User login
Elevated low-density lipoprotein cholesterol (LDL-C) is a major risk factor for the development of coronary artery disease (CAD). For the past decade, lowering LDL-C has been the main focus in the treatment of dyslipidemia.1-3 Significant evidence also exists that hypertriglyceridemia is related to complications, including pancreatitis, and may also be independently linked to cardiovascular risk.4,5
Current treatment guidelines, published by the National Cholesterol Education Program Adult Treatment Panel III (NCEP ATP III), establish LDL-C reduction as the primary goal and triglyceride (TG) reduction as the secondary goal. If TG levels are significantly elevated (> 500 mg/dL), then TG becomes the primary goal due to increased risk of pancreatitis.3
Simvastatin, a 3-hydroxy-3-methylglutaryl-coenzyme (HMG-CoA) reductase inhibitor, has long been the drug of choice in the VA system for the treatment of dyslipidemia, despite its risks, which include myalgias, myopathy, and rhabdomyolysis.6 Incidence of true statin-induced myopathy or rhabdomyolysis is very low, estimated to occur in < 1% of high-dose simvastatin users; the benefits of statin use are often thought to outweigh the risks of therapy.6,7
In the Helsinki Heart Study and the Veterans Affairs High-Density Lipoprotein Cholesterol Intervention Trial (VA-HIT), gemfibrozil reduced TG concentrations by 20% to 50%. Gemfibrozil was shown to be useful in both the primary and secondary prevention of CAD and demonstrated mortality reduction in patients with CAD.8,9 The combination of gemfibrozil with simvastatin has been discouraged due to the increased risk of muscle-related complications; however, in practice, the medications are often prescribed concomitantly. A pharmacokinetic study reported that gemfibrozil increased the measured area under the curve concentration of simvastatin 2-fold, likely the reason that rates of myopathy and rhabdomyolysis are 6 times greater when simvastatin is used in combination with gemfibrozil.10
In June 2011, the FDA released new recommendations on the use of simvastatin, which included a dose limit of 40 mg (previously the maximum simvastatin dose was 80 mg) and new drug combination contraindications.11 These recommendations were made in light of the SEARCH (Study of the Effectiveness of Additional Reductions in Cholesterol and Homocysteine) trial, a large, randomized, placebo-controlled trial that revealed a significantly higher rate of myopathy and rhabdomyolysis with simvastatin than had other previous studies.12 Medications cited to increase risk of simvastatin-induced muscle injury included gemfibrozil, and combination therapy is now considered a contraindicated treatment option.10-13
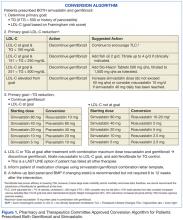
In August 2011, the Pharmacy and Therapeutics (P&T) Committee at the Ralph H. Johnson VA Medical Center in Charleston, South Carolina, reviewed the FDA warning and approved an automatic conversion protocol to be applied to patients prescribed both gemfibrozil and simvastatin. First, the primary goal of therapy (either TG or LDL-C reduction) was determined. TG reduction was considered the primary goal if patients had a history of TG level of > 500 mg/dL or TG-induced pancreatitis; LDL-C was the primary goal in all other patients.
Once the goal of therapy was determined, options for intervention included discontinuation of gemfibrozil, statin dose escalation, and/or addition of niacin or fish oil. In patients whose TG concentration met NCEP goal < 150 mg/dL, gemfibrozil was discontinued. Statin dose was escalated if needed for further LDL-C reduction. Per P&T recommendations, a clinical pharmacist evaluated each patient, made an intervention based on the P&T approved protocol, and sent a letter, which described the intervention and the reason for the action, to each patient.
This study evaluated the outcome of the P&T committee-approved automatic conversion protocol in subgroups of patients prescribed simvastatin and gemfibrozil with a TG serum concentration ≤ 150 mg/dL at baseline (Figure 1).
Methods
A retrospective chart review was conducted on all patients who had prescriptions for gemfibrozil and simvastatin, prescribed in the 52 weeks before August 15, 2011; patient records were reviewed between September 1, 2011, and April 30, 2012. Patients were included for study analysis if they underwent a P&T committee-approved conversion algorithm between September 1, 2011, and December 31, 2011, were aged 18 to 88 years, and their most recent TG measurement was ≤ 150 mg/dL.
Only those patients who had a fasting lipid panel documented in the 56 weeks preceding the study intervention and who returned for a follow-up lipid panel within 6 to 24 weeks following the intervention were included in the study analysis. Patients were excluded from the analysis if they received prescriptions for simvastatin or gemfibrozil from pharmacies outside the VA system, if their cholesterol medications were adjusted during the observation period outside of the initial intervention, if they had any lifetime history of TG > 500 mg/dL or pancreatitis, or if the subjects were incarcerated or pregnant at any time during the study period.
Relevant data were collected, using the VistA (Veterans Health Information Systems and Technology Architecture) and CPRS medical record documentation systems. Patient demographic data, including age, gender, treatment indication, past medical history, adverse drug reaction history, and prescription history were collected for analysis of the study population baseline characteristics. In addition, pertinent laboratory data were examined, including lipid parameters (total cholesterol [TC]; LDL-C; high-density lipoprotein cholesterol [HDL-C]; and TG) and liver function (LF) markers (aspartate aminotransferase [AST]/alanine aminotransferase [ALT]), both before and after the intervention. Patient charts were manually reviewed at follow-up for any adverse events (AEs) attributed to the cholesterol medications.
The primary endpoints included the average change from baseline in lipid parameters, including TC, LDL-C, HDL-C, and TG in each group. Secondary endpoints included descriptions of any safety concerns or AEs. The study was approved on December 15, 2011, by the Medical University of South Carolina internal review board and the VA research and development committee. Statistical analysis was completed, using the paired Student t test for continuous data, and a descriptive analysis was performed for AEs.
Results
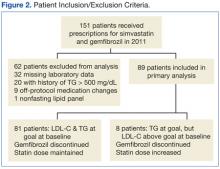
Initial patient retrieval included 151 patients who had received prescriptions for gemfibrozil and simvastatin during 2010-2011 and who also had a TG level of ≤ 150 mg/dL obtained in the previous year. Of these patients, 32 patients were missing laboratory data, 20 patients had a lifetime history of a TG level of > 500 mg/dL or pancreatitis, 9 patients had off protocol medication changes, and 1 patient had a nonfasting lipid panel. A total of 62 patients were excluded from the final analysis (Figure 2).
Eighty-nine patients were included in the primary analysis; 8 of those patients were meeting their TG goal but had an LDL-C elevated from goal at baseline, and 81 patients were meeting both their TG and LDL-C goals at baseline. The baseline demographics were reflective of a typical VA population: 99% male, average age 67.3 years, 38% white and 10% African American (52% did not disclose a racial identification).
