User login
Throughout the 20th century, the management of ectopic pregnancy evolved from preserving the life of the mother to preserving fertility by utilizing the conservative treatment of methotrexate and/or tubal surgery. I make this, seemingly obscure, reference to managing ectopic pregnancy to consider an analogous shift over time in the management of patients with cancer. Over the next decade, the number of people who have lived 5 or more years after their cancer diagnosis is projected to increase approximately 30%, to 16.3 million. Due to the improved survival rates following a cancer diagnosis,1 revolutionary developments have been made in fertility preservation to obviate the impact of gonadotoxic therapy. We have evolved, however, from shielding and transposing ovaries to ovarian tissue cryopreservation,2 with rapid implementation.
While advances in reproductive cryopreservation have allowed for the delay, or even potential “prevention” of infertility, assisted reproductive technology (ART) cannot yet claim a “cure” in ensuring procreation. Nevertheless, fertility preservation is a burgeoning field that has transitioned from an experimental label to a standard of care in 2012, as designated by the American Society for Reproductive Medicine (ASRM).3 From the original intention of offering oocyte cryopreservation to women at risk of ovarian failure from impending gonadotoxic cancer treatment, fertility preservation has accelerated to include freezing for nonmedical reasons—eg, planned oocyte cryopreservation (POC), or “social” egg freezing, to ovarian tissue cryopreservation to accommodate the expediency needed for the treatment of certain cancer treatments. Additionally, across the United States, the number of donor egg banks, which allow women an easily accessible option, is rivaling enduring sperm banks. Due to the advanced methodology of vitrification and growing demand for the technology due to increasing IVF cycles, cryopreservation has become a specialized area of reproductive medicine, and a target of venture capital and private equity commercialization. This article will review the latest techniques, appropriate counseling, and cost/benefit ratio of fertility preservation, with an emphasis on POC.
CASE 1 Fertility preservation options for patient with breast cancer
A 37-year-old woman with newly diagnosed hormone receptor−positive breast cancer is referred for a fertility preservation consultation prior to initiating treatment. Her oncologist plans chemotherapy, followed by radiation and a minimum of 5 years of tamoxifen therapy.
What is the best consultation approach for this patient?
Consultation involves understanding several factors
The consultation approach to this patient involves ascertaining her medical, social, and family history, along with her reproductive plans.
Medical history. For the medical component, we must focus on her diagnosis, anticipated treatment with timeline, risks of gonadal toxicity with planned treatments, her current medical stability, and prognosis for expected survival.
Social history. Her age, relationship status, and desired family size address her social history.
Family history. Given that her cancer affects the breast, there is the risk of genetic susceptibility and potential for embryo testing for the BRCA gene.
Reproductive plans. These include her and her partner’s, if applicable, number of desired children and their risk factors for infertility.
Regarding the reproductive timeline, the antihormonal therapy that may be required for her treatment may improve overall survival, but it would delay the time to pregnancy. Consequently, the pursuit of fertility preservation prior to cancer treatment is a multidisciplinary approach that can involve medical oncology, radiation oncology, REI, medical genetics, and often, psychology. Fortunately, evidence continues to support fertility preservation, with or without hormonal ovarian stimulation, for patients with breast cancer. Data, with up to 5 years of follow-up, has indicated that it is safe.4
Continue to: Oncofertility...
Oncofertility
To address the need to maximize the reproductive potential of patients with newly diagnosed cancer, the field of oncofertility combines the specialties of oncology and reproductive medicine. The reproductive risk of cancer treatment is gonadotoxicity, with subsequent iatrogenic primary ovarian insufficiency (POI) and infertility. Alkylating agents (including cyclosphosphamide) have the highest risk for amenorrhea, while antimetabolites (including methotrexate, 5–fluorouracil) have the lowest risk.5 Treating bone marrow/stem cell transplantation using high-dose alkylating agents, with or without whole body irradiation, results in ≥80% amenorrhea. The minimum radiation dose to induce ovarian failure decreases with advancing age, from 18.4 Gy at age 10 years to 6 Gy at age 40 years, due to biologically diminishing ovarian reserve and an increase in the radiosensitivity of oocytes.6 An online tool—using varying factors including age, chemotherapy dose, prior treatment, smoking, and baseline diminished ovarian reserve—is available to help predict the chance of ovarian failure following chemotherapy.7
Since 2006, the American Society of Clinical Oncology recommended, as part of the consent prior to therapy, oncologists should address the possibility of infertility with patients “as early in treatment planning as possible” and “...Fertility preservation is an important, if not necessary, consideration when planning cancer treatment in reproductive-age patients.”
Reference
1. Lee SJ, Schover LR, Partridge AH, et al. American Society of Clinical Oncology recommendations on fertility preservation in cancer patients. J Clin Oncol. 2006;24:2917-2931.
Cryopreservation to the rescue
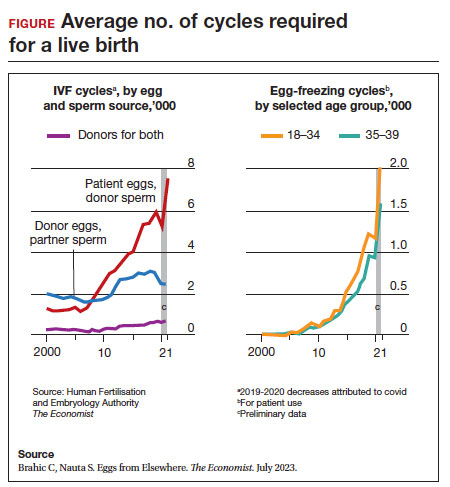
Since 2012, when ASRM removed the experimental designation on oocyte cryopreservation (OC), the number of cycles offered for fertility preservation has increased dramatically (FIGURE),8 initially being used for patients with cancer and now also including women desiring POC.
Ovarian and embryo cryopreservation. Ovarian stimulation and egg retrieval for OC can now occur within 2 weeks due to a random start protocol whereby women can begin ovarian stimulation any day in their cycle (ie, preovulation or postovulation).9
OC followed by thawing for subsequent fertilization and embryo transfer is employed as a matter of routine when patients with infertility utilize frozen eggs from a donor. While there remains debate over better live birth rates with frozen eggs versus fresh eggs, clinic experience may be a critical factor.10
Ovarian tissue cryopreservation. In addition to the fertility preservation procedures of oocytes and embryo cryopreservation, ovarian tissue cryopreservation became a standard option in 2019 when ASRM removed its experimental designation.11 Given the potential time constraints of urgent cancer treatment, ovarian tissue cryopreservation has the advantage of not requiring ovarian stimulation or sexual maturity and is able to be performed while patients are receiving chemotherapy. If successful, ovarian tissue cryopreservation followed by orthotopic transplantation has the potential to restore natural ovarian function and natural conceptions.12 However, despite first successfully being described in 2004, ovarian tissue cryopreservation, which does require subsequent thawing and tissue transplantation, remains less available to patients due to low usage rates, which have resulted in few clinics having adequate proficiency.13,14
Ovarian tissue cryopreservation involves obtaining ovarian cortical tissue, dissecting the tissue into small fragments, and cryopreserving it using either a slow-cool technique or vitrification. Orthotopic transplantation has been the most successful method for using ovarian tissue in humans. Live birth rates are modest.15 In all cancer survivors, particularly those with leukemia, autologous ovarian tissue transplantation may contain malignant cells that could lead to the reintroduction of cancer as the tissue is removed prior to treatment.16
Pregnancy outcomes using embryos created from ovaries recently exposed to chemotherapy in humans is not known, but animal studies suggest that there may be higher rates of miscarriage and birth defects given the severe DNA damage to oocytes of developing follicles.17 Hence, ovarian stimulation should be initiated and completed before the start of chemotherapy.
Continue to: Planned oocyte cryopreservation...
