User login
Vaccinations have substantially reduced morbidity and mortality from many infectious diseases. Despite the clear value of vaccinations in public health, efforts to better understand adverse events (AEs) following immunization are important to sustain public trust and vaccine confidence. Noninfectious inflammation of the heart may manifest as myocarditis or pericarditis, or occasionally, with shared signs and symptoms of each, as myopericarditis. This is a rare AE following some immunizations. Vaccine-associated myocarditis, pericarditis, or myopericarditis (VAMP) has been most clearly associated with smallpox vaccines and mRNA COVID-19 vaccines.1-6 Although extremely rare, VAMP also has been associated with other vaccines.7,8 Limited information exists to guide shared clinical decision making on COVID-19 vaccination in persons with a history of VAMP. It is unknown whether individuals with a history of VAMP are at higher risk for developing a recurrence or experiencing a more severe outcome following COVID-19 vaccination.
Methods
As part of the collaborative public health mission with the Centers for Disease Control and Prevention (CDC) for enhanced vaccine AE surveillance, the Defense Health Agency Immunization Healthcare Division (IHD) maintains a clinical database of service members and beneficiaries referred for suspected AEs following immunizations. A review of all AEs following immunization cases in this database from January 1, 2003, through February 28, 2022, identified individuals meeting the following criteria: (a) VAMP prior to receipt of COVID-19 vaccine; (b) receipt of COVID-19 vaccine in 2021; and (c) medical documentation in available electronic health records sufficient to describe health status at least 30 days following COVID-19 vaccination.9 If medical entries suggested cardiac symptoms following a COVID-19 vaccine, additional information was sought to verify VAMP based on current published criteria.10,11 Both the initial VAMP cases and the suspected COVID-19 VAMP cases were adjudicated by a team of vaccine experts and specialists in immunology, cardiology, and preventive medicine.
This retrospective review was approved and conducted in accordance with the Walter Reed National Military Medical Center Institutional Review Board protocol #20664. All individuals with recurrent VAMP consented to share their health records and clinical details.
Results
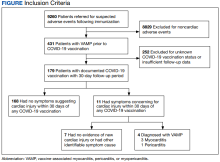
Among 9260 cases in the IHD database, 431 met the case definition for VAMP.
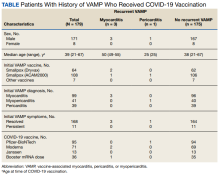
Among the 179 patients included in this analysis, 171 (96%) were male. Their median age was 39 years at the time of COVID-19 vaccination.
Within 1 month of receipt of any COVID-19 vaccine, 11 individuals had documented symptoms suggesting cardiac involvement, specifically, chest pain, palpitations, or dyspnea. After cardiac evaluation, 4 patients met the criteria for VAMP after COVID-19 vaccination.10,11 Seven patients either did not meet the criteria for VAMP or had alternative causes for their symptoms.
Two men aged 49 and 50 years with a history of vaccine-associated myocarditis following smallpox vaccination (Dryvax and ACAM2000) developed myocarditis 3 days after their second dose of the Moderna vaccine. One of these patients received a Pfizer-BioNTech booster 10 months later with no recurrence of symptoms. A 55-year-old man with a history of vaccine-associated myocarditis following Dryvax vaccination developed myocarditis 2 days after his Pfizer-BioNTech booster. None of the patients who developed post-COVID-19 VAMP reported residual symptoms from their initial VAMP episode, which occurred 12 to 18 years earlier. All were hospitalized briefly for observation and had complete symptom resolution within 6 weeks.
A 25-year-old man developed pericarditis 4 days after his second Pfizer-BioNTech vaccination. His previous ACAM2000 vaccine-associated myocarditis occurred 3 years earlier, with no residual symptoms. Of note, he had a mild COVID-19 infection 78 days before the onset of his pericarditis. After the onset of his COVID-19 vaccine-associated pericarditis, he continued to experience transient bouts of chest pressure and exertional dyspnea that resolved within 7 months of onset.
The median interval between COVID-19 vaccine doses in those who developed post-COVID-19 VAMP was within the recommended mRNA vaccine dosing intervals of 3 to 4 weeks and was consistent with the median mRNA vaccine dosing intervals among the entire cohort.
Due to the small cohort size and other limitations of this study, the suggested rate of cardiac injury in this review (4 cases in 179 persons, or 2.2%) is an imprecise estimate of risk in a small population (95% CI, 0.1%-4.4%). While this rate may seem higher than expected within the general population after COVID-19 vaccination, it is lower than the estimated lifetime risk of recurrent myocarditis from any cause.6,12
Discussion
To our knowledge, this is the first report describing cardiac outcomes after COVID-19 vaccination among a cohort of individuals with prior history of VAMP. Four cases of COVID-19 VAMP were identified among 179 patients with previous VAMP. All cases had experienced VAMP after the smallpox vaccine several years earlier, with complete resolution of symptoms. Three cases presented with recurrent VAMP after their second dose of an mRNA COVID-19 vaccine, and one after an mRNA booster dose. All fully recovered over the course of several months.
