User login
Lu W, Chen W, Zhou Y, et al. A model to predict the prognosis of diffuse large B-cell lymphoma based on ultrasound images. Sci Rep. 2023;13(1):3346. doi:10.1038/s41598-023-30533-y
Leukemia - Chronic lymphocytic - CLL: statistics. Cancer.net. Published February 2023. Accessed January 24, 2024. https://www.cancer.net/cancer-types/leukemia-chronic-lymphocytic-cll/statistics
Harmanen M, Hujo M, Sund R, et al. Survival of patients with mantle cell lymphoma in the rituximab era: retrospective binational analysis between 2000 and 2020. Br J Haematol. 2023;201(1):64-74. doi:10.1111/bjh.18597
Romancik JT, Cohen JB. Management of older adults with mantle cell lymphoma. Drugs Aging. 2020;37(7):469-481. doi:10.1007/s40266-020-00765-y
Marginal zone lymphoma (MZL). Leukemia and Lymphoma Society. Accessed January 24, 2024. https://www.lls.org/research/marginal-zone-lymphoma-mzl
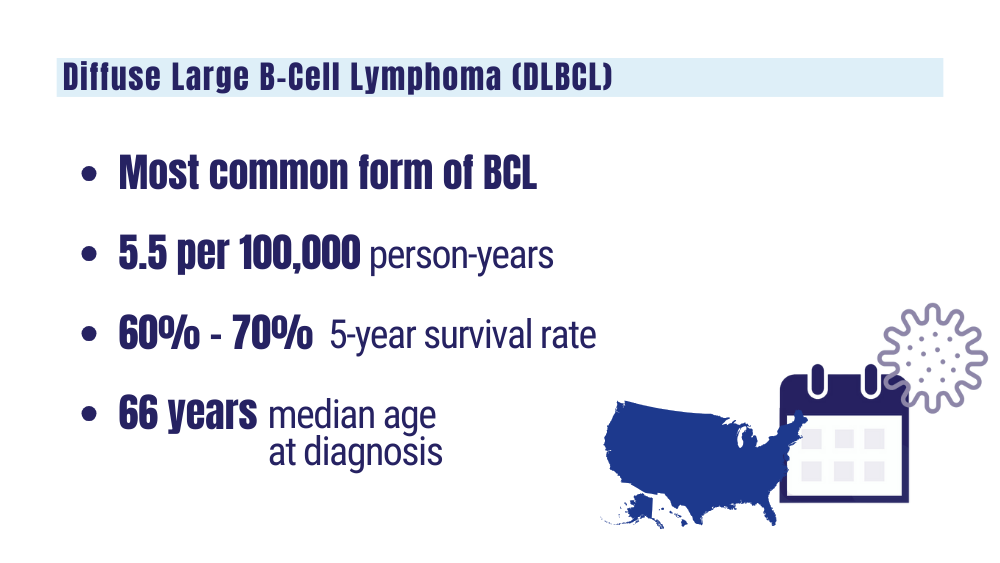
Understanding lymphoma: diffuse large B-cell lymphoma. Lymphoma Research Foundation fact sheet. Updated 2023. Accessed January 24, 2024 https://lymphoma.org/wp-content/uploads/2023/10/LRF_Understanding_Lymphoma_Diffuse_Large_B_Cell_Lymphoma_Fact_Sheet.pdf
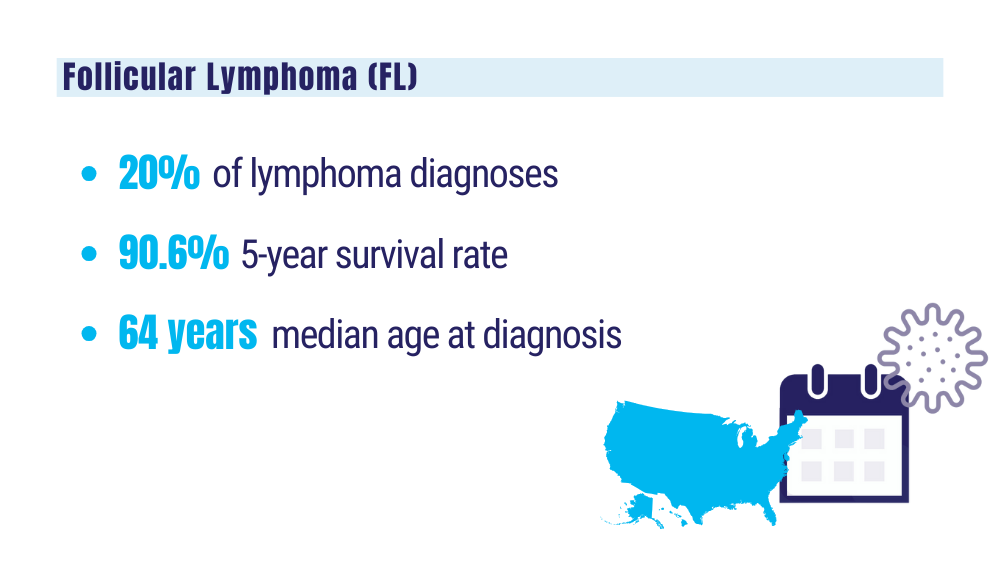
Key statistics for non-Hodgkin lymphoma. American Cancer Society. Updated January 17, 2024. Accessed January 24, 2024. https://www.cancer.org/cancer/types/non-hodgkin-lymphoma/about/key-statistics.html
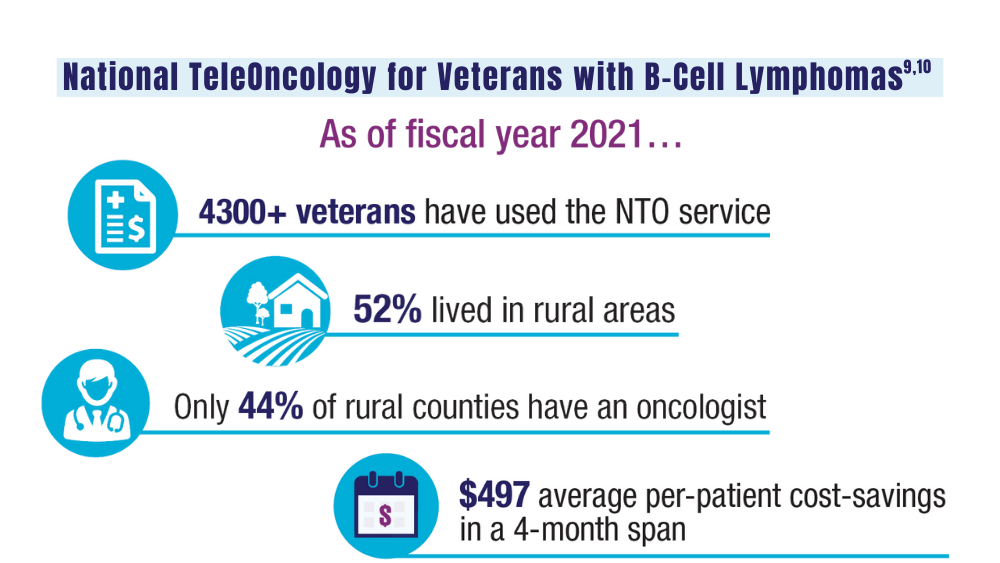
Zullig LL, Raska W, McWhirter G, et al. Veterans Health Administration National TeleOncology Service. JCO Oncol Pract. 2023;19(4):e504-e510. doi:10.1200/OP.22.00455