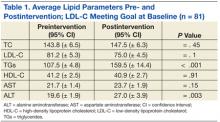
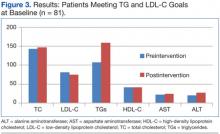
The primary efficacy goal of change in average lipid parameters after intervention was assessed at 6 to 24 weeks postintervention. Eighty-one patients who met both TG and LDL-C goals at baseline were evaluated (Table 1, Figure 3). Average TC, LDL-C, HDL-C, and AST were not significantly different before and after intervention. There was a statistically significant difference in the average TG levels preintervention and postintervention (107.5 mg/dL vs 159.5 mg/dL; P < .001). Average ALT was significantly higher in the postintervention group (19.6 vs 27; P < .001).
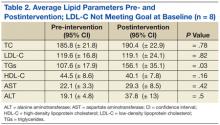
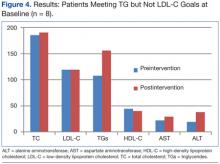
In patients with TGs ≤ 150 mg/dL who did not meet the LDL-C goal at baseline, there was also a statistically significant difference in average TG levels pre- and postintervention (107.6 mg/dL vs 156.1 mg/dL; P = .01). Average LDL-C did not significantly change pre- and postintervention (119.6 mg/dL vs 119.1 mg/dL; P = .82), nor did TC, HDL-C, or AST/ALT (Table 2, Figure 4).
There were no AEs reported in the 6 to 24 weeks following the intervention that could have been attributed to the cholesterol medications. In addition, there were no new diagnoses of pancreatitis entered in the patient’s medical records in the follow-up period. Of note, the range of TG levels after discontinuation of gemfibrozil was 38 mg/dL to 341 mg/dL. After the intervention, 17 of 89 patients (19%) had TG levels of > 200 mg/dL; however, only 1 of 89 patients had a TG level of > 300 mg/dL. No patients had a measured TG level of > 500 mg/dL after the intervention.
Discussion
There was a statistically significant increase in TG concentrations in both groups after discontinuation of gemfibrozil, regardless of change in statin dose. However, the clinical significance of this increase is debatable. The NCEP ATP III guidelines define a TG goal of < 150 mg/dL, and in this study, the average TG level after discontinuation of gemfibrozil was 159.5 mg/dL in the group meeting LDL-C goal and 156.1 mg/dL in the group not meeting LDL-C goal. Following this strict definition, patients did not maintain TG control after the study intervention. Nevertheless, 80% of patients moved from the “normal” category (TG < 150 mg/dL) preintervention to the “borderline high” category (TG 150-200 mg/dL) postintervention per ATP III definitions.3
The importance of TGs as an independent marker for cardiovascular risk has been debated for decades. Data from the Copenhagen City Heart Study indicated that plasma TG levels were significantly associated with increased risk of nonhemorrhagic ischemic events. The relative risk (RR) of ischemic stroke was increased 1.12 (95% confidence interval [CI], 1.07 – 1.16) for every 88.6 mg/dL increase in TGs in that population.14
A meta-analysis of 17 prospective studies of Western subjects found TGs to be an independent risk factor for cardiovascular disease (CVD) endpoints; RR was 1.14 in men (95% CI, 1.05 – 1.28) and 1.37 in women (95% CI, 1.13 – 1.66) per 88.6 mg/dL increase in TGs.15 The association of increased TG concentrations with increased risk of stroke has been validated in 2 other meta-analyses.16,17 Based on these data, there is a correlation between elevated TGs and CVD; however, as Dr. Jerzy-Roch Nofer discusses in an editorial published in 2011 in Current Opinion in Lipidology, the importance of a risk factor is contingent on finding benefit with treatment.18
Two large interventional studies, ACCORD (Action to Control Cardiovascular Risk in Diabetes trial) and FIELDS (Fenofibrate Intervention and Event Lowering in Diabetes trial), did not show a beneficial effect on cardiovascular morbidity and mortality after a clear TG reduction.19,20 The ACCORD and FIELDS studies suggest that although elevated TGs may be associated with elevated cardiovascular risk, no clear protective relationship exists when TGs are reduced to the goal level. Patients in this study who had gemfibrozil discontinued exhibited an increase in TG concentrations; however, the clinical significance of the change is minimal.
Additionally, none of the patients in this study had a TG concentration ≥ 500 mg/dL after the intervention; only 1 of 89 had a TG ≥ 300 mg/dL. This information indicates that most of the patients who were receiving both gemfibrozil and simvastatin likely did not need medical management of their TG levels to begin with. As mentioned earlier, numerous studies did not find a direct causal relationship between the reduction of cardiovascular risk after treating TG concentrations and the NCEP ATP III goal < 150 mg/dL.
There was a small yet significant increase in average ALT in the group of patients who met both LDL-C and TG goals at baseline at follow-up. The increase in ALT met criteria for statistical significance, but both values were maintained within the range of normal values (defined as 7 to 55 units/L by the Mayo Clinic).21 Additionally, the FDA no longer recommends routine monitoring of LF tests during statin therapy in patients with no history of abnormal results.22 The authors concluded that the change in ALT was an incidental finding and did not require further investigation.
The retrospective study design and small patient population limit the external validity of the study. Additionally, the authors did not assess patient compliance with therapy before or after the intervention, which may have skewed the results. Future studies may be designed in a randomized, controlled fashion and would ideally include a broader patient population.
This study provides evidence that can be used in future clinical decisions. Patients in this study may have had slightly elevated TG levels when gemfibrozil was initiated; however, all patients with a history of TG ≥ 500 mg/dL were excluded in this study. None of the patients reached the critical threshold at study follow-up despite a history of TG ≥150 mg/dL (but ≤ 500 mg/dL). This study provides further compelling information that practitioners should aggressively focus on reaching LDL-C and non−HDL-C goals before addressing TG concentrations.
Conclusions
Implementation of an automatic conversion protocol in patients prescribed both simvastatin and gemfibrozil with a baseline TG ≤ 150 mg/dL did not adversely affect lipid control. Patients whose LDL-C met goal preintervention maintained their LDL-C goal at follow-up. Additionally, patients who were not meeting LDL-C goals did not have an increase in LDL-C after the intervention, although there was not a significant improvement in LDL-C either. Both groups demonstrated a statistically significant increase in TG levels after discontinuation of gemfibrozil; however, the clinical significance of the TG change was limited. The results of this study support eliminating gemfibrozil from a statin-containing regimen in patients with low TG who are prescribed the combination.
Author disclosures
The authors report no actual or potential conflicts of interest with regard to this article.
Disclaimer
The opinions expressed herein are those of the authors and do not necessarily reflect those of Federal Practitioner, Frontline Medical Communications Inc., the U.S. Government, or any of its agencies. This article may discuss unlabeled or investigational use of certain drugs. Please review the complete prescribing information for specific drugs or drug combinations—including indications, contraindications, warnings, and adverse effects—before administering pharmacologic therapy to patients.
1. Heart Protection Study Collaborative Group. MRC/BHF Heart Protection Study of cholesterol lowering with simvastatin in 20,536 high-risk individuals: A randomised placebo-controlled trial. Lancet. 2002;360(9326):7-22.
