User login
As soon as next year, the human papillomavirus (HPV) vaccine could transform clinical practice more than anything since the Pap smear was introduced 60 years ago.
A second large trial has shown extraordinary efficacy, and now 2 manufacturers, GlaxoSmithKline and Merck, are conducting late-phase clinical trials and working toward registering their vaccines for clinical use in 2006. Only last month, Merck and GSK signed an agreement that resolves their competing intellectual property claims—removing one more barrier to rapid commercialization.
But there are other important new developments that apply to practice now:
- Colposcopy, it appears, is far less reliable for identifying cervical intraepithelial neoplasia (CIN) 2,3 than we thought.
- The long-term risk of preterm delivery with loop electrosurgical excision procedures (LEEP) points to a need to counsel patients and consider all management options for women with CIN 1.
- The high rates of spontaneous regression of low-grade squamous intraepithelial lesion (LSIL) cytologic changes in young women are now better defined, and indicate colposcopy is not always needed.
Colposcopy not as sensitive as we thought
Pretorius R, Zhang W, Bellinson J, et al. Colposcopically directed biopsy, random cervical biopsy, and endocervical curettage in the diagnosis of cervical intraepithelial neoplasia II or worse. Am J Obstet Gynecol. 2004; 191:430–434.
We need to carefully follow up whenever colposcopy does not identify a CIN 2,3 lesion. This study also reinforces the need for diagnostic excisional procedures in women with an HSIL Pap result, and who are found after colposcopy to have CIN 1 or less (FIGURE 1).
Unfortunately, colposcopy is highly subjective. Accuracy depends on training and experience. Nevertheless, it is the standard of care for identifying CIN 2,3 and invasive cervical cancer in women with abnormal Pap results. Colposcopy was thought to be a sensitive but rather nonspecific method for identifying high-grade neoplasia. A 1998 comprehensive meta-analysis estimated that colposcopy had a weighted mean sensitivity for distinguishing normal tissue from abnormal tissue of 0.96 (95% confidence interval [CI], 0.95-0.97) and a weighted mean specificity of 0.48 (95% CI, 0.47-0.49).1 This means that colposcopy would miss a biopsy-confirmed cervical abnormality in only about 4% of patients. However, more recent follow-up studies have reported much higher false negative rates for colposcopy.
Pretorius and colleagues studied women enrolled in a cervical cancer screening trial conducted in Shanxi, China. The colposcopy in this study was performed by attending gynecologic oncologists who worked closely with a team of US-based gynecologic oncologists. The women in the study had biopsies taken of all areas classified as abnormal by colposcopy. In addition, random 4-quadrant cervical biopsies were obtained from colposcopically normal regions of the cervix.
A total of 364 women with a satisfactory colposcopy and biopsy-confirmed CIN 2 or greater lesions were identified. Even though all 364 women had a satisfactory colposcopic examination, only 57.1% of the women with biopsy-confirmed CIN 2 or worse were detected by the colposcopically-directed biopsy; the remaining 42.9% were detected by the random biopsies of colposcopically normal-appearing tissue. The lesions that were missed by colposcopy tended to be smaller than those identified by colposcopy and were more frequently CIN 2 rather than CIN 3 lesions.
This study also evaluated the role of endocervical curettage, and found that even among women with a satisfactory colposcopic examination, a significant proportion (5.5%) of cases of CIN 2,3 or worse were detected only by using endocervical curettage.
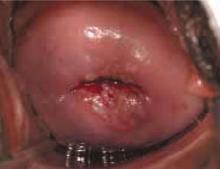
FIGURE 1 Repeat colposcopy and biopsy may reveal high-grade lesion
Low-grade cervical intraepithelial neoplasia (CIN 1) of the cervix. This young woman has a well-defined acetowhite lesion of her cervix that was diagnosed as a CIN 1 on cervical biopsy. In many such cases, repeat colposcopy and biopsy identifies an area of high-grade lesion that was missed at the initial colposcopy.
REFERENCE
1. Mitchell MF, Schottenfeld D, Tortolero-Luna G, Cantor SB, Richards-Kortum R. Colposcopy for the diagnosis of squamous intraepithelial lesions: a meta-analysis. Obstet Gynecol. 1998;91:626-631.
LEEP raises risk of preterm birth
Sadler L, Saftlas A, Wang W, et al. Treatment for cervical intraepithelial neoplasia and risk of preterm delivery. JAMA. 2004;291:2100-2106.
We need to counsel women that LEEP will increase their risk for preterm premature rupture of membranes (PPROM) and preterm delivery. We must recognize that it is desirable to follow, rather than treat, biopsy-confirmed CIN 1, and to limit the depth of excision to 1 cm or less whenever possible.
Although Consensus Guidelines state that both ablative and excisional methods are acceptable forms of managing women with satisfactory colposcopy and CIN 2,3, for most clinicians, LEEP has completely replaced laser ablation and cryotherapy for treatment of CIN.1 Because LEEP is so widely utilized, its effects on fertility and preterm delivery, as well as other adverse pregnancy outcomes, are of great concern.
