User login
Individuals spend close to half of their lives preventing, or planning for, pregnancy. As such, contraception plays a major role in patient-provider interactions. Contraception counseling and management is a common scenario encountered in the general gynecologist’s practice. Luckily, we have 2 evidence-based guidelines developed by the US Centers for Disease Control and Prevention (CDC) that support the provision of contraceptive care:
- US Medical Eligibility for Contraceptive Use (US-MEC),1 which provides guidance on which patients can safely use a method
- US Selected Practice Recommendations for Contraceptive Use (US-SPR),2 which provides method-specific guidance on how to use a method (including how to: initiate or start a method; manage adherence issues, such as a missed pill, etc; and manage common issues like breakthrough bleeding).
Both of these guidelines are updated routinely and are publicly available online or for free, through smartphone applications.
While most contraceptive care is straightforward, there are circumstances that require additional consideration. In the concluding part of this series on contraceptive conundrums, we review 2 clinical cases, existing evidence to guide management decisions, and our recommendations.
CASE 1 Patient presents with hard-to-remove implant
A 44-year-old patient (G2P2) with a new diagnosis of estrogen and progesterone-receptor–positive breast cancer is undergoing her evaluation with her oncologist who recommends removal of her contraceptive implant, which has been in place for 2 years. She presents to your office for removal; however, the device is no longer palpable.
What are your next steps?
Conundrum 1. Should you attempt to remove it?
No, never attempt implant removal if you cannot palpate or localize it. Localization of the implant needs to occur prior to any attempt. However, we recommend checking the contra-lateral arm before sending the patient to obtain imaging, especially if you have no formal documentation regarding in which arm the implant was placed. The next step is identifying what type of implant the patient likely has so you can correctly interpret imaging studies.
Conundrum 2. What type of subdermal contraceptive device is it likely to be?
Currently, the only subdermal contraceptive device available for placement in the United States is the 68-mg etonogestrel implant, marketed with the brand name Nexplanon. This device was initially approved by the US Food and Drug Administration in 2001 and measures 4 cm in length by 2 mm in diameter. It is placed in the medial upper arm, about 8 cm proximal to the medial epicondyle and 3 cm posterior to the sulcus between the biceps and triceps muscles. (The implant should no longer be placed over the bicipital groove.) The implant is impregnated with 15 mg of barium sulfate, making it radiopaque and able to be seen on imaging modalities such as ultrasonography (10–18 mHz high frequency transducer) and x-ray (arm anteroposterior and lateral) for localization in cases in which the device becomes nonpalpable.3
Clinicians also may encounter devices which are no longer marketed in the United States, or which are only available in other countries, and thus should be aware of the appearance and imaging characteristics. It is important to let your imaging team know these characteristics as well:
- From 2006–2010, a 68-mg etonogestrel implant marketed under the name Implanon was available in the United States.4 It has the same dimensions and general placement recommendations as the Nexplanon etonogestrel device but is not able to be seen via imaging.
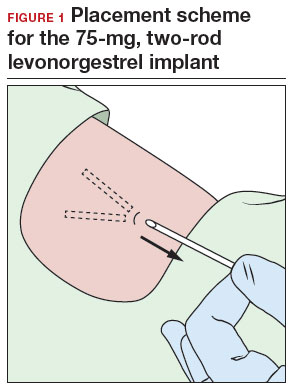
- A 2-arm, 75-mg levonorgestrel (LNG) device known as Jadelle (or, Norplant II; FIGURE 1) received FDA approval in 1996 and is currently only available overseas.5 It is also placed in the upper, inner arm in a V-shape using a single incision, and has dimensions similar to the etonogestrel implants.
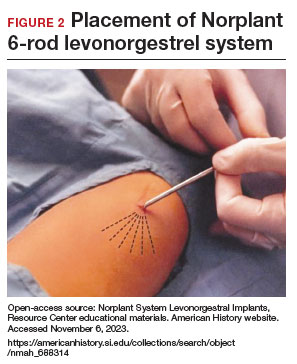
- From 1990– 2002, the 6-rod device known as Norplant was available in the United States. Each rod measured 3.4 cm in length and contained 36 mg of LNG (FIGURE 2).
Continue to: How do you approach removal of a deep contraceptive implant?...
How do you approach removal of a deep contraceptive implant?
Clinicians who are not trained in deep or difficult implant removal should refer patients to a trained provider (eg, a complex family planning subspecialist), or if not available, partner with a health care practitioner that has expertise in the anatomy of the upper arm (eg, vascular surgery, orthopedics, or interventional radiology). A resource for finding a nearby trained provider is the Organon Information Center (1-877-467-5266). However, when these services are not readily available, consider the following 3-step approach to complex implant removal.
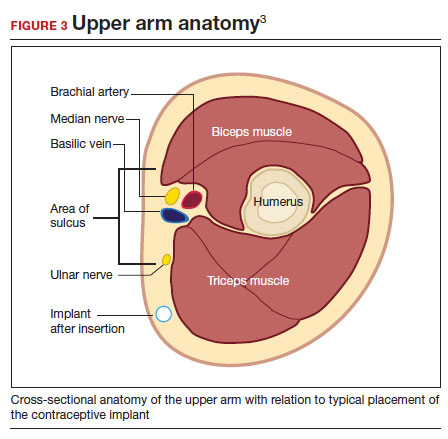
- Be familiar with the anatomy of the upper arm (FIGURE 3). Nonpalpable implants may be close to or under the biceps or triceps fascia or be near critically important and fragile structures like the neurovascular bundle of the upper arm. Prior to attempting a difficult implant removal, ensure that you are well acquainted with critical structures in the upper arm.