Planned oocyte cryopreservation
With advances in ART, POC offers patients the opportunity to preserve fertility until desired. However, despite its potential benefits, POC compels the discussion of various considerations in addition to oncofertility, such as ethical concerns and insurance coverage.
CASE 2 Woman plans for elective egg freezing
A 32-year-old single, professional woman is advancing in her career and wishes to delay childbearing. She is concerned about the potential for age-related fertility decline and wants to explore the option of elective egg freezing. Emily has no medical conditions that would impair her fertility, but she wants to ensure that she has the option of having biological children in the future. She is unsure about the potential financial burden of the procedure and whether her employer’s insurance covers such elective procedures.
How do you counsel her about her options?
Medical considerations
Approximately 25% of reproductive-aged women have considered POC.18 An analysis revealed POC was more cost-effective than delaying procreation and undergoing IVF with preimplantation genetic testing for aneuploidies at an advanced reproductive age.19
The process of planned oocyte cryopreservation. POC involves ovarian stimulation, usually with parenteral gonadotropins, to produce multiple mature oocytes for same-day cryopreservation following transvaginal retrieval, typically in an office-based surgery center as an outpatient procedure while the patient is under IV sedation. While the procedure has been proven effective, there are inherent risks and limitations. The success rates of subsequent fertility treatments using the cryopreserved eggs are influenced by the woman’s age at the time of freezing, the number of mature oocytes retrieved and vitrified, and the quality of the oocytes following thaw. A recent study reported a 70% live-birth rate in women aged less than 38 years who cryopreserved ≥ 20 mature eggs.20 To increase the number of cryopreserved oocytes, multiple egg retrievals or “batching” may be of benefit for women with diminished ovarian reserve.21
It is important for clinicians to thoroughly assess a patient’s medical history, ovarian reserve (by antral follicle count and levels of anti-müllerian hormone [AMH]), and reproductive goals before recommending proceeding with POC. Of note, AMH is a useful marker for ovarian reserve but has not been shown to predict natural fertility. Its value is in providing a guide to the dosage of ovarian stimulation and an estimation of the number of oocytes to be retrieved. Per ASRM, “Extremely low AMH values should not be used to refuse treatment in IVF.” AMH levels and antral follicle count have only a weak association with such qualitative outcomes as oocyte quality, clinical pregnancy rates, and live birth rates. Complications from egg retrieval, both short and long term, are rare. The inherent risk from POC is the lack of a guaranteed subsequent live birth.22
Ethical and social considerations
POC raises several ethical considerations, including concerns of perpetuating societal pressure on women to defer procreation to prioritize their careers over family planning.23 Despite controversies, POC appears as a chosen strategy against age-related infertility and may allow women to feel that they are more socially, psychologically, and financially stable before pursuing motherhood.24 Open and honest discussions between clinicians and patients are crucial to ensure informed decision making and address these ethical concerns.
Per an ACOG statement from February 2023 (https://www.acog.org/womens-health/faqs/having-a-baby-after-age-35-how-aging-affects-fertility-and-pregnancy) “...egg freezing is recommended mainly for patients having cancer treatment that will affect their future fertility. There is not enough research to recommend routine egg freezing for the sole purpose of delaying childbearing.”
A recent survey of patients who had elected egg freezing at some point included more than 80% who were aged 35 or older, and revealed that 93% of the survey participants had not yet returned to use their frozen oocytes.25 The most common reason cited in the survey for a delay in attempted procreation was lack of a partner. Another reason was undergoing oocyte cryopreservation after an optimal reproductive age, with participants concluding that they felt they had improved their reproductive future after undergoing oocyte cryopreservation and feeling empowered by the process. As part of counseling, women should be informed of the possibility of not utilizing their frozen eggs in the future, whether due to natural conception or other personal reasons.
Continue to: Employer insurance coverage...
Employer insurance coverage
Access to elective egg freezing is largely influenced by insurance coverage. Currently, employer-provided insurance coverage for this procedure varies widely. While some companies offer comprehensive coverage, others provide limited or no coverage at all. The cost of elective egg freezing can range from $10,000 to $15,000, excluding additional expenses such as medications and annual storage fees. The financial burden can create a gap between patients who desire POC and those with an ability to implement the process. The cost can be a significant barrier for many patients considering this option and perpetuates the lack of universal diversity, equity, and inclusion.
CASE 3 Gender dysphoria and fertility preservation
A 22-year-old transgender man is preparing to undergo gender-affirming hormone therapy and surgery. He is concerned about the potential impact of testosterone therapy on his oocytes and wishes to explore options for fertility preservation prior to oophorectomy.26
What are the patient’s options for fertility preservation?
The patient has the fertility preservation options of OC following ovarian stimulation or ovarian tissue cryopreservation at the time of oophorectomy. Preliminary evidence does not demonstrate impairment of ovarian stimulation and oocyte retrieval number with concurrent testosterone exposure. Ethical considerations, in this case, involve respecting the patient’s autonomy, addressing potential conflicts between gender-affirming care and fertility preservation (eg, a risk of dysphoria in transgender patients preserving biological gametes from a prior assigned gender), and ensuring access to fertility preservation services without discrimination. It is essential to provide the patient in this case with comprehensive information regarding the impact of hormone therapy on fertility, the available options, and the potential financial costs involved. Supportive counseling should also be offered to address any psychological or emotional aspects related to fertility preservation for all patients considering this option.
A call for diversity, equity, and inclusion
To improve access to POC, advocating for employer-offered insurance coverage is paramount. Women’s health providers can encourage dialogue between employers, insurers, and policymakers, which can lead to policy changes that prioritize coverage for fertilitypreservation options. This could include mandating coverage for POC as part of comprehensive health care plans or providing tax incentives to employers who offer coverage for these procedures. Furthermore, public awareness campaigns and advocacy efforts can help educate employers about the importance of including fertility preservation coverage in their employee benefits packages.
Conclusion
Just as physicians must recognize their responsibility to patients to distinguish unproven yet promising science from evidence-based and clinically established science, so too must they advise their patients to consider fertility preservation services in a way that is both clinically justified and ethically appropriate. Informed decisions must be made by appropriate counseling of evidence-based medicine to protect the interest of patients. POC provides patients with an opportunity to preserve their fertility and exercise reproductive autonomy. However, access to this procedure is often hindered by limited or nonexistent employer insurance coverage. By recognizing the medical, ethical, and social implications of POC and implementing strategies to improve coverage, collaborative efforts may increase accessibility and defray costs to provide patients with the option of deferring childbearing and preserving their reproductive potential. ●
1. Promptly offer fertility preservation treatment options with sensitivity and clarity.
2. Dedicate ample time and exercise patience during the consultation.
3. Provide education using multiple modalities to help patients assimilate information.
4. Encourage consultation with mental health professionals.
Special considerations for hematologic malignancies:
- Treatment can be associated with significant gonadal toxicity and premature ovarian failure.
- Patients are frequently ill at the time of presentation and ineligible for certain fertility preservation options.
References
1. Ethics Committee of the American Society for Reproductive Medicine. Fertility preservation and reproduction in patients facing gonadotoxic therapies: a committee opinion. Fertil Steril. 2018;110:380-386. doi:10.1016/j.fertnstert.2018.06.012
2. Kim SS, Klemp J, Fabian C. Breast cancer and fertility preservation. Fertil Steril. 2011;95:15351543. doi: 10.1016/j.fertnstert.2011.01.003
- American Cancer Society. Cancer Treatment & Survivorship Facts & Figures 2022-2024. Atlanta, Georgia: American Cancer Society; 2022.