LEEP became widely adopted since its introduction in the early 1990s because it yields a tissue specimen for histological evaluation and is less expensive and easier to perform than laser ablation.
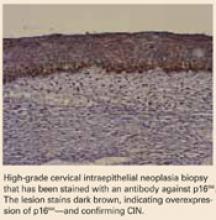
Many consider CIN 2,3 biomarkers the next step away from the Pap smear, toward more accurate molecular testing. One of the more promising biomarkers is p16INK4A, a cyclin-dependent kinase inhibitor involved in control of the cell cycle. Wang et al took tissue blocks from a large population-based screening study and evaluated the performance of p16INK4A on the full diagnostic spectrum of lesions. A very strong correlation was seen between identification of p16INK4A in the lesion and CIN 2,3; 100% of CIN 3 lesions showed diffuse staining with p16INK4A.
Wang S, Trunk M, Schiffman, M et al. Validation of p16INK4a as a marker of oncogenic human papillomavirus infection in cervical biopsies from a population-based cohort in Costa Rica. Cancer Epidemiol Biomarkers Prev. 2004;13:1355–1360.
Unfortunately, most studies of the impact of LEEP on fertility and pregnancy have been limited or inconclusive, and most lacked statistical power to detect a doubling of risk. The New Zealand study conducted by Sadler and colleagues—a large retrospective cohort study—compared delivery outcomes of 426 untreated women with 652 women treated by laser conization, laser ablation, or LEEP. Women who had LEEP or laser cone treatment were at significantly increased risk of rupture of membranes before 37 weeks’ gestation. Notably, in women who had undergone a LEEP, the adjusted relative risk (RR) for PPROM was 1.9 (95% CI, 1.0-3.8) compared to the untreated women. Laser ablation did not increase risk (RR 1.1). This study demonstrate that women who have undergone LEEP have almost twice the risk for PPROM as untreated women, should be of concern to all gynecologists.
Risk of both PPROM and preterm delivery increased as depth of cervical tissue removed increased. Women in whom 1 cm or less of tissue was excised had no increased risk of PPROM or preterm birth; women in whom more than 1.7 cm of tissue was excised had an adjusted relative risk of 3.6 (95% CI, 1.8-7.5).
In a Canadian study published only last month, Samson and colleagues found PPROM was almost 4 times more common among women who had had a LEEP.2
REFERENCES
1. Wright TC, Jr, Cox JT, Massad LS, Carlson J, Twiggs LB, Wilkinson EJ. 2001 consensus guidelines for the management of women with cervical intraepithelial neoplasia. Am J Obstet Gynecol. 2003;189:295-304.
2. Samson S, Bentley JR, Fahey T, McKay D, Gill G. The effect of loop electrosurgical excision procedure on future pregnancy outcome. Obstet Gynecol. 2005;105:325-332.
LSIL cytology meaningless?
Moscicki A, Shiboski S, Hills N, et al. Regression of low-grade squamous intraepithelial lesions in young women. Lancet. 2004;364:1678–1683.
This study shows just how meaningless LSIL cytology is in young women—and it portends changes in the next Consensus Guidelines. Colposcopy for all adolescents and young women is unwarranted, the authors stated. They recommend monitoring with repeat cytology instead.
For over a decade it has been widely appreciated that many CIN 1 lesions spontaneously regress in the absence of therapy.1 Based on what we recently learned from natural history studies of HPV, we know that the majority of LSIL cytology results and biopsy-confirmed CIN 1 lesions represent nothing more than the morphological manifestation of a productive HPV infection.2 HPV infections, including those with high-risk types of HPV, are typically self-limited (FIGURE 2). In approximately 90% of women, HPV shedding stops spontaneously within 24 months.
However, in the United States, most women with LSIL undergo colposcopy, and many clinicians continue to treat women with biopsy-confirmed CIN 1. These approaches do correspond to the most recent Consensus Guidelines, which recommend colposcopy for women with LSIL, and state that follow-up with treatment, as well as treatment with ablative or excisional methods, are acceptable management options for women with CIN 1.3
Regarding adolescents with LSIL, the guidelines made an exception to performing a colposcopy. For these patients, an acceptable management option is follow-up without initial colposcopy, using a protocol of repeat cytological testing at 6 and 12 months, or HPV testing at 12 months.
To better define the best way to manage young women with LSIL, Moscicki and colleagues followed a cohort of 204 young women (ages 13 to 22 years), who had an LSIL Pap result, for up to 80 months (median 61 months). HSIL cytology (N=6) or biopsy-confirmed CIN 2,3 (N=17) was found in only 11.3% of the women. After 36 months, only 6% had persistent LSIL.