- Locate the device. Prior to attempting removal, localize the device using either x-ray or ultrasonography, depending on local availability. Ultrasound offers the advantage of mapping the location in 3 dimensions, with the ability to map the device with skin markings immediately prior to removal. Typically, a highfrequency transducer (15- or 18-MHz) is used, such as for breast imaging, either in a clinician’s office or in coordination with radiology. If device removal is attempted the same day, the proximal, midportion, and distal aspects of the device should be marked with a skin pen, and it should be noted what position the arm is in when the device is marked (eg, arm flexed at elbow and externally rotated so that the wrist is parallel to the ear).
Rarely, if a device is not seen in the expected extremity, imaging of the contralateral arm or a chest x-ray can be undertaken to rule out mis-documented laterality or a migrated device. Lastly, if no device is seen, and the patient has no memory of device removal, you can obtain the patient’s etonogestrel levels. (Resource: Merck National Service Center, 1-877-888-4231.)
Removal procedure. For nonpalpable implants, strong consideration should be given to performing the procedure with ultrasonography guidance. Rarely, fluoroscopic guidance may be useful for orientation in challenging cases, which may require coordination with other services, such as interventional radiology.
Cleaning and anesthetizing the site is similar to routine removal of a palpable implant. A 2- to 3-mm skin incision is made, either at the distal end of the implant (if one end is amenable to traditional pop-out technique) or over the midportion of the device (if a clinician has experience using the “U” technique).6 The incision should be parallel to the long axis of the implant and not perpendicular, to facilitate extension of the incision if needed during the procedure. Straight or curved hemostat clamps can then be used for blunt dissection of the subcutaneous tissues and to grasp the end of the device. Experienced clinicians may have access to a modified vasectomy clamp (with a
Indications for referral. Typically, referral to a complex family planning specialist or vascular surgeon is required for cases that involve dissection of the muscular fascia or where dissection would be in close proximity to critical neurologic or vascular structures.
CASE 1 Conclusion
Ultrasonography of the patient’s extremity demonstrated a
CASE 2 Patient enquires about immediate IUD insertion
A 28-year-old patient (G1P0) arrives at your clinic for a contraceptive consultation. They report a condom break during intercourse 4 days ago. Prior to that they used condoms consistently with each act of intercourse. They have used combined hormonal contraceptive pills in the past but had difficulty remembering to take them consistently. The patient and their partner have been mutually monogamous for 6 months and have no plans for pregnancy. Last menstrual period was 12 days ago. Their cycles are regular but heavy and painful. They are interested in using a hormonal IUD for contraception and would love to get it today.
- Do not attempt removal of a nonpalpable implant without prior localization via imaging
- Ultrasound-guided removal procedures using a “U” technique are successful for many deep implant removals but require specialized equipment and training
- Referral to a complex family planning specialist or other specialist is highly recommended for implants located below the triceps fascia or close to the nerves and vessels of the upper arm
- Never attempt to remove a nonpalpable implant prior to determining its location via imaging
Continue to: Is same-day IUD an option?...
Is same-day IUD an option?
Yes. This patient needs EC given the recent condom break, but they are still eligible for having an IUD placed today if their pregnancy test is negative and after counseling of the potential risks and benefits. According to the US-SPR it is reasonable to insert an IUD at any time during the cycle as long as you are reasonably certain the patient is not pregnant.7
Options for EC are:
- 1.5-mg oral LNG pill
- 30-mg oral UPA pill
- copper IUD (cu-IUD).
If they are interested in the cu-IUD for long-term contraception, by having a cu-IUD placed they can get both their needs met—EC and an ongoing method of contraception. Any patient receiving EC, whether a pill or an IUD, should be counseled to repeat a home urine pregnancy test in 2 to 4 weeks.
Given the favorable non–contraceptive benefits associated with 52-mg LNG-IUDs, many clinicians and patients have advocated for additional evidence regarding the use of hormonal IUDs alone for EC.
What is the evidence concerning LNG-IUD placement as EC?
The 52-mg LNG-IUD has not been mechanistically proven to work as an EC, but growing evidence exists showing that it is safe for same-day or “quick start” placement even in a population seeking EC—if their pregnancy test result is negative at the time of presentation.
Turok and colleagues performed a noninferiority trial comparing 1-month pregnancy rates after placement of either an LNG-IUD or a cu-IUD for EC.8 This study concluded that the LNG-IUD (which resulted in 1 pregnancy in 317 users; pregnancy rate, 0.3%; 95% confidence interval [CI], 0.01–1.70) is noninferior to cu-IUD (0 pregnancies in 321 users; pregnancy rate, 0%; 95% CI, 0.0–1.1) for EC. Although encouraging, only a small percentage of the study population seeking EC who received an IUD were actually at high risk of pregnancy (eg, they were not mid-cycle or were recently using contraception), which is why it is difficult to determine if the LNG-IUD actually works mechanistically as an EC. More likely, the LNG-IUD helps prevent pregnancy due to its ongoing contraceptive effect.9 Ongoing acts of intercourse post–oral EC initiation without starting a method of contraception is one of the main reasons for EC failure, which is why starting a method immediately is so effective at preventing pregnancy.10
A systematic review conducted by Ramanadhan and colleagues concluded that Turok’s 2021 trial is the only relevant study specific to 52-mg LNG-IUD use as EC, but they also mention that its results are limited in the strength of its conclusions due to biases in randomization, including11:
- the study groups were not balanced in that there was a 10% difference in reported use of contraception at last intercourse, which means that the LNG-IUD group had a lower baseline risk of pregnancy
- and a rare primary outcome (ie, pregnancy, which requires a larger sample size to know if the method works as an EC).
