User login
Chronic obstructive pulmonary disease (COPD) usually is not diagnosed until clinically apparent and moderately advanced. Patients might not notice chronic dyspnea and smoker’s cough, or might consider their symptoms “normal” and not seek medical care. Delayed diagnosis is particularly prevalent in the psychiatric population, in which co-existing medical problems tend to remain unrecognized and untreated.1
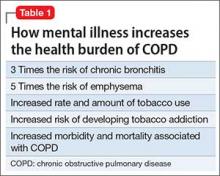
Life expectancy of people with serious mental illness (SMI) is 13 to 30 years less than that of the general population—a gap that has widened over time.2 Pulmonary disease is a leading cause of elevated mortality risk in SMI, along with cardiovascular and infectious disease, diabetes, and barriers to care. Having a comorbid mental illness triples the mortality risk of chronic lower respiratory disease (Table 1).3
This article describes how you can intervene and improve quality of life for your patients with COPD by:
- asking all patients, especially smokers, if they are experiencing classic symptoms of COPD
- advocating for and supporting smoking cessation efforts
- avoiding drug interactions and off-target dosing related to COPD and nicotine replacement therapy
- considering, if feasible, a switch from typical to atypical antipsychotic therapy, which could reduce smoking behavior.
What is COPD?
COPD is preventable and treatable. It is characterized by “persistent airflow limitation that is usually progressive and associated with an enhanced chronic inflammatory response in the airways and the lungs to inhaled noxious particles or gases.”4
Smoking tobacco is the greatest risk factor for developing COPD.5 An estimated 50% to 80% of people with schizophrenia are smokers, as are 55% of people with bipolar disorder.6 COPD is a leading cause of morbidity and mortality worldwide,7,8 and its prevalence is projected to increase as the global population and smoking rates grow.9
A simplified schema of the pathophysiology of COPD implicates 4 lung areas: parenchyma, pulmonary vasculature, central airways, and peripheral airways.10 Variation in the areas affected and severity of change contributes to the disease’s heterogeneous presentation, which can include pulmonary hypertension, hypersecretion of mucus, ciliary dysfunction, airway hyperinflation, and impaired gas exchange.11,12 Many of these features lead to systemic effects as well, particularly on cardiac function.
When to test a patient for COPD
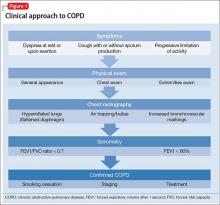
Early diagnosis and treatment can substantially improve quality-of-life outcomes for patients with COPD. The clinical approach (Figure 1) begins with recognizing classic symptoms. Consider COPD in any patient with:
- dyspnea (particularly if becoming worse, persistent, or associated with exercise)
- chronic cough
- chronic sputum production
- history of risk-factor exposure (particularly tobacco smoke)
- family history of COPD.4
If the history and physical exam suggest COPD (Table 2), spirometry is the most reliable test to quantify and characterize lung dysfunction. It is not indicated as a screening tool for healthy adults or appropriate when a patient is acutely ill. Forced expiratory volume in the first second of expiration divided by the measured forced vital capacity (FEV1/FVC) < 0.7 defines clinical COPD and determines the need for pharmacologic intervention. Laboratory studies could be useful in certain clinical scenarios, such as serum testing for alpha1-antitrypsin deficiency in patients age <45 with emphysema. Plain film imaging might be useful to support a COPD diagnosis or rule out alternate diagnoses.
Psychopharmacology issues with comorbid COPD
Pharmacotherapy for psychiatric disorders can exacerbate comorbid COPD. For example, long-term use of phenothiazine-related typical antipsychotics for schizophrenia has been linked to an increased incidence of COPD.13 Antipsychotic side effects such as acute laryngeal dystonia and tardive dyskinesia, most commonly seen with first-generation antipsychotic use, can aggravate dyspnea caused by COPD. Opioids and most hypnotics, sedatives, and anxiolytics suppress the respiratory drive, and therefore should be used with caution in patients with COPD.
Carefully monitor serum levels of medications before and during attempts at smoking cessation. Nicotine’s induction of the cytochrome P450 1A2 system increases the metabolism of antipsychotics such as clozapine, fluvoxamine, olanzapine, and haloperidol. As a result, potentially toxic drug levels can occur when a smoker tries to quit.14
Screen patients with COPD for comorbid psychiatric conditions. New psychiatric symptoms can emerge after COPD has been diagnosed, even in patients without pre-existing psychopathology.
Anxiety is a particularly common COPD comorbidity that can be difficult to manage. Selective serotonin reuptake inhibitors, buspirone, cognitive-behavioral therapy, and pulmonary rehabilitation can be helpful, although the effect of antidepressants on respiration is controversial. Nortriptyline has been shown to be effective in treating both anxiety and depressive symptoms in patients with COPD.15 Avoid using hypnotics to manage sleep problems related to COPD; instead, focus on minimizing sleep disturbance by limiting cough and dyspnea.
Antipsychotics and nicotine metabolism
Multiple studies have focused on the interplay among nicotine, dopamine, and antipsychotic agents. Nicotine receptors are present in the ventral tegmental dopaminergic cell bodies, which induce the release of dopamine and other neurotransmitters when stimulated. Smoking has been noted to increase in patients administered haloperidol (a dopamine antagonist) and to decrease with administration of bromocriptine (a dopamine agonist).16 This suggests that psychiatric patients might smoke to overcome the dopamine blockade caused by most typical antipsychotics, therefore alleviating their negative and extrapyramidal side effects.17
Alternatively, some studies suggest that a difference in dopamine receptor occupancy between typical and atypical antipsychotics leads to different effects on smoking behavior.18 When used long term, typical antipsychotics might increase dopamine receptors or dopamine sensitivity, and thus reinforce the positive effect of nicotine by increasing the number of receptors that can be stimulated, whereas atypical antipsychotics help stimulate the release of dopamine directly through partial agonist of serotonin 5-HT1A receptors.19,20 Atypical antipsychotics also appear to decrease cue-elicited cravings in people who are not mentally ill, whereas haloperidol does not.21
Based on these findings, switching patients with COPD from a typical to an atypical antipsychotic, if feasible, might make smoking cessation more manageable.22 Multiple studies have shown that clozapine is the preferred atypical antipsychotic because it is associated with the most significant decrease in smoking behaviors.23
First-line therapy: Nicotine replacement
Smoking cessation slows the progression of COPD and leads to marked improvements in cough, expectoration, breathlessness, and wheezing.24,25 Nicotine replacement therapy (NRT)—gum, inhaler, lozenges, nasal spray, and skin patch—is considered first-line pharmacotherapy. These nicotine substitutes can decrease withdrawal symptoms, although they do not appear to be as effective for light smokers (eg, <10 cigarettes/d), compared with heavy smokers (eg, ≥20 cigarettes/d).26
Long-term smoking abstinence can be improved with combination therapies. A nicotine patch, kept in place for as long as 24 hours, often is used with a nicotine gum or nasal spray. Another option combines the patch with a first-line, non-NRT intervention, such as sustained-release bupropion. Use bupropion with caution in psychiatric patients, however. Do not combine it with a monoamine oxidase inhibitor, and do not prescribe it to patients with an eating disorder or history of seizures.26 Bupropion could induce mania in patients with bipolar disorder.