2. Scandinavian Simvastatin Survival Study Group. Randomised trial of cholesterol lowering in 4444 patients with coronary heart disease: The Scandinavian simvastatin survival study (4S). Lancet. 1994;344(8934):1383-1389.
3. Expert Panel on Detection, Evaluation and Treatment of High Blood Cholesterol in Adults. Executive summary of the third report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III). JAMA. 2001;285(19):2486-2497.
4. Hokanson JE, Austin MA. Plasma triglyceride level is a risk factor for cardiovascular disease independent of high density lipoprotein cholesterol level: A meta-analysis of population based prospective studies. J Cardiovasc Risk. 1996;3(2):213-219.
5 Gotto AM Jr. High-density lipoprotein cholesterol and triglycerides as therapeutic targets for preventing and treating coronary artery disease. Am Heart J. 2002;144(suppl 6):S33-S42.
6 Pedersen TR, Tobert JA. Benefits and risks of HMG-CoA reductase inhibitors in the prevention of coronary heart disease: A reappraisal. Drug Saf. 1996;14(1):11-24.
7. Zocor [package insert]. Whitehouse Station, NJ: Merck & Co, Inc; 2014.
8. Rubins HB, Robins SJ, Collins D, et al; for the Veterans Affairs High-Density Lipoprotein Cholesterol Intervention Trial Study Group. Gemfibrozil for the secondary prevention of coronary heart disease in men with low levels of high-density lipoprotein cholesterol. N Engl J Med. 1999;341(6):410-418.
9. Frick MH, Elo O, Haapa K, et al. Helsinki Heart Study: Primary-prevention trial with gemfibrozil in middle-aged men with dyslipidemia. Safety of treatment, changes in risk factors, and incidence of coronary heart disease. N Engl J Med. 1987;317(20):1237-1245.
10. Backman JT, Kyrklund C, Kivistö KT, Wang JS, Neuvonen PJ. Plasma concentrations of active simvastatin acid are increased by gemfibrozil. Clin Pharmacol Ther. 2000;68(2):122-129.
11. U.S. Food and Drug Administration. FDA Drug Safety Communication: High Dose Simvastatin. U.S. Food and Drug Administration Website. http://www.fda.gov/Drugs/DrugSafety/ucm256581.htm. Updated January 3, 2013. Accessed June 13, 2014.
12. SEARCH Collaborative Group, Armitage J, Bowman L, Wallendszus K, et al. Intensive lowering of LDL cholesterol with 80 mg versus 20 mg simvastatin daily in 12,064 survivors of myocardial infarction: A double-blind, randomised trial. Lancet. 2010;376(9753):1658-1669.
13. Gaist D, Rodríguez LA, Huerta C, Hallas J, Sindrup SH. Lipid-lowering drugs and risk of myopathy: A population based follow-up study. Epidemiology. 2001;12(1):565-569.
14. Lindenstrøm E, Boysen G, Nyboe J. Influence of total cholesterol, high density lipoprotein cholesterol, and triglycerides on risk of cerebrovascular disease: The Copenhagen City Heart Study. BMJ. 1994;309(6946):11-15.
15. Hokanson JE, Austin MA. Plasma triglyceride level is a risk factor for cardiovascular disease independent of high-density lipoprotein cholesterol level: A meta-analysis of population-based prospective studies.J Cardiovasc Risk. 1996;3(2):213-219.
16. Labreuche J, Deplanque D, Touboul PJ, Bruckert E, Amarenco P. Association between change in plasma triglyceride levels and risk of stroke and carotid atherosclerosis: Systematic review and meta-regression analysis. Atherosclerosis. 2010;212(1):9-15.
17. De Caterina R, Scarano M, Marfisi R, et al. Cholesterol-lowering interventions and stroke: Insights from a meta-analysis of randomized controlled trials. J Am Coll Cardiol. 2010;55(3):198-211.
18. Nofer JR. Hyperlipidemia and cardiovascular disease: Triglycerides—a revival of cardiovascular risk factor? Curr Opin Lipidol. 2011;22(4):319-321.
19. ACCORD Study Group; Ginsberg HN, Elam MB, Lovato LC, et al. Effects of combination lipid therapy in type 2 diabetes mellitus. N Eng J Med. 2010;362(17):1563-1574.
20. Keech A, Simes RJ, Barter P, et al; FIELD Study Investigators. Effects of long term fenofibrate therapy on cardiovascular events in 9795 people with type 2 diabetes mellitus (the FIELD study): Randomised controlled trial. Lancet. 2005;366(9500):1849-1861.
21. Mayo Clinic. Liver function tests. Mayo Clinic Website. http://www.mayoclinic.org/tests-procedures/liver-function-tests/basics/results/prc-20012602. Accessed May 30, 2014.
22. U.S. Food and Drug Administration. FDA drug safety communication: Important safety label changes to cholesterol-lowering statin drugs. U.S. Food and Drug Administration Website. http://www.fda.gov/Drugs/DrugSafety/ucm293101.htm#sa. Update July 7, 2012. Accessed May 30, 2014.
Elevated low-density lipoprotein cholesterol (LDL-C) is a major risk factor for the development of coronary artery disease (CAD). For the past decade, lowering LDL-C has been the main focus in the treatment of dyslipidemia.1-3 Significant evidence also exists that hypertriglyceridemia is related to complications, including pancreatitis, and may also be independently linked to cardiovascular risk.4,5
Current treatment guidelines, published by the National Cholesterol Education Program Adult Treatment Panel III (NCEP ATP III), establish LDL-C reduction as the primary goal and triglyceride (TG) reduction as the secondary goal. If TG levels are significantly elevated (> 500 mg/dL), then TG becomes the primary goal due to increased risk of pancreatitis.3
Simvastatin, a 3-hydroxy-3-methylglutaryl-coenzyme (HMG-CoA) reductase inhibitor, has long been the drug of choice in the VA system for the treatment of dyslipidemia, despite its risks, which include myalgias, myopathy, and rhabdomyolysis.6 Incidence of true statin-induced myopathy or rhabdomyolysis is very low, estimated to occur in < 1% of high-dose simvastatin users; the benefits of statin use are often thought to outweigh the risks of therapy.6,7
In the Helsinki Heart Study and the Veterans Affairs High-Density Lipoprotein Cholesterol Intervention Trial (VA-HIT), gemfibrozil reduced TG concentrations by 20% to 50%. Gemfibrozil was shown to be useful in both the primary and secondary prevention of CAD and demonstrated mortality reduction in patients with CAD.8,9 The combination of gemfibrozil with simvastatin has been discouraged due to the increased risk of muscle-related complications; however, in practice, the medications are often prescribed concomitantly. A pharmacokinetic study reported that gemfibrozil increased the measured area under the curve concentration of simvastatin 2-fold, likely the reason that rates of myopathy and rhabdomyolysis are 6 times greater when simvastatin is used in combination with gemfibrozil.10
In June 2011, the FDA released new recommendations on the use of simvastatin, which included a dose limit of 40 mg (previously the maximum simvastatin dose was 80 mg) and new drug combination contraindications.11 These recommendations were made in light of the SEARCH (Study of the Effectiveness of Additional Reductions in Cholesterol and Homocysteine) trial, a large, randomized, placebo-controlled trial that revealed a significantly higher rate of myopathy and rhabdomyolysis with simvastatin than had other previous studies.12 Medications cited to increase risk of simvastatin-induced muscle injury included gemfibrozil, and combination therapy is now considered a contraindicated treatment option.10-13
In August 2011, the Pharmacy and Therapeutics (P&T) Committee at the Ralph H. Johnson VA Medical Center in Charleston, South Carolina, reviewed the FDA warning and approved an automatic conversion protocol to be applied to patients prescribed both gemfibrozil and simvastatin. First, the primary goal of therapy (either TG or LDL-C reduction) was determined. TG reduction was considered the primary goal if patients had a history of TG level of > 500 mg/dL or TG-induced pancreatitis; LDL-C was the primary goal in all other patients.