Myocarditis is a heterogeneous inflammatory injury with diverse, sometimes idiopathic, etiologies.13 In contrast to infection-related cardiac injury, prior reports of vaccine-associated myocarditis have suggested a hypersensitivity reaction characterized by patchy eosinophilic infiltrates, a benign clinical course, and good prognosis.2,3
There are several common features between VAMP after smallpox and COVID-19 vaccination. Cases occur predominantly in young men. The onset of symptoms after smallpox vaccine (mean, 10 days) and after mRNA COVID-19 vaccine (mean, 3 days) appears to correspond to the timing of peak postvaccination pro-inflammatory cytokine elevation.14 While all VAMP cases are serious events, the majority of patients appear to have a relatively benign clinical course with rapid and full recovery.13
Patients who have experienced an inflammatory cardiac injury may be at higher risk for recurrence, but quantifying risk of this rare phenomenon is challenging. Cases of VAMP after the COVID-19 vaccine have occasionally been reported in patients with previous cardiac injury unrelated to vaccination.15-17 The cases presented here represent the first report of recurrent VAMP following prior non-COVID-19 vaccinations.
Most patients with prior VAMP in this cohort did not experience cardiac-suggestive symptoms following COVID-19 vaccination. Among 11 patients who developed symptoms, 3 had confirmed myocarditis and 1 had confirmed pericarditis. The clinical course for these patients with recurrent VAMP was observed to be no different in severity or duration from those who experience new-onset VAMP.4 All other patients not meeting criteria for VAMP or having alternative explanations for their symptoms also had a benign clinical course. Nonetheless, of the study cohort of 179, recurrent VAMP was diagnosed in 4 of the 11 who developed cardiac-suggestive symptoms following COVID-19 vaccination. The importance of cardiac evaluation should be emphasized for any patient presenting with chest pain, dyspnea, or other cardiac-suggestive symptoms following vaccination.
Strengths and Limitations
The strength of this review of VAMP recurrence associated with COVID-19 vaccination derives from our large and unique longitudinal database of VAMP among current and prior service members. Additionally, the IHD’s ongoing enhanced vaccine AEs surveillance provides the opportunity to contact patients and review their electronic health records over an extended interval of time.
When interpreting this report’s implications, limitations inherent to any retrospective case review should be considered. The cohort of cases of prior VAMP included primarily healthy, fit, young service members; this population is not representative of the general population. The cohort included prior VAMP cases that generally occurred after smallpox vaccination. Experiences after smallpox vaccine may not apply to cardiac injury from other vaccines or etiologies. By the nature of this review, the population studied at the time of COVID-19 vaccination was somewhat older than those most likely to develop an initial bout of VAMP.2 This review was limited by information available in the electronic health records of a small number of patients. Subclinical cases of VAMP and cases without adequate clinical evaluation also could not be included.
Conclusions
Noninfectious inflammation of the heart (myocarditis, pericarditis, or myopericarditis) is a rare AE following certain vaccines, especially live replicating smallpox vaccine and mRNA COVID-19 vaccines. In this observational analysis, the majority of patients with previous VAMP successfully received a COVID-19 vaccine without recurrence. The 4 patients who were identified with recurrent VAMP following COVID-19 vaccination all recovered with supportive care. While the CDC endorses that individuals with a history of infectious myocarditis may receive COVID-19 vaccine after symptoms have resolved, there is currently insufficient safety data regarding COVID-19 vaccination of those with prior non-COVID-19 VAMP or following subsequent COVID-19 vaccination in those with prior VAMP related to COVID-19.10 For these individuals, COVID-19 vaccination is a precaution.10 Although insufficient to determine a precise level of risk, this report does provide data on which to base the CDC-recommended shared decision-making counseling of these patients. More research is needed to better define factors that increase risk for, or protection from, immune-mediated AEs following immunization, including VAMP. While benefits of vaccination have clearly outweighed risks during the COVID-19 pandemic, such research may optimize future vaccine recommendations.18
1. Decker MD, Garman PM, Hughes H, et al. Enhanced safety surveillance study of ACAM2000 smallpox vaccine among US military service members. Vaccine. 2021;39(39):5541-5547. doi:10.1016/j.vaccine.2021.08.041
2. Engler RJ, Nelson MR, Collins LC Jr, et al. A prospective study of the incidence of myocarditis/pericarditis and new onset cardiac symptoms following smallpox and influenza vaccination. PLoS One. 2015;10(3):e0118283. doi:10.1371/journal.pone.0118283
3. Faix DJ, Gordon DM, Perry LN, et al. Prospective safety surveillance study of ACAM2000 smallpox vaccine in deploying military personnel. Vaccine. 2020;38(46):7323-7330. doi:10.1016/j.vaccine.2020.09.037
4. Montgomery J, Ryan M, Engler R, et al. Myocarditis following immunization with mRNA COVID-19 vaccines in members of the US military. JAMA Cardiol. 2021;6(10):1202-1206. doi:10.1001/jamacardio.2021.2833
5. Witberg G, Barda N, Hoss S, et al. Myocarditis after Covid-19 vaccination in a large health care organization. N Engl J Med. 2021;385(23):2132-2139. doi:10.1056/NEJMoa2110737
6. Oster ME, Shay DK, Su JR, et al. Myocarditis cases reported after mRNA-based COVID-19 vaccination in the US from December 2020 to August 2021. JAMA. 2022;327(4):331-340. doi:10.1001/jama.2021.24110
7. Su JR, McNeil MM, Welsh KJ, et al. Myopericarditis after vaccination, Vaccine Adverse Event Reporting System (VAERS), 1990-2018. Vaccine. 2021;39(5):839-845. doi:10.1016/j.vaccine.2020.12.046
8. Mei R, Raschi E, Forcesi E, Diemberger I, De Ponti F, Poluzzi E. Myocarditis and pericarditis after immunization: gaining insights through the Vaccine Adverse Event Reporting System. Int J Cardiol. 2018;273:183-186. doi:10.1016/j.ijcard.2018.09.054
9. Centers for Disease Control and Prevention (CDC). Update: cardiac-related events during the civilian smallpox vaccination program—United States, 2003. MMWR Morb Mortal Wkly Rep. 2003;52(21):492-496.