The review authors concluded that more studies are needed to further validate the effectiveness of using the 52-mg LNG-IUD as EC. Thus, for those at highest risk of pregnancy from recent unprotected sex and desiring a 52-mg IUD, it is probably best to continue combining oral EC with a 52-mg LNG-IUD and utilizing the LNG-IUD only as EC on a limited, case-by-case basis.
What we recommend
For anyone with a negative pregnancy test on the day of presentation, the studies mentioned further support the practice of same-day placement of a 52-mg LNG-IUD. However, those seeking EC who are at highest risk for an unplanned pregnancy (ie, the unprotected sex was mid-cycle), we recommend co-administering the LNG-IUD with oral LNG for EC.
CASE 2 Conclusion
After a conversation with the patient about all contraceptive options, through shared decision making the patient decided to take 1.5 mg of oral LNG and have a 52-mg LNG-IUD placed in the office today. They do not wish to be pregnant at this time and would choose termination if they became pregnant. They understood their pregnancy risk and opted to plan a urine pregnancy test at home in 2 weeks with a clear understanding that they should return to clinic immediately if the test is positive. ●
- A copper IUD is the most effective method of emergency contraception (EC).
- 52-mg LNG-IUDs are an emerging consideration for EC, but evidence is still lacking that they work as EC (or whether they just prevent pregnancy after placement for subsequent acts of intercourse). Clinicians should utilize shared decision making and advise patients to repeat a pregnancy test at home in 2 to 4 weeks
- Any patient receiving EC, whether a pill or an IUD, should be counseled to repeat a home urine pregnancy test in 2 to 4 weeks
- Any type of IUD can be placed same day if the clinician is reasonably sure the patient is not pregnant
- It appears safe to co-administer the 52-mg LNG-IUD with oral EC for those seeking emergency contraception but also want to use an LNG-IUD for contraception going forward
- Curtis KM, Jatlaoui TC, Tepper NK, et al. U.S. Selected Practice Recommendations for Contraceptive Use, 2016. Morb Mortal Wkly Rep. 2016;65:1-66. https://doi .org/10.15585/mmwr .rr6504a1
- Centers for Disease Control and Prevention. National Center for Chronic Disease Prevention and Health Promotion, Division of Reproductive Health. US Selected Practice Recommendations for Contraceptive Use (US-SPR). Accessed October 11, 2023. https://www.cdc.gov/reproductivehealth /contraception/mmwr/spr/summary.html
- Nexplanon [package insert]. Whitehouse Station, NJ: Merck; 2018.
- US Food and Drug Administration. Implanon (etonogestrel implant) 2006. Accessed November 6, 2023. https://www .accessdata.fda.gov/drugsatfda_docs/nda/2006 /021529s000_Lbl.pdf
- US Food and Drug Administration. Jadelle (levonorgestrel implant) 2016. Accessed November 6, 2023. https://www. accessdata.fda.gov/drugsatfda_docs/label/2016/020544s 010lbl.pdf
- Chen MJ, Creinin MD. Removal of a nonpalpable etonogestrel implant with preprocedure ultrasonography and modified vasectomy clamp. Obstet Gynecol. 2015;126:935-938.
- Curtis KM, Jatlaoui TC, Tepper NK, et al. U.S. Selected Practice Recommendations for Contraceptive Use, 2016. MMWR Recomm Rep Morb Mortal Wkly. 2016;65:1-66. https://doi .org/10.15585/mmwr.rr6504a1
- Turok DK, Gero A, Simmons RG, et al. Levonorgestrel vs. copper intrauterine devices for emergency contraception. N Engl J Med. 2021;384:335-344. https://pubmed.ncbi.nlm .nih.gov/33503342/
- Kaiser JE, Turok DK, Gero A, et al. One-year pregnancy and continuation rates after placement of levonorgestrel or copper intrauterine devices for emergency contraception: a randomized controlled trial. Am J Obstet Gynecol. 2023;228:438.e1-438.e10. https://doi.org/10.1016/j.ajog.2022 .11.1296
- Sander PM, Raymond EG, Weaver MA. Emergency contraceptive use as a marker of future risky sex, pregnancy, and sexually transmitted infection. Am J Obstet Gynecol. 2009;201:146.e1-e6.
- Ramanadhan S, Goldstuck N, Henderson JT, et al. Progestin intrauterine devices versus copper intrauterine devices for emergency contraception. Cochrane Database Syst Rev. 2023;2:CD013744. https://doi.org/10.1002/14651858 .CD013744.pub2
Individuals spend close to half of their lives preventing, or planning for, pregnancy. As such, contraception plays a major role in patient-provider interactions. Contraception counseling and management is a common scenario encountered in the general gynecologist’s practice. Luckily, we have 2 evidence-based guidelines developed by the US Centers for Disease Control and Prevention (CDC) that support the provision of contraceptive care:
- US Medical Eligibility for Contraceptive Use (US-MEC),1 which provides guidance on which patients can safely use a method
- US Selected Practice Recommendations for Contraceptive Use (US-SPR),2 which provides method-specific guidance on how to use a method (including how to: initiate or start a method; manage adherence issues, such as a missed pill, etc; and manage common issues like breakthrough bleeding).
Both of these guidelines are updated routinely and are publicly available online or for free, through smartphone applications.
While most contraceptive care is straightforward, there are circumstances that require additional consideration. In the concluding part of this series on contraceptive conundrums, we review 2 clinical cases, existing evidence to guide management decisions, and our recommendations.
