User login
In her presentation at The North American Menopause Society (NAMS) 2021 annual meeting (September 22–25, 2021, in Washington, DC), Dr. Holly J. Pederson offered her expert perspectives on breast cancer prevention in at-risk women in “Chemoprevention for risk reduction: Women’s health clinicians have a role.”
Which patients would benefit from chemoprevention?
Holly J. Pederson, MD: Obviously, women with significant family history are at risk. And approximately 10% of biopsies that are done for other reasons incidentally show atypical hyperplasia (AH) or lobular carcinoma in situ (LCIS)—which are not precancers or cancers but are markers for the development of the disease—and they markedly increase risk. Atypical hyperplasia confers a 30% risk for developing breast cancer over the next 25 years, and LCIS is associated with up to a 2% per year risk. In this setting, preventive medication has been shown to cut risk by 56% to 86%; this is a targeted population that is often overlooked.
Mathematical risk models can be used to assess risk by assessing women’s risk factors. The United States Preventive Services Task Force (USPSTF) has set forth a threshold at which they believe the benefits outweigh the risks of preventive medications. That threshold is 3% or greater over the next 5 years using the Gail breast cancer risk assessment tool.1 The American Society of Clinical Oncology (ASCO) uses the Tyrer-Cuzick breast cancer risk evaluation model with a threshold of 5% over the next 10 years.2 In general, those are the situations in which chemoprevention is a no-brainer.
Certain genetic mutations also predispose to estrogen-sensitive breast cancer. While preventive medications specifically have not been studied in large groups of gene carriers, chemoprevention makes sense because these medications prevent estrogen-sensitive breast cancers that those patients are prone to. Examples would be patients with ATM and CHEK2 gene mutations, which are very common, and patients with BRCA2 and even BRCA1 variants in the postmenopausal years. Those are the big targets.
Risk assessment models
Dr. Pederson: Yes, I almost exclusively use the Tyrer-Cuzick risk model, version 8, which incorporates breast density. This model is intimidating to some practitioners initially, but once you get used to it, you can complete it very quickly.
The Gail model is very limited. It assesses only first-degree relatives, so you don’t get the paternal information at all, and you don’t use age at diagnosis, family structure, genetic testing, results of breast density, or body mass index (BMI). There are many limitations of the Gail model, but most people use it because it is so easy and they are familiar with it.
Possibly the best model is the CanRisk tool, which incorporates the Breast and Ovarian Analysis of Disease Incidence and Carrier Estimation Algorithm (BOADICEA), but it takes too much time to use in clinic; it’s too complicated. The Tyrer-Cuzick model is easy to use once you get used to it.
Dr. Pederson: Risk doesn’t always need to be formally calculated, which can be time-consuming. It’s one of those situations where most practitioners know it when they see it. Benign atypical biopsies, a strong family history, or, obviously, the presence of a genetic mutation are huge red flags.
If a practitioner has a nearby high-risk center where they can refer patients, that can be so useful, even for a one-time consultation to guide management. For example, with the virtual world now, I do a lot of consultations for patients and outline a plan, and then the referring practitioner can carry out the plan with confidence and then send the patient back periodically. There are so many more options now that previously did not exist for the busy ObGyn or primary care provider to rely on.
Continue to: Chemoprevention uptake in at-risk women...
Chemoprevention uptake in at-risk women
Dr. Pederson: We really never practice medicine using numbers. We use clinical judgment, and we use relationships with patients in terms of developing confidence and trust. I think that the uptake that we exhibit in our center probably is more based on the patients’ perception that we are confident in our recommendations. I think that many practitioners simply are not comfortable with explaining medications, explaining and managing adverse effects, and using alternative medications. While the modeling helps, I think the personal expertise really makes the difference.
Going forward, the addition of the polygenic risk score to the mathematical risk models is going to make a big difference. Right now, the mathematical risk model is simply that: it takes the traditional risk factors that a patient has and spits out a number. But adding the patient’s genomic data—that is, a weighted summation of SNPs, or single nucleotide polymorphisms, now numbering over 300 for breast cancer—can explain more about their personalized risk, which is going to be more powerful in influencing a woman to take medication or not to take medication, in my opinion. Knowing their actual genomic risk will be a big step forward in individualized risk stratification and increased medication uptake as well as vigilance with high risk screening and attention to diet, exercise, and drinking alcohol in moderation.