10. Gargano JW, Wallace M, Hadler SC, et al. Use of mRNA COVID-19 vaccine after reports of myocarditis among vaccine recipients: update from the Advisory Committee on Immunization Practices—United States, June 2021. MMWR Morb Mortal Wkly Rep. 2021;70(27):977-982. doi:10.15585/mmwr.mm7027e2
11. Sexson Tejtel SK, Munoz FM, Al-Ammouri I, et al. Myocarditis and pericarditis: case definition and guidelines for data collection, analysis, and presentation of immunization safety data. Vaccine. 2022;40(10):1499-1511. doi:10.1016/j.vaccine.2021.11.074
12. Sagar S, Liu PP, Cooper LT Jr. Myocarditis. Lancet. 2012;379(9817):738-747. doi:10.1016/S0140-6736(11) 60648-X
13. Heymans S, Cooper LT. Myocarditis after COVID-19 mRNA vaccination: clinical observations and potential mechanisms. Nat Rev Cardiol. 2022;19(2):75-77. doi:10.1038/s41569-021-00662-w
14. Cohen JI, Hohman P, Fulton R, et al. Kinetics of serum cytokines after primary or repeat vaccination with the smallpox vaccine. J Infect Dis. 2010;201(8):1183-1191. doi:10.1086/651453
15. Minocha PK, Better D, Singh RK, Hoque T. Recurrence of acute myocarditis temporally associated with receipt of the mRNA COVID-19 vaccine in an adolescent male. J Pediatr. 2021;238:321-323. doi:10.1016/j.jpeds.2021.06.035
16. Umei TC, Kishino Y, Watanabe K, et al. Recurrence of myopericarditis following mRNA COVID-19 vaccination in a male adolescent. CJC Open. 2022;4(3):350-352. doi:10.1016/j.cjco.2021.12.002
17. Pasha MA, Isaac S, Khan Z. Recurrent myocarditis following COVID-19 infection and the mRNA vaccine. Cureus. 2022;14(7):e26650. doi:10.7759/cureus.26650
18. Block JP, Boehmer TK, Forrest CB, et al. Cardiac complications after SARS-CoV-2 infection and mRNA COVID-19 vaccination—PCORnet, United States, January 2021-January 2022. MMWR Morb Mortal Wkly Rep. 2022;71(14):517-523. Published 2022 Apr 8. doi:10.15585/mmwr.mm7114e1
Vaccinations have substantially reduced morbidity and mortality from many infectious diseases. Despite the clear value of vaccinations in public health, efforts to better understand adverse events (AEs) following immunization are important to sustain public trust and vaccine confidence. Noninfectious inflammation of the heart may manifest as myocarditis or pericarditis, or occasionally, with shared signs and symptoms of each, as myopericarditis. This is a rare AE following some immunizations. Vaccine-associated myocarditis, pericarditis, or myopericarditis (VAMP) has been most clearly associated with smallpox vaccines and mRNA COVID-19 vaccines.1-6 Although extremely rare, VAMP also has been associated with other vaccines.7,8 Limited information exists to guide shared clinical decision making on COVID-19 vaccination in persons with a history of VAMP. It is unknown whether individuals with a history of VAMP are at higher risk for developing a recurrence or experiencing a more severe outcome following COVID-19 vaccination.
Methods
As part of the collaborative public health mission with the Centers for Disease Control and Prevention (CDC) for enhanced vaccine AE surveillance, the Defense Health Agency Immunization Healthcare Division (IHD) maintains a clinical database of service members and beneficiaries referred for suspected AEs following immunizations. A review of all AEs following immunization cases in this database from January 1, 2003, through February 28, 2022, identified individuals meeting the following criteria: (a) VAMP prior to receipt of COVID-19 vaccine; (b) receipt of COVID-19 vaccine in 2021; and (c) medical documentation in available electronic health records sufficient to describe health status at least 30 days following COVID-19 vaccination.9 If medical entries suggested cardiac symptoms following a COVID-19 vaccine, additional information was sought to verify VAMP based on current published criteria.10,11 Both the initial VAMP cases and the suspected COVID-19 VAMP cases were adjudicated by a team of vaccine experts and specialists in immunology, cardiology, and preventive medicine.