CASE 1 Patient presents with hard-to-remove implant
A 44-year-old patient (G2P2) with a new diagnosis of estrogen and progesterone-receptor–positive breast cancer is undergoing her evaluation with her oncologist who recommends removal of her contraceptive implant, which has been in place for 2 years. She presents to your office for removal; however, the device is no longer palpable.
What are your next steps?
Conundrum 1. Should you attempt to remove it?
No, never attempt implant removal if you cannot palpate or localize it. Localization of the implant needs to occur prior to any attempt. However, we recommend checking the contra-lateral arm before sending the patient to obtain imaging, especially if you have no formal documentation regarding in which arm the implant was placed. The next step is identifying what type of implant the patient likely has so you can correctly interpret imaging studies.
Conundrum 2. What type of subdermal contraceptive device is it likely to be?
Currently, the only subdermal contraceptive device available for placement in the United States is the 68-mg etonogestrel implant, marketed with the brand name Nexplanon. This device was initially approved by the US Food and Drug Administration in 2001 and measures 4 cm in length by 2 mm in diameter. It is placed in the medial upper arm, about 8 cm proximal to the medial epicondyle and 3 cm posterior to the sulcus between the biceps and triceps muscles. (The implant should no longer be placed over the bicipital groove.) The implant is impregnated with 15 mg of barium sulfate, making it radiopaque and able to be seen on imaging modalities such as ultrasonography (10–18 mHz high frequency transducer) and x-ray (arm anteroposterior and lateral) for localization in cases in which the device becomes nonpalpable.3
Clinicians also may encounter devices which are no longer marketed in the United States, or which are only available in other countries, and thus should be aware of the appearance and imaging characteristics. It is important to let your imaging team know these characteristics as well:
- From 2006–2010, a 68-mg etonogestrel implant marketed under the name Implanon was available in the United States.4 It has the same dimensions and general placement recommendations as the Nexplanon etonogestrel device but is not able to be seen via imaging.
- A 2-arm, 75-mg levonorgestrel (LNG) device known as Jadelle (or, Norplant II; FIGURE 1) received FDA approval in 1996 and is currently only available overseas.5 It is also placed in the upper, inner arm in a V-shape using a single incision, and has dimensions similar to the etonogestrel implants.
- From 1990– 2002, the 6-rod device known as Norplant was available in the United States. Each rod measured 3.4 cm in length and contained 36 mg of LNG (FIGURE 2).


Continue to: How do you approach removal of a deep contraceptive implant?...
How do you approach removal of a deep contraceptive implant?
Clinicians who are not trained in deep or difficult implant removal should refer patients to a trained provider (eg, a complex family planning subspecialist), or if not available, partner with a health care practitioner that has expertise in the anatomy of the upper arm (eg, vascular surgery, orthopedics, or interventional radiology). A resource for finding a nearby trained provider is the Organon Information Center (1-877-467-5266). However, when these services are not readily available, consider the following 3-step approach to complex implant removal.
- Be familiar with the anatomy of the upper arm (FIGURE 3). Nonpalpable implants may be close to or under the biceps or triceps fascia or be near critically important and fragile structures like the neurovascular bundle of the upper arm. Prior to attempting a difficult implant removal, ensure that you are well acquainted with critical structures in the upper arm.
- Locate the device. Prior to attempting removal, localize the device using either x-ray or ultrasonography, depending on local availability. Ultrasound offers the advantage of mapping the location in 3 dimensions, with the ability to map the device with skin markings immediately prior to removal. Typically, a highfrequency transducer (15- or 18-MHz) is used, such as for breast imaging, either in a clinician’s office or in coordination with radiology. If device removal is attempted the same day, the proximal, midportion, and distal aspects of the device should be marked with a skin pen, and it should be noted what position the arm is in when the device is marked (eg, arm flexed at elbow and externally rotated so that the wrist is parallel to the ear).

Rarely, if a device is not seen in the expected extremity, imaging of the contralateral arm or a chest x-ray can be undertaken to rule out mis-documented laterality or a migrated device. Lastly, if no device is seen, and the patient has no memory of device removal, you can obtain the patient’s etonogestrel levels. (Resource: Merck National Service Center, 1-877-888-4231.)
Removal procedure. For nonpalpable implants, strong consideration should be given to performing the procedure with ultrasonography guidance. Rarely, fluoroscopic guidance may be useful for orientation in challenging cases, which may require coordination with other services, such as interventional radiology.
Cleaning and anesthetizing the site is similar to routine removal of a palpable implant. A 2- to 3-mm skin incision is made, either at the distal end of the implant (if one end is amenable to traditional pop-out technique) or over the midportion of the device (if a clinician has experience using the “U” technique).6 The incision should be parallel to the long axis of the implant and not perpendicular, to facilitate extension of the incision if needed during the procedure. Straight or curved hemostat clamps can then be used for blunt dissection of the subcutaneous tissues and to grasp the end of the device. Experienced clinicians may have access to a modified vasectomy clamp (with a
Indications for referral. Typically, referral to a complex family planning specialist or vascular surgeon is required for cases that involve dissection of the muscular fascia or where dissection would be in close proximity to critical neurologic or vascular structures.
CASE 1 Conclusion
Ultrasonography of the patient’s extremity demonstrated a
CASE 2 Patient enquires about immediate IUD insertion
A 28-year-old patient (G1P0) arrives at your clinic for a contraceptive consultation. They report a condom break during intercourse 4 days ago. Prior to that they used condoms consistently with each act of intercourse. They have used combined hormonal contraceptive pills in the past but had difficulty remembering to take them consistently. The patient and their partner have been mutually monogamous for 6 months and have no plans for pregnancy. Last menstrual period was 12 days ago. Their cycles are regular but heavy and painful. They are interested in using a hormonal IUD for contraception and would love to get it today.