The remainder had had 3 consecutive negative Pap results, and the median time to developing the first of 3 negative Pap results was only 8 months.
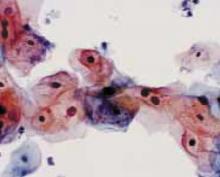
FIGURE 2 Even high-risk HPV types usually abate in young women
Liquid-based cytology specimen diagnosed as low-grade squamous intraepithelial lesion (LSIL), with marked koilocytosis with multinucleation, perinuclear halos, and nuclear atypia. These features typify productive HPV infection that usually regresses spontaneously in young women.
REFERENCES
1. Melnikow J, Nuovo J, Willan AR, Chan BK, Howell LP. Natural history of cervical squamous intraepithelial lesions: a meta-analysis. Obstet Gynecol. 1998;92:727-735.
2. Wright TC, Schiffman M. Adding a test for human papillomavirus DNA to cervical-cancer screening. N Engl J Med 2003;348:489-490.
3. Wright TC, Jr, Cox JT, Massad LS, Twiggs LB, Wilkinson EJ. 2001 consensus guidelines for the management of women with cervical cytological abnormalities. JAMA. 2002;287:2120-2129.
Bivalent vaccine vanquishes HPV
Harper D, Franco E, Wheeler C, et al. Efficacy of a bivalent L1 virus-like particle vaccine in prevention of infection with human papillomavirus types 16 and 18 in young women: a randomized controlled trial. Lancet. 2004;364:1757–1765.
HPV vaccine may be registered for clinical use next year. Since two-thirds of cervical cancers are caused by only 2 types of high-risk HPV—HPV 16 and HPV 18—a vaccine that prevents infection with HPV 16 and 18 could reduce cervical cancer and high-grade precursor lesions by more than half.
Extraordinary efficacy—100% against persistent infections and 91.6% against incident HPV 16 or 18 infections—was found in this Phase II trial of a bivalent HPV vaccine made by GlaxoSmithKline—the second such trial to show high efficacy for an HPV vaccine. Merck found high efficacy for its monovalent vaccine. Both companies are conducting Phase III registration trials.
Harper and colleagues observed these efficacy rates in women who took all their scheduled vaccinations. They used bivalent HPV 16 and 18 vaccine in a study of 1,113 women randomized to receive 3 doses of vaccine or placebo over a 6-month period. All were followed for up to 27 months.
The vaccine was also highly effective against cytological abnormalities associated with HPV 16 or 18 and was generally safe, well tolerated, and highly immunogenic.
In 2002, a Phase II trial of a monovalent HPV 16 vaccine produced by Merck demonstrated efficacy of 100% over 18 months in preventing persistent HPV 16 infection or CIN associated with HPV 16.1
Both companies’ vaccines consist of viral-like particles that are made by producing recombinant L1 capsid protein of the specific HPV type and then allowing the recombinant L1 capsid proteins to assemble into a structure that appears identical to the native virus, but lacks infectious DNA.
Each year, 470,000 women develop invasive cervical cancer, and 230,000 die, globally. Vaccination is a particularly attractive strategy for preventing cervical cancer in developing countries, where less than 5% of women have ever been screened.
Yet these numbers do not begin to take into account the huge costs and burden of disease due to noninvasive cervical cancer precursors and abnormal screening cytology. In the United States alone, we spend up to $6 billion a year on prevention and treatment of cervical cancer.
The author reports no financial relationships relevant to this article.
REFERENCE
1. Koutsky LA, Ault KA, Wheeler CM, et al. A controlled trial of a human papillomavirus type 16 vaccine. N Engl J Med. 2002;347:1645-1651.
As soon as next year, the human papillomavirus (HPV) vaccine could transform clinical practice more than anything since the Pap smear was introduced 60 years ago.
A second large trial has shown extraordinary efficacy, and now 2 manufacturers, GlaxoSmithKline and Merck, are conducting late-phase clinical trials and working toward registering their vaccines for clinical use in 2006. Only last month, Merck and GSK signed an agreement that resolves their competing intellectual property claims—removing one more barrier to rapid commercialization.
But there are other important new developments that apply to practice now:
- Colposcopy, it appears, is far less reliable for identifying cervical intraepithelial neoplasia (CIN) 2,3 than we thought.
- The long-term risk of preterm delivery with loop electrosurgical excision procedures (LEEP) points to a need to counsel patients and consider all management options for women with CIN 1.
- The high rates of spontaneous regression of low-grade squamous intraepithelial lesion (LSIL) cytologic changes in young women are now better defined, and indicate colposcopy is not always needed.
Colposcopy not as sensitive as we thought
Pretorius R, Zhang W, Bellinson J, et al. Colposcopically directed biopsy, random cervical biopsy, and endocervical curettage in the diagnosis of cervical intraepithelial neoplasia II or worse. Am J Obstet Gynecol. 2004; 191:430–434.
