User login
Implantation of a deep brain stimulator is the most common surgical procedure performed in the United States and industrialized world for the management of advanced movement disorders. These procedures are US Food and Drug Administration (FDA)–approved for the management of the symptoms of Parkinson disease (PD) and essential tremor. Deep brain stimulation (DBS) is also approved for managing primary generalized dystonia and torticollis under a humanitarian device exemption.
Deep brain stimulation has largely replaced ablative procedures such as thalamotomy and pallidotomy. While ablative procedures can be effective for the symptoms of movement disorders, they cause a permanent lesion in the targeted nuclei and are therefore not reversible. DBS is considered safer because it can be adjusted over time and the location of the leads can be revised.1 On the other hand, regular maintenance of implanted hardware may be considered a disadvantage of DBS.
HARDWARE AND TARGETS
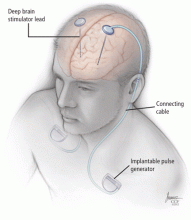
While ablative procedures do not require implantable hardware, DBS consists of permanently implanted neurostimulation systems. The battery-powered pulse generators typically last for several years but require multiple replacements during a lifetime. In addition, if other hardware components fail, surgical revision may be required to maintain treatment efficacy. Surgery involving implantation of hardware carries a higher risk of infection than does a nonimplantation procedure. If infections occur, removal of the hardware is often required, with reimplantation performed after the infection clears. In addition, the expense of DBS hardware may limit availability in some cases.
Three components
Target nuclei
Several nodes or nuclei can serve as targets for DBS. In patients with PD, the most common surgical target is the subthalamic nucleus (STN), either unilaterally or bilaterally.2 The globus pallidus pars interna (GPi) is also a viable target and is preferred for some patients with PD. The most common target for managing essential tremor is the ventral intermediate nucleus (VIM) of the thalamus, which can also be the target of choice for patients with tremor-predominant PD. However, the GPi and STN are usually preferred over the VIM in patients with PD because stimulation of these targets can relieve symptoms other than tremor, such as rigidity and bradykinesia. Bilateral stimulation of the GPi is the most frequent approach in patients with generalized torsion dystonia and torticollis, although the STN and thalamic nuclei (off-label) are also considered options.
PATIENT SELECTION
Patients are evaluated in our center at Cleveland Clinic by a multidisciplinary team that includes a movement disorder neurologist, a subspecialized neurosurgeon, a movement disorder neuropsychologist, and a psychiatrist with special interest in the behavioral comorbidities of movement disorders.3 Neuroimaging is included in this assessment. We have also included physical therapy as part of the initial evaluation in order to gain insight into the patient’s limitations and develop rehabilitation strategies that may enhance the outcomes of surgery or provide alternatives should surgery not be indicated. This evaluation provides extensive data that are then reviewed by the team in a conference dedicated to discussing candidacy for DBS or options for managing the symptoms of advanced movement disorders. Behavioral and cognitive issues are assessed in detail and, in our experience, are the most common reasons for not recommending DBS.
An important part of the evaluation of patients with PD is a formal test with rating of the motor section of the Unified Parkinson’s Disease Rating Scale (UPDRS) with the patient off medications for 8 to 12 hours and then after a test dose of levodopa. At our center, this off/on test is videotaped so that the responsiveness of individual symptoms to levodopa can be reviewed later in conference.
Risk of cognitive decline
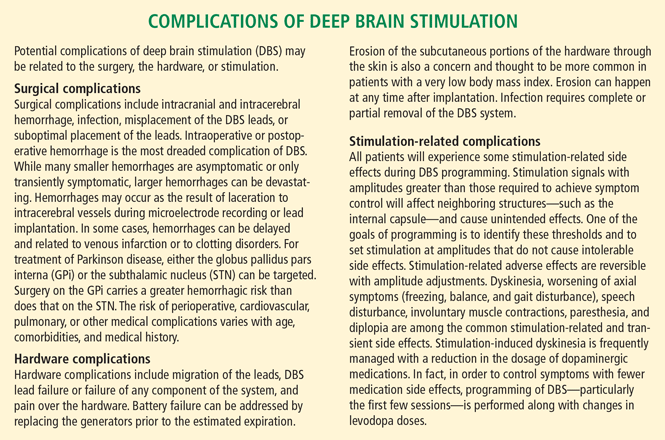
While DBS is considered safe and effective, there is a risk of cognitive decline in some patients. In most patients, long-term stimulation-related cognitive decline may be detected with formal measures but is not clinically significant and is outweighed by the motor and quality-of-life benefits of surgery. In some patients, long-term cognitive decline can be significant and can limit function. Cognitive neuropsychologic testing provides valuable information in this regard. Patients with preserved cognitive function seldom experience significant decline with DBS while those with substantial baseline impairment are thought to be at greater risk. Patients who meet criteria for dementia are usually not considered candidates for DBS, but exceptions exist. Transient perioperative cognitive difficulties are more common than persistent deficits, and typically resolve within a few weeks (see “Complications of deep brain stimulation”).