- Do not attempt removal of a nonpalpable implant without prior localization via imaging
- Ultrasound-guided removal procedures using a “U” technique are successful for many deep implant removals but require specialized equipment and training
- Referral to a complex family planning specialist or other specialist is highly recommended for implants located below the triceps fascia or close to the nerves and vessels of the upper arm
- Never attempt to remove a nonpalpable implant prior to determining its location via imaging
Continue to: Is same-day IUD an option?...
Is same-day IUD an option?
Yes. This patient needs EC given the recent condom break, but they are still eligible for having an IUD placed today if their pregnancy test is negative and after counseling of the potential risks and benefits. According to the US-SPR it is reasonable to insert an IUD at any time during the cycle as long as you are reasonably certain the patient is not pregnant.7
Options for EC are:
- 1.5-mg oral LNG pill
- 30-mg oral UPA pill
- copper IUD (cu-IUD).
If they are interested in the cu-IUD for long-term contraception, by having a cu-IUD placed they can get both their needs met—EC and an ongoing method of contraception. Any patient receiving EC, whether a pill or an IUD, should be counseled to repeat a home urine pregnancy test in 2 to 4 weeks.
Given the favorable non–contraceptive benefits associated with 52-mg LNG-IUDs, many clinicians and patients have advocated for additional evidence regarding the use of hormonal IUDs alone for EC.
What is the evidence concerning LNG-IUD placement as EC?
The 52-mg LNG-IUD has not been mechanistically proven to work as an EC, but growing evidence exists showing that it is safe for same-day or “quick start” placement even in a population seeking EC—if their pregnancy test result is negative at the time of presentation.
Turok and colleagues performed a noninferiority trial comparing 1-month pregnancy rates after placement of either an LNG-IUD or a cu-IUD for EC.8 This study concluded that the LNG-IUD (which resulted in 1 pregnancy in 317 users; pregnancy rate, 0.3%; 95% confidence interval [CI], 0.01–1.70) is noninferior to cu-IUD (0 pregnancies in 321 users; pregnancy rate, 0%; 95% CI, 0.0–1.1) for EC. Although encouraging, only a small percentage of the study population seeking EC who received an IUD were actually at high risk of pregnancy (eg, they were not mid-cycle or were recently using contraception), which is why it is difficult to determine if the LNG-IUD actually works mechanistically as an EC. More likely, the LNG-IUD helps prevent pregnancy due to its ongoing contraceptive effect.9 Ongoing acts of intercourse post–oral EC initiation without starting a method of contraception is one of the main reasons for EC failure, which is why starting a method immediately is so effective at preventing pregnancy.10
A systematic review conducted by Ramanadhan and colleagues concluded that Turok’s 2021 trial is the only relevant study specific to 52-mg LNG-IUD use as EC, but they also mention that its results are limited in the strength of its conclusions due to biases in randomization, including11:
- the study groups were not balanced in that there was a 10% difference in reported use of contraception at last intercourse, which means that the LNG-IUD group had a lower baseline risk of pregnancy
- and a rare primary outcome (ie, pregnancy, which requires a larger sample size to know if the method works as an EC).
The review authors concluded that more studies are needed to further validate the effectiveness of using the 52-mg LNG-IUD as EC. Thus, for those at highest risk of pregnancy from recent unprotected sex and desiring a 52-mg IUD, it is probably best to continue combining oral EC with a 52-mg LNG-IUD and utilizing the LNG-IUD only as EC on a limited, case-by-case basis.
What we recommend
For anyone with a negative pregnancy test on the day of presentation, the studies mentioned further support the practice of same-day placement of a 52-mg LNG-IUD. However, those seeking EC who are at highest risk for an unplanned pregnancy (ie, the unprotected sex was mid-cycle), we recommend co-administering the LNG-IUD with oral LNG for EC.
CASE 2 Conclusion
After a conversation with the patient about all contraceptive options, through shared decision making the patient decided to take 1.5 mg of oral LNG and have a 52-mg LNG-IUD placed in the office today. They do not wish to be pregnant at this time and would choose termination if they became pregnant. They understood their pregnancy risk and opted to plan a urine pregnancy test at home in 2 weeks with a clear understanding that they should return to clinic immediately if the test is positive. ●
- A copper IUD is the most effective method of emergency contraception (EC).
- 52-mg LNG-IUDs are an emerging consideration for EC, but evidence is still lacking that they work as EC (or whether they just prevent pregnancy after placement for subsequent acts of intercourse). Clinicians should utilize shared decision making and advise patients to repeat a pregnancy test at home in 2 to 4 weeks
- Any patient receiving EC, whether a pill or an IUD, should be counseled to repeat a home urine pregnancy test in 2 to 4 weeks
- Any type of IUD can be placed same day if the clinician is reasonably sure the patient is not pregnant
- It appears safe to co-administer the 52-mg LNG-IUD with oral EC for those seeking emergency contraception but also want to use an LNG-IUD for contraception going forward
Individuals spend close to half of their lives preventing, or planning for, pregnancy. As such, contraception plays a major role in patient-provider interactions. Contraception counseling and management is a common scenario encountered in the general gynecologist’s practice. Luckily, we have 2 evidence-based guidelines developed by the US Centers for Disease Control and Prevention (CDC) that support the provision of contraceptive care:
- US Medical Eligibility for Contraceptive Use (US-MEC),1 which provides guidance on which patients can safely use a method
- US Selected Practice Recommendations for Contraceptive Use (US-SPR),2 which provides method-specific guidance on how to use a method (including how to: initiate or start a method; manage adherence issues, such as a missed pill, etc; and manage common issues like breakthrough bleeding).
