
OBG Management: Is VIN associated with any particular type of HPV?
Dr. Massad: Yes, more than 80% of VIN lesions are associated with HPV 16.
OBG Management: One study from 2005 noted that the mean age of women with VIN decreased from 50 years before 1980 to 39 years in subsequent years.4 Why are more younger women developing VIN?
Dr. Massad: The study that showed that age shift was from New Zealand. The authors speculated that the change was due to earlier sexual activity among women who smoke: HPV, especially HPV 16, and smoking are important risk factors for VIN. The question hasn’t been definitively answered.
OBG Management: What are the risk factors for VIN?
Dr. Massad: Smoking is a big one. More than 50% of women who have VIN are smokers. Thirty percent have concurrent or prior cervical intraepithelial neoplasia (CIN) or vaginal intraepithelial neoplasia (VAIN). The risk of invasion rises with age at the time of the initial diagnosis and with longer follow-up. I can talk about surveillance a little later.
Are some lesions more worrisome than others?
OBG Management: Are VIN lesions categorized similarly to CIN lesions—that is, using three different grades of severity?
Dr. Massad: Until recently, that was the case, but it is no longer so. Broadly, there are now two classes of VIN, according to the International Society for the Study of Vulvovaginal Disease (ISSVD): usual-type VIN and differentiated VIN.
ACOG and ASCCP have embraced this classification system, although not all pathologists have done so, and clinicians may still see reports using the old three-tier system.
Usual-type VIN is associated with infection with high-risk types of HPV—most notably, HPV 16, as I mentioned. Histologically, usual-type VIN can mimic common genital warts, and the warty subtype shows keratosis at the surface, a spiky or undulating surface, and vertical maturation of cells in the lesion but with pleomorphic cells filling half or more of the epithelial thickness. The basaloid subtype of usual VIN shows little maturation.
Differentiated VIN exhibits more subtle atypia, with keratin pearls and an eosinophilic cytoplasm.
Biologically, usual-type VIN is associated with HPV and linked to smoking and sexual activity, as we discussed. As its name suggests, it is found more frequently than differentiated VIN. It is most common in women in their late 30s to early 50s.
In contrast, differentiated VIN is not associated with HPV and is more common in postmenopausal women; it is frequently seen with lichen sclerosus.
OBG Management: When did this new way of classifying VIN—as usual-type and differentiated—originate?
Dr. Massad: The ISVVD classification system changed in 2004. Before then, it paralleled the CIN classification system, with three grades of intraepithelial neoplasia corresponding to the thickness of the epithelium filled by dysplastic cells: VIN 1, 2, and 3. However, VIN 1 was not really neoplastic. It reflected infection with HPV, and although it might progress to higher-grade dysplasia or cancer, the risk was minimal. So the ISVVD revised the classification system to include only high-grade VIN—the old VIN 2 and VIN 3. HPV-associated lesions with dysplastic cells confined to the lower third of the epithelium are managed like genital warts, with observation for spontaneous regression or treatment with topical therapy or surgery.
How to screen for VIN
OBG Management: What screening strategy is recommended for VIN?
Dr. Massad: There is no such recommendation. Screening for VIN hasn’t been implemented for several reasons. Most important, other than inspection of the vulva by a clinician, there is no good screening test. VIN isn’t very common, so mass inspection for lesions is unlikely to be cost-effective. The sensitivity and specificity of inspection by a clinician aren’t known. Most lesions are found by women, their partners, or clinicians before cancer develops. And most disease is treated before cancer arises.
OBG Management: Isn’t there a need for heightened scrutiny of the vulva?
Dr. Massad: Yes. Women should examine their genitalia several times a year and seek attention if anything changes. That’s especially true for women who have risk factors, such as smoking, immunosuppression, and a history of being treated for cervical dysplasia. It’s the same concept we employ when teaching women to identify early breast lesions through self-examination.
The biggest challenges in detecting VIN are educating women to report vulvar skin changes to their clinicians for assessment and educating clinicians to examine the vulva before inserting the speculum for cervical screening and vaginal inspection.
OBG Management: Is another challenge distinguishing some forms of VIN from genital warts?
Dr. Massad: It can be a challenge, but clinicians should recall that warts are most common among women around the time of the onset of sexual activity. Older women sometimes develop warts with a new sexual partner. However, when women in their 40s and older develop new warty lesions, always suspect VIN. A woman in her 60s or older who has a new, warty-appearing vulvar lesion should be assumed to have VIN or cancer.