Once the goal of therapy was determined, options for intervention included discontinuation of gemfibrozil, statin dose escalation, and/or addition of niacin or fish oil. In patients whose TG concentration met NCEP goal < 150 mg/dL, gemfibrozil was discontinued. Statin dose was escalated if needed for further LDL-C reduction. Per P&T recommendations, a clinical pharmacist evaluated each patient, made an intervention based on the P&T approved protocol, and sent a letter, which described the intervention and the reason for the action, to each patient.
This study evaluated the outcome of the P&T committee-approved automatic conversion protocol in subgroups of patients prescribed simvastatin and gemfibrozil with a TG serum concentration ≤ 150 mg/dL at baseline (Figure 1).
Methods
A retrospective chart review was conducted on all patients who had prescriptions for gemfibrozil and simvastatin, prescribed in the 52 weeks before August 15, 2011; patient records were reviewed between September 1, 2011, and April 30, 2012. Patients were included for study analysis if they underwent a P&T committee-approved conversion algorithm between September 1, 2011, and December 31, 2011, were aged 18 to 88 years, and their most recent TG measurement was ≤ 150 mg/dL.
Only those patients who had a fasting lipid panel documented in the 56 weeks preceding the study intervention and who returned for a follow-up lipid panel within 6 to 24 weeks following the intervention were included in the study analysis. Patients were excluded from the analysis if they received prescriptions for simvastatin or gemfibrozil from pharmacies outside the VA system, if their cholesterol medications were adjusted during the observation period outside of the initial intervention, if they had any lifetime history of TG > 500 mg/dL or pancreatitis, or if the subjects were incarcerated or pregnant at any time during the study period.
Relevant data were collected, using the VistA (Veterans Health Information Systems and Technology Architecture) and CPRS medical record documentation systems. Patient demographic data, including age, gender, treatment indication, past medical history, adverse drug reaction history, and prescription history were collected for analysis of the study population baseline characteristics. In addition, pertinent laboratory data were examined, including lipid parameters (total cholesterol [TC]; LDL-C; high-density lipoprotein cholesterol [HDL-C]; and TG) and liver function (LF) markers (aspartate aminotransferase [AST]/alanine aminotransferase [ALT]), both before and after the intervention. Patient charts were manually reviewed at follow-up for any adverse events (AEs) attributed to the cholesterol medications.
The primary endpoints included the average change from baseline in lipid parameters, including TC, LDL-C, HDL-C, and TG in each group. Secondary endpoints included descriptions of any safety concerns or AEs. The study was approved on December 15, 2011, by the Medical University of South Carolina internal review board and the VA research and development committee. Statistical analysis was completed, using the paired Student t test for continuous data, and a descriptive analysis was performed for AEs.
Results
Initial patient retrieval included 151 patients who had received prescriptions for gemfibrozil and simvastatin during 2010-2011 and who also had a TG level of ≤ 150 mg/dL obtained in the previous year. Of these patients, 32 patients were missing laboratory data, 20 patients had a lifetime history of a TG level of > 500 mg/dL or pancreatitis, 9 patients had off protocol medication changes, and 1 patient had a nonfasting lipid panel. A total of 62 patients were excluded from the final analysis (Figure 2).
Eighty-nine patients were included in the primary analysis; 8 of those patients were meeting their TG goal but had an LDL-C elevated from goal at baseline, and 81 patients were meeting both their TG and LDL-C goals at baseline. The baseline demographics were reflective of a typical VA population: 99% male, average age 67.3 years, 38% white and 10% African American (52% did not disclose a racial identification).
The primary efficacy goal of change in average lipid parameters after intervention was assessed at 6 to 24 weeks postintervention. Eighty-one patients who met both TG and LDL-C goals at baseline were evaluated (Table 1, Figure 3). Average TC, LDL-C, HDL-C, and AST were not significantly different before and after intervention. There was a statistically significant difference in the average TG levels preintervention and postintervention (107.5 mg/dL vs 159.5 mg/dL; P < .001). Average ALT was significantly higher in the postintervention group (19.6 vs 27; P < .001).
In patients with TGs ≤ 150 mg/dL who did not meet the LDL-C goal at baseline, there was also a statistically significant difference in average TG levels pre- and postintervention (107.6 mg/dL vs 156.1 mg/dL; P = .01). Average LDL-C did not significantly change pre- and postintervention (119.6 mg/dL vs 119.1 mg/dL; P = .82), nor did TC, HDL-C, or AST/ALT (Table 2, Figure 4).
There were no AEs reported in the 6 to 24 weeks following the intervention that could have been attributed to the cholesterol medications. In addition, there were no new diagnoses of pancreatitis entered in the patient’s medical records in the follow-up period. Of note, the range of TG levels after discontinuation of gemfibrozil was 38 mg/dL to 341 mg/dL. After the intervention, 17 of 89 patients (19%) had TG levels of > 200 mg/dL; however, only 1 of 89 patients had a TG level of > 300 mg/dL. No patients had a measured TG level of > 500 mg/dL after the intervention.
