User login
Multiple sclerosis (MS) is a lifelong disease that affects about a million people in the United States.1,2 Since 1998 more than 45,000 veterans have been diagnosed with MS and about 20,000 are evaluated in the Veterans Health Administration (VHA) annually.3
Magnetic resonance imaging (MRI) is a cornerstone for the assessment of persons with multiple sclerosis (pwMS).4-6 MRI assists with disease diagnosis, allowing for timely therapeutic interventions and withthe evaluation of its progression, treatment effect, and safety. 4,5 MRI-based outcomes also are used as primary endpoints in clinical trials.4,5
MS has its clinical onset in early adulthood in most individuals and is diagnosed at a mean age of 30 years.7 As a result, pwMS may receive care and MRIs in different facilities during their lifetime. Mitigating interscan variabilities that can challenge intra- and interperson comparisons is crucial for accurate care. Radiologists may find it difficult to compare scans acquired in different facilities, as dissimilarities in acquisition protocols may mask or uncover focal disease, creating false negative or false positive findings. Moreover, lack of a standardized method to report MRI changes may compromise neurologists’ ability to correctly interpret scans and disease progression.
Accordingly, in October 2019, an international task force of neurologists, radiologists, MRI technologists, and imaging scientists with expertise in MS, including representatives from the VHA, worked together to update guidelines for imaging the brain, spinal cord, and optic nerve in pwMS.8,9 Recognizing the importance of this effort, the VHA Multiple Sclerosis Centers of Excellence (MSCoE), in collaboration with a team of subject matter expert neuroradiologists promptly committed to this effort, advocating the updated consensus recommendations, and favoring their dissemination within the VHA.10
As part of this commitment and dissemination effort, in this report we summarize the core points of the newly proposed MRI guidelines and ways to adapt them for use within the VHA. We then discuss key elements for their successful implementation and dissemination, specifically regarding the clinical operations of VHA.
Updated Guidelines
MRI Scan at Different Timepoints of MS
There are 3 crucial milestones within a the lifespan of a pwMS that require an MRI to reach appropriate conclusions and avoid clinical errors. These include the initial diagnosis, the follow-up to monitor disease and/or treatment effect, and the assessment of medication safety.
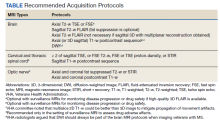
In the interest of efficiency, MRI protocols may vary slightly depending on these clinical indications. The Table lists core sequences of the updated 2021 consensus recommendations at each timepoint along with the proposed alternatives or preferences from the VHA workgroup.
At the time of diagnosis, both brain and spine (cervical and thoracic) MRIs are recommended. Routine MRI of the optic nerve is considered optional at diagnosis. However, imaging the optic nerve may be useful in specific clinical scenarios when the optic nerve is selectively involved, and the diagnosis or etiology of an optic neuritis is not clear. A repeat brain MRI is advised every 6 to 12 months in patients with clinically or radiologically isolated syndrome who do not fulfill the diagnostic criteria of MS but present risk factors for conversion to MS or paraclinical features of it.
Once the diagnosis is established, brain MRI is recommended for follow-up and for surveillance of drug safety. Spinal cord and optic nerve MRIs are desirable but optional in the follow-up of pwMS and are not required for drug surveillance. Spinal cord MRIs are required at follow-up for patients whose progression cannot be explained by brain MRI features, or who manifest with recurrent spinal cord symptoms, or have spinal cord comorbidities. In these cases, spinal cord MRI also may assist with treatment decisions. Similarly, optic nerve MRI is necessary during follow-up only when optic nerve comorbidities are suspected or when there is progression or reoccurrence of optic nerve–related symptoms.
Brain MRIs are recommended for monitoring drug effect yearly (or at longer intervals, after a few years of disease stability). Conversely, a repeat brain MRI is advised after 6 months if nonsymptomatic radiological disease activity is discovered on surveillance scans.
