User login
From Saint Luke’s Mid America Heart Institute/University of Missouri–Kansas City, Kansas City, MO.
Abstract
- Objective: To outline the tools available to help understand the risk of transcatheter aortic valve replacement (TAVR) and the gaps in knowledge regarding TAVR risk estimation.
- Methods: Review of the literature.
- Results: Two models developed and validated by the American College of Cardiology can be used to estimate the risk of short-term mortality, a 6-variable in-hospital model designed for clinical use and a 41-variable 30-day model designed primarily for site comparisons and quality improvement. Importantly, neither model should be used to inform the choice of TAVR versus surgical aortic valve replacement. Regarding long-term outcomes, a risk model to estimate risk of dying or having a persistently poor quality of life at 1 year after TAVR has been developed and validated. Factors that most significantly increase a patient’s risk for poor outcomes are very poor functional status prior to TAVR, requiring home oxygen, chronic renal insufficiency, atrial fibrillation, dependencies in activities of daily living, and dementia. If a patient has ≥ 2 or 3 major risk factors for a poor outcome, this risk and the uncertainty about the degree of recovery expected after TAVR should be discussed with the patient (and family).
- Conclusion: It is important to understand the patient factors that most strongly drive risk of poor outcomes after TAVR and use this information to set appropriate expectations for recovery.
Keywords: aortic valve stenosis; risk factors; postoperative complications; TAVR.
Among patients with severe aortic stenosis,
As with any emerging technology, selecting the appropriate patients for TAVR—a procedure with high initial costs10—has been an area of active investigation. As TAVR was first introduced in patients who were considered inoperable, initial efforts focused on trying to identify the patients who did not improve functionally or live longer following TAVR. Termed Cohort C patients, these patients were thought to have too many comorbidities, be too sick, and have too little reserve to recover from TAVR, and in the early trials, represented a substantial minority of the patients. For example, in pivotal clinical trials of patients at high or extreme surgical risk, approximately 1 in 4 patients who were treated with TAVR were dead at 1 year.1,3,11 Furthermore, a number of patients who received TAVR were alive at 1 year but continued to have significant heart failure symptoms and functional limitations.2,4 Practitioners,12,13 regulators,14 and third-party payers15 have recommended that TAVR should not be offered to patients in whom valve replacement would not be expected to positively impact either their survival or quality of life, but how best to identify these patients has been less clear.
More recently, as the use of TAVR has moved down the risk spectrum, patient selection for TAVR has shifted to understanding which patients should be preferentially treated with TAVR versus SAVR. While patients often prefer a less invasive treatment option with faster recovery—which is what TAVR offers—there are lingering questions about valve longevity, need for a pacemaker (and the associated long-term implications), and the ability to treat other cardiovascular conditions (eg, Maze, mitral valve repair) that potentially make a patient a more appropriate candidate for valve surgery. This review
Short-Term Outcomes
When TAVR was initially introduced, the 30-day mortality rate was 5% to 8%.1,11,16 This high mortality rate was a function of treating very ill patients and more invasive procedures with larger sheath sizes and routine use of general anesthesia, transesophageal echocardiography, pulmonary artery catheterization, and so on. Over time, however, this rate has gone down substantially, with the 30-day mortality rate in intermediate- and low-risk patients now ranging from 0.5% to 1%.8,17-19 Although this low mortality rate indicates that the vast majority of patients will survive to discharge from the hospital,
While 30 days is a better time frame for assessment because outcome is less impacted by differences in local post-acute care facilities, we explicitly did not create a parsimonious 30-day mortality model for clinical use due to concern that having such a model would allow for indirect comparisons with estimated risk of SAVR using the Society of Thoracic Surgeons risk model (http://riskcalc.sts.org/stswebriskcalc). It would be tempting to estimate a patient’s risk of mortality with the TAVR calculator and the SAVR calculator and use those risk estimates to
The decision of selecting SAVR over TAVR is typically driven by factors other than short- or long-term mortality (eg, whether TAVR will be covered by insurance, very young age and concern about durability, need to treat concomitant mitral regurgitation or aortopathy), as clinical trials have shown that survival and quality of life outcomes are at least as good with TAVR compared with SAVR.6,7,9,23 In fact, in an analysis that compared similar patients treated with TAVR versus SAVR and specifically looked for patient factors that might make one treatment preferable to the other, patients who had a prior cardiac operation and those on home oxygen were more likely to do better with TAVR, whereas no patient factors that favored SAVR were found.24 The majority of patients, however, were expected to have similar long-term outcomes regardless of treatment choice, and as such, the benefit of TAVR appears mostly to be an earlier and easier recovery.