Both of these guidelines are updated routinely and are publicly available online or for free, through smartphone applications.
While most contraceptive care is straightforward, there are circumstances that require additional consideration. In the concluding part of this series on contraceptive conundrums, we review 2 clinical cases, existing evidence to guide management decisions, and our recommendations.
CASE 1 Patient presents with hard-to-remove implant
A 44-year-old patient (G2P2) with a new diagnosis of estrogen and progesterone-receptor–positive breast cancer is undergoing her evaluation with her oncologist who recommends removal of her contraceptive implant, which has been in place for 2 years. She presents to your office for removal; however, the device is no longer palpable.
What are your next steps?
Conundrum 1. Should you attempt to remove it?
No, never attempt implant removal if you cannot palpate or localize it. Localization of the implant needs to occur prior to any attempt. However, we recommend checking the contra-lateral arm before sending the patient to obtain imaging, especially if you have no formal documentation regarding in which arm the implant was placed. The next step is identifying what type of implant the patient likely has so you can correctly interpret imaging studies.
Conundrum 2. What type of subdermal contraceptive device is it likely to be?
Currently, the only subdermal contraceptive device available for placement in the United States is the 68-mg etonogestrel implant, marketed with the brand name Nexplanon. This device was initially approved by the US Food and Drug Administration in 2001 and measures 4 cm in length by 2 mm in diameter. It is placed in the medial upper arm, about 8 cm proximal to the medial epicondyle and 3 cm posterior to the sulcus between the biceps and triceps muscles. (The implant should no longer be placed over the bicipital groove.) The implant is impregnated with 15 mg of barium sulfate, making it radiopaque and able to be seen on imaging modalities such as ultrasonography (10–18 mHz high frequency transducer) and x-ray (arm anteroposterior and lateral) for localization in cases in which the device becomes nonpalpable.3
Clinicians also may encounter devices which are no longer marketed in the United States, or which are only available in other countries, and thus should be aware of the appearance and imaging characteristics. It is important to let your imaging team know these characteristics as well:
- From 2006–2010, a 68-mg etonogestrel implant marketed under the name Implanon was available in the United States.4 It has the same dimensions and general placement recommendations as the Nexplanon etonogestrel device but is not able to be seen via imaging.
- A 2-arm, 75-mg levonorgestrel (LNG) device known as Jadelle (or, Norplant II; FIGURE 1) received FDA approval in 1996 and is currently only available overseas.5 It is also placed in the upper, inner arm in a V-shape using a single incision, and has dimensions similar to the etonogestrel implants.
- From 1990– 2002, the 6-rod device known as Norplant was available in the United States. Each rod measured 3.4 cm in length and contained 36 mg of LNG (FIGURE 2).


Continue to: How do you approach removal of a deep contraceptive implant?...
How do you approach removal of a deep contraceptive implant?
Clinicians who are not trained in deep or difficult implant removal should refer patients to a trained provider (eg, a complex family planning subspecialist), or if not available, partner with a health care practitioner that has expertise in the anatomy of the upper arm (eg, vascular surgery, orthopedics, or interventional radiology). A resource for finding a nearby trained provider is the Organon Information Center (1-877-467-5266). However, when these services are not readily available, consider the following 3-step approach to complex implant removal.
- Be familiar with the anatomy of the upper arm (FIGURE 3). Nonpalpable implants may be close to or under the biceps or triceps fascia or be near critically important and fragile structures like the neurovascular bundle of the upper arm. Prior to attempting a difficult implant removal, ensure that you are well acquainted with critical structures in the upper arm.
- Locate the device. Prior to attempting removal, localize the device using either x-ray or ultrasonography, depending on local availability. Ultrasound offers the advantage of mapping the location in 3 dimensions, with the ability to map the device with skin markings immediately prior to removal. Typically, a highfrequency transducer (15- or 18-MHz) is used, such as for breast imaging, either in a clinician’s office or in coordination with radiology. If device removal is attempted the same day, the proximal, midportion, and distal aspects of the device should be marked with a skin pen, and it should be noted what position the arm is in when the device is marked (eg, arm flexed at elbow and externally rotated so that the wrist is parallel to the ear).

Rarely, if a device is not seen in the expected extremity, imaging of the contralateral arm or a chest x-ray can be undertaken to rule out mis-documented laterality or a migrated device. Lastly, if no device is seen, and the patient has no memory of device removal, you can obtain the patient’s etonogestrel levels. (Resource: Merck National Service Center, 1-877-888-4231.)
Removal procedure. For nonpalpable implants, strong consideration should be given to performing the procedure with ultrasonography guidance. Rarely, fluoroscopic guidance may be useful for orientation in challenging cases, which may require coordination with other services, such as interventional radiology.
Cleaning and anesthetizing the site is similar to routine removal of a palpable implant. A 2- to 3-mm skin incision is made, either at the distal end of the implant (if one end is amenable to traditional pop-out technique) or over the midportion of the device (if a clinician has experience using the “U” technique).6 The incision should be parallel to the long axis of the implant and not perpendicular, to facilitate extension of the incision if needed during the procedure. Straight or curved hemostat clamps can then be used for blunt dissection of the subcutaneous tissues and to grasp the end of the device. Experienced clinicians may have access to a modified vasectomy clamp (with a
Indications for referral. Typically, referral to a complex family planning specialist or vascular surgeon is required for cases that involve dissection of the muscular fascia or where dissection would be in close proximity to critical neurologic or vascular structures.
