User login
Endometrial cancer is the most common malignancy of the female reproductive tract in the United States, and its incidence continues to rise, with an estimated 49,560 new cases predicted for 2013.1 If we are to successfully traverse the pathway from molecular cell genetics to the development of targeted therapies and personalized cancer care, we need to meet a few benchmarks:
- We need to enhance our understanding of the molecular changes that lead to endometrial cancer. Of particular interest are nonendometrioid tumors. Increased mortality from endometrial cancer appears to be related to the growing number of uterine papillary serous carcinomas and clear-cell cancers. Although these cancers constitute less than 10% of all endometrial cancers, they account for a disproportionately high number of recurrences and cancer-related deaths.2,3 Do recent studies validate the original classification of endometrial cancers as Type I (endometrioid) or Type II (serous and clear cell), or is there more heterogeneity than was originally thought? How do recent studies affect treatment options?
- We need to establish a genomic characterization of endometrial cancer to supplement clinical research data. The identification of novel mutations specific to each histologic type has the potential to improve adjuvant therapy. How close are we to performing a comprehensive genomic analysis of endometrial cancer?
- We need to develop new adjuvant treatment options for recurrent and advanced disease. When clinical symptoms of endometrial cancer are overt, as they often are, early diagnosis is possible, with a 5-year survival rate of 80% to 90%. The prognosis declines dramatically in women with advanced-stage disease or high-risk histologies, with a 5-year survival rate of 57% and 19% for Stage III and Stage IV disease, respectively.1 Adjuvant treatment options are limited in the setting of recurrent or advanced disease. Do any biologic agents increase survival?
In this article, we highlight the historical foundation and newest advances in the field of endometrial cancer, focusing on:
- histologic classification
- etiologic heterogeneity and molecular biology
- genome-guided clinical trials involving targeted therapy, with the ultimate goal of achieving individualized cancer care.
Should we reclassify endometrial cancers to reflect molecular characteristics of tumors?
Brinton LA, Felix AS, McMeekin DS, et al. Etiologic heterogeneity in endometrial cancer: evidence from a Gynecologic Oncology Group trial. Gynecol Oncol. 2013;129(2):277–284.
Cancer Genome Atlas Research Network, et al. Integrated genomic characterization of endometrial carcinoma. Nature. 2013;497(7447):67–73.
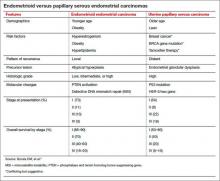
Uterine papillary serous carcinoma (UPSC) was first established as a distinct subtype of endometrial cancer in the early 1980s, when teams led by Lauchlan4 and Hendrickson5 described it as histologically similar to serous epithelial ovarian carcinoma. Shortly thereafter, Bokhman proposed two broad categories of endometrial carcinoma characterized by distinct microscopic appearance, epidemiology, and endocrine and metabolic functioning (TABLE, page 28).6
More recently, research has focused on expanding this histologic classification system to encompass molecular differences. Brinton and colleagues conducted a study within Gynecologic Oncology Group 210, investigating the etiologic heterogeneity of endometrial cancers by comparing risk factors for different histologies. They found that risk factors for aggressive endometrial cancers, including Grade 3 endometrioid and nonendometrioid tumors, appear to differ from those of lower-grade endometrioid carcinomas.
Details of the study by Brinton and colleagues
A total of 3,434 women were included, representing endometrioid (78%) and serous (9%) carcinomas. Grade 3 endometrioid tumors resembled Type II endometrial cancers more closely than did Grade 1–2 endometrioid tumors. Patients with Grade 3 endometrioid and Type II cancers were diagnosed at a significantly older age than patients with Grades 1–2 endometrioid cancers (eg, diagnosis of serous cancers: median age, 67.4 years; Grade 3 endometrioid cancers: median age, 61.9 years; Grade 1–2 endometrioid cancers: median age, 59.6 years). They also were more likely to be nonwhite than patients with Grades 1–2 endometrioid histology. Specifically, black patients were rarely diagnosed with Grades 1–2 endometrioid cancers (5% vs 9% for Grade 3 endometrioid cancers; 20% for serous cancers, 23% for carcinosarcomas, and 12% for clear-cell cancers).
After adjustments for age, enrollment year, and race, patients with Type II tumors (serous, carcinosarcomas, or clear-cell tumors) were much more likely to be multiparous or smokers or to have a history of breast cancer treated with tamoxifen, compared with women with Grade 1–2 endometrioid cancers. An adequately powered subanalysis of serous carcinomas and Grades 1–2 endometrioid cancers revealed that associations persisted between serous carcinomas and multiparity, body mass index, and a history of breast cancer treated with tamoxifen.
Related article: The future of the Pap test: Identifying endometrial and ovarian cancers (Janelle Yates, August 2013)
Overall, this study provides some of the strongest epidemiologic support we have that endometrial cancers are heterogeneous, with evidence to suggest that we might classify Grade 3 endometrioid carcinomas as Type II cancers. These findings paved the way for molecular profiling of endometrial cancers.
Details of the study by the Cancer Genome Atlas Research Network
The Cancer Genome Atlas Research Network recently published an integrated genomic, transcriptomic, and proteomic characterization of 373 endometrial carcinomas using array- and sequencing-based technologies. The goal was to provide better insight into disease biology and tumor classification to help guide clinical trials and drug development, with the ultimate goal of achieving personalized cancer care.
The group classified endometrial cancers into four new categories:
- polymerase (DNA-directed) epsilon catalytic subunit (POLE) ultramutated
- microsatellite instability (MSI) hypermutated
- somatic copy number alterations (SCNA) low
- SCNA high.