Long-Term Outcomes: Estimating the Risk for Failure to Recover
While many patients who undergo TAVR are quite ill prior to the procedure, with substantial limitations due to the fatigue and shortness of breath associated with severe aortic stenosis, most patients recover well after the procedure, with marked improvement in symptoms and functional capacity. Approximately 25% to 35% of patients currently treated with TAVR commercially (ie, intermediate- and high-surgical-risk patients) either die or do not recover a reasonable quality of life after the procedure. Identifying those patients prior to the procedure can be challenging. We have previously developed and externally validated
Beyond clinical factors, frailty negatively impacts both survival and quality of life after TAVR. Frailty is a geriatric syndrome of impaired physiologic reserve and decreased resistance to stressors27 that is characterized by weakness, slowness, exhaustion, wasting, and low activity level. Across a wide variety of clinical situations (eg, pneumonia,28 myocardial infarction,29 general30,31 and cardiac surgery32,33), frailty increases the risk of morbidity and mortality after nearly any intervention34 or clinical insult, independent of traditional demographic and clinical risk factors. Frail patients often do better with less invasive interventions such as TAVR compared with traditional surgery, but nonetheless remain at increased risk for death35-37 or failure to recover quality of life and functional status25,37 after TAVR. However, there are unique challenges in both assessing and managing frailty in patients who are considered potential candidates for TAVR. One challenge is the lack of a laboratory or radiologic test for frailty; instead, the lack of physiologic reserve of frailty is identified through a combination of factors, such as slow gait speed, weak grip strength, and unintentional weight loss. While these factors readily identify frail patients in general elderly populations, in patients with severe symptomatic aortic stenosis, these metrics can be impacted by the disease process itself. This distinction is important as slow gait speed that is due to aortic stenosis will be “fixed” by TAVR, but slow gait speed from frailty would identify a patient who will have a difficult time recovering from the procedure. For example, in the CoreValve High Risk Pivotal Trial, 80% of patients had a slow gait speed and 67% had a weak grip strength,5 and yet 58% of patients in this trial were alive and with a reasonable quality of life 1 year after TAVR.6 A number of studies have attempted to define true frailty within the pre-TAVR population, that which represents decreased physiologic reserve and an impaired ability to recover from an insult, and the factors that appear to be most prognostically important are malnutrition38 or unintentional weight loss25 and the inability to be independent in activities of daily living (eg, dressing, feeding, transferring).25,37
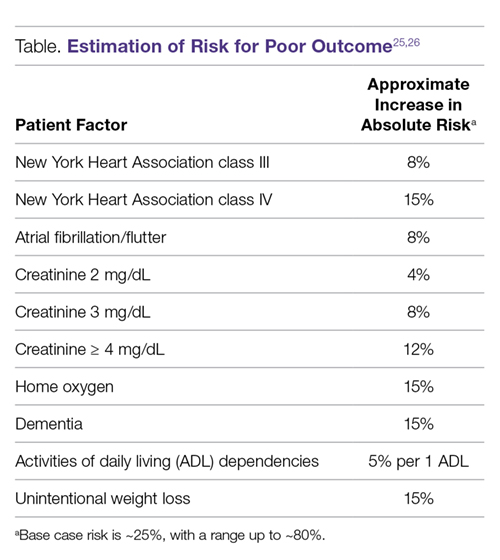
Even with frailty assessments, the ability to predict who is or is not going to have a poor outcome after TAVR (ie, to use pre-procedural factors to identify patients who perhaps should not be offered TAVR because he or she will not recover from the procedure) is exceedingly difficult. The Table shows how to grossly estimate risk using the major factors that impact risk based on the more precise estimates from our models.25,26
The model shown in the Table can be used to estimate a patient’s risk for a poor outcome, but it should be noted that even at the extreme high end of risk, there will be some patients who still do well after TAVR. Furthermore, being high risk for a poor outcome after TAVR does not imply anything about how the patient would do without TAVR, as many of these patients would likely die even sooner or have worse quality of life with medical therapy only. However,
Conclusion
Calculating the risk of TAVR can be complicated. In patients who are electively treated using transfemoral access and a less invasive approach, the short-term risk of mortality is very low. Risk calculators can be used to estimate short-term risk, but the patients who are high risk for in-hospital mortality are often fairly easy to recognize, as the factors that drive that risk are not subtle (eg, the patient is in shock at the time of the procedure). The true risk of TAVR lies in the inability to recover from the procedure—being chronically ill, frail, or debilitated to a degree that the patient either dies or fails to recover a reasonable quality of life. Given
Corresponding author: Suzanne V. Arnold, MD, MHA, 4401 Wornall Rd., Kansas City, MO 64111.
Financial disclosures: This work was funded in part by grant K23HL116799 from the National Institutes of Health.
1. Leon MB, Smith CR, Mack M, et al. Transcatheter aortic-valve implantation for aortic stenosis in patients who cannot undergo surgery. N Engl J Med. 2010;363:1597-1607.
2. Reynolds MR, Magnuson EA, Lei Y, et al. Health-related quality of life after transcatheter aortic valve replacement in inoperable patients with severe aortic stenosis. Circulation. 2011;124(:1964-1972.
3. Smith CR, Leon MB, Mack MJ, et al. Transcatheter versus surgical aortic-valve replacement in high-risk patients. N Engl J Med. 2011;364:2187-2198.
4. Reynolds MR, Magnuson EA, Wang K, et al. Health-related quality of life after transcatheter or surgical aortic valve replacement in high-risk patients with severe aortic stenosis: results from the PARTNER (Placement of AoRTic TraNscathetER Valve) trial (Cohort A). J Am Coll Cardiol. 2012;60:548-558.
5. Adams DH, Popma JJ, Reardon MJ, et al. Transcatheter aortic-valve replacement with a self-expanding prosthesis. N Engl J Med. 2014;370:1790-1798.
6. Arnold SV, Reynolds MR, Wang K, et al. Health status after transcatheter or surgical aortic valve replacement in patients with severe aortic stenosis at increased surgical risk: results from the CoreValve US Pivotal trial. JACC Cardiovasc Interv. 2015;8:1207-1217.
7. Leon MB, Smith CR, Mack MJ, et al. Transcatheter or surgical aortic-valve replacement in intermediate-risk patients. N Engl J Med. 2016;374:1609-1620.
8. Reardon MJ, Van Mieghem NM, Popma JJ, et al. Surgical or transcatheter aortic-valve replacement in intermediate-risk patients. N Engl J Med. 2017;376:1321-1331.
9. Baron SJ, Arnold SV, Wang K, et al. Health status benefits of transcatheter vs surgical aortic valve replacement in patients with severe aortic stenosis at intermediate surgical risk: results from the PARTNER 2 randomized clinical trial. JAMA Cardiol. 2017;2:837-845.
10. Reynolds MR, Magnuson EA, Wang K, et al. Cost-effectiveness of transcatheter aortic valve replacement compared with standard care among inoperable patients with severe aortic stenosis: results from the placement of aortic transcatheter valves (PARTNER) trial (Cohort B). Circulation. 2012;125:1102-1109.
11. Popma JJ, Adams DH, Reardon MJ, et al. Transcatheter aortic valve replacement using a self-expanding bioprosthesis in patients with severe aortic stenosis at extreme risk for surgery. J Am Coll Cardiol. 2014;63:1972-1981.
12. Vahanian A, Alfieri O, Al-Attar N, et al. Transcatheter valve implantation for patients with aortic stenosis: a position statement from the European Association of Cardio-Thoracic Surgery (EACTS) and the European Society of Cardiology (ESC), in collaboration with the European Association of Percutaneous Cardiovascular Interventions (EAPCI). Eur Heart J. 2008;29:1463-1470.
13. Holmes DR Jr, Mack MJ, Kaul S, et al. 2012 ACCF/AATS/SCAI/STS expert consensus document on transcatheter aortic valve replacement. J Am Coll Cardiol. 2012;59:1200-1254.
14. US Food and Drug Administration. FDA Executive Summary: Edwards SAPIEN™ Transcatheter Heart Valve. Presented July 20, 2011, Gaithersburg, MD.