Benefits in Parkinson disease
Deep brain stimulation can address several symptoms of PD but with varying effects. Tremor, rigidity, and bradykinesia usually improve substantially. Gait has a more variable response, and balance is typically refractory. A general rule is that symptoms that improve with a single dose of levodopa should also improve with DBS. (Tremor, however, will most often respond to DBS even if refractory to medication.) Good candidates for surgery typically have a greater than 30% improvement in UPDRS motor score with levodopa challenge, but sometimes, improvement in the total score is less informative than evaluation of the effects of levodopa on particular symptoms. Treatment effects can be compared with the patient’s expectations for surgery in order to infer whether the goals for symptom improvement are realistic.
Treatment outcomes depend on etiology
After programming, DBS can provide PD symptom control similar to that of medication “on time,” but with fewer on-off fluctuations and less on-time dyskinesia. Good surgical candidates are patients who once responded well to dopaminergic medications but who, after several years with the disease, present with increased duration of “off time,” unpredictable duration of on time, and medication side effects such as on-time dyskinesia. Patients who do not respond well to levodopa even in subscores of the UPDRS may not be good candidates for DBS, and in some cases the diagnosis itself needs to be reviewed.
Deep brain stimulation can improve quality of life and alleviate symptoms of essential tremor. Tremor control is best for the upper extremities and tends to be better for distal tremors than for proximal ones. Patients who are good candidates for surgery often have severe tremors. A substantial improvement in these symptoms often has a dramatic, positive effect on work and quality of life. In some patients, surgery is considered for mild tremor if it seriously disrupts the patient’s lifestyle or occupation and cannot be well controlled with medications. Often, in these cases, tremor that appears relatively mild to the examiner is significantly limiting for the patient.
Very severe and proximal tremor is more refractory, though it may also improve. The changes can be well documented with objective measures. In these cases, however, residual tremor can still be moderate to severe and can be functionally limiting. Head or vocal tremors are typically refractory. They may be improved with bilateral implantation, but this cannot be accurately predicted. Patients who present with head-only or head-predominant tremor are thought to be less likely to benefit than those with limb tremor. Nonetheless, tremors of the head can severely impair quality of life. Because there are few other treatment options, some patients choose DBS with the understanding that the outcome is uncertain and the benefit may be limited.
Tremor resulting from multiple sclerosis or other causes can be medically refractory and disabling. In our experience, DBS can be an off-label option for managing secondary tremors and good outcomes have been observed. However, outcomes are much less predictable and tremor control less effective than in patients with essential tremor.
Patients with primary generalized dystonia can be considered candidates for DBS and may experience improved symptom control and quality of life.4 Patients with the DYT1 mutation are more likely to respond well to DBS, as are those with other forms of primary generalized dystonia. In contrast to that seen in patients with PD and tremor, symptomatic improvement is frequently not observed during intraoperative testing. Several months of stimulation and programming may be required before significant improvements are detected.5 Surgery can also be considered for off-label use in the treatment of patients with secondary dystonia—such as that following injury or associated with cerebral palsy—but outcomes are less predictable and usually more limited. A possible exception may be seen in cases of tardive dystonia, for which there is increasing evidence6 for the effectiveness of DBS. This remains an off-label use of DBS.
Realistic expectations
An important aspect of the multidisciplinary evaluation includes a discussion of the expectations for surgery, the risks, and the requirements for postoperative care. As discussed above, DBS is reversible and adjustable, so outcomes depend not only on accurate implantation of the hardware but also on postoperative programming. Also, monitoring and maintenance of the implanted hardware are required in these patients. It is important that patients and families appreciate the fact that specialized, long-term postoperative follow-up is as much a part of the treatment as is the implantation itself.
UNILATERAL VERSUS BILATERAL DBS
Most patients with generalized dystonia undergo bilateral DBS. However, patients with PD or essential tremor may receive bilateral, staged, or unilateral implants. Some patients with PD present with either near-complete predominance of symptoms on one side or with symptoms that affect mostly the dominant extremity. In these patients, unilateral implantation is often recommended because it has less risk than the bilateral approach and may be sufficient to address the most limiting symptoms.
As the disease advances, an additional surgery may be required to accomplish bilateral symptom control. Nevertheless, we do not routinely recommend preventive implantation because it is not known whether second-side symptoms will become severe enough to require it. This strategy allows for deferring surgical risk, which is in itself advantageous. In our experience, bilateral implantation is often recommended to PD patients who present with symptoms such as freezing of gait.
Patients who have essential tremor often present with bilateral symptoms. Although many patients will indicate that they need symptom relief on both upper extremities in order to perform activities of daily living, our practice is to recommend surgery on one side at first and to suggest the patient consider contralateral implantation after weeks or months. Bilateral implantation may carry a risk for dysarthria and the risk is thought to be reduced if bilateral procedures are staged. Although high rates of dysarthria have been reported following bilateral surgery for tremor, its occurrence has been infrequent in our experience with bilateral staged DBS. Benefits of treating tremor in the dominant extremity usually exceed those of treating nondominant tremor, so most patients prefer that the dominant side be the first one treated.
TECHNICAL OPTIONS
There are several technical options for implantation of DBS systems. Stereotactic procedures rely on co-registration of preoperative imaging with external and internal fiducials, or points of reference. Targeting of the intended structures is performed by combining direct and indirect methods. Direct methods rely on identification of the target structures with imaging, such as visualization of the STN and GPi on preoperative magnetic resonance imaging (MRI). Indirect targeting relies on cadaveric anatomic atlases and coordinate systems that infer the location of the intended structures in relation to anatomical points of reference.