OBG Management: Does VIN ever regress spontaneously?
Dr. Massad: Yes. There have been reports of spontaneous regression of VIN, especially in young women. Regrettably, there are also reports of progression to cancer during observation. There are no characteristics that allow us to distinguish lesions that are going to progress from those that will regress. The ACOG-ASCCP Committee Opinion recommends treatment of all VIN.1
Can VIN be prevented?
OBG Management: The Committee Opinion recommends that the quadrivalent HPV vaccine be offered to women “in target populations” because it can decrease the risk of VIN. What are those target populations?
Dr. Massad: The target population for HPV vaccination is 11- and 12-year-old girls, but catch-up vaccination is acceptable in patients as old as 26 years.
OBG Management: Why isn’t the bivalent vaccine recommended?
Dr. Massad: Only the quadrivalent vaccine has been approved by the US Food and Drug Administration (FDA) for prevention of VIN, although, in theory, the bivalent vaccine ought to be effective as well.
When to biopsy
OBG Management: Do you recommend that any suspect lesion on the vulva be biopsied?
Dr. Massad: The decision to biopsy should be individualized. However, women who have apparent warts that fail to respond to topical therapy should undergo biopsy, as should older women with warty lesions. Keep in mind that older women may develop verrucous carcinomas and may benefit from excision of enlarging warty lesions even if a biopsy is reported as only condylomata. Clinicians should not biopsy varicosities or obvious flat nevi.
OBG Management: Is colposcopy ever helpful in assessing vulvar lesions?
Dr. Massad: Most vulvar lesions can be identified without colposcopy, but colposcopy is useful in determining the extent of lesions. It often reveals subclinical disease not evident at the time of vulvar inspection.
OBG Management: When colposcopy is used, is the procedure the same as for cervical examination?
Dr. Massad: Not exactly. The clinician should apply 5% acetic acid for 5 minutes using a gauze sponge, but the magnification should be 63 to 103—not 153, as it is for cervical examination. It’s important to distinguish hyperplasia from VIN. In general, hyperplastic lesions are faint, gray, diffuse, and flat, whereas VIN lesions are raised and irregular in shape, with sharp borders.
OBG Management: What about toluidine blue? Is it useful in inspection of lesions?
Dr. Massad: Toluidine blue stains skin that is irritated. It isn’t very specific for VIN or vulvar cancer, and it can make colposcopy difficult, so experts no longer recommend it.
OBG Management: What are the treatment options for VIN?
Dr. Massad: They include surgical excision, laser ablation, and topical therapy with 5% imiquimod. All are potentially effective. The Committee Opinion doesn’t specify a preference, except to say that excision is advised when there is any suspicion of cancer to preserve a sample for pathologic analysis. Ablation destroys the lesion, making assessment of possible invasion impossible, and imiquimod may allow disease to progress during observation.
OBG Management: The Committee Opinion recommends wide local excision when cancer is suspected. What size of margin is optimal?
Dr. Massad: In general, a margin of 5 to 10 mm around the lesion is recommended. Vulvectomy isn’t needed because close follow-up usually identifies recurrence before invasion occurs.
OBG Management: When is laser ablation a good choice?
Dr. Massad: Whenever a biopsy shows VIN and cancer is not suspected. Laser ablation is ideal when lesions are multifocal or extensive, although repeated treatments may be required to resolve small foci of residual disease. Done with careful attention to power density and depth of ablation, laser therapy can be less disfiguring than excision.
OBG Management: You mentioned 5% imiquimod. Is there evidence that it’s effective in the treatment of VIN?
Dr. Massad: Multiple randomized, controlled trials have shown 5% imiquimod to be effective against VIN, although the agent does not have approval from the FDA for that indication.5,6 Lower concentrations of imiquimod have not been studied in the treatment of VIN. Women treated with this topical therapy should be followed every 4 weeks with colposcopy because progression to cancer has been reported during imiquimod therapy. Lesions that fail to respond completely after a full course of imiquimod should be treated with excision or laser ablation.
Surveillance is critical
OBG Management: According to the Committee Opinion, the recurrence rate of VIN can reach 30% to 50%.7 Why so high?
Dr. Massad: Usual-type VIN reflects exposure to carcinogenic HPV, and differentiated VIN arises from a vulvar dystrophy. In both situations, treatments destroy VIN and arrest progress to cancer, but the entire vulvar skin remains subject to the inciting condition.