Varenicline, a nicotinic receptor partial agonist indicated to aid in smoking cessation, has been shown to reduce pleasure gained from tobacco as well as cravings. It can increase the likelihood of abstinence from smoking for as long as 1 year, but it also can provoke behavioral changes, depressed mood, and suicidal ideation. These risks—described in an FDA black-box warning of serious neuropsychiatric events—warrant due caution when prescribing varenicline to patients with depression. The FDA also has warned that varenicline could lead to decreased alcohol tolerance and atypically aggressive behavior during intoxication, which is of particular concern because of the high rate of alcohol use among people with SMI.
Motivating and supporting change
When counseling patients with mental illness about smoking cessation, consider unique motivations that, if disregarded, could undermine your efforts. As described above, smoking can ameliorate negative and extrapyramidal symptoms associated with typical antipsychotics. This could explain the significantly higher rates of smoking associated with typical antipsychotics, compared with atypical antipsychotics.27 Patients also could use smoking as self-medication for depression and anxiety. Therefore, take care to offer alternate methods for coping, along with smoking cessation recommendations.22
Screen all adult patients for tobacco use, and offer prompt cessation counseling and pharmacologic interventions.28As a motivational intervention, the “5 As” framework—ask, advise, assess, assist, arrange—can help gauge patients’ smoking status and willingness to quit, as well as emphasize the importance of establishing a concrete, manageable plan.29
Keep in mind the barriers all patients face in their fight to quit smoking, such as nicotine withdrawal, weight gain, and loss of a coping mechanism for stress.29 Patients with schizophrenia can be motivated to quit smoking and participate in treatment for nicotine dependence.30
Besides encouraging smoking cessation, you can educate patients in behaviors that will improve COPD symptoms and management. These include:
- reducing the risk of lung infections through vaccinations (influenza yearly, pneumonia once in adulthood) and avoiding crowds during peak cold and influenza season
- participating in physical activity, which could slow lung function decline
- adhering to prescribed medication
- eating a balanced diet
- seeking medical care early during an exacerbation.
Coaching patients in symptom control
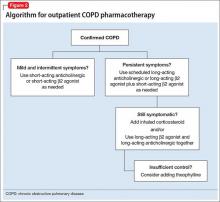
Smoking cessation may have the greatest long-term benefit for patients with COPD, but symptom management is important as well (Figure 2). Pharmacotherapy for COPD usually is advanced in steps, but a more aggressive approach may be necessary for patients presenting with severe symptoms.
Mainstays of COPD therapy are inhaled bronchodilators, consisting of β2 agonists and anticholinergics, alone or in combination. Short-acting formulations are used for mild and intermittent symptoms; long-acting bronchodilators are added if symptoms persist.4 When dyspnea, wheezing, and activity intolerance are not well-controlled with bronchodilators, an inhaled corticosteroid can be tried, either alone or in combination with a long-acting bronchodilator.4
Adherence to medical recommendations is critical for successful COPD management, but inhaled therapy can be difficult for psychiatric patients—especially patients with cognitive or functional impairment. Asking them to demonstrate their inhaler technique can help assess treatment effectiveness.31
Referral to a pulmonologist is strongly advised in cases of:
- advanced, end-stage COPD (FEV1 <50% predicted value despite adherence to recommended treatment, or rapid decline of FEV1)
- COPD in patients age <50
- frequent exacerbations
- possible complications related to chronic heart failure
- indications for oxygen treatment (eg, resting or ambulatory oxygen saturation ≤88% or PaO2 ≤55 mm Hg).32
1. Miller BJ, Paschall CB 3rd, Svendsen DP. Mortality and medical comorbidity among patients with serious mental illness. Psychiatr Serv. 2006;57(10):1482-1487.
2. Saha S, Chant D, McGrath J. A systematic review of mortality in schizophrenia: is the differential mortality gap worsening over time? Arch Gen Psychiatry. 2007;64(10):1123-1131.
3. Freeman E, Yoe JT. The poor health status of consumers of mental healthcare: behavioral disorders and chronic disease. Paper presented at: the National Association of State Mental Health Program Directors Medical Directors Workgroup; May 2006; Alexandria, VA.
4. Global Initiative for Chronic Obstructive Lung Disease. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease. http://www.goldcopd.org/uploads/users/files/GOLD_Report_2013_Feb20.pdf. Published February 20, 2013. Accessed March 2, 2016.
5. AntÒ JM, Vermeire P, Vestbo J, et al. Epidemiology of chronic obstructive pulmonary disease. Eur Respir J. 2001;17(5):982-994.
6. Newcomer JW. Antipsychotic medications: metabolic and cardiovascular risk. J Clin Psychiatry. 2007;68(suppl 4):8-13.
7. Calverley PM, Walker P. Chronic obstructive pulmonary disease. Lancet. 2003;362(9389):1053-1061.
8. Centers for Disease Control and Prevention. Chronic obstructive pulmonary disease among adults—United States, 2011. MMWR Morb Mortal Wkly Rep. 2012;61:938-943.
9. Feenstra TL, van Genugten ML, Hoogenveen RT, et al. The impact of aging and smoking on the future burden of chronic obstructive pulmonary disease: a model analysis in the Netherlands. Am J Respir Crit Care Med. 2001;164(4):590-596.