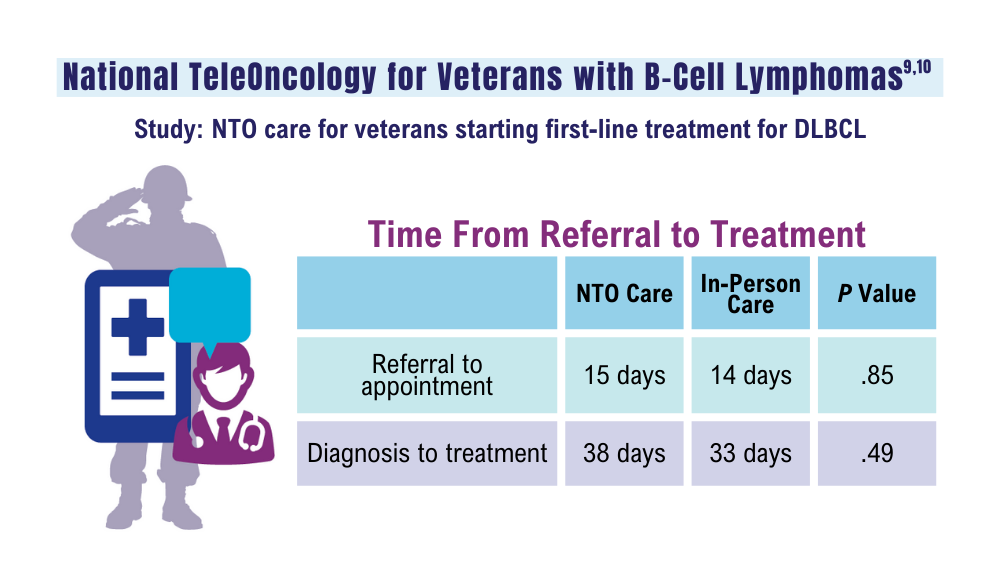
Lin C, Zhou KI, Burningham ZR, et al. Telemedicine-supervised cancer therapy for patients with an aggressive lymphoma and metastatic lung cancer in the U.S. Veterans Affairs National TeleOncology Service. J Clin Oncol. 2023;41(16 suppl)16:Abstract 1602. doi:10.1200/JCO.2023.41.16_suppl.1602
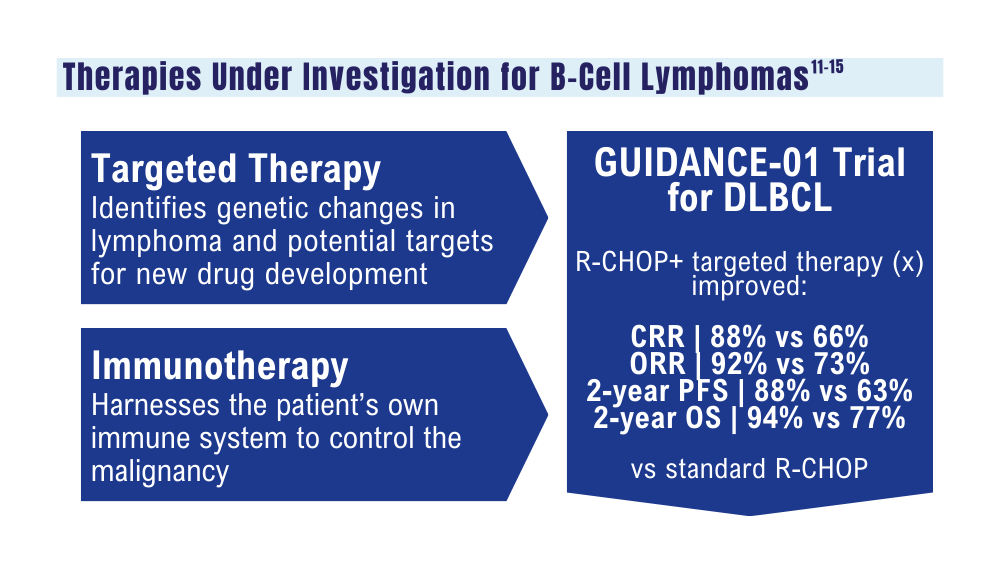
Zhang MC, Tian S, Fu D, et al. Genetic subtype-guided immunochemotherapy in diffuse large B-cell lymphoma: the randomized GUIDANCE-01 trial. Cancer Cell. 2023;41(10):1705-1716.e5. doi:10.1016/j.ccell.2023.09.004
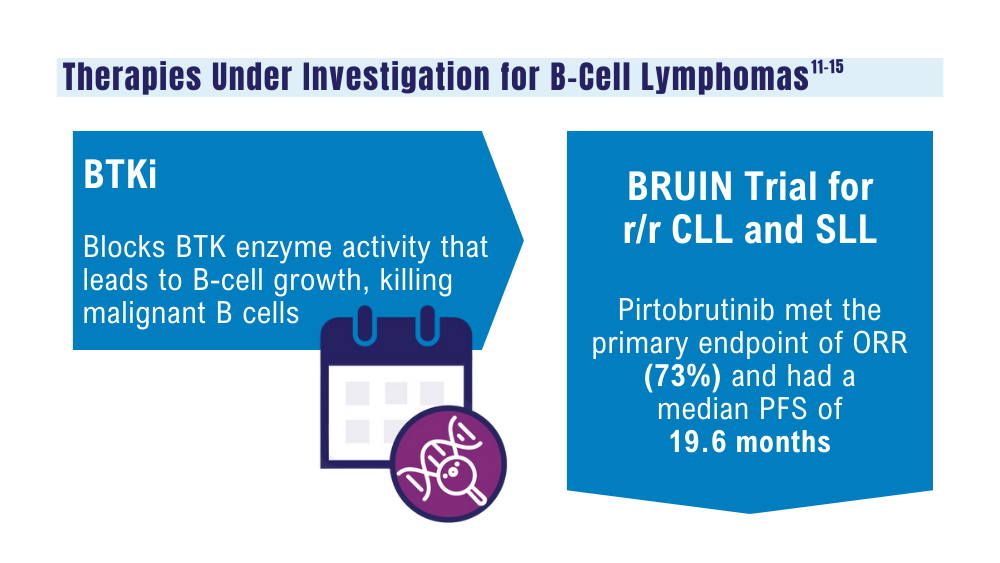
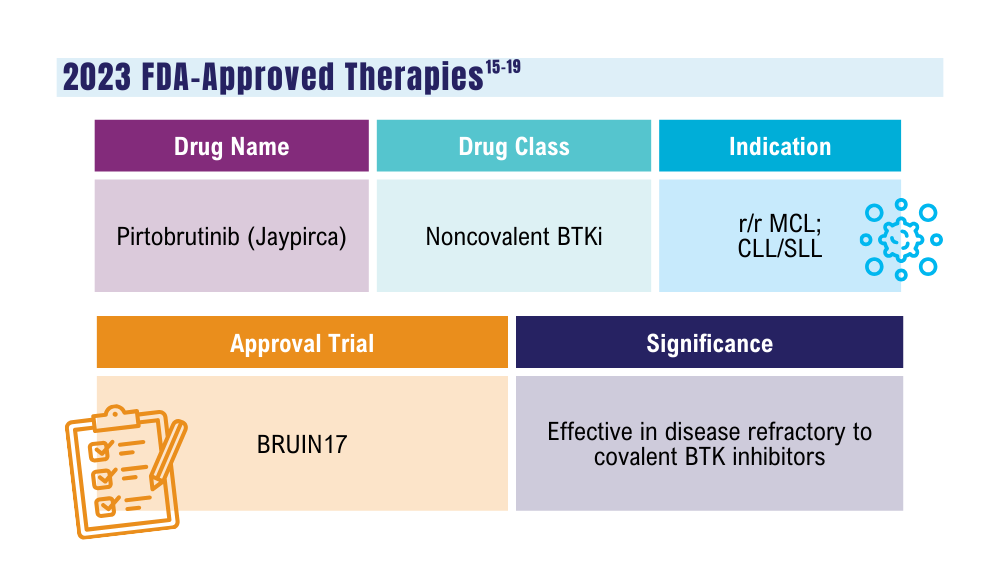
Mato AR, Woyach JA, Brown JR, et al. Pirtobrutinib after a covalent BTK inhibitor in chronic lymphocytic leukemia. N Engl J Med. 2023;389(1):33-44. doi:10.1056/NEJMoa2300696
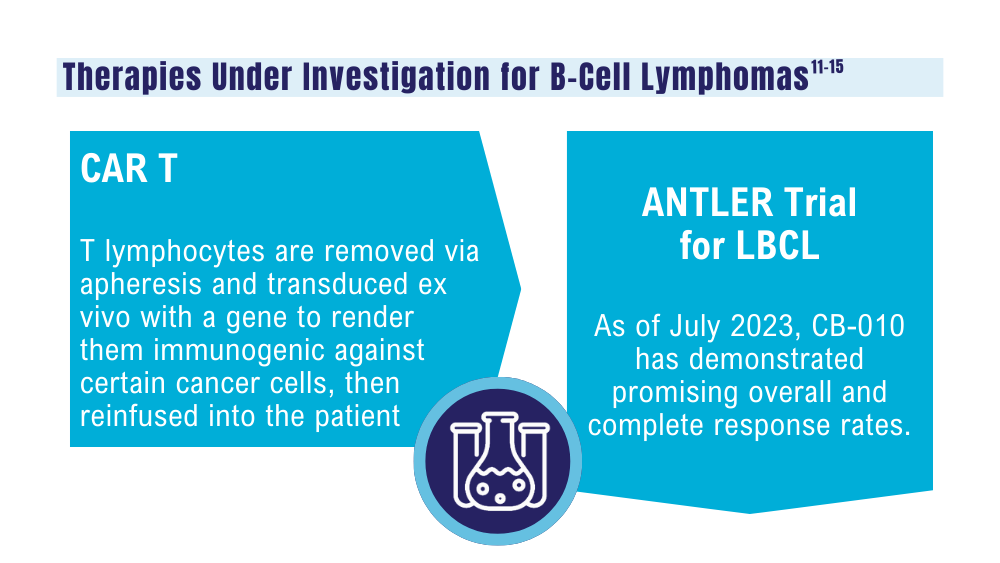
Sterner RC, Sterner RM. CAR-T cell therapy: current limitations and potential strategies. Blood Cancer J. 2021;11(4):69. doi:10.1038/s41408-021-00459-7
Caribou Biosciences reports positive clinical data from dose escalation of CB-010 ANTLER phase 1 trial in r/r B-NHL [news release]. Globalnewswire.com. Published July 13, 2023. Accessed January 24, 2024. https://www.globenewswire.com/news-release/2023/07/13/2704702/0/en/Caribou-Biosciences-Reports-Positive-Clinical-Data-from-Dose-Escalation-of-CB-010-ANTLER-Phase-1-Trial-in-r-r-B-NHL.html
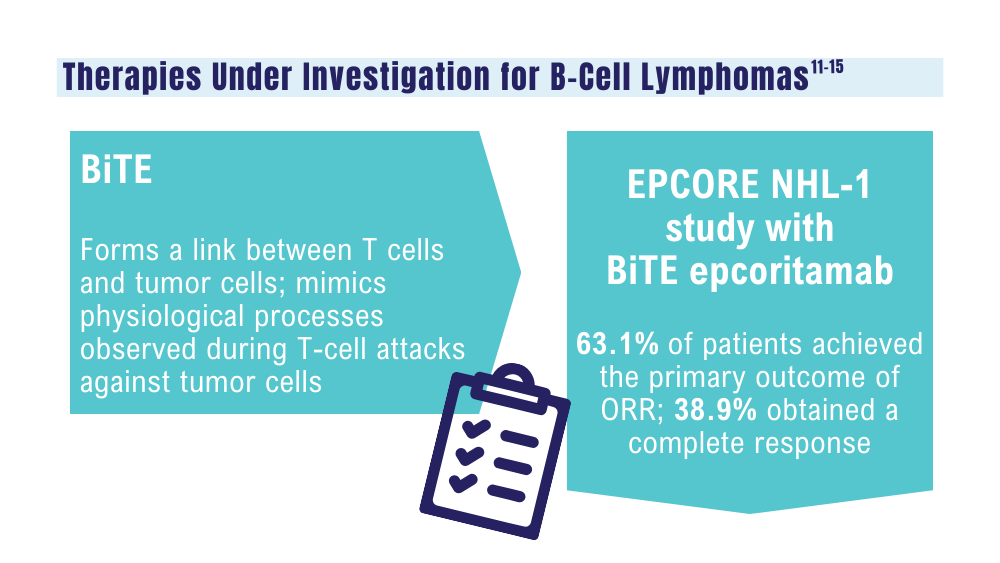
Ma J, Mo Y, Tang M, et al. Bispecific antibodies: from research to clinical application. Front Immunol. 2021;12:626616. doi:10.3389/fimmu.2021.626616