Abbreviated but more frequent serial brain MRI protocols (eg, every 3 to 4 months) are recommended for pwMS treated with natalizumab and at high risk of developing progressive multifocal leukoencephalopathy (eg, pwMS who are John Cunningham virus [JCV]–positive, and have been treated with natalizumabfor ≥ 18 months, have a JCV antibody index > 0.9, or have a history of immunosuppression). A similar approach is recommended for carryover cases, such as those with high JCV antibody index who are switched to other immunosuppressive treatments.
MRI Field, Scan Resolution, and Coverage
Both 1.5-Tesla (1.5-T) and 3-T scans are believed to be equally effective in imaging pwMS, providing that the 1.5-T scans are good quality. Although imaging at < 1.5 T is not recommended due to suboptimal disease detection, the use of scanners > 3 T is equally discouraged outside the supervision of trained investigators. Signal-to-noise ratio and resolution are key factors impacting scan quality, and their optimization is prioritized over the number of sequences in the updated 2021 consensus recommendations. For brain imaging, a resolution of 1 mm3 isotropic is preferred for 3-dimensional (3D) imaging and slice thickness ≤ 3 mm without gap (≤ 5 mm with 10-30% gaps for diffusion-weighted imaging only) is recommended for 2D sequences. Images should cover the entire brain and as much of the cervical spine as possible; images should be prescribed axial for 2D or reformatted axial oblique for 3D using the subcallosal plane as reference. For spine imaging, sites should aim at an in-plane resolution of 1 mm2; using sagittal slices ≤ 3 mm thick and axial slices ≤ 5 mm thick, both with no gap. Scans should cover the entire cervical and thoracolumbar region inclusive of the conus. For the optic nerve images, slices should be ≤ 2 or 3 mm thick with an in-plane resolution of 1 mm2. Images should be aligned to the orientation of the optic nerve and chiasms, both of which should be entirely covered.
Postgadolinium Images Use
The discovery of the higher sensitivity of post-gadolinium (Gd) T1-weighted (T1-w) MRI relative to high iodine (88.1 g I) computed tomography scans in demonstrating contrast-enhancing MS lesions has revolutionized the way clinicians diagnose and monitor this disease.11 However, in recent years the role of postcontrast MRI has been debated, considering the potential safety concerns secondary to Gd tissue deposition. For this reason, an intentionally more judicious use of postcontrast MRI is proposed by the consensus recommendations. At disease diagnosis, the use of Gd is advisable to (1) show disease dissemination in time; (2) differentiate the diagnosis based on the Gd pattern; (3) predict short-term disease activity; and (4) characterize activity in the setting of progression. When monitoring pwMS, the use of Gd may be useful in the first year of follow-up, particularly if in the setting of low potency medications or for patients for whom the detection of one or more active lesions would lead to a change in disease-modifying agents. Gd also should be used to first, confirm a clinical exacerbation (if needed); second, further characterize a lesion suggestive of progressive multifocal encephalopathy or monitor this disease over time; and third, monitor lesion burden change in patients with large confluent lesions, the count of which otherwise may be difficult.
MRI During Pregnancy and Lactation
The consensus recommendations state that Gd contrast–enhanced MRI is not absolutely contraindicated during pregnancy, although its use should be limited to strictly necessary situations, particularly those involving differential diagnosis, such as cerebral venous thrombosis or monitoring of possibly enlarging lesion burden. The use of Gd is not contraindicated during lactation, as only a small proportion (< 0.4%) passes into the breast milk, leading to an exposure to < 1% of the permitted Gd dose for neonates.12,13
Harmonizing MRI Reports
The consensus recommendations propose reporting the exact lesion count on T2-weighted (T2-w) images when lesions are < 20, or specifying if the number of T2 lesions is between 20 and 50, between 50 and 100, or uncountable, eg, confluent large lesions. Similarly, for the spinal cord, the consensus recommendations propose reporting the exact lesion count on T2-w images when lesions are < 10, or otherwise report that > 10 lesions are seen.