- Oktay K, Karlikaya G. Ovarian function after autologous transplantation of frozen-banked human ovarian tissue. N Engl J Med. 2000;342:1919
- Practice Committees of the American Society for Reproductive Medicine and the Society for Assisted Reproductive Technology. Mature oocyte cryopreservation: a guideline. Fertil Steril. 2013;99:37-43. doi: 10.1016 /j.fertnstert.2012.09.028
- Marklund A, Lekberg T, Hedayati E, et al. Relapse rates and diseasespecific mortality following procedures for fertility preservation at time of breast cancer diagnosis. JAMA Oncol. 2022;8:1438-1446. doi:10.1001 /jamaoncol.2022.3677
- Zhao J, Liu J, Chen K, et al. What lies behind chemotherapy-induced amenorrhea for breast cancer patients: a meta-analysis. Breast Cancer Res Treat. 2014;145:113-128. https://doi.org/10.1007/s10549-014-2914-x
- Wallace WH, Thomson AB, Saran F, et al. Predicting age of ovarian failure after radiation to a field that includes the ovaries. Int J Radiat Oncol Biol Phys. 2005;62:738-744. http://doi.org10.1016/j.ijrobp.2004.11.038
- Chung EH, Acharya CR, Harris BS, et al. Development of a fertility risk calculator to predict individualized chance of hovarian failure after chemotherapy. J Assist Reprod Genetics. 2021;38:3047-3055. https://doi .org/10.1007/s10815-021-02311-0
- Brahic C, Nauta S. Eggs From Elsewhere. The Economist. July 2023.
- Cakmak H, Rosen MP. Random-start ovarian stimulation in patients with cancer. Curr Opin Obstet Gynecol. 2015;27:215-221. doi: 10.1097/ GCO.0000000000000180
- Eaton JL, Truong T, Li YJ, et al. Prevalence of a good perinatal outcome with cryopreserved compared with fresh donor oocytes. Obstet Gynecol. 2020;135:709-716. doi: 10.1097/AOG.0000000000003695
- Practice Committee of the American Society for Reproductive Medicine. Fertility preservation in patients undergoing gonadotoxic therapy or gonadectomy: a committee opinion. Fertil Steril. 2019;112:1022-1033. doi: 10.1016/j.fertnstert.2019.09.013
- Oktay K, Marin L, Bedoschi G, et al. Ovarian transplantation with robotic surgery and a neovascularizing human extracellular matrix scaffold: a case series in comparison to meta-analytic data. Fertil Steril. 2021. doi:https ://doi.org/10.1016/j.fertnstert.2021.08.034
- Donnez J, Dolmans MM, Demylle D, et al. Livebirth after orthotopic transplantation of cryopreserved ovarian tissue. Lancet. 2004;364:1405-1410.
- Hoekman EJ, Louwe LA, Rooijers M, et al. Ovarian tissue cryopreservation: low usage rates and high live-birth rate after transplantation. Acta Obstet Gynecol Scand. 2020;99:213-221. doi: 10.1111/aogs.13735
- Donnez J, Dolmans MM, Diaz C, et al. Ovarian cortex transplantation: time to move on from experimental studies to open clinical application. Fertil Steril. 2015;104:1097-1098. doi: 10.1016/j.fertnstert.2015.08.005
- Rosendahl M, Greve T, Andersen CY. The safety of transplanting cryopreserved ovarian tissue in cancer patients: a review of the literature. J Assist Reprod Genet. 2013;30, 11-24. https://doi.org/10.1007/s10815-012-9912-x
- Soleimani R, Heytens E, Darzynkiewicz Z, et al. Mechanisms of chemotherapyinduced human ovarian aging: double strand DNA breaks and microvascular compromise. Aging (Albany NY). 2011;3:782-793.
- Milman LW, Senapati S, Sammel MD, et al. Assessing reproductive choices of women and the likelihood of oocyte cryopreservation in the era of elective oocyte freezing. Fertil Steril. 2017;107:1214-1222.e3. doi: 10.1016 /j.fertnstert.2017.03.010
- Bakkensen JB, Flannagan KSJ, Mumford SL, et al. A SART data cost-effectiveness analysis of planned oocyte cryopreservation versus in vitro fertilization with preimplantation genetic testing for aneuploidy considering ideal family size. Fertil Steril. 2022;118:875-884. https://doi.org/10.1016/j.fertnstert.2022.07.022
- Cascante SD, Blakemore JK, DeVore S. Fifteen years of autologous oocyte thaw outcomes from a large university-based fertility center. Fertil Steril. 2022;118:158-166. doi: 10.1016/j.fertnstert.2022.04.013
- Cobo A, Garrido N, Crespo J, et al. Accumulation of oocytes: a new strategy for managing low-responder patients. Reprod BioMedicine Online. 2018;37:669675. doi:10.1016/j.rbmo.2018.07.004
- Practice Committee of the American Society for Reproductive Medicine. Testing and interpreting measures of ovarian reserve: a committee opinion. Fertil Steril. 2020;114:1151-1157. doi: 10.1016/j.fertnstert.2020.09
- What you need to know about egg-freezing, the hot new perk at Google, Apple, and Facebook. Business Insider. September 17, 2017. Accessed August 9, 2023. https://www.businessinsider.com/egg-freezing-at-facebook-apple -google-hot-new-perk-2017-9
- Varlas VN, Bors RG, Albu D, et al. Social freezing: pressing pause on fertility. Int J Environ Res Public Health. 2021;18:8088. doi: 10.3390/ijerph18158088
- Hodes-Wertz B, Druckenmiller S, Smith M, et al. What do reproductive-age women who undergo oocyte cryopreservation think about the process as a means to preserve fertility? Fertil Steril. 2013;100:1343-1349. doi: 10.1016 /j.fertnstert.2013.07.201
- Moravek MB, Dixon M, Pena SM, et al. Management of testosterone around ovarian stimulation in transmasculine patients: challenging common practices to meet patient needs-2 case reports. Hum Reprod. 2023;38:482-488. doi: 10.1093/humrep/dead003
Throughout the 20th century, the management of ectopic pregnancy evolved from preserving the life of the mother to preserving fertility by utilizing the conservative treatment of methotrexate and/or tubal surgery. I make this, seemingly obscure, reference to managing ectopic pregnancy to consider an analogous shift over time in the management of patients with cancer. Over the next decade, the number of people who have lived 5 or more years after their cancer diagnosis is projected to increase approximately 30%, to 16.3 million. Due to the improved survival rates following a cancer diagnosis,1 revolutionary developments have been made in fertility preservation to obviate the impact of gonadotoxic therapy. We have evolved, however, from shielding and transposing ovaries to ovarian tissue cryopreservation,2 with rapid implementation.
While advances in reproductive cryopreservation have allowed for the delay, or even potential “prevention” of infertility, assisted reproductive technology (ART) cannot yet claim a “cure” in ensuring procreation. Nevertheless, fertility preservation is a burgeoning field that has transitioned from an experimental label to a standard of care in 2012, as designated by the American Society for Reproductive Medicine (ASRM).3 From the original intention of offering oocyte cryopreservation to women at risk of ovarian failure from impending gonadotoxic cancer treatment, fertility preservation has accelerated to include freezing for nonmedical reasons—eg, planned oocyte cryopreservation (POC), or “social” egg freezing, to ovarian tissue cryopreservation to accommodate the expediency needed for the treatment of certain cancer treatments. Additionally, across the United States, the number of donor egg banks, which allow women an easily accessible option, is rivaling enduring sperm banks. Due to the advanced methodology of vitrification and growing demand for the technology due to increasing IVF cycles, cryopreservation has become a specialized area of reproductive medicine, and a target of venture capital and private equity commercialization. This article will review the latest techniques, appropriate counseling, and cost/benefit ratio of fertility preservation, with an emphasis on POC.
CASE 1 Fertility preservation options for patient with breast cancer
A 37-year-old woman with newly diagnosed hormone receptor−positive breast cancer is referred for a fertility preservation consultation prior to initiating treatment. Her oncologist plans chemotherapy, followed by radiation and a minimum of 5 years of tamoxifen therapy.