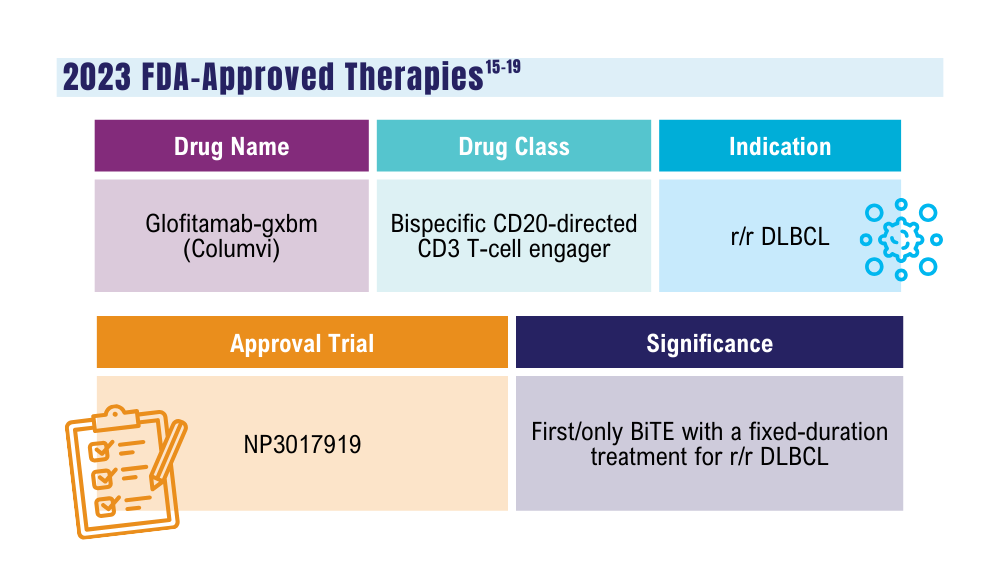
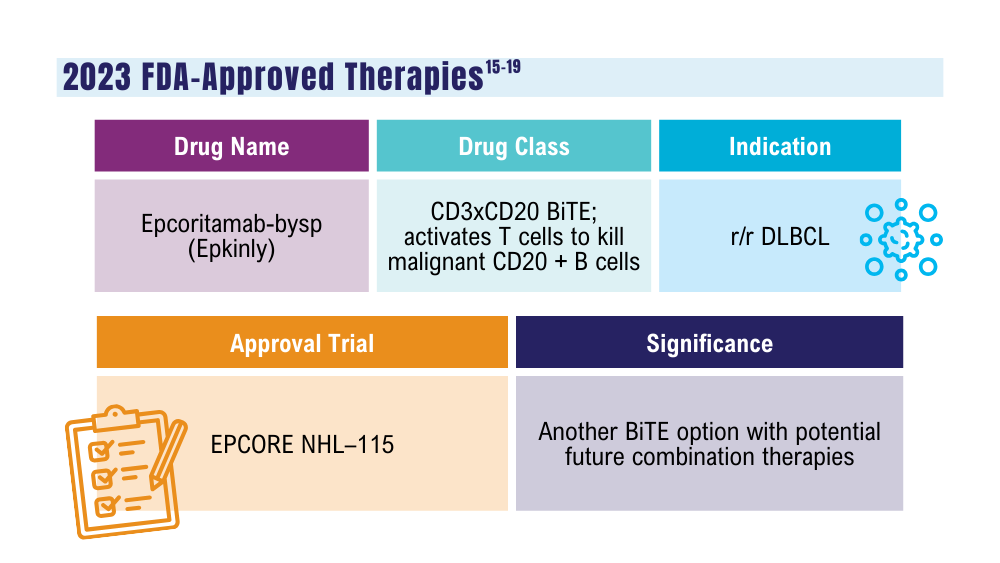
Davis JA, Granger K, Sakowski A, et al. Dual target dilemma: navigating epcoritamab vs. glofitamab in relapsed refractory diffuse large B-cell lymphoma. Expert Rev Hematol. 2023;16(12):915-918. doi:10.1080/17474086.2023.2285978
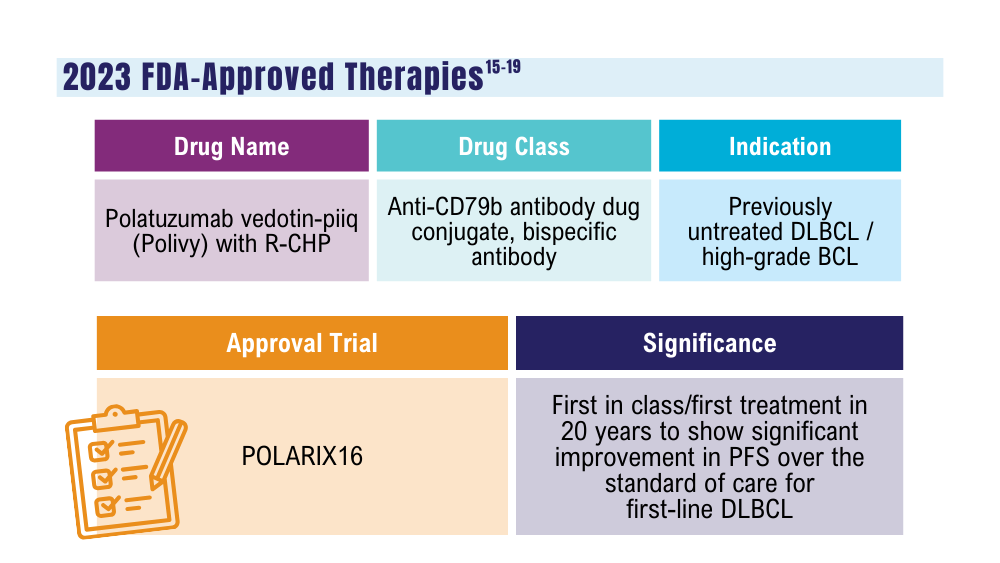
Lynch RC, Poh C, Ujjani CS, et al. Polatuzumab vedotin with infusional chemotherapy for untreated aggressive B-cell non-Hodgkin lymphomas. Blood Adv. 2023;7(11):2449-2458. doi:10.1182/bloodadvances.2022009145
Thompson PA, Tam CS. Pirtobrutinib: a new hope for patients with BTK inhibitor-refractory lymphoproliferative disorders. Blood. 2023;141(26):3137-3142. doi:10.1182/blood.2023020240
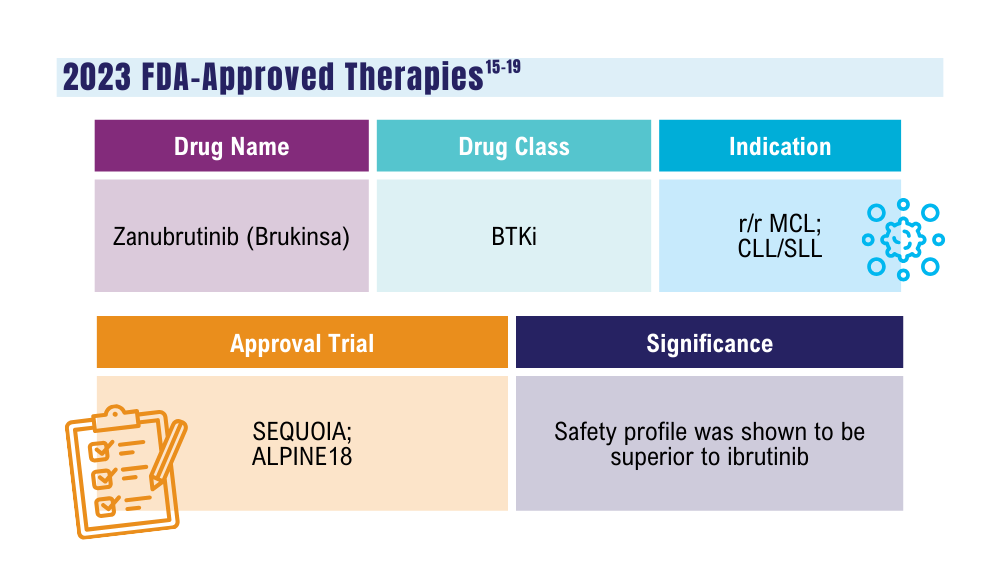
US Food and Drug Administration. FDA approves zanubrutinib for chronic lymphocytic leukemia or small lymphocytic lymphoma. Published January 19, 2023. Accessed January 24, 2024. https://www.fda.gov/drugs/resources-information-approved-drugs/fda-approves-zanubrutinib-chronic-lymphocytic-leukemia-or-small-lymphocytic-lymphoma
US Food and Drug Administration. FDA grants accelerated approval to glofitamab-gxbm for selected relapsed or refractory large B-cell lymphomas. Published June 16, 2023. Accessed January 24, 2024. https://www.fda.gov/drugs/drug-approvals-and-databases/fda-grants-accelerated-approval-glofitamab-gxbm-selected-relapsed-or-refractory-large-b-cell
Lu W, Chen W, Zhou Y, et al. A model to predict the prognosis of diffuse large B-cell lymphoma based on ultrasound images. Sci Rep. 2023;13(1):3346. doi:10.1038/s41598-023-30533-y