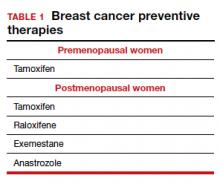
Dr. Pederson: The only drug that can be used in the premenopausal setting is tamoxifen (TABLE 1). Women can’t take it if they are pregnant, planning to become pregnant, or if they don’t use a reliable form of birth control because it is teratogenic. Women also cannot take tamoxifen if they have had a history of blood clots, stroke, or transient ischemic attack; if they are on warfarin or estrogen preparations; or if they have had atypical endometrial biopsies or endometrial cancer. Those are the absolute contraindications for tamoxifen use.
Tamoxifen is generally very well tolerated in most women; some women experience hot flashes and night sweats that often will subside (or become tolerable) over the first 90 days. In addition, some women experience vaginal discharge rather than dryness, but it is not as bothersome to patients as dryness can be.
Tamoxifen can be used in the pre- or postmenopausal setting. In healthy premenopausal women, there’s no increased risk of the serious adverse effects that are seen with tamoxifen use in postmenopausal women, such as the 1% risk of blood clots and the 1% risk of endometrial cancer.
In postmenopausal women who still have their uterus, I’ll preferentially use raloxifene over tamoxifen. If they don’t have their uterus, tamoxifen is slightly more effective than the raloxifene, and I’ll use that.
Tamoxifen and raloxifene are both selective estrogen receptor modulators, or SERMs, which means that they stimulate receptors in some tissues, like bone, keeping bones strong, and block the receptors in other tissues, like the breast, reducing risk. And so you get kind of a two-for-one in terms of breast cancer risk reduction and osteoporosis prevention.
Another class of preventive drugs is the aromatase inhibitors (AIs). They block the enzyme aromatase, which converts androgens to estrogens peripherally; that is, the androgens that are produced primarily in the adrenal gland, but in part in postmenopausal ovaries.
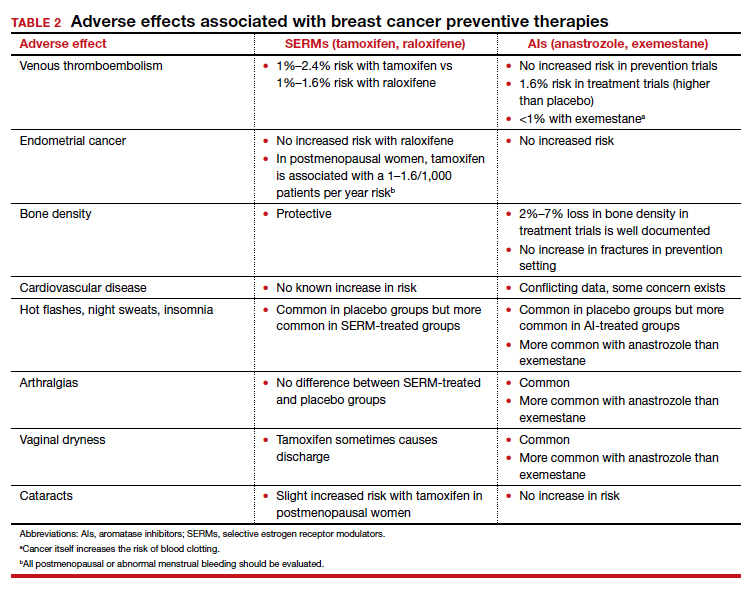
In general, AIs are less well tolerated. There are generally more hot flashes and night sweats, and more vaginal dryness than with the SERMs. Anastrozole use is associated with arthralgias; and with exemestane use, there can be some hair loss (TABLE 2). Relative contraindications to SERMs become more important in the postmenopausal setting because of the increased frequency of both blood clots and uterine cancer in the postmenopausal years. I won’t give it to smokers. I won’t give tamoxifen to smokers in the premenopausal period either. With obese women, care must be taken because of the risk of blood clots with the SERMS, so then I’ll resort to the AIs. In the postmenopausal setting, you have to think a lot harder about the choices you use for preventive medication. Preferentially, I’ll use the SERMS if possible as they have fewer adverse effects.
Dr. Pederson: All of them are recommended to be given for 5 years, but the MAP.3 trial, which studied exemestane compared with placebo, showed a 65% risk reduction with 3 years of therapy.3 So occasionally, we’ll use 3 years of therapy. Why the treatment recommendation is universally 5 years is unclear, given that the trial with that particular drug was done in 3 years. And with low-dose tamoxifen, the recommended duration is 3 years. That study was done in Italy with 5 mg daily for 3 years.4 In the United States we use 10 mg every other day for 3 years because the 5-mg tablet is not available here.
Continue to: Counseling points...
