User login
CASE Young woman with discharge from one nipple
A 26-year-old African American woman presents with a 10-month history of left nipple discharge. The patient describes the discharge as spontaneous, colored dark brown to yellow, and occurring from a single opening in the nipple. The discharge is associated with left breast pain and fullness, without a palpable lump. The patient has no family or personal history of breast cancer.
Nipple discharge is the third most common breast-related symptom (after palpable masses and breast pain), with an estimated prevalence of 5% to 8% among premenopausal women.1 While most causes of nipple discharge reflect benign issues, approximately 5% to 12% of breast cancers have nipple discharge as the only symptom.2 Not surprisingly, nipple discharge creates anxiety for both patients and clinicians.
In this article, we—a breast imaging radiologist, gynecologist, and breast surgeon—outline key steps for evaluating and managing patients with nipple discharge.
Two types of nipple discharge
Nipple discharge can be characterized as physiologic or pathologic. The distinction is based on the patient’s history in conjunction with the clinical breast exam.
Physiologic nipple discharge often is bilateral, nonspontaneous, and white, yellow, green, or brown (TABLE).3 It often is due to nipple stimulation, and the patient can elicit discharge by manually manipulating the breast. Usually, multiple ducts are involved. Galactorrhea refers specifically to milky discharge and occurs most commonly during pregnancy or lactation.2 Galactorrhea that is not associated with pregnancy or lactation often is related to elevated prolactin or thyroid-stimulating hormone levels or to medications. One study reported that no cancers were found when discharge was nonspontaneous and colored or milky.4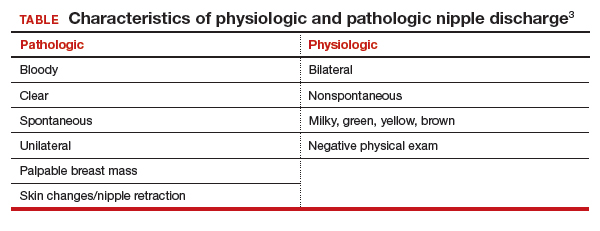
Pathologic nipple discharge is defined as a spontaneous, bloody, clear, or single-duct discharge. A palpable mass in the same breast automatically increases the suspicion of the discharge, regardless of its color or spontaneity.2 The most common cause of pathologic nipple discharge is an intraductal papilloma, a benign epithelial tumor, which accounts for approximately 57% of cases.5
Although the risk of malignancy is low for all patients with nipple discharge, increasing age is associated with increased risk of breast cancer. One study demonstrated that among women aged 40 to 60 years presenting with nipple discharge, the prevalence of invasive cancer is 10%, and the percentage jumps to 32% among women older than 60.6
Breast exam. For any patient with nonlactational nipple discharge, we recommend a thorough breast examination. Deep palpation of all quadrants of the symptomatic breast, especially near the nipple areolar complex, should elicit nipple discharge without any direct squeezing of the nipple. If the patient’s history and physical exam are consistent with physiologic discharge, no further workup is needed. Reassure the patient and recommend appropriate breast cancer screening. Encourage the patient to decrease stimulation or manual manipulation of the nipples if the discharge bothers her.
Continue to: CASE Continued: Workup...
CASE Continued: Workup
On physical exam, the patient’s breasts are noted to be cup size DDD and asymmetric, with the left breast larger than the right; there is no contour deformity. There is no skin or nipple retraction, skin rash, swelling, or nipple changes bilaterally. No dominant masses are appreciated bilaterally. Manual compression elicits no nipple discharge.
Although the discharge is nonbloody, its spontaneity, unilaterality, and single-duct/orifice origin suggest a pathologic cause. The patient is referred for breast imaging.
Imaging workup for pathologic discharge
The American College of Radiology (ACR) Appropriateness Criteria is a useful tool that provides an evidence-based, easy-to-use algorithm for breast imaging in the patient with pathologic nipple discharge (FIGURE 1).6 The algorithm is categorized by patient age, with diagnostic mammography recommended for women aged 30 and older.6 Diagnostic mammography is recommended if the patient has not had a mammogram study in the last 6 months.6 For patients with no prior mammograms, we recommend bilateral diagnostic mammography to compare symmetry of the breasts.
Currently, no studies show that digital breast tomosynthesis (3-D mammography) has a benefit compared with standard 2-D mammography in women with pathologic nipple discharge.6 Given the increased sensitivity of digital breast tomosynthesis for cancer detection, however, in our practice it is standard to use tomosynthesis in the diagnostic evaluation of most patients.
Mammography
On mammography, ductal carcinoma in situ (DCIS) usually presents as calcifications. Both the morphology and distribution of calcifications are used to characterize them as suspicious or, typically, benign. DCIS usually presents as fine pleomorphic or fine linear branching calcifications in a segmental or linear distribution. In patients with pathologic nipple discharge and no other symptoms, the radiologist must closely examine the retroareolar region of the breast to assess for faint calcifications. Magnification views also can be performed to better characterize calcifications.
The sensitivity of mammography for nipple discharge varies in the literature, ranging from approximately 15% to 68%, with a specificity range of 38% to 98%.6 This results in a relatively low positive predictive value but a high negative predictive value of 90%.7 Mammographic sensitivity largely is limited by increased breast density. As more data emerge on the utility of digital breast tomosynthesis in dense breasts, mammographic sensitivity for nipple discharge will likely increase.