15. Centers for Medicare & Medicaid Services. Decision Memo for Transcatheter Aortic Valve Replacement (TAVR) (CAG-00430N). May 5, 2012.
16. Mack MJ, Brennan JM, Brindis R, et al. Outcomes following transcatheter aortic valve replacement in the United States. JAMA. 2013;310:2069-2077.
17. Thourani VH, Kodali S, Makkar RR, et al. Transcatheter aortic valve replacement versus surgical valve replacement in intermediate-risk patients: a propensity score analysis. Lancet. 2016;387:2218-2225.
18. Mack MJ, Leon MB, Thourani VH, et al. Transcatheter aortic-valve replacement with a balloon-expandable valve in low-risk patients. N Engl J Med. 2019;380:1695-1705.
19. Popma JJ, Deeb GM, Yakubov SJ, et al. Transcatheter aortic-valve replacement with a self-expanding valve in low-risk patients. N Engl J Med. 2019;380:1706-1715.
20. Edwards FH, Peterson ED, Coombs LP, et al. Prediction of operative mortality after valve replacement surgery. J Am Coll Cardiol. 2001;37:885-892.
21. Arnold SV, O’Brien SM, Vemulapalli S, et al. Inclusion of functional status measures in the risk adjustment of 30-day mortality after transcatheter aortic valve replacement: a report from the Society of Thoracic Surgeons/American College of Cardiology TVT Registry. JACC Cardiovasc Interv. 2018;11:581-589.
22. Brennan JM, Thomas L, Cohen DJ, et al. Transcatheter versus surgical aortic valve replacement: propensity-matched comparison. J Am Coll Cardiol. 2017;70:439-450.
23. Reardon MJ, Adams DH, Kleiman NS, et al. 2-year outcomes in patients undergoing surgical or self-expanding transcatheter aortic valve replacement. J Am Coll Cardiol. 2015;66:113-121.
24. Baron SJ, Cohen DJ, Suchindran S, et al. Development of a risk prediction model for 1-year mortality after surgical vs. transcatheter aortic valve replacement in patients with severe aortic stenosis. Circulation. 2016;134(A20166).
25. Arnold SV, Afilalo J, Spertus JA, et al. Prediction of poor outcome after transcatheter aortic valve replacement. J Am Coll Cardiol. 2016;68:1868-1877.
26. Arnold SV, Reynolds MR, Lei Y, et al. Predictors of poor outcomes after transcatheter aortic valve replacement: results from the PARTNER (Placement of Aortic Transcatheter Valve) trial. Circulation. 2014;129:2682-2690.
27. Fried LP, Hadley EC, Walston JD, et al. From bedside to bench: research agenda for frailty. Sci Aging Knowledge Environ. 2005;2005:pe24.
28. Torres OH, Munoz J, Ruiz D, et al. Outcome predictors of pneumonia in elderly patients: importance of functional assessment. J Am Geriatr Soc. 2004;52:1603-1609.
29. Ekerstad N, Swahn E, Janzon M, et al. Frailty is independently associated with short-term outcomes for elderly patients with non-ST-segment elevation myocardial infarction. Circulation. 2011;124:2397-2404.
30. Makary MA, Segev DL, Pronovost PJ, et al. Frailty as a predictor of surgical outcomes in older patients. J Am Coll Surg. 2010;210:901-908.
31. Hewitt J, Moug SJ, Middleton M, et al. Prevalence of frailty and its association with mortality in general surgery. Am J Surg. 2015;209:254-259.
32. Sundermann S, Dademasch A, Praetorius J, et al. Comprehensive assessment of frailty for elderly high-risk patients undergoing cardiac surgery. Eur J Cardiothorac Surg. 2011;39:33-37.
33. Afilalo J, Mottillo S, Eisenberg MJ, et al. Addition of frailty and disability to cardiac surgery risk scores identifies elderly patients at high risk of mortality or major morbidity. Circ Cardiovasc Qual Outcomes. 2012;5:222-228.
34. Lin HS, Watts JN, Peel NM, Hubbard RE. Frailty and post-operative outcomes in older surgical patients: a systematic review. BMC Geriatr. 2016;16:157.
35. Stortecky S, Schoenenberger AW, Moser A, et al. Evaluation of multidimensional geriatric assessment as a predictor of mortality and cardiovascular events after transcatheter aortic valve implantation. JACC Cardiovasc Interv. 2012;5:489-496.
36. Schoenenberger AW, Stortecky S, Neumann S, et al. Predictors of functional decline in elderly patients undergoing transcatheter aortic valve implantation (TAVI). Eur Heart J. 2013;34:684-689.
37. Green P, Arnold SV, Cohen DJ, et al. Relation of frailty to outcomes after transcatheter aortic valve replacement (from the PARTNER trial). Am J Cardiol. 2015;116:264-269.
38. Goldfarb M, Lauck S, Webb J, et al. Malnutrition and mortality in frail and non-frail older adults undergoing aortic valve replacement. Circulation. 2018;138:2202-2211.
From Saint Luke’s Mid America Heart Institute/University of Missouri–Kansas City, Kansas City, MO.
Abstract
- Objective: To outline the tools available to help understand the risk of transcatheter aortic valve replacement (TAVR) and the gaps in knowledge regarding TAVR risk estimation.
- Methods: Review of the literature.
- Results: Two models developed and validated by the American College of Cardiology can be used to estimate the risk of short-term mortality, a 6-variable in-hospital model designed for clinical use and a 41-variable 30-day model designed primarily for site comparisons and quality improvement. Importantly, neither model should be used to inform the choice of TAVR versus surgical aortic valve replacement. Regarding long-term outcomes, a risk model to estimate risk of dying or having a persistently poor quality of life at 1 year after TAVR has been developed and validated. Factors that most significantly increase a patient’s risk for poor outcomes are very poor functional status prior to TAVR, requiring home oxygen, chronic renal insufficiency, atrial fibrillation, dependencies in activities of daily living, and dementia. If a patient has ≥ 2 or 3 major risk factors for a poor outcome, this risk and the uncertainty about the degree of recovery expected after TAVR should be discussed with the patient (and family).
- Conclusion: It is important to understand the patient factors that most strongly drive risk of poor outcomes after TAVR and use this information to set appropriate expectations for recovery.
Keywords: aortic valve stenosis; risk factors; postoperative complications; TAVR.