The VHA workgroup proposed reporting a mild, moderate, or severe T2-lesion burden for a T2-lesion count < 20, between 20 and 50, and > 50, respectively. For follow-up MRIs, notation should be made if there is any change in lesion number, indicating the number of new lesions whenever possible. At each timepoint, the presence of active lesions on postcontrast images should be accurately defined.
Dissemination and Implementation
To implement and disseminate these proposed recommendations within the VHA, a workgroup of neurologists and radiologists was formed in late 2020. A review and discussion of the importance of each of the proposed MRI protocols for veterans with MS was held along with possible modifications to balance the intent of meeting standards of care with resources of individual US Department of Veterans Affairs (VA) medical centers and veterans’ needs. The final protocol recommendations were agreed on by group consensus.
In general, this VHA workgroup felt that the current adopted MRI protocols in several VA medical centers (based on previously proposed recommendations) were similar to the ones newly proposed and that implementing changes to meet the 2021 criteria would not be a major challenge.14,15 Possible regional and nonregional barriers were discussed. The result of these discussions led to a modified version of what could be considered more stringent guidelines to accommodate medical centers that had fewer imaging resources. This modified protocol offers a viable alternative that allows for minimizing heterogeneities while recognizing the capabilities of the available scanner fleet and meeting the needs of specific centers or veterans. Finally, the workgroup recognized a fundamental obstacle toward this harmonization process in the heterogeneity in vendors and scanner field strength, factors that have previously limited implementation.
The guidelines and proposed changes were then presented to the VA National Radiology Program Office, examined, and discussed for consensus. No changes were felt to be needed, and the recommendation to implement these guidelines in MS regional programs, whenever possible, was deemed appropriate.
At this time, a focused communication plan has been implemented to diffuse the use of this protocol at MS regional programs in the MSCoE network. We will work iteratively with individual sites to practically apply the guidelines, learn about challenges, and work through them to optimize local implementation.
Conclusions
Standardized MRI protocols are fundamental for the care of veterans with MS. Mitigating interscan variabilities should be recognized as a priority by scientific and clinical expert committees. Several guidelines have been developed over the years to standardize MRI acquisition protocols and interpretations, while updating the same to the latest discoveries.4,5,8,14,15 The VHA has been historically committed to these international efforts, with the goal to excel in the care of veterans with MS by providing access to state-of-the-art technologies. To this end, the initial Consortium of MS Centers MRI protocol was implemented in several MSCoE VA Regional Program sites a decade ago.14 Efforts continue to update protocol recommendations as needed and to promote their dissemination across the VHA enterprise.
This commentary is part of the continuous effort of the MSCoE to align with contemporary guidelines, apply the highest scientific standards, and achieve consistent outcomes for veterans with MS. For more important details of the clinical scenarios when additional/optional sequences or scans can be acquired, we advise the reader to refer to the 2021 MAGNIMS-CMSC-NAIMS Consensus Recommendations on the Use of MRI in Patients With Multiple Sclerosis.8
1. Wallin MT, Culpepper WJ, Campbell JD, et al. The prevalence of MS in the United States: A population-based estimate using health claims data. Neurology. 2019;92(10):e1029-e1040. doi:10.1212/WNL.0000000000007035
2. Nelson LM, Wallin MT, Marrie RA, et al. A new way to estimate neurologic disease prevalence in the United States: Illustrated with MS. Neurology. 2019;92(10):469-480. doi:10.1212/WNL.0000000000007044