Counseling points
Dr. Pederson: Patients’ fears about adverse effects are often worse than the adverse effects themselves. Women will fester over, Should I take it? Should I take it possibly for years? And then they take the medication and they tell me, “I don’t even notice that I’m taking it, and I know I’m being proactive.” The majority of patients who take these medications don’t have a lot of significant adverse effects.
Severe hot flashes can be managed in a number of ways, primarily and most effectively with certain antidepressants. Oxybutynin use is another good way to manage vasomotor symptoms. Sometimes we use local vaginal estrogen if a patient has vaginal dryness. In general, however, I would say at least 80% of my patients who take preventive medications do not require management of adverse side effects, that they are tolerable.
I counsel women this way, “Don’t think of this as a 5-year course of medication. Think of it as a 90-day trial, and let’s see how you do. If you hate it, then we don’t do it.” They often are pleasantly surprised that the medication is much easier to tolerate than they thought it would be.
Dr. Pederson: It would be neat if a trial would directly compare lifestyle interventions with medications, because probably lifestyle change is as effective as medication is—but we don’t know that and probably will never have that data. We do know that alcohol consumption, every drink per day, increases risk by 10%. We know that obesity is responsible for 30% of breast cancers in this country, and that hormone replacement probably is overrated as a significant risk factor. Updated data from the Women’s Health Initiative study suggest that hormone replacement may actually reduce both breast cancer and cardiovascular risk in women in their 50s, but that’s in average-risk women and not in high-risk women, so we can’t generalize. We do recommend lifestyle measures including weight loss, exercise, and limiting alcohol consumption for all of our patients and certainly for our high-risk patients.
The only 2 things a woman can do to reduce the risk of triple negative breast cancer are to achieve and maintain ideal body weight and to breastfeed. The medications that I have mentioned don’t reduce the risk of triple negative breast cancer. Staying thin and breastfeeding do. It’s a problem in this country because at least 35% of all women and 58% of Black women are obese in America, and Black women tend to be prone to triple-negative breast cancer. That’s a real public health issue that we need to address. If we were going to focus on one thing, it would be focusing on obesity in terms of risk reduction.
Final thoughts
Dr. Pederson: I would like to direct attention to the American Heart Association scientific statement published at the end of 2020 that reported that hormone replacement in average-risk women reduced both cardiovascular events and overall mortality in women in their 50s by 30%.5 While that’s not directly related to what we are talking about, we need to weigh the pros and cons of estrogen versus estrogen blockade in women in terms of breast cancer risk management discussions. Part of shared decision making now needs to include cardiovascular risk factors and how estrogen is going to play into that.
In women with atypical hyperplasia or LCIS, they may benefit from the preventive medications we discussed. But in women with family history or in women with genetic mutations who have not had benign atypical biopsies, they may choose to consider estrogen during their 50s and perhaps take tamoxifen either beforehand or raloxifene afterward.
We need to look at patients holistically and consider all their risk factors together. We can’t look at one dimension alone.
- US Preventive Services Task Force. Medication use to reduce risk of breast cancer: US Preventive Services Task Force Recommendation Statement. JAMA. 2019;322:857-867.
- Visvanathan K, Fabian CJ, Bantug E, et al. Use of endocrine therapy for breast cancer risk reduction: ASCO clinical practice guideline update. J Clin Oncol. 2019;37:3152-3165.
- Goss PE, Ingle JN, Alex-Martinez JE, et al. Exemestane for breast-cancer prevention in postmenopausal women. N Engl J Med. 2011;364:2381-2391.
- DeCensi A, Puntoni M, Guerrieri-Gonzaga A, et al. Randomized placebo controlled trial of low-dose tamoxifen to prevent local and contralateral recurrence in breast intraepithelial neoplasia. J Clin Oncol. 2019;37:1629-1637.
- El Khoudary SR, Aggarwal B, Beckie TM, et al; American Heart Association Prevention Science Committee of the Council on Epidemiology and Prevention, and Council on Cardiovascular and Stroke Nursing. Menopause transition and cardiovascular disease risk: implications for timing of early prevention: a scientific statement from the American Heart Association. Circulation. 2020;142:e506-e532.
In her presentation at The North American Menopause Society (NAMS) 2021 annual meeting (September 22–25, 2021, in Washington, DC), Dr. Holly J. Pederson offered her expert perspectives on breast cancer prevention in at-risk women in “Chemoprevention for risk reduction: Women’s health clinicians have a role.”
Which patients would benefit from chemoprevention?