Leukemia - Chronic lymphocytic - CLL: statistics. Cancer.net. Published February 2023. Accessed January 24, 2024. https://www.cancer.net/cancer-types/leukemia-chronic-lymphocytic-cll/statistics
Harmanen M, Hujo M, Sund R, et al. Survival of patients with mantle cell lymphoma in the rituximab era: retrospective binational analysis between 2000 and 2020. Br J Haematol. 2023;201(1):64-74. doi:10.1111/bjh.18597
Romancik JT, Cohen JB. Management of older adults with mantle cell lymphoma. Drugs Aging. 2020;37(7):469-481. doi:10.1007/s40266-020-00765-y
Marginal zone lymphoma (MZL). Leukemia and Lymphoma Society. Accessed January 24, 2024. https://www.lls.org/research/marginal-zone-lymphoma-mzl
Understanding lymphoma: diffuse large B-cell lymphoma. Lymphoma Research Foundation fact sheet. Updated 2023. Accessed January 24, 2024 https://lymphoma.org/wp-content/uploads/2023/10/LRF_Understanding_Lymphoma_Diffuse_Large_B_Cell_Lymphoma_Fact_Sheet.pdf
Key statistics for non-Hodgkin lymphoma. American Cancer Society. Updated January 17, 2024. Accessed January 24, 2024. https://www.cancer.org/cancer/types/non-hodgkin-lymphoma/about/key-statistics.html
Zullig LL, Raska W, McWhirter G, et al. Veterans Health Administration National TeleOncology Service. JCO Oncol Pract. 2023;19(4):e504-e510. doi:10.1200/OP.22.00455
Lin C, Zhou KI, Burningham ZR, et al. Telemedicine-supervised cancer therapy for patients with an aggressive lymphoma and metastatic lung cancer in the U.S. Veterans Affairs National TeleOncology Service. J Clin Oncol. 2023;41(16 suppl)16:Abstract 1602. doi:10.1200/JCO.2023.41.16_suppl.1602