OBG Management: Would skinning vulvectomy eliminate the risk of recurrence?
Dr. Massad: Full vulvectomy is crippling and usually unnecessary. Most patients and clinicians accept the risk of recurrence of VIN to avoid the side effects of radical treatment.
OBG Management: What kind of surveillance is recommended after treatment?
Dr. Massad: Patients should perform vulvar self-examination every few months. They should also be examined 6 and 12 months after initial treatment and annually thereafter because the risk of recurrence may persist for years.
Because VIN is associated with carcinogenic HPV, women with VIN should undergo an annual Pap test.
OBG Management: Thank you, Dr. Massad. Let’s hope the incidence of this precancer begins to decline.
- Recommend the quadrivalent HPV vaccine for girls in the target age range (11 and 12 years old) to reduce the risk of VIN.
- Encourage smoking cessation.
- Make it a practice to inspect the vulva before inserting the speculum for cervical examination.
- Biopsy most pigmented lesions on the vulva. Biopsy all warty lesions in postmenopausal women and in women who fail topical treatment for genital warts.
- Treat all VIN lesions. When cancer is suspected, use wide local excision with a margin of 5 to 10 mm.
- Keep in mind that dysplastic cells can extend into hair follicles and sweat glands.
- Closely follow up all women treated for VIN (6 and 12 months after treatment and annually thereafter) and encourage them to examine their vulva several times every year. Perform an annual Pap test for any woman found to have VIN.
We want to hear from you! Tell us what you think.
How should you evaluate a patient who has a cytologic diagnosis of atypical glandular cells (AGC)?
Charles J. Dunton, MD (Examining the Evidence; August 2011)
What is optimal surveillance after treatment for high-grade cervical intraepithelial neoplasia (CIN)?
Alan G. Waxman, MD, MPH (Examining the Evidence; June 2011)
2 HPV vaccines, 7 questions that you need answered
Neal M. Lonky, MD, MPH, and an expert panel (August 2010)
Dr. Massad reports no financial relationships relevant to this article.
The societal shifts of the 1960s generated many changes—among them, permanently altered sexual mores. That may be a primary reason why the incidence of vulvar intraepithelial neoplasia (VIN) increased more than 400% between 1973 and 2000, says L. Stewart Massad, Jr, MD, chairman of the Practice Committee of the American Society for Colposcopy and Cervical Pathology (ASCCP) and member of the ACOG Committee on Gynecologic Practice—and one of the authors of a new joint Committee Opinion on the management of VIN.1 This precancer is often associated with carcinogenic types of human papillomavirus (HPV), the most common sexually transmitted disease in the nation.
The 400% statistic caught the attention of OBG Management. The editors invited Dr. Massad to discuss the subject of VIN at length, elaborating on key issues such as its prevention, identification, treatment, and surveillance.
How to identify a VIN lesion
OBG Management: What is VIN? What does it look like?
Dr. Massad: VIN is a premalignant condition of the vulva that may present as unifocal or multifocal lesions. These lesions may be flesh-colored, hypopigmented, or hyperpigmented (FIGURE). They also may be erythematous, flat, or raised. They can be found on any part of the vulva. The dysplastic cells may extend into hair shafts or sweat glands; they don’t penetrate the basement membrane, however, so, by definition, they aren’t invasive.
Usual-type VIN
A. This warty lesion is hyperpigmented around the periphery, hypopigmented in the center. B. Another warty lesion. Both images reflect the application of acetic acid.OBG Management: Why has the incidence increased so considerably?
Dr. Massad: The data we have on incidence comes from the Surveillance, Epidemiology and End Results (SEER) program of the National Cancer Institute, as reported by Judson and colleagues.2 Although better reporting of findings of VIN may play a role, the rising incidence seems to be attributable to changes in sexual behavior over the past half century. The incidence of vulvar cancer rose during the same period—about 20%.3 The much slower growth in the incidence of vulvar cancer suggests that treatment of VIN has blunted the risk of cancer.
OBG Management: Is VIN associated with any particular type of HPV?
Dr. Massad: Yes, more than 80% of VIN lesions are associated with HPV 16.
OBG Management: One study from 2005 noted that the mean age of women with VIN decreased from 50 years before 1980 to 39 years in subsequent years.4 Why are more younger women developing VIN?