Discussion
There was a statistically significant increase in TG concentrations in both groups after discontinuation of gemfibrozil, regardless of change in statin dose. However, the clinical significance of this increase is debatable. The NCEP ATP III guidelines define a TG goal of < 150 mg/dL, and in this study, the average TG level after discontinuation of gemfibrozil was 159.5 mg/dL in the group meeting LDL-C goal and 156.1 mg/dL in the group not meeting LDL-C goal. Following this strict definition, patients did not maintain TG control after the study intervention. Nevertheless, 80% of patients moved from the “normal” category (TG < 150 mg/dL) preintervention to the “borderline high” category (TG 150-200 mg/dL) postintervention per ATP III definitions.3
The importance of TGs as an independent marker for cardiovascular risk has been debated for decades. Data from the Copenhagen City Heart Study indicated that plasma TG levels were significantly associated with increased risk of nonhemorrhagic ischemic events. The relative risk (RR) of ischemic stroke was increased 1.12 (95% confidence interval [CI], 1.07 – 1.16) for every 88.6 mg/dL increase in TGs in that population.14
A meta-analysis of 17 prospective studies of Western subjects found TGs to be an independent risk factor for cardiovascular disease (CVD) endpoints; RR was 1.14 in men (95% CI, 1.05 – 1.28) and 1.37 in women (95% CI, 1.13 – 1.66) per 88.6 mg/dL increase in TGs.15 The association of increased TG concentrations with increased risk of stroke has been validated in 2 other meta-analyses.16,17 Based on these data, there is a correlation between elevated TGs and CVD; however, as Dr. Jerzy-Roch Nofer discusses in an editorial published in 2011 in Current Opinion in Lipidology, the importance of a risk factor is contingent on finding benefit with treatment.18
Two large interventional studies, ACCORD (Action to Control Cardiovascular Risk in Diabetes trial) and FIELDS (Fenofibrate Intervention and Event Lowering in Diabetes trial), did not show a beneficial effect on cardiovascular morbidity and mortality after a clear TG reduction.19,20 The ACCORD and FIELDS studies suggest that although elevated TGs may be associated with elevated cardiovascular risk, no clear protective relationship exists when TGs are reduced to the goal level. Patients in this study who had gemfibrozil discontinued exhibited an increase in TG concentrations; however, the clinical significance of the change is minimal.
Additionally, none of the patients in this study had a TG concentration ≥ 500 mg/dL after the intervention; only 1 of 89 had a TG ≥ 300 mg/dL. This information indicates that most of the patients who were receiving both gemfibrozil and simvastatin likely did not need medical management of their TG levels to begin with. As mentioned earlier, numerous studies did not find a direct causal relationship between the reduction of cardiovascular risk after treating TG concentrations and the NCEP ATP III goal < 150 mg/dL.
There was a small yet significant increase in average ALT in the group of patients who met both LDL-C and TG goals at baseline at follow-up. The increase in ALT met criteria for statistical significance, but both values were maintained within the range of normal values (defined as 7 to 55 units/L by the Mayo Clinic).21 Additionally, the FDA no longer recommends routine monitoring of LF tests during statin therapy in patients with no history of abnormal results.22 The authors concluded that the change in ALT was an incidental finding and did not require further investigation.
The retrospective study design and small patient population limit the external validity of the study. Additionally, the authors did not assess patient compliance with therapy before or after the intervention, which may have skewed the results. Future studies may be designed in a randomized, controlled fashion and would ideally include a broader patient population.
This study provides evidence that can be used in future clinical decisions. Patients in this study may have had slightly elevated TG levels when gemfibrozil was initiated; however, all patients with a history of TG ≥ 500 mg/dL were excluded in this study. None of the patients reached the critical threshold at study follow-up despite a history of TG ≥150 mg/dL (but ≤ 500 mg/dL). This study provides further compelling information that practitioners should aggressively focus on reaching LDL-C and non−HDL-C goals before addressing TG concentrations.
Conclusions
Implementation of an automatic conversion protocol in patients prescribed both simvastatin and gemfibrozil with a baseline TG ≤ 150 mg/dL did not adversely affect lipid control. Patients whose LDL-C met goal preintervention maintained their LDL-C goal at follow-up. Additionally, patients who were not meeting LDL-C goals did not have an increase in LDL-C after the intervention, although there was not a significant improvement in LDL-C either. Both groups demonstrated a statistically significant increase in TG levels after discontinuation of gemfibrozil; however, the clinical significance of the TG change was limited. The results of this study support eliminating gemfibrozil from a statin-containing regimen in patients with low TG who are prescribed the combination.
Author disclosures
The authors report no actual or potential conflicts of interest with regard to this article.
Disclaimer
The opinions expressed herein are those of the authors and do not necessarily reflect those of Federal Practitioner, Frontline Medical Communications Inc., the U.S. Government, or any of its agencies. This article may discuss unlabeled or investigational use of certain drugs. Please review the complete prescribing information for specific drugs or drug combinations—including indications, contraindications, warnings, and adverse effects—before administering pharmacologic therapy to patients.
Elevated low-density lipoprotein cholesterol (LDL-C) is a major risk factor for the development of coronary artery disease (CAD). For the past decade, lowering LDL-C has been the main focus in the treatment of dyslipidemia.1-3 Significant evidence also exists that hypertriglyceridemia is related to complications, including pancreatitis, and may also be independently linked to cardiovascular risk.4,5
Current treatment guidelines, published by the National Cholesterol Education Program Adult Treatment Panel III (NCEP ATP III), establish LDL-C reduction as the primary goal and triglyceride (TG) reduction as the secondary goal. If TG levels are significantly elevated (> 500 mg/dL), then TG becomes the primary goal due to increased risk of pancreatitis.3
Simvastatin, a 3-hydroxy-3-methylglutaryl-coenzyme (HMG-CoA) reductase inhibitor, has long been the drug of choice in the VA system for the treatment of dyslipidemia, despite its risks, which include myalgias, myopathy, and rhabdomyolysis.6 Incidence of true statin-induced myopathy or rhabdomyolysis is very low, estimated to occur in < 1% of high-dose simvastatin users; the benefits of statin use are often thought to outweigh the risks of therapy.6,7
In the Helsinki Heart Study and the Veterans Affairs High-Density Lipoprotein Cholesterol Intervention Trial (VA-HIT), gemfibrozil reduced TG concentrations by 20% to 50%. Gemfibrozil was shown to be useful in both the primary and secondary prevention of CAD and demonstrated mortality reduction in patients with CAD.8,9 The combination of gemfibrozil with simvastatin has been discouraged due to the increased risk of muscle-related complications; however, in practice, the medications are often prescribed concomitantly. A pharmacokinetic study reported that gemfibrozil increased the measured area under the curve concentration of simvastatin 2-fold, likely the reason that rates of myopathy and rhabdomyolysis are 6 times greater when simvastatin is used in combination with gemfibrozil.10
In June 2011, the FDA released new recommendations on the use of simvastatin, which included a dose limit of 40 mg (previously the maximum simvastatin dose was 80 mg) and new drug combination contraindications.11 These recommendations were made in light of the SEARCH (Study of the Effectiveness of Additional Reductions in Cholesterol and Homocysteine) trial, a large, randomized, placebo-controlled trial that revealed a significantly higher rate of myopathy and rhabdomyolysis with simvastatin than had other previous studies.12 Medications cited to increase risk of simvastatin-induced muscle injury included gemfibrozil, and combination therapy is now considered a contraindicated treatment option.10-13
In August 2011, the Pharmacy and Therapeutics (P&T) Committee at the Ralph H. Johnson VA Medical Center in Charleston, South Carolina, reviewed the FDA warning and approved an automatic conversion protocol to be applied to patients prescribed both gemfibrozil and simvastatin. First, the primary goal of therapy (either TG or LDL-C reduction) was determined. TG reduction was considered the primary goal if patients had a history of TG level of > 500 mg/dL or TG-induced pancreatitis; LDL-C was the primary goal in all other patients.