Among patients with severe aortic stenosis,
As with any emerging technology, selecting the appropriate patients for TAVR—a procedure with high initial costs10—has been an area of active investigation. As TAVR was first introduced in patients who were considered inoperable, initial efforts focused on trying to identify the patients who did not improve functionally or live longer following TAVR. Termed Cohort C patients, these patients were thought to have too many comorbidities, be too sick, and have too little reserve to recover from TAVR, and in the early trials, represented a substantial minority of the patients. For example, in pivotal clinical trials of patients at high or extreme surgical risk, approximately 1 in 4 patients who were treated with TAVR were dead at 1 year.1,3,11 Furthermore, a number of patients who received TAVR were alive at 1 year but continued to have significant heart failure symptoms and functional limitations.2,4 Practitioners,12,13 regulators,14 and third-party payers15 have recommended that TAVR should not be offered to patients in whom valve replacement would not be expected to positively impact either their survival or quality of life, but how best to identify these patients has been less clear.
More recently, as the use of TAVR has moved down the risk spectrum, patient selection for TAVR has shifted to understanding which patients should be preferentially treated with TAVR versus SAVR. While patients often prefer a less invasive treatment option with faster recovery—which is what TAVR offers—there are lingering questions about valve longevity, need for a pacemaker (and the associated long-term implications), and the ability to treat other cardiovascular conditions (eg, Maze, mitral valve repair) that potentially make a patient a more appropriate candidate for valve surgery. This review
Short-Term Outcomes
When TAVR was initially introduced, the 30-day mortality rate was 5% to 8%.1,11,16 This high mortality rate was a function of treating very ill patients and more invasive procedures with larger sheath sizes and routine use of general anesthesia, transesophageal echocardiography, pulmonary artery catheterization, and so on. Over time, however, this rate has gone down substantially, with the 30-day mortality rate in intermediate- and low-risk patients now ranging from 0.5% to 1%.8,17-19 Although this low mortality rate indicates that the vast majority of patients will survive to discharge from the hospital,
While 30 days is a better time frame for assessment because outcome is less impacted by differences in local post-acute care facilities, we explicitly did not create a parsimonious 30-day mortality model for clinical use due to concern that having such a model would allow for indirect comparisons with estimated risk of SAVR using the Society of Thoracic Surgeons risk model (http://riskcalc.sts.org/stswebriskcalc). It would be tempting to estimate a patient’s risk of mortality with the TAVR calculator and the SAVR calculator and use those risk estimates to
The decision of selecting SAVR over TAVR is typically driven by factors other than short- or long-term mortality (eg, whether TAVR will be covered by insurance, very young age and concern about durability, need to treat concomitant mitral regurgitation or aortopathy), as clinical trials have shown that survival and quality of life outcomes are at least as good with TAVR compared with SAVR.6,7,9,23 In fact, in an analysis that compared similar patients treated with TAVR versus SAVR and specifically looked for patient factors that might make one treatment preferable to the other, patients who had a prior cardiac operation and those on home oxygen were more likely to do better with TAVR, whereas no patient factors that favored SAVR were found.24 The majority of patients, however, were expected to have similar long-term outcomes regardless of treatment choice, and as such, the benefit of TAVR appears mostly to be an earlier and easier recovery.
Long-Term Outcomes: Estimating the Risk for Failure to Recover
While many patients who undergo TAVR are quite ill prior to the procedure, with substantial limitations due to the fatigue and shortness of breath associated with severe aortic stenosis, most patients recover well after the procedure, with marked improvement in symptoms and functional capacity. Approximately 25% to 35% of patients currently treated with TAVR commercially (ie, intermediate- and high-surgical-risk patients) either die or do not recover a reasonable quality of life after the procedure. Identifying those patients prior to the procedure can be challenging. We have previously developed and externally validated
Beyond clinical factors, frailty negatively impacts both survival and quality of life after TAVR. Frailty is a geriatric syndrome of impaired physiologic reserve and decreased resistance to stressors27 that is characterized by weakness, slowness, exhaustion, wasting, and low activity level. Across a wide variety of clinical situations (eg, pneumonia,28 myocardial infarction,29 general30,31 and cardiac surgery32,33), frailty increases the risk of morbidity and mortality after nearly any intervention34 or clinical insult, independent of traditional demographic and clinical risk factors. Frail patients often do better with less invasive interventions such as TAVR compared with traditional surgery, but nonetheless remain at increased risk for death35-37 or failure to recover quality of life and functional status25,37 after TAVR. However, there are unique challenges in both assessing and managing frailty in patients who are considered potential candidates for TAVR. One challenge is the lack of a laboratory or radiologic test for frailty; instead, the lack of physiologic reserve of frailty is identified through a combination of factors, such as slow gait speed, weak grip strength, and unintentional weight loss. While these factors readily identify frail patients in general elderly populations, in patients with severe symptomatic aortic stenosis, these metrics can be impacted by the disease process itself. This distinction is important as slow gait speed that is due to aortic stenosis will be “fixed” by TAVR, but slow gait speed from frailty would identify a patient who will have a difficult time recovering from the procedure. For example, in the CoreValve High Risk Pivotal Trial, 80% of patients had a slow gait speed and 67% had a weak grip strength,5 and yet 58% of patients in this trial were alive and with a reasonable quality of life 1 year after TAVR.6 A number of studies have attempted to define true frailty within the pre-TAVR population, that which represents decreased physiologic reserve and an impaired ability to recover from an insult, and the factors that appear to be most prognostically important are malnutrition38 or unintentional weight loss25 and the inability to be independent in activities of daily living (eg, dressing, feeding, transferring).25,37
Even with frailty assessments, the ability to predict who is or is not going to have a poor outcome after TAVR (ie, to use pre-procedural factors to identify patients who perhaps should not be offered TAVR because he or she will not recover from the procedure) is exceedingly difficult. The Table shows how to grossly estimate risk using the major factors that impact risk based on the more precise estimates from our models.25,26
The model shown in the Table can be used to estimate a patient’s risk for a poor outcome, but it should be noted that even at the extreme high end of risk, there will be some patients who still do well after TAVR. Furthermore, being high risk for a poor outcome after TAVR does not imply anything about how the patient would do without TAVR, as many of these patients would likely die even sooner or have worse quality of life with medical therapy only. However,
Conclusion
Calculating the risk of TAVR can be complicated. In patients who are electively treated using transfemoral access and a less invasive approach, the short-term risk of mortality is very low. Risk calculators can be used to estimate short-term risk, but the patients who are high risk for in-hospital mortality are often fairly easy to recognize, as the factors that drive that risk are not subtle (eg, the patient is in shock at the time of the procedure). The true risk of TAVR lies in the inability to recover from the procedure—being chronically ill, frail, or debilitated to a degree that the patient either dies or fails to recover a reasonable quality of life. Given
Corresponding author: Suzanne V. Arnold, MD, MHA, 4401 Wornall Rd., Kansas City, MO 64111.