Ultrasonography
As an adjunct to mammography, the ACR Appropriateness Criteria recommends targeted (or “limited”) ultrasonography of the retroareolar region of the affected breast for patients aged 30 and older. Ultrasonography is useful to assess for intraductal masses and architectural distortion, and it has higher sensitivity but lower specificity than mammography. The sensitivity of ultrasonography for detecting breast cancer in patients presenting with nipple discharge is reported to be 56% to 80%.6 Ultrasonography can identify lesions not visible mammographically in 63% to 69% of cases.8 Although DCIS usually presents as calcifications, it also can present as an intraductal mass on ultrasonography.
The ACR recommends targeted ultrasonography for patients with nipple discharge and a negative mammogram, or to evaluate a suspicious mammographic abnormality such as architectural distortion, focal asymmetry, or a mass.6 For patient comfort, ultrasonography is the preferred modality for image-guided biopsy.
For women younger than 30 years, targeted ultrasonography is the initial imaging study of choice, according to the ACR criteria.6 Women younger than 30 years with pathologic nipple discharge have a very low risk of breast cancer and tend to have higher breast density, making mammography less useful. Although the radiation dose from mammography is negligible given technological improvements and dose-reduction techniques, ultrasonography remains the preferred initial imaging modality in young women, not only for nipple discharge but also for palpable lumps and focal breast pain.
Mammography is used as an adjunct to ultrasonography in women younger than 30 years when a suspicious abnormality is detected on ultrasonography, such as an intraductal mass or architectural distortion. In these cases, mammography can be used to assess for extent of disease or to visualize suspicious calcifications not well seen on ultrasonography.
For practical purposes regarding which imaging study to order for a patient, it is most efficient to order both a diagnostic mammogram (with tomosynthesis, if possible) and a targeted ultrasound scan of the affected breast. Even if both orders are not needed, having them available increases efficiency for both the radiologist and the ordering physician.
Continue to: CASE Continued: Imaging findings...
CASE Continued: Imaging findings
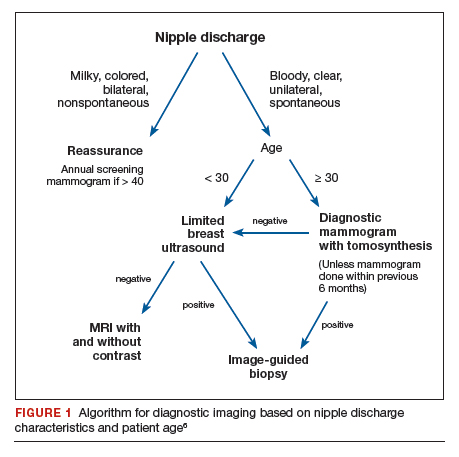
Given her age, the patient initially undergoes targeted ultrasonography. The grayscale image (FIGURE 2) demonstrates multiple mildly dilated ducts (white arrows) with surrounding hyperechogenicity of the fat (red arrows), indicating soft tissue edema. No intraductal mass is imaged. Given that the ultrasonography findings are not completely negative and are equivocal for malignancy, bilateral diagnostic mammography (FIGURE 3, left breast only) is performed. Standard full-field craniocaudal (FIGURE 3A) and mediolateral oblique (FIGURE 3B) mammographic views demonstrate a heterogeneously dense breast with a few calcifications in the retroareolar left breast (red ovals). No associated mass or architectural distortions are noted. The mammographic and sonographic findings do not reveal a definitive biopsy target.

Ductography
When a suspicious abnormality is visualized on either mammography or ultrasonography, the standard of care is to perform an image-guided biopsy of the abnormality. When the standard workup is negative or equivocal, the standard of care historically was to perform ductography.
Ductography is an invasive procedure that involves cannulating the suspicious duct with a small catheter and injecting radiopaque dye into the duct under mammographic guidance. While the sensitivity of ductography is higher than that of both mammography and ultrasonography, its specificity is lower than that of either modality.
Most cases of pathologic discharge are spontaneous and are not reproducible on the day of the procedure. If the procedural radiologist cannot visualize the duct that is producing the discharge, the procedure cannot be performed. Although most patients tolerate the procedure well, ductography produces patient discomfort from cannulation of the duct and injection of contrast.
Magnetic resonance imaging
Dynamic contrast-enhanced magnetic resonance imaging (MRI) is the most sensitive imaging study for evaluating pathologic nipple discharge, and it has largely replaced ductography as an adjunct to mammography and ultrasonography. MRI’s sensitivity for detecting breast cancer ranges from 93% to 100%.6 In addition, MRI allows visualization of the entire breast and areas peripheral to the field of view of a standard ductogram or ultrasound scan.9
Clinicians commonly ask, “Why not skip the mammogram and ultrasound scan and go straight to MRI, since it is so much more sensitive?” Breast MRI has several limitations, including relatively low specificity, cost, use of intravenous contrast, and patient discomfort (that is, claustrophobia, prone positioning). MRI should be utilized for pathologic discharge only when the mammogram and/or targeted ultrasound scans are negative or equivocal.