Once the goal of therapy was determined, options for intervention included discontinuation of gemfibrozil, statin dose escalation, and/or addition of niacin or fish oil. In patients whose TG concentration met NCEP goal < 150 mg/dL, gemfibrozil was discontinued. Statin dose was escalated if needed for further LDL-C reduction. Per P&T recommendations, a clinical pharmacist evaluated each patient, made an intervention based on the P&T approved protocol, and sent a letter, which described the intervention and the reason for the action, to each patient.
This study evaluated the outcome of the P&T committee-approved automatic conversion protocol in subgroups of patients prescribed simvastatin and gemfibrozil with a TG serum concentration ≤ 150 mg/dL at baseline (Figure 1).
Methods
A retrospective chart review was conducted on all patients who had prescriptions for gemfibrozil and simvastatin, prescribed in the 52 weeks before August 15, 2011; patient records were reviewed between September 1, 2011, and April 30, 2012. Patients were included for study analysis if they underwent a P&T committee-approved conversion algorithm between September 1, 2011, and December 31, 2011, were aged 18 to 88 years, and their most recent TG measurement was ≤ 150 mg/dL.
Only those patients who had a fasting lipid panel documented in the 56 weeks preceding the study intervention and who returned for a follow-up lipid panel within 6 to 24 weeks following the intervention were included in the study analysis. Patients were excluded from the analysis if they received prescriptions for simvastatin or gemfibrozil from pharmacies outside the VA system, if their cholesterol medications were adjusted during the observation period outside of the initial intervention, if they had any lifetime history of TG > 500 mg/dL or pancreatitis, or if the subjects were incarcerated or pregnant at any time during the study period.
Relevant data were collected, using the VistA (Veterans Health Information Systems and Technology Architecture) and CPRS medical record documentation systems. Patient demographic data, including age, gender, treatment indication, past medical history, adverse drug reaction history, and prescription history were collected for analysis of the study population baseline characteristics. In addition, pertinent laboratory data were examined, including lipid parameters (total cholesterol [TC]; LDL-C; high-density lipoprotein cholesterol [HDL-C]; and TG) and liver function (LF) markers (aspartate aminotransferase [AST]/alanine aminotransferase [ALT]), both before and after the intervention. Patient charts were manually reviewed at follow-up for any adverse events (AEs) attributed to the cholesterol medications.
The primary endpoints included the average change from baseline in lipid parameters, including TC, LDL-C, HDL-C, and TG in each group. Secondary endpoints included descriptions of any safety concerns or AEs. The study was approved on December 15, 2011, by the Medical University of South Carolina internal review board and the VA research and development committee. Statistical analysis was completed, using the paired Student t test for continuous data, and a descriptive analysis was performed for AEs.
Results
Initial patient retrieval included 151 patients who had received prescriptions for gemfibrozil and simvastatin during 2010-2011 and who also had a TG level of ≤ 150 mg/dL obtained in the previous year. Of these patients, 32 patients were missing laboratory data, 20 patients had a lifetime history of a TG level of > 500 mg/dL or pancreatitis, 9 patients had off protocol medication changes, and 1 patient had a nonfasting lipid panel. A total of 62 patients were excluded from the final analysis (Figure 2).
Eighty-nine patients were included in the primary analysis; 8 of those patients were meeting their TG goal but had an LDL-C elevated from goal at baseline, and 81 patients were meeting both their TG and LDL-C goals at baseline. The baseline demographics were reflective of a typical VA population: 99% male, average age 67.3 years, 38% white and 10% African American (52% did not disclose a racial identification).
The primary efficacy goal of change in average lipid parameters after intervention was assessed at 6 to 24 weeks postintervention. Eighty-one patients who met both TG and LDL-C goals at baseline were evaluated (Table 1, Figure 3). Average TC, LDL-C, HDL-C, and AST were not significantly different before and after intervention. There was a statistically significant difference in the average TG levels preintervention and postintervention (107.5 mg/dL vs 159.5 mg/dL; P < .001). Average ALT was significantly higher in the postintervention group (19.6 vs 27; P < .001).
In patients with TGs ≤ 150 mg/dL who did not meet the LDL-C goal at baseline, there was also a statistically significant difference in average TG levels pre- and postintervention (107.6 mg/dL vs 156.1 mg/dL; P = .01). Average LDL-C did not significantly change pre- and postintervention (119.6 mg/dL vs 119.1 mg/dL; P = .82), nor did TC, HDL-C, or AST/ALT (Table 2, Figure 4).
There were no AEs reported in the 6 to 24 weeks following the intervention that could have been attributed to the cholesterol medications. In addition, there were no new diagnoses of pancreatitis entered in the patient’s medical records in the follow-up period. Of note, the range of TG levels after discontinuation of gemfibrozil was 38 mg/dL to 341 mg/dL. After the intervention, 17 of 89 patients (19%) had TG levels of > 200 mg/dL; however, only 1 of 89 patients had a TG level of > 300 mg/dL. No patients had a measured TG level of > 500 mg/dL after the intervention.
Discussion
There was a statistically significant increase in TG concentrations in both groups after discontinuation of gemfibrozil, regardless of change in statin dose. However, the clinical significance of this increase is debatable. The NCEP ATP III guidelines define a TG goal of < 150 mg/dL, and in this study, the average TG level after discontinuation of gemfibrozil was 159.5 mg/dL in the group meeting LDL-C goal and 156.1 mg/dL in the group not meeting LDL-C goal. Following this strict definition, patients did not maintain TG control after the study intervention. Nevertheless, 80% of patients moved from the “normal” category (TG < 150 mg/dL) preintervention to the “borderline high” category (TG 150-200 mg/dL) postintervention per ATP III definitions.3
The importance of TGs as an independent marker for cardiovascular risk has been debated for decades. Data from the Copenhagen City Heart Study indicated that plasma TG levels were significantly associated with increased risk of nonhemorrhagic ischemic events. The relative risk (RR) of ischemic stroke was increased 1.12 (95% confidence interval [CI], 1.07 – 1.16) for every 88.6 mg/dL increase in TGs in that population.14
A meta-analysis of 17 prospective studies of Western subjects found TGs to be an independent risk factor for cardiovascular disease (CVD) endpoints; RR was 1.14 in men (95% CI, 1.05 – 1.28) and 1.37 in women (95% CI, 1.13 – 1.66) per 88.6 mg/dL increase in TGs.15 The association of increased TG concentrations with increased risk of stroke has been validated in 2 other meta-analyses.16,17 Based on these data, there is a correlation between elevated TGs and CVD; however, as Dr. Jerzy-Roch Nofer discusses in an editorial published in 2011 in Current Opinion in Lipidology, the importance of a risk factor is contingent on finding benefit with treatment.18
Two large interventional studies, ACCORD (Action to Control Cardiovascular Risk in Diabetes trial) and FIELDS (Fenofibrate Intervention and Event Lowering in Diabetes trial), did not show a beneficial effect on cardiovascular morbidity and mortality after a clear TG reduction.19,20 The ACCORD and FIELDS studies suggest that although elevated TGs may be associated with elevated cardiovascular risk, no clear protective relationship exists when TGs are reduced to the goal level. Patients in this study who had gemfibrozil discontinued exhibited an increase in TG concentrations; however, the clinical significance of the change is minimal.