Most endometrioid tumors had few SCNA or P53 mutations but frequent mutations in PTEN, CTNNB1, PIK3CA, ARID1A, and KRAS genes. Novel mutations also were discovered in the SWI/SNF chromatin remodeling complex gene ARID5B. About 10% of endometrioid tumors had markedly increased transversion mutations and newly identified mutations in POLE, a gene involved in nuclear DNA replication and repair.
As expected, serous tumors had significantly worse progression-free survival (PFS) than endometrioid tumors (P = .003, log-rank). A subset (25%) of high-grade endometrioid tumors had SCNAs and mutation spectra similar to those of uterine serous carcinomas, suggesting that patients with such tumors might benefit from treatment options that parallel those for serous tumors.
Other overlapping treatment paradigms existed between different organ systems. For example, some molecular features were similar in uterine serous carcinomas, basal-like breast carcinomas, and high-grade serous ovarian carcinomas. All three carcinomas displayed a high frequency of P53 mutations, very low frequency of PTEN mutations, similar focal SCNA patterns, and minimal DNA methylation changes. However, investigators also found several mutations that are unique to uterine serous carcinomas (eg, PIK3CA, FBXW7, PPP2R1A, and ARID1A), providing potential opportunities for targeted pharmacotherapy.
What this evidence means for practice
These studies highlight the etiologic heterogeneity of endometrial cancer. The histologic groundwork laid by Bokhman was not only correct but provided a foundation for molecular characterization of endometrial cancers to drive translational science into targeted therapeutics.
The similar molecular phenotypes of high-grade endometrioid carcinomas and serous endometrial carcinomas strengthens existing evidence that chemotherapy may be preferable to adjuvant radiotherapy for patients with highly mutated endometrioid cancers (eg, SCNA). Current chemotherapy regimens for serous endometrial cancers remain appropriate, given the compelling similarities between these cancers and serous ovarian and basal-like breast cancers. However, the identification of unique molecular features not shared by breast or ovarian cancer may expand standard options to include more targeted therapy.
Overall, this type of research, which encompasses proper histologic classification refined by genomics, has the potential to achieve personalized cancer care.
How we are achieving individualized cancer care: 3 genome-guided clinical trials
Oza AM, Elit L, Tsao MS, et al. Phase II study of temsirolimus in women with recurrent or metastatic endometrial cancer: a trial of the NCIC Clinical Trials Group. J Clin Oncol. 2011;29(24):3278–3285.
Aghajanian C, Sill MW, Darcy KM, et al. Phase II trial of bevacizumab in recurrent or persistent endometrial cancer: a Gynecologic Oncology Group study. J Clin Oncol. 2011;29(16):2259–2265.
Alvarez EA, Brady WE, Walker JL, et al. Phase II trial of combination bevacizumab and temsirolimus in the treatment of recurrent or persistent endometrial carcinoma: a Gynecologic Oncology Group study. Gynecol Oncol. 2013;129(1):22–27.
Women with locally recurrent, advanced, or metastatic endometrial cancer have limited options for treatment. Hormonal therapies have modest effects at best, with a median survival rate of 7 to 12 months.7–9 To address this lack of options, researchers have begun to focus on targeted therapies directed at molecular pathways of cellular proliferation. These therapies include but are not limited to inhibitors of:
- mammalian target of rapamycin (mTOR)
- human epidermal growth factor receptor 2
- epidermal growth factor receptor
- vascular endothelial growth factor (VEGF).
Several studies have produced promising findings in recent years. They involve investigations of temsirolimus and bevacizumab as single agents in two independent clinical trials, and a study of the drugs in combination in women with recurrent or persistent endometrial carcinoma.
mTOR inhibitors elicited a greater response in chemotherapy-naïve women
Phosphatase and tensin homolog (PTEN) is a tumor-suppressor gene more commonly associated with endometrioid endometrial cancers (26%–80%) than with Type II cancers.10 Loss of PTEN expression leads to deregulated signaling of the phosphatidylinositol-3 kinase (PI3K)/serine-threonine kinase (Akt)/mTOR pathway. Disruption of this pathway is thought to provide cells with a selective survival advantage by enhancing angiogenesis, protein translation, and cell-cycle progression.10
Temsirolimus is an mTOR inhibitor recently explored by Oza and colleagues. They performed a multicenter, Phase II study involving 62 patients with recurrent and/or metastatic endometrial cancer as part of the National Cancer Institute of Canada (NCIC) Clinical Trials Group. Patients were divided into two groups on the basis of their treatment history:
- chemotherapy-naïve women, with no more than one prior hormonal treatment (n = 33)
- chemotherapy-treated women (n = 27).
Temsirolimus was given weekly in 4-week cycles at an intravenous (IV) dose of 25 mg over 30 minutes.
The drug elicited a response regardless of the histologic type of cancer. That response was more pronounced in chemotherapy-naïve women and not limited to patients with PTEN loss. In the chemotherapy-naïve group, four women (14%) had a partial response, 20 (69%) had stable disease, and five (18%) had progressive disease. Median PFS was 7.33 months (95% confidence interval [CI], 3.61–9.86), compared with 3.25 months (95% CI, 1.97–3.84) in chemotherapy-treated women. Among chemotherapy-treated women, one (4%) had a partial response and 12 (48%) had stable disease.
An angiogenesis inhibitor alone was well tolerated
VEGF is the principal growth factor responsible for angiogenesis, initiating the process of neovascularization. Bevacizumab is a humanized monoclonal antibody that binds to circulating VEGF-A, stimulating clinical effects in multiple tumor types, including persistent or recurrent ovarian and cervical cancers.