Zhang MC, Tian S, Fu D, et al. Genetic subtype-guided immunochemotherapy in diffuse large B-cell lymphoma: the randomized GUIDANCE-01 trial. Cancer Cell. 2023;41(10):1705-1716.e5. doi:10.1016/j.ccell.2023.09.004
Mato AR, Woyach JA, Brown JR, et al. Pirtobrutinib after a covalent BTK inhibitor in chronic lymphocytic leukemia. N Engl J Med. 2023;389(1):33-44. doi:10.1056/NEJMoa2300696
Sterner RC, Sterner RM. CAR-T cell therapy: current limitations and potential strategies. Blood Cancer J. 2021;11(4):69. doi:10.1038/s41408-021-00459-7
Caribou Biosciences reports positive clinical data from dose escalation of CB-010 ANTLER phase 1 trial in r/r B-NHL [news release]. Globalnewswire.com. Published July 13, 2023. Accessed January 24, 2024. https://www.globenewswire.com/news-release/2023/07/13/2704702/0/en/Caribou-Biosciences-Reports-Positive-Clinical-Data-from-Dose-Escalation-of-CB-010-ANTLER-Phase-1-Trial-in-r-r-B-NHL.html
Ma J, Mo Y, Tang M, et al. Bispecific antibodies: from research to clinical application. Front Immunol. 2021;12:626616. doi:10.3389/fimmu.2021.626616
Davis JA, Granger K, Sakowski A, et al. Dual target dilemma: navigating epcoritamab vs. glofitamab in relapsed refractory diffuse large B-cell lymphoma. Expert Rev Hematol. 2023;16(12):915-918. doi:10.1080/17474086.2023.2285978
Lynch RC, Poh C, Ujjani CS, et al. Polatuzumab vedotin with infusional chemotherapy for untreated aggressive B-cell non-Hodgkin lymphomas. Blood Adv. 2023;7(11):2449-2458. doi:10.1182/bloodadvances.2022009145
Thompson PA, Tam CS. Pirtobrutinib: a new hope for patients with BTK inhibitor-refractory lymphoproliferative disorders. Blood. 2023;141(26):3137-3142. doi:10.1182/blood.2023020240