We need to carefully follow up whenever colposcopy does not identify a CIN 2,3 lesion. This study also reinforces the need for diagnostic excisional procedures in women with an HSIL Pap result, and who are found after colposcopy to have CIN 1 or less (FIGURE 1).
Unfortunately, colposcopy is highly subjective. Accuracy depends on training and experience. Nevertheless, it is the standard of care for identifying CIN 2,3 and invasive cervical cancer in women with abnormal Pap results. Colposcopy was thought to be a sensitive but rather nonspecific method for identifying high-grade neoplasia. A 1998 comprehensive meta-analysis estimated that colposcopy had a weighted mean sensitivity for distinguishing normal tissue from abnormal tissue of 0.96 (95% confidence interval [CI], 0.95-0.97) and a weighted mean specificity of 0.48 (95% CI, 0.47-0.49).1 This means that colposcopy would miss a biopsy-confirmed cervical abnormality in only about 4% of patients. However, more recent follow-up studies have reported much higher false negative rates for colposcopy.
Pretorius and colleagues studied women enrolled in a cervical cancer screening trial conducted in Shanxi, China. The colposcopy in this study was performed by attending gynecologic oncologists who worked closely with a team of US-based gynecologic oncologists. The women in the study had biopsies taken of all areas classified as abnormal by colposcopy. In addition, random 4-quadrant cervical biopsies were obtained from colposcopically normal regions of the cervix.
A total of 364 women with a satisfactory colposcopy and biopsy-confirmed CIN 2 or greater lesions were identified. Even though all 364 women had a satisfactory colposcopic examination, only 57.1% of the women with biopsy-confirmed CIN 2 or worse were detected by the colposcopically-directed biopsy; the remaining 42.9% were detected by the random biopsies of colposcopically normal-appearing tissue. The lesions that were missed by colposcopy tended to be smaller than those identified by colposcopy and were more frequently CIN 2 rather than CIN 3 lesions.
This study also evaluated the role of endocervical curettage, and found that even among women with a satisfactory colposcopic examination, a significant proportion (5.5%) of cases of CIN 2,3 or worse were detected only by using endocervical curettage.
FIGURE 1 Repeat colposcopy and biopsy may reveal high-grade lesion
Low-grade cervical intraepithelial neoplasia (CIN 1) of the cervix. This young woman has a well-defined acetowhite lesion of her cervix that was diagnosed as a CIN 1 on cervical biopsy. In many such cases, repeat colposcopy and biopsy identifies an area of high-grade lesion that was missed at the initial colposcopy.
REFERENCE
1. Mitchell MF, Schottenfeld D, Tortolero-Luna G, Cantor SB, Richards-Kortum R. Colposcopy for the diagnosis of squamous intraepithelial lesions: a meta-analysis. Obstet Gynecol. 1998;91:626-631.
LEEP raises risk of preterm birth
Sadler L, Saftlas A, Wang W, et al. Treatment for cervical intraepithelial neoplasia and risk of preterm delivery. JAMA. 2004;291:2100-2106.
We need to counsel women that LEEP will increase their risk for preterm premature rupture of membranes (PPROM) and preterm delivery. We must recognize that it is desirable to follow, rather than treat, biopsy-confirmed CIN 1, and to limit the depth of excision to 1 cm or less whenever possible.
Although Consensus Guidelines state that both ablative and excisional methods are acceptable forms of managing women with satisfactory colposcopy and CIN 2,3, for most clinicians, LEEP has completely replaced laser ablation and cryotherapy for treatment of CIN.1 Because LEEP is so widely utilized, its effects on fertility and preterm delivery, as well as other adverse pregnancy outcomes, are of great concern.
LEEP became widely adopted since its introduction in the early 1990s because it yields a tissue specimen for histological evaluation and is less expensive and easier to perform than laser ablation.
Many consider CIN 2,3 biomarkers the next step away from the Pap smear, toward more accurate molecular testing. One of the more promising biomarkers is p16INK4A, a cyclin-dependent kinase inhibitor involved in control of the cell cycle. Wang et al took tissue blocks from a large population-based screening study and evaluated the performance of p16INK4A on the full diagnostic spectrum of lesions. A very strong correlation was seen between identification of p16INK4A in the lesion and CIN 2,3; 100% of CIN 3 lesions showed diffuse staining with p16INK4A.
Wang S, Trunk M, Schiffman, M et al. Validation of p16INK4a as a marker of oncogenic human papillomavirus infection in cervical biopsies from a population-based cohort in Costa Rica. Cancer Epidemiol Biomarkers Prev. 2004;13:1355–1360.