3. Culpepper WJ, Wallin MT, Magder LS, et al. VHA Multiple Sclerosis Surveillance Registry and its similarities to other contemporary multiple sclerosis cohorts. J Rehabil Res Dev. 2015;52(3):263-272. doi:10.1682/JRRD.2014.07.0172
4. Wattjes MP, Rovira À, Miller D, et al. Evidence-based guidelines: MAGNIMS consensus guidelines on the use of MRI in multiple sclerosis--establishing disease prognosis and monitoring patients. Nat Rev Neurol. 2015;11(10):597-606. doi:10.1038/nrneurol.2015.157
5. Rovira À, Wattjes MP, Tintoré M, et al. Evidence-based guidelines: MAGNIMS consensus guidelines on the use of MRI in multiple sclerosis-clinical implementation in the diagnostic process. Nat Rev Neurol. 2015;11(8):471-482. doi:10.1038/nrneurol.2015.106
6. Thompson AJ, Banwell BL, Barkhof F, et al. Diagnosis of multiple sclerosis: 2017 revisions of the McDonald criteria. Lancet Neurol. 2018;17(2):162-173. doi:10.1016/S1474-4422(17)30470-2
7. Reich DS, Lucchinetti CF, Calabresi PA. Multiple sclerosis. N Engl J Med. 2018;378(2):169-180. doi:10.1056/NEJMra1401483
8. Wattjes MP, Ciccarelli O, Reich DS, et al. 2021 MAGNIMS-CMSC-NAIMS consensus recommendations on the use of MRI in patients with multiple sclerosis. Lancet Neurol. 2021;20(8):653-670. doi:10.1016/S1474-4422(21)00095-8
9. Saslow L, Li DKB, Halper J, et al. An International Standardized Magnetic Resonance Imaging Protocol for Diagnosis and Follow-up of Patients with Multiple Sclerosis: Advocacy, Dissemination, and Implementation Strategies. Int J MS Care. 2020;22(5):226-232. doi:10.7224/1537-2073.2020-094
10. Cameron MH, Haselkorn JK, Wallin MT. The Multiple Sclerosis Centers of Excellence: a model of excellence in the VA. Fed Pract. 2020;37(suppl 1):S6-S10.
11. Grossman RI, Gonzalez-Scarano F, Atlas SW, Galetta S, Silberberg DH. Multiple sclerosis: gadolinium enhancement in MR imaging. Radiology. 1986;161(3):721-725. doi:10.1148/radiology.161.3.3786722
12. European Society of Urogenital Radiology. ESUR guidelines on contrast agent, 10.0. March 2018. Accessed March 11, 2022. https://www.esur.org/fileadmin/content/2019/ESUR_Guidelines_10.0_Final_Version.pdf
13. Sundgren PC, Leander P. Is administration of gadolinium-based contrast media to pregnant women and small children justified?. J Magn Reson Imaging. 2011;34(4):750-757. doi:10.1002/jmri.22413
14. Simon JH, Li D, Traboulsee A, et al. Standardized MR imaging protocol for multiple sclerosis: Consortium of MS Centers consensus guidelines. AJNR Am J Neuroradiol. 2006;27(2):455-461.
15. Traboulsee A, Simon JH, Stone L, et al. Revised Recommendations of the Consortium of MS Centers Task Force for a Standardized MRI Protocol and Clinical Guidelines for the Diagnosis and Follow-Up of Multiple Sclerosis. AJNR Am J Neuroradiol. 2016;37(3):394-401. doi:10.3174/ajnr.A4539
Multiple sclerosis (MS) is a lifelong disease that affects about a million people in the United States.1,2 Since 1998 more than 45,000 veterans have been diagnosed with MS and about 20,000 are evaluated in the Veterans Health Administration (VHA) annually.3
Magnetic resonance imaging (MRI) is a cornerstone for the assessment of persons with multiple sclerosis (pwMS).4-6 MRI assists with disease diagnosis, allowing for timely therapeutic interventions and withthe evaluation of its progression, treatment effect, and safety. 4,5 MRI-based outcomes also are used as primary endpoints in clinical trials.4,5
MS has its clinical onset in early adulthood in most individuals and is diagnosed at a mean age of 30 years.7 As a result, pwMS may receive care and MRIs in different facilities during their lifetime. Mitigating interscan variabilities that can challenge intra- and interperson comparisons is crucial for accurate care. Radiologists may find it difficult to compare scans acquired in different facilities, as dissimilarities in acquisition protocols may mask or uncover focal disease, creating false negative or false positive findings. Moreover, lack of a standardized method to report MRI changes may compromise neurologists’ ability to correctly interpret scans and disease progression.