US Food and Drug Administration. FDA approves zanubrutinib for chronic lymphocytic leukemia or small lymphocytic lymphoma. Published January 19, 2023. Accessed January 24, 2024. https://www.fda.gov/drugs/resources-information-approved-drugs/fda-approves-zanubrutinib-chronic-lymphocytic-leukemia-or-small-lymphocytic-lymphoma
US Food and Drug Administration. FDA grants accelerated approval to glofitamab-gxbm for selected relapsed or refractory large B-cell lymphomas. Published June 16, 2023. Accessed January 24, 2024. https://www.fda.gov/drugs/drug-approvals-and-databases/fda-grants-accelerated-approval-glofitamab-gxbm-selected-relapsed-or-refractory-large-b-cell
Lu W, Chen W, Zhou Y, et al. A model to predict the prognosis of diffuse large B-cell lymphoma based on ultrasound images. Sci Rep. 2023;13(1):3346. doi:10.1038/s41598-023-30533-y
Leukemia - Chronic lymphocytic - CLL: statistics. Cancer.net. Published February 2023. Accessed January 24, 2024. https://www.cancer.net/cancer-types/leukemia-chronic-lymphocytic-cll/statistics
Harmanen M, Hujo M, Sund R, et al. Survival of patients with mantle cell lymphoma in the rituximab era: retrospective binational analysis between 2000 and 2020. Br J Haematol. 2023;201(1):64-74. doi:10.1111/bjh.18597
Romancik JT, Cohen JB. Management of older adults with mantle cell lymphoma. Drugs Aging. 2020;37(7):469-481. doi:10.1007/s40266-020-00765-y
Marginal zone lymphoma (MZL). Leukemia and Lymphoma Society. Accessed January 24, 2024. https://www.lls.org/research/marginal-zone-lymphoma-mzl