Unfortunately, most studies of the impact of LEEP on fertility and pregnancy have been limited or inconclusive, and most lacked statistical power to detect a doubling of risk. The New Zealand study conducted by Sadler and colleagues—a large retrospective cohort study—compared delivery outcomes of 426 untreated women with 652 women treated by laser conization, laser ablation, or LEEP. Women who had LEEP or laser cone treatment were at significantly increased risk of rupture of membranes before 37 weeks’ gestation. Notably, in women who had undergone a LEEP, the adjusted relative risk (RR) for PPROM was 1.9 (95% CI, 1.0-3.8) compared to the untreated women. Laser ablation did not increase risk (RR 1.1). This study demonstrate that women who have undergone LEEP have almost twice the risk for PPROM as untreated women, should be of concern to all gynecologists.
Risk of both PPROM and preterm delivery increased as depth of cervical tissue removed increased. Women in whom 1 cm or less of tissue was excised had no increased risk of PPROM or preterm birth; women in whom more than 1.7 cm of tissue was excised had an adjusted relative risk of 3.6 (95% CI, 1.8-7.5).
In a Canadian study published only last month, Samson and colleagues found PPROM was almost 4 times more common among women who had had a LEEP.2
REFERENCES
1. Wright TC, Jr, Cox JT, Massad LS, Carlson J, Twiggs LB, Wilkinson EJ. 2001 consensus guidelines for the management of women with cervical intraepithelial neoplasia. Am J Obstet Gynecol. 2003;189:295-304.
2. Samson S, Bentley JR, Fahey T, McKay D, Gill G. The effect of loop electrosurgical excision procedure on future pregnancy outcome. Obstet Gynecol. 2005;105:325-332.
LSIL cytology meaningless?
Moscicki A, Shiboski S, Hills N, et al. Regression of low-grade squamous intraepithelial lesions in young women. Lancet. 2004;364:1678–1683.
This study shows just how meaningless LSIL cytology is in young women—and it portends changes in the next Consensus Guidelines. Colposcopy for all adolescents and young women is unwarranted, the authors stated. They recommend monitoring with repeat cytology instead.
For over a decade it has been widely appreciated that many CIN 1 lesions spontaneously regress in the absence of therapy.1 Based on what we recently learned from natural history studies of HPV, we know that the majority of LSIL cytology results and biopsy-confirmed CIN 1 lesions represent nothing more than the morphological manifestation of a productive HPV infection.2 HPV infections, including those with high-risk types of HPV, are typically self-limited (FIGURE 2). In approximately 90% of women, HPV shedding stops spontaneously within 24 months.
However, in the United States, most women with LSIL undergo colposcopy, and many clinicians continue to treat women with biopsy-confirmed CIN 1. These approaches do correspond to the most recent Consensus Guidelines, which recommend colposcopy for women with LSIL, and state that follow-up with treatment, as well as treatment with ablative or excisional methods, are acceptable management options for women with CIN 1.3
Regarding adolescents with LSIL, the guidelines made an exception to performing a colposcopy. For these patients, an acceptable management option is follow-up without initial colposcopy, using a protocol of repeat cytological testing at 6 and 12 months, or HPV testing at 12 months.
To better define the best way to manage young women with LSIL, Moscicki and colleagues followed a cohort of 204 young women (ages 13 to 22 years), who had an LSIL Pap result, for up to 80 months (median 61 months). HSIL cytology (N=6) or biopsy-confirmed CIN 2,3 (N=17) was found in only 11.3% of the women. After 36 months, only 6% had persistent LSIL.
The remainder had had 3 consecutive negative Pap results, and the median time to developing the first of 3 negative Pap results was only 8 months.
FIGURE 2 Even high-risk HPV types usually abate in young women
Liquid-based cytology specimen diagnosed as low-grade squamous intraepithelial lesion (LSIL), with marked koilocytosis with multinucleation, perinuclear halos, and nuclear atypia. These features typify productive HPV infection that usually regresses spontaneously in young women.
REFERENCES
1. Melnikow J, Nuovo J, Willan AR, Chan BK, Howell LP. Natural history of cervical squamous intraepithelial lesions: a meta-analysis. Obstet Gynecol. 1998;92:727-735.
2. Wright TC, Schiffman M. Adding a test for human papillomavirus DNA to cervical-cancer screening. N Engl J Med 2003;348:489-490.
3. Wright TC, Jr, Cox JT, Massad LS, Twiggs LB, Wilkinson EJ. 2001 consensus guidelines for the management of women with cervical cytological abnormalities. JAMA. 2002;287:2120-2129.
Bivalent vaccine vanquishes HPV
Harper D, Franco E, Wheeler C, et al. Efficacy of a bivalent L1 virus-like particle vaccine in prevention of infection with human papillomavirus types 16 and 18 in young women: a randomized controlled trial. Lancet. 2004;364:1757–1765.
HPV vaccine may be registered for clinical use next year. Since two-thirds of cervical cancers are caused by only 2 types of high-risk HPV—HPV 16 and HPV 18—a vaccine that prevents infection with HPV 16 and 18 could reduce cervical cancer and high-grade precursor lesions by more than half.