Aghajanian and colleagues conducted a Phase II trial of single-agent bevacizumab in women with recurrent or persistent endometrial cancer to assess the drug’s activity and tolerability. Eligible patients had histologic confirmation by central pathology review, recurrent or persistent disease after one or two cytotoxic regimens, and a Gynecologic Oncology Group performance score of 2 or lower. They received IV bevacizumab 15 mg/kg every 3 weeks until the disease progressed or toxicity became prohibitive. Fifty-two women participated.
Seven women (13.5%) experienced a clinical response (one complete response and six partial responses), and 21 (40%) had PFS of at least 6 months. Median PFS and overall survival were 4.2 and 10.5 months, respectively.
Combined with temsirolimus, bevacizumab increased overall survival
Alvarez and colleagues conducted a Phase II trial of combination bevacizumab and temsirolimus in women with recurrent or persistent endometrial carcinoma. Forty-nine patients participated.
These women had undergone earlier treatment with one (82%) or two (18%) chemotherapy regimens and radiation (41%). Median PFS and overall survival were 5.6 and 16.9 months, respectively. Toxicity was significant, with 38.8% of women withdrawn from the study due to toxicity.
What this evidence means for practice
Given the findings regarding temsirolimus and bevacizumab as single agents, their use in combination was expected to produce a robust effect. The 6-month PFS rate is similar for all three regimens, but for overall survival, combination therapy had a 6.4-month advantage over bevacizumab alone.
Given the significant toxicity associated with the combination of bevacizumab and temsirolimus, further study is needed to develop biomarkers to predict response and toxicity. Other areas meriting future research include optimal timing of angiogenesis and mTOR pathway inhibitors, different combinations of agents, and the identification, through genomic analysis, of patient populations most likely to benefit from these drugs with minimal toxicity.
The studies presented here show promise in the area of genomics and represent the beginning of our movement toward personalized cancer care.
We want to hear from you! Tell us what you think.
- Siegel R, Naishadham D, Jemal A. Cancer statistics, 2013. CA Cancer J Clin. 2013;63(1):11–30.
- Boruta DM 2nd, Gehrig PA, Fader AN, Olawaiye AB. Management of women with uterine papillary serous cancer: a Society of Gynecologic Oncology (SGO) review. Gynecol Oncol. 2009;115(1):142–153.
- Ueda SM, Kapp DS, Cheung MK, et al. Trends in demographic and clinical characteristics in women diagnosed with corpus cancer and their potential impact on the increasing number of deaths. Am J Obstet Gynecol. 2008;198(2):218.e1–e6.
- Lauchlan SC. Tubal (serous) carcinoma of the endometrium. Arch Pathol Lab Med. 1981;105(11):615–618.
- Hendrickson M, Ross J, Eifel P, Martinez A, Kempson R. Uterine papillary serous carcinoma: a highly malignant form of endometrial adenocarcinoma. Am J Surg Pathol. 1982;6(2):93–108.
- Bokhman JV. Two pathogenetic types of endometrial
carcinoma. Gynecol Oncol. 1983;15(1):10–17. - Bellone S, Shah HR, McKenney JK, Stone PJ, Santin AD. Recurrent endometrial carcinoma regression with the use of the aromatase inhibitor anastrozole. Am J Obstet Gynecol. 2008;199(3):e7–e10.
- Rose PG, Brunetto VL, VanLe L, Bell J, Walker JL, Lee
RB. A phase II trial of anastrozole in advanced recurrent
or persistent endometrial carcinoma: a Gynecologic Oncology Group study. Gynecol Oncol. 2000;78(2):212–216. - Thigpen T, Brady MF, Homesley HD, Soper JT, Bell J. Tamoxifen in the treatment of advanced or recurrent endometrial carcinoma: a Gynecologic Oncology Group study. J Clin Oncol. 2001;19(2):364–367.
- Kanamori Y, Kigawa J, Itamochi H, et al. Correlation between loss of PTEN expression and Akt phosphorylation in endometrial carcinoma. Clin Cancer Res. 2001; 7(4):892–895.
Endometrial cancer is the most common malignancy of the female reproductive tract in the United States, and its incidence continues to rise, with an estimated 49,560 new cases predicted for 2013.1 If we are to successfully traverse the pathway from molecular cell genetics to the development of targeted therapies and personalized cancer care, we need to meet a few benchmarks:
- We need to enhance our understanding of the molecular changes that lead to endometrial cancer. Of particular interest are nonendometrioid tumors. Increased mortality from endometrial cancer appears to be related to the growing number of uterine papillary serous carcinomas and clear-cell cancers. Although these cancers constitute less than 10% of all endometrial cancers, they account for a disproportionately high number of recurrences and cancer-related deaths.2,3 Do recent studies validate the original classification of endometrial cancers as Type I (endometrioid) or Type II (serous and clear cell), or is there more heterogeneity than was originally thought? How do recent studies affect treatment options?
- We need to establish a genomic characterization of endometrial cancer to supplement clinical research data. The identification of novel mutations specific to each histologic type has the potential to improve adjuvant therapy. How close are we to performing a comprehensive genomic analysis of endometrial cancer?
- We need to develop new adjuvant treatment options for recurrent and advanced disease. When clinical symptoms of endometrial cancer are overt, as they often are, early diagnosis is possible, with a 5-year survival rate of 80% to 90%. The prognosis declines dramatically in women with advanced-stage disease or high-risk histologies, with a 5-year survival rate of 57% and 19% for Stage III and Stage IV disease, respectively.1 Adjuvant treatment options are limited in the setting of recurrent or advanced disease. Do any biologic agents increase survival?
In this article, we highlight the historical foundation and newest advances in the field of endometrial cancer, focusing on:
- histologic classification
- etiologic heterogeneity and molecular biology
- genome-guided clinical trials involving targeted therapy, with the ultimate goal of achieving individualized cancer care.