What is the best consultation approach for this patient?
Consultation involves understanding several factors
The consultation approach to this patient involves ascertaining her medical, social, and family history, along with her reproductive plans.
Medical history. For the medical component, we must focus on her diagnosis, anticipated treatment with timeline, risks of gonadal toxicity with planned treatments, her current medical stability, and prognosis for expected survival.
Social history. Her age, relationship status, and desired family size address her social history.
Family history. Given that her cancer affects the breast, there is the risk of genetic susceptibility and potential for embryo testing for the BRCA gene.
Reproductive plans. These include her and her partner’s, if applicable, number of desired children and their risk factors for infertility.
Regarding the reproductive timeline, the antihormonal therapy that may be required for her treatment may improve overall survival, but it would delay the time to pregnancy. Consequently, the pursuit of fertility preservation prior to cancer treatment is a multidisciplinary approach that can involve medical oncology, radiation oncology, REI, medical genetics, and often, psychology. Fortunately, evidence continues to support fertility preservation, with or without hormonal ovarian stimulation, for patients with breast cancer. Data, with up to 5 years of follow-up, has indicated that it is safe.4
Continue to: Oncofertility...
Oncofertility
To address the need to maximize the reproductive potential of patients with newly diagnosed cancer, the field of oncofertility combines the specialties of oncology and reproductive medicine. The reproductive risk of cancer treatment is gonadotoxicity, with subsequent iatrogenic primary ovarian insufficiency (POI) and infertility. Alkylating agents (including cyclosphosphamide) have the highest risk for amenorrhea, while antimetabolites (including methotrexate, 5–fluorouracil) have the lowest risk.5 Treating bone marrow/stem cell transplantation using high-dose alkylating agents, with or without whole body irradiation, results in ≥80% amenorrhea. The minimum radiation dose to induce ovarian failure decreases with advancing age, from 18.4 Gy at age 10 years to 6 Gy at age 40 years, due to biologically diminishing ovarian reserve and an increase in the radiosensitivity of oocytes.6 An online tool—using varying factors including age, chemotherapy dose, prior treatment, smoking, and baseline diminished ovarian reserve—is available to help predict the chance of ovarian failure following chemotherapy.7
Since 2006, the American Society of Clinical Oncology recommended, as part of the consent prior to therapy, oncologists should address the possibility of infertility with patients “as early in treatment planning as possible” and “...Fertility preservation is an important, if not necessary, consideration when planning cancer treatment in reproductive-age patients.”
Reference
1. Lee SJ, Schover LR, Partridge AH, et al. American Society of Clinical Oncology recommendations on fertility preservation in cancer patients. J Clin Oncol. 2006;24:2917-2931.
Cryopreservation to the rescue
Since 2012, when ASRM removed the experimental designation on oocyte cryopreservation (OC), the number of cycles offered for fertility preservation has increased dramatically (FIGURE),8 initially being used for patients with cancer and now also including women desiring POC.

Ovarian and embryo cryopreservation. Ovarian stimulation and egg retrieval for OC can now occur within 2 weeks due to a random start protocol whereby women can begin ovarian stimulation any day in their cycle (ie, preovulation or postovulation).9
OC followed by thawing for subsequent fertilization and embryo transfer is employed as a matter of routine when patients with infertility utilize frozen eggs from a donor. While there remains debate over better live birth rates with frozen eggs versus fresh eggs, clinic experience may be a critical factor.10
Ovarian tissue cryopreservation. In addition to the fertility preservation procedures of oocytes and embryo cryopreservation, ovarian tissue cryopreservation became a standard option in 2019 when ASRM removed its experimental designation.11 Given the potential time constraints of urgent cancer treatment, ovarian tissue cryopreservation has the advantage of not requiring ovarian stimulation or sexual maturity and is able to be performed while patients are receiving chemotherapy. If successful, ovarian tissue cryopreservation followed by orthotopic transplantation has the potential to restore natural ovarian function and natural conceptions.12 However, despite first successfully being described in 2004, ovarian tissue cryopreservation, which does require subsequent thawing and tissue transplantation, remains less available to patients due to low usage rates, which have resulted in few clinics having adequate proficiency.13,14
Ovarian tissue cryopreservation involves obtaining ovarian cortical tissue, dissecting the tissue into small fragments, and cryopreserving it using either a slow-cool technique or vitrification. Orthotopic transplantation has been the most successful method for using ovarian tissue in humans. Live birth rates are modest.15 In all cancer survivors, particularly those with leukemia, autologous ovarian tissue transplantation may contain malignant cells that could lead to the reintroduction of cancer as the tissue is removed prior to treatment.16
Pregnancy outcomes using embryos created from ovaries recently exposed to chemotherapy in humans is not known, but animal studies suggest that there may be higher rates of miscarriage and birth defects given the severe DNA damage to oocytes of developing follicles.17 Hence, ovarian stimulation should be initiated and completed before the start of chemotherapy.
Continue to: Planned oocyte cryopreservation...
Planned oocyte cryopreservation
With advances in ART, POC offers patients the opportunity to preserve fertility until desired. However, despite its potential benefits, POC compels the discussion of various considerations in addition to oncofertility, such as ethical concerns and insurance coverage.
CASE 2 Woman plans for elective egg freezing
A 32-year-old single, professional woman is advancing in her career and wishes to delay childbearing. She is concerned about the potential for age-related fertility decline and wants to explore the option of elective egg freezing. Emily has no medical conditions that would impair her fertility, but she wants to ensure that she has the option of having biological children in the future. She is unsure about the potential financial burden of the procedure and whether her employer’s insurance covers such elective procedures.
How do you counsel her about her options?
Medical considerations
Approximately 25% of reproductive-aged women have considered POC.18 An analysis revealed POC was more cost-effective than delaying procreation and undergoing IVF with preimplantation genetic testing for aneuploidies at an advanced reproductive age.19
The process of planned oocyte cryopreservation. POC involves ovarian stimulation, usually with parenteral gonadotropins, to produce multiple mature oocytes for same-day cryopreservation following transvaginal retrieval, typically in an office-based surgery center as an outpatient procedure while the patient is under IV sedation. While the procedure has been proven effective, there are inherent risks and limitations. The success rates of subsequent fertility treatments using the cryopreserved eggs are influenced by the woman’s age at the time of freezing, the number of mature oocytes retrieved and vitrified, and the quality of the oocytes following thaw. A recent study reported a 70% live-birth rate in women aged less than 38 years who cryopreserved ≥ 20 mature eggs.20 To increase the number of cryopreserved oocytes, multiple egg retrievals or “batching” may be of benefit for women with diminished ovarian reserve.21
It is important for clinicians to thoroughly assess a patient’s medical history, ovarian reserve (by antral follicle count and levels of anti-müllerian hormone [AMH]), and reproductive goals before recommending proceeding with POC. Of note, AMH is a useful marker for ovarian reserve but has not been shown to predict natural fertility. Its value is in providing a guide to the dosage of ovarian stimulation and an estimation of the number of oocytes to be retrieved. Per ASRM, “Extremely low AMH values should not be used to refuse treatment in IVF.” AMH levels and antral follicle count have only a weak association with such qualitative outcomes as oocyte quality, clinical pregnancy rates, and live birth rates. Complications from egg retrieval, both short and long term, are rare. The inherent risk from POC is the lack of a guaranteed subsequent live birth.22
Ethical and social considerations
POC raises several ethical considerations, including concerns of perpetuating societal pressure on women to defer procreation to prioritize their careers over family planning.23 Despite controversies, POC appears as a chosen strategy against age-related infertility and may allow women to feel that they are more socially, psychologically, and financially stable before pursuing motherhood.24 Open and honest discussions between clinicians and patients are crucial to ensure informed decision making and address these ethical concerns.