Extraordinary efficacy—100% against persistent infections and 91.6% against incident HPV 16 or 18 infections—was found in this Phase II trial of a bivalent HPV vaccine made by GlaxoSmithKline—the second such trial to show high efficacy for an HPV vaccine. Merck found high efficacy for its monovalent vaccine. Both companies are conducting Phase III registration trials.
Harper and colleagues observed these efficacy rates in women who took all their scheduled vaccinations. They used bivalent HPV 16 and 18 vaccine in a study of 1,113 women randomized to receive 3 doses of vaccine or placebo over a 6-month period. All were followed for up to 27 months.
The vaccine was also highly effective against cytological abnormalities associated with HPV 16 or 18 and was generally safe, well tolerated, and highly immunogenic.
In 2002, a Phase II trial of a monovalent HPV 16 vaccine produced by Merck demonstrated efficacy of 100% over 18 months in preventing persistent HPV 16 infection or CIN associated with HPV 16.1
Both companies’ vaccines consist of viral-like particles that are made by producing recombinant L1 capsid protein of the specific HPV type and then allowing the recombinant L1 capsid proteins to assemble into a structure that appears identical to the native virus, but lacks infectious DNA.
Each year, 470,000 women develop invasive cervical cancer, and 230,000 die, globally. Vaccination is a particularly attractive strategy for preventing cervical cancer in developing countries, where less than 5% of women have ever been screened.
Yet these numbers do not begin to take into account the huge costs and burden of disease due to noninvasive cervical cancer precursors and abnormal screening cytology. In the United States alone, we spend up to $6 billion a year on prevention and treatment of cervical cancer.
The author reports no financial relationships relevant to this article.
REFERENCE
1. Koutsky LA, Ault KA, Wheeler CM, et al. A controlled trial of a human papillomavirus type 16 vaccine. N Engl J Med. 2002;347:1645-1651.
As soon as next year, the human papillomavirus (HPV) vaccine could transform clinical practice more than anything since the Pap smear was introduced 60 years ago.
A second large trial has shown extraordinary efficacy, and now 2 manufacturers, GlaxoSmithKline and Merck, are conducting late-phase clinical trials and working toward registering their vaccines for clinical use in 2006. Only last month, Merck and GSK signed an agreement that resolves their competing intellectual property claims—removing one more barrier to rapid commercialization.
But there are other important new developments that apply to practice now:
- Colposcopy, it appears, is far less reliable for identifying cervical intraepithelial neoplasia (CIN) 2,3 than we thought.
- The long-term risk of preterm delivery with loop electrosurgical excision procedures (LEEP) points to a need to counsel patients and consider all management options for women with CIN 1.
- The high rates of spontaneous regression of low-grade squamous intraepithelial lesion (LSIL) cytologic changes in young women are now better defined, and indicate colposcopy is not always needed.
Colposcopy not as sensitive as we thought
Pretorius R, Zhang W, Bellinson J, et al. Colposcopically directed biopsy, random cervical biopsy, and endocervical curettage in the diagnosis of cervical intraepithelial neoplasia II or worse. Am J Obstet Gynecol. 2004; 191:430–434.
We need to carefully follow up whenever colposcopy does not identify a CIN 2,3 lesion. This study also reinforces the need for diagnostic excisional procedures in women with an HSIL Pap result, and who are found after colposcopy to have CIN 1 or less (FIGURE 1).
Unfortunately, colposcopy is highly subjective. Accuracy depends on training and experience. Nevertheless, it is the standard of care for identifying CIN 2,3 and invasive cervical cancer in women with abnormal Pap results. Colposcopy was thought to be a sensitive but rather nonspecific method for identifying high-grade neoplasia. A 1998 comprehensive meta-analysis estimated that colposcopy had a weighted mean sensitivity for distinguishing normal tissue from abnormal tissue of 0.96 (95% confidence interval [CI], 0.95-0.97) and a weighted mean specificity of 0.48 (95% CI, 0.47-0.49).1 This means that colposcopy would miss a biopsy-confirmed cervical abnormality in only about 4% of patients. However, more recent follow-up studies have reported much higher false negative rates for colposcopy.
Pretorius and colleagues studied women enrolled in a cervical cancer screening trial conducted in Shanxi, China. The colposcopy in this study was performed by attending gynecologic oncologists who worked closely with a team of US-based gynecologic oncologists. The women in the study had biopsies taken of all areas classified as abnormal by colposcopy. In addition, random 4-quadrant cervical biopsies were obtained from colposcopically normal regions of the cervix.
A total of 364 women with a satisfactory colposcopy and biopsy-confirmed CIN 2 or greater lesions were identified. Even though all 364 women had a satisfactory colposcopic examination, only 57.1% of the women with biopsy-confirmed CIN 2 or worse were detected by the colposcopically-directed biopsy; the remaining 42.9% were detected by the random biopsies of colposcopically normal-appearing tissue. The lesions that were missed by colposcopy tended to be smaller than those identified by colposcopy and were more frequently CIN 2 rather than CIN 3 lesions.