Financial disclosures: This work was funded in part by grant K23HL116799 from the National Institutes of Health.
From Saint Luke’s Mid America Heart Institute/University of Missouri–Kansas City, Kansas City, MO.
Abstract
- Objective: To outline the tools available to help understand the risk of transcatheter aortic valve replacement (TAVR) and the gaps in knowledge regarding TAVR risk estimation.
- Methods: Review of the literature.
- Results: Two models developed and validated by the American College of Cardiology can be used to estimate the risk of short-term mortality, a 6-variable in-hospital model designed for clinical use and a 41-variable 30-day model designed primarily for site comparisons and quality improvement. Importantly, neither model should be used to inform the choice of TAVR versus surgical aortic valve replacement. Regarding long-term outcomes, a risk model to estimate risk of dying or having a persistently poor quality of life at 1 year after TAVR has been developed and validated. Factors that most significantly increase a patient’s risk for poor outcomes are very poor functional status prior to TAVR, requiring home oxygen, chronic renal insufficiency, atrial fibrillation, dependencies in activities of daily living, and dementia. If a patient has ≥ 2 or 3 major risk factors for a poor outcome, this risk and the uncertainty about the degree of recovery expected after TAVR should be discussed with the patient (and family).
- Conclusion: It is important to understand the patient factors that most strongly drive risk of poor outcomes after TAVR and use this information to set appropriate expectations for recovery.
Keywords: aortic valve stenosis; risk factors; postoperative complications; TAVR.
Among patients with severe aortic stenosis,
As with any emerging technology, selecting the appropriate patients for TAVR—a procedure with high initial costs10—has been an area of active investigation. As TAVR was first introduced in patients who were considered inoperable, initial efforts focused on trying to identify the patients who did not improve functionally or live longer following TAVR. Termed Cohort C patients, these patients were thought to have too many comorbidities, be too sick, and have too little reserve to recover from TAVR, and in the early trials, represented a substantial minority of the patients. For example, in pivotal clinical trials of patients at high or extreme surgical risk, approximately 1 in 4 patients who were treated with TAVR were dead at 1 year.1,3,11 Furthermore, a number of patients who received TAVR were alive at 1 year but continued to have significant heart failure symptoms and functional limitations.2,4 Practitioners,12,13 regulators,14 and third-party payers15 have recommended that TAVR should not be offered to patients in whom valve replacement would not be expected to positively impact either their survival or quality of life, but how best to identify these patients has been less clear.
More recently, as the use of TAVR has moved down the risk spectrum, patient selection for TAVR has shifted to understanding which patients should be preferentially treated with TAVR versus SAVR. While patients often prefer a less invasive treatment option with faster recovery—which is what TAVR offers—there are lingering questions about valve longevity, need for a pacemaker (and the associated long-term implications), and the ability to treat other cardiovascular conditions (eg, Maze, mitral valve repair) that potentially make a patient a more appropriate candidate for valve surgery. This review
Short-Term Outcomes
When TAVR was initially introduced, the 30-day mortality rate was 5% to 8%.1,11,16 This high mortality rate was a function of treating very ill patients and more invasive procedures with larger sheath sizes and routine use of general anesthesia, transesophageal echocardiography, pulmonary artery catheterization, and so on. Over time, however, this rate has gone down substantially, with the 30-day mortality rate in intermediate- and low-risk patients now ranging from 0.5% to 1%.8,17-19 Although this low mortality rate indicates that the vast majority of patients will survive to discharge from the hospital,
While 30 days is a better time frame for assessment because outcome is less impacted by differences in local post-acute care facilities, we explicitly did not create a parsimonious 30-day mortality model for clinical use due to concern that having such a model would allow for indirect comparisons with estimated risk of SAVR using the Society of Thoracic Surgeons risk model (http://riskcalc.sts.org/stswebriskcalc). It would be tempting to estimate a patient’s risk of mortality with the TAVR calculator and the SAVR calculator and use those risk estimates to
The decision of selecting SAVR over TAVR is typically driven by factors other than short- or long-term mortality (eg, whether TAVR will be covered by insurance, very young age and concern about durability, need to treat concomitant mitral regurgitation or aortopathy), as clinical trials have shown that survival and quality of life outcomes are at least as good with TAVR compared with SAVR.6,7,9,23 In fact, in an analysis that compared similar patients treated with TAVR versus SAVR and specifically looked for patient factors that might make one treatment preferable to the other, patients who had a prior cardiac operation and those on home oxygen were more likely to do better with TAVR, whereas no patient factors that favored SAVR were found.24 The majority of patients, however, were expected to have similar long-term outcomes regardless of treatment choice, and as such, the benefit of TAVR appears mostly to be an earlier and easier recovery.