Additionally, none of the patients in this study had a TG concentration ≥ 500 mg/dL after the intervention; only 1 of 89 had a TG ≥ 300 mg/dL. This information indicates that most of the patients who were receiving both gemfibrozil and simvastatin likely did not need medical management of their TG levels to begin with. As mentioned earlier, numerous studies did not find a direct causal relationship between the reduction of cardiovascular risk after treating TG concentrations and the NCEP ATP III goal < 150 mg/dL.
There was a small yet significant increase in average ALT in the group of patients who met both LDL-C and TG goals at baseline at follow-up. The increase in ALT met criteria for statistical significance, but both values were maintained within the range of normal values (defined as 7 to 55 units/L by the Mayo Clinic).21 Additionally, the FDA no longer recommends routine monitoring of LF tests during statin therapy in patients with no history of abnormal results.22 The authors concluded that the change in ALT was an incidental finding and did not require further investigation.
The retrospective study design and small patient population limit the external validity of the study. Additionally, the authors did not assess patient compliance with therapy before or after the intervention, which may have skewed the results. Future studies may be designed in a randomized, controlled fashion and would ideally include a broader patient population.
This study provides evidence that can be used in future clinical decisions. Patients in this study may have had slightly elevated TG levels when gemfibrozil was initiated; however, all patients with a history of TG ≥ 500 mg/dL were excluded in this study. None of the patients reached the critical threshold at study follow-up despite a history of TG ≥150 mg/dL (but ≤ 500 mg/dL). This study provides further compelling information that practitioners should aggressively focus on reaching LDL-C and non−HDL-C goals before addressing TG concentrations.
Conclusions
Implementation of an automatic conversion protocol in patients prescribed both simvastatin and gemfibrozil with a baseline TG ≤ 150 mg/dL did not adversely affect lipid control. Patients whose LDL-C met goal preintervention maintained their LDL-C goal at follow-up. Additionally, patients who were not meeting LDL-C goals did not have an increase in LDL-C after the intervention, although there was not a significant improvement in LDL-C either. Both groups demonstrated a statistically significant increase in TG levels after discontinuation of gemfibrozil; however, the clinical significance of the TG change was limited. The results of this study support eliminating gemfibrozil from a statin-containing regimen in patients with low TG who are prescribed the combination.
Author disclosures
The authors report no actual or potential conflicts of interest with regard to this article.
Disclaimer
The opinions expressed herein are those of the authors and do not necessarily reflect those of Federal Practitioner, Frontline Medical Communications Inc., the U.S. Government, or any of its agencies. This article may discuss unlabeled or investigational use of certain drugs. Please review the complete prescribing information for specific drugs or drug combinations—including indications, contraindications, warnings, and adverse effects—before administering pharmacologic therapy to patients.
1. Heart Protection Study Collaborative Group. MRC/BHF Heart Protection Study of cholesterol lowering with simvastatin in 20,536 high-risk individuals: A randomised placebo-controlled trial. Lancet. 2002;360(9326):7-22.
2. Scandinavian Simvastatin Survival Study Group. Randomised trial of cholesterol lowering in 4444 patients with coronary heart disease: The Scandinavian simvastatin survival study (4S). Lancet. 1994;344(8934):1383-1389.
3. Expert Panel on Detection, Evaluation and Treatment of High Blood Cholesterol in Adults. Executive summary of the third report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III). JAMA. 2001;285(19):2486-2497.
4. Hokanson JE, Austin MA. Plasma triglyceride level is a risk factor for cardiovascular disease independent of high density lipoprotein cholesterol level: A meta-analysis of population based prospective studies. J Cardiovasc Risk. 1996;3(2):213-219.
5 Gotto AM Jr. High-density lipoprotein cholesterol and triglycerides as therapeutic targets for preventing and treating coronary artery disease. Am Heart J. 2002;144(suppl 6):S33-S42.
6 Pedersen TR, Tobert JA. Benefits and risks of HMG-CoA reductase inhibitors in the prevention of coronary heart disease: A reappraisal. Drug Saf. 1996;14(1):11-24.
7. Zocor [package insert]. Whitehouse Station, NJ: Merck & Co, Inc; 2014.
8. Rubins HB, Robins SJ, Collins D, et al; for the Veterans Affairs High-Density Lipoprotein Cholesterol Intervention Trial Study Group. Gemfibrozil for the secondary prevention of coronary heart disease in men with low levels of high-density lipoprotein cholesterol. N Engl J Med. 1999;341(6):410-418.
9. Frick MH, Elo O, Haapa K, et al. Helsinki Heart Study: Primary-prevention trial with gemfibrozil in middle-aged men with dyslipidemia. Safety of treatment, changes in risk factors, and incidence of coronary heart disease. N Engl J Med. 1987;317(20):1237-1245.
10. Backman JT, Kyrklund C, Kivistö KT, Wang JS, Neuvonen PJ. Plasma concentrations of active simvastatin acid are increased by gemfibrozil. Clin Pharmacol Ther. 2000;68(2):122-129.
11. U.S. Food and Drug Administration. FDA Drug Safety Communication: High Dose Simvastatin. U.S. Food and Drug Administration Website. http://www.fda.gov/Drugs/DrugSafety/ucm256581.htm. Updated January 3, 2013. Accessed June 13, 2014.
12. SEARCH Collaborative Group, Armitage J, Bowman L, Wallendszus K, et al. Intensive lowering of LDL cholesterol with 80 mg versus 20 mg simvastatin daily in 12,064 survivors of myocardial infarction: A double-blind, randomised trial. Lancet. 2010;376(9753):1658-1669.
13. Gaist D, Rodríguez LA, Huerta C, Hallas J, Sindrup SH. Lipid-lowering drugs and risk of myopathy: A population based follow-up study. Epidemiology. 2001;12(1):565-569.
14. Lindenstrøm E, Boysen G, Nyboe J. Influence of total cholesterol, high density lipoprotein cholesterol, and triglycerides on risk of cerebrovascular disease: The Copenhagen City Heart Study. BMJ. 1994;309(6946):11-15.
15. Hokanson JE, Austin MA. Plasma triglyceride level is a risk factor for cardiovascular disease independent of high-density lipoprotein cholesterol level: A meta-analysis of population-based prospective studies.J Cardiovasc Risk. 1996;3(2):213-219.