10. Celli BR, MacNee W; ATS/ERS Task Force. Standards for the diagnosis and treatment of patients with COPD: a summary of the ATS/ERS position paper [Erratum in: Eur Respir J. 2006;27(1):242]. Eur Respir J. 2004;23(6):932-946.
11. Matsuba K, Wright JL, Wiggs BR, et al. The changes in airways structure associated with reduced forced expiratory volume in one second. Eur Respir J. 1989;2(9):834-839.
12. O’Donnell DE, Revill SM, Webb KA. Dynamic hyperinflation and exercise intolerance in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2001;164(5):770-777.
13. Volkov VP. Respiratory diseases as a cause of death in schizophrenia [article in Russian]. Probl Tuberk Bolezn Legk. 2009;(6):24-27.
14. Kroon LA. Drug interactions and smoking: raising awareness for acute and critical care providers. Crit Care Nurs Clin North Am. 2006;18(1):53-62, xii.
15. Borson S, McDonald GJ, Gayle T, et al. Improvement in mood, physical symptoms, and function with nortriptyline for depression in patients with chronic obstructive pulmonary disease. Psychosomatics. 1992;33(2):190-201.
16. Caskey NH, Jarvik ME, Wirshing WC. The effects of dopaminergic D2 stimulation and blockade on smoking behavior. Exp Clin Psychopharmacol. 1999;7(1):72-78.
17. Dawe S, Gerada C, Russell MA, et al. Nicotine intake in smokers increases following a single dose of haloperidol. Psychopharmacol (Berl). 1995;117(1):110-115.
18. de Haan L, Booji J, Lavalaye J, et al. Occupancy of dopamine D2 receptors by antipsychotic drugs is related to nicotine addiction in young patients with schizophrenia. Psychopharmacology (Berl). 2006;183(4):500-505.
19. Hertel P, Nomikos GG, Iurlo M, et al. Risperidone: regional effects in vivo on release and metabolism of dopamine and serotonin in the rat brain. Psychopharmacology (Berl). 1996;124(1-2):74-86.
20. Silvestri S, Seeman MV, Negrete JC, et al. Increased dopamine D2 receptor binding after long-term treatment with antipsychotics in humans: a clinical PET study. Psychopharmacology (Berl). 2000;152(2):174-180.
21. Hutchison KE, Rutter MC, Niaura R, et al. Olanzapine attenuates cue-elicited craving for tobacco. Psychopharmacology (Berl). 2004;175(4):407-413.
22. Kumari V, Postma P. Nicotine use in schizophrenia: the self medication hypotheses. Neurosci Biobehav Rev. 2005;29(6):1021-1034.
23. Procyshyn RM, Tse G, Sin O, et al. Concomitant clozapine reduces smoking in patients treated with risperidone. Eur Neuropsychopharmacol. 2002;12(1):77-80.
24. Anthonisen NR, Connett JE, Kiley JP, et al. Effects of smoking intervention and the use of an inhaled anticholinergic bronchodilator on the rate of decline of FEV1. The Lung Health Study. JAMA. 1994;272(19):1497-1505.
25. Pisinger C, Godtfredsen NS. Is there a health benefit of reduced tobacco consumption? A systematic review. Nicotine Tob Res. 2007;9(6):631-646.
26. Fiore MC, Jaén CR, Baker TB, et al. Treating tobacco use and dependence: 2008 update. Clinical Practice Guideline. Rockville, MD: Public Health Service, US Department of Health and Human Services; 2008.
27. Barnes M, Lawford BR, Burton SC, et al. Smoking and schizophrenia: is symptom profile related to smoking and which antipsychotic medication is of benefit in reducing cigarette use? Aust N Z J Psychiatry. 2006;40(6-7):575-580.
28. Screening for chronic obstructive pulmonary disease using spirometry: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med. 2008;148(7):529-534.
29. Agency for Healthcare Research and Quality. Five major steps to intervention (The “5 A’s”). http://www.ahrq.gov/professionals/clinicians-providers/guidelines-recommendations/tobacco/5steps.html. Published 2012. Accessed March 2, 2016.
30. Addington J, el-Guebaly N, Campbell W, et al. Smoking cessation treatment for patients with schizophrenia. Am J Psychiatry. 1998;155(7):974-976.
31. Zarowitz BJ, O’Shea T. Chronic obstructive pulmonary disease: prevalence, characteristics, and pharmacologic treatment in nursing home residents with cognitive impairment. J Manag Care Pharm. 2012;18(8):598-606.
32. Schermer T, Smeenk F, van Weel C. Referral and consultation in asthma and COPD: an exploration of pulmonologists’ views. Neth J Med. 2003;61(3):71-81.
Chronic obstructive pulmonary disease (COPD) usually is not diagnosed until clinically apparent and moderately advanced. Patients might not notice chronic dyspnea and smoker’s cough, or might consider their symptoms “normal” and not seek medical care. Delayed diagnosis is particularly prevalent in the psychiatric population, in which co-existing medical problems tend to remain unrecognized and untreated.1
Life expectancy of people with serious mental illness (SMI) is 13 to 30 years less than that of the general population—a gap that has widened over time.2 Pulmonary disease is a leading cause of elevated mortality risk in SMI, along with cardiovascular and infectious disease, diabetes, and barriers to care. Having a comorbid mental illness triples the mortality risk of chronic lower respiratory disease (Table 1).3
This article describes how you can intervene and improve quality of life for your patients with COPD by:
- asking all patients, especially smokers, if they are experiencing classic symptoms of COPD
- advocating for and supporting smoking cessation efforts
- avoiding drug interactions and off-target dosing related to COPD and nicotine replacement therapy
- considering, if feasible, a switch from typical to atypical antipsychotic therapy, which could reduce smoking behavior.
What is COPD?
COPD is preventable and treatable. It is characterized by “persistent airflow limitation that is usually progressive and associated with an enhanced chronic inflammatory response in the airways and the lungs to inhaled noxious particles or gases.”4
Smoking tobacco is the greatest risk factor for developing COPD.5 An estimated 50% to 80% of people with schizophrenia are smokers, as are 55% of people with bipolar disorder.6 COPD is a leading cause of morbidity and mortality worldwide,7,8 and its prevalence is projected to increase as the global population and smoking rates grow.9
A simplified schema of the pathophysiology of COPD implicates 4 lung areas: parenchyma, pulmonary vasculature, central airways, and peripheral airways.10 Variation in the areas affected and severity of change contributes to the disease’s heterogeneous presentation, which can include pulmonary hypertension, hypersecretion of mucus, ciliary dysfunction, airway hyperinflation, and impaired gas exchange.11,12 Many of these features lead to systemic effects as well, particularly on cardiac function.