Accordingly, in October 2019, an international task force of neurologists, radiologists, MRI technologists, and imaging scientists with expertise in MS, including representatives from the VHA, worked together to update guidelines for imaging the brain, spinal cord, and optic nerve in pwMS.8,9 Recognizing the importance of this effort, the VHA Multiple Sclerosis Centers of Excellence (MSCoE), in collaboration with a team of subject matter expert neuroradiologists promptly committed to this effort, advocating the updated consensus recommendations, and favoring their dissemination within the VHA.10
As part of this commitment and dissemination effort, in this report we summarize the core points of the newly proposed MRI guidelines and ways to adapt them for use within the VHA. We then discuss key elements for their successful implementation and dissemination, specifically regarding the clinical operations of VHA.
Updated Guidelines
MRI Scan at Different Timepoints of MS
There are 3 crucial milestones within a the lifespan of a pwMS that require an MRI to reach appropriate conclusions and avoid clinical errors. These include the initial diagnosis, the follow-up to monitor disease and/or treatment effect, and the assessment of medication safety.
In the interest of efficiency, MRI protocols may vary slightly depending on these clinical indications. The Table lists core sequences of the updated 2021 consensus recommendations at each timepoint along with the proposed alternatives or preferences from the VHA workgroup.
At the time of diagnosis, both brain and spine (cervical and thoracic) MRIs are recommended. Routine MRI of the optic nerve is considered optional at diagnosis. However, imaging the optic nerve may be useful in specific clinical scenarios when the optic nerve is selectively involved, and the diagnosis or etiology of an optic neuritis is not clear. A repeat brain MRI is advised every 6 to 12 months in patients with clinically or radiologically isolated syndrome who do not fulfill the diagnostic criteria of MS but present risk factors for conversion to MS or paraclinical features of it.
Once the diagnosis is established, brain MRI is recommended for follow-up and for surveillance of drug safety. Spinal cord and optic nerve MRIs are desirable but optional in the follow-up of pwMS and are not required for drug surveillance. Spinal cord MRIs are required at follow-up for patients whose progression cannot be explained by brain MRI features, or who manifest with recurrent spinal cord symptoms, or have spinal cord comorbidities. In these cases, spinal cord MRI also may assist with treatment decisions. Similarly, optic nerve MRI is necessary during follow-up only when optic nerve comorbidities are suspected or when there is progression or reoccurrence of optic nerve–related symptoms.
Brain MRIs are recommended for monitoring drug effect yearly (or at longer intervals, after a few years of disease stability). Conversely, a repeat brain MRI is advised after 6 months if nonsymptomatic radiological disease activity is discovered on surveillance scans.
Abbreviated but more frequent serial brain MRI protocols (eg, every 3 to 4 months) are recommended for pwMS treated with natalizumab and at high risk of developing progressive multifocal leukoencephalopathy (eg, pwMS who are John Cunningham virus [JCV]–positive, and have been treated with natalizumabfor ≥ 18 months, have a JCV antibody index > 0.9, or have a history of immunosuppression). A similar approach is recommended for carryover cases, such as those with high JCV antibody index who are switched to other immunosuppressive treatments.
MRI Field, Scan Resolution, and Coverage
Both 1.5-Tesla (1.5-T) and 3-T scans are believed to be equally effective in imaging pwMS, providing that the 1.5-T scans are good quality. Although imaging at < 1.5 T is not recommended due to suboptimal disease detection, the use of scanners > 3 T is equally discouraged outside the supervision of trained investigators. Signal-to-noise ratio and resolution are key factors impacting scan quality, and their optimization is prioritized over the number of sequences in the updated 2021 consensus recommendations. For brain imaging, a resolution of 1 mm3 isotropic is preferred for 3-dimensional (3D) imaging and slice thickness ≤ 3 mm without gap (≤ 5 mm with 10-30% gaps for diffusion-weighted imaging only) is recommended for 2D sequences. Images should cover the entire brain and as much of the cervical spine as possible; images should be prescribed axial for 2D or reformatted axial oblique for 3D using the subcallosal plane as reference. For spine imaging, sites should aim at an in-plane resolution of 1 mm2; using sagittal slices ≤ 3 mm thick and axial slices ≤ 5 mm thick, both with no gap. Scans should cover the entire cervical and thoracolumbar region inclusive of the conus. For the optic nerve images, slices should be ≤ 2 or 3 mm thick with an in-plane resolution of 1 mm2. Images should be aligned to the orientation of the optic nerve and chiasms, both of which should be entirely covered.