CASE Continued: Additional imaging
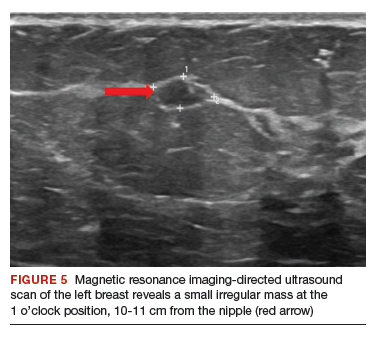
A contrast-enhanced MRI of the breasts (FIGURE 4) demonstrates a large area of non-mass enhancement (red oval) in the left breast, which involves most of the upper breast extending from the nipple to the posterior breast tissue; it measures approximately 7.3 x 14 x 9.1 cm in transverse, anteroposterior, and craniocaudal dimensions, respectively. There is no evidence of left pectoralis muscle involvement. An MRI-directed second look left breast ultrasonography (FIGURE 5) is performed, revealing a small irregular mass in the left breast 1 o’clock position, 10 to 11 cm from the nipple (red arrow). This area had not been imaged in the prior ultrasound scan due to its posterior location far from the nipple. Ultrasound-guided core needle biopsy is performed; moderately differentiated invasive ductal carcinoma (IDC) with high-grade DCIS is found.

Continue to: When to refer for surgery...
When to refer for surgery
No surgical evaluation or intervention is needed for physiologic nipple discharge. As mentioned previously, reassure the patient and recommend appropriate breast cancer screening. In the setting of pathologic discharge, however, referral to a breast surgeon may be indicated after appropriate imaging workup has been done.
Since abnormal imaging almost always results in a recommendation for image-guided biopsy, typically the biopsy is performed prior to the surgical consultation. Once the pathology report from the biopsy is available, the radiologist makes a radiologic-pathologic concordance statement and recommends surgical consultation. This process allows the surgeon to have all the necessary information at the initial visit.
However, in the setting of pathologic nipple discharge with normal breast imaging, the surgeon and patient may opt for close observation or surgery for definitive diagnosis. Surgical options include single-duct excision when nipple discharge is localized to one duct or central duct excision when nipple discharge cannot be localized to one duct.
CASE Continued: Follow-up
The patient was referred to a breast surgeon. Given the extent of disease in the left breast, breast conservation was not possible. The patient underwent left breast simple mastectomy with sentinel lymph node biopsy and prophylactic right simple mastectomy. Final pathology results revealed stage IA IDC with DCIS. Sentinel lymph nodes were negative for malignancy. The patient underwent adjuvant left chest wall radiation, endocrine therapy with tamoxifen, and implant reconstruction. After 2 years of follow-up, she is disease free.
In summary
Nipple discharge can be classified as physiologic or pathologic. For pathologic discharge, a thorough physical examination should be performed with subsequent imaging evaluation. First-line tools, based on patient age, include diagnostic mammography and targeted ultrasonography. Contrast-enhanced MRI is then recommended for negative or equivocal cases. All patients with pathologic nipple discharge should be referred to a breast surgeon following appropriate imaging evaluation. ●
- Alcock C, Layer GT. Predicting occult malignancy in nipple discharge. ANZ J Surg. 2010;80:646-649.
- Patel BK, Falcon S, Drukteinis J. Management of nipple discharge and the associated imaging findings. Am J Med. 2015;128:353-360.
- Mazzarello S, Arnaout A. Five things to know about nipple discharge. CMAJ. 2015;187:599.
- Goksel HA, Yagmurdur MC, Demirhan B, et al. Management strategies for patients with nipple discharge. Langenbecks Arch Surg. 2005;390:52-58.
- Vargas HI, Vargas MP, Eldrageely K, et al. Outcomes of clinical and surgical assessment of women with pathological nipple discharge. Am Surg. 2006;72:124-128.
- Expert Panel on Breast Imaging; Lee S, Tikha S, Moy L, et al. American College of Radiology Appropriateness Criteria: Evaluation of nipple discharge. https://acsearch.acr.org /docs/3099312/Narrative/. Accessed February 2, 2020.
- Cabioglu N, Hunt KK, Singletary SE, et al. Surgical decision making and factors determining a diagnosis of breast carcinoma in women presenting with nipple discharge. J Am Coll Surg. 2003;196:354-364.
- Morrogh M, Park A, Elkin EB, et al. Lessons learned from 416 cases of nipple discharge of the breast. Am J Surg. 2010;200:73-80.
- Morrogh M, Morris EA, Liberman L, et al. The predictive value of ductography and magnetic resonance imaging in the management of nipple discharge. Ann Surg Oncol. 2007;14:3369-3377.
CASE Young woman with discharge from one nipple
A 26-year-old African American woman presents with a 10-month history of left nipple discharge. The patient describes the discharge as spontaneous, colored dark brown to yellow, and occurring from a single opening in the nipple. The discharge is associated with left breast pain and fullness, without a palpable lump. The patient has no family or personal history of breast cancer.
Nipple discharge is the third most common breast-related symptom (after palpable masses and breast pain), with an estimated prevalence of 5% to 8% among premenopausal women.1 While most causes of nipple discharge reflect benign issues, approximately 5% to 12% of breast cancers have nipple discharge as the only symptom.2 Not surprisingly, nipple discharge creates anxiety for both patients and clinicians.