Long-Term Outcomes: Estimating the Risk for Failure to Recover
While many patients who undergo TAVR are quite ill prior to the procedure, with substantial limitations due to the fatigue and shortness of breath associated with severe aortic stenosis, most patients recover well after the procedure, with marked improvement in symptoms and functional capacity. Approximately 25% to 35% of patients currently treated with TAVR commercially (ie, intermediate- and high-surgical-risk patients) either die or do not recover a reasonable quality of life after the procedure. Identifying those patients prior to the procedure can be challenging. We have previously developed and externally validated
Beyond clinical factors, frailty negatively impacts both survival and quality of life after TAVR. Frailty is a geriatric syndrome of impaired physiologic reserve and decreased resistance to stressors27 that is characterized by weakness, slowness, exhaustion, wasting, and low activity level. Across a wide variety of clinical situations (eg, pneumonia,28 myocardial infarction,29 general30,31 and cardiac surgery32,33), frailty increases the risk of morbidity and mortality after nearly any intervention34 or clinical insult, independent of traditional demographic and clinical risk factors. Frail patients often do better with less invasive interventions such as TAVR compared with traditional surgery, but nonetheless remain at increased risk for death35-37 or failure to recover quality of life and functional status25,37 after TAVR. However, there are unique challenges in both assessing and managing frailty in patients who are considered potential candidates for TAVR. One challenge is the lack of a laboratory or radiologic test for frailty; instead, the lack of physiologic reserve of frailty is identified through a combination of factors, such as slow gait speed, weak grip strength, and unintentional weight loss. While these factors readily identify frail patients in general elderly populations, in patients with severe symptomatic aortic stenosis, these metrics can be impacted by the disease process itself. This distinction is important as slow gait speed that is due to aortic stenosis will be “fixed” by TAVR, but slow gait speed from frailty would identify a patient who will have a difficult time recovering from the procedure. For example, in the CoreValve High Risk Pivotal Trial, 80% of patients had a slow gait speed and 67% had a weak grip strength,5 and yet 58% of patients in this trial were alive and with a reasonable quality of life 1 year after TAVR.6 A number of studies have attempted to define true frailty within the pre-TAVR population, that which represents decreased physiologic reserve and an impaired ability to recover from an insult, and the factors that appear to be most prognostically important are malnutrition38 or unintentional weight loss25 and the inability to be independent in activities of daily living (eg, dressing, feeding, transferring).25,37
Even with frailty assessments, the ability to predict who is or is not going to have a poor outcome after TAVR (ie, to use pre-procedural factors to identify patients who perhaps should not be offered TAVR because he or she will not recover from the procedure) is exceedingly difficult. The Table shows how to grossly estimate risk using the major factors that impact risk based on the more precise estimates from our models.25,26
The model shown in the Table can be used to estimate a patient’s risk for a poor outcome, but it should be noted that even at the extreme high end of risk, there will be some patients who still do well after TAVR. Furthermore, being high risk for a poor outcome after TAVR does not imply anything about how the patient would do without TAVR, as many of these patients would likely die even sooner or have worse quality of life with medical therapy only. However,
Conclusion
Calculating the risk of TAVR can be complicated. In patients who are electively treated using transfemoral access and a less invasive approach, the short-term risk of mortality is very low. Risk calculators can be used to estimate short-term risk, but the patients who are high risk for in-hospital mortality are often fairly easy to recognize, as the factors that drive that risk are not subtle (eg, the patient is in shock at the time of the procedure). The true risk of TAVR lies in the inability to recover from the procedure—being chronically ill, frail, or debilitated to a degree that the patient either dies or fails to recover a reasonable quality of life. Given
Corresponding author: Suzanne V. Arnold, MD, MHA, 4401 Wornall Rd., Kansas City, MO 64111.
Financial disclosures: This work was funded in part by grant K23HL116799 from the National Institutes of Health.
1. Leon MB, Smith CR, Mack M, et al. Transcatheter aortic-valve implantation for aortic stenosis in patients who cannot undergo surgery. N Engl J Med. 2010;363:1597-1607.
2. Reynolds MR, Magnuson EA, Lei Y, et al. Health-related quality of life after transcatheter aortic valve replacement in inoperable patients with severe aortic stenosis. Circulation. 2011;124(:1964-1972.
3. Smith CR, Leon MB, Mack MJ, et al. Transcatheter versus surgical aortic-valve replacement in high-risk patients. N Engl J Med. 2011;364:2187-2198.
4. Reynolds MR, Magnuson EA, Wang K, et al. Health-related quality of life after transcatheter or surgical aortic valve replacement in high-risk patients with severe aortic stenosis: results from the PARTNER (Placement of AoRTic TraNscathetER Valve) trial (Cohort A). J Am Coll Cardiol. 2012;60:548-558.
5. Adams DH, Popma JJ, Reardon MJ, et al. Transcatheter aortic-valve replacement with a self-expanding prosthesis. N Engl J Med. 2014;370:1790-1798.
6. Arnold SV, Reynolds MR, Wang K, et al. Health status after transcatheter or surgical aortic valve replacement in patients with severe aortic stenosis at increased surgical risk: results from the CoreValve US Pivotal trial. JACC Cardiovasc Interv. 2015;8:1207-1217.
7. Leon MB, Smith CR, Mack MJ, et al. Transcatheter or surgical aortic-valve replacement in intermediate-risk patients. N Engl J Med. 2016;374:1609-1620.
8. Reardon MJ, Van Mieghem NM, Popma JJ, et al. Surgical or transcatheter aortic-valve replacement in intermediate-risk patients. N Engl J Med. 2017;376:1321-1331.
9. Baron SJ, Arnold SV, Wang K, et al. Health status benefits of transcatheter vs surgical aortic valve replacement in patients with severe aortic stenosis at intermediate surgical risk: results from the PARTNER 2 randomized clinical trial. JAMA Cardiol. 2017;2:837-845.
10. Reynolds MR, Magnuson EA, Wang K, et al. Cost-effectiveness of transcatheter aortic valve replacement compared with standard care among inoperable patients with severe aortic stenosis: results from the placement of aortic transcatheter valves (PARTNER) trial (Cohort B). Circulation. 2012;125:1102-1109.
11. Popma JJ, Adams DH, Reardon MJ, et al. Transcatheter aortic valve replacement using a self-expanding bioprosthesis in patients with severe aortic stenosis at extreme risk for surgery. J Am Coll Cardiol. 2014;63:1972-1981.
12. Vahanian A, Alfieri O, Al-Attar N, et al. Transcatheter valve implantation for patients with aortic stenosis: a position statement from the European Association of Cardio-Thoracic Surgery (EACTS) and the European Society of Cardiology (ESC), in collaboration with the European Association of Percutaneous Cardiovascular Interventions (EAPCI). Eur Heart J. 2008;29:1463-1470.
13. Holmes DR Jr, Mack MJ, Kaul S, et al. 2012 ACCF/AATS/SCAI/STS expert consensus document on transcatheter aortic valve replacement. J Am Coll Cardiol. 2012;59:1200-1254.
14. US Food and Drug Administration. FDA Executive Summary: Edwards SAPIEN™ Transcatheter Heart Valve. Presented July 20, 2011, Gaithersburg, MD.
15. Centers for Medicare & Medicaid Services. Decision Memo for Transcatheter Aortic Valve Replacement (TAVR) (CAG-00430N). May 5, 2012.
16. Mack MJ, Brennan JM, Brindis R, et al. Outcomes following transcatheter aortic valve replacement in the United States. JAMA. 2013;310:2069-2077.
17. Thourani VH, Kodali S, Makkar RR, et al. Transcatheter aortic valve replacement versus surgical valve replacement in intermediate-risk patients: a propensity score analysis. Lancet. 2016;387:2218-2225.