Holly J. Pederson, MD: Obviously, women with significant family history are at risk. And approximately 10% of biopsies that are done for other reasons incidentally show atypical hyperplasia (AH) or lobular carcinoma in situ (LCIS)—which are not precancers or cancers but are markers for the development of the disease—and they markedly increase risk. Atypical hyperplasia confers a 30% risk for developing breast cancer over the next 25 years, and LCIS is associated with up to a 2% per year risk. In this setting, preventive medication has been shown to cut risk by 56% to 86%; this is a targeted population that is often overlooked.
Mathematical risk models can be used to assess risk by assessing women’s risk factors. The United States Preventive Services Task Force (USPSTF) has set forth a threshold at which they believe the benefits outweigh the risks of preventive medications. That threshold is 3% or greater over the next 5 years using the Gail breast cancer risk assessment tool.1 The American Society of Clinical Oncology (ASCO) uses the Tyrer-Cuzick breast cancer risk evaluation model with a threshold of 5% over the next 10 years.2 In general, those are the situations in which chemoprevention is a no-brainer.
Certain genetic mutations also predispose to estrogen-sensitive breast cancer. While preventive medications specifically have not been studied in large groups of gene carriers, chemoprevention makes sense because these medications prevent estrogen-sensitive breast cancers that those patients are prone to. Examples would be patients with ATM and CHEK2 gene mutations, which are very common, and patients with BRCA2 and even BRCA1 variants in the postmenopausal years. Those are the big targets.
Risk assessment models
Dr. Pederson: Yes, I almost exclusively use the Tyrer-Cuzick risk model, version 8, which incorporates breast density. This model is intimidating to some practitioners initially, but once you get used to it, you can complete it very quickly.
The Gail model is very limited. It assesses only first-degree relatives, so you don’t get the paternal information at all, and you don’t use age at diagnosis, family structure, genetic testing, results of breast density, or body mass index (BMI). There are many limitations of the Gail model, but most people use it because it is so easy and they are familiar with it.
Possibly the best model is the CanRisk tool, which incorporates the Breast and Ovarian Analysis of Disease Incidence and Carrier Estimation Algorithm (BOADICEA), but it takes too much time to use in clinic; it’s too complicated. The Tyrer-Cuzick model is easy to use once you get used to it.
Dr. Pederson: Risk doesn’t always need to be formally calculated, which can be time-consuming. It’s one of those situations where most practitioners know it when they see it. Benign atypical biopsies, a strong family history, or, obviously, the presence of a genetic mutation are huge red flags.
If a practitioner has a nearby high-risk center where they can refer patients, that can be so useful, even for a one-time consultation to guide management. For example, with the virtual world now, I do a lot of consultations for patients and outline a plan, and then the referring practitioner can carry out the plan with confidence and then send the patient back periodically. There are so many more options now that previously did not exist for the busy ObGyn or primary care provider to rely on.
Continue to: Chemoprevention uptake in at-risk women...
Chemoprevention uptake in at-risk women
Dr. Pederson: We really never practice medicine using numbers. We use clinical judgment, and we use relationships with patients in terms of developing confidence and trust. I think that the uptake that we exhibit in our center probably is more based on the patients’ perception that we are confident in our recommendations. I think that many practitioners simply are not comfortable with explaining medications, explaining and managing adverse effects, and using alternative medications. While the modeling helps, I think the personal expertise really makes the difference.
Going forward, the addition of the polygenic risk score to the mathematical risk models is going to make a big difference. Right now, the mathematical risk model is simply that: it takes the traditional risk factors that a patient has and spits out a number. But adding the patient’s genomic data—that is, a weighted summation of SNPs, or single nucleotide polymorphisms, now numbering over 300 for breast cancer—can explain more about their personalized risk, which is going to be more powerful in influencing a woman to take medication or not to take medication, in my opinion. Knowing their actual genomic risk will be a big step forward in individualized risk stratification and increased medication uptake as well as vigilance with high risk screening and attention to diet, exercise, and drinking alcohol in moderation.
Dr. Pederson: The only drug that can be used in the premenopausal setting is tamoxifen (TABLE 1). Women can’t take it if they are pregnant, planning to become pregnant, or if they don’t use a reliable form of birth control because it is teratogenic. Women also cannot take tamoxifen if they have had a history of blood clots, stroke, or transient ischemic attack; if they are on warfarin or estrogen preparations; or if they have had atypical endometrial biopsies or endometrial cancer. Those are the absolute contraindications for tamoxifen use.