In this article, we—a breast imaging radiologist, gynecologist, and breast surgeon—outline key steps for evaluating and managing patients with nipple discharge.
Two types of nipple discharge
Nipple discharge can be characterized as physiologic or pathologic. The distinction is based on the patient’s history in conjunction with the clinical breast exam.
Physiologic nipple discharge often is bilateral, nonspontaneous, and white, yellow, green, or brown (TABLE).3 It often is due to nipple stimulation, and the patient can elicit discharge by manually manipulating the breast. Usually, multiple ducts are involved. Galactorrhea refers specifically to milky discharge and occurs most commonly during pregnancy or lactation.2 Galactorrhea that is not associated with pregnancy or lactation often is related to elevated prolactin or thyroid-stimulating hormone levels or to medications. One study reported that no cancers were found when discharge was nonspontaneous and colored or milky.4
Pathologic nipple discharge is defined as a spontaneous, bloody, clear, or single-duct discharge. A palpable mass in the same breast automatically increases the suspicion of the discharge, regardless of its color or spontaneity.2 The most common cause of pathologic nipple discharge is an intraductal papilloma, a benign epithelial tumor, which accounts for approximately 57% of cases.5
Although the risk of malignancy is low for all patients with nipple discharge, increasing age is associated with increased risk of breast cancer. One study demonstrated that among women aged 40 to 60 years presenting with nipple discharge, the prevalence of invasive cancer is 10%, and the percentage jumps to 32% among women older than 60.6
Breast exam. For any patient with nonlactational nipple discharge, we recommend a thorough breast examination. Deep palpation of all quadrants of the symptomatic breast, especially near the nipple areolar complex, should elicit nipple discharge without any direct squeezing of the nipple. If the patient’s history and physical exam are consistent with physiologic discharge, no further workup is needed. Reassure the patient and recommend appropriate breast cancer screening. Encourage the patient to decrease stimulation or manual manipulation of the nipples if the discharge bothers her.
Continue to: CASE Continued: Workup...
CASE Continued: Workup
On physical exam, the patient’s breasts are noted to be cup size DDD and asymmetric, with the left breast larger than the right; there is no contour deformity. There is no skin or nipple retraction, skin rash, swelling, or nipple changes bilaterally. No dominant masses are appreciated bilaterally. Manual compression elicits no nipple discharge.
Although the discharge is nonbloody, its spontaneity, unilaterality, and single-duct/orifice origin suggest a pathologic cause. The patient is referred for breast imaging.
Imaging workup for pathologic discharge
The American College of Radiology (ACR) Appropriateness Criteria is a useful tool that provides an evidence-based, easy-to-use algorithm for breast imaging in the patient with pathologic nipple discharge (FIGURE 1).6 The algorithm is categorized by patient age, with diagnostic mammography recommended for women aged 30 and older.6 Diagnostic mammography is recommended if the patient has not had a mammogram study in the last 6 months.6 For patients with no prior mammograms, we recommend bilateral diagnostic mammography to compare symmetry of the breasts.

Currently, no studies show that digital breast tomosynthesis (3-D mammography) has a benefit compared with standard 2-D mammography in women with pathologic nipple discharge.6 Given the increased sensitivity of digital breast tomosynthesis for cancer detection, however, in our practice it is standard to use tomosynthesis in the diagnostic evaluation of most patients.
Mammography
On mammography, ductal carcinoma in situ (DCIS) usually presents as calcifications. Both the morphology and distribution of calcifications are used to characterize them as suspicious or, typically, benign. DCIS usually presents as fine pleomorphic or fine linear branching calcifications in a segmental or linear distribution. In patients with pathologic nipple discharge and no other symptoms, the radiologist must closely examine the retroareolar region of the breast to assess for faint calcifications. Magnification views also can be performed to better characterize calcifications.
The sensitivity of mammography for nipple discharge varies in the literature, ranging from approximately 15% to 68%, with a specificity range of 38% to 98%.6 This results in a relatively low positive predictive value but a high negative predictive value of 90%.7 Mammographic sensitivity largely is limited by increased breast density. As more data emerge on the utility of digital breast tomosynthesis in dense breasts, mammographic sensitivity for nipple discharge will likely increase.
Ultrasonography
As an adjunct to mammography, the ACR Appropriateness Criteria recommends targeted (or “limited”) ultrasonography of the retroareolar region of the affected breast for patients aged 30 and older. Ultrasonography is useful to assess for intraductal masses and architectural distortion, and it has higher sensitivity but lower specificity than mammography. The sensitivity of ultrasonography for detecting breast cancer in patients presenting with nipple discharge is reported to be 56% to 80%.6 Ultrasonography can identify lesions not visible mammographically in 63% to 69% of cases.8 Although DCIS usually presents as calcifications, it also can present as an intraductal mass on ultrasonography.
The ACR recommends targeted ultrasonography for patients with nipple discharge and a negative mammogram, or to evaluate a suspicious mammographic abnormality such as architectural distortion, focal asymmetry, or a mass.6 For patient comfort, ultrasonography is the preferred modality for image-guided biopsy.