Postgadolinium Images Use
The discovery of the higher sensitivity of post-gadolinium (Gd) T1-weighted (T1-w) MRI relative to high iodine (88.1 g I) computed tomography scans in demonstrating contrast-enhancing MS lesions has revolutionized the way clinicians diagnose and monitor this disease.11 However, in recent years the role of postcontrast MRI has been debated, considering the potential safety concerns secondary to Gd tissue deposition. For this reason, an intentionally more judicious use of postcontrast MRI is proposed by the consensus recommendations. At disease diagnosis, the use of Gd is advisable to (1) show disease dissemination in time; (2) differentiate the diagnosis based on the Gd pattern; (3) predict short-term disease activity; and (4) characterize activity in the setting of progression. When monitoring pwMS, the use of Gd may be useful in the first year of follow-up, particularly if in the setting of low potency medications or for patients for whom the detection of one or more active lesions would lead to a change in disease-modifying agents. Gd also should be used to first, confirm a clinical exacerbation (if needed); second, further characterize a lesion suggestive of progressive multifocal encephalopathy or monitor this disease over time; and third, monitor lesion burden change in patients with large confluent lesions, the count of which otherwise may be difficult.
MRI During Pregnancy and Lactation
The consensus recommendations state that Gd contrast–enhanced MRI is not absolutely contraindicated during pregnancy, although its use should be limited to strictly necessary situations, particularly those involving differential diagnosis, such as cerebral venous thrombosis or monitoring of possibly enlarging lesion burden. The use of Gd is not contraindicated during lactation, as only a small proportion (< 0.4%) passes into the breast milk, leading to an exposure to < 1% of the permitted Gd dose for neonates.12,13
Harmonizing MRI Reports
The consensus recommendations propose reporting the exact lesion count on T2-weighted (T2-w) images when lesions are < 20, or specifying if the number of T2 lesions is between 20 and 50, between 50 and 100, or uncountable, eg, confluent large lesions. Similarly, for the spinal cord, the consensus recommendations propose reporting the exact lesion count on T2-w images when lesions are < 10, or otherwise report that > 10 lesions are seen.
The VHA workgroup proposed reporting a mild, moderate, or severe T2-lesion burden for a T2-lesion count < 20, between 20 and 50, and > 50, respectively. For follow-up MRIs, notation should be made if there is any change in lesion number, indicating the number of new lesions whenever possible. At each timepoint, the presence of active lesions on postcontrast images should be accurately defined.
Dissemination and Implementation
To implement and disseminate these proposed recommendations within the VHA, a workgroup of neurologists and radiologists was formed in late 2020. A review and discussion of the importance of each of the proposed MRI protocols for veterans with MS was held along with possible modifications to balance the intent of meeting standards of care with resources of individual US Department of Veterans Affairs (VA) medical centers and veterans’ needs. The final protocol recommendations were agreed on by group consensus.
In general, this VHA workgroup felt that the current adopted MRI protocols in several VA medical centers (based on previously proposed recommendations) were similar to the ones newly proposed and that implementing changes to meet the 2021 criteria would not be a major challenge.14,15 Possible regional and nonregional barriers were discussed. The result of these discussions led to a modified version of what could be considered more stringent guidelines to accommodate medical centers that had fewer imaging resources. This modified protocol offers a viable alternative that allows for minimizing heterogeneities while recognizing the capabilities of the available scanner fleet and meeting the needs of specific centers or veterans. Finally, the workgroup recognized a fundamental obstacle toward this harmonization process in the heterogeneity in vendors and scanner field strength, factors that have previously limited implementation.