When to test a patient for COPD
Early diagnosis and treatment can substantially improve quality-of-life outcomes for patients with COPD. The clinical approach (Figure 1) begins with recognizing classic symptoms. Consider COPD in any patient with:
- dyspnea (particularly if becoming worse, persistent, or associated with exercise)
- chronic cough
- chronic sputum production
- history of risk-factor exposure (particularly tobacco smoke)
- family history of COPD.4
If the history and physical exam suggest COPD (Table 2), spirometry is the most reliable test to quantify and characterize lung dysfunction. It is not indicated as a screening tool for healthy adults or appropriate when a patient is acutely ill. Forced expiratory volume in the first second of expiration divided by the measured forced vital capacity (FEV1/FVC) < 0.7 defines clinical COPD and determines the need for pharmacologic intervention. Laboratory studies could be useful in certain clinical scenarios, such as serum testing for alpha1-antitrypsin deficiency in patients age <45 with emphysema. Plain film imaging might be useful to support a COPD diagnosis or rule out alternate diagnoses.
Psychopharmacology issues with comorbid COPD
Pharmacotherapy for psychiatric disorders can exacerbate comorbid COPD. For example, long-term use of phenothiazine-related typical antipsychotics for schizophrenia has been linked to an increased incidence of COPD.13 Antipsychotic side effects such as acute laryngeal dystonia and tardive dyskinesia, most commonly seen with first-generation antipsychotic use, can aggravate dyspnea caused by COPD. Opioids and most hypnotics, sedatives, and anxiolytics suppress the respiratory drive, and therefore should be used with caution in patients with COPD.
Carefully monitor serum levels of medications before and during attempts at smoking cessation. Nicotine’s induction of the cytochrome P450 1A2 system increases the metabolism of antipsychotics such as clozapine, fluvoxamine, olanzapine, and haloperidol. As a result, potentially toxic drug levels can occur when a smoker tries to quit.14
Screen patients with COPD for comorbid psychiatric conditions. New psychiatric symptoms can emerge after COPD has been diagnosed, even in patients without pre-existing psychopathology.
Anxiety is a particularly common COPD comorbidity that can be difficult to manage. Selective serotonin reuptake inhibitors, buspirone, cognitive-behavioral therapy, and pulmonary rehabilitation can be helpful, although the effect of antidepressants on respiration is controversial. Nortriptyline has been shown to be effective in treating both anxiety and depressive symptoms in patients with COPD.15 Avoid using hypnotics to manage sleep problems related to COPD; instead, focus on minimizing sleep disturbance by limiting cough and dyspnea.
Antipsychotics and nicotine metabolism
Multiple studies have focused on the interplay among nicotine, dopamine, and antipsychotic agents. Nicotine receptors are present in the ventral tegmental dopaminergic cell bodies, which induce the release of dopamine and other neurotransmitters when stimulated. Smoking has been noted to increase in patients administered haloperidol (a dopamine antagonist) and to decrease with administration of bromocriptine (a dopamine agonist).16 This suggests that psychiatric patients might smoke to overcome the dopamine blockade caused by most typical antipsychotics, therefore alleviating their negative and extrapyramidal side effects.17
Alternatively, some studies suggest that a difference in dopamine receptor occupancy between typical and atypical antipsychotics leads to different effects on smoking behavior.18 When used long term, typical antipsychotics might increase dopamine receptors or dopamine sensitivity, and thus reinforce the positive effect of nicotine by increasing the number of receptors that can be stimulated, whereas atypical antipsychotics help stimulate the release of dopamine directly through partial agonist of serotonin 5-HT1A receptors.19,20 Atypical antipsychotics also appear to decrease cue-elicited cravings in people who are not mentally ill, whereas haloperidol does not.21
Based on these findings, switching patients with COPD from a typical to an atypical antipsychotic, if feasible, might make smoking cessation more manageable.22 Multiple studies have shown that clozapine is the preferred atypical antipsychotic because it is associated with the most significant decrease in smoking behaviors.23
First-line therapy: Nicotine replacement
Smoking cessation slows the progression of COPD and leads to marked improvements in cough, expectoration, breathlessness, and wheezing.24,25 Nicotine replacement therapy (NRT)—gum, inhaler, lozenges, nasal spray, and skin patch—is considered first-line pharmacotherapy. These nicotine substitutes can decrease withdrawal symptoms, although they do not appear to be as effective for light smokers (eg, <10 cigarettes/d), compared with heavy smokers (eg, ≥20 cigarettes/d).26
Long-term smoking abstinence can be improved with combination therapies. A nicotine patch, kept in place for as long as 24 hours, often is used with a nicotine gum or nasal spray. Another option combines the patch with a first-line, non-NRT intervention, such as sustained-release bupropion. Use bupropion with caution in psychiatric patients, however. Do not combine it with a monoamine oxidase inhibitor, and do not prescribe it to patients with an eating disorder or history of seizures.26 Bupropion could induce mania in patients with bipolar disorder.
Varenicline, a nicotinic receptor partial agonist indicated to aid in smoking cessation, has been shown to reduce pleasure gained from tobacco as well as cravings. It can increase the likelihood of abstinence from smoking for as long as 1 year, but it also can provoke behavioral changes, depressed mood, and suicidal ideation. These risks—described in an FDA black-box warning of serious neuropsychiatric events—warrant due caution when prescribing varenicline to patients with depression. The FDA also has warned that varenicline could lead to decreased alcohol tolerance and atypically aggressive behavior during intoxication, which is of particular concern because of the high rate of alcohol use among people with SMI.