For women younger than 30 years, targeted ultrasonography is the initial imaging study of choice, according to the ACR criteria.6 Women younger than 30 years with pathologic nipple discharge have a very low risk of breast cancer and tend to have higher breast density, making mammography less useful. Although the radiation dose from mammography is negligible given technological improvements and dose-reduction techniques, ultrasonography remains the preferred initial imaging modality in young women, not only for nipple discharge but also for palpable lumps and focal breast pain.
Mammography is used as an adjunct to ultrasonography in women younger than 30 years when a suspicious abnormality is detected on ultrasonography, such as an intraductal mass or architectural distortion. In these cases, mammography can be used to assess for extent of disease or to visualize suspicious calcifications not well seen on ultrasonography.
For practical purposes regarding which imaging study to order for a patient, it is most efficient to order both a diagnostic mammogram (with tomosynthesis, if possible) and a targeted ultrasound scan of the affected breast. Even if both orders are not needed, having them available increases efficiency for both the radiologist and the ordering physician.
Continue to: CASE Continued: Imaging findings...
CASE Continued: Imaging findings
Given her age, the patient initially undergoes targeted ultrasonography. The grayscale image (FIGURE 2) demonstrates multiple mildly dilated ducts (white arrows) with surrounding hyperechogenicity of the fat (red arrows), indicating soft tissue edema. No intraductal mass is imaged. Given that the ultrasonography findings are not completely negative and are equivocal for malignancy, bilateral diagnostic mammography (FIGURE 3, left breast only) is performed. Standard full-field craniocaudal (FIGURE 3A) and mediolateral oblique (FIGURE 3B) mammographic views demonstrate a heterogeneously dense breast with a few calcifications in the retroareolar left breast (red ovals). No associated mass or architectural distortions are noted. The mammographic and sonographic findings do not reveal a definitive biopsy target.


Ductography
When a suspicious abnormality is visualized on either mammography or ultrasonography, the standard of care is to perform an image-guided biopsy of the abnormality. When the standard workup is negative or equivocal, the standard of care historically was to perform ductography.
Ductography is an invasive procedure that involves cannulating the suspicious duct with a small catheter and injecting radiopaque dye into the duct under mammographic guidance. While the sensitivity of ductography is higher than that of both mammography and ultrasonography, its specificity is lower than that of either modality.
Most cases of pathologic discharge are spontaneous and are not reproducible on the day of the procedure. If the procedural radiologist cannot visualize the duct that is producing the discharge, the procedure cannot be performed. Although most patients tolerate the procedure well, ductography produces patient discomfort from cannulation of the duct and injection of contrast.
Magnetic resonance imaging
Dynamic contrast-enhanced magnetic resonance imaging (MRI) is the most sensitive imaging study for evaluating pathologic nipple discharge, and it has largely replaced ductography as an adjunct to mammography and ultrasonography. MRI’s sensitivity for detecting breast cancer ranges from 93% to 100%.6 In addition, MRI allows visualization of the entire breast and areas peripheral to the field of view of a standard ductogram or ultrasound scan.9
Clinicians commonly ask, “Why not skip the mammogram and ultrasound scan and go straight to MRI, since it is so much more sensitive?” Breast MRI has several limitations, including relatively low specificity, cost, use of intravenous contrast, and patient discomfort (that is, claustrophobia, prone positioning). MRI should be utilized for pathologic discharge only when the mammogram and/or targeted ultrasound scans are negative or equivocal.
CASE Continued: Additional imaging
A contrast-enhanced MRI of the breasts (FIGURE 4) demonstrates a large area of non-mass enhancement (red oval) in the left breast, which involves most of the upper breast extending from the nipple to the posterior breast tissue; it measures approximately 7.3 x 14 x 9.1 cm in transverse, anteroposterior, and craniocaudal dimensions, respectively. There is no evidence of left pectoralis muscle involvement. An MRI-directed second look left breast ultrasonography (FIGURE 5) is performed, revealing a small irregular mass in the left breast 1 o’clock position, 10 to 11 cm from the nipple (red arrow). This area had not been imaged in the prior ultrasound scan due to its posterior location far from the nipple. Ultrasound-guided core needle biopsy is performed; moderately differentiated invasive ductal carcinoma (IDC) with high-grade DCIS is found.


Continue to: When to refer for surgery...
When to refer for surgery
No surgical evaluation or intervention is needed for physiologic nipple discharge. As mentioned previously, reassure the patient and recommend appropriate breast cancer screening. In the setting of pathologic discharge, however, referral to a breast surgeon may be indicated after appropriate imaging workup has been done.
Since abnormal imaging almost always results in a recommendation for image-guided biopsy, typically the biopsy is performed prior to the surgical consultation. Once the pathology report from the biopsy is available, the radiologist makes a radiologic-pathologic concordance statement and recommends surgical consultation. This process allows the surgeon to have all the necessary information at the initial visit.
However, in the setting of pathologic nipple discharge with normal breast imaging, the surgeon and patient may opt for close observation or surgery for definitive diagnosis. Surgical options include single-duct excision when nipple discharge is localized to one duct or central duct excision when nipple discharge cannot be localized to one duct.