16. Labreuche J, Deplanque D, Touboul PJ, Bruckert E, Amarenco P. Association between change in plasma triglyceride levels and risk of stroke and carotid atherosclerosis: Systematic review and meta-regression analysis. Atherosclerosis. 2010;212(1):9-15.
17. De Caterina R, Scarano M, Marfisi R, et al. Cholesterol-lowering interventions and stroke: Insights from a meta-analysis of randomized controlled trials. J Am Coll Cardiol. 2010;55(3):198-211.
18. Nofer JR. Hyperlipidemia and cardiovascular disease: Triglycerides—a revival of cardiovascular risk factor? Curr Opin Lipidol. 2011;22(4):319-321.
19. ACCORD Study Group; Ginsberg HN, Elam MB, Lovato LC, et al. Effects of combination lipid therapy in type 2 diabetes mellitus. N Eng J Med. 2010;362(17):1563-1574.
20. Keech A, Simes RJ, Barter P, et al; FIELD Study Investigators. Effects of long term fenofibrate therapy on cardiovascular events in 9795 people with type 2 diabetes mellitus (the FIELD study): Randomised controlled trial. Lancet. 2005;366(9500):1849-1861.
21. Mayo Clinic. Liver function tests. Mayo Clinic Website. http://www.mayoclinic.org/tests-procedures/liver-function-tests/basics/results/prc-20012602. Accessed May 30, 2014.
22. U.S. Food and Drug Administration. FDA drug safety communication: Important safety label changes to cholesterol-lowering statin drugs. U.S. Food and Drug Administration Website. http://www.fda.gov/Drugs/DrugSafety/ucm293101.htm#sa. Update July 7, 2012. Accessed May 30, 2014.
1. Heart Protection Study Collaborative Group. MRC/BHF Heart Protection Study of cholesterol lowering with simvastatin in 20,536 high-risk individuals: A randomised placebo-controlled trial. Lancet. 2002;360(9326):7-22.
2. Scandinavian Simvastatin Survival Study Group. Randomised trial of cholesterol lowering in 4444 patients with coronary heart disease: The Scandinavian simvastatin survival study (4S). Lancet. 1994;344(8934):1383-1389.
3. Expert Panel on Detection, Evaluation and Treatment of High Blood Cholesterol in Adults. Executive summary of the third report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III). JAMA. 2001;285(19):2486-2497.
4. Hokanson JE, Austin MA. Plasma triglyceride level is a risk factor for cardiovascular disease independent of high density lipoprotein cholesterol level: A meta-analysis of population based prospective studies. J Cardiovasc Risk. 1996;3(2):213-219.
5 Gotto AM Jr. High-density lipoprotein cholesterol and triglycerides as therapeutic targets for preventing and treating coronary artery disease. Am Heart J. 2002;144(suppl 6):S33-S42.
6 Pedersen TR, Tobert JA. Benefits and risks of HMG-CoA reductase inhibitors in the prevention of coronary heart disease: A reappraisal. Drug Saf. 1996;14(1):11-24.
7. Zocor [package insert]. Whitehouse Station, NJ: Merck & Co, Inc; 2014.
8. Rubins HB, Robins SJ, Collins D, et al; for the Veterans Affairs High-Density Lipoprotein Cholesterol Intervention Trial Study Group. Gemfibrozil for the secondary prevention of coronary heart disease in men with low levels of high-density lipoprotein cholesterol. N Engl J Med. 1999;341(6):410-418.
9. Frick MH, Elo O, Haapa K, et al. Helsinki Heart Study: Primary-prevention trial with gemfibrozil in middle-aged men with dyslipidemia. Safety of treatment, changes in risk factors, and incidence of coronary heart disease. N Engl J Med. 1987;317(20):1237-1245.
10. Backman JT, Kyrklund C, Kivistö KT, Wang JS, Neuvonen PJ. Plasma concentrations of active simvastatin acid are increased by gemfibrozil. Clin Pharmacol Ther. 2000;68(2):122-129.
11. U.S. Food and Drug Administration. FDA Drug Safety Communication: High Dose Simvastatin. U.S. Food and Drug Administration Website. http://www.fda.gov/Drugs/DrugSafety/ucm256581.htm. Updated January 3, 2013. Accessed June 13, 2014.
12. SEARCH Collaborative Group, Armitage J, Bowman L, Wallendszus K, et al. Intensive lowering of LDL cholesterol with 80 mg versus 20 mg simvastatin daily in 12,064 survivors of myocardial infarction: A double-blind, randomised trial. Lancet. 2010;376(9753):1658-1669.
13. Gaist D, Rodríguez LA, Huerta C, Hallas J, Sindrup SH. Lipid-lowering drugs and risk of myopathy: A population based follow-up study. Epidemiology. 2001;12(1):565-569.
14. Lindenstrøm E, Boysen G, Nyboe J. Influence of total cholesterol, high density lipoprotein cholesterol, and triglycerides on risk of cerebrovascular disease: The Copenhagen City Heart Study. BMJ. 1994;309(6946):11-15.
15. Hokanson JE, Austin MA. Plasma triglyceride level is a risk factor for cardiovascular disease independent of high-density lipoprotein cholesterol level: A meta-analysis of population-based prospective studies.J Cardiovasc Risk. 1996;3(2):213-219.
16. Labreuche J, Deplanque D, Touboul PJ, Bruckert E, Amarenco P. Association between change in plasma triglyceride levels and risk of stroke and carotid atherosclerosis: Systematic review and meta-regression analysis. Atherosclerosis. 2010;212(1):9-15.
17. De Caterina R, Scarano M, Marfisi R, et al. Cholesterol-lowering interventions and stroke: Insights from a meta-analysis of randomized controlled trials. J Am Coll Cardiol. 2010;55(3):198-211.
18. Nofer JR. Hyperlipidemia and cardiovascular disease: Triglycerides—a revival of cardiovascular risk factor? Curr Opin Lipidol. 2011;22(4):319-321.
19. ACCORD Study Group; Ginsberg HN, Elam MB, Lovato LC, et al. Effects of combination lipid therapy in type 2 diabetes mellitus. N Eng J Med. 2010;362(17):1563-1574.
20. Keech A, Simes RJ, Barter P, et al; FIELD Study Investigators. Effects of long term fenofibrate therapy on cardiovascular events in 9795 people with type 2 diabetes mellitus (the FIELD study): Randomised controlled trial. Lancet. 2005;366(9500):1849-1861.
21. Mayo Clinic. Liver function tests. Mayo Clinic Website. http://www.mayoclinic.org/tests-procedures/liver-function-tests/basics/results/prc-20012602. Accessed May 30, 2014.
22. U.S. Food and Drug Administration. FDA drug safety communication: Important safety label changes to cholesterol-lowering statin drugs. U.S. Food and Drug Administration Website. http://www.fda.gov/Drugs/DrugSafety/ucm293101.htm#sa. Update July 7, 2012. Accessed May 30, 2014.