Motivating and supporting change
When counseling patients with mental illness about smoking cessation, consider unique motivations that, if disregarded, could undermine your efforts. As described above, smoking can ameliorate negative and extrapyramidal symptoms associated with typical antipsychotics. This could explain the significantly higher rates of smoking associated with typical antipsychotics, compared with atypical antipsychotics.27 Patients also could use smoking as self-medication for depression and anxiety. Therefore, take care to offer alternate methods for coping, along with smoking cessation recommendations.22
Screen all adult patients for tobacco use, and offer prompt cessation counseling and pharmacologic interventions.28As a motivational intervention, the “5 As” framework—ask, advise, assess, assist, arrange—can help gauge patients’ smoking status and willingness to quit, as well as emphasize the importance of establishing a concrete, manageable plan.29
Keep in mind the barriers all patients face in their fight to quit smoking, such as nicotine withdrawal, weight gain, and loss of a coping mechanism for stress.29 Patients with schizophrenia can be motivated to quit smoking and participate in treatment for nicotine dependence.30
Besides encouraging smoking cessation, you can educate patients in behaviors that will improve COPD symptoms and management. These include:
- reducing the risk of lung infections through vaccinations (influenza yearly, pneumonia once in adulthood) and avoiding crowds during peak cold and influenza season
- participating in physical activity, which could slow lung function decline
- adhering to prescribed medication
- eating a balanced diet
- seeking medical care early during an exacerbation.
Coaching patients in symptom control
Smoking cessation may have the greatest long-term benefit for patients with COPD, but symptom management is important as well (Figure 2). Pharmacotherapy for COPD usually is advanced in steps, but a more aggressive approach may be necessary for patients presenting with severe symptoms.
Mainstays of COPD therapy are inhaled bronchodilators, consisting of β2 agonists and anticholinergics, alone or in combination. Short-acting formulations are used for mild and intermittent symptoms; long-acting bronchodilators are added if symptoms persist.4 When dyspnea, wheezing, and activity intolerance are not well-controlled with bronchodilators, an inhaled corticosteroid can be tried, either alone or in combination with a long-acting bronchodilator.4
Adherence to medical recommendations is critical for successful COPD management, but inhaled therapy can be difficult for psychiatric patients—especially patients with cognitive or functional impairment. Asking them to demonstrate their inhaler technique can help assess treatment effectiveness.31
Referral to a pulmonologist is strongly advised in cases of:
- advanced, end-stage COPD (FEV1 <50% predicted value despite adherence to recommended treatment, or rapid decline of FEV1)
- COPD in patients age <50
- frequent exacerbations
- possible complications related to chronic heart failure
- indications for oxygen treatment (eg, resting or ambulatory oxygen saturation ≤88% or PaO2 ≤55 mm Hg).32
Chronic obstructive pulmonary disease (COPD) usually is not diagnosed until clinically apparent and moderately advanced. Patients might not notice chronic dyspnea and smoker’s cough, or might consider their symptoms “normal” and not seek medical care. Delayed diagnosis is particularly prevalent in the psychiatric population, in which co-existing medical problems tend to remain unrecognized and untreated.1
Life expectancy of people with serious mental illness (SMI) is 13 to 30 years less than that of the general population—a gap that has widened over time.2 Pulmonary disease is a leading cause of elevated mortality risk in SMI, along with cardiovascular and infectious disease, diabetes, and barriers to care. Having a comorbid mental illness triples the mortality risk of chronic lower respiratory disease (Table 1).3
This article describes how you can intervene and improve quality of life for your patients with COPD by:
- asking all patients, especially smokers, if they are experiencing classic symptoms of COPD
- advocating for and supporting smoking cessation efforts
- avoiding drug interactions and off-target dosing related to COPD and nicotine replacement therapy
- considering, if feasible, a switch from typical to atypical antipsychotic therapy, which could reduce smoking behavior.
What is COPD?
COPD is preventable and treatable. It is characterized by “persistent airflow limitation that is usually progressive and associated with an enhanced chronic inflammatory response in the airways and the lungs to inhaled noxious particles or gases.”4
Smoking tobacco is the greatest risk factor for developing COPD.5 An estimated 50% to 80% of people with schizophrenia are smokers, as are 55% of people with bipolar disorder.6 COPD is a leading cause of morbidity and mortality worldwide,7,8 and its prevalence is projected to increase as the global population and smoking rates grow.9
A simplified schema of the pathophysiology of COPD implicates 4 lung areas: parenchyma, pulmonary vasculature, central airways, and peripheral airways.10 Variation in the areas affected and severity of change contributes to the disease’s heterogeneous presentation, which can include pulmonary hypertension, hypersecretion of mucus, ciliary dysfunction, airway hyperinflation, and impaired gas exchange.11,12 Many of these features lead to systemic effects as well, particularly on cardiac function.
When to test a patient for COPD
Early diagnosis and treatment can substantially improve quality-of-life outcomes for patients with COPD. The clinical approach (Figure 1) begins with recognizing classic symptoms. Consider COPD in any patient with:
- dyspnea (particularly if becoming worse, persistent, or associated with exercise)
- chronic cough
- chronic sputum production
- history of risk-factor exposure (particularly tobacco smoke)
- family history of COPD.4
If the history and physical exam suggest COPD (Table 2), spirometry is the most reliable test to quantify and characterize lung dysfunction. It is not indicated as a screening tool for healthy adults or appropriate when a patient is acutely ill. Forced expiratory volume in the first second of expiration divided by the measured forced vital capacity (FEV1/FVC) < 0.7 defines clinical COPD and determines the need for pharmacologic intervention. Laboratory studies could be useful in certain clinical scenarios, such as serum testing for alpha1-antitrypsin deficiency in patients age <45 with emphysema. Plain film imaging might be useful to support a COPD diagnosis or rule out alternate diagnoses.
Psychopharmacology issues with comorbid COPD
Pharmacotherapy for psychiatric disorders can exacerbate comorbid COPD. For example, long-term use of phenothiazine-related typical antipsychotics for schizophrenia has been linked to an increased incidence of COPD.13 Antipsychotic side effects such as acute laryngeal dystonia and tardive dyskinesia, most commonly seen with first-generation antipsychotic use, can aggravate dyspnea caused by COPD. Opioids and most hypnotics, sedatives, and anxiolytics suppress the respiratory drive, and therefore should be used with caution in patients with COPD.
Carefully monitor serum levels of medications before and during attempts at smoking cessation. Nicotine’s induction of the cytochrome P450 1A2 system increases the metabolism of antipsychotics such as clozapine, fluvoxamine, olanzapine, and haloperidol. As a result, potentially toxic drug levels can occur when a smoker tries to quit.14
Screen patients with COPD for comorbid psychiatric conditions. New psychiatric symptoms can emerge after COPD has been diagnosed, even in patients without pre-existing psychopathology.
Anxiety is a particularly common COPD comorbidity that can be difficult to manage. Selective serotonin reuptake inhibitors, buspirone, cognitive-behavioral therapy, and pulmonary rehabilitation can be helpful, although the effect of antidepressants on respiration is controversial. Nortriptyline has been shown to be effective in treating both anxiety and depressive symptoms in patients with COPD.15 Avoid using hypnotics to manage sleep problems related to COPD; instead, focus on minimizing sleep disturbance by limiting cough and dyspnea.
Antipsychotics and nicotine metabolism
Multiple studies have focused on the interplay among nicotine, dopamine, and antipsychotic agents. Nicotine receptors are present in the ventral tegmental dopaminergic cell bodies, which induce the release of dopamine and other neurotransmitters when stimulated. Smoking has been noted to increase in patients administered haloperidol (a dopamine antagonist) and to decrease with administration of bromocriptine (a dopamine agonist).16 This suggests that psychiatric patients might smoke to overcome the dopamine blockade caused by most typical antipsychotics, therefore alleviating their negative and extrapyramidal side effects.17
Alternatively, some studies suggest that a difference in dopamine receptor occupancy between typical and atypical antipsychotics leads to different effects on smoking behavior.18 When used long term, typical antipsychotics might increase dopamine receptors or dopamine sensitivity, and thus reinforce the positive effect of nicotine by increasing the number of receptors that can be stimulated, whereas atypical antipsychotics help stimulate the release of dopamine directly through partial agonist of serotonin 5-HT1A receptors.19,20 Atypical antipsychotics also appear to decrease cue-elicited cravings in people who are not mentally ill, whereas haloperidol does not.21
Based on these findings, switching patients with COPD from a typical to an atypical antipsychotic, if feasible, might make smoking cessation more manageable.22 Multiple studies have shown that clozapine is the preferred atypical antipsychotic because it is associated with the most significant decrease in smoking behaviors.23
First-line therapy: Nicotine replacement
Smoking cessation slows the progression of COPD and leads to marked improvements in cough, expectoration, breathlessness, and wheezing.24,25 Nicotine replacement therapy (NRT)—gum, inhaler, lozenges, nasal spray, and skin patch—is considered first-line pharmacotherapy. These nicotine substitutes can decrease withdrawal symptoms, although they do not appear to be as effective for light smokers (eg, <10 cigarettes/d), compared with heavy smokers (eg, ≥20 cigarettes/d).26
Long-term smoking abstinence can be improved with combination therapies. A nicotine patch, kept in place for as long as 24 hours, often is used with a nicotine gum or nasal spray. Another option combines the patch with a first-line, non-NRT intervention, such as sustained-release bupropion. Use bupropion with caution in psychiatric patients, however. Do not combine it with a monoamine oxidase inhibitor, and do not prescribe it to patients with an eating disorder or history of seizures.26 Bupropion could induce mania in patients with bipolar disorder.
Varenicline, a nicotinic receptor partial agonist indicated to aid in smoking cessation, has been shown to reduce pleasure gained from tobacco as well as cravings. It can increase the likelihood of abstinence from smoking for as long as 1 year, but it also can provoke behavioral changes, depressed mood, and suicidal ideation. These risks—described in an FDA black-box warning of serious neuropsychiatric events—warrant due caution when prescribing varenicline to patients with depression. The FDA also has warned that varenicline could lead to decreased alcohol tolerance and atypically aggressive behavior during intoxication, which is of particular concern because of the high rate of alcohol use among people with SMI.
Motivating and supporting change
When counseling patients with mental illness about smoking cessation, consider unique motivations that, if disregarded, could undermine your efforts. As described above, smoking can ameliorate negative and extrapyramidal symptoms associated with typical antipsychotics. This could explain the significantly higher rates of smoking associated with typical antipsychotics, compared with atypical antipsychotics.27 Patients also could use smoking as self-medication for depression and anxiety. Therefore, take care to offer alternate methods for coping, along with smoking cessation recommendations.22
Screen all adult patients for tobacco use, and offer prompt cessation counseling and pharmacologic interventions.28As a motivational intervention, the “5 As” framework—ask, advise, assess, assist, arrange—can help gauge patients’ smoking status and willingness to quit, as well as emphasize the importance of establishing a concrete, manageable plan.29
Keep in mind the barriers all patients face in their fight to quit smoking, such as nicotine withdrawal, weight gain, and loss of a coping mechanism for stress.29 Patients with schizophrenia can be motivated to quit smoking and participate in treatment for nicotine dependence.30
Besides encouraging smoking cessation, you can educate patients in behaviors that will improve COPD symptoms and management. These include:
- reducing the risk of lung infections through vaccinations (influenza yearly, pneumonia once in adulthood) and avoiding crowds during peak cold and influenza season
- participating in physical activity, which could slow lung function decline
- adhering to prescribed medication
- eating a balanced diet
- seeking medical care early during an exacerbation.
Coaching patients in symptom control
Smoking cessation may have the greatest long-term benefit for patients with COPD, but symptom management is important as well (Figure 2). Pharmacotherapy for COPD usually is advanced in steps, but a more aggressive approach may be necessary for patients presenting with severe symptoms.
Mainstays of COPD therapy are inhaled bronchodilators, consisting of β2 agonists and anticholinergics, alone or in combination. Short-acting formulations are used for mild and intermittent symptoms; long-acting bronchodilators are added if symptoms persist.4 When dyspnea, wheezing, and activity intolerance are not well-controlled with bronchodilators, an inhaled corticosteroid can be tried, either alone or in combination with a long-acting bronchodilator.4
Adherence to medical recommendations is critical for successful COPD management, but inhaled therapy can be difficult for psychiatric patients—especially patients with cognitive or functional impairment. Asking them to demonstrate their inhaler technique can help assess treatment effectiveness.31
Referral to a pulmonologist is strongly advised in cases of:
- advanced, end-stage COPD (FEV1 <50% predicted value despite adherence to recommended treatment, or rapid decline of FEV1)
- COPD in patients age <50
- frequent exacerbations
- possible complications related to chronic heart failure
- indications for oxygen treatment (eg, resting or ambulatory oxygen saturation ≤88% or PaO2 ≤55 mm Hg).32
1. Miller BJ, Paschall CB 3rd, Svendsen DP. Mortality and medical comorbidity among patients with serious mental illness. Psychiatr Serv. 2006;57(10):1482-1487.
2. Saha S, Chant D, McGrath J. A systematic review of mortality in schizophrenia: is the differential mortality gap worsening over time? Arch Gen Psychiatry. 2007;64(10):1123-1131.
3. Freeman E, Yoe JT. The poor health status of consumers of mental healthcare: behavioral disorders and chronic disease. Paper presented at: the National Association of State Mental Health Program Directors Medical Directors Workgroup; May 2006; Alexandria, VA.
4. Global Initiative for Chronic Obstructive Lung Disease. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease. http://www.goldcopd.org/uploads/users/files/GOLD_Report_2013_Feb20.pdf. Published February 20, 2013. Accessed March 2, 2016.
5. AntÒ JM, Vermeire P, Vestbo J, et al. Epidemiology of chronic obstructive pulmonary disease. Eur Respir J. 2001;17(5):982-994.
6. Newcomer JW. Antipsychotic medications: metabolic and cardiovascular risk. J Clin Psychiatry. 2007;68(suppl 4):8-13.
7. Calverley PM, Walker P. Chronic obstructive pulmonary disease. Lancet. 2003;362(9389):1053-1061.
8. Centers for Disease Control and Prevention. Chronic obstructive pulmonary disease among adults—United States, 2011. MMWR Morb Mortal Wkly Rep. 2012;61:938-943.
9. Feenstra TL, van Genugten ML, Hoogenveen RT, et al. The impact of aging and smoking on the future burden of chronic obstructive pulmonary disease: a model analysis in the Netherlands. Am J Respir Crit Care Med. 2001;164(4):590-596.
10. Celli BR, MacNee W; ATS/ERS Task Force. Standards for the diagnosis and treatment of patients with COPD: a summary of the ATS/ERS position paper [Erratum in: Eur Respir J. 2006;27(1):242]. Eur Respir J. 2004;23(6):932-946.
11. Matsuba K, Wright JL, Wiggs BR, et al. The changes in airways structure associated with reduced forced expiratory volume in one second. Eur Respir J. 1989;2(9):834-839.
12. O’Donnell DE, Revill SM, Webb KA. Dynamic hyperinflation and exercise intolerance in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2001;164(5):770-777.
13. Volkov VP. Respiratory diseases as a cause of death in schizophrenia [article in Russian]. Probl Tuberk Bolezn Legk. 2009;(6):24-27.
14. Kroon LA. Drug interactions and smoking: raising awareness for acute and critical care providers. Crit Care Nurs Clin North Am. 2006;18(1):53-62, xii.
15. Borson S, McDonald GJ, Gayle T, et al. Improvement in mood, physical symptoms, and function with nortriptyline for depression in patients with chronic obstructive pulmonary disease. Psychosomatics. 1992;33(2):190-201.
16. Caskey NH, Jarvik ME, Wirshing WC. The effects of dopaminergic D2 stimulation and blockade on smoking behavior. Exp Clin Psychopharmacol. 1999;7(1):72-78.
17. Dawe S, Gerada C, Russell MA, et al. Nicotine intake in smokers increases following a single dose of haloperidol. Psychopharmacol (Berl). 1995;117(1):110-115.
18. de Haan L, Booji J, Lavalaye J, et al. Occupancy of dopamine D2 receptors by antipsychotic drugs is related to nicotine addiction in young patients with schizophrenia. Psychopharmacology (Berl). 2006;183(4):500-505.
19. Hertel P, Nomikos GG, Iurlo M, et al. Risperidone: regional effects in vivo on release and metabolism of dopamine and serotonin in the rat brain. Psychopharmacology (Berl). 1996;124(1-2):74-86.
20. Silvestri S, Seeman MV, Negrete JC, et al. Increased dopamine D2 receptor binding after long-term treatment with antipsychotics in humans: a clinical PET study. Psychopharmacology (Berl). 2000;152(2):174-180.
21. Hutchison KE, Rutter MC, Niaura R, et al. Olanzapine attenuates cue-elicited craving for tobacco. Psychopharmacology (Berl). 2004;175(4):407-413.
22. Kumari V, Postma P. Nicotine use in schizophrenia: the self medication hypotheses. Neurosci Biobehav Rev. 2005;29(6):1021-1034.
23. Procyshyn RM, Tse G, Sin O, et al. Concomitant clozapine reduces smoking in patients treated with risperidone. Eur Neuropsychopharmacol. 2002;12(1):77-80.
24. Anthonisen NR, Connett JE, Kiley JP, et al. Effects of smoking intervention and the use of an inhaled anticholinergic bronchodilator on the rate of decline of FEV1. The Lung Health Study. JAMA. 1994;272(19):1497-1505.
25. Pisinger C, Godtfredsen NS. Is there a health benefit of reduced tobacco consumption? A systematic review. Nicotine Tob Res. 2007;9(6):631-646.
26. Fiore MC, Jaén CR, Baker TB, et al. Treating tobacco use and dependence: 2008 update. Clinical Practice Guideline. Rockville, MD: Public Health Service, US Department of Health and Human Services; 2008.
27. Barnes M, Lawford BR, Burton SC, et al. Smoking and schizophrenia: is symptom profile related to smoking and which antipsychotic medication is of benefit in reducing cigarette use? Aust N Z J Psychiatry. 2006;40(6-7):575-580.
28. Screening for chronic obstructive pulmonary disease using spirometry: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med. 2008;148(7):529-534.
29. Agency for Healthcare Research and Quality. Five major steps to intervention (The “5 A’s”). http://www.ahrq.gov/professionals/clinicians-providers/guidelines-recommendations/tobacco/5steps.html. Published 2012. Accessed March 2, 2016.
30. Addington J, el-Guebaly N, Campbell W, et al. Smoking cessation treatment for patients with schizophrenia. Am J Psychiatry. 1998;155(7):974-976.
31. Zarowitz BJ, O’Shea T. Chronic obstructive pulmonary disease: prevalence, characteristics, and pharmacologic treatment in nursing home residents with cognitive impairment. J Manag Care Pharm. 2012;18(8):598-606.
32. Schermer T, Smeenk F, van Weel C. Referral and consultation in asthma and COPD: an exploration of pulmonologists’ views. Neth J Med. 2003;61(3):71-81.