This study also evaluated the role of endocervical curettage, and found that even among women with a satisfactory colposcopic examination, a significant proportion (5.5%) of cases of CIN 2,3 or worse were detected only by using endocervical curettage.
FIGURE 1 Repeat colposcopy and biopsy may reveal high-grade lesion
Low-grade cervical intraepithelial neoplasia (CIN 1) of the cervix. This young woman has a well-defined acetowhite lesion of her cervix that was diagnosed as a CIN 1 on cervical biopsy. In many such cases, repeat colposcopy and biopsy identifies an area of high-grade lesion that was missed at the initial colposcopy.
REFERENCE
1. Mitchell MF, Schottenfeld D, Tortolero-Luna G, Cantor SB, Richards-Kortum R. Colposcopy for the diagnosis of squamous intraepithelial lesions: a meta-analysis. Obstet Gynecol. 1998;91:626-631.
LEEP raises risk of preterm birth
Sadler L, Saftlas A, Wang W, et al. Treatment for cervical intraepithelial neoplasia and risk of preterm delivery. JAMA. 2004;291:2100-2106.
We need to counsel women that LEEP will increase their risk for preterm premature rupture of membranes (PPROM) and preterm delivery. We must recognize that it is desirable to follow, rather than treat, biopsy-confirmed CIN 1, and to limit the depth of excision to 1 cm or less whenever possible.
Although Consensus Guidelines state that both ablative and excisional methods are acceptable forms of managing women with satisfactory colposcopy and CIN 2,3, for most clinicians, LEEP has completely replaced laser ablation and cryotherapy for treatment of CIN.1 Because LEEP is so widely utilized, its effects on fertility and preterm delivery, as well as other adverse pregnancy outcomes, are of great concern.
LEEP became widely adopted since its introduction in the early 1990s because it yields a tissue specimen for histological evaluation and is less expensive and easier to perform than laser ablation.
Many consider CIN 2,3 biomarkers the next step away from the Pap smear, toward more accurate molecular testing. One of the more promising biomarkers is p16INK4A, a cyclin-dependent kinase inhibitor involved in control of the cell cycle. Wang et al took tissue blocks from a large population-based screening study and evaluated the performance of p16INK4A on the full diagnostic spectrum of lesions. A very strong correlation was seen between identification of p16INK4A in the lesion and CIN 2,3; 100% of CIN 3 lesions showed diffuse staining with p16INK4A.
Wang S, Trunk M, Schiffman, M et al. Validation of p16INK4a as a marker of oncogenic human papillomavirus infection in cervical biopsies from a population-based cohort in Costa Rica. Cancer Epidemiol Biomarkers Prev. 2004;13:1355–1360.
Unfortunately, most studies of the impact of LEEP on fertility and pregnancy have been limited or inconclusive, and most lacked statistical power to detect a doubling of risk. The New Zealand study conducted by Sadler and colleagues—a large retrospective cohort study—compared delivery outcomes of 426 untreated women with 652 women treated by laser conization, laser ablation, or LEEP. Women who had LEEP or laser cone treatment were at significantly increased risk of rupture of membranes before 37 weeks’ gestation. Notably, in women who had undergone a LEEP, the adjusted relative risk (RR) for PPROM was 1.9 (95% CI, 1.0-3.8) compared to the untreated women. Laser ablation did not increase risk (RR 1.1). This study demonstrate that women who have undergone LEEP have almost twice the risk for PPROM as untreated women, should be of concern to all gynecologists.
Risk of both PPROM and preterm delivery increased as depth of cervical tissue removed increased. Women in whom 1 cm or less of tissue was excised had no increased risk of PPROM or preterm birth; women in whom more than 1.7 cm of tissue was excised had an adjusted relative risk of 3.6 (95% CI, 1.8-7.5).
In a Canadian study published only last month, Samson and colleagues found PPROM was almost 4 times more common among women who had had a LEEP.2
REFERENCES
1. Wright TC, Jr, Cox JT, Massad LS, Carlson J, Twiggs LB, Wilkinson EJ. 2001 consensus guidelines for the management of women with cervical intraepithelial neoplasia. Am J Obstet Gynecol. 2003;189:295-304.
2. Samson S, Bentley JR, Fahey T, McKay D, Gill G. The effect of loop electrosurgical excision procedure on future pregnancy outcome. Obstet Gynecol. 2005;105:325-332.
LSIL cytology meaningless?
Moscicki A, Shiboski S, Hills N, et al. Regression of low-grade squamous intraepithelial lesions in young women. Lancet. 2004;364:1678–1683.
This study shows just how meaningless LSIL cytology is in young women—and it portends changes in the next Consensus Guidelines. Colposcopy for all adolescents and young women is unwarranted, the authors stated. They recommend monitoring with repeat cytology instead.