18. Mack MJ, Leon MB, Thourani VH, et al. Transcatheter aortic-valve replacement with a balloon-expandable valve in low-risk patients. N Engl J Med. 2019;380:1695-1705.
19. Popma JJ, Deeb GM, Yakubov SJ, et al. Transcatheter aortic-valve replacement with a self-expanding valve in low-risk patients. N Engl J Med. 2019;380:1706-1715.
20. Edwards FH, Peterson ED, Coombs LP, et al. Prediction of operative mortality after valve replacement surgery. J Am Coll Cardiol. 2001;37:885-892.
21. Arnold SV, O’Brien SM, Vemulapalli S, et al. Inclusion of functional status measures in the risk adjustment of 30-day mortality after transcatheter aortic valve replacement: a report from the Society of Thoracic Surgeons/American College of Cardiology TVT Registry. JACC Cardiovasc Interv. 2018;11:581-589.
22. Brennan JM, Thomas L, Cohen DJ, et al. Transcatheter versus surgical aortic valve replacement: propensity-matched comparison. J Am Coll Cardiol. 2017;70:439-450.
23. Reardon MJ, Adams DH, Kleiman NS, et al. 2-year outcomes in patients undergoing surgical or self-expanding transcatheter aortic valve replacement. J Am Coll Cardiol. 2015;66:113-121.
24. Baron SJ, Cohen DJ, Suchindran S, et al. Development of a risk prediction model for 1-year mortality after surgical vs. transcatheter aortic valve replacement in patients with severe aortic stenosis. Circulation. 2016;134(A20166).
25. Arnold SV, Afilalo J, Spertus JA, et al. Prediction of poor outcome after transcatheter aortic valve replacement. J Am Coll Cardiol. 2016;68:1868-1877.
26. Arnold SV, Reynolds MR, Lei Y, et al. Predictors of poor outcomes after transcatheter aortic valve replacement: results from the PARTNER (Placement of Aortic Transcatheter Valve) trial. Circulation. 2014;129:2682-2690.
27. Fried LP, Hadley EC, Walston JD, et al. From bedside to bench: research agenda for frailty. Sci Aging Knowledge Environ. 2005;2005:pe24.
28. Torres OH, Munoz J, Ruiz D, et al. Outcome predictors of pneumonia in elderly patients: importance of functional assessment. J Am Geriatr Soc. 2004;52:1603-1609.
29. Ekerstad N, Swahn E, Janzon M, et al. Frailty is independently associated with short-term outcomes for elderly patients with non-ST-segment elevation myocardial infarction. Circulation. 2011;124:2397-2404.
30. Makary MA, Segev DL, Pronovost PJ, et al. Frailty as a predictor of surgical outcomes in older patients. J Am Coll Surg. 2010;210:901-908.
31. Hewitt J, Moug SJ, Middleton M, et al. Prevalence of frailty and its association with mortality in general surgery. Am J Surg. 2015;209:254-259.
32. Sundermann S, Dademasch A, Praetorius J, et al. Comprehensive assessment of frailty for elderly high-risk patients undergoing cardiac surgery. Eur J Cardiothorac Surg. 2011;39:33-37.
33. Afilalo J, Mottillo S, Eisenberg MJ, et al. Addition of frailty and disability to cardiac surgery risk scores identifies elderly patients at high risk of mortality or major morbidity. Circ Cardiovasc Qual Outcomes. 2012;5:222-228.
34. Lin HS, Watts JN, Peel NM, Hubbard RE. Frailty and post-operative outcomes in older surgical patients: a systematic review. BMC Geriatr. 2016;16:157.
35. Stortecky S, Schoenenberger AW, Moser A, et al. Evaluation of multidimensional geriatric assessment as a predictor of mortality and cardiovascular events after transcatheter aortic valve implantation. JACC Cardiovasc Interv. 2012;5:489-496.
36. Schoenenberger AW, Stortecky S, Neumann S, et al. Predictors of functional decline in elderly patients undergoing transcatheter aortic valve implantation (TAVI). Eur Heart J. 2013;34:684-689.
37. Green P, Arnold SV, Cohen DJ, et al. Relation of frailty to outcomes after transcatheter aortic valve replacement (from the PARTNER trial). Am J Cardiol. 2015;116:264-269.
38. Goldfarb M, Lauck S, Webb J, et al. Malnutrition and mortality in frail and non-frail older adults undergoing aortic valve replacement. Circulation. 2018;138:2202-2211.
1. Leon MB, Smith CR, Mack M, et al. Transcatheter aortic-valve implantation for aortic stenosis in patients who cannot undergo surgery. N Engl J Med. 2010;363:1597-1607.
2. Reynolds MR, Magnuson EA, Lei Y, et al. Health-related quality of life after transcatheter aortic valve replacement in inoperable patients with severe aortic stenosis. Circulation. 2011;124(:1964-1972.
3. Smith CR, Leon MB, Mack MJ, et al. Transcatheter versus surgical aortic-valve replacement in high-risk patients. N Engl J Med. 2011;364:2187-2198.
4. Reynolds MR, Magnuson EA, Wang K, et al. Health-related quality of life after transcatheter or surgical aortic valve replacement in high-risk patients with severe aortic stenosis: results from the PARTNER (Placement of AoRTic TraNscathetER Valve) trial (Cohort A). J Am Coll Cardiol. 2012;60:548-558.
5. Adams DH, Popma JJ, Reardon MJ, et al. Transcatheter aortic-valve replacement with a self-expanding prosthesis. N Engl J Med. 2014;370:1790-1798.
6. Arnold SV, Reynolds MR, Wang K, et al. Health status after transcatheter or surgical aortic valve replacement in patients with severe aortic stenosis at increased surgical risk: results from the CoreValve US Pivotal trial. JACC Cardiovasc Interv. 2015;8:1207-1217.
7. Leon MB, Smith CR, Mack MJ, et al. Transcatheter or surgical aortic-valve replacement in intermediate-risk patients. N Engl J Med. 2016;374:1609-1620.
8. Reardon MJ, Van Mieghem NM, Popma JJ, et al. Surgical or transcatheter aortic-valve replacement in intermediate-risk patients. N Engl J Med. 2017;376:1321-1331.
9. Baron SJ, Arnold SV, Wang K, et al. Health status benefits of transcatheter vs surgical aortic valve replacement in patients with severe aortic stenosis at intermediate surgical risk: results from the PARTNER 2 randomized clinical trial. JAMA Cardiol. 2017;2:837-845.
