User login
The first artificial valve for percutaneous placement in stenotic aortic valves was approved today by the Food and Drug Administration.
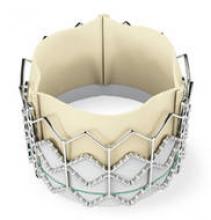
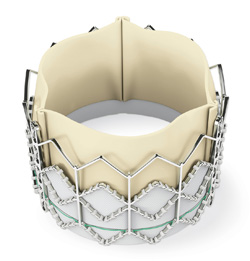
The Sapien transcatheter heart valve, made by Edwards Lifesciences, could be a treatment option for patients who have severe aortic stenosis and are deemed too sick to undergo surgical heart valve replacement.
The long-term durability of the valve is not known, and the information about restrictions on which centers are allowed to perform the procedure is yet to emerge.
In anticipation of the FDA approval, the Centers for Medicare and Medicaid Services opened a national coverage determination analysis for transcatheter valve replacement. CMS opened the coverage analysis in response to a joint request by the Society of Thoracic Surgeons and the American College of Cardiology. The groups argue that only cardiologists with appropriate training and credentials, working at specialized heart centers, should be performing transcatheter valve replacement.
So far, one of the clear requirements has been the presence and collaboration of interventional cardiologists and cardiac surgeons in patient selection as well as the procedure. The valve-stent system is placed in the patient’s beating heart by a multidisciplinary team at a hybrid operating room or a hybrid catheterization laboratory.
The device’s label was not available at the time of the FDA announcement. According to the agency’s statement, "The Sapien THV is approved for patients who are not eligible for open-heart surgery for replacement of their aortic valve and have a calcified aortic annulus. ... The product label advises that a heart surgeon should be involved in determining if the Sapien THV is an appropriate treatment for the patient. It is not approved for patients who can be treated by open-heart surgery. Patients who have congenital heart valve anomalies, have masses or an infection in their hearts, or cannot tolerate anticoagulation/antiplatelet therapy should not receive the Sapien THV."
Transcatheter aortic valve replacement, or TAVR, has been commercially available in Europe since 2007, and has been undergoing clinical trials in the United States. The first trial to launch in the United States was Edwards’ Placement of Aortic Transcatheter Valve Trial, or PARTNER.
Another trial is underway for Medtronic’s CoreValve.
The Sapien valve was approved on the basis of results from the Cohort B of PARTNER, which showed that TAVR improved patients’ quality of life and decreased mortality at 30 days and 1 year.
However, results also showed that patients who received TAVR had a 2.5-fold higher rate of strokes and an 8-fold higher rate of vascular and bleeding complications as compared to patients who did not receive the implant, according to an FDA statement.
Edwards is also pursuing an indication for patients with severe aortic stenosis who are at high risk for surgical complications (Cohort A of the PARTNER trial), on the basis of positive results released in March.
The first artificial valve for percutaneous placement in stenotic aortic valves was approved today by the Food and Drug Administration.
The Sapien transcatheter heart valve, made by Edwards Lifesciences, could be a treatment option for patients who have severe aortic stenosis and are deemed too sick to undergo surgical heart valve replacement.
The long-term durability of the valve is not known, and the information about restrictions on which centers are allowed to perform the procedure is yet to emerge.
In anticipation of the FDA approval, the Centers for Medicare and Medicaid Services opened a national coverage determination analysis for transcatheter valve replacement. CMS opened the coverage analysis in response to a joint request by the Society of Thoracic Surgeons and the American College of Cardiology. The groups argue that only cardiologists with appropriate training and credentials, working at specialized heart centers, should be performing transcatheter valve replacement.
So far, one of the clear requirements has been the presence and collaboration of interventional cardiologists and cardiac surgeons in patient selection as well as the procedure. The valve-stent system is placed in the patient’s beating heart by a multidisciplinary team at a hybrid operating room or a hybrid catheterization laboratory.
The device’s label was not available at the time of the FDA announcement. According to the agency’s statement, "The Sapien THV is approved for patients who are not eligible for open-heart surgery for replacement of their aortic valve and have a calcified aortic annulus. ... The product label advises that a heart surgeon should be involved in determining if the Sapien THV is an appropriate treatment for the patient. It is not approved for patients who can be treated by open-heart surgery. Patients who have congenital heart valve anomalies, have masses or an infection in their hearts, or cannot tolerate anticoagulation/antiplatelet therapy should not receive the Sapien THV."
Transcatheter aortic valve replacement, or TAVR, has been commercially available in Europe since 2007, and has been undergoing clinical trials in the United States. The first trial to launch in the United States was Edwards’ Placement of Aortic Transcatheter Valve Trial, or PARTNER.
Another trial is underway for Medtronic’s CoreValve.
The Sapien valve was approved on the basis of results from the Cohort B of PARTNER, which showed that TAVR improved patients’ quality of life and decreased mortality at 30 days and 1 year.
However, results also showed that patients who received TAVR had a 2.5-fold higher rate of strokes and an 8-fold higher rate of vascular and bleeding complications as compared to patients who did not receive the implant, according to an FDA statement.
Edwards is also pursuing an indication for patients with severe aortic stenosis who are at high risk for surgical complications (Cohort A of the PARTNER trial), on the basis of positive results released in March.
The first artificial valve for percutaneous placement in stenotic aortic valves was approved today by the Food and Drug Administration.
The Sapien transcatheter heart valve, made by Edwards Lifesciences, could be a treatment option for patients who have severe aortic stenosis and are deemed too sick to undergo surgical heart valve replacement.
The long-term durability of the valve is not known, and the information about restrictions on which centers are allowed to perform the procedure is yet to emerge.
In anticipation of the FDA approval, the Centers for Medicare and Medicaid Services opened a national coverage determination analysis for transcatheter valve replacement. CMS opened the coverage analysis in response to a joint request by the Society of Thoracic Surgeons and the American College of Cardiology. The groups argue that only cardiologists with appropriate training and credentials, working at specialized heart centers, should be performing transcatheter valve replacement.
So far, one of the clear requirements has been the presence and collaboration of interventional cardiologists and cardiac surgeons in patient selection as well as the procedure. The valve-stent system is placed in the patient’s beating heart by a multidisciplinary team at a hybrid operating room or a hybrid catheterization laboratory.
The device’s label was not available at the time of the FDA announcement. According to the agency’s statement, "The Sapien THV is approved for patients who are not eligible for open-heart surgery for replacement of their aortic valve and have a calcified aortic annulus. ... The product label advises that a heart surgeon should be involved in determining if the Sapien THV is an appropriate treatment for the patient. It is not approved for patients who can be treated by open-heart surgery. Patients who have congenital heart valve anomalies, have masses or an infection in their hearts, or cannot tolerate anticoagulation/antiplatelet therapy should not receive the Sapien THV."
Transcatheter aortic valve replacement, or TAVR, has been commercially available in Europe since 2007, and has been undergoing clinical trials in the United States. The first trial to launch in the United States was Edwards’ Placement of Aortic Transcatheter Valve Trial, or PARTNER.
Another trial is underway for Medtronic’s CoreValve.
The Sapien valve was approved on the basis of results from the Cohort B of PARTNER, which showed that TAVR improved patients’ quality of life and decreased mortality at 30 days and 1 year.
However, results also showed that patients who received TAVR had a 2.5-fold higher rate of strokes and an 8-fold higher rate of vascular and bleeding complications as compared to patients who did not receive the implant, according to an FDA statement.
Edwards is also pursuing an indication for patients with severe aortic stenosis who are at high risk for surgical complications (Cohort A of the PARTNER trial), on the basis of positive results released in March.