1. Miller BJ, Paschall CB 3rd, Svendsen DP. Mortality and medical comorbidity among patients with serious mental illness. Psychiatr Serv. 2006;57(10):1482-1487.
2. Saha S, Chant D, McGrath J. A systematic review of mortality in schizophrenia: is the differential mortality gap worsening over time? Arch Gen Psychiatry. 2007;64(10):1123-1131.
3. Freeman E, Yoe JT. The poor health status of consumers of mental healthcare: behavioral disorders and chronic disease. Paper presented at: the National Association of State Mental Health Program Directors Medical Directors Workgroup; May 2006; Alexandria, VA.
4. Global Initiative for Chronic Obstructive Lung Disease. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease. http://www.goldcopd.org/uploads/users/files/GOLD_Report_2013_Feb20.pdf. Published February 20, 2013. Accessed March 2, 2016.
5. AntÒ JM, Vermeire P, Vestbo J, et al. Epidemiology of chronic obstructive pulmonary disease. Eur Respir J. 2001;17(5):982-994.
6. Newcomer JW. Antipsychotic medications: metabolic and cardiovascular risk. J Clin Psychiatry. 2007;68(suppl 4):8-13.
7. Calverley PM, Walker P. Chronic obstructive pulmonary disease. Lancet. 2003;362(9389):1053-1061.
8. Centers for Disease Control and Prevention. Chronic obstructive pulmonary disease among adults—United States, 2011. MMWR Morb Mortal Wkly Rep. 2012;61:938-943.
9. Feenstra TL, van Genugten ML, Hoogenveen RT, et al. The impact of aging and smoking on the future burden of chronic obstructive pulmonary disease: a model analysis in the Netherlands. Am J Respir Crit Care Med. 2001;164(4):590-596.
10. Celli BR, MacNee W; ATS/ERS Task Force. Standards for the diagnosis and treatment of patients with COPD: a summary of the ATS/ERS position paper [Erratum in: Eur Respir J. 2006;27(1):242]. Eur Respir J. 2004;23(6):932-946.
11. Matsuba K, Wright JL, Wiggs BR, et al. The changes in airways structure associated with reduced forced expiratory volume in one second. Eur Respir J. 1989;2(9):834-839.
12. O’Donnell DE, Revill SM, Webb KA. Dynamic hyperinflation and exercise intolerance in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2001;164(5):770-777.
13. Volkov VP. Respiratory diseases as a cause of death in schizophrenia [article in Russian]. Probl Tuberk Bolezn Legk. 2009;(6):24-27.
14. Kroon LA. Drug interactions and smoking: raising awareness for acute and critical care providers. Crit Care Nurs Clin North Am. 2006;18(1):53-62, xii.
15. Borson S, McDonald GJ, Gayle T, et al. Improvement in mood, physical symptoms, and function with nortriptyline for depression in patients with chronic obstructive pulmonary disease. Psychosomatics. 1992;33(2):190-201.
16. Caskey NH, Jarvik ME, Wirshing WC. The effects of dopaminergic D2 stimulation and blockade on smoking behavior. Exp Clin Psychopharmacol. 1999;7(1):72-78.
17. Dawe S, Gerada C, Russell MA, et al. Nicotine intake in smokers increases following a single dose of haloperidol. Psychopharmacol (Berl). 1995;117(1):110-115.
18. de Haan L, Booji J, Lavalaye J, et al. Occupancy of dopamine D2 receptors by antipsychotic drugs is related to nicotine addiction in young patients with schizophrenia. Psychopharmacology (Berl). 2006;183(4):500-505.
19. Hertel P, Nomikos GG, Iurlo M, et al. Risperidone: regional effects in vivo on release and metabolism of dopamine and serotonin in the rat brain. Psychopharmacology (Berl). 1996;124(1-2):74-86.
20. Silvestri S, Seeman MV, Negrete JC, et al. Increased dopamine D2 receptor binding after long-term treatment with antipsychotics in humans: a clinical PET study. Psychopharmacology (Berl). 2000;152(2):174-180.
21. Hutchison KE, Rutter MC, Niaura R, et al. Olanzapine attenuates cue-elicited craving for tobacco. Psychopharmacology (Berl). 2004;175(4):407-413.
22. Kumari V, Postma P. Nicotine use in schizophrenia: the self medication hypotheses. Neurosci Biobehav Rev. 2005;29(6):1021-1034.
23. Procyshyn RM, Tse G, Sin O, et al. Concomitant clozapine reduces smoking in patients treated with risperidone. Eur Neuropsychopharmacol. 2002;12(1):77-80.
24. Anthonisen NR, Connett JE, Kiley JP, et al. Effects of smoking intervention and the use of an inhaled anticholinergic bronchodilator on the rate of decline of FEV1. The Lung Health Study. JAMA. 1994;272(19):1497-1505.
25. Pisinger C, Godtfredsen NS. Is there a health benefit of reduced tobacco consumption? A systematic review. Nicotine Tob Res. 2007;9(6):631-646.
26. Fiore MC, Jaén CR, Baker TB, et al. Treating tobacco use and dependence: 2008 update. Clinical Practice Guideline. Rockville, MD: Public Health Service, US Department of Health and Human Services; 2008.
27. Barnes M, Lawford BR, Burton SC, et al. Smoking and schizophrenia: is symptom profile related to smoking and which antipsychotic medication is of benefit in reducing cigarette use? Aust N Z J Psychiatry. 2006;40(6-7):575-580.
28. Screening for chronic obstructive pulmonary disease using spirometry: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med. 2008;148(7):529-534.
29. Agency for Healthcare Research and Quality. Five major steps to intervention (The “5 A’s”). http://www.ahrq.gov/professionals/clinicians-providers/guidelines-recommendations/tobacco/5steps.html. Published 2012. Accessed March 2, 2016.
30. Addington J, el-Guebaly N, Campbell W, et al. Smoking cessation treatment for patients with schizophrenia. Am J Psychiatry. 1998;155(7):974-976.
31. Zarowitz BJ, O’Shea T. Chronic obstructive pulmonary disease: prevalence, characteristics, and pharmacologic treatment in nursing home residents with cognitive impairment. J Manag Care Pharm. 2012;18(8):598-606.
32. Schermer T, Smeenk F, van Weel C. Referral and consultation in asthma and COPD: an exploration of pulmonologists’ views. Neth J Med. 2003;61(3):71-81.