Should we reclassify endometrial cancers to reflect molecular characteristics of tumors?
Brinton LA, Felix AS, McMeekin DS, et al. Etiologic heterogeneity in endometrial cancer: evidence from a Gynecologic Oncology Group trial. Gynecol Oncol. 2013;129(2):277–284.
Cancer Genome Atlas Research Network, et al. Integrated genomic characterization of endometrial carcinoma. Nature. 2013;497(7447):67–73.
Uterine papillary serous carcinoma (UPSC) was first established as a distinct subtype of endometrial cancer in the early 1980s, when teams led by Lauchlan4 and Hendrickson5 described it as histologically similar to serous epithelial ovarian carcinoma. Shortly thereafter, Bokhman proposed two broad categories of endometrial carcinoma characterized by distinct microscopic appearance, epidemiology, and endocrine and metabolic functioning (TABLE, page 28).6
More recently, research has focused on expanding this histologic classification system to encompass molecular differences. Brinton and colleagues conducted a study within Gynecologic Oncology Group 210, investigating the etiologic heterogeneity of endometrial cancers by comparing risk factors for different histologies. They found that risk factors for aggressive endometrial cancers, including Grade 3 endometrioid and nonendometrioid tumors, appear to differ from those of lower-grade endometrioid carcinomas.
Details of the study by Brinton and colleagues
A total of 3,434 women were included, representing endometrioid (78%) and serous (9%) carcinomas. Grade 3 endometrioid tumors resembled Type II endometrial cancers more closely than did Grade 1–2 endometrioid tumors. Patients with Grade 3 endometrioid and Type II cancers were diagnosed at a significantly older age than patients with Grades 1–2 endometrioid cancers (eg, diagnosis of serous cancers: median age, 67.4 years; Grade 3 endometrioid cancers: median age, 61.9 years; Grade 1–2 endometrioid cancers: median age, 59.6 years). They also were more likely to be nonwhite than patients with Grades 1–2 endometrioid histology. Specifically, black patients were rarely diagnosed with Grades 1–2 endometrioid cancers (5% vs 9% for Grade 3 endometrioid cancers; 20% for serous cancers, 23% for carcinosarcomas, and 12% for clear-cell cancers).
After adjustments for age, enrollment year, and race, patients with Type II tumors (serous, carcinosarcomas, or clear-cell tumors) were much more likely to be multiparous or smokers or to have a history of breast cancer treated with tamoxifen, compared with women with Grade 1–2 endometrioid cancers. An adequately powered subanalysis of serous carcinomas and Grades 1–2 endometrioid cancers revealed that associations persisted between serous carcinomas and multiparity, body mass index, and a history of breast cancer treated with tamoxifen.
Related article: The future of the Pap test: Identifying endometrial and ovarian cancers (Janelle Yates, August 2013)
Overall, this study provides some of the strongest epidemiologic support we have that endometrial cancers are heterogeneous, with evidence to suggest that we might classify Grade 3 endometrioid carcinomas as Type II cancers. These findings paved the way for molecular profiling of endometrial cancers.
Details of the study by the Cancer Genome Atlas Research Network
The Cancer Genome Atlas Research Network recently published an integrated genomic, transcriptomic, and proteomic characterization of 373 endometrial carcinomas using array- and sequencing-based technologies. The goal was to provide better insight into disease biology and tumor classification to help guide clinical trials and drug development, with the ultimate goal of achieving personalized cancer care.
The group classified endometrial cancers into four new categories:
- polymerase (DNA-directed) epsilon catalytic subunit (POLE) ultramutated
- microsatellite instability (MSI) hypermutated
- somatic copy number alterations (SCNA) low
- SCNA high.
Most endometrioid tumors had few SCNA or P53 mutations but frequent mutations in PTEN, CTNNB1, PIK3CA, ARID1A, and KRAS genes. Novel mutations also were discovered in the SWI/SNF chromatin remodeling complex gene ARID5B. About 10% of endometrioid tumors had markedly increased transversion mutations and newly identified mutations in POLE, a gene involved in nuclear DNA replication and repair.
As expected, serous tumors had significantly worse progression-free survival (PFS) than endometrioid tumors (P = .003, log-rank). A subset (25%) of high-grade endometrioid tumors had SCNAs and mutation spectra similar to those of uterine serous carcinomas, suggesting that patients with such tumors might benefit from treatment options that parallel those for serous tumors.
Other overlapping treatment paradigms existed between different organ systems. For example, some molecular features were similar in uterine serous carcinomas, basal-like breast carcinomas, and high-grade serous ovarian carcinomas. All three carcinomas displayed a high frequency of P53 mutations, very low frequency of PTEN mutations, similar focal SCNA patterns, and minimal DNA methylation changes. However, investigators also found several mutations that are unique to uterine serous carcinomas (eg, PIK3CA, FBXW7, PPP2R1A, and ARID1A), providing potential opportunities for targeted pharmacotherapy.
What this evidence means for practice
These studies highlight the etiologic heterogeneity of endometrial cancer. The histologic groundwork laid by Bokhman was not only correct but provided a foundation for molecular characterization of endometrial cancers to drive translational science into targeted therapeutics.
The similar molecular phenotypes of high-grade endometrioid carcinomas and serous endometrial carcinomas strengthens existing evidence that chemotherapy may be preferable to adjuvant radiotherapy for patients with highly mutated endometrioid cancers (eg, SCNA). Current chemotherapy regimens for serous endometrial cancers remain appropriate, given the compelling similarities between these cancers and serous ovarian and basal-like breast cancers. However, the identification of unique molecular features not shared by breast or ovarian cancer may expand standard options to include more targeted therapy.