Per an ACOG statement from February 2023 (https://www.acog.org/womens-health/faqs/having-a-baby-after-age-35-how-aging-affects-fertility-and-pregnancy) “...egg freezing is recommended mainly for patients having cancer treatment that will affect their future fertility. There is not enough research to recommend routine egg freezing for the sole purpose of delaying childbearing.”
A recent survey of patients who had elected egg freezing at some point included more than 80% who were aged 35 or older, and revealed that 93% of the survey participants had not yet returned to use their frozen oocytes.25 The most common reason cited in the survey for a delay in attempted procreation was lack of a partner. Another reason was undergoing oocyte cryopreservation after an optimal reproductive age, with participants concluding that they felt they had improved their reproductive future after undergoing oocyte cryopreservation and feeling empowered by the process. As part of counseling, women should be informed of the possibility of not utilizing their frozen eggs in the future, whether due to natural conception or other personal reasons.
Continue to: Employer insurance coverage...
Employer insurance coverage
Access to elective egg freezing is largely influenced by insurance coverage. Currently, employer-provided insurance coverage for this procedure varies widely. While some companies offer comprehensive coverage, others provide limited or no coverage at all. The cost of elective egg freezing can range from $10,000 to $15,000, excluding additional expenses such as medications and annual storage fees. The financial burden can create a gap between patients who desire POC and those with an ability to implement the process. The cost can be a significant barrier for many patients considering this option and perpetuates the lack of universal diversity, equity, and inclusion.
CASE 3 Gender dysphoria and fertility preservation
A 22-year-old transgender man is preparing to undergo gender-affirming hormone therapy and surgery. He is concerned about the potential impact of testosterone therapy on his oocytes and wishes to explore options for fertility preservation prior to oophorectomy.26
What are the patient’s options for fertility preservation?
The patient has the fertility preservation options of OC following ovarian stimulation or ovarian tissue cryopreservation at the time of oophorectomy. Preliminary evidence does not demonstrate impairment of ovarian stimulation and oocyte retrieval number with concurrent testosterone exposure. Ethical considerations, in this case, involve respecting the patient’s autonomy, addressing potential conflicts between gender-affirming care and fertility preservation (eg, a risk of dysphoria in transgender patients preserving biological gametes from a prior assigned gender), and ensuring access to fertility preservation services without discrimination. It is essential to provide the patient in this case with comprehensive information regarding the impact of hormone therapy on fertility, the available options, and the potential financial costs involved. Supportive counseling should also be offered to address any psychological or emotional aspects related to fertility preservation for all patients considering this option.
A call for diversity, equity, and inclusion
To improve access to POC, advocating for employer-offered insurance coverage is paramount. Women’s health providers can encourage dialogue between employers, insurers, and policymakers, which can lead to policy changes that prioritize coverage for fertilitypreservation options. This could include mandating coverage for POC as part of comprehensive health care plans or providing tax incentives to employers who offer coverage for these procedures. Furthermore, public awareness campaigns and advocacy efforts can help educate employers about the importance of including fertility preservation coverage in their employee benefits packages.
Conclusion
Just as physicians must recognize their responsibility to patients to distinguish unproven yet promising science from evidence-based and clinically established science, so too must they advise their patients to consider fertility preservation services in a way that is both clinically justified and ethically appropriate. Informed decisions must be made by appropriate counseling of evidence-based medicine to protect the interest of patients. POC provides patients with an opportunity to preserve their fertility and exercise reproductive autonomy. However, access to this procedure is often hindered by limited or nonexistent employer insurance coverage. By recognizing the medical, ethical, and social implications of POC and implementing strategies to improve coverage, collaborative efforts may increase accessibility and defray costs to provide patients with the option of deferring childbearing and preserving their reproductive potential. ●
1. Promptly offer fertility preservation treatment options with sensitivity and clarity.
2. Dedicate ample time and exercise patience during the consultation.
3. Provide education using multiple modalities to help patients assimilate information.
4. Encourage consultation with mental health professionals.
Special considerations for hematologic malignancies:
- Treatment can be associated with significant gonadal toxicity and premature ovarian failure.
- Patients are frequently ill at the time of presentation and ineligible for certain fertility preservation options.
References
1. Ethics Committee of the American Society for Reproductive Medicine. Fertility preservation and reproduction in patients facing gonadotoxic therapies: a committee opinion. Fertil Steril. 2018;110:380-386. doi:10.1016/j.fertnstert.2018.06.012
2. Kim SS, Klemp J, Fabian C. Breast cancer and fertility preservation. Fertil Steril. 2011;95:15351543. doi: 10.1016/j.fertnstert.2011.01.003
Throughout the 20th century, the management of ectopic pregnancy evolved from preserving the life of the mother to preserving fertility by utilizing the conservative treatment of methotrexate and/or tubal surgery. I make this, seemingly obscure, reference to managing ectopic pregnancy to consider an analogous shift over time in the management of patients with cancer. Over the next decade, the number of people who have lived 5 or more years after their cancer diagnosis is projected to increase approximately 30%, to 16.3 million. Due to the improved survival rates following a cancer diagnosis,1 revolutionary developments have been made in fertility preservation to obviate the impact of gonadotoxic therapy. We have evolved, however, from shielding and transposing ovaries to ovarian tissue cryopreservation,2 with rapid implementation.
While advances in reproductive cryopreservation have allowed for the delay, or even potential “prevention” of infertility, assisted reproductive technology (ART) cannot yet claim a “cure” in ensuring procreation. Nevertheless, fertility preservation is a burgeoning field that has transitioned from an experimental label to a standard of care in 2012, as designated by the American Society for Reproductive Medicine (ASRM).3 From the original intention of offering oocyte cryopreservation to women at risk of ovarian failure from impending gonadotoxic cancer treatment, fertility preservation has accelerated to include freezing for nonmedical reasons—eg, planned oocyte cryopreservation (POC), or “social” egg freezing, to ovarian tissue cryopreservation to accommodate the expediency needed for the treatment of certain cancer treatments. Additionally, across the United States, the number of donor egg banks, which allow women an easily accessible option, is rivaling enduring sperm banks. Due to the advanced methodology of vitrification and growing demand for the technology due to increasing IVF cycles, cryopreservation has become a specialized area of reproductive medicine, and a target of venture capital and private equity commercialization. This article will review the latest techniques, appropriate counseling, and cost/benefit ratio of fertility preservation, with an emphasis on POC.
CASE 1 Fertility preservation options for patient with breast cancer
A 37-year-old woman with newly diagnosed hormone receptor−positive breast cancer is referred for a fertility preservation consultation prior to initiating treatment. Her oncologist plans chemotherapy, followed by radiation and a minimum of 5 years of tamoxifen therapy.
What is the best consultation approach for this patient?
Consultation involves understanding several factors
The consultation approach to this patient involves ascertaining her medical, social, and family history, along with her reproductive plans.
Medical history. For the medical component, we must focus on her diagnosis, anticipated treatment with timeline, risks of gonadal toxicity with planned treatments, her current medical stability, and prognosis for expected survival.
Social history. Her age, relationship status, and desired family size address her social history.
Family history. Given that her cancer affects the breast, there is the risk of genetic susceptibility and potential for embryo testing for the BRCA gene.
Reproductive plans. These include her and her partner’s, if applicable, number of desired children and their risk factors for infertility.
Regarding the reproductive timeline, the antihormonal therapy that may be required for her treatment may improve overall survival, but it would delay the time to pregnancy. Consequently, the pursuit of fertility preservation prior to cancer treatment is a multidisciplinary approach that can involve medical oncology, radiation oncology, REI, medical genetics, and often, psychology. Fortunately, evidence continues to support fertility preservation, with or without hormonal ovarian stimulation, for patients with breast cancer. Data, with up to 5 years of follow-up, has indicated that it is safe.4
Continue to: Oncofertility...