The guidelines and proposed changes were then presented to the VA National Radiology Program Office, examined, and discussed for consensus. No changes were felt to be needed, and the recommendation to implement these guidelines in MS regional programs, whenever possible, was deemed appropriate.
At this time, a focused communication plan has been implemented to diffuse the use of this protocol at MS regional programs in the MSCoE network. We will work iteratively with individual sites to practically apply the guidelines, learn about challenges, and work through them to optimize local implementation.
Conclusions
Standardized MRI protocols are fundamental for the care of veterans with MS. Mitigating interscan variabilities should be recognized as a priority by scientific and clinical expert committees. Several guidelines have been developed over the years to standardize MRI acquisition protocols and interpretations, while updating the same to the latest discoveries.4,5,8,14,15 The VHA has been historically committed to these international efforts, with the goal to excel in the care of veterans with MS by providing access to state-of-the-art technologies. To this end, the initial Consortium of MS Centers MRI protocol was implemented in several MSCoE VA Regional Program sites a decade ago.14 Efforts continue to update protocol recommendations as needed and to promote their dissemination across the VHA enterprise.
This commentary is part of the continuous effort of the MSCoE to align with contemporary guidelines, apply the highest scientific standards, and achieve consistent outcomes for veterans with MS. For more important details of the clinical scenarios when additional/optional sequences or scans can be acquired, we advise the reader to refer to the 2021 MAGNIMS-CMSC-NAIMS Consensus Recommendations on the Use of MRI in Patients With Multiple Sclerosis.8
Multiple sclerosis (MS) is a lifelong disease that affects about a million people in the United States.1,2 Since 1998 more than 45,000 veterans have been diagnosed with MS and about 20,000 are evaluated in the Veterans Health Administration (VHA) annually.3
Magnetic resonance imaging (MRI) is a cornerstone for the assessment of persons with multiple sclerosis (pwMS).4-6 MRI assists with disease diagnosis, allowing for timely therapeutic interventions and withthe evaluation of its progression, treatment effect, and safety. 4,5 MRI-based outcomes also are used as primary endpoints in clinical trials.4,5
MS has its clinical onset in early adulthood in most individuals and is diagnosed at a mean age of 30 years.7 As a result, pwMS may receive care and MRIs in different facilities during their lifetime. Mitigating interscan variabilities that can challenge intra- and interperson comparisons is crucial for accurate care. Radiologists may find it difficult to compare scans acquired in different facilities, as dissimilarities in acquisition protocols may mask or uncover focal disease, creating false negative or false positive findings. Moreover, lack of a standardized method to report MRI changes may compromise neurologists’ ability to correctly interpret scans and disease progression.
Accordingly, in October 2019, an international task force of neurologists, radiologists, MRI technologists, and imaging scientists with expertise in MS, including representatives from the VHA, worked together to update guidelines for imaging the brain, spinal cord, and optic nerve in pwMS.8,9 Recognizing the importance of this effort, the VHA Multiple Sclerosis Centers of Excellence (MSCoE), in collaboration with a team of subject matter expert neuroradiologists promptly committed to this effort, advocating the updated consensus recommendations, and favoring their dissemination within the VHA.10
As part of this commitment and dissemination effort, in this report we summarize the core points of the newly proposed MRI guidelines and ways to adapt them for use within the VHA. We then discuss key elements for their successful implementation and dissemination, specifically regarding the clinical operations of VHA.
Updated Guidelines
MRI Scan at Different Timepoints of MS
There are 3 crucial milestones within a the lifespan of a pwMS that require an MRI to reach appropriate conclusions and avoid clinical errors. These include the initial diagnosis, the follow-up to monitor disease and/or treatment effect, and the assessment of medication safety.