Tamoxifen is generally very well tolerated in most women; some women experience hot flashes and night sweats that often will subside (or become tolerable) over the first 90 days. In addition, some women experience vaginal discharge rather than dryness, but it is not as bothersome to patients as dryness can be.
Tamoxifen can be used in the pre- or postmenopausal setting. In healthy premenopausal women, there’s no increased risk of the serious adverse effects that are seen with tamoxifen use in postmenopausal women, such as the 1% risk of blood clots and the 1% risk of endometrial cancer.
In postmenopausal women who still have their uterus, I’ll preferentially use raloxifene over tamoxifen. If they don’t have their uterus, tamoxifen is slightly more effective than the raloxifene, and I’ll use that.
Tamoxifen and raloxifene are both selective estrogen receptor modulators, or SERMs, which means that they stimulate receptors in some tissues, like bone, keeping bones strong, and block the receptors in other tissues, like the breast, reducing risk. And so you get kind of a two-for-one in terms of breast cancer risk reduction and osteoporosis prevention.
Another class of preventive drugs is the aromatase inhibitors (AIs). They block the enzyme aromatase, which converts androgens to estrogens peripherally; that is, the androgens that are produced primarily in the adrenal gland, but in part in postmenopausal ovaries.
In general, AIs are less well tolerated. There are generally more hot flashes and night sweats, and more vaginal dryness than with the SERMs. Anastrozole use is associated with arthralgias; and with exemestane use, there can be some hair loss (TABLE 2). Relative contraindications to SERMs become more important in the postmenopausal setting because of the increased frequency of both blood clots and uterine cancer in the postmenopausal years. I won’t give it to smokers. I won’t give tamoxifen to smokers in the premenopausal period either. With obese women, care must be taken because of the risk of blood clots with the SERMS, so then I’ll resort to the AIs. In the postmenopausal setting, you have to think a lot harder about the choices you use for preventive medication. Preferentially, I’ll use the SERMS if possible as they have fewer adverse effects.

Dr. Pederson: All of them are recommended to be given for 5 years, but the MAP.3 trial, which studied exemestane compared with placebo, showed a 65% risk reduction with 3 years of therapy.3 So occasionally, we’ll use 3 years of therapy. Why the treatment recommendation is universally 5 years is unclear, given that the trial with that particular drug was done in 3 years. And with low-dose tamoxifen, the recommended duration is 3 years. That study was done in Italy with 5 mg daily for 3 years.4 In the United States we use 10 mg every other day for 3 years because the 5-mg tablet is not available here.
Continue to: Counseling points...
Counseling points
Dr. Pederson: Patients’ fears about adverse effects are often worse than the adverse effects themselves. Women will fester over, Should I take it? Should I take it possibly for years? And then they take the medication and they tell me, “I don’t even notice that I’m taking it, and I know I’m being proactive.” The majority of patients who take these medications don’t have a lot of significant adverse effects.
Severe hot flashes can be managed in a number of ways, primarily and most effectively with certain antidepressants. Oxybutynin use is another good way to manage vasomotor symptoms. Sometimes we use local vaginal estrogen if a patient has vaginal dryness. In general, however, I would say at least 80% of my patients who take preventive medications do not require management of adverse side effects, that they are tolerable.
I counsel women this way, “Don’t think of this as a 5-year course of medication. Think of it as a 90-day trial, and let’s see how you do. If you hate it, then we don’t do it.” They often are pleasantly surprised that the medication is much easier to tolerate than they thought it would be.
Dr. Pederson: It would be neat if a trial would directly compare lifestyle interventions with medications, because probably lifestyle change is as effective as medication is—but we don’t know that and probably will never have that data. We do know that alcohol consumption, every drink per day, increases risk by 10%. We know that obesity is responsible for 30% of breast cancers in this country, and that hormone replacement probably is overrated as a significant risk factor. Updated data from the Women’s Health Initiative study suggest that hormone replacement may actually reduce both breast cancer and cardiovascular risk in women in their 50s, but that’s in average-risk women and not in high-risk women, so we can’t generalize. We do recommend lifestyle measures including weight loss, exercise, and limiting alcohol consumption for all of our patients and certainly for our high-risk patients.
The only 2 things a woman can do to reduce the risk of triple negative breast cancer are to achieve and maintain ideal body weight and to breastfeed. The medications that I have mentioned don’t reduce the risk of triple negative breast cancer. Staying thin and breastfeeding do. It’s a problem in this country because at least 35% of all women and 58% of Black women are obese in America, and Black women tend to be prone to triple-negative breast cancer. That’s a real public health issue that we need to address. If we were going to focus on one thing, it would be focusing on obesity in terms of risk reduction.
Final thoughts
Dr. Pederson: I would like to direct attention to the American Heart Association scientific statement published at the end of 2020 that reported that hormone replacement in average-risk women reduced both cardiovascular events and overall mortality in women in their 50s by 30%.5 While that’s not directly related to what we are talking about, we need to weigh the pros and cons of estrogen versus estrogen blockade in women in terms of breast cancer risk management discussions. Part of shared decision making now needs to include cardiovascular risk factors and how estrogen is going to play into that.
In women with atypical hyperplasia or LCIS, they may benefit from the preventive medications we discussed. But in women with family history or in women with genetic mutations who have not had benign atypical biopsies, they may choose to consider estrogen during their 50s and perhaps take tamoxifen either beforehand or raloxifene afterward.
We need to look at patients holistically and consider all their risk factors together. We can’t look at one dimension alone.
In her presentation at The North American Menopause Society (NAMS) 2021 annual meeting (September 22–25, 2021, in Washington, DC), Dr. Holly J. Pederson offered her expert perspectives on breast cancer prevention in at-risk women in “Chemoprevention for risk reduction: Women’s health clinicians have a role.”
Which patients would benefit from chemoprevention?
Holly J. Pederson, MD: Obviously, women with significant family history are at risk. And approximately 10% of biopsies that are done for other reasons incidentally show atypical hyperplasia (AH) or lobular carcinoma in situ (LCIS)—which are not precancers or cancers but are markers for the development of the disease—and they markedly increase risk. Atypical hyperplasia confers a 30% risk for developing breast cancer over the next 25 years, and LCIS is associated with up to a 2% per year risk. In this setting, preventive medication has been shown to cut risk by 56% to 86%; this is a targeted population that is often overlooked.
Mathematical risk models can be used to assess risk by assessing women’s risk factors. The United States Preventive Services Task Force (USPSTF) has set forth a threshold at which they believe the benefits outweigh the risks of preventive medications. That threshold is 3% or greater over the next 5 years using the Gail breast cancer risk assessment tool.1 The American Society of Clinical Oncology (ASCO) uses the Tyrer-Cuzick breast cancer risk evaluation model with a threshold of 5% over the next 10 years.2 In general, those are the situations in which chemoprevention is a no-brainer.
Certain genetic mutations also predispose to estrogen-sensitive breast cancer. While preventive medications specifically have not been studied in large groups of gene carriers, chemoprevention makes sense because these medications prevent estrogen-sensitive breast cancers that those patients are prone to. Examples would be patients with ATM and CHEK2 gene mutations, which are very common, and patients with BRCA2 and even BRCA1 variants in the postmenopausal years. Those are the big targets.
Risk assessment models
Dr. Pederson: Yes, I almost exclusively use the Tyrer-Cuzick risk model, version 8, which incorporates breast density. This model is intimidating to some practitioners initially, but once you get used to it, you can complete it very quickly.
The Gail model is very limited. It assesses only first-degree relatives, so you don’t get the paternal information at all, and you don’t use age at diagnosis, family structure, genetic testing, results of breast density, or body mass index (BMI). There are many limitations of the Gail model, but most people use it because it is so easy and they are familiar with it.
Possibly the best model is the CanRisk tool, which incorporates the Breast and Ovarian Analysis of Disease Incidence and Carrier Estimation Algorithm (BOADICEA), but it takes too much time to use in clinic; it’s too complicated. The Tyrer-Cuzick model is easy to use once you get used to it.
Dr. Pederson: Risk doesn’t always need to be formally calculated, which can be time-consuming. It’s one of those situations where most practitioners know it when they see it. Benign atypical biopsies, a strong family history, or, obviously, the presence of a genetic mutation are huge red flags.
If a practitioner has a nearby high-risk center where they can refer patients, that can be so useful, even for a one-time consultation to guide management. For example, with the virtual world now, I do a lot of consultations for patients and outline a plan, and then the referring practitioner can carry out the plan with confidence and then send the patient back periodically. There are so many more options now that previously did not exist for the busy ObGyn or primary care provider to rely on.
Continue to: Chemoprevention uptake in at-risk women...
Chemoprevention uptake in at-risk women
Dr. Pederson: We really never practice medicine using numbers. We use clinical judgment, and we use relationships with patients in terms of developing confidence and trust. I think that the uptake that we exhibit in our center probably is more based on the patients’ perception that we are confident in our recommendations. I think that many practitioners simply are not comfortable with explaining medications, explaining and managing adverse effects, and using alternative medications. While the modeling helps, I think the personal expertise really makes the difference.
Going forward, the addition of the polygenic risk score to the mathematical risk models is going to make a big difference. Right now, the mathematical risk model is simply that: it takes the traditional risk factors that a patient has and spits out a number. But adding the patient’s genomic data—that is, a weighted summation of SNPs, or single nucleotide polymorphisms, now numbering over 300 for breast cancer—can explain more about their personalized risk, which is going to be more powerful in influencing a woman to take medication or not to take medication, in my opinion. Knowing their actual genomic risk will be a big step forward in individualized risk stratification and increased medication uptake as well as vigilance with high risk screening and attention to diet, exercise, and drinking alcohol in moderation.
Dr. Pederson: The only drug that can be used in the premenopausal setting is tamoxifen (TABLE 1). Women can’t take it if they are pregnant, planning to become pregnant, or if they don’t use a reliable form of birth control because it is teratogenic. Women also cannot take tamoxifen if they have had a history of blood clots, stroke, or transient ischemic attack; if they are on warfarin or estrogen preparations; or if they have had atypical endometrial biopsies or endometrial cancer. Those are the absolute contraindications for tamoxifen use.
Tamoxifen is generally very well tolerated in most women; some women experience hot flashes and night sweats that often will subside (or become tolerable) over the first 90 days. In addition, some women experience vaginal discharge rather than dryness, but it is not as bothersome to patients as dryness can be.
Tamoxifen can be used in the pre- or postmenopausal setting. In healthy premenopausal women, there’s no increased risk of the serious adverse effects that are seen with tamoxifen use in postmenopausal women, such as the 1% risk of blood clots and the 1% risk of endometrial cancer.
In postmenopausal women who still have their uterus, I’ll preferentially use raloxifene over tamoxifen. If they don’t have their uterus, tamoxifen is slightly more effective than the raloxifene, and I’ll use that.
Tamoxifen and raloxifene are both selective estrogen receptor modulators, or SERMs, which means that they stimulate receptors in some tissues, like bone, keeping bones strong, and block the receptors in other tissues, like the breast, reducing risk. And so you get kind of a two-for-one in terms of breast cancer risk reduction and osteoporosis prevention.
Another class of preventive drugs is the aromatase inhibitors (AIs). They block the enzyme aromatase, which converts androgens to estrogens peripherally; that is, the androgens that are produced primarily in the adrenal gland, but in part in postmenopausal ovaries.
In general, AIs are less well tolerated. There are generally more hot flashes and night sweats, and more vaginal dryness than with the SERMs. Anastrozole use is associated with arthralgias; and with exemestane use, there can be some hair loss (TABLE 2). Relative contraindications to SERMs become more important in the postmenopausal setting because of the increased frequency of both blood clots and uterine cancer in the postmenopausal years. I won’t give it to smokers. I won’t give tamoxifen to smokers in the premenopausal period either. With obese women, care must be taken because of the risk of blood clots with the SERMS, so then I’ll resort to the AIs. In the postmenopausal setting, you have to think a lot harder about the choices you use for preventive medication. Preferentially, I’ll use the SERMS if possible as they have fewer adverse effects.

Dr. Pederson: All of them are recommended to be given for 5 years, but the MAP.3 trial, which studied exemestane compared with placebo, showed a 65% risk reduction with 3 years of therapy.3 So occasionally, we’ll use 3 years of therapy. Why the treatment recommendation is universally 5 years is unclear, given that the trial with that particular drug was done in 3 years. And with low-dose tamoxifen, the recommended duration is 3 years. That study was done in Italy with 5 mg daily for 3 years.4 In the United States we use 10 mg every other day for 3 years because the 5-mg tablet is not available here.
Continue to: Counseling points...
Counseling points
Dr. Pederson: Patients’ fears about adverse effects are often worse than the adverse effects themselves. Women will fester over, Should I take it? Should I take it possibly for years? And then they take the medication and they tell me, “I don’t even notice that I’m taking it, and I know I’m being proactive.” The majority of patients who take these medications don’t have a lot of significant adverse effects.
Severe hot flashes can be managed in a number of ways, primarily and most effectively with certain antidepressants. Oxybutynin use is another good way to manage vasomotor symptoms. Sometimes we use local vaginal estrogen if a patient has vaginal dryness. In general, however, I would say at least 80% of my patients who take preventive medications do not require management of adverse side effects, that they are tolerable.
I counsel women this way, “Don’t think of this as a 5-year course of medication. Think of it as a 90-day trial, and let’s see how you do. If you hate it, then we don’t do it.” They often are pleasantly surprised that the medication is much easier to tolerate than they thought it would be.
Dr. Pederson: It would be neat if a trial would directly compare lifestyle interventions with medications, because probably lifestyle change is as effective as medication is—but we don’t know that and probably will never have that data. We do know that alcohol consumption, every drink per day, increases risk by 10%. We know that obesity is responsible for 30% of breast cancers in this country, and that hormone replacement probably is overrated as a significant risk factor. Updated data from the Women’s Health Initiative study suggest that hormone replacement may actually reduce both breast cancer and cardiovascular risk in women in their 50s, but that’s in average-risk women and not in high-risk women, so we can’t generalize. We do recommend lifestyle measures including weight loss, exercise, and limiting alcohol consumption for all of our patients and certainly for our high-risk patients.
The only 2 things a woman can do to reduce the risk of triple negative breast cancer are to achieve and maintain ideal body weight and to breastfeed. The medications that I have mentioned don’t reduce the risk of triple negative breast cancer. Staying thin and breastfeeding do. It’s a problem in this country because at least 35% of all women and 58% of Black women are obese in America, and Black women tend to be prone to triple-negative breast cancer. That’s a real public health issue that we need to address. If we were going to focus on one thing, it would be focusing on obesity in terms of risk reduction.
Final thoughts
Dr. Pederson: I would like to direct attention to the American Heart Association scientific statement published at the end of 2020 that reported that hormone replacement in average-risk women reduced both cardiovascular events and overall mortality in women in their 50s by 30%.5 While that’s not directly related to what we are talking about, we need to weigh the pros and cons of estrogen versus estrogen blockade in women in terms of breast cancer risk management discussions. Part of shared decision making now needs to include cardiovascular risk factors and how estrogen is going to play into that.
In women with atypical hyperplasia or LCIS, they may benefit from the preventive medications we discussed. But in women with family history or in women with genetic mutations who have not had benign atypical biopsies, they may choose to consider estrogen during their 50s and perhaps take tamoxifen either beforehand or raloxifene afterward.
We need to look at patients holistically and consider all their risk factors together. We can’t look at one dimension alone.
- US Preventive Services Task Force. Medication use to reduce risk of breast cancer: US Preventive Services Task Force Recommendation Statement. JAMA. 2019;322:857-867.
- Visvanathan K, Fabian CJ, Bantug E, et al. Use of endocrine therapy for breast cancer risk reduction: ASCO clinical practice guideline update. J Clin Oncol. 2019;37:3152-3165.
- Goss PE, Ingle JN, Alex-Martinez JE, et al. Exemestane for breast-cancer prevention in postmenopausal women. N Engl J Med. 2011;364:2381-2391.
- DeCensi A, Puntoni M, Guerrieri-Gonzaga A, et al. Randomized placebo controlled trial of low-dose tamoxifen to prevent local and contralateral recurrence in breast intraepithelial neoplasia. J Clin Oncol. 2019;37:1629-1637.
- El Khoudary SR, Aggarwal B, Beckie TM, et al; American Heart Association Prevention Science Committee of the Council on Epidemiology and Prevention, and Council on Cardiovascular and Stroke Nursing. Menopause transition and cardiovascular disease risk: implications for timing of early prevention: a scientific statement from the American Heart Association. Circulation. 2020;142:e506-e532.
- US Preventive Services Task Force. Medication use to reduce risk of breast cancer: US Preventive Services Task Force Recommendation Statement. JAMA. 2019;322:857-867.
- Visvanathan K, Fabian CJ, Bantug E, et al. Use of endocrine therapy for breast cancer risk reduction: ASCO clinical practice guideline update. J Clin Oncol. 2019;37:3152-3165.
- Goss PE, Ingle JN, Alex-Martinez JE, et al. Exemestane for breast-cancer prevention in postmenopausal women. N Engl J Med. 2011;364:2381-2391.
- DeCensi A, Puntoni M, Guerrieri-Gonzaga A, et al. Randomized placebo controlled trial of low-dose tamoxifen to prevent local and contralateral recurrence in breast intraepithelial neoplasia. J Clin Oncol. 2019;37:1629-1637.
- El Khoudary SR, Aggarwal B, Beckie TM, et al; American Heart Association Prevention Science Committee of the Council on Epidemiology and Prevention, and Council on Cardiovascular and Stroke Nursing. Menopause transition and cardiovascular disease risk: implications for timing of early prevention: a scientific statement from the American Heart Association. Circulation. 2020;142:e506-e532.