Oncofertility
To address the need to maximize the reproductive potential of patients with newly diagnosed cancer, the field of oncofertility combines the specialties of oncology and reproductive medicine. The reproductive risk of cancer treatment is gonadotoxicity, with subsequent iatrogenic primary ovarian insufficiency (POI) and infertility. Alkylating agents (including cyclosphosphamide) have the highest risk for amenorrhea, while antimetabolites (including methotrexate, 5–fluorouracil) have the lowest risk.5 Treating bone marrow/stem cell transplantation using high-dose alkylating agents, with or without whole body irradiation, results in ≥80% amenorrhea. The minimum radiation dose to induce ovarian failure decreases with advancing age, from 18.4 Gy at age 10 years to 6 Gy at age 40 years, due to biologically diminishing ovarian reserve and an increase in the radiosensitivity of oocytes.6 An online tool—using varying factors including age, chemotherapy dose, prior treatment, smoking, and baseline diminished ovarian reserve—is available to help predict the chance of ovarian failure following chemotherapy.7
Since 2006, the American Society of Clinical Oncology recommended, as part of the consent prior to therapy, oncologists should address the possibility of infertility with patients “as early in treatment planning as possible” and “...Fertility preservation is an important, if not necessary, consideration when planning cancer treatment in reproductive-age patients.”
Reference
1. Lee SJ, Schover LR, Partridge AH, et al. American Society of Clinical Oncology recommendations on fertility preservation in cancer patients. J Clin Oncol. 2006;24:2917-2931.
Cryopreservation to the rescue
Since 2012, when ASRM removed the experimental designation on oocyte cryopreservation (OC), the number of cycles offered for fertility preservation has increased dramatically (FIGURE),8 initially being used for patients with cancer and now also including women desiring POC.

Ovarian and embryo cryopreservation. Ovarian stimulation and egg retrieval for OC can now occur within 2 weeks due to a random start protocol whereby women can begin ovarian stimulation any day in their cycle (ie, preovulation or postovulation).9
OC followed by thawing for subsequent fertilization and embryo transfer is employed as a matter of routine when patients with infertility utilize frozen eggs from a donor. While there remains debate over better live birth rates with frozen eggs versus fresh eggs, clinic experience may be a critical factor.10
Ovarian tissue cryopreservation. In addition to the fertility preservation procedures of oocytes and embryo cryopreservation, ovarian tissue cryopreservation became a standard option in 2019 when ASRM removed its experimental designation.11 Given the potential time constraints of urgent cancer treatment, ovarian tissue cryopreservation has the advantage of not requiring ovarian stimulation or sexual maturity and is able to be performed while patients are receiving chemotherapy. If successful, ovarian tissue cryopreservation followed by orthotopic transplantation has the potential to restore natural ovarian function and natural conceptions.12 However, despite first successfully being described in 2004, ovarian tissue cryopreservation, which does require subsequent thawing and tissue transplantation, remains less available to patients due to low usage rates, which have resulted in few clinics having adequate proficiency.13,14
Ovarian tissue cryopreservation involves obtaining ovarian cortical tissue, dissecting the tissue into small fragments, and cryopreserving it using either a slow-cool technique or vitrification. Orthotopic transplantation has been the most successful method for using ovarian tissue in humans. Live birth rates are modest.15 In all cancer survivors, particularly those with leukemia, autologous ovarian tissue transplantation may contain malignant cells that could lead to the reintroduction of cancer as the tissue is removed prior to treatment.16
Pregnancy outcomes using embryos created from ovaries recently exposed to chemotherapy in humans is not known, but animal studies suggest that there may be higher rates of miscarriage and birth defects given the severe DNA damage to oocytes of developing follicles.17 Hence, ovarian stimulation should be initiated and completed before the start of chemotherapy.
Continue to: Planned oocyte cryopreservation...
Planned oocyte cryopreservation
With advances in ART, POC offers patients the opportunity to preserve fertility until desired. However, despite its potential benefits, POC compels the discussion of various considerations in addition to oncofertility, such as ethical concerns and insurance coverage.
CASE 2 Woman plans for elective egg freezing
A 32-year-old single, professional woman is advancing in her career and wishes to delay childbearing. She is concerned about the potential for age-related fertility decline and wants to explore the option of elective egg freezing. Emily has no medical conditions that would impair her fertility, but she wants to ensure that she has the option of having biological children in the future. She is unsure about the potential financial burden of the procedure and whether her employer’s insurance covers such elective procedures.
How do you counsel her about her options?
Medical considerations
Approximately 25% of reproductive-aged women have considered POC.18 An analysis revealed POC was more cost-effective than delaying procreation and undergoing IVF with preimplantation genetic testing for aneuploidies at an advanced reproductive age.19
The process of planned oocyte cryopreservation. POC involves ovarian stimulation, usually with parenteral gonadotropins, to produce multiple mature oocytes for same-day cryopreservation following transvaginal retrieval, typically in an office-based surgery center as an outpatient procedure while the patient is under IV sedation. While the procedure has been proven effective, there are inherent risks and limitations. The success rates of subsequent fertility treatments using the cryopreserved eggs are influenced by the woman’s age at the time of freezing, the number of mature oocytes retrieved and vitrified, and the quality of the oocytes following thaw. A recent study reported a 70% live-birth rate in women aged less than 38 years who cryopreserved ≥ 20 mature eggs.20 To increase the number of cryopreserved oocytes, multiple egg retrievals or “batching” may be of benefit for women with diminished ovarian reserve.21
It is important for clinicians to thoroughly assess a patient’s medical history, ovarian reserve (by antral follicle count and levels of anti-müllerian hormone [AMH]), and reproductive goals before recommending proceeding with POC. Of note, AMH is a useful marker for ovarian reserve but has not been shown to predict natural fertility. Its value is in providing a guide to the dosage of ovarian stimulation and an estimation of the number of oocytes to be retrieved. Per ASRM, “Extremely low AMH values should not be used to refuse treatment in IVF.” AMH levels and antral follicle count have only a weak association with such qualitative outcomes as oocyte quality, clinical pregnancy rates, and live birth rates. Complications from egg retrieval, both short and long term, are rare. The inherent risk from POC is the lack of a guaranteed subsequent live birth.22
Ethical and social considerations
POC raises several ethical considerations, including concerns of perpetuating societal pressure on women to defer procreation to prioritize their careers over family planning.23 Despite controversies, POC appears as a chosen strategy against age-related infertility and may allow women to feel that they are more socially, psychologically, and financially stable before pursuing motherhood.24 Open and honest discussions between clinicians and patients are crucial to ensure informed decision making and address these ethical concerns.
Per an ACOG statement from February 2023 (https://www.acog.org/womens-health/faqs/having-a-baby-after-age-35-how-aging-affects-fertility-and-pregnancy) “...egg freezing is recommended mainly for patients having cancer treatment that will affect their future fertility. There is not enough research to recommend routine egg freezing for the sole purpose of delaying childbearing.”
A recent survey of patients who had elected egg freezing at some point included more than 80% who were aged 35 or older, and revealed that 93% of the survey participants had not yet returned to use their frozen oocytes.25 The most common reason cited in the survey for a delay in attempted procreation was lack of a partner. Another reason was undergoing oocyte cryopreservation after an optimal reproductive age, with participants concluding that they felt they had improved their reproductive future after undergoing oocyte cryopreservation and feeling empowered by the process. As part of counseling, women should be informed of the possibility of not utilizing their frozen eggs in the future, whether due to natural conception or other personal reasons.
Continue to: Employer insurance coverage...
Employer insurance coverage
Access to elective egg freezing is largely influenced by insurance coverage. Currently, employer-provided insurance coverage for this procedure varies widely. While some companies offer comprehensive coverage, others provide limited or no coverage at all. The cost of elective egg freezing can range from $10,000 to $15,000, excluding additional expenses such as medications and annual storage fees. The financial burden can create a gap between patients who desire POC and those with an ability to implement the process. The cost can be a significant barrier for many patients considering this option and perpetuates the lack of universal diversity, equity, and inclusion.