Dr. Massad: The study that showed that age shift was from New Zealand. The authors speculated that the change was due to earlier sexual activity among women who smoke: HPV, especially HPV 16, and smoking are important risk factors for VIN. The question hasn’t been definitively answered.
OBG Management: What are the risk factors for VIN?
Dr. Massad: Smoking is a big one. More than 50% of women who have VIN are smokers. Thirty percent have concurrent or prior cervical intraepithelial neoplasia (CIN) or vaginal intraepithelial neoplasia (VAIN). The risk of invasion rises with age at the time of the initial diagnosis and with longer follow-up. I can talk about surveillance a little later.
Are some lesions more worrisome than others?
OBG Management: Are VIN lesions categorized similarly to CIN lesions—that is, using three different grades of severity?
Dr. Massad: Until recently, that was the case, but it is no longer so. Broadly, there are now two classes of VIN, according to the International Society for the Study of Vulvovaginal Disease (ISSVD): usual-type VIN and differentiated VIN.
ACOG and ASCCP have embraced this classification system, although not all pathologists have done so, and clinicians may still see reports using the old three-tier system.
Usual-type VIN is associated with infection with high-risk types of HPV—most notably, HPV 16, as I mentioned. Histologically, usual-type VIN can mimic common genital warts, and the warty subtype shows keratosis at the surface, a spiky or undulating surface, and vertical maturation of cells in the lesion but with pleomorphic cells filling half or more of the epithelial thickness. The basaloid subtype of usual VIN shows little maturation.
Differentiated VIN exhibits more subtle atypia, with keratin pearls and an eosinophilic cytoplasm.
Biologically, usual-type VIN is associated with HPV and linked to smoking and sexual activity, as we discussed. As its name suggests, it is found more frequently than differentiated VIN. It is most common in women in their late 30s to early 50s.
In contrast, differentiated VIN is not associated with HPV and is more common in postmenopausal women; it is frequently seen with lichen sclerosus.
OBG Management: When did this new way of classifying VIN—as usual-type and differentiated—originate?
Dr. Massad: The ISVVD classification system changed in 2004. Before then, it paralleled the CIN classification system, with three grades of intraepithelial neoplasia corresponding to the thickness of the epithelium filled by dysplastic cells: VIN 1, 2, and 3. However, VIN 1 was not really neoplastic. It reflected infection with HPV, and although it might progress to higher-grade dysplasia or cancer, the risk was minimal. So the ISVVD revised the classification system to include only high-grade VIN—the old VIN 2 and VIN 3. HPV-associated lesions with dysplastic cells confined to the lower third of the epithelium are managed like genital warts, with observation for spontaneous regression or treatment with topical therapy or surgery.
How to screen for VIN
OBG Management: What screening strategy is recommended for VIN?
Dr. Massad: There is no such recommendation. Screening for VIN hasn’t been implemented for several reasons. Most important, other than inspection of the vulva by a clinician, there is no good screening test. VIN isn’t very common, so mass inspection for lesions is unlikely to be cost-effective. The sensitivity and specificity of inspection by a clinician aren’t known. Most lesions are found by women, their partners, or clinicians before cancer develops. And most disease is treated before cancer arises.
OBG Management: Isn’t there a need for heightened scrutiny of the vulva?
Dr. Massad: Yes. Women should examine their genitalia several times a year and seek attention if anything changes. That’s especially true for women who have risk factors, such as smoking, immunosuppression, and a history of being treated for cervical dysplasia. It’s the same concept we employ when teaching women to identify early breast lesions through self-examination.
The biggest challenges in detecting VIN are educating women to report vulvar skin changes to their clinicians for assessment and educating clinicians to examine the vulva before inserting the speculum for cervical screening and vaginal inspection.
OBG Management: Is another challenge distinguishing some forms of VIN from genital warts?
Dr. Massad: It can be a challenge, but clinicians should recall that warts are most common among women around the time of the onset of sexual activity. Older women sometimes develop warts with a new sexual partner. However, when women in their 40s and older develop new warty lesions, always suspect VIN. A woman in her 60s or older who has a new, warty-appearing vulvar lesion should be assumed to have VIN or cancer.
OBG Management: Does VIN ever regress spontaneously?
Dr. Massad: Yes. There have been reports of spontaneous regression of VIN, especially in young women. Regrettably, there are also reports of progression to cancer during observation. There are no characteristics that allow us to distinguish lesions that are going to progress from those that will regress. The ACOG-ASCCP Committee Opinion recommends treatment of all VIN.1
Can VIN be prevented?