10. Reynolds MR, Magnuson EA, Wang K, et al. Cost-effectiveness of transcatheter aortic valve replacement compared with standard care among inoperable patients with severe aortic stenosis: results from the placement of aortic transcatheter valves (PARTNER) trial (Cohort B). Circulation. 2012;125:1102-1109.
11. Popma JJ, Adams DH, Reardon MJ, et al. Transcatheter aortic valve replacement using a self-expanding bioprosthesis in patients with severe aortic stenosis at extreme risk for surgery. J Am Coll Cardiol. 2014;63:1972-1981.
12. Vahanian A, Alfieri O, Al-Attar N, et al. Transcatheter valve implantation for patients with aortic stenosis: a position statement from the European Association of Cardio-Thoracic Surgery (EACTS) and the European Society of Cardiology (ESC), in collaboration with the European Association of Percutaneous Cardiovascular Interventions (EAPCI). Eur Heart J. 2008;29:1463-1470.
13. Holmes DR Jr, Mack MJ, Kaul S, et al. 2012 ACCF/AATS/SCAI/STS expert consensus document on transcatheter aortic valve replacement. J Am Coll Cardiol. 2012;59:1200-1254.
14. US Food and Drug Administration. FDA Executive Summary: Edwards SAPIEN™ Transcatheter Heart Valve. Presented July 20, 2011, Gaithersburg, MD.
15. Centers for Medicare & Medicaid Services. Decision Memo for Transcatheter Aortic Valve Replacement (TAVR) (CAG-00430N). May 5, 2012.
16. Mack MJ, Brennan JM, Brindis R, et al. Outcomes following transcatheter aortic valve replacement in the United States. JAMA. 2013;310:2069-2077.
17. Thourani VH, Kodali S, Makkar RR, et al. Transcatheter aortic valve replacement versus surgical valve replacement in intermediate-risk patients: a propensity score analysis. Lancet. 2016;387:2218-2225.
18. Mack MJ, Leon MB, Thourani VH, et al. Transcatheter aortic-valve replacement with a balloon-expandable valve in low-risk patients. N Engl J Med. 2019;380:1695-1705.
19. Popma JJ, Deeb GM, Yakubov SJ, et al. Transcatheter aortic-valve replacement with a self-expanding valve in low-risk patients. N Engl J Med. 2019;380:1706-1715.
20. Edwards FH, Peterson ED, Coombs LP, et al. Prediction of operative mortality after valve replacement surgery. J Am Coll Cardiol. 2001;37:885-892.
21. Arnold SV, O’Brien SM, Vemulapalli S, et al. Inclusion of functional status measures in the risk adjustment of 30-day mortality after transcatheter aortic valve replacement: a report from the Society of Thoracic Surgeons/American College of Cardiology TVT Registry. JACC Cardiovasc Interv. 2018;11:581-589.
22. Brennan JM, Thomas L, Cohen DJ, et al. Transcatheter versus surgical aortic valve replacement: propensity-matched comparison. J Am Coll Cardiol. 2017;70:439-450.
23. Reardon MJ, Adams DH, Kleiman NS, et al. 2-year outcomes in patients undergoing surgical or self-expanding transcatheter aortic valve replacement. J Am Coll Cardiol. 2015;66:113-121.
24. Baron SJ, Cohen DJ, Suchindran S, et al. Development of a risk prediction model for 1-year mortality after surgical vs. transcatheter aortic valve replacement in patients with severe aortic stenosis. Circulation. 2016;134(A20166).
25. Arnold SV, Afilalo J, Spertus JA, et al. Prediction of poor outcome after transcatheter aortic valve replacement. J Am Coll Cardiol. 2016;68:1868-1877.
26. Arnold SV, Reynolds MR, Lei Y, et al. Predictors of poor outcomes after transcatheter aortic valve replacement: results from the PARTNER (Placement of Aortic Transcatheter Valve) trial. Circulation. 2014;129:2682-2690.
27. Fried LP, Hadley EC, Walston JD, et al. From bedside to bench: research agenda for frailty. Sci Aging Knowledge Environ. 2005;2005:pe24.
28. Torres OH, Munoz J, Ruiz D, et al. Outcome predictors of pneumonia in elderly patients: importance of functional assessment. J Am Geriatr Soc. 2004;52:1603-1609.
29. Ekerstad N, Swahn E, Janzon M, et al. Frailty is independently associated with short-term outcomes for elderly patients with non-ST-segment elevation myocardial infarction. Circulation. 2011;124:2397-2404.
30. Makary MA, Segev DL, Pronovost PJ, et al. Frailty as a predictor of surgical outcomes in older patients. J Am Coll Surg. 2010;210:901-908.
31. Hewitt J, Moug SJ, Middleton M, et al. Prevalence of frailty and its association with mortality in general surgery. Am J Surg. 2015;209:254-259.
32. Sundermann S, Dademasch A, Praetorius J, et al. Comprehensive assessment of frailty for elderly high-risk patients undergoing cardiac surgery. Eur J Cardiothorac Surg. 2011;39:33-37.
33. Afilalo J, Mottillo S, Eisenberg MJ, et al. Addition of frailty and disability to cardiac surgery risk scores identifies elderly patients at high risk of mortality or major morbidity. Circ Cardiovasc Qual Outcomes. 2012;5:222-228.
34. Lin HS, Watts JN, Peel NM, Hubbard RE. Frailty and post-operative outcomes in older surgical patients: a systematic review. BMC Geriatr. 2016;16:157.
35. Stortecky S, Schoenenberger AW, Moser A, et al. Evaluation of multidimensional geriatric assessment as a predictor of mortality and cardiovascular events after transcatheter aortic valve implantation. JACC Cardiovasc Interv. 2012;5:489-496.
36. Schoenenberger AW, Stortecky S, Neumann S, et al. Predictors of functional decline in elderly patients undergoing transcatheter aortic valve implantation (TAVI). Eur Heart J. 2013;34:684-689.
37. Green P, Arnold SV, Cohen DJ, et al. Relation of frailty to outcomes after transcatheter aortic valve replacement (from the PARTNER trial). Am J Cardiol. 2015;116:264-269.
38. Goldfarb M, Lauck S, Webb J, et al. Malnutrition and mortality in frail and non-frail older adults undergoing aortic valve replacement. Circulation. 2018;138:2202-2211.