CASE 3 Gender dysphoria and fertility preservation
A 22-year-old transgender man is preparing to undergo gender-affirming hormone therapy and surgery. He is concerned about the potential impact of testosterone therapy on his oocytes and wishes to explore options for fertility preservation prior to oophorectomy.26
What are the patient’s options for fertility preservation?
The patient has the fertility preservation options of OC following ovarian stimulation or ovarian tissue cryopreservation at the time of oophorectomy. Preliminary evidence does not demonstrate impairment of ovarian stimulation and oocyte retrieval number with concurrent testosterone exposure. Ethical considerations, in this case, involve respecting the patient’s autonomy, addressing potential conflicts between gender-affirming care and fertility preservation (eg, a risk of dysphoria in transgender patients preserving biological gametes from a prior assigned gender), and ensuring access to fertility preservation services without discrimination. It is essential to provide the patient in this case with comprehensive information regarding the impact of hormone therapy on fertility, the available options, and the potential financial costs involved. Supportive counseling should also be offered to address any psychological or emotional aspects related to fertility preservation for all patients considering this option.
A call for diversity, equity, and inclusion
To improve access to POC, advocating for employer-offered insurance coverage is paramount. Women’s health providers can encourage dialogue between employers, insurers, and policymakers, which can lead to policy changes that prioritize coverage for fertilitypreservation options. This could include mandating coverage for POC as part of comprehensive health care plans or providing tax incentives to employers who offer coverage for these procedures. Furthermore, public awareness campaigns and advocacy efforts can help educate employers about the importance of including fertility preservation coverage in their employee benefits packages.
Conclusion
Just as physicians must recognize their responsibility to patients to distinguish unproven yet promising science from evidence-based and clinically established science, so too must they advise their patients to consider fertility preservation services in a way that is both clinically justified and ethically appropriate. Informed decisions must be made by appropriate counseling of evidence-based medicine to protect the interest of patients. POC provides patients with an opportunity to preserve their fertility and exercise reproductive autonomy. However, access to this procedure is often hindered by limited or nonexistent employer insurance coverage. By recognizing the medical, ethical, and social implications of POC and implementing strategies to improve coverage, collaborative efforts may increase accessibility and defray costs to provide patients with the option of deferring childbearing and preserving their reproductive potential. ●
1. Promptly offer fertility preservation treatment options with sensitivity and clarity.
2. Dedicate ample time and exercise patience during the consultation.
3. Provide education using multiple modalities to help patients assimilate information.
4. Encourage consultation with mental health professionals.
Special considerations for hematologic malignancies:
- Treatment can be associated with significant gonadal toxicity and premature ovarian failure.
- Patients are frequently ill at the time of presentation and ineligible for certain fertility preservation options.
References
1. Ethics Committee of the American Society for Reproductive Medicine. Fertility preservation and reproduction in patients facing gonadotoxic therapies: a committee opinion. Fertil Steril. 2018;110:380-386. doi:10.1016/j.fertnstert.2018.06.012
2. Kim SS, Klemp J, Fabian C. Breast cancer and fertility preservation. Fertil Steril. 2011;95:15351543. doi: 10.1016/j.fertnstert.2011.01.003
- American Cancer Society. Cancer Treatment & Survivorship Facts & Figures 2022-2024. Atlanta, Georgia: American Cancer Society; 2022.
- Oktay K, Karlikaya G. Ovarian function after autologous transplantation of frozen-banked human ovarian tissue. N Engl J Med. 2000;342:1919
- Practice Committees of the American Society for Reproductive Medicine and the Society for Assisted Reproductive Technology. Mature oocyte cryopreservation: a guideline. Fertil Steril. 2013;99:37-43. doi: 10.1016 /j.fertnstert.2012.09.028
- Marklund A, Lekberg T, Hedayati E, et al. Relapse rates and diseasespecific mortality following procedures for fertility preservation at time of breast cancer diagnosis. JAMA Oncol. 2022;8:1438-1446. doi:10.1001 /jamaoncol.2022.3677
- Zhao J, Liu J, Chen K, et al. What lies behind chemotherapy-induced amenorrhea for breast cancer patients: a meta-analysis. Breast Cancer Res Treat. 2014;145:113-128. https://doi.org/10.1007/s10549-014-2914-x
- Wallace WH, Thomson AB, Saran F, et al. Predicting age of ovarian failure after radiation to a field that includes the ovaries. Int J Radiat Oncol Biol Phys. 2005;62:738-744. http://doi.org10.1016/j.ijrobp.2004.11.038
- Chung EH, Acharya CR, Harris BS, et al. Development of a fertility risk calculator to predict individualized chance of hovarian failure after chemotherapy. J Assist Reprod Genetics. 2021;38:3047-3055. https://doi .org/10.1007/s10815-021-02311-0
- Brahic C, Nauta S. Eggs From Elsewhere. The Economist. July 2023.
- Cakmak H, Rosen MP. Random-start ovarian stimulation in patients with cancer. Curr Opin Obstet Gynecol. 2015;27:215-221. doi: 10.1097/ GCO.0000000000000180
- Eaton JL, Truong T, Li YJ, et al. Prevalence of a good perinatal outcome with cryopreserved compared with fresh donor oocytes. Obstet Gynecol. 2020;135:709-716. doi: 10.1097/AOG.0000000000003695
- Practice Committee of the American Society for Reproductive Medicine. Fertility preservation in patients undergoing gonadotoxic therapy or gonadectomy: a committee opinion. Fertil Steril. 2019;112:1022-1033. doi: 10.1016/j.fertnstert.2019.09.013
- Oktay K, Marin L, Bedoschi G, et al. Ovarian transplantation with robotic surgery and a neovascularizing human extracellular matrix scaffold: a case series in comparison to meta-analytic data. Fertil Steril. 2021. doi:https ://doi.org/10.1016/j.fertnstert.2021.08.034
- Donnez J, Dolmans MM, Demylle D, et al. Livebirth after orthotopic transplantation of cryopreserved ovarian tissue. Lancet. 2004;364:1405-1410.
- Hoekman EJ, Louwe LA, Rooijers M, et al. Ovarian tissue cryopreservation: low usage rates and high live-birth rate after transplantation. Acta Obstet Gynecol Scand. 2020;99:213-221. doi: 10.1111/aogs.13735
- Donnez J, Dolmans MM, Diaz C, et al. Ovarian cortex transplantation: time to move on from experimental studies to open clinical application. Fertil Steril. 2015;104:1097-1098. doi: 10.1016/j.fertnstert.2015.08.005
- Rosendahl M, Greve T, Andersen CY. The safety of transplanting cryopreserved ovarian tissue in cancer patients: a review of the literature. J Assist Reprod Genet. 2013;30, 11-24. https://doi.org/10.1007/s10815-012-9912-x
- Soleimani R, Heytens E, Darzynkiewicz Z, et al. Mechanisms of chemotherapyinduced human ovarian aging: double strand DNA breaks and microvascular compromise. Aging (Albany NY). 2011;3:782-793.
