User login
The Food and Drug Administration has approved a new implantable cardioverter defibrillator that is implanted subcutaneously as a treatment for ventricular tachyarrhythmias.
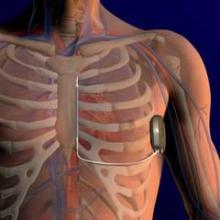
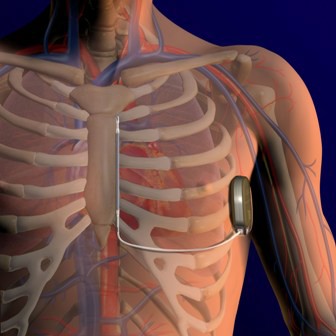
The agency approved the Subcutaneous Implantable Defibrillator (S-ICD) System only for patients who do not require a pacemaker or pacing therapy.
The S-ICD System, made by Cameron Health of San Clemente, Calif., is the first such device that can be placed without transvenous leads. Instead, the leads are placed under the skin and then connected to the heart.
"The S-ICD System provides an alternative for treating patients with life-threatening heart arrhythmias for whom the routine ICD placement procedure is not ideal," said Christy Foreman, director of the Office of Device Evaluation at FDA’s Center for Devices and Radiological Health, in a statement. "Some patients with anatomy that makes it challenging to place one of the implantable defibrillators currently on the market may especially benefit from this device."
The device won near-unanimous backing of the FDA’s Circulatory System Devices Panel in April. The panel voted 7-1 that the benefits of the S-ICD system outweighed its risks in patients who meet the criteria in the indication proposed by Cameron Health: to provide "defibrillation therapy for the treatment of life-threatening ventricular tachyarrhythmias in patients who do not have symptomatic bradycardia; incessant ventricular tachycardia; or spontaneous, frequently recurring ventricular tachycardia that is reliably terminated with antitachycardia pacing."
Safety and efficacy data for the approval came from a 321-patient study, which was reported at the Heart Rhythm Society in May. In that trial, 304 patients were successfully implanted and the device converted all abnormal heart rhythms that it detected back to normal rhythms, according to the FDA. In the 6 months postimplantation, the S-ICD detected and recorded 78 spontaneous arrhythmias in 21 patients that were either all converted or resolved on their own.
The FDA said that the most common complications included inappropriate shocks, discomfort, system infection, and electrode movement. Eight patients died during the study, but the S-ICD was not definitively seen as the cause. There were 11 explants. Six months after implantation, 90% of the device recipients had no complications.
Cameron Health reported in May that the S-ICD is distributed in 10 other countries and that more than 1,200 patients have been implanted worldwide.
The company proposed a postmarketing study when it submitted its application for approval. The FDA will require that study, seeking assessment of how it performs over the long term, and across genders. The study will follow 1,616 patients for 5 years, according to the agency.
The Food and Drug Administration has approved a new implantable cardioverter defibrillator that is implanted subcutaneously as a treatment for ventricular tachyarrhythmias.
The agency approved the Subcutaneous Implantable Defibrillator (S-ICD) System only for patients who do not require a pacemaker or pacing therapy.
The S-ICD System, made by Cameron Health of San Clemente, Calif., is the first such device that can be placed without transvenous leads. Instead, the leads are placed under the skin and then connected to the heart.
"The S-ICD System provides an alternative for treating patients with life-threatening heart arrhythmias for whom the routine ICD placement procedure is not ideal," said Christy Foreman, director of the Office of Device Evaluation at FDA’s Center for Devices and Radiological Health, in a statement. "Some patients with anatomy that makes it challenging to place one of the implantable defibrillators currently on the market may especially benefit from this device."
The device won near-unanimous backing of the FDA’s Circulatory System Devices Panel in April. The panel voted 7-1 that the benefits of the S-ICD system outweighed its risks in patients who meet the criteria in the indication proposed by Cameron Health: to provide "defibrillation therapy for the treatment of life-threatening ventricular tachyarrhythmias in patients who do not have symptomatic bradycardia; incessant ventricular tachycardia; or spontaneous, frequently recurring ventricular tachycardia that is reliably terminated with antitachycardia pacing."
Safety and efficacy data for the approval came from a 321-patient study, which was reported at the Heart Rhythm Society in May. In that trial, 304 patients were successfully implanted and the device converted all abnormal heart rhythms that it detected back to normal rhythms, according to the FDA. In the 6 months postimplantation, the S-ICD detected and recorded 78 spontaneous arrhythmias in 21 patients that were either all converted or resolved on their own.
The FDA said that the most common complications included inappropriate shocks, discomfort, system infection, and electrode movement. Eight patients died during the study, but the S-ICD was not definitively seen as the cause. There were 11 explants. Six months after implantation, 90% of the device recipients had no complications.
Cameron Health reported in May that the S-ICD is distributed in 10 other countries and that more than 1,200 patients have been implanted worldwide.
The company proposed a postmarketing study when it submitted its application for approval. The FDA will require that study, seeking assessment of how it performs over the long term, and across genders. The study will follow 1,616 patients for 5 years, according to the agency.
The Food and Drug Administration has approved a new implantable cardioverter defibrillator that is implanted subcutaneously as a treatment for ventricular tachyarrhythmias.
The agency approved the Subcutaneous Implantable Defibrillator (S-ICD) System only for patients who do not require a pacemaker or pacing therapy.
The S-ICD System, made by Cameron Health of San Clemente, Calif., is the first such device that can be placed without transvenous leads. Instead, the leads are placed under the skin and then connected to the heart.
"The S-ICD System provides an alternative for treating patients with life-threatening heart arrhythmias for whom the routine ICD placement procedure is not ideal," said Christy Foreman, director of the Office of Device Evaluation at FDA’s Center for Devices and Radiological Health, in a statement. "Some patients with anatomy that makes it challenging to place one of the implantable defibrillators currently on the market may especially benefit from this device."
The device won near-unanimous backing of the FDA’s Circulatory System Devices Panel in April. The panel voted 7-1 that the benefits of the S-ICD system outweighed its risks in patients who meet the criteria in the indication proposed by Cameron Health: to provide "defibrillation therapy for the treatment of life-threatening ventricular tachyarrhythmias in patients who do not have symptomatic bradycardia; incessant ventricular tachycardia; or spontaneous, frequently recurring ventricular tachycardia that is reliably terminated with antitachycardia pacing."
Safety and efficacy data for the approval came from a 321-patient study, which was reported at the Heart Rhythm Society in May. In that trial, 304 patients were successfully implanted and the device converted all abnormal heart rhythms that it detected back to normal rhythms, according to the FDA. In the 6 months postimplantation, the S-ICD detected and recorded 78 spontaneous arrhythmias in 21 patients that were either all converted or resolved on their own.
The FDA said that the most common complications included inappropriate shocks, discomfort, system infection, and electrode movement. Eight patients died during the study, but the S-ICD was not definitively seen as the cause. There were 11 explants. Six months after implantation, 90% of the device recipients had no complications.
Cameron Health reported in May that the S-ICD is distributed in 10 other countries and that more than 1,200 patients have been implanted worldwide.
The company proposed a postmarketing study when it submitted its application for approval. The FDA will require that study, seeking assessment of how it performs over the long term, and across genders. The study will follow 1,616 patients for 5 years, according to the agency.