CASE 1 Conclusion
Ultrasonography of the patient’s extremity demonstrated a
CASE 2 Patient enquires about immediate IUD insertion
A 28-year-old patient (G1P0) arrives at your clinic for a contraceptive consultation. They report a condom break during intercourse 4 days ago. Prior to that they used condoms consistently with each act of intercourse. They have used combined hormonal contraceptive pills in the past but had difficulty remembering to take them consistently. The patient and their partner have been mutually monogamous for 6 months and have no plans for pregnancy. Last menstrual period was 12 days ago. Their cycles are regular but heavy and painful. They are interested in using a hormonal IUD for contraception and would love to get it today.
- Do not attempt removal of a nonpalpable implant without prior localization via imaging
- Ultrasound-guided removal procedures using a “U” technique are successful for many deep implant removals but require specialized equipment and training
- Referral to a complex family planning specialist or other specialist is highly recommended for implants located below the triceps fascia or close to the nerves and vessels of the upper arm
- Never attempt to remove a nonpalpable implant prior to determining its location via imaging
Continue to: Is same-day IUD an option?...
Is same-day IUD an option?
Yes. This patient needs EC given the recent condom break, but they are still eligible for having an IUD placed today if their pregnancy test is negative and after counseling of the potential risks and benefits. According to the US-SPR it is reasonable to insert an IUD at any time during the cycle as long as you are reasonably certain the patient is not pregnant.7
Options for EC are:
- 1.5-mg oral LNG pill
- 30-mg oral UPA pill
- copper IUD (cu-IUD).
If they are interested in the cu-IUD for long-term contraception, by having a cu-IUD placed they can get both their needs met—EC and an ongoing method of contraception. Any patient receiving EC, whether a pill or an IUD, should be counseled to repeat a home urine pregnancy test in 2 to 4 weeks.
Given the favorable non–contraceptive benefits associated with 52-mg LNG-IUDs, many clinicians and patients have advocated for additional evidence regarding the use of hormonal IUDs alone for EC.
What is the evidence concerning LNG-IUD placement as EC?
The 52-mg LNG-IUD has not been mechanistically proven to work as an EC, but growing evidence exists showing that it is safe for same-day or “quick start” placement even in a population seeking EC—if their pregnancy test result is negative at the time of presentation.
Turok and colleagues performed a noninferiority trial comparing 1-month pregnancy rates after placement of either an LNG-IUD or a cu-IUD for EC.8 This study concluded that the LNG-IUD (which resulted in 1 pregnancy in 317 users; pregnancy rate, 0.3%; 95% confidence interval [CI], 0.01–1.70) is noninferior to cu-IUD (0 pregnancies in 321 users; pregnancy rate, 0%; 95% CI, 0.0–1.1) for EC. Although encouraging, only a small percentage of the study population seeking EC who received an IUD were actually at high risk of pregnancy (eg, they were not mid-cycle or were recently using contraception), which is why it is difficult to determine if the LNG-IUD actually works mechanistically as an EC. More likely, the LNG-IUD helps prevent pregnancy due to its ongoing contraceptive effect.9 Ongoing acts of intercourse post–oral EC initiation without starting a method of contraception is one of the main reasons for EC failure, which is why starting a method immediately is so effective at preventing pregnancy.10
A systematic review conducted by Ramanadhan and colleagues concluded that Turok’s 2021 trial is the only relevant study specific to 52-mg LNG-IUD use as EC, but they also mention that its results are limited in the strength of its conclusions due to biases in randomization, including11:
- the study groups were not balanced in that there was a 10% difference in reported use of contraception at last intercourse, which means that the LNG-IUD group had a lower baseline risk of pregnancy
- and a rare primary outcome (ie, pregnancy, which requires a larger sample size to know if the method works as an EC).
The review authors concluded that more studies are needed to further validate the effectiveness of using the 52-mg LNG-IUD as EC. Thus, for those at highest risk of pregnancy from recent unprotected sex and desiring a 52-mg IUD, it is probably best to continue combining oral EC with a 52-mg LNG-IUD and utilizing the LNG-IUD only as EC on a limited, case-by-case basis.
What we recommend
For anyone with a negative pregnancy test on the day of presentation, the studies mentioned further support the practice of same-day placement of a 52-mg LNG-IUD. However, those seeking EC who are at highest risk for an unplanned pregnancy (ie, the unprotected sex was mid-cycle), we recommend co-administering the LNG-IUD with oral LNG for EC.
CASE 2 Conclusion
After a conversation with the patient about all contraceptive options, through shared decision making the patient decided to take 1.5 mg of oral LNG and have a 52-mg LNG-IUD placed in the office today. They do not wish to be pregnant at this time and would choose termination if they became pregnant. They understood their pregnancy risk and opted to plan a urine pregnancy test at home in 2 weeks with a clear understanding that they should return to clinic immediately if the test is positive. ●
- A copper IUD is the most effective method of emergency contraception (EC).