OBG Management: The Committee Opinion recommends that the quadrivalent HPV vaccine be offered to women “in target populations” because it can decrease the risk of VIN. What are those target populations?
Dr. Massad: The target population for HPV vaccination is 11- and 12-year-old girls, but catch-up vaccination is acceptable in patients as old as 26 years.
OBG Management: Why isn’t the bivalent vaccine recommended?
Dr. Massad: Only the quadrivalent vaccine has been approved by the US Food and Drug Administration (FDA) for prevention of VIN, although, in theory, the bivalent vaccine ought to be effective as well.
When to biopsy
OBG Management: Do you recommend that any suspect lesion on the vulva be biopsied?
Dr. Massad: The decision to biopsy should be individualized. However, women who have apparent warts that fail to respond to topical therapy should undergo biopsy, as should older women with warty lesions. Keep in mind that older women may develop verrucous carcinomas and may benefit from excision of enlarging warty lesions even if a biopsy is reported as only condylomata. Clinicians should not biopsy varicosities or obvious flat nevi.
OBG Management: Is colposcopy ever helpful in assessing vulvar lesions?
Dr. Massad: Most vulvar lesions can be identified without colposcopy, but colposcopy is useful in determining the extent of lesions. It often reveals subclinical disease not evident at the time of vulvar inspection.
OBG Management: When colposcopy is used, is the procedure the same as for cervical examination?
Dr. Massad: Not exactly. The clinician should apply 5% acetic acid for 5 minutes using a gauze sponge, but the magnification should be 63 to 103—not 153, as it is for cervical examination. It’s important to distinguish hyperplasia from VIN. In general, hyperplastic lesions are faint, gray, diffuse, and flat, whereas VIN lesions are raised and irregular in shape, with sharp borders.
OBG Management: What about toluidine blue? Is it useful in inspection of lesions?
Dr. Massad: Toluidine blue stains skin that is irritated. It isn’t very specific for VIN or vulvar cancer, and it can make colposcopy difficult, so experts no longer recommend it.
OBG Management: What are the treatment options for VIN?
Dr. Massad: They include surgical excision, laser ablation, and topical therapy with 5% imiquimod. All are potentially effective. The Committee Opinion doesn’t specify a preference, except to say that excision is advised when there is any suspicion of cancer to preserve a sample for pathologic analysis. Ablation destroys the lesion, making assessment of possible invasion impossible, and imiquimod may allow disease to progress during observation.
OBG Management: The Committee Opinion recommends wide local excision when cancer is suspected. What size of margin is optimal?
Dr. Massad: In general, a margin of 5 to 10 mm around the lesion is recommended. Vulvectomy isn’t needed because close follow-up usually identifies recurrence before invasion occurs.
OBG Management: When is laser ablation a good choice?
Dr. Massad: Whenever a biopsy shows VIN and cancer is not suspected. Laser ablation is ideal when lesions are multifocal or extensive, although repeated treatments may be required to resolve small foci of residual disease. Done with careful attention to power density and depth of ablation, laser therapy can be less disfiguring than excision.
OBG Management: You mentioned 5% imiquimod. Is there evidence that it’s effective in the treatment of VIN?
Dr. Massad: Multiple randomized, controlled trials have shown 5% imiquimod to be effective against VIN, although the agent does not have approval from the FDA for that indication.5,6 Lower concentrations of imiquimod have not been studied in the treatment of VIN. Women treated with this topical therapy should be followed every 4 weeks with colposcopy because progression to cancer has been reported during imiquimod therapy. Lesions that fail to respond completely after a full course of imiquimod should be treated with excision or laser ablation.
Surveillance is critical
OBG Management: According to the Committee Opinion, the recurrence rate of VIN can reach 30% to 50%.7 Why so high?
Dr. Massad: Usual-type VIN reflects exposure to carcinogenic HPV, and differentiated VIN arises from a vulvar dystrophy. In both situations, treatments destroy VIN and arrest progress to cancer, but the entire vulvar skin remains subject to the inciting condition.
OBG Management: Would skinning vulvectomy eliminate the risk of recurrence?
Dr. Massad: Full vulvectomy is crippling and usually unnecessary. Most patients and clinicians accept the risk of recurrence of VIN to avoid the side effects of radical treatment.