For over a decade it has been widely appreciated that many CIN 1 lesions spontaneously regress in the absence of therapy.1 Based on what we recently learned from natural history studies of HPV, we know that the majority of LSIL cytology results and biopsy-confirmed CIN 1 lesions represent nothing more than the morphological manifestation of a productive HPV infection.2 HPV infections, including those with high-risk types of HPV, are typically self-limited (FIGURE 2). In approximately 90% of women, HPV shedding stops spontaneously within 24 months.
However, in the United States, most women with LSIL undergo colposcopy, and many clinicians continue to treat women with biopsy-confirmed CIN 1. These approaches do correspond to the most recent Consensus Guidelines, which recommend colposcopy for women with LSIL, and state that follow-up with treatment, as well as treatment with ablative or excisional methods, are acceptable management options for women with CIN 1.3
Regarding adolescents with LSIL, the guidelines made an exception to performing a colposcopy. For these patients, an acceptable management option is follow-up without initial colposcopy, using a protocol of repeat cytological testing at 6 and 12 months, or HPV testing at 12 months.
To better define the best way to manage young women with LSIL, Moscicki and colleagues followed a cohort of 204 young women (ages 13 to 22 years), who had an LSIL Pap result, for up to 80 months (median 61 months). HSIL cytology (N=6) or biopsy-confirmed CIN 2,3 (N=17) was found in only 11.3% of the women. After 36 months, only 6% had persistent LSIL.
The remainder had had 3 consecutive negative Pap results, and the median time to developing the first of 3 negative Pap results was only 8 months.
FIGURE 2 Even high-risk HPV types usually abate in young women
Liquid-based cytology specimen diagnosed as low-grade squamous intraepithelial lesion (LSIL), with marked koilocytosis with multinucleation, perinuclear halos, and nuclear atypia. These features typify productive HPV infection that usually regresses spontaneously in young women.
REFERENCES
1. Melnikow J, Nuovo J, Willan AR, Chan BK, Howell LP. Natural history of cervical squamous intraepithelial lesions: a meta-analysis. Obstet Gynecol. 1998;92:727-735.
2. Wright TC, Schiffman M. Adding a test for human papillomavirus DNA to cervical-cancer screening. N Engl J Med 2003;348:489-490.
3. Wright TC, Jr, Cox JT, Massad LS, Twiggs LB, Wilkinson EJ. 2001 consensus guidelines for the management of women with cervical cytological abnormalities. JAMA. 2002;287:2120-2129.
Bivalent vaccine vanquishes HPV
Harper D, Franco E, Wheeler C, et al. Efficacy of a bivalent L1 virus-like particle vaccine in prevention of infection with human papillomavirus types 16 and 18 in young women: a randomized controlled trial. Lancet. 2004;364:1757–1765.
HPV vaccine may be registered for clinical use next year. Since two-thirds of cervical cancers are caused by only 2 types of high-risk HPV—HPV 16 and HPV 18—a vaccine that prevents infection with HPV 16 and 18 could reduce cervical cancer and high-grade precursor lesions by more than half.
Extraordinary efficacy—100% against persistent infections and 91.6% against incident HPV 16 or 18 infections—was found in this Phase II trial of a bivalent HPV vaccine made by GlaxoSmithKline—the second such trial to show high efficacy for an HPV vaccine. Merck found high efficacy for its monovalent vaccine. Both companies are conducting Phase III registration trials.
Harper and colleagues observed these efficacy rates in women who took all their scheduled vaccinations. They used bivalent HPV 16 and 18 vaccine in a study of 1,113 women randomized to receive 3 doses of vaccine or placebo over a 6-month period. All were followed for up to 27 months.
The vaccine was also highly effective against cytological abnormalities associated with HPV 16 or 18 and was generally safe, well tolerated, and highly immunogenic.
In 2002, a Phase II trial of a monovalent HPV 16 vaccine produced by Merck demonstrated efficacy of 100% over 18 months in preventing persistent HPV 16 infection or CIN associated with HPV 16.1
Both companies’ vaccines consist of viral-like particles that are made by producing recombinant L1 capsid protein of the specific HPV type and then allowing the recombinant L1 capsid proteins to assemble into a structure that appears identical to the native virus, but lacks infectious DNA.
Each year, 470,000 women develop invasive cervical cancer, and 230,000 die, globally. Vaccination is a particularly attractive strategy for preventing cervical cancer in developing countries, where less than 5% of women have ever been screened.
Yet these numbers do not begin to take into account the huge costs and burden of disease due to noninvasive cervical cancer precursors and abnormal screening cytology. In the United States alone, we spend up to $6 billion a year on prevention and treatment of cervical cancer.
The author reports no financial relationships relevant to this article.
REFERENCE
1. Koutsky LA, Ault KA, Wheeler CM, et al. A controlled trial of a human papillomavirus type 16 vaccine. N Engl J Med. 2002;347:1645-1651.