This retrospective review was approved and conducted in accordance with the Walter Reed National Military Medical Center Institutional Review Board protocol #20664. All individuals with recurrent VAMP consented to share their health records and clinical details.
Results
Among 9260 cases in the IHD database, 431 met the case definition for VAMP.
Among the 179 patients included in this analysis, 171 (96%) were male. Their median age was 39 years at the time of COVID-19 vaccination.
Within 1 month of receipt of any COVID-19 vaccine, 11 individuals had documented symptoms suggesting cardiac involvement, specifically, chest pain, palpitations, or dyspnea. After cardiac evaluation, 4 patients met the criteria for VAMP after COVID-19 vaccination.10,11 Seven patients either did not meet the criteria for VAMP or had alternative causes for their symptoms.
Two men aged 49 and 50 years with a history of vaccine-associated myocarditis following smallpox vaccination (Dryvax and ACAM2000) developed myocarditis 3 days after their second dose of the Moderna vaccine. One of these patients received a Pfizer-BioNTech booster 10 months later with no recurrence of symptoms. A 55-year-old man with a history of vaccine-associated myocarditis following Dryvax vaccination developed myocarditis 2 days after his Pfizer-BioNTech booster. None of the patients who developed post-COVID-19 VAMP reported residual symptoms from their initial VAMP episode, which occurred 12 to 18 years earlier. All were hospitalized briefly for observation and had complete symptom resolution within 6 weeks.
A 25-year-old man developed pericarditis 4 days after his second Pfizer-BioNTech vaccination. His previous ACAM2000 vaccine-associated myocarditis occurred 3 years earlier, with no residual symptoms. Of note, he had a mild COVID-19 infection 78 days before the onset of his pericarditis. After the onset of his COVID-19 vaccine-associated pericarditis, he continued to experience transient bouts of chest pressure and exertional dyspnea that resolved within 7 months of onset.
The median interval between COVID-19 vaccine doses in those who developed post-COVID-19 VAMP was within the recommended mRNA vaccine dosing intervals of 3 to 4 weeks and was consistent with the median mRNA vaccine dosing intervals among the entire cohort.
Due to the small cohort size and other limitations of this study, the suggested rate of cardiac injury in this review (4 cases in 179 persons, or 2.2%) is an imprecise estimate of risk in a small population (95% CI, 0.1%-4.4%). While this rate may seem higher than expected within the general population after COVID-19 vaccination, it is lower than the estimated lifetime risk of recurrent myocarditis from any cause.6,12
Discussion
To our knowledge, this is the first report describing cardiac outcomes after COVID-19 vaccination among a cohort of individuals with prior history of VAMP. Four cases of COVID-19 VAMP were identified among 179 patients with previous VAMP. All cases had experienced VAMP after the smallpox vaccine several years earlier, with complete resolution of symptoms. Three cases presented with recurrent VAMP after their second dose of an mRNA COVID-19 vaccine, and one after an mRNA booster dose. All fully recovered over the course of several months.
Myocarditis is a heterogeneous inflammatory injury with diverse, sometimes idiopathic, etiologies.13 In contrast to infection-related cardiac injury, prior reports of vaccine-associated myocarditis have suggested a hypersensitivity reaction characterized by patchy eosinophilic infiltrates, a benign clinical course, and good prognosis.2,3
There are several common features between VAMP after smallpox and COVID-19 vaccination. Cases occur predominantly in young men. The onset of symptoms after smallpox vaccine (mean, 10 days) and after mRNA COVID-19 vaccine (mean, 3 days) appears to correspond to the timing of peak postvaccination pro-inflammatory cytokine elevation.14 While all VAMP cases are serious events, the majority of patients appear to have a relatively benign clinical course with rapid and full recovery.13
Patients who have experienced an inflammatory cardiac injury may be at higher risk for recurrence, but quantifying risk of this rare phenomenon is challenging. Cases of VAMP after the COVID-19 vaccine have occasionally been reported in patients with previous cardiac injury unrelated to vaccination.15-17 The cases presented here represent the first report of recurrent VAMP following prior non-COVID-19 vaccinations.
Most patients with prior VAMP in this cohort did not experience cardiac-suggestive symptoms following COVID-19 vaccination. Among 11 patients who developed symptoms, 3 had confirmed myocarditis and 1 had confirmed pericarditis. The clinical course for these patients with recurrent VAMP was observed to be no different in severity or duration from those who experience new-onset VAMP.4 All other patients not meeting criteria for VAMP or having alternative explanations for their symptoms also had a benign clinical course. Nonetheless, of the study cohort of 179, recurrent VAMP was diagnosed in 4 of the 11 who developed cardiac-suggestive symptoms following COVID-19 vaccination. The importance of cardiac evaluation should be emphasized for any patient presenting with chest pain, dyspnea, or other cardiac-suggestive symptoms following vaccination.