Overall, this type of research, which encompasses proper histologic classification refined by genomics, has the potential to achieve personalized cancer care.
How we are achieving individualized cancer care: 3 genome-guided clinical trials
Oza AM, Elit L, Tsao MS, et al. Phase II study of temsirolimus in women with recurrent or metastatic endometrial cancer: a trial of the NCIC Clinical Trials Group. J Clin Oncol. 2011;29(24):3278–3285.
Aghajanian C, Sill MW, Darcy KM, et al. Phase II trial of bevacizumab in recurrent or persistent endometrial cancer: a Gynecologic Oncology Group study. J Clin Oncol. 2011;29(16):2259–2265.
Alvarez EA, Brady WE, Walker JL, et al. Phase II trial of combination bevacizumab and temsirolimus in the treatment of recurrent or persistent endometrial carcinoma: a Gynecologic Oncology Group study. Gynecol Oncol. 2013;129(1):22–27.
Women with locally recurrent, advanced, or metastatic endometrial cancer have limited options for treatment. Hormonal therapies have modest effects at best, with a median survival rate of 7 to 12 months.7–9 To address this lack of options, researchers have begun to focus on targeted therapies directed at molecular pathways of cellular proliferation. These therapies include but are not limited to inhibitors of:
- mammalian target of rapamycin (mTOR)
- human epidermal growth factor receptor 2
- epidermal growth factor receptor
- vascular endothelial growth factor (VEGF).
Several studies have produced promising findings in recent years. They involve investigations of temsirolimus and bevacizumab as single agents in two independent clinical trials, and a study of the drugs in combination in women with recurrent or persistent endometrial carcinoma.
mTOR inhibitors elicited a greater response in chemotherapy-naïve women
Phosphatase and tensin homolog (PTEN) is a tumor-suppressor gene more commonly associated with endometrioid endometrial cancers (26%–80%) than with Type II cancers.10 Loss of PTEN expression leads to deregulated signaling of the phosphatidylinositol-3 kinase (PI3K)/serine-threonine kinase (Akt)/mTOR pathway. Disruption of this pathway is thought to provide cells with a selective survival advantage by enhancing angiogenesis, protein translation, and cell-cycle progression.10
Temsirolimus is an mTOR inhibitor recently explored by Oza and colleagues. They performed a multicenter, Phase II study involving 62 patients with recurrent and/or metastatic endometrial cancer as part of the National Cancer Institute of Canada (NCIC) Clinical Trials Group. Patients were divided into two groups on the basis of their treatment history:
- chemotherapy-naïve women, with no more than one prior hormonal treatment (n = 33)
- chemotherapy-treated women (n = 27).
Temsirolimus was given weekly in 4-week cycles at an intravenous (IV) dose of 25 mg over 30 minutes.
The drug elicited a response regardless of the histologic type of cancer. That response was more pronounced in chemotherapy-naïve women and not limited to patients with PTEN loss. In the chemotherapy-naïve group, four women (14%) had a partial response, 20 (69%) had stable disease, and five (18%) had progressive disease. Median PFS was 7.33 months (95% confidence interval [CI], 3.61–9.86), compared with 3.25 months (95% CI, 1.97–3.84) in chemotherapy-treated women. Among chemotherapy-treated women, one (4%) had a partial response and 12 (48%) had stable disease.
An angiogenesis inhibitor alone was well tolerated
VEGF is the principal growth factor responsible for angiogenesis, initiating the process of neovascularization. Bevacizumab is a humanized monoclonal antibody that binds to circulating VEGF-A, stimulating clinical effects in multiple tumor types, including persistent or recurrent ovarian and cervical cancers.
Aghajanian and colleagues conducted a Phase II trial of single-agent bevacizumab in women with recurrent or persistent endometrial cancer to assess the drug’s activity and tolerability. Eligible patients had histologic confirmation by central pathology review, recurrent or persistent disease after one or two cytotoxic regimens, and a Gynecologic Oncology Group performance score of 2 or lower. They received IV bevacizumab 15 mg/kg every 3 weeks until the disease progressed or toxicity became prohibitive. Fifty-two women participated.
Seven women (13.5%) experienced a clinical response (one complete response and six partial responses), and 21 (40%) had PFS of at least 6 months. Median PFS and overall survival were 4.2 and 10.5 months, respectively.
Combined with temsirolimus, bevacizumab increased overall survival
Alvarez and colleagues conducted a Phase II trial of combination bevacizumab and temsirolimus in women with recurrent or persistent endometrial carcinoma. Forty-nine patients participated.
These women had undergone earlier treatment with one (82%) or two (18%) chemotherapy regimens and radiation (41%). Median PFS and overall survival were 5.6 and 16.9 months, respectively. Toxicity was significant, with 38.8% of women withdrawn from the study due to toxicity.
What this evidence means for practice
Given the findings regarding temsirolimus and bevacizumab as single agents, their use in combination was expected to produce a robust effect. The 6-month PFS rate is similar for all three regimens, but for overall survival, combination therapy had a 6.4-month advantage over bevacizumab alone.
Given the significant toxicity associated with the combination of bevacizumab and temsirolimus, further study is needed to develop biomarkers to predict response and toxicity. Other areas meriting future research include optimal timing of angiogenesis and mTOR pathway inhibitors, different combinations of agents, and the identification, through genomic analysis, of patient populations most likely to benefit from these drugs with minimal toxicity.
The studies presented here show promise in the area of genomics and represent the beginning of our movement toward personalized cancer care.
We want to hear from you! Tell us what you think.
Endometrial cancer is the most common malignancy of the female reproductive tract in the United States, and its incidence continues to rise, with an estimated 49,560 new cases predicted for 2013.1 If we are to successfully traverse the pathway from molecular cell genetics to the development of targeted therapies and personalized cancer care, we need to meet a few benchmarks:
- We need to enhance our understanding of the molecular changes that lead to endometrial cancer. Of particular interest are nonendometrioid tumors. Increased mortality from endometrial cancer appears to be related to the growing number of uterine papillary serous carcinomas and clear-cell cancers. Although these cancers constitute less than 10% of all endometrial cancers, they account for a disproportionately high number of recurrences and cancer-related deaths.2,3 Do recent studies validate the original classification of endometrial cancers as Type I (endometrioid) or Type II (serous and clear cell), or is there more heterogeneity than was originally thought? How do recent studies affect treatment options?
- We need to establish a genomic characterization of endometrial cancer to supplement clinical research data. The identification of novel mutations specific to each histologic type has the potential to improve adjuvant therapy. How close are we to performing a comprehensive genomic analysis of endometrial cancer?
- We need to develop new adjuvant treatment options for recurrent and advanced disease. When clinical symptoms of endometrial cancer are overt, as they often are, early diagnosis is possible, with a 5-year survival rate of 80% to 90%. The prognosis declines dramatically in women with advanced-stage disease or high-risk histologies, with a 5-year survival rate of 57% and 19% for Stage III and Stage IV disease, respectively.1 Adjuvant treatment options are limited in the setting of recurrent or advanced disease. Do any biologic agents increase survival?
In this article, we highlight the historical foundation and newest advances in the field of endometrial cancer, focusing on:
- histologic classification
- etiologic heterogeneity and molecular biology
- genome-guided clinical trials involving targeted therapy, with the ultimate goal of achieving individualized cancer care.
Should we reclassify endometrial cancers to reflect molecular characteristics of tumors?
Brinton LA, Felix AS, McMeekin DS, et al. Etiologic heterogeneity in endometrial cancer: evidence from a Gynecologic Oncology Group trial. Gynecol Oncol. 2013;129(2):277–284.
Cancer Genome Atlas Research Network, et al. Integrated genomic characterization of endometrial carcinoma. Nature. 2013;497(7447):67–73.
Uterine papillary serous carcinoma (UPSC) was first established as a distinct subtype of endometrial cancer in the early 1980s, when teams led by Lauchlan4 and Hendrickson5 described it as histologically similar to serous epithelial ovarian carcinoma. Shortly thereafter, Bokhman proposed two broad categories of endometrial carcinoma characterized by distinct microscopic appearance, epidemiology, and endocrine and metabolic functioning (TABLE, page 28).6
More recently, research has focused on expanding this histologic classification system to encompass molecular differences. Brinton and colleagues conducted a study within Gynecologic Oncology Group 210, investigating the etiologic heterogeneity of endometrial cancers by comparing risk factors for different histologies. They found that risk factors for aggressive endometrial cancers, including Grade 3 endometrioid and nonendometrioid tumors, appear to differ from those of lower-grade endometrioid carcinomas.
Details of the study by Brinton and colleagues
A total of 3,434 women were included, representing endometrioid (78%) and serous (9%) carcinomas. Grade 3 endometrioid tumors resembled Type II endometrial cancers more closely than did Grade 1–2 endometrioid tumors. Patients with Grade 3 endometrioid and Type II cancers were diagnosed at a significantly older age than patients with Grades 1–2 endometrioid cancers (eg, diagnosis of serous cancers: median age, 67.4 years; Grade 3 endometrioid cancers: median age, 61.9 years; Grade 1–2 endometrioid cancers: median age, 59.6 years). They also were more likely to be nonwhite than patients with Grades 1–2 endometrioid histology. Specifically, black patients were rarely diagnosed with Grades 1–2 endometrioid cancers (5% vs 9% for Grade 3 endometrioid cancers; 20% for serous cancers, 23% for carcinosarcomas, and 12% for clear-cell cancers).
After adjustments for age, enrollment year, and race, patients with Type II tumors (serous, carcinosarcomas, or clear-cell tumors) were much more likely to be multiparous or smokers or to have a history of breast cancer treated with tamoxifen, compared with women with Grade 1–2 endometrioid cancers. An adequately powered subanalysis of serous carcinomas and Grades 1–2 endometrioid cancers revealed that associations persisted between serous carcinomas and multiparity, body mass index, and a history of breast cancer treated with tamoxifen.
Related article: The future of the Pap test: Identifying endometrial and ovarian cancers (Janelle Yates, August 2013)
Overall, this study provides some of the strongest epidemiologic support we have that endometrial cancers are heterogeneous, with evidence to suggest that we might classify Grade 3 endometrioid carcinomas as Type II cancers. These findings paved the way for molecular profiling of endometrial cancers.
Details of the study by the Cancer Genome Atlas Research Network
The Cancer Genome Atlas Research Network recently published an integrated genomic, transcriptomic, and proteomic characterization of 373 endometrial carcinomas using array- and sequencing-based technologies. The goal was to provide better insight into disease biology and tumor classification to help guide clinical trials and drug development, with the ultimate goal of achieving personalized cancer care.
The group classified endometrial cancers into four new categories:
- polymerase (DNA-directed) epsilon catalytic subunit (POLE) ultramutated
- microsatellite instability (MSI) hypermutated
- somatic copy number alterations (SCNA) low
- SCNA high.
Most endometrioid tumors had few SCNA or P53 mutations but frequent mutations in PTEN, CTNNB1, PIK3CA, ARID1A, and KRAS genes. Novel mutations also were discovered in the SWI/SNF chromatin remodeling complex gene ARID5B. About 10% of endometrioid tumors had markedly increased transversion mutations and newly identified mutations in POLE, a gene involved in nuclear DNA replication and repair.
As expected, serous tumors had significantly worse progression-free survival (PFS) than endometrioid tumors (P = .003, log-rank). A subset (25%) of high-grade endometrioid tumors had SCNAs and mutation spectra similar to those of uterine serous carcinomas, suggesting that patients with such tumors might benefit from treatment options that parallel those for serous tumors.
Other overlapping treatment paradigms existed between different organ systems. For example, some molecular features were similar in uterine serous carcinomas, basal-like breast carcinomas, and high-grade serous ovarian carcinomas. All three carcinomas displayed a high frequency of P53 mutations, very low frequency of PTEN mutations, similar focal SCNA patterns, and minimal DNA methylation changes. However, investigators also found several mutations that are unique to uterine serous carcinomas (eg, PIK3CA, FBXW7, PPP2R1A, and ARID1A), providing potential opportunities for targeted pharmacotherapy.
What this evidence means for practice
These studies highlight the etiologic heterogeneity of endometrial cancer. The histologic groundwork laid by Bokhman was not only correct but provided a foundation for molecular characterization of endometrial cancers to drive translational science into targeted therapeutics.
The similar molecular phenotypes of high-grade endometrioid carcinomas and serous endometrial carcinomas strengthens existing evidence that chemotherapy may be preferable to adjuvant radiotherapy for patients with highly mutated endometrioid cancers (eg, SCNA). Current chemotherapy regimens for serous endometrial cancers remain appropriate, given the compelling similarities between these cancers and serous ovarian and basal-like breast cancers. However, the identification of unique molecular features not shared by breast or ovarian cancer may expand standard options to include more targeted therapy.
Overall, this type of research, which encompasses proper histologic classification refined by genomics, has the potential to achieve personalized cancer care.
How we are achieving individualized cancer care: 3 genome-guided clinical trials
Oza AM, Elit L, Tsao MS, et al. Phase II study of temsirolimus in women with recurrent or metastatic endometrial cancer: a trial of the NCIC Clinical Trials Group. J Clin Oncol. 2011;29(24):3278–3285.
Aghajanian C, Sill MW, Darcy KM, et al. Phase II trial of bevacizumab in recurrent or persistent endometrial cancer: a Gynecologic Oncology Group study. J Clin Oncol. 2011;29(16):2259–2265.
Alvarez EA, Brady WE, Walker JL, et al. Phase II trial of combination bevacizumab and temsirolimus in the treatment of recurrent or persistent endometrial carcinoma: a Gynecologic Oncology Group study. Gynecol Oncol. 2013;129(1):22–27.
Women with locally recurrent, advanced, or metastatic endometrial cancer have limited options for treatment. Hormonal therapies have modest effects at best, with a median survival rate of 7 to 12 months.7–9 To address this lack of options, researchers have begun to focus on targeted therapies directed at molecular pathways of cellular proliferation. These therapies include but are not limited to inhibitors of:
- mammalian target of rapamycin (mTOR)
- human epidermal growth factor receptor 2
- epidermal growth factor receptor
- vascular endothelial growth factor (VEGF).
Several studies have produced promising findings in recent years. They involve investigations of temsirolimus and bevacizumab as single agents in two independent clinical trials, and a study of the drugs in combination in women with recurrent or persistent endometrial carcinoma.
mTOR inhibitors elicited a greater response in chemotherapy-naïve women
Phosphatase and tensin homolog (PTEN) is a tumor-suppressor gene more commonly associated with endometrioid endometrial cancers (26%–80%) than with Type II cancers.10 Loss of PTEN expression leads to deregulated signaling of the phosphatidylinositol-3 kinase (PI3K)/serine-threonine kinase (Akt)/mTOR pathway. Disruption of this pathway is thought to provide cells with a selective survival advantage by enhancing angiogenesis, protein translation, and cell-cycle progression.10
Temsirolimus is an mTOR inhibitor recently explored by Oza and colleagues. They performed a multicenter, Phase II study involving 62 patients with recurrent and/or metastatic endometrial cancer as part of the National Cancer Institute of Canada (NCIC) Clinical Trials Group. Patients were divided into two groups on the basis of their treatment history:
- chemotherapy-naïve women, with no more than one prior hormonal treatment (n = 33)
- chemotherapy-treated women (n = 27).
Temsirolimus was given weekly in 4-week cycles at an intravenous (IV) dose of 25 mg over 30 minutes.
The drug elicited a response regardless of the histologic type of cancer. That response was more pronounced in chemotherapy-naïve women and not limited to patients with PTEN loss. In the chemotherapy-naïve group, four women (14%) had a partial response, 20 (69%) had stable disease, and five (18%) had progressive disease. Median PFS was 7.33 months (95% confidence interval [CI], 3.61–9.86), compared with 3.25 months (95% CI, 1.97–3.84) in chemotherapy-treated women. Among chemotherapy-treated women, one (4%) had a partial response and 12 (48%) had stable disease.
An angiogenesis inhibitor alone was well tolerated
VEGF is the principal growth factor responsible for angiogenesis, initiating the process of neovascularization. Bevacizumab is a humanized monoclonal antibody that binds to circulating VEGF-A, stimulating clinical effects in multiple tumor types, including persistent or recurrent ovarian and cervical cancers.
Aghajanian and colleagues conducted a Phase II trial of single-agent bevacizumab in women with recurrent or persistent endometrial cancer to assess the drug’s activity and tolerability. Eligible patients had histologic confirmation by central pathology review, recurrent or persistent disease after one or two cytotoxic regimens, and a Gynecologic Oncology Group performance score of 2 or lower. They received IV bevacizumab 15 mg/kg every 3 weeks until the disease progressed or toxicity became prohibitive. Fifty-two women participated.
Seven women (13.5%) experienced a clinical response (one complete response and six partial responses), and 21 (40%) had PFS of at least 6 months. Median PFS and overall survival were 4.2 and 10.5 months, respectively.
Combined with temsirolimus, bevacizumab increased overall survival
Alvarez and colleagues conducted a Phase II trial of combination bevacizumab and temsirolimus in women with recurrent or persistent endometrial carcinoma. Forty-nine patients participated.
These women had undergone earlier treatment with one (82%) or two (18%) chemotherapy regimens and radiation (41%). Median PFS and overall survival were 5.6 and 16.9 months, respectively. Toxicity was significant, with 38.8% of women withdrawn from the study due to toxicity.
What this evidence means for practice
Given the findings regarding temsirolimus and bevacizumab as single agents, their use in combination was expected to produce a robust effect. The 6-month PFS rate is similar for all three regimens, but for overall survival, combination therapy had a 6.4-month advantage over bevacizumab alone.
Given the significant toxicity associated with the combination of bevacizumab and temsirolimus, further study is needed to develop biomarkers to predict response and toxicity. Other areas meriting future research include optimal timing of angiogenesis and mTOR pathway inhibitors, different combinations of agents, and the identification, through genomic analysis, of patient populations most likely to benefit from these drugs with minimal toxicity.
The studies presented here show promise in the area of genomics and represent the beginning of our movement toward personalized cancer care.
We want to hear from you! Tell us what you think.
- Siegel R, Naishadham D, Jemal A. Cancer statistics, 2013. CA Cancer J Clin. 2013;63(1):11–30.
- Boruta DM 2nd, Gehrig PA, Fader AN, Olawaiye AB. Management of women with uterine papillary serous cancer: a Society of Gynecologic Oncology (SGO) review. Gynecol Oncol. 2009;115(1):142–153.
- Ueda SM, Kapp DS, Cheung MK, et al. Trends in demographic and clinical characteristics in women diagnosed with corpus cancer and their potential impact on the increasing number of deaths. Am J Obstet Gynecol. 2008;198(2):218.e1–e6.
- Lauchlan SC. Tubal (serous) carcinoma of the endometrium. Arch Pathol Lab Med. 1981;105(11):615–618.
- Hendrickson M, Ross J, Eifel P, Martinez A, Kempson R. Uterine papillary serous carcinoma: a highly malignant form of endometrial adenocarcinoma. Am J Surg Pathol. 1982;6(2):93–108.
- Bokhman JV. Two pathogenetic types of endometrial
carcinoma. Gynecol Oncol. 1983;15(1):10–17. - Bellone S, Shah HR, McKenney JK, Stone PJ, Santin AD. Recurrent endometrial carcinoma regression with the use of the aromatase inhibitor anastrozole. Am J Obstet Gynecol. 2008;199(3):e7–e10.
- Rose PG, Brunetto VL, VanLe L, Bell J, Walker JL, Lee
RB. A phase II trial of anastrozole in advanced recurrent
or persistent endometrial carcinoma: a Gynecologic Oncology Group study. Gynecol Oncol. 2000;78(2):212–216. - Thigpen T, Brady MF, Homesley HD, Soper JT, Bell J. Tamoxifen in the treatment of advanced or recurrent endometrial carcinoma: a Gynecologic Oncology Group study. J Clin Oncol. 2001;19(2):364–367.
- Kanamori Y, Kigawa J, Itamochi H, et al. Correlation between loss of PTEN expression and Akt phosphorylation in endometrial carcinoma. Clin Cancer Res. 2001; 7(4):892–895.
- Siegel R, Naishadham D, Jemal A. Cancer statistics, 2013. CA Cancer J Clin. 2013;63(1):11–30.
- Boruta DM 2nd, Gehrig PA, Fader AN, Olawaiye AB. Management of women with uterine papillary serous cancer: a Society of Gynecologic Oncology (SGO) review. Gynecol Oncol. 2009;115(1):142–153.
- Ueda SM, Kapp DS, Cheung MK, et al. Trends in demographic and clinical characteristics in women diagnosed with corpus cancer and their potential impact on the increasing number of deaths. Am J Obstet Gynecol. 2008;198(2):218.e1–e6.
- Lauchlan SC. Tubal (serous) carcinoma of the endometrium. Arch Pathol Lab Med. 1981;105(11):615–618.
- Hendrickson M, Ross J, Eifel P, Martinez A, Kempson R. Uterine papillary serous carcinoma: a highly malignant form of endometrial adenocarcinoma. Am J Surg Pathol. 1982;6(2):93–108.
- Bokhman JV. Two pathogenetic types of endometrial
carcinoma. Gynecol Oncol. 1983;15(1):10–17. - Bellone S, Shah HR, McKenney JK, Stone PJ, Santin AD. Recurrent endometrial carcinoma regression with the use of the aromatase inhibitor anastrozole. Am J Obstet Gynecol. 2008;199(3):e7–e10.
- Rose PG, Brunetto VL, VanLe L, Bell J, Walker JL, Lee
RB. A phase II trial of anastrozole in advanced recurrent
or persistent endometrial carcinoma: a Gynecologic Oncology Group study. Gynecol Oncol. 2000;78(2):212–216. - Thigpen T, Brady MF, Homesley HD, Soper JT, Bell J. Tamoxifen in the treatment of advanced or recurrent endometrial carcinoma: a Gynecologic Oncology Group study. J Clin Oncol. 2001;19(2):364–367.
- Kanamori Y, Kigawa J, Itamochi H, et al. Correlation between loss of PTEN expression and Akt phosphorylation in endometrial carcinoma. Clin Cancer Res. 2001; 7(4):892–895.