OBG Management: What kind of surveillance is recommended after treatment?
Dr. Massad: Patients should perform vulvar self-examination every few months. They should also be examined 6 and 12 months after initial treatment and annually thereafter because the risk of recurrence may persist for years.
Because VIN is associated with carcinogenic HPV, women with VIN should undergo an annual Pap test.
OBG Management: Thank you, Dr. Massad. Let’s hope the incidence of this precancer begins to decline.
- Recommend the quadrivalent HPV vaccine for girls in the target age range (11 and 12 years old) to reduce the risk of VIN.
- Encourage smoking cessation.
- Make it a practice to inspect the vulva before inserting the speculum for cervical examination.
- Biopsy most pigmented lesions on the vulva. Biopsy all warty lesions in postmenopausal women and in women who fail topical treatment for genital warts.
- Treat all VIN lesions. When cancer is suspected, use wide local excision with a margin of 5 to 10 mm.
- Keep in mind that dysplastic cells can extend into hair follicles and sweat glands.
- Closely follow up all women treated for VIN (6 and 12 months after treatment and annually thereafter) and encourage them to examine their vulva several times every year. Perform an annual Pap test for any woman found to have VIN.
We want to hear from you! Tell us what you think.
1. Committee on Gynecologic Practice; American College of Obstetricians and Gynecologists. Committee Opinion #509: Management of vulvar intraepithelial neoplasia. Obstet Gynecol. 2011;118(5):1192-1194.
2. Judson PL, Habermann EB, Baxter NN, Durham SB, Virnig BA. Trends in the incidence of invasive and in situ vulvar carcinoma. Obstet Gynecol. 2006;107(5):1018-1022.
3. Markowitz LE, Dunne EF, Saraiya M, Lawson HW, Chesson H, Unger ER. Quadrivalent human papillomavirus vaccine: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep. 2007;56(RR-2):1-24.
4. Jones RW, Rowan DM, Stewart AW. Vulvar intraepithelial neoplasia: aspects of the natural history and outcome in 405 women. Obstet Gynecol. 2005;106(6):1319-1326.
5. Van Seters M, van Beurden M, ten Kate FJW, et al. Treatment of vulvar intraepithelial neoplasia with topical imiquimod. N Engl J Med. 2008;358(14):1465-1473.
6. Terlou A, van Seters M, Ewing PC, et al. Treatment of vaginal intraepithelial neoplasia with topical imiquimod: seven years median follow-up of a randomized clinical trial. Gynecol Oncol. 2011;121(1):157-162.
7. Hillemanns P, Wang X, Staehle S, Michels W, Dannecker C. Evaluation of different treatment modalities for vulvar intraepithelial neoplasia (VIN): CO2 laser vaporization, photodynamic therapy, excision and vulvectomy. Gynecol Oncol. 2006;100(2):271-275.
1. Committee on Gynecologic Practice; American College of Obstetricians and Gynecologists. Committee Opinion #509: Management of vulvar intraepithelial neoplasia. Obstet Gynecol. 2011;118(5):1192-1194.
2. Judson PL, Habermann EB, Baxter NN, Durham SB, Virnig BA. Trends in the incidence of invasive and in situ vulvar carcinoma. Obstet Gynecol. 2006;107(5):1018-1022.
3. Markowitz LE, Dunne EF, Saraiya M, Lawson HW, Chesson H, Unger ER. Quadrivalent human papillomavirus vaccine: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep. 2007;56(RR-2):1-24.
4. Jones RW, Rowan DM, Stewart AW. Vulvar intraepithelial neoplasia: aspects of the natural history and outcome in 405 women. Obstet Gynecol. 2005;106(6):1319-1326.
5. Van Seters M, van Beurden M, ten Kate FJW, et al. Treatment of vulvar intraepithelial neoplasia with topical imiquimod. N Engl J Med. 2008;358(14):1465-1473.
6. Terlou A, van Seters M, Ewing PC, et al. Treatment of vaginal intraepithelial neoplasia with topical imiquimod: seven years median follow-up of a randomized clinical trial. Gynecol Oncol. 2011;121(1):157-162.
7. Hillemanns P, Wang X, Staehle S, Michels W, Dannecker C. Evaluation of different treatment modalities for vulvar intraepithelial neoplasia (VIN): CO2 laser vaporization, photodynamic therapy, excision and vulvectomy. Gynecol Oncol. 2006;100(2):271-275.