- 52-mg LNG-IUDs are an emerging consideration for EC, but evidence is still lacking that they work as EC (or whether they just prevent pregnancy after placement for subsequent acts of intercourse). Clinicians should utilize shared decision making and advise patients to repeat a pregnancy test at home in 2 to 4 weeks
- Any patient receiving EC, whether a pill or an IUD, should be counseled to repeat a home urine pregnancy test in 2 to 4 weeks
- Any type of IUD can be placed same day if the clinician is reasonably sure the patient is not pregnant
- It appears safe to co-administer the 52-mg LNG-IUD with oral EC for those seeking emergency contraception but also want to use an LNG-IUD for contraception going forward
- Curtis KM, Jatlaoui TC, Tepper NK, et al. U.S. Selected Practice Recommendations for Contraceptive Use, 2016. Morb Mortal Wkly Rep. 2016;65:1-66. https://doi .org/10.15585/mmwr .rr6504a1
- Centers for Disease Control and Prevention. National Center for Chronic Disease Prevention and Health Promotion, Division of Reproductive Health. US Selected Practice Recommendations for Contraceptive Use (US-SPR). Accessed October 11, 2023. https://www.cdc.gov/reproductivehealth /contraception/mmwr/spr/summary.html
- Nexplanon [package insert]. Whitehouse Station, NJ: Merck; 2018.
- US Food and Drug Administration. Implanon (etonogestrel implant) 2006. Accessed November 6, 2023. https://www .accessdata.fda.gov/drugsatfda_docs/nda/2006 /021529s000_Lbl.pdf
- US Food and Drug Administration. Jadelle (levonorgestrel implant) 2016. Accessed November 6, 2023. https://www. accessdata.fda.gov/drugsatfda_docs/label/2016/020544s 010lbl.pdf
- Chen MJ, Creinin MD. Removal of a nonpalpable etonogestrel implant with preprocedure ultrasonography and modified vasectomy clamp. Obstet Gynecol. 2015;126:935-938.
- Curtis KM, Jatlaoui TC, Tepper NK, et al. U.S. Selected Practice Recommendations for Contraceptive Use, 2016. MMWR Recomm Rep Morb Mortal Wkly. 2016;65:1-66. https://doi .org/10.15585/mmwr.rr6504a1
- Turok DK, Gero A, Simmons RG, et al. Levonorgestrel vs. copper intrauterine devices for emergency contraception. N Engl J Med. 2021;384:335-344. https://pubmed.ncbi.nlm .nih.gov/33503342/
- Kaiser JE, Turok DK, Gero A, et al. One-year pregnancy and continuation rates after placement of levonorgestrel or copper intrauterine devices for emergency contraception: a randomized controlled trial. Am J Obstet Gynecol. 2023;228:438.e1-438.e10. https://doi.org/10.1016/j.ajog.2022 .11.1296
- Sander PM, Raymond EG, Weaver MA. Emergency contraceptive use as a marker of future risky sex, pregnancy, and sexually transmitted infection. Am J Obstet Gynecol. 2009;201:146.e1-e6.
- Ramanadhan S, Goldstuck N, Henderson JT, et al. Progestin intrauterine devices versus copper intrauterine devices for emergency contraception. Cochrane Database Syst Rev. 2023;2:CD013744. https://doi.org/10.1002/14651858 .CD013744.pub2
- Curtis KM, Jatlaoui TC, Tepper NK, et al. U.S. Selected Practice Recommendations for Contraceptive Use, 2016. Morb Mortal Wkly Rep. 2016;65:1-66. https://doi .org/10.15585/mmwr .rr6504a1
- Centers for Disease Control and Prevention. National Center for Chronic Disease Prevention and Health Promotion, Division of Reproductive Health. US Selected Practice Recommendations for Contraceptive Use (US-SPR). Accessed October 11, 2023. https://www.cdc.gov/reproductivehealth /contraception/mmwr/spr/summary.html
- Nexplanon [package insert]. Whitehouse Station, NJ: Merck; 2018.
- US Food and Drug Administration. Implanon (etonogestrel implant) 2006. Accessed November 6, 2023. https://www .accessdata.fda.gov/drugsatfda_docs/nda/2006 /021529s000_Lbl.pdf
- US Food and Drug Administration. Jadelle (levonorgestrel implant) 2016. Accessed November 6, 2023. https://www. accessdata.fda.gov/drugsatfda_docs/label/2016/020544s 010lbl.pdf
- Chen MJ, Creinin MD. Removal of a nonpalpable etonogestrel implant with preprocedure ultrasonography and modified vasectomy clamp. Obstet Gynecol. 2015;126:935-938.
- Curtis KM, Jatlaoui TC, Tepper NK, et al. U.S. Selected Practice Recommendations for Contraceptive Use, 2016. MMWR Recomm Rep Morb Mortal Wkly. 2016;65:1-66. https://doi .org/10.15585/mmwr.rr6504a1
- Turok DK, Gero A, Simmons RG, et al. Levonorgestrel vs. copper intrauterine devices for emergency contraception. N Engl J Med. 2021;384:335-344. https://pubmed.ncbi.nlm .nih.gov/33503342/
- Kaiser JE, Turok DK, Gero A, et al. One-year pregnancy and continuation rates after placement of levonorgestrel or copper intrauterine devices for emergency contraception: a randomized controlled trial. Am J Obstet Gynecol. 2023;228:438.e1-438.e10. https://doi.org/10.1016/j.ajog.2022 .11.1296
- Sander PM, Raymond EG, Weaver MA. Emergency contraceptive use as a marker of future risky sex, pregnancy, and sexually transmitted infection. Am J Obstet Gynecol. 2009;201:146.e1-e6.
- Ramanadhan S, Goldstuck N, Henderson JT, et al. Progestin intrauterine devices versus copper intrauterine devices for emergency contraception. Cochrane Database Syst Rev. 2023;2:CD013744. https://doi.org/10.1002/14651858 .CD013744.pub2