Understanding lymphoma: diffuse large B-cell lymphoma. Lymphoma Research Foundation fact sheet. Updated 2023. Accessed January 24, 2024 https://lymphoma.org/wp-content/uploads/2023/10/LRF_Understanding_Lymphoma_Diffuse_Large_B_Cell_Lymphoma_Fact_Sheet.pdf
Key statistics for non-Hodgkin lymphoma. American Cancer Society. Updated January 17, 2024. Accessed January 24, 2024. https://www.cancer.org/cancer/types/non-hodgkin-lymphoma/about/key-statistics.html
Zullig LL, Raska W, McWhirter G, et al. Veterans Health Administration National TeleOncology Service. JCO Oncol Pract. 2023;19(4):e504-e510. doi:10.1200/OP.22.00455
Lin C, Zhou KI, Burningham ZR, et al. Telemedicine-supervised cancer therapy for patients with an aggressive lymphoma and metastatic lung cancer in the U.S. Veterans Affairs National TeleOncology Service. J Clin Oncol. 2023;41(16 suppl)16:Abstract 1602. doi:10.1200/JCO.2023.41.16_suppl.1602
Zhang MC, Tian S, Fu D, et al. Genetic subtype-guided immunochemotherapy in diffuse large B-cell lymphoma: the randomized GUIDANCE-01 trial. Cancer Cell. 2023;41(10):1705-1716.e5. doi:10.1016/j.ccell.2023.09.004
Mato AR, Woyach JA, Brown JR, et al. Pirtobrutinib after a covalent BTK inhibitor in chronic lymphocytic leukemia. N Engl J Med. 2023;389(1):33-44. doi:10.1056/NEJMoa2300696
Sterner RC, Sterner RM. CAR-T cell therapy: current limitations and potential strategies. Blood Cancer J. 2021;11(4):69. doi:10.1038/s41408-021-00459-7
Caribou Biosciences reports positive clinical data from dose escalation of CB-010 ANTLER phase 1 trial in r/r B-NHL [news release]. Globalnewswire.com. Published July 13, 2023. Accessed January 24, 2024. https://www.globenewswire.com/news-release/2023/07/13/2704702/0/en/Caribou-Biosciences-Reports-Positive-Clinical-Data-from-Dose-Escalation-of-CB-010-ANTLER-Phase-1-Trial-in-r-r-B-NHL.html