Strengths and Limitations
The strength of this review of VAMP recurrence associated with COVID-19 vaccination derives from our large and unique longitudinal database of VAMP among current and prior service members. Additionally, the IHD’s ongoing enhanced vaccine AEs surveillance provides the opportunity to contact patients and review their electronic health records over an extended interval of time.
When interpreting this report’s implications, limitations inherent to any retrospective case review should be considered. The cohort of cases of prior VAMP included primarily healthy, fit, young service members; this population is not representative of the general population. The cohort included prior VAMP cases that generally occurred after smallpox vaccination. Experiences after smallpox vaccine may not apply to cardiac injury from other vaccines or etiologies. By the nature of this review, the population studied at the time of COVID-19 vaccination was somewhat older than those most likely to develop an initial bout of VAMP.2 This review was limited by information available in the electronic health records of a small number of patients. Subclinical cases of VAMP and cases without adequate clinical evaluation also could not be included.
Conclusions
Noninfectious inflammation of the heart (myocarditis, pericarditis, or myopericarditis) is a rare AE following certain vaccines, especially live replicating smallpox vaccine and mRNA COVID-19 vaccines. In this observational analysis, the majority of patients with previous VAMP successfully received a COVID-19 vaccine without recurrence. The 4 patients who were identified with recurrent VAMP following COVID-19 vaccination all recovered with supportive care. While the CDC endorses that individuals with a history of infectious myocarditis may receive COVID-19 vaccine after symptoms have resolved, there is currently insufficient safety data regarding COVID-19 vaccination of those with prior non-COVID-19 VAMP or following subsequent COVID-19 vaccination in those with prior VAMP related to COVID-19.10 For these individuals, COVID-19 vaccination is a precaution.10 Although insufficient to determine a precise level of risk, this report does provide data on which to base the CDC-recommended shared decision-making counseling of these patients. More research is needed to better define factors that increase risk for, or protection from, immune-mediated AEs following immunization, including VAMP. While benefits of vaccination have clearly outweighed risks during the COVID-19 pandemic, such research may optimize future vaccine recommendations.18
Vaccinations have substantially reduced morbidity and mortality from many infectious diseases. Despite the clear value of vaccinations in public health, efforts to better understand adverse events (AEs) following immunization are important to sustain public trust and vaccine confidence. Noninfectious inflammation of the heart may manifest as myocarditis or pericarditis, or occasionally, with shared signs and symptoms of each, as myopericarditis. This is a rare AE following some immunizations. Vaccine-associated myocarditis, pericarditis, or myopericarditis (VAMP) has been most clearly associated with smallpox vaccines and mRNA COVID-19 vaccines.1-6 Although extremely rare, VAMP also has been associated with other vaccines.7,8 Limited information exists to guide shared clinical decision making on COVID-19 vaccination in persons with a history of VAMP. It is unknown whether individuals with a history of VAMP are at higher risk for developing a recurrence or experiencing a more severe outcome following COVID-19 vaccination.
Methods
As part of the collaborative public health mission with the Centers for Disease Control and Prevention (CDC) for enhanced vaccine AE surveillance, the Defense Health Agency Immunization Healthcare Division (IHD) maintains a clinical database of service members and beneficiaries referred for suspected AEs following immunizations. A review of all AEs following immunization cases in this database from January 1, 2003, through February 28, 2022, identified individuals meeting the following criteria: (a) VAMP prior to receipt of COVID-19 vaccine; (b) receipt of COVID-19 vaccine in 2021; and (c) medical documentation in available electronic health records sufficient to describe health status at least 30 days following COVID-19 vaccination.9 If medical entries suggested cardiac symptoms following a COVID-19 vaccine, additional information was sought to verify VAMP based on current published criteria.10,11 Both the initial VAMP cases and the suspected COVID-19 VAMP cases were adjudicated by a team of vaccine experts and specialists in immunology, cardiology, and preventive medicine.
This retrospective review was approved and conducted in accordance with the Walter Reed National Military Medical Center Institutional Review Board protocol #20664. All individuals with recurrent VAMP consented to share their health records and clinical details.
Results
Among 9260 cases in the IHD database, 431 met the case definition for VAMP.
Among the 179 patients included in this analysis, 171 (96%) were male. Their median age was 39 years at the time of COVID-19 vaccination.
Within 1 month of receipt of any COVID-19 vaccine, 11 individuals had documented symptoms suggesting cardiac involvement, specifically, chest pain, palpitations, or dyspnea. After cardiac evaluation, 4 patients met the criteria for VAMP after COVID-19 vaccination.10,11 Seven patients either did not meet the criteria for VAMP or had alternative causes for their symptoms.