CASE Continued: Follow-up
The patient was referred to a breast surgeon. Given the extent of disease in the left breast, breast conservation was not possible. The patient underwent left breast simple mastectomy with sentinel lymph node biopsy and prophylactic right simple mastectomy. Final pathology results revealed stage IA IDC with DCIS. Sentinel lymph nodes were negative for malignancy. The patient underwent adjuvant left chest wall radiation, endocrine therapy with tamoxifen, and implant reconstruction. After 2 years of follow-up, she is disease free.
In summary
Nipple discharge can be classified as physiologic or pathologic. For pathologic discharge, a thorough physical examination should be performed with subsequent imaging evaluation. First-line tools, based on patient age, include diagnostic mammography and targeted ultrasonography. Contrast-enhanced MRI is then recommended for negative or equivocal cases. All patients with pathologic nipple discharge should be referred to a breast surgeon following appropriate imaging evaluation. ●
CASE Young woman with discharge from one nipple
A 26-year-old African American woman presents with a 10-month history of left nipple discharge. The patient describes the discharge as spontaneous, colored dark brown to yellow, and occurring from a single opening in the nipple. The discharge is associated with left breast pain and fullness, without a palpable lump. The patient has no family or personal history of breast cancer.
Nipple discharge is the third most common breast-related symptom (after palpable masses and breast pain), with an estimated prevalence of 5% to 8% among premenopausal women.1 While most causes of nipple discharge reflect benign issues, approximately 5% to 12% of breast cancers have nipple discharge as the only symptom.2 Not surprisingly, nipple discharge creates anxiety for both patients and clinicians.
In this article, we—a breast imaging radiologist, gynecologist, and breast surgeon—outline key steps for evaluating and managing patients with nipple discharge.
Two types of nipple discharge
Nipple discharge can be characterized as physiologic or pathologic. The distinction is based on the patient’s history in conjunction with the clinical breast exam.
Physiologic nipple discharge often is bilateral, nonspontaneous, and white, yellow, green, or brown (TABLE).3 It often is due to nipple stimulation, and the patient can elicit discharge by manually manipulating the breast. Usually, multiple ducts are involved. Galactorrhea refers specifically to milky discharge and occurs most commonly during pregnancy or lactation.2 Galactorrhea that is not associated with pregnancy or lactation often is related to elevated prolactin or thyroid-stimulating hormone levels or to medications. One study reported that no cancers were found when discharge was nonspontaneous and colored or milky.4
Pathologic nipple discharge is defined as a spontaneous, bloody, clear, or single-duct discharge. A palpable mass in the same breast automatically increases the suspicion of the discharge, regardless of its color or spontaneity.2 The most common cause of pathologic nipple discharge is an intraductal papilloma, a benign epithelial tumor, which accounts for approximately 57% of cases.5
Although the risk of malignancy is low for all patients with nipple discharge, increasing age is associated with increased risk of breast cancer. One study demonstrated that among women aged 40 to 60 years presenting with nipple discharge, the prevalence of invasive cancer is 10%, and the percentage jumps to 32% among women older than 60.6
Breast exam. For any patient with nonlactational nipple discharge, we recommend a thorough breast examination. Deep palpation of all quadrants of the symptomatic breast, especially near the nipple areolar complex, should elicit nipple discharge without any direct squeezing of the nipple. If the patient’s history and physical exam are consistent with physiologic discharge, no further workup is needed. Reassure the patient and recommend appropriate breast cancer screening. Encourage the patient to decrease stimulation or manual manipulation of the nipples if the discharge bothers her.
Continue to: CASE Continued: Workup...
CASE Continued: Workup
On physical exam, the patient’s breasts are noted to be cup size DDD and asymmetric, with the left breast larger than the right; there is no contour deformity. There is no skin or nipple retraction, skin rash, swelling, or nipple changes bilaterally. No dominant masses are appreciated bilaterally. Manual compression elicits no nipple discharge.
Although the discharge is nonbloody, its spontaneity, unilaterality, and single-duct/orifice origin suggest a pathologic cause. The patient is referred for breast imaging.
Imaging workup for pathologic discharge
The American College of Radiology (ACR) Appropriateness Criteria is a useful tool that provides an evidence-based, easy-to-use algorithm for breast imaging in the patient with pathologic nipple discharge (FIGURE 1).6 The algorithm is categorized by patient age, with diagnostic mammography recommended for women aged 30 and older.6 Diagnostic mammography is recommended if the patient has not had a mammogram study in the last 6 months.6 For patients with no prior mammograms, we recommend bilateral diagnostic mammography to compare symmetry of the breasts.

Currently, no studies show that digital breast tomosynthesis (3-D mammography) has a benefit compared with standard 2-D mammography in women with pathologic nipple discharge.6 Given the increased sensitivity of digital breast tomosynthesis for cancer detection, however, in our practice it is standard to use tomosynthesis in the diagnostic evaluation of most patients.
Mammography
On mammography, ductal carcinoma in situ (DCIS) usually presents as calcifications. Both the morphology and distribution of calcifications are used to characterize them as suspicious or, typically, benign. DCIS usually presents as fine pleomorphic or fine linear branching calcifications in a segmental or linear distribution. In patients with pathologic nipple discharge and no other symptoms, the radiologist must closely examine the retroareolar region of the breast to assess for faint calcifications. Magnification views also can be performed to better characterize calcifications.