In the interest of efficiency, MRI protocols may vary slightly depending on these clinical indications. The Table lists core sequences of the updated 2021 consensus recommendations at each timepoint along with the proposed alternatives or preferences from the VHA workgroup.
At the time of diagnosis, both brain and spine (cervical and thoracic) MRIs are recommended. Routine MRI of the optic nerve is considered optional at diagnosis. However, imaging the optic nerve may be useful in specific clinical scenarios when the optic nerve is selectively involved, and the diagnosis or etiology of an optic neuritis is not clear. A repeat brain MRI is advised every 6 to 12 months in patients with clinically or radiologically isolated syndrome who do not fulfill the diagnostic criteria of MS but present risk factors for conversion to MS or paraclinical features of it.
Once the diagnosis is established, brain MRI is recommended for follow-up and for surveillance of drug safety. Spinal cord and optic nerve MRIs are desirable but optional in the follow-up of pwMS and are not required for drug surveillance. Spinal cord MRIs are required at follow-up for patients whose progression cannot be explained by brain MRI features, or who manifest with recurrent spinal cord symptoms, or have spinal cord comorbidities. In these cases, spinal cord MRI also may assist with treatment decisions. Similarly, optic nerve MRI is necessary during follow-up only when optic nerve comorbidities are suspected or when there is progression or reoccurrence of optic nerve–related symptoms.
Brain MRIs are recommended for monitoring drug effect yearly (or at longer intervals, after a few years of disease stability). Conversely, a repeat brain MRI is advised after 6 months if nonsymptomatic radiological disease activity is discovered on surveillance scans.
Abbreviated but more frequent serial brain MRI protocols (eg, every 3 to 4 months) are recommended for pwMS treated with natalizumab and at high risk of developing progressive multifocal leukoencephalopathy (eg, pwMS who are John Cunningham virus [JCV]–positive, and have been treated with natalizumabfor ≥ 18 months, have a JCV antibody index > 0.9, or have a history of immunosuppression). A similar approach is recommended for carryover cases, such as those with high JCV antibody index who are switched to other immunosuppressive treatments.
MRI Field, Scan Resolution, and Coverage
Both 1.5-Tesla (1.5-T) and 3-T scans are believed to be equally effective in imaging pwMS, providing that the 1.5-T scans are good quality. Although imaging at < 1.5 T is not recommended due to suboptimal disease detection, the use of scanners > 3 T is equally discouraged outside the supervision of trained investigators. Signal-to-noise ratio and resolution are key factors impacting scan quality, and their optimization is prioritized over the number of sequences in the updated 2021 consensus recommendations. For brain imaging, a resolution of 1 mm3 isotropic is preferred for 3-dimensional (3D) imaging and slice thickness ≤ 3 mm without gap (≤ 5 mm with 10-30% gaps for diffusion-weighted imaging only) is recommended for 2D sequences. Images should cover the entire brain and as much of the cervical spine as possible; images should be prescribed axial for 2D or reformatted axial oblique for 3D using the subcallosal plane as reference. For spine imaging, sites should aim at an in-plane resolution of 1 mm2; using sagittal slices ≤ 3 mm thick and axial slices ≤ 5 mm thick, both with no gap. Scans should cover the entire cervical and thoracolumbar region inclusive of the conus. For the optic nerve images, slices should be ≤ 2 or 3 mm thick with an in-plane resolution of 1 mm2. Images should be aligned to the orientation of the optic nerve and chiasms, both of which should be entirely covered.
Postgadolinium Images Use
The discovery of the higher sensitivity of post-gadolinium (Gd) T1-weighted (T1-w) MRI relative to high iodine (88.1 g I) computed tomography scans in demonstrating contrast-enhancing MS lesions has revolutionized the way clinicians diagnose and monitor this disease.11 However, in recent years the role of postcontrast MRI has been debated, considering the potential safety concerns secondary to Gd tissue deposition. For this reason, an intentionally more judicious use of postcontrast MRI is proposed by the consensus recommendations. At disease diagnosis, the use of Gd is advisable to (1) show disease dissemