User login
Inhaled corticosteroids (ICSs) are frequently prescribed for the treatment of chronic obstructive pulmonary disease (COPD) to reduce exacerbations in a specific subset of patients. The long-term use of ICSs, however, is associated with several potential systemic adverse effects, including adrenal suppression, decreased bone mineral density, and immunosuppression.1 The concern for immunosuppression is particularly notable and leads to a known increased risk for developing pneumonia in patients with COPD. These patients frequently have other concurrent risk factors for pneumonia (eg, history of tobacco use, older age, and severe airway limitations) and are at higher risk for more severe outcomes in the setting of pneumonia.2,3
Primarily due to the concern of pneumonia risks, the Global Initiative for Chronic Obstructive Lung Disease (GOLD) guidelines have recommended ICS discontinuation in patients who are less likely to receive significant benefits from therapy.4 Likely due to an anti-inflammatory mechanism of action, ICSs have been shown to reduce COPD exacerbation rates in patients with comorbid asthma or who have evidence of a strong inflammatory component to their COPD. The strongest indicator of an inflammatory component is an elevated blood eosinophil (EOS) count; those with EOS > 300 cells/µL are most likely to benefit from ICSs, whereas those with a count < 100 cells/µL are unlikely to have a significant response. In addition to the inflammatory component consideration, prior studies have shown improvements in lung function and reduction of exacerbations with ICS use in patients with frequent moderate-to-severe COPD exacerbations.5 Although the GOLD guidelines provide recommendations about who is appropriate to discontinue ICS use, clinicians have no clear guidance on the risks or the best discontinuation strategy.
Based primarily on data from a prior randomized controlled trial, the Veterans Integrated Services Network (VISN) 17, which includes the Veterans Affairs North Texas Health Care System (VANTHCS) in Dallas, established a recommended ICS de-escalation strategy.6,7 The strategy included a 12-week stepwise taper using a mometasone inhaler for all patients discontinuing a moderate or high dose ICS. The lack of substantial clinical trial data or expert consensus guideline recommendations has left open the question of whether a taper is necessary. To answer that question, this study was conducted to evaluate whether there is a difference in the rate of COPD exacerbations following abrupt discontinuation vs gradual taper of ICS therapy.
Methods
This single-center, retrospective cohort study was conducted at VANTHCS. Patient electronic health records between January 10, 2021, and September 1, 2021, were reviewed for the last documented fill date of any inhaler containing a steroid component. This time frame was chosen to coincide with a VANTHCS initiative to follow GOLD guidelines for ICS discontinuation. Patients were followed for outcomes until November 1, 2022.
To be included in this study, patients had to have active prescriptions at VANTHCS, have a documented diagnosis of COPD in their chart, and be prescribed a stable dose of ICS for ≥ 1 year prior to their latest refill. The inhaler used could contain an ICS as monotherapy, in combination with a long-acting β-agonist (LABA), or as part of triple therapy with an additional long-acting muscarinic antagonist (LAMA). The inhaler needed to be discontinued during the study period of interest.
Patients were excluded if they had a diagnosis of asthma, were aged < 40 years, had active prescriptions for multiple ICS inhalers or nebulizers, or had significant oral steroid use (≥ 5 mg/d prednisone or an equivalent steroid for > 6 weeks) within 1 year of their ICS discontinuation date. In addition, to reduce the risk of future events being misclassified as COPD exacerbations, patients were excluded if they had a congestive heart failure exacerbation up to 2 years before ICS discontinuation or a diagnosis of COVID-19 infection up to 1 year before or 6 months after ICS discontinuation. Patients with a COPD exacerbation requiring an emergency department or hospital visit within 2 years prior to ICS discontinuation were also excluded, as de-escalation of ICS therapy was likely inappropriate in these cases. Finally, patients were excluded if they were started on a different ICS immediately following the discontinuation of their first ICS.
The primary outcome for this study was COPD exacerbations requiring an emergency department visit or hospitalization within 6 months of ICS discontinuation. A secondary outcome examining the rates of COPD exacerbations within 12 months also was used. The original study design called for the use of inferential statistics to compare the rates of primary and secondary outcomes in patients whose ICS was abruptly discontinued with those who were tapered slowly. After data collection, however, the small sample size and low event rate meant that the planned statistical tests were no longer appropriate. Instead, we decided to analyze the planned outcomes using descriptive statistics and look at an additional number of post hoc outcomes to provide deeper insight into clinical practice. We examined the association between relevant demographic factors, such as age, comorbidity burden, ICS potency, duration of ICS therapy, and EOS count and the clinician decision whether to taper the ICS. These same factors were also evaluated for potential association with the increased risk of COPD exacerbations following ICS discontinuation.
Results
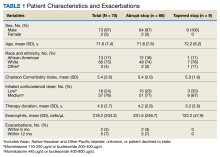
A total of 75 patients were included. Most patients were White race and male with a mean (SD) age of 71.6 (7.4) years. Charlson Comorbidity Index scores were calculated for all included patients with a mean (SD) score of 5.4 (2.0). Of note, scores > 5 are considered a severe comorbidity burden and have an estimated mean 10-year survival rate < 21%. The overwhelming majority of patients were receiving budesonide/formoterol as their ICS inhaler with 1 receiving mometasone monotherapy. When evaluating the steroid dose, 18 (24%) patients received a low dose ICS (200-400 µg of budesonide or 110-220 µg of mometasone), while 57 (76%) received a medium dose (400-800 µg of budesonide or 440 µg of mometasone). No patients received a high ICS dose. The mean (SD) duration of therapy before discontinuation was 4.0 (2.7) years (Table 1).
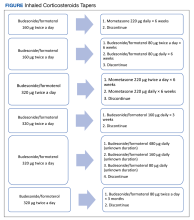
Nine (12%) patients had their ICS slowly tapered, while therapy was abruptly discontinued in the other 66 (88%) patients. A variety of taper types were used (Figure) without a strong preference for a particular dosing strategy. The primary outcome of COPD exacerbation requiring emergency department visit or hospitalization within 6 months occurred in 2 patients. When the time frame was extended to 12 months for the secondary outcome, an additional 3 patients experienced an event. The mean time to event was 172 days following ICS discontinuation. All the events occurred in patients whose ICS was discontinued without any type of taper.
In a post hoc analysis, we examined the relationship between specific variables and the clinician choice whether to taper an ICS. There was no discernable impact of age, race and ethnicity, comorbidity score, or ICS dose on whether an ICS was tapered. We observed a slight association between shorter duration of therapy and lower EOS count and use of a taper. When evaluating the relationship between these same factors and exacerbation occurrence, we saw comparable trends (Table 2). Patients with an exacerbation had a slightly longer mean duration of ICS therapy and lower mean EOS count.
Discussion
Despite facility guidance recommending tapering of therapy when discontinuing a moderate- or high-dose ICS, most patients in this study discontinued the ICS abruptly. The clinician may have been concerned with patients being able to adhere to a taper regimen, skeptical of the actual need to taper, or unaware of the VANTHCS recommendations for a specific taper method. Shared decision making with patients may have also played a role in prescribing patterns. Currently, there is not sufficient data to support the use of any one particular type of taper over another, which accounts for the variability seen in practice.
The decision to taper ICSs did not seem to be strongly associated with any specific demographic factor, although the ability to examine the impact of factors (eg, race and ethnicity) was limited due to the largely homogenous population. One may have expected a taper to be more common in older patients or in those with more comorbidities; however, this was not observed in this study. The only discernible trends seen were a lower frequency of tapering in patients who had a shorter duration of ICS therapy and those with lower EOS counts. These patients were at lower risk of repeat COPD exacerbations compared with those with longer ICS therapy duration and higher EOS counts; therefore, this finding was unexpected. This suggests that patient-specific factors may not be the primary driving force in the ICS tapering decision; instead it may be based on general clinician preferences or shared decision making with individual patients.
Overall, we noted very low rates of COPD exacerbations. As ICS discontinuation was occurring in stable patients without any recent exacerbations, lower rates of future exacerbations were expected compared with the population of patients with COPD as a whole. This suggests that ICS therapy can be safely stopped in stable patients with COPD who are not likely to receive significant benefits as defined in the GOLD guidelines. All of the exacerbations that occurred were in patients whose ICS was abruptly discontinued; however, given the small number of patients who had a taper, it is difficult to draw conclusions. The low overall rate of exacerbations suggests that a taper may not be necessary to ensure safety while stopping a low- or moderate-intensity ICS.
Several randomized controlled trials have attempted to evaluate the need for an ICS taper; however, results remain mixed. The COSMIC study showed a decline in lung function following ICS discontinuation in patients with ≥ 2 COPD exacerbations in the previous year.8 Similar results were seen in the SUNSET study with increased exacerbation rates after ICS discontinuation in patients with elevated EOS counts.9 However, these studies included patients for whom ICS discontinuation is currently not recommended. Alternatively, the INSTEAD trial looked at patients without frequent recent exacerbations and found no difference in lung function, exacerbation rates, or rescue inhaler use in patients that continued combination ICS plus bronchodilator use vs those de-escalated to bronchodilator monotherapy.10
All 3 studies chose to abruptly stop the ICS when discontinuing therapy; however, using a slow, stepwise taper similar to that used after long periods of oral steroid use may reduce the risk of worsening exacerbations. The WISDOM trial is the only major randomized trial to date that stopped ICS therapy using a stepwise withdrawal of therapy.7 In patients who were continued on triple inhaled therapy (2 bronchodilators plus ICS) vs those who were de-escalated to dual bronchodilator therapy, de-escalation was noninferior to continuation of therapy in time to first COPD exacerbation. Both the WISDOM and INSTEAD trials were consistent with the results found in our real-world retrospective evaluation.
There did not seem to be an increased exacerbation risk following ICS discontinuation in any patient subpopulation based on sex, age, race and ethnicity, or comorbidity burden. We noted a trend toward more exacerbations in patients with a longer duration of ICS therapy, suggesting that additional caution may be needed when stopping ICS therapy for these patients. We also noted a trend toward more exacerbations in patients with a lower mean EOS count; however, given the low event rate and wide variability in observed patient EOS counts, this is likely a spurious finding.
Limitations
The small sample size, resulting from the strict exclusion criteria, limits the generalizability of the results. Although the low number of events seen in this study supports safety in ICS discontinuation, there may have been higher rates observed in a larger population. The most common reason for patient exclusion was the initiation of another ICS immediately following discontinuation of the original ICS. During the study period, VANTHCS underwent a change to its formulary: Fluticasone/salmeterol replaced budesonide/formoterol as the preferred ICS/LABA combination. As a result, many patients had their budesonide/formoterol discontinued during the study period solely to initiate fluticasone/salmeterol therapy. As these patients did not truly have their ICS discontinued or have a significant period without ICS therapy, they were not included in the results, and the total patient population available to analyze was relatively limited.
The low event rate also limits the ability to compare various factors influencing exacerbation risk, particularly taper vs abrupt ICS discontinuation. This is further compounded by the small number of patients who had a taper performed and the lack of consistency in the method of tapering used. Statistical significance could not be determined for any outcome, and all findings were purely hypothesis generating. Finally, data were only collected for moderate or severe COPD exacerbations that resulted in an emergency department visit or hospitalization, so there may have been mild exacerbations treated in the outpatient setting that were not captured.
Despite these limitations, this study adds data to an area of COPD management that currently lacks strong clinical guidance. Since investigators had access to clinician notes, we were able to capture ICS tapers even if patients did not receive a prescription with specific taper instructions. The extended follow-up period of 12 months evaluated a longer potential time to impact of ICS discontinuation than is done in most COPD clinical trials.
Conclusions
Overall, very low rates of COPD exacerbations occurred following ICS discontinuation, regardless of whether a taper
1. Yang IA, Clarke MS, Sim EH, Fong KM. Inhaled corticosteroids for stable chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2012;7(7):CD002991. doi:10.1002/14651858.CD002991.pub3
2. Crim C, Dransfield MT, Bourbeau J, et al. Pneumonia risk with inhaled fluticasone furoate and vilanterol compared with vilanterol alone in patients with COPD. Ann Am Thorac Soc. 2015;12(1):27-34. doi:10.1513/AnnalsATS.201409-413OC
3. Crim C, Calverley PMA, Anderson JA, et al. Pneumonia risk with inhaled fluticasone furoate and vilanterol in COPD patients with moderate airflow limitation: The SUMMIT trial. Respir Med. 2017;131:27-34. doi:10.1016/j.rmed.2017.07.060
4. Global Initiative for Chronic Obstructive Lung Disease. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease (2023 Report). Accessed November 3, 2023. https://goldcopd.org/wp-content/uploads/2023/03/GOLD-2023-ver-1.3-17Feb2023_WMV.pdf
5. Nannini LJ, Lasserson TJ, Poole P. Combined corticosteroid and long-acting beta(2)-agonist in one inhaler versus long-acting beta(2)-agonists for chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2012;9(9):CD006829. doi:10.1002/14651858.CD006826.pub2
6. Kaplan AG. Applying the wisdom of stepping down inhaled corticosteroids in patients with COPD: a proposed algorithm for clinical practice. Int J Chron Obstruct Pulmon Dis. 2015;10:2535-2548. doi:10.2147/COPD.S93321
7. Magnussen H, Disse B, Rodriguez-Roisin R, et al; WISDOM Investigators. Withdrawal of inhaled glucocorticoids and exacerbations of COPD. N Engl J Med. 2014;371(14):1285-1294. doi:10.1056/NEJMoa1407154
8. Wouters EFM, Postma DS, Fokkens B. COSMIC (COPD and Seretide: a Multi-Center Intervention and Characterization) Study Group. Withdrawal of fluticasone propionate from combined salmeterol/fluticasone treatment in patients with COPD causes immediate and sustained disease deterioration: a randomized controlled trial. Thorax. 2005;60(6):480-487. doi:10.1136/thx.2004.034280
9. Chapman KR, Hurst JR, Frent S-M, et al. Long-term triple therapy de-escalation to indacaterol/glycopyrronium in patients with chronic obstructive pulmonary disease (SUNSET): a randomized, double-blind, triple-dummy clinical trial. Am J Respir Crit Care Med. 2018;198(3):329-339. doi:10.1164/rccm.201803-0405OC
10. Rossi A, van der Molen T, del Olmo R, et al. INSTEAD: a randomized switch trial of indacaterol versus salmeterol/fluticasone in moderate COPD. Eur Respir J. 2014;44(6):1548-1556. doi:10.1183/09031936.00126814
Inhaled corticosteroids (ICSs) are frequently prescribed for the treatment of chronic obstructive pulmonary disease (COPD) to reduce exacerbations in a specific subset of patients. The long-term use of ICSs, however, is associated with several potential systemic adverse effects, including adrenal suppression, decreased bone mineral density, and immunosuppression.1 The concern for immunosuppression is particularly notable and leads to a known increased risk for developing pneumonia in patients with COPD. These patients frequently have other concurrent risk factors for pneumonia (eg, history of tobacco use, older age, and severe airway limitations) and are at higher risk for more severe outcomes in the setting of pneumonia.2,3
Primarily due to the concern of pneumonia risks, the Global Initiative for Chronic Obstructive Lung Disease (GOLD) guidelines have recommended ICS discontinuation in patients who are less likely to receive significant benefits from therapy.4 Likely due to an anti-inflammatory mechanism of action, ICSs have been shown to reduce COPD exacerbation rates in patients with comorbid asthma or who have evidence of a strong inflammatory component to their COPD. The strongest indicator of an inflammatory component is an elevated blood eosinophil (EOS) count; those with EOS > 300 cells/µL are most likely to benefit from ICSs, whereas those with a count < 100 cells/µL are unlikely to have a significant response. In addition to the inflammatory component consideration, prior studies have shown improvements in lung function and reduction of exacerbations with ICS use in patients with frequent moderate-to-severe COPD exacerbations.5 Although the GOLD guidelines provide recommendations about who is appropriate to discontinue ICS use, clinicians have no clear guidance on the risks or the best discontinuation strategy.
Based primarily on data from a prior randomized controlled trial, the Veterans Integrated Services Network (VISN) 17, which includes the Veterans Affairs North Texas Health Care System (VANTHCS) in Dallas, established a recommended ICS de-escalation strategy.6,7 The strategy included a 12-week stepwise taper using a mometasone inhaler for all patients discontinuing a moderate or high dose ICS. The lack of substantial clinical trial data or expert consensus guideline recommendations has left open the question of whether a taper is necessary. To answer that question, this study was conducted to evaluate whether there is a difference in the rate of COPD exacerbations following abrupt discontinuation vs gradual taper of ICS therapy.
Methods
This single-center, retrospective cohort study was conducted at VANTHCS. Patient electronic health records between January 10, 2021, and September 1, 2021, were reviewed for the last documented fill date of any inhaler containing a steroid component. This time frame was chosen to coincide with a VANTHCS initiative to follow GOLD guidelines for ICS discontinuation. Patients were followed for outcomes until November 1, 2022.
To be included in this study, patients had to have active prescriptions at VANTHCS, have a documented diagnosis of COPD in their chart, and be prescribed a stable dose of ICS for ≥ 1 year prior to their latest refill. The inhaler used could contain an ICS as monotherapy, in combination with a long-acting β-agonist (LABA), or as part of triple therapy with an additional long-acting muscarinic antagonist (LAMA). The inhaler needed to be discontinued during the study period of interest.
Patients were excluded if they had a diagnosis of asthma, were aged < 40 years, had active prescriptions for multiple ICS inhalers or nebulizers, or had significant oral steroid use (≥ 5 mg/d prednisone or an equivalent steroid for > 6 weeks) within 1 year of their ICS discontinuation date. In addition, to reduce the risk of future events being misclassified as COPD exacerbations, patients were excluded if they had a congestive heart failure exacerbation up to 2 years before ICS discontinuation or a diagnosis of COVID-19 infection up to 1 year before or 6 months after ICS discontinuation. Patients with a COPD exacerbation requiring an emergency department or hospital visit within 2 years prior to ICS discontinuation were also excluded, as de-escalation of ICS therapy was likely inappropriate in these cases. Finally, patients were excluded if they were started on a different ICS immediately following the discontinuation of their first ICS.
The primary outcome for this study was COPD exacerbations requiring an emergency department visit or hospitalization within 6 months of ICS discontinuation. A secondary outcome examining the rates of COPD exacerbations within 12 months also was used. The original study design called for the use of inferential statistics to compare the rates of primary and secondary outcomes in patients whose ICS was abruptly discontinued with those who were tapered slowly. After data collection, however, the small sample size and low event rate meant that the planned statistical tests were no longer appropriate. Instead, we decided to analyze the planned outcomes using descriptive statistics and look at an additional number of post hoc outcomes to provide deeper insight into clinical practice. We examined the association between relevant demographic factors, such as age, comorbidity burden, ICS potency, duration of ICS therapy, and EOS count and the clinician decision whether to taper the ICS. These same factors were also evaluated for potential association with the increased risk of COPD exacerbations following ICS discontinuation.
Results
A total of 75 patients were included. Most patients were White race and male with a mean (SD) age of 71.6 (7.4) years. Charlson Comorbidity Index scores were calculated for all included patients with a mean (SD) score of 5.4 (2.0). Of note, scores > 5 are considered a severe comorbidity burden and have an estimated mean 10-year survival rate < 21%. The overwhelming majority of patients were receiving budesonide/formoterol as their ICS inhaler with 1 receiving mometasone monotherapy. When evaluating the steroid dose, 18 (24%) patients received a low dose ICS (200-400 µg of budesonide or 110-220 µg of mometasone), while 57 (76%) received a medium dose (400-800 µg of budesonide or 440 µg of mometasone). No patients received a high ICS dose. The mean (SD) duration of therapy before discontinuation was 4.0 (2.7) years (Table 1).
Nine (12%) patients had their ICS slowly tapered, while therapy was abruptly discontinued in the other 66 (88%) patients. A variety of taper types were used (Figure) without a strong preference for a particular dosing strategy. The primary outcome of COPD exacerbation requiring emergency department visit or hospitalization within 6 months occurred in 2 patients. When the time frame was extended to 12 months for the secondary outcome, an additional 3 patients experienced an event. The mean time to event was 172 days following ICS discontinuation. All the events occurred in patients whose ICS was discontinued without any type of taper.
In a post hoc analysis, we examined the relationship between specific variables and the clinician choice whether to taper an ICS. There was no discernable impact of age, race and ethnicity, comorbidity score, or ICS dose on whether an ICS was tapered. We observed a slight association between shorter duration of therapy and lower EOS count and use of a taper. When evaluating the relationship between these same factors and exacerbation occurrence, we saw comparable trends (Table 2). Patients with an exacerbation had a slightly longer mean duration of ICS therapy and lower mean EOS count.
Discussion
Despite facility guidance recommending tapering of therapy when discontinuing a moderate- or high-dose ICS, most patients in this study discontinued the ICS abruptly. The clinician may have been concerned with patients being able to adhere to a taper regimen, skeptical of the actual need to taper, or unaware of the VANTHCS recommendations for a specific taper method. Shared decision making with patients may have also played a role in prescribing patterns. Currently, there is not sufficient data to support the use of any one particular type of taper over another, which accounts for the variability seen in practice.
The decision to taper ICSs did not seem to be strongly associated with any specific demographic factor, although the ability to examine the impact of factors (eg, race and ethnicity) was limited due to the largely homogenous population. One may have expected a taper to be more common in older patients or in those with more comorbidities; however, this was not observed in this study. The only discernible trends seen were a lower frequency of tapering in patients who had a shorter duration of ICS therapy and those with lower EOS counts. These patients were at lower risk of repeat COPD exacerbations compared with those with longer ICS therapy duration and higher EOS counts; therefore, this finding was unexpected. This suggests that patient-specific factors may not be the primary driving force in the ICS tapering decision; instead it may be based on general clinician preferences or shared decision making with individual patients.
Overall, we noted very low rates of COPD exacerbations. As ICS discontinuation was occurring in stable patients without any recent exacerbations, lower rates of future exacerbations were expected compared with the population of patients with COPD as a whole. This suggests that ICS therapy can be safely stopped in stable patients with COPD who are not likely to receive significant benefits as defined in the GOLD guidelines. All of the exacerbations that occurred were in patients whose ICS was abruptly discontinued; however, given the small number of patients who had a taper, it is difficult to draw conclusions. The low overall rate of exacerbations suggests that a taper may not be necessary to ensure safety while stopping a low- or moderate-intensity ICS.
Several randomized controlled trials have attempted to evaluate the need for an ICS taper; however, results remain mixed. The COSMIC study showed a decline in lung function following ICS discontinuation in patients with ≥ 2 COPD exacerbations in the previous year.8 Similar results were seen in the SUNSET study with increased exacerbation rates after ICS discontinuation in patients with elevated EOS counts.9 However, these studies included patients for whom ICS discontinuation is currently not recommended. Alternatively, the INSTEAD trial looked at patients without frequent recent exacerbations and found no difference in lung function, exacerbation rates, or rescue inhaler use in patients that continued combination ICS plus bronchodilator use vs those de-escalated to bronchodilator monotherapy.10
All 3 studies chose to abruptly stop the ICS when discontinuing therapy; however, using a slow, stepwise taper similar to that used after long periods of oral steroid use may reduce the risk of worsening exacerbations. The WISDOM trial is the only major randomized trial to date that stopped ICS therapy using a stepwise withdrawal of therapy.7 In patients who were continued on triple inhaled therapy (2 bronchodilators plus ICS) vs those who were de-escalated to dual bronchodilator therapy, de-escalation was noninferior to continuation of therapy in time to first COPD exacerbation. Both the WISDOM and INSTEAD trials were consistent with the results found in our real-world retrospective evaluation.
There did not seem to be an increased exacerbation risk following ICS discontinuation in any patient subpopulation based on sex, age, race and ethnicity, or comorbidity burden. We noted a trend toward more exacerbations in patients with a longer duration of ICS therapy, suggesting that additional caution may be needed when stopping ICS therapy for these patients. We also noted a trend toward more exacerbations in patients with a lower mean EOS count; however, given the low event rate and wide variability in observed patient EOS counts, this is likely a spurious finding.
Limitations
The small sample size, resulting from the strict exclusion criteria, limits the generalizability of the results. Although the low number of events seen in this study supports safety in ICS discontinuation, there may have been higher rates observed in a larger population. The most common reason for patient exclusion was the initiation of another ICS immediately following discontinuation of the original ICS. During the study period, VANTHCS underwent a change to its formulary: Fluticasone/salmeterol replaced budesonide/formoterol as the preferred ICS/LABA combination. As a result, many patients had their budesonide/formoterol discontinued during the study period solely to initiate fluticasone/salmeterol therapy. As these patients did not truly have their ICS discontinued or have a significant period without ICS therapy, they were not included in the results, and the total patient population available to analyze was relatively limited.
The low event rate also limits the ability to compare various factors influencing exacerbation risk, particularly taper vs abrupt ICS discontinuation. This is further compounded by the small number of patients who had a taper performed and the lack of consistency in the method of tapering used. Statistical significance could not be determined for any outcome, and all findings were purely hypothesis generating. Finally, data were only collected for moderate or severe COPD exacerbations that resulted in an emergency department visit or hospitalization, so there may have been mild exacerbations treated in the outpatient setting that were not captured.
Despite these limitations, this study adds data to an area of COPD management that currently lacks strong clinical guidance. Since investigators had access to clinician notes, we were able to capture ICS tapers even if patients did not receive a prescription with specific taper instructions. The extended follow-up period of 12 months evaluated a longer potential time to impact of ICS discontinuation than is done in most COPD clinical trials.
Conclusions
Overall, very low rates of COPD exacerbations occurred following ICS discontinuation, regardless of whether a taper
Inhaled corticosteroids (ICSs) are frequently prescribed for the treatment of chronic obstructive pulmonary disease (COPD) to reduce exacerbations in a specific subset of patients. The long-term use of ICSs, however, is associated with several potential systemic adverse effects, including adrenal suppression, decreased bone mineral density, and immunosuppression.1 The concern for immunosuppression is particularly notable and leads to a known increased risk for developing pneumonia in patients with COPD. These patients frequently have other concurrent risk factors for pneumonia (eg, history of tobacco use, older age, and severe airway limitations) and are at higher risk for more severe outcomes in the setting of pneumonia.2,3
Primarily due to the concern of pneumonia risks, the Global Initiative for Chronic Obstructive Lung Disease (GOLD) guidelines have recommended ICS discontinuation in patients who are less likely to receive significant benefits from therapy.4 Likely due to an anti-inflammatory mechanism of action, ICSs have been shown to reduce COPD exacerbation rates in patients with comorbid asthma or who have evidence of a strong inflammatory component to their COPD. The strongest indicator of an inflammatory component is an elevated blood eosinophil (EOS) count; those with EOS > 300 cells/µL are most likely to benefit from ICSs, whereas those with a count < 100 cells/µL are unlikely to have a significant response. In addition to the inflammatory component consideration, prior studies have shown improvements in lung function and reduction of exacerbations with ICS use in patients with frequent moderate-to-severe COPD exacerbations.5 Although the GOLD guidelines provide recommendations about who is appropriate to discontinue ICS use, clinicians have no clear guidance on the risks or the best discontinuation strategy.
Based primarily on data from a prior randomized controlled trial, the Veterans Integrated Services Network (VISN) 17, which includes the Veterans Affairs North Texas Health Care System (VANTHCS) in Dallas, established a recommended ICS de-escalation strategy.6,7 The strategy included a 12-week stepwise taper using a mometasone inhaler for all patients discontinuing a moderate or high dose ICS. The lack of substantial clinical trial data or expert consensus guideline recommendations has left open the question of whether a taper is necessary. To answer that question, this study was conducted to evaluate whether there is a difference in the rate of COPD exacerbations following abrupt discontinuation vs gradual taper of ICS therapy.
Methods
This single-center, retrospective cohort study was conducted at VANTHCS. Patient electronic health records between January 10, 2021, and September 1, 2021, were reviewed for the last documented fill date of any inhaler containing a steroid component. This time frame was chosen to coincide with a VANTHCS initiative to follow GOLD guidelines for ICS discontinuation. Patients were followed for outcomes until November 1, 2022.
To be included in this study, patients had to have active prescriptions at VANTHCS, have a documented diagnosis of COPD in their chart, and be prescribed a stable dose of ICS for ≥ 1 year prior to their latest refill. The inhaler used could contain an ICS as monotherapy, in combination with a long-acting β-agonist (LABA), or as part of triple therapy with an additional long-acting muscarinic antagonist (LAMA). The inhaler needed to be discontinued during the study period of interest.
Patients were excluded if they had a diagnosis of asthma, were aged < 40 years, had active prescriptions for multiple ICS inhalers or nebulizers, or had significant oral steroid use (≥ 5 mg/d prednisone or an equivalent steroid for > 6 weeks) within 1 year of their ICS discontinuation date. In addition, to reduce the risk of future events being misclassified as COPD exacerbations, patients were excluded if they had a congestive heart failure exacerbation up to 2 years before ICS discontinuation or a diagnosis of COVID-19 infection up to 1 year before or 6 months after ICS discontinuation. Patients with a COPD exacerbation requiring an emergency department or hospital visit within 2 years prior to ICS discontinuation were also excluded, as de-escalation of ICS therapy was likely inappropriate in these cases. Finally, patients were excluded if they were started on a different ICS immediately following the discontinuation of their first ICS.
The primary outcome for this study was COPD exacerbations requiring an emergency department visit or hospitalization within 6 months of ICS discontinuation. A secondary outcome examining the rates of COPD exacerbations within 12 months also was used. The original study design called for the use of inferential statistics to compare the rates of primary and secondary outcomes in patients whose ICS was abruptly discontinued with those who were tapered slowly. After data collection, however, the small sample size and low event rate meant that the planned statistical tests were no longer appropriate. Instead, we decided to analyze the planned outcomes using descriptive statistics and look at an additional number of post hoc outcomes to provide deeper insight into clinical practice. We examined the association between relevant demographic factors, such as age, comorbidity burden, ICS potency, duration of ICS therapy, and EOS count and the clinician decision whether to taper the ICS. These same factors were also evaluated for potential association with the increased risk of COPD exacerbations following ICS discontinuation.
Results
A total of 75 patients were included. Most patients were White race and male with a mean (SD) age of 71.6 (7.4) years. Charlson Comorbidity Index scores were calculated for all included patients with a mean (SD) score of 5.4 (2.0). Of note, scores > 5 are considered a severe comorbidity burden and have an estimated mean 10-year survival rate < 21%. The overwhelming majority of patients were receiving budesonide/formoterol as their ICS inhaler with 1 receiving mometasone monotherapy. When evaluating the steroid dose, 18 (24%) patients received a low dose ICS (200-400 µg of budesonide or 110-220 µg of mometasone), while 57 (76%) received a medium dose (400-800 µg of budesonide or 440 µg of mometasone). No patients received a high ICS dose. The mean (SD) duration of therapy before discontinuation was 4.0 (2.7) years (Table 1).
Nine (12%) patients had their ICS slowly tapered, while therapy was abruptly discontinued in the other 66 (88%) patients. A variety of taper types were used (Figure) without a strong preference for a particular dosing strategy. The primary outcome of COPD exacerbation requiring emergency department visit or hospitalization within 6 months occurred in 2 patients. When the time frame was extended to 12 months for the secondary outcome, an additional 3 patients experienced an event. The mean time to event was 172 days following ICS discontinuation. All the events occurred in patients whose ICS was discontinued without any type of taper.
In a post hoc analysis, we examined the relationship between specific variables and the clinician choice whether to taper an ICS. There was no discernable impact of age, race and ethnicity, comorbidity score, or ICS dose on whether an ICS was tapered. We observed a slight association between shorter duration of therapy and lower EOS count and use of a taper. When evaluating the relationship between these same factors and exacerbation occurrence, we saw comparable trends (Table 2). Patients with an exacerbation had a slightly longer mean duration of ICS therapy and lower mean EOS count.
Discussion
Despite facility guidance recommending tapering of therapy when discontinuing a moderate- or high-dose ICS, most patients in this study discontinued the ICS abruptly. The clinician may have been concerned with patients being able to adhere to a taper regimen, skeptical of the actual need to taper, or unaware of the VANTHCS recommendations for a specific taper method. Shared decision making with patients may have also played a role in prescribing patterns. Currently, there is not sufficient data to support the use of any one particular type of taper over another, which accounts for the variability seen in practice.
The decision to taper ICSs did not seem to be strongly associated with any specific demographic factor, although the ability to examine the impact of factors (eg, race and ethnicity) was limited due to the largely homogenous population. One may have expected a taper to be more common in older patients or in those with more comorbidities; however, this was not observed in this study. The only discernible trends seen were a lower frequency of tapering in patients who had a shorter duration of ICS therapy and those with lower EOS counts. These patients were at lower risk of repeat COPD exacerbations compared with those with longer ICS therapy duration and higher EOS counts; therefore, this finding was unexpected. This suggests that patient-specific factors may not be the primary driving force in the ICS tapering decision; instead it may be based on general clinician preferences or shared decision making with individual patients.
Overall, we noted very low rates of COPD exacerbations. As ICS discontinuation was occurring in stable patients without any recent exacerbations, lower rates of future exacerbations were expected compared with the population of patients with COPD as a whole. This suggests that ICS therapy can be safely stopped in stable patients with COPD who are not likely to receive significant benefits as defined in the GOLD guidelines. All of the exacerbations that occurred were in patients whose ICS was abruptly discontinued; however, given the small number of patients who had a taper, it is difficult to draw conclusions. The low overall rate of exacerbations suggests that a taper may not be necessary to ensure safety while stopping a low- or moderate-intensity ICS.
Several randomized controlled trials have attempted to evaluate the need for an ICS taper; however, results remain mixed. The COSMIC study showed a decline in lung function following ICS discontinuation in patients with ≥ 2 COPD exacerbations in the previous year.8 Similar results were seen in the SUNSET study with increased exacerbation rates after ICS discontinuation in patients with elevated EOS counts.9 However, these studies included patients for whom ICS discontinuation is currently not recommended. Alternatively, the INSTEAD trial looked at patients without frequent recent exacerbations and found no difference in lung function, exacerbation rates, or rescue inhaler use in patients that continued combination ICS plus bronchodilator use vs those de-escalated to bronchodilator monotherapy.10
All 3 studies chose to abruptly stop the ICS when discontinuing therapy; however, using a slow, stepwise taper similar to that used after long periods of oral steroid use may reduce the risk of worsening exacerbations. The WISDOM trial is the only major randomized trial to date that stopped ICS therapy using a stepwise withdrawal of therapy.7 In patients who were continued on triple inhaled therapy (2 bronchodilators plus ICS) vs those who were de-escalated to dual bronchodilator therapy, de-escalation was noninferior to continuation of therapy in time to first COPD exacerbation. Both the WISDOM and INSTEAD trials were consistent with the results found in our real-world retrospective evaluation.
There did not seem to be an increased exacerbation risk following ICS discontinuation in any patient subpopulation based on sex, age, race and ethnicity, or comorbidity burden. We noted a trend toward more exacerbations in patients with a longer duration of ICS therapy, suggesting that additional caution may be needed when stopping ICS therapy for these patients. We also noted a trend toward more exacerbations in patients with a lower mean EOS count; however, given the low event rate and wide variability in observed patient EOS counts, this is likely a spurious finding.
Limitations
The small sample size, resulting from the strict exclusion criteria, limits the generalizability of the results. Although the low number of events seen in this study supports safety in ICS discontinuation, there may have been higher rates observed in a larger population. The most common reason for patient exclusion was the initiation of another ICS immediately following discontinuation of the original ICS. During the study period, VANTHCS underwent a change to its formulary: Fluticasone/salmeterol replaced budesonide/formoterol as the preferred ICS/LABA combination. As a result, many patients had their budesonide/formoterol discontinued during the study period solely to initiate fluticasone/salmeterol therapy. As these patients did not truly have their ICS discontinued or have a significant period without ICS therapy, they were not included in the results, and the total patient population available to analyze was relatively limited.
The low event rate also limits the ability to compare various factors influencing exacerbation risk, particularly taper vs abrupt ICS discontinuation. This is further compounded by the small number of patients who had a taper performed and the lack of consistency in the method of tapering used. Statistical significance could not be determined for any outcome, and all findings were purely hypothesis generating. Finally, data were only collected for moderate or severe COPD exacerbations that resulted in an emergency department visit or hospitalization, so there may have been mild exacerbations treated in the outpatient setting that were not captured.
Despite these limitations, this study adds data to an area of COPD management that currently lacks strong clinical guidance. Since investigators had access to clinician notes, we were able to capture ICS tapers even if patients did not receive a prescription with specific taper instructions. The extended follow-up period of 12 months evaluated a longer potential time to impact of ICS discontinuation than is done in most COPD clinical trials.
Conclusions
Overall, very low rates of COPD exacerbations occurred following ICS discontinuation, regardless of whether a taper
1. Yang IA, Clarke MS, Sim EH, Fong KM. Inhaled corticosteroids for stable chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2012;7(7):CD002991. doi:10.1002/14651858.CD002991.pub3
2. Crim C, Dransfield MT, Bourbeau J, et al. Pneumonia risk with inhaled fluticasone furoate and vilanterol compared with vilanterol alone in patients with COPD. Ann Am Thorac Soc. 2015;12(1):27-34. doi:10.1513/AnnalsATS.201409-413OC
3. Crim C, Calverley PMA, Anderson JA, et al. Pneumonia risk with inhaled fluticasone furoate and vilanterol in COPD patients with moderate airflow limitation: The SUMMIT trial. Respir Med. 2017;131:27-34. doi:10.1016/j.rmed.2017.07.060
4. Global Initiative for Chronic Obstructive Lung Disease. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease (2023 Report). Accessed November 3, 2023. https://goldcopd.org/wp-content/uploads/2023/03/GOLD-2023-ver-1.3-17Feb2023_WMV.pdf
5. Nannini LJ, Lasserson TJ, Poole P. Combined corticosteroid and long-acting beta(2)-agonist in one inhaler versus long-acting beta(2)-agonists for chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2012;9(9):CD006829. doi:10.1002/14651858.CD006826.pub2
6. Kaplan AG. Applying the wisdom of stepping down inhaled corticosteroids in patients with COPD: a proposed algorithm for clinical practice. Int J Chron Obstruct Pulmon Dis. 2015;10:2535-2548. doi:10.2147/COPD.S93321
7. Magnussen H, Disse B, Rodriguez-Roisin R, et al; WISDOM Investigators. Withdrawal of inhaled glucocorticoids and exacerbations of COPD. N Engl J Med. 2014;371(14):1285-1294. doi:10.1056/NEJMoa1407154
8. Wouters EFM, Postma DS, Fokkens B. COSMIC (COPD and Seretide: a Multi-Center Intervention and Characterization) Study Group. Withdrawal of fluticasone propionate from combined salmeterol/fluticasone treatment in patients with COPD causes immediate and sustained disease deterioration: a randomized controlled trial. Thorax. 2005;60(6):480-487. doi:10.1136/thx.2004.034280
9. Chapman KR, Hurst JR, Frent S-M, et al. Long-term triple therapy de-escalation to indacaterol/glycopyrronium in patients with chronic obstructive pulmonary disease (SUNSET): a randomized, double-blind, triple-dummy clinical trial. Am J Respir Crit Care Med. 2018;198(3):329-339. doi:10.1164/rccm.201803-0405OC
10. Rossi A, van der Molen T, del Olmo R, et al. INSTEAD: a randomized switch trial of indacaterol versus salmeterol/fluticasone in moderate COPD. Eur Respir J. 2014;44(6):1548-1556. doi:10.1183/09031936.00126814
1. Yang IA, Clarke MS, Sim EH, Fong KM. Inhaled corticosteroids for stable chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2012;7(7):CD002991. doi:10.1002/14651858.CD002991.pub3
2. Crim C, Dransfield MT, Bourbeau J, et al. Pneumonia risk with inhaled fluticasone furoate and vilanterol compared with vilanterol alone in patients with COPD. Ann Am Thorac Soc. 2015;12(1):27-34. doi:10.1513/AnnalsATS.201409-413OC
3. Crim C, Calverley PMA, Anderson JA, et al. Pneumonia risk with inhaled fluticasone furoate and vilanterol in COPD patients with moderate airflow limitation: The SUMMIT trial. Respir Med. 2017;131:27-34. doi:10.1016/j.rmed.2017.07.060
4. Global Initiative for Chronic Obstructive Lung Disease. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease (2023 Report). Accessed November 3, 2023. https://goldcopd.org/wp-content/uploads/2023/03/GOLD-2023-ver-1.3-17Feb2023_WMV.pdf
5. Nannini LJ, Lasserson TJ, Poole P. Combined corticosteroid and long-acting beta(2)-agonist in one inhaler versus long-acting beta(2)-agonists for chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2012;9(9):CD006829. doi:10.1002/14651858.CD006826.pub2
6. Kaplan AG. Applying the wisdom of stepping down inhaled corticosteroids in patients with COPD: a proposed algorithm for clinical practice. Int J Chron Obstruct Pulmon Dis. 2015;10:2535-2548. doi:10.2147/COPD.S93321
7. Magnussen H, Disse B, Rodriguez-Roisin R, et al; WISDOM Investigators. Withdrawal of inhaled glucocorticoids and exacerbations of COPD. N Engl J Med. 2014;371(14):1285-1294. doi:10.1056/NEJMoa1407154
8. Wouters EFM, Postma DS, Fokkens B. COSMIC (COPD and Seretide: a Multi-Center Intervention and Characterization) Study Group. Withdrawal of fluticasone propionate from combined salmeterol/fluticasone treatment in patients with COPD causes immediate and sustained disease deterioration: a randomized controlled trial. Thorax. 2005;60(6):480-487. doi:10.1136/thx.2004.034280
9. Chapman KR, Hurst JR, Frent S-M, et al. Long-term triple therapy de-escalation to indacaterol/glycopyrronium in patients with chronic obstructive pulmonary disease (SUNSET): a randomized, double-blind, triple-dummy clinical trial. Am J Respir Crit Care Med. 2018;198(3):329-339. doi:10.1164/rccm.201803-0405OC
10. Rossi A, van der Molen T, del Olmo R, et al. INSTEAD: a randomized switch trial of indacaterol versus salmeterol/fluticasone in moderate COPD. Eur Respir J. 2014;44(6):1548-1556. doi:10.1183/09031936.00126814