The sensitivity of mammography for nipple discharge varies in the literature, ranging from approximately 15% to 68%, with a specificity range of 38% to 98%.6 This results in a relatively low positive predictive value but a high negative predictive value of 90%.7 Mammographic sensitivity largely is limited by increased breast density. As more data emerge on the utility of digital breast tomosynthesis in dense breasts, mammographic sensitivity for nipple discharge will likely increase.
Ultrasonography
As an adjunct to mammography, the ACR Appropriateness Criteria recommends targeted (or “limited”) ultrasonography of the retroareolar region of the affected breast for patients aged 30 and older. Ultrasonography is useful to assess for intraductal masses and architectural distortion, and it has higher sensitivity but lower specificity than mammography. The sensitivity of ultrasonography for detecting breast cancer in patients presenting with nipple discharge is reported to be 56% to 80%.6 Ultrasonography can identify lesions not visible mammographically in 63% to 69% of cases.8 Although DCIS usually presents as calcifications, it also can present as an intraductal mass on ultrasonography.
The ACR recommends targeted ultrasonography for patients with nipple discharge and a negative mammogram, or to evaluate a suspicious mammographic abnormality such as architectural distortion, focal asymmetry, or a mass.6 For patient comfort, ultrasonography is the preferred modality for image-guided biopsy.
For women younger than 30 years, targeted ultrasonography is the initial imaging study of choice, according to the ACR criteria.6 Women younger than 30 years with pathologic nipple discharge have a very low risk of breast cancer and tend to have higher breast density, making mammography less useful. Although the radiation dose from mammography is negligible given technological improvements and dose-reduction techniques, ultrasonography remains the preferred initial imaging modality in young women, not only for nipple discharge but also for palpable lumps and focal breast pain.
Mammography is used as an adjunct to ultrasonography in women younger than 30 years when a suspicious abnormality is detected on ultrasonography, such as an intraductal mass or architectural distortion. In these cases, mammography can be used to assess for extent of disease or to visualize suspicious calcifications not well seen on ultrasonography.
For practical purposes regarding which imaging study to order for a patient, it is most efficient to order both a diagnostic mammogram (with tomosynthesis, if possible) and a targeted ultrasound scan of the affected breast. Even if both orders are not needed, having them available increases efficiency for both the radiologist and the ordering physician.
Continue to: CASE Continued: Imaging findings...
CASE Continued: Imaging findings
Given her age, the patient initially undergoes targeted ultrasonography. The grayscale image (FIGURE 2) demonstrates multiple mildly dilated ducts (white arrows) with surrounding hyperechogenicity of the fat (red arrows), indicating soft tissue edema. No intraductal mass is imaged. Given that the ultrasonography findings are not completely negative and are equivocal for malignancy, bilateral diagnostic mammography (FIGURE 3, left breast only) is performed. Standard full-field craniocaudal (FIGURE 3A) and mediolateral oblique (FIGURE 3B) mammographic views demonstrate a heterogeneously dense breast with a few calcifications in the retroareolar left breast (red ovals). No associated mass or architectural distortions are noted. The mammographic and sonographic findings do not reveal a definitive biopsy target.


Ductography
When a suspicious abnormality is visualized on either mammography or ultrasonography, the standard of care is to perform an image-guided biopsy of the abnormality. When the standard workup is negative or equivocal, the standard of care historically was to perform ductography.
Ductography is an invasive procedure that involves cannulating the suspicious duct with a small catheter and injecting radiopaque dye into the duct under mammographic guidance. While the sensitivity of ductography is higher than that of both mammography and ultrasonography, its specificity is lower than that of either modality.
Most cases of pathologic discharge are spontaneous and are not reproducible on the day of the procedure. If the procedural radiologist cannot visualize the duct that is producing the discharge, the procedure cannot be performed. Although most patients tolerate the procedure well, ductography produces patient discomfort from cannulation of the duct and injection of contrast.
Magnetic resonance imaging
Dynamic contrast-enhanced magnetic resonance imaging (MRI) is the most sensitive imaging study for evaluating pathologic nipple discharge, and it has largely replaced ductography as an adjunct to mammography and ultrasonography. MRI’s sensitivity for detecting breast cancer ranges from 93% to 100%.6 In addition, MRI allows visualization of the entire breast and areas peripheral to the field of view of a standard ductogram or ultrasound scan.9
Clinicians commonly ask, “Why not skip the mammogram and ultrasound scan and go straight to MRI, since it is so much more sensitive?” Breast MRI has several limitations, including relatively low specificity, cost, use of intravenous contrast, and patient discomfort (that is, claustrophobia, prone positioning). MRI should be utilized for pathologic discharge only when the mammogram and/or targeted ultrasound scans are negative or equivocal.
CASE Continued: Additional imaging
A contrast-enhanced MRI of the breasts (FIGURE 4) demonstrates a large area of non-mass enhancement (red oval) in the left breast, which involves most of the upper breast extending from the nipple to the posterior breast tissue; it measures approximately 7.3 x 14 x 9.1 cm in transverse, anteroposterior, and craniocaudal dimensions, respectively. There is no evidence of left pectoralis muscle involvement. An MRI-directed second look left breast ultrasonography (FIGURE 5) is performed, revealing a small irregular mass in the left breast 1 o’clock position, 10 to 11 cm from the nipple (red arrow). This area had not been imaged in the prior ultrasound scan due to its posterior location far from the nipple. Ultrasound-guided core needle biopsy is performed; moderately differentiated invasive ductal carcinoma (IDC) with high-grade DCIS is found.