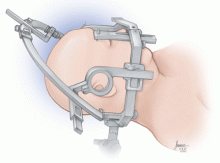
Frame-based systems
Frameless systems
The key advantage of the frameless system over the frame-based system is greater mobility of the head. Another important advantage is easier access to the airway, should an emergency situation occur. In our practice, patients with experience of both frameless and frame-based systems did not report significantly less discomfort with the frameless system.
The frameless system also has disadvantages, including less secure fixation of the head, which can add risk to the procedure. In addition, because of its lightweight, plastic construction, it provides less robust support to the instrumentation entering the brain than do metallic head frames and, in some cases, there is less flexibility for adjusting targets if needed during surgery. In addition, frameless systems are nonreusable and represent a substantial additional cost.
Microelectrode recording
Physiologic verification of anatomic targets identified by imaging can be accomplished with microelectrode recording (MER). This technique involves placing fine, high-impedance electrodes through the target area, so that anatomic structures can be recognized by characteristic electrical activity of individual neurons or groups of neurons. The locations of the structures are identified and the lengths of the electrode trajectories through the different structures—as well as the gaps between these structures—are recorded. The distances are then compared with the anatomy and a best-fit model is created to infer the location of the trajectory in the target area. Additional MER penetrations are made in order to further delineate the anatomy. Once a location for implantation has been selected, the DBS lead is inserted into the target area.
Electrode implantation
Lead implantation is often performed under fluoroscopic guidance in order to ensure accuracy and stability. When implanted, the electrode may cause a microlesional effect, manifested by transient improvement in symptoms.
The DBS leads are then connected to external pulse generators and assessed for clinical benefits and side effects. Amplitude, pulse width, and frequency are adjusted to test the therapeutic window of stimulation (clinical improvement thresholds versus side effect thresholds). Some PD patients develop dyskinesia during test stimulation, which may be a positive indicator for lead location. If good effects and a therapeutic window are observed, the location of the lead is considered to be satisfactory and the procedure is completed.
Pulse generator implantation
During the final step of surgery, performed under general anesthesia, the pulse generator is implanted. The extension cable that connects the DBS lead to the implantable pulse generator is tunneled subcutaneously, connecting the DBS lead to the pulse generator in the chest.
Intraoperative, real-time MRI stereotaxis
Real-time intraoperative MRI has become available for DBS implantation with devices recently cleared for use by the FDA. The procedure, typically performed in a diagnostic MRI suite, uses MR images acquired during surgery to guide DBS lead implantation in the target area and to verify implantation accuracy.8
- Rezai AR, Machado AG, Azmi H, Kubu C, Boulis NM. Surgery for movement disorders. Neurosurg 2008; 62(SHC suppl 2):SHC809–SHC839.
- Krack P, Batir A, Van Blercom N, et al. Five-year follow-up of bilateral stimulation of the subthalamic nucleus in advanced Parkinson’s disease. N Engl J Med 2003; 349:1925–1934.
- Machado A, Fernandez HH, Deogaonkar M. Deep brain stimulation: what can patients expect from it? Cleve Clin J Med 2012; 79:113–120.
- Vidailhet M, Vercueil L, Houeto JL, et al. Bilateral deep-brain stimulation of the globus pallidus in primary generalized dystonia. N Engl J Med 2005; 352:459–467.
- Kupsch A, Benecke R, Müller J, et al. Pallidal deep-brain stimulation in primary generalized or segmental dystonia. N Engl J Med 2006; 355:1978–1990.
- Gruber D, Trottenberg T, Kivi A, et al. Long-term effects of pallidal deep brain stimulation in tardive dystonia. Neurology 2009; 73:53–58.
- Gross RE, Sharan AD, Benabid AL. Deep brain stimulation for Parkinson’s disease: surgical technique and perioperative management. Mov Disord 2006; 21( suppl 14):S247–S258.
- Starr PA, Martin AJ, Ostrem JL, et al. Subthalamic nucleus deep brain stimulator placement using high-field interventional magnetic resonance imaging and a skull-mounted aiming device: technique and application accuracy. J Neurosurg 2010; 112:479–490.
Implantation of a deep brain stimulator is the most common surgical procedure performed in the United States and industrialized world for the management of advanced movement disorders. These procedures are US Food and Drug Administration (FDA)–approved for the management of the symptoms of Parkinson disease (PD) and essential tremor. Deep brain stimulation (DBS) is also approved for managing primary generalized dystonia and torticollis under a humanitarian device exemption.
Deep brain stimulation has largely replaced ablative procedures such as thalamotomy and pallidotomy. While ablative procedures can be effective for the symptoms of movement disorders, they cause a permanent lesion in the targeted nuclei and are therefore not reversible. DBS is considered safer because it can be adjusted over time and the location of the leads can be revised.1 On the other hand, regular maintenance of implanted hardware may be considered a disadvantage of DBS.
HARDWARE AND TARGETS
While ablative procedures do not require implantable hardware, DBS consists of permanently implanted neurostimulation systems. The battery-powered pulse generators typically last for several years but require multiple replacements during a lifetime. In addition, if other hardware components fail, surgical revision may be required to maintain treatment efficacy. Surgery involving implantation of hardware carries a higher risk of infection than does a nonimplantation procedure. If infections occur, removal of the hardware is often required, with reimplantation performed after the infection clears. In addition, the expense of DBS hardware may limit availability in some cases.
Three components
Target nuclei
Several nodes or nuclei can serve as targets for DBS. In patients with PD, the most common surgical target is the subthalamic nucleus (STN), either unilaterally or bilaterally.2 The globus pallidus pars interna (GPi) is also a viable target and is preferred for some patients with PD. The most common target for managing essential tremor is the ventral intermediate nucleus (VIM) of the thalamus, which can also be the target of choice for patients with tremor-predominant PD. However, the GPi and STN are usually preferred over the VIM in patients with PD because stimulation of these targets can relieve symptoms other than tremor, such as rigidity and bradykinesia. Bilateral stimulation of the GPi is the most frequent approach in patients with generalized torsion dystonia and torticollis, although the STN and thalamic nuclei (off-label) are also considered options.
PATIENT SELECTION
Patients are evaluated in our center at Cleveland Clinic by a multidisciplinary team that includes a movement disorder neurologist, a subspecialized neurosurgeon, a movement disorder neuropsychologist, and a psychiatrist with special interest in the behavioral comorbidities of movement disorders.3 Neuroimaging is included in this assessment. We have also included physical therapy as part of the initial evaluation in order to gain insight into the patient’s limitations and develop rehabilitation strategies that may enhance the outcomes of surgery or provide alternatives should surgery not be indicated. This evaluation provides extensive data that are then reviewed by the team in a conference dedicated to discussing candidacy for DBS or options for managing the symptoms of advanced movement disorders. Behavioral and cognitive issues are assessed in detail and, in our experience, are the most common reasons for not recommending DBS.
An important part of the evaluation of patients with PD is a formal test with rating of the motor section of the Unified Parkinson’s Disease Rating Scale (UPDRS) with the patient off medications for 8 to 12 hours and then after a test dose of levodopa. At our center, this off/on test is videotaped so that the responsiveness of individual symptoms to levodopa can be reviewed later in conference.
Risk of cognitive decline
While DBS is considered safe and effective, there is a risk of cognitive decline in some patients. In most patients, long-term stimulation-related cognitive decline may be detected with formal measures but is not clinically significant and is outweighed by the motor and quality-of-life benefits of surgery. In some patients, long-term cognitive decline can be significant and can limit function. Cognitive neuropsychologic testing provides valuable information in this regard. Patients with preserved cognitive function seldom experience significant decline with DBS while those with substantial baseline impairment are thought to be at greater risk. Patients who meet criteria for dementia are usually not considered candidates for DBS, but exceptions exist. Transient perioperative cognitive difficulties are more common than persistent deficits, and typically resolve within a few weeks (see “Complications of deep brain stimulation”).
Benefits in Parkinson disease
Deep brain stimulation can address several symptoms of PD but with varying effects. Tremor, rigidity, and bradykinesia usually improve substantially. Gait has a more variable response, and balance is typically refractory. A general rule is that symptoms that improve with a single dose of levodopa should also improve with DBS. (Tremor, however, will most often respond to DBS even if refractory to medication.) Good candidates for surgery typically have a greater than 30% improvement in UPDRS motor score with levodopa challenge, but sometimes, improvement in the total score is less informative than evaluation of the effects of levodopa on particular symptoms. Treatment effects can be compared with the patient’s expectations for surgery in order to infer whether the goals for symptom improvement are realistic.
Treatment outcomes depend on etiology
After programming, DBS can provide PD symptom control similar to that of medication “on time,” but with fewer on-off fluctuations and less on-time dyskinesia. Good surgical candidates are patients who once responded well to dopaminergic medications but who, after several years with the disease, present with increased duration of “off time,” unpredictable duration of on time, and medication side effects such as on-time dyskinesia. Patients who do not respond well to levodopa even in subscores of the UPDRS may not be good candidates for DBS, and in some cases the diagnosis itself needs to be reviewed.
Deep brain stimulation can improve quality of life and alleviate symptoms of essential tremor. Tremor control is best for the upper extremities and tends to be better for distal tremors than for proximal ones. Patients who are good candidates for surgery often have severe tremors. A substantial improvement in these symptoms often has a dramatic, positive effect on work and quality of life. In some patients, surgery is considered for mild tremor if it seriously disrupts the patient’s lifestyle or occupation and cannot be well controlled with medications. Often, in these cases, tremor that appears relatively mild to the examiner is significantly limiting for the patient.
Very severe and proximal tremor is more refractory, though it may also improve. The changes can be well documented with objective measures. In these cases, however, residual tremor can still be moderate to severe and can be functionally limiting. Head or vocal tremors are typically refractory. They may be improved with bilateral implantation, but this cannot be accurately predicted. Patients who present with head-only or head-predominant tremor are thought to be less likely to benefit than those with limb tremor. Nonetheless, tremors of the head can severely impair quality of life. Because there are few other treatment options, some patients choose DBS with the understanding that the outcome is uncertain and the benefit may be limited.
Tremor resulting from multiple sclerosis or other causes can be medically refractory and disabling. In our experience, DBS can be an off-label option for managing secondary tremors and good outcomes have been observed. However, outcomes are much less predictable and tremor control less effective than in patients with essential tremor.
Patients with primary generalized dystonia can be considered candidates for DBS and may experience improved symptom control and quality of life.4 Patients with the DYT1 mutation are more likely to respond well to DBS, as are those with other forms of primary generalized dystonia. In contrast to that seen in patients with PD and tremor, symptomatic improvement is frequently not observed during intraoperative testing. Several months of stimulation and programming may be required before significant improvements are detected.5 Surgery can also be considered for off-label use in the treatment of patients with secondary dystonia—such as that following injury or associated with cerebral palsy—but outcomes are less predictable and usually more limited. A possible exception may be seen in cases of tardive dystonia, for which there is increasing evidence6 for the effectiveness of DBS. This remains an off-label use of DBS.
Realistic expectations
An important aspect of the multidisciplinary evaluation includes a discussion of the expectations for surgery, the risks, and the requirements for postoperative care. As discussed above, DBS is reversible and adjustable, so outcomes depend not only on accurate implantation of the hardware but also on postoperative programming. Also, monitoring and maintenance of the implanted hardware are required in these patients. It is important that patients and families appreciate the fact that specialized, long-term postoperative follow-up is as much a part of the treatment as is the implantation itself.

UNILATERAL VERSUS BILATERAL DBS
Most patients with generalized dystonia undergo bilateral DBS. However, patients with PD or essential tremor may receive bilateral, staged, or unilateral implants. Some patients with PD present with either near-complete predominance of symptoms on one side or with symptoms that affect mostly the dominant extremity. In these patients, unilateral implantation is often recommended because it has less risk than the bilateral approach and may be sufficient to address the most limiting symptoms.
As the disease advances, an additional surgery may be required to accomplish bilateral symptom control. Nevertheless, we do not routinely recommend preventive implantation because it is not known whether second-side symptoms will become severe enough to require it. This strategy allows for deferring surgical risk, which is in itself advantageous. In our experience, bilateral implantation is often recommended to PD patients who present with symptoms such as freezing of gait.
Patients who have essential tremor often present with bilateral symptoms. Although many patients will indicate that they need symptom relief on both upper extremities in order to perform activities of daily living, our practice is to recommend surgery on one side at first and to suggest the patient consider contralateral implantation after weeks or months. Bilateral implantation may carry a risk for dysarthria and the risk is thought to be reduced if bilateral procedures are staged. Although high rates of dysarthria have been reported following bilateral surgery for tremor, its occurrence has been infrequent in our experience with bilateral staged DBS. Benefits of treating tremor in the dominant extremity usually exceed those of treating nondominant tremor, so most patients prefer that the dominant side be the first one treated.
TECHNICAL OPTIONS
There are several technical options for implantation of DBS systems. Stereotactic procedures rely on co-registration of preoperative imaging with external and internal fiducials, or points of reference. Targeting of the intended structures is performed by combining direct and indirect methods. Direct methods rely on identification of the target structures with imaging, such as visualization of the STN and GPi on preoperative magnetic resonance imaging (MRI). Indirect targeting relies on cadaveric anatomic atlases and coordinate systems that infer the location of the intended structures in relation to anatomical points of reference.
Frame-based systems
Frameless systems
The key advantage of the frameless system over the frame-based system is greater mobility of the head. Another important advantage is easier access to the airway, should an emergency situation occur. In our practice, patients with experience of both frameless and frame-based systems did not report significantly less discomfort with the frameless system.
The frameless system also has disadvantages, including less secure fixation of the head, which can add risk to the procedure. In addition, because of its lightweight, plastic construction, it provides less robust support to the instrumentation entering the brain than do metallic head frames and, in some cases, there is less flexibility for adjusting targets if needed during surgery. In addition, frameless systems are nonreusable and represent a substantial additional cost.
Microelectrode recording
Physiologic verification of anatomic targets identified by imaging can be accomplished with microelectrode recording (MER). This technique involves placing fine, high-impedance electrodes through the target area, so that anatomic structures can be recognized by characteristic electrical activity of individual neurons or groups of neurons. The locations of the structures are identified and the lengths of the electrode trajectories through the different structures—as well as the gaps between these structures—are recorded. The distances are then compared with the anatomy and a best-fit model is created to infer the location of the trajectory in the target area. Additional MER penetrations are made in order to further delineate the anatomy. Once a location for implantation has been selected, the DBS lead is inserted into the target area.
Electrode implantation
Lead implantation is often performed under fluoroscopic guidance in order to ensure accuracy and stability. When implanted, the electrode may cause a microlesional effect, manifested by transient improvement in symptoms.
The DBS leads are then connected to external pulse generators and assessed for clinical benefits and side effects. Amplitude, pulse width, and frequency are adjusted to test the therapeutic window of stimulation (clinical improvement thresholds versus side effect thresholds). Some PD patients develop dyskinesia during test stimulation, which may be a positive indicator for lead location. If good effects and a therapeutic window are observed, the location of the lead is considered to be satisfactory and the procedure is completed.
Pulse generator implantation
During the final step of surgery, performed under general anesthesia, the pulse generator is implanted. The extension cable that connects the DBS lead to the implantable pulse generator is tunneled subcutaneously, connecting the DBS lead to the pulse generator in the chest.
Intraoperative, real-time MRI stereotaxis
Real-time intraoperative MRI has become available for DBS implantation with devices recently cleared for use by the FDA. The procedure, typically performed in a diagnostic MRI suite, uses MR images acquired during surgery to guide DBS lead implantation in the target area and to verify implantation accuracy.8
Implantation of a deep brain stimulator is the most common surgical procedure performed in the United States and industrialized world for the management of advanced movement disorders. These procedures are US Food and Drug Administration (FDA)–approved for the management of the symptoms of Parkinson disease (PD) and essential tremor. Deep brain stimulation (DBS) is also approved for managing primary generalized dystonia and torticollis under a humanitarian device exemption.
Deep brain stimulation has largely replaced ablative procedures such as thalamotomy and pallidotomy. While ablative procedures can be effective for the symptoms of movement disorders, they cause a permanent lesion in the targeted nuclei and are therefore not reversible. DBS is considered safer because it can be adjusted over time and the location of the leads can be revised.1 On the other hand, regular maintenance of implanted hardware may be considered a disadvantage of DBS.
HARDWARE AND TARGETS
While ablative procedures do not require implantable hardware, DBS consists of permanently implanted neurostimulation systems. The battery-powered pulse generators typically last for several years but require multiple replacements during a lifetime. In addition, if other hardware components fail, surgical revision may be required to maintain treatment efficacy. Surgery involving implantation of hardware carries a higher risk of infection than does a nonimplantation procedure. If infections occur, removal of the hardware is often required, with reimplantation performed after the infection clears. In addition, the expense of DBS hardware may limit availability in some cases.
Three components
Target nuclei
Several nodes or nuclei can serve as targets for DBS. In patients with PD, the most common surgical target is the subthalamic nucleus (STN), either unilaterally or bilaterally.2 The globus pallidus pars interna (GPi) is also a viable target and is preferred for some patients with PD. The most common target for managing essential tremor is the ventral intermediate nucleus (VIM) of the thalamus, which can also be the target of choice for patients with tremor-predominant PD. However, the GPi and STN are usually preferred over the VIM in patients with PD because stimulation of these targets can relieve symptoms other than tremor, such as rigidity and bradykinesia. Bilateral stimulation of the GPi is the most frequent approach in patients with generalized torsion dystonia and torticollis, although the STN and thalamic nuclei (off-label) are also considered options.
PATIENT SELECTION
Patients are evaluated in our center at Cleveland Clinic by a multidisciplinary team that includes a movement disorder neurologist, a subspecialized neurosurgeon, a movement disorder neuropsychologist, and a psychiatrist with special interest in the behavioral comorbidities of movement disorders.3 Neuroimaging is included in this assessment. We have also included physical therapy as part of the initial evaluation in order to gain insight into the patient’s limitations and develop rehabilitation strategies that may enhance the outcomes of surgery or provide alternatives should surgery not be indicated. This evaluation provides extensive data that are then reviewed by the team in a conference dedicated to discussing candidacy for DBS or options for managing the symptoms of advanced movement disorders. Behavioral and cognitive issues are assessed in detail and, in our experience, are the most common reasons for not recommending DBS.
An important part of the evaluation of patients with PD is a formal test with rating of the motor section of the Unified Parkinson’s Disease Rating Scale (UPDRS) with the patient off medications for 8 to 12 hours and then after a test dose of levodopa. At our center, this off/on test is videotaped so that the responsiveness of individual symptoms to levodopa can be reviewed later in conference.
Risk of cognitive decline
While DBS is considered safe and effective, there is a risk of cognitive decline in some patients. In most patients, long-term stimulation-related cognitive decline may be detected with formal measures but is not clinically significant and is outweighed by the motor and quality-of-life benefits of surgery. In some patients, long-term cognitive decline can be significant and can limit function. Cognitive neuropsychologic testing provides valuable information in this regard. Patients with preserved cognitive function seldom experience significant decline with DBS while those with substantial baseline impairment are thought to be at greater risk. Patients who meet criteria for dementia are usually not considered candidates for DBS, but exceptions exist. Transient perioperative cognitive difficulties are more common than persistent deficits, and typically resolve within a few weeks (see “Complications of deep brain stimulation”).
Benefits in Parkinson disease
Deep brain stimulation can address several symptoms of PD but with varying effects. Tremor, rigidity, and bradykinesia usually improve substantially. Gait has a more variable response, and balance is typically refractory. A general rule is that symptoms that improve with a single dose of levodopa should also improve with DBS. (Tremor, however, will most often respond to DBS even if refractory to medication.) Good candidates for surgery typically have a greater than 30% improvement in UPDRS motor score with levodopa challenge, but sometimes, improvement in the total score is less informative than evaluation of the effects of levodopa on particular symptoms. Treatment effects can be compared with the patient’s expectations for surgery in order to infer whether the goals for symptom improvement are realistic.
Treatment outcomes depend on etiology
After programming, DBS can provide PD symptom control similar to that of medication “on time,” but with fewer on-off fluctuations and less on-time dyskinesia. Good surgical candidates are patients who once responded well to dopaminergic medications but who, after several years with the disease, present with increased duration of “off time,” unpredictable duration of on time, and medication side effects such as on-time dyskinesia. Patients who do not respond well to levodopa even in subscores of the UPDRS may not be good candidates for DBS, and in some cases the diagnosis itself needs to be reviewed.
Deep brain stimulation can improve quality of life and alleviate symptoms of essential tremor. Tremor control is best for the upper extremities and tends to be better for distal tremors than for proximal ones. Patients who are good candidates for surgery often have severe tremors. A substantial improvement in these symptoms often has a dramatic, positive effect on work and quality of life. In some patients, surgery is considered for mild tremor if it seriously disrupts the patient’s lifestyle or occupation and cannot be well controlled with medications. Often, in these cases, tremor that appears relatively mild to the examiner is significantly limiting for the patient.
Very severe and proximal tremor is more refractory, though it may also improve. The changes can be well documented with objective measures. In these cases, however, residual tremor can still be moderate to severe and can be functionally limiting. Head or vocal tremors are typically refractory. They may be improved with bilateral implantation, but this cannot be accurately predicted. Patients who present with head-only or head-predominant tremor are thought to be less likely to benefit than those with limb tremor. Nonetheless, tremors of the head can severely impair quality of life. Because there are few other treatment options, some patients choose DBS with the understanding that the outcome is uncertain and the benefit may be limited.
Tremor resulting from multiple sclerosis or other causes can be medically refractory and disabling. In our experience, DBS can be an off-label option for managing secondary tremors and good outcomes have been observed. However, outcomes are much less predictable and tremor control less effective than in patients with essential tremor.
Patients with primary generalized dystonia can be considered candidates for DBS and may experience improved symptom control and quality of life.4 Patients with the DYT1 mutation are more likely to respond well to DBS, as are those with other forms of primary generalized dystonia. In contrast to that seen in patients with PD and tremor, symptomatic improvement is frequently not observed during intraoperative testing. Several months of stimulation and programming may be required before significant improvements are detected.5 Surgery can also be considered for off-label use in the treatment of patients with secondary dystonia—such as that following injury or associated with cerebral palsy—but outcomes are less predictable and usually more limited. A possible exception may be seen in cases of tardive dystonia, for which there is increasing evidence6 for the effectiveness of DBS. This remains an off-label use of DBS.
Realistic expectations
An important aspect of the multidisciplinary evaluation includes a discussion of the expectations for surgery, the risks, and the requirements for postoperative care. As discussed above, DBS is reversible and adjustable, so outcomes depend not only on accurate implantation of the hardware but also on postoperative programming. Also, monitoring and maintenance of the implanted hardware are required in these patients. It is important that patients and families appreciate the fact that specialized, long-term postoperative follow-up is as much a part of the treatment as is the implantation itself.

UNILATERAL VERSUS BILATERAL DBS
Most patients with generalized dystonia undergo bilateral DBS. However, patients with PD or essential tremor may receive bilateral, staged, or unilateral implants. Some patients with PD present with either near-complete predominance of symptoms on one side or with symptoms that affect mostly the dominant extremity. In these patients, unilateral implantation is often recommended because it has less risk than the bilateral approach and may be sufficient to address the most limiting symptoms.
As the disease advances, an additional surgery may be required to accomplish bilateral symptom control. Nevertheless, we do not routinely recommend preventive implantation because it is not known whether second-side symptoms will become severe enough to require it. This strategy allows for deferring surgical risk, which is in itself advantageous. In our experience, bilateral implantation is often recommended to PD patients who present with symptoms such as freezing of gait.
Patients who have essential tremor often present with bilateral symptoms. Although many patients will indicate that they need symptom relief on both upper extremities in order to perform activities of daily living, our practice is to recommend surgery on one side at first and to suggest the patient consider contralateral implantation after weeks or months. Bilateral implantation may carry a risk for dysarthria and the risk is thought to be reduced if bilateral procedures are staged. Although high rates of dysarthria have been reported following bilateral surgery for tremor, its occurrence has been infrequent in our experience with bilateral staged DBS. Benefits of treating tremor in the dominant extremity usually exceed those of treating nondominant tremor, so most patients prefer that the dominant side be the first one treated.
TECHNICAL OPTIONS
There are several technical options for implantation of DBS systems. Stereotactic procedures rely on co-registration of preoperative imaging with external and internal fiducials, or points of reference. Targeting of the intended structures is performed by combining direct and indirect methods. Direct methods rely on identification of the target structures with imaging, such as visualization of the STN and GPi on preoperative magnetic resonance imaging (MRI). Indirect targeting relies on cadaveric anatomic atlases and coordinate systems that infer the location of the intended structures in relation to anatomical points of reference.
Frame-based systems
Frameless systems
The key advantage of the frameless system over the frame-based system is greater mobility of the head. Another important advantage is easier access to the airway, should an emergency situation occur. In our practice, patients with experience of both frameless and frame-based systems did not report significantly less discomfort with the frameless system.
The frameless system also has disadvantages, including less secure fixation of the head, which can add risk to the procedure. In addition, because of its lightweight, plastic construction, it provides less robust support to the instrumentation entering the brain than do metallic head frames and, in some cases, there is less flexibility for adjusting targets if needed during surgery. In addition, frameless systems are nonreusable and represent a substantial additional cost.
Microelectrode recording
Physiologic verification of anatomic targets identified by imaging can be accomplished with microelectrode recording (MER). This technique involves placing fine, high-impedance electrodes through the target area, so that anatomic structures can be recognized by characteristic electrical activity of individual neurons or groups of neurons. The locations of the structures are identified and the lengths of the electrode trajectories through the different structures—as well as the gaps between these structures—are recorded. The distances are then compared with the anatomy and a best-fit model is created to infer the location of the trajectory in the target area. Additional MER penetrations are made in order to further delineate the anatomy. Once a location for implantation has been selected, the DBS lead is inserted into the target area.
Electrode implantation
Lead implantation is often performed under fluoroscopic guidance in order to ensure accuracy and stability. When implanted, the electrode may cause a microlesional effect, manifested by transient improvement in symptoms.
The DBS leads are then connected to external pulse generators and assessed for clinical benefits and side effects. Amplitude, pulse width, and frequency are adjusted to test the therapeutic window of stimulation (clinical improvement thresholds versus side effect thresholds). Some PD patients develop dyskinesia during test stimulation, which may be a positive indicator for lead location. If good effects and a therapeutic window are observed, the location of the lead is considered to be satisfactory and the procedure is completed.
Pulse generator implantation
During the final step of surgery, performed under general anesthesia, the pulse generator is implanted. The extension cable that connects the DBS lead to the implantable pulse generator is tunneled subcutaneously, connecting the DBS lead to the pulse generator in the chest.
Intraoperative, real-time MRI stereotaxis
Real-time intraoperative MRI has become available for DBS implantation with devices recently cleared for use by the FDA. The procedure, typically performed in a diagnostic MRI suite, uses MR images acquired during surgery to guide DBS lead implantation in the target area and to verify implantation accuracy.8
- Rezai AR, Machado AG, Azmi H, Kubu C, Boulis NM. Surgery for movement disorders. Neurosurg 2008; 62(SHC suppl 2):SHC809–SHC839.
- Krack P, Batir A, Van Blercom N, et al. Five-year follow-up of bilateral stimulation of the subthalamic nucleus in advanced Parkinson’s disease. N Engl J Med 2003; 349:1925–1934.
- Machado A, Fernandez HH, Deogaonkar M. Deep brain stimulation: what can patients expect from it? Cleve Clin J Med 2012; 79:113–120.
- Vidailhet M, Vercueil L, Houeto JL, et al. Bilateral deep-brain stimulation of the globus pallidus in primary generalized dystonia. N Engl J Med 2005; 352:459–467.
- Kupsch A, Benecke R, Müller J, et al. Pallidal deep-brain stimulation in primary generalized or segmental dystonia. N Engl J Med 2006; 355:1978–1990.
- Gruber D, Trottenberg T, Kivi A, et al. Long-term effects of pallidal deep brain stimulation in tardive dystonia. Neurology 2009; 73:53–58.
- Gross RE, Sharan AD, Benabid AL. Deep brain stimulation for Parkinson’s disease: surgical technique and perioperative management. Mov Disord 2006; 21( suppl 14):S247–S258.
- Starr PA, Martin AJ, Ostrem JL, et al. Subthalamic nucleus deep brain stimulator placement using high-field interventional magnetic resonance imaging and a skull-mounted aiming device: technique and application accuracy. J Neurosurg 2010; 112:479–490.
- Rezai AR, Machado AG, Azmi H, Kubu C, Boulis NM. Surgery for movement disorders. Neurosurg 2008; 62(SHC suppl 2):SHC809–SHC839.
- Krack P, Batir A, Van Blercom N, et al. Five-year follow-up of bilateral stimulation of the subthalamic nucleus in advanced Parkinson’s disease. N Engl J Med 2003; 349:1925–1934.
- Machado A, Fernandez HH, Deogaonkar M. Deep brain stimulation: what can patients expect from it? Cleve Clin J Med 2012; 79:113–120.
- Vidailhet M, Vercueil L, Houeto JL, et al. Bilateral deep-brain stimulation of the globus pallidus in primary generalized dystonia. N Engl J Med 2005; 352:459–467.
- Kupsch A, Benecke R, Müller J, et al. Pallidal deep-brain stimulation in primary generalized or segmental dystonia. N Engl J Med 2006; 355:1978–1990.
- Gruber D, Trottenberg T, Kivi A, et al. Long-term effects of pallidal deep brain stimulation in tardive dystonia. Neurology 2009; 73:53–58.
- Gross RE, Sharan AD, Benabid AL. Deep brain stimulation for Parkinson’s disease: surgical technique and perioperative management. Mov Disord 2006; 21( suppl 14):S247–S258.
- Starr PA, Martin AJ, Ostrem JL, et al. Subthalamic nucleus deep brain stimulator placement using high-field interventional magnetic resonance imaging and a skull-mounted aiming device: technique and application accuracy. J Neurosurg 2010; 112:479–490.