- Milman LW, Senapati S, Sammel MD, et al. Assessing reproductive choices of women and the likelihood of oocyte cryopreservation in the era of elective oocyte freezing. Fertil Steril. 2017;107:1214-1222.e3. doi: 10.1016 /j.fertnstert.2017.03.010
- Bakkensen JB, Flannagan KSJ, Mumford SL, et al. A SART data cost-effectiveness analysis of planned oocyte cryopreservation versus in vitro fertilization with preimplantation genetic testing for aneuploidy considering ideal family size. Fertil Steril. 2022;118:875-884. https://doi.org/10.1016/j.fertnstert.2022.07.022
- Cascante SD, Blakemore JK, DeVore S. Fifteen years of autologous oocyte thaw outcomes from a large university-based fertility center. Fertil Steril. 2022;118:158-166. doi: 10.1016/j.fertnstert.2022.04.013
- Cobo A, Garrido N, Crespo J, et al. Accumulation of oocytes: a new strategy for managing low-responder patients. Reprod BioMedicine Online. 2018;37:669675. doi:10.1016/j.rbmo.2018.07.004
- Practice Committee of the American Society for Reproductive Medicine. Testing and interpreting measures of ovarian reserve: a committee opinion. Fertil Steril. 2020;114:1151-1157. doi: 10.1016/j.fertnstert.2020.09
- What you need to know about egg-freezing, the hot new perk at Google, Apple, and Facebook. Business Insider. September 17, 2017. Accessed August 9, 2023. https://www.businessinsider.com/egg-freezing-at-facebook-apple -google-hot-new-perk-2017-9
- Varlas VN, Bors RG, Albu D, et al. Social freezing: pressing pause on fertility. Int J Environ Res Public Health. 2021;18:8088. doi: 10.3390/ijerph18158088
- Hodes-Wertz B, Druckenmiller S, Smith M, et al. What do reproductive-age women who undergo oocyte cryopreservation think about the process as a means to preserve fertility? Fertil Steril. 2013;100:1343-1349. doi: 10.1016 /j.fertnstert.2013.07.201
- Moravek MB, Dixon M, Pena SM, et al. Management of testosterone around ovarian stimulation in transmasculine patients: challenging common practices to meet patient needs-2 case reports. Hum Reprod. 2023;38:482-488. doi: 10.1093/humrep/dead003
- American Cancer Society. Cancer Treatment & Survivorship Facts & Figures 2022-2024. Atlanta, Georgia: American Cancer Society; 2022.
- Oktay K, Karlikaya G. Ovarian function after autologous transplantation of frozen-banked human ovarian tissue. N Engl J Med. 2000;342:1919
- Practice Committees of the American Society for Reproductive Medicine and the Society for Assisted Reproductive Technology. Mature oocyte cryopreservation: a guideline. Fertil Steril. 2013;99:37-43. doi: 10.1016 /j.fertnstert.2012.09.028
- Marklund A, Lekberg T, Hedayati E, et al. Relapse rates and diseasespecific mortality following procedures for fertility preservation at time of breast cancer diagnosis. JAMA Oncol. 2022;8:1438-1446. doi:10.1001 /jamaoncol.2022.3677
- Zhao J, Liu J, Chen K, et al. What lies behind chemotherapy-induced amenorrhea for breast cancer patients: a meta-analysis. Breast Cancer Res Treat. 2014;145:113-128. https://doi.org/10.1007/s10549-014-2914-x
- Wallace WH, Thomson AB, Saran F, et al. Predicting age of ovarian failure after radiation to a field that includes the ovaries. Int J Radiat Oncol Biol Phys. 2005;62:738-744. http://doi.org10.1016/j.ijrobp.2004.11.038
- Chung EH, Acharya CR, Harris BS, et al. Development of a fertility risk calculator to predict individualized chance of hovarian failure after chemotherapy. J Assist Reprod Genetics. 2021;38:3047-3055. https://doi .org/10.1007/s10815-021-02311-0
- Brahic C, Nauta S. Eggs From Elsewhere. The Economist. July 2023.
- Cakmak H, Rosen MP. Random-start ovarian stimulation in patients with cancer. Curr Opin Obstet Gynecol. 2015;27:215-221. doi: 10.1097/ GCO.0000000000000180
- Eaton JL, Truong T, Li YJ, et al. Prevalence of a good perinatal outcome with cryopreserved compared with fresh donor oocytes. Obstet Gynecol. 2020;135:709-716. doi: 10.1097/AOG.0000000000003695
- Practice Committee of the American Society for Reproductive Medicine. Fertility preservation in patients undergoing gonadotoxic therapy or gonadectomy: a committee opinion. Fertil Steril. 2019;112:1022-1033. doi: 10.1016/j.fertnstert.2019.09.013
- Oktay K, Marin L, Bedoschi G, et al. Ovarian transplantation with robotic surgery and a neovascularizing human extracellular matrix scaffold: a case series in comparison to meta-analytic data. Fertil Steril. 2021. doi:https ://doi.org/10.1016/j.fertnstert.2021.08.034
- Donnez J, Dolmans MM, Demylle D, et al. Livebirth after orthotopic transplantation of cryopreserved ovarian tissue. Lancet. 2004;364:1405-1410.
- Hoekman EJ, Louwe LA, Rooijers M, et al. Ovarian tissue cryopreservation: low usage rates and high live-birth rate after transplantation. Acta Obstet Gynecol Scand. 2020;99:213-221. doi: 10.1111/aogs.13735
- Donnez J, Dolmans MM, Diaz C, et al. Ovarian cortex transplantation: time to move on from experimental studies to open clinical application. Fertil Steril. 2015;104:1097-1098. doi: 10.1016/j.fertnstert.2015.08.005
- Rosendahl M, Greve T, Andersen CY. The safety of transplanting cryopreserved ovarian tissue in cancer patients: a review of the literature. J Assist Reprod Genet. 2013;30, 11-24. https://doi.org/10.1007/s10815-012-9912-x
- Soleimani R, Heytens E, Darzynkiewicz Z, et al. Mechanisms of chemotherapyinduced human ovarian aging: double strand DNA breaks and microvascular compromise. Aging (Albany NY). 2011;3:782-793.
- Milman LW, Senapati S, Sammel MD, et al. Assessing reproductive choices of women and the likelihood of oocyte cryopreservation in the era of elective oocyte freezing. Fertil Steril. 2017;107:1214-1222.e3. doi: 10.1016 /j.fertnstert.2017.03.010
- Bakkensen JB, Flannagan KSJ, Mumford SL, et al. A SART data cost-effectiveness analysis of planned oocyte cryopreservation versus in vitro fertilization with preimplantation genetic testing for aneuploidy considering ideal family size. Fertil Steril. 2022;118:875-884. https://doi.org/10.1016/j.fertnstert.2022.07.022
- Cascante SD, Blakemore JK, DeVore S. Fifteen years of autologous oocyte thaw outcomes from a large university-based fertility center. Fertil Steril. 2022;118:158-166. doi: 10.1016/j.fertnstert.2022.04.013
- Cobo A, Garrido N, Crespo J, et al. Accumulation of oocytes: a new strategy for managing low-responder patients. Reprod BioMedicine Online. 2018;37:669675. doi:10.1016/j.rbmo.2018.07.004
- Practice Committee of the American Society for Reproductive Medicine. Testing and interpreting measures of ovarian reserve: a committee opinion. Fertil Steril. 2020;114:1151-1157. doi: 10.1016/j.fertnstert.2020.09
- What you need to know about egg-freezing, the hot new perk at Google, Apple, and Facebook. Business Insider. September 17, 2017. Accessed August 9, 2023. https://www.businessinsider.com/egg-freezing-at-facebook-apple -google-hot-new-perk-2017-9
- Varlas VN, Bors RG, Albu D, et al. Social freezing: pressing pause on fertility. Int J Environ Res Public Health. 2021;18:8088. doi: 10.3390/ijerph18158088
- Hodes-Wertz B, Druckenmiller S, Smith M, et al. What do reproductive-age women who undergo oocyte cryopreservation think about the process as a means to preserve fertility? Fertil Steril. 2013;100:1343-1349. doi: 10.1016 /j.fertnstert.2013.07.201
- Moravek MB, Dixon M, Pena SM, et al. Management of testosterone around ovarian stimulation in transmasculine patients: challenging common practices to meet patient needs-2 case reports. Hum Reprod. 2023;38:482-488. doi: 10.1093/humrep/dead003