Continue to: When to refer for surgery...
When to refer for surgery
No surgical evaluation or intervention is needed for physiologic nipple discharge. As mentioned previously, reassure the patient and recommend appropriate breast cancer screening. In the setting of pathologic discharge, however, referral to a breast surgeon may be indicated after appropriate imaging workup has been done.
Since abnormal imaging almost always results in a recommendation for image-guided biopsy, typically the biopsy is performed prior to the surgical consultation. Once the pathology report from the biopsy is available, the radiologist makes a radiologic-pathologic concordance statement and recommends surgical consultation. This process allows the surgeon to have all the necessary information at the initial visit.
However, in the setting of pathologic nipple discharge with normal breast imaging, the surgeon and patient may opt for close observation or surgery for definitive diagnosis. Surgical options include single-duct excision when nipple discharge is localized to one duct or central duct excision when nipple discharge cannot be localized to one duct.
CASE Continued: Follow-up
The patient was referred to a breast surgeon. Given the extent of disease in the left breast, breast conservation was not possible. The patient underwent left breast simple mastectomy with sentinel lymph node biopsy and prophylactic right simple mastectomy. Final pathology results revealed stage IA IDC with DCIS. Sentinel lymph nodes were negative for malignancy. The patient underwent adjuvant left chest wall radiation, endocrine therapy with tamoxifen, and implant reconstruction. After 2 years of follow-up, she is disease free.
In summary
Nipple discharge can be classified as physiologic or pathologic. For pathologic discharge, a thorough physical examination should be performed with subsequent imaging evaluation. First-line tools, based on patient age, include diagnostic mammography and targeted ultrasonography. Contrast-enhanced MRI is then recommended for negative or equivocal cases. All patients with pathologic nipple discharge should be referred to a breast surgeon following appropriate imaging evaluation. ●
- Alcock C, Layer GT. Predicting occult malignancy in nipple discharge. ANZ J Surg. 2010;80:646-649.
- Patel BK, Falcon S, Drukteinis J. Management of nipple discharge and the associated imaging findings. Am J Med. 2015;128:353-360.
- Mazzarello S, Arnaout A. Five things to know about nipple discharge. CMAJ. 2015;187:599.
- Goksel HA, Yagmurdur MC, Demirhan B, et al. Management strategies for patients with nipple discharge. Langenbecks Arch Surg. 2005;390:52-58.
- Vargas HI, Vargas MP, Eldrageely K, et al. Outcomes of clinical and surgical assessment of women with pathological nipple discharge. Am Surg. 2006;72:124-128.
- Expert Panel on Breast Imaging; Lee S, Tikha S, Moy L, et al. American College of Radiology Appropriateness Criteria: Evaluation of nipple discharge. https://acsearch.acr.org /docs/3099312/Narrative/. Accessed February 2, 2020.
- Cabioglu N, Hunt KK, Singletary SE, et al. Surgical decision making and factors determining a diagnosis of breast carcinoma in women presenting with nipple discharge. J Am Coll Surg. 2003;196:354-364.
- Morrogh M, Park A, Elkin EB, et al. Lessons learned from 416 cases of nipple discharge of the breast. Am J Surg. 2010;200:73-80.
- Morrogh M, Morris EA, Liberman L, et al. The predictive value of ductography and magnetic resonance imaging in the management of nipple discharge. Ann Surg Oncol. 2007;14:3369-3377.
- Alcock C, Layer GT. Predicting occult malignancy in nipple discharge. ANZ J Surg. 2010;80:646-649.
- Patel BK, Falcon S, Drukteinis J. Management of nipple discharge and the associated imaging findings. Am J Med. 2015;128:353-360.
- Mazzarello S, Arnaout A. Five things to know about nipple discharge. CMAJ. 2015;187:599.
- Goksel HA, Yagmurdur MC, Demirhan B, et al. Management strategies for patients with nipple discharge. Langenbecks Arch Surg. 2005;390:52-58.
- Vargas HI, Vargas MP, Eldrageely K, et al. Outcomes of clinical and surgical assessment of women with pathological nipple discharge. Am Surg. 2006;72:124-128.
- Expert Panel on Breast Imaging; Lee S, Tikha S, Moy L, et al. American College of Radiology Appropriateness Criteria: Evaluation of nipple discharge. https://acsearch.acr.org /docs/3099312/Narrative/. Accessed February 2, 2020.
- Cabioglu N, Hunt KK, Singletary SE, et al. Surgical decision making and factors determining a diagnosis of breast carcinoma in women presenting with nipple discharge. J Am Coll Surg. 2003;196:354-364.
- Morrogh M, Park A, Elkin EB, et al. Lessons learned from 416 cases of nipple discharge of the breast. Am J Surg. 2010;200:73-80.
- Morrogh M, Morris EA, Liberman L, et al. The predictive value of ductography and magnetic resonance imaging in the management of nipple discharge. Ann Surg Oncol. 2007;14:3369-3377.