Two men aged 49 and 50 years with a history of vaccine-associated myocarditis following smallpox vaccination (Dryvax and ACAM2000) developed myocarditis 3 days after their second dose of the Moderna vaccine. One of these patients received a Pfizer-BioNTech booster 10 months later with no recurrence of symptoms. A 55-year-old man with a history of vaccine-associated myocarditis following Dryvax vaccination developed myocarditis 2 days after his Pfizer-BioNTech booster. None of the patients who developed post-COVID-19 VAMP reported residual symptoms from their initial VAMP episode, which occurred 12 to 18 years earlier. All were hospitalized briefly for observation and had complete symptom resolution within 6 weeks.
A 25-year-old man developed pericarditis 4 days after his second Pfizer-BioNTech vaccination. His previous ACAM2000 vaccine-associated myocarditis occurred 3 years earlier, with no residual symptoms. Of note, he had a mild COVID-19 infection 78 days before the onset of his pericarditis. After the onset of his COVID-19 vaccine-associated pericarditis, he continued to experience transient bouts of chest pressure and exertional dyspnea that resolved within 7 months of onset.
The median interval between COVID-19 vaccine doses in those who developed post-COVID-19 VAMP was within the recommended mRNA vaccine dosing intervals of 3 to 4 weeks and was consistent with the median mRNA vaccine dosing intervals among the entire cohort.
Due to the small cohort size and other limitations of this study, the suggested rate of cardiac injury in this review (4 cases in 179 persons, or 2.2%) is an imprecise estimate of risk in a small population (95% CI, 0.1%-4.4%). While this rate may seem higher than expected within the general population after COVID-19 vaccination, it is lower than the estimated lifetime risk of recurrent myocarditis from any cause.6,12
Discussion
To our knowledge, this is the first report describing cardiac outcomes after COVID-19 vaccination among a cohort of individuals with prior history of VAMP. Four cases of COVID-19 VAMP were identified among 179 patients with previous VAMP. All cases had experienced VAMP after the smallpox vaccine several years earlier, with complete resolution of symptoms. Three cases presented with recurrent VAMP after their second dose of an mRNA COVID-19 vaccine, and one after an mRNA booster dose. All fully recovered over the course of several months.
Myocarditis is a heterogeneous inflammatory injury with diverse, sometimes idiopathic, etiologies.13 In contrast to infection-related cardiac injury, prior reports of vaccine-associated myocarditis have suggested a hypersensitivity reaction characterized by patchy eosinophilic infiltrates, a benign clinical course, and good prognosis.2,3
There are several common features between VAMP after smallpox and COVID-19 vaccination. Cases occur predominantly in young men. The onset of symptoms after smallpox vaccine (mean, 10 days) and after mRNA COVID-19 vaccine (mean, 3 days) appears to correspond to the timing of peak postvaccination pro-inflammatory cytokine elevation.14 While all VAMP cases are serious events, the majority of patients appear to have a relatively benign clinical course with rapid and full recovery.13
Patients who have experienced an inflammatory cardiac injury may be at higher risk for recurrence, but quantifying risk of this rare phenomenon is challenging. Cases of VAMP after the COVID-19 vaccine have occasionally been reported in patients with previous cardiac injury unrelated to vaccination.15-17 The cases presented here represent the first report of recurrent VAMP following prior non-COVID-19 vaccinations.
Most patients with prior VAMP in this cohort did not experience cardiac-suggestive symptoms following COVID-19 vaccination. Among 11 patients who developed symptoms, 3 had confirmed myocarditis and 1 had confirmed pericarditis. The clinical course for these patients with recurrent VAMP was observed to be no different in severity or duration from those who experience new-onset VAMP.4 All other patients not meeting criteria for VAMP or having alternative explanations for their symptoms also had a benign clinical course. Nonetheless, of the study cohort of 179, recurrent VAMP was diagnosed in 4 of the 11 who developed cardiac-suggestive symptoms following COVID-19 vaccination. The importance of cardiac evaluation should be emphasized for any patient presenting with chest pain, dyspnea, or other cardiac-suggestive symptoms following vaccination.
Strengths and Limitations
The strength of this review of VAMP recurrence associated with COVID-19 vaccination derives from our large and unique longitudinal database of VAMP among current and prior service members. Additionally, the IHD’s ongoing enhanced vaccine AEs surveillance provides the opportunity to contact patients and review their electronic health records over an extended interval of time.
When interpreting this report’s implications, limitations inherent to any retrospective case review should be considered. The cohort of cases of prior VAMP included primarily healthy, fit, young service members; this population is not representative of the general population. The cohort included prior VAMP cases that generally occurred after smallpox vaccination. Experiences after smallpox vaccine may not apply to cardiac injury from other vaccines or etiologies. By the nature of this review, the population studied at the time of COVID-19 vaccination was somewhat older than those most likely to develop an initial bout of VAMP.2 This review was limited by information available in the electronic health records of a small number of patients. Subclinical cases of VAMP and cases without adequate clinical evaluation also could not be included.