Ma J, Mo Y, Tang M, et al. Bispecific antibodies: from research to clinical application. Front Immunol. 2021;12:626616. doi:10.3389/fimmu.2021.626616
Davis JA, Granger K, Sakowski A, et al. Dual target dilemma: navigating epcoritamab vs. glofitamab in relapsed refractory diffuse large B-cell lymphoma. Expert Rev Hematol. 2023;16(12):915-918. doi:10.1080/17474086.2023.2285978
Lynch RC, Poh C, Ujjani CS, et al. Polatuzumab vedotin with infusional chemotherapy for untreated aggressive B-cell non-Hodgkin lymphomas. Blood Adv. 2023;7(11):2449-2458. doi:10.1182/bloodadvances.2022009145
Thompson PA, Tam CS. Pirtobrutinib: a new hope for patients with BTK inhibitor-refractory lymphoproliferative disorders. Blood. 2023;141(26):3137-3142. doi:10.1182/blood.2023020240
US Food and Drug Administration. FDA approves zanubrutinib for chronic lymphocytic leukemia or small lymphocytic lymphoma. Published January 19, 2023. Accessed January 24, 2024. https://www.fda.gov/drugs/resources-information-approved-drugs/fda-approves-zanubrutinib-chronic-lymphocytic-leukemia-or-small-lymphocytic-lymphoma
US Food and Drug Administration. FDA grants accelerated approval to glofitamab-gxbm for selected relapsed or refractory large B-cell lymphomas. Published June 16, 2023. Accessed January 24, 2024. https://www.fda.gov/drugs/drug-approvals-and-databases/fda-grants-accelerated-approval-glofitamab-gxbm-selected-relapsed-or-refractory-large-b-cell