Conclusions
Noninfectious inflammation of the heart (myocarditis, pericarditis, or myopericarditis) is a rare AE following certain vaccines, especially live replicating smallpox vaccine and mRNA COVID-19 vaccines. In this observational analysis, the majority of patients with previous VAMP successfully received a COVID-19 vaccine without recurrence. The 4 patients who were identified with recurrent VAMP following COVID-19 vaccination all recovered with supportive care. While the CDC endorses that individuals with a history of infectious myocarditis may receive COVID-19 vaccine after symptoms have resolved, there is currently insufficient safety data regarding COVID-19 vaccination of those with prior non-COVID-19 VAMP or following subsequent COVID-19 vaccination in those with prior VAMP related to COVID-19.10 For these individuals, COVID-19 vaccination is a precaution.10 Although insufficient to determine a precise level of risk, this report does provide data on which to base the CDC-recommended shared decision-making counseling of these patients. More research is needed to better define factors that increase risk for, or protection from, immune-mediated AEs following immunization, including VAMP. While benefits of vaccination have clearly outweighed risks during the COVID-19 pandemic, such research may optimize future vaccine recommendations.18
1. Decker MD, Garman PM, Hughes H, et al. Enhanced safety surveillance study of ACAM2000 smallpox vaccine among US military service members. Vaccine. 2021;39(39):5541-5547. doi:10.1016/j.vaccine.2021.08.041
2. Engler RJ, Nelson MR, Collins LC Jr, et al. A prospective study of the incidence of myocarditis/pericarditis and new onset cardiac symptoms following smallpox and influenza vaccination. PLoS One. 2015;10(3):e0118283. doi:10.1371/journal.pone.0118283
3. Faix DJ, Gordon DM, Perry LN, et al. Prospective safety surveillance study of ACAM2000 smallpox vaccine in deploying military personnel. Vaccine. 2020;38(46):7323-7330. doi:10.1016/j.vaccine.2020.09.037
4. Montgomery J, Ryan M, Engler R, et al. Myocarditis following immunization with mRNA COVID-19 vaccines in members of the US military. JAMA Cardiol. 2021;6(10):1202-1206. doi:10.1001/jamacardio.2021.2833
5. Witberg G, Barda N, Hoss S, et al. Myocarditis after Covid-19 vaccination in a large health care organization. N Engl J Med. 2021;385(23):2132-2139. doi:10.1056/NEJMoa2110737
6. Oster ME, Shay DK, Su JR, et al. Myocarditis cases reported after mRNA-based COVID-19 vaccination in the US from December 2020 to August 2021. JAMA. 2022;327(4):331-340. doi:10.1001/jama.2021.24110
7. Su JR, McNeil MM, Welsh KJ, et al. Myopericarditis after vaccination, Vaccine Adverse Event Reporting System (VAERS), 1990-2018. Vaccine. 2021;39(5):839-845. doi:10.1016/j.vaccine.2020.12.046
8. Mei R, Raschi E, Forcesi E, Diemberger I, De Ponti F, Poluzzi E. Myocarditis and pericarditis after immunization: gaining insights through the Vaccine Adverse Event Reporting System. Int J Cardiol. 2018;273:183-186. doi:10.1016/j.ijcard.2018.09.054
9. Centers for Disease Control and Prevention (CDC). Update: cardiac-related events during the civilian smallpox vaccination program—United States, 2003. MMWR Morb Mortal Wkly Rep. 2003;52(21):492-496.
10. Gargano JW, Wallace M, Hadler SC, et al. Use of mRNA COVID-19 vaccine after reports of myocarditis among vaccine recipients: update from the Advisory Committee on Immunization Practices—United States, June 2021. MMWR Morb Mortal Wkly Rep. 2021;70(27):977-982. doi:10.15585/mmwr.mm7027e2
11. Sexson Tejtel SK, Munoz FM, Al-Ammouri I, et al. Myocarditis and pericarditis: case definition and guidelines for data collection, analysis, and presentation of immunization safety data. Vaccine. 2022;40(10):1499-1511. doi:10.1016/j.vaccine.2021.11.074
12. Sagar S, Liu PP, Cooper LT Jr. Myocarditis. Lancet. 2012;379(9817):738-747. doi:10.1016/S0140-6736(11) 60648-X
13. Heymans S, Cooper LT. Myocarditis after COVID-19 mRNA vaccination: clinical observations and potential mechanisms. Nat Rev Cardiol. 2022;19(2):75-77. doi:10.1038/s41569-021-00662-w
14. Cohen JI, Hohman P, Fulton R, et al. Kinetics of serum cytokines after primary or repeat vaccination with the smallpox vaccine. J Infect Dis. 2010;201(8):1183-1191. doi:10.1086/651453
15. Minocha PK, Better D, Singh RK, Hoque T. Recurrence of acute myocarditis temporally associated with receipt of the mRNA COVID-19 vaccine in an adolescent male. J Pediatr. 2021;238:321-323. doi:10.1016/j.jpeds.2021.06.035
16. Umei TC, Kishino Y, Watanabe K, et al. Recurrence of myopericarditis following mRNA COVID-19 vaccination in a male adolescent. CJC Open. 2022;4(3):350-352. doi:10.1016/j.cjco.2021.12.002
17. Pasha MA, Isaac S, Khan Z. Recurrent myocarditis following COVID-19 infection and the mRNA vaccine. Cureus. 2022;14(7):e26650. doi:10.7759/cureus.26650
18. Block JP, Boehmer TK, Forrest CB, et al. Cardiac complications after SARS-CoV-2 infection and mRNA COVID-19 vaccination—PCORnet, United States, January 2021-January 2022. MMWR Morb Mortal Wkly Rep. 2022;71(14):517-523. Published 2022 Apr 8. doi:10.15585/mmwr.mm7114e1
1. Decker MD, Garman PM, Hughes H, et al. Enhanced safety surveillance study of ACAM2000 smallpox vaccine among US military service members. Vaccine. 2021;39(39):5541-5547. doi:10.1016/j.vaccine.2021.08.041
2. Engler RJ, Nelson MR, Collins LC Jr, et al. A prospective study of the incidence of myocarditis/pericarditis and new onset cardiac symptoms following smallpox and influenza vaccination. PLoS One. 2015;10(3):e0118283. doi:10.1371/journal.pone.0118283
3. Faix DJ, Gordon DM, Perry LN, et al. Prospective safety surveillance study of ACAM2000 smallpox vaccine in deploying military personnel. Vaccine. 2020;38(46):7323-7330. doi:10.1016/j.vaccine.2020.09.037
4. Montgomery J, Ryan M, Engler R, et al. Myocarditis following immunization with mRNA COVID-19 vaccines in members of the US military. JAMA Cardiol. 2021;6(10):1202-1206. doi:10.1001/jamacardio.2021.2833
5. Witberg G, Barda N, Hoss S, et al. Myocarditis after Covid-19 vaccination in a large health care organization. N Engl J Med. 2021;385(23):2132-2139. doi:10.1056/NEJMoa2110737
6. Oster ME, Shay DK, Su JR, et al. Myocarditis cases reported after mRNA-based COVID-19 vaccination in the US from December 2020 to August 2021. JAMA. 2022;327(4):331-340. doi:10.1001/jama.2021.24110
7. Su JR, McNeil MM, Welsh KJ, et al. Myopericarditis after vaccination, Vaccine Adverse Event Reporting System (VAERS), 1990-2018. Vaccine. 2021;39(5):839-845. doi:10.1016/j.vaccine.2020.12.046
8. Mei R, Raschi E, Forcesi E, Diemberger I, De Ponti F, Poluzzi E. Myocarditis and pericarditis after immunization: gaining insights through the Vaccine Adverse Event Reporting System. Int J Cardiol. 2018;273:183-186. doi:10.1016/j.ijcard.2018.09.054
9. Centers for Disease Control and Prevention (CDC). Update: cardiac-related events during the civilian smallpox vaccination program—United States, 2003. MMWR Morb Mortal Wkly Rep. 2003;52(21):492-496.
10. Gargano JW, Wallace M, Hadler SC, et al. Use of mRNA COVID-19 vaccine after reports of myocarditis among vaccine recipients: update from the Advisory Committee on Immunization Practices—United States, June 2021. MMWR Morb Mortal Wkly Rep. 2021;70(27):977-982. doi:10.15585/mmwr.mm7027e2
11. Sexson Tejtel SK, Munoz FM, Al-Ammouri I, et al. Myocarditis and pericarditis: case definition and guidelines for data collection, analysis, and presentation of immunization safety data. Vaccine. 2022;40(10):1499-1511. doi:10.1016/j.vaccine.2021.11.074
12. Sagar S, Liu PP, Cooper LT Jr. Myocarditis. Lancet. 2012;379(9817):738-747. doi:10.1016/S0140-6736(11) 60648-X
13. Heymans S, Cooper LT. Myocarditis after COVID-19 mRNA vaccination: clinical observations and potential mechanisms. Nat Rev Cardiol. 2022;19(2):75-77. doi:10.1038/s41569-021-00662-w
14. Cohen JI, Hohman P, Fulton R, et al. Kinetics of serum cytokines after primary or repeat vaccination with the smallpox vaccine. J Infect Dis. 2010;201(8):1183-1191. doi:10.1086/651453
15. Minocha PK, Better D, Singh RK, Hoque T. Recurrence of acute myocarditis temporally associated with receipt of the mRNA COVID-19 vaccine in an adolescent male. J Pediatr. 2021;238:321-323. doi:10.1016/j.jpeds.2021.06.035
16. Umei TC, Kishino Y, Watanabe K, et al. Recurrence of myopericarditis following mRNA COVID-19 vaccination in a male adolescent. CJC Open. 2022;4(3):350-352. doi:10.1016/j.cjco.2021.12.002
17. Pasha MA, Isaac S, Khan Z. Recurrent myocarditis following COVID-19 infection and the mRNA vaccine. Cureus. 2022;14(7):e26650. doi:10.7759/cureus.26650
18. Block JP, Boehmer TK, Forrest CB, et al. Cardiac complications after SARS-CoV-2 infection and mRNA COVID-19 vaccination—PCORnet, United States, January 2021-January 2022. MMWR Morb Mortal Wkly Rep. 2022;71(14):517-523. Published 2022 Apr 8. doi:10.15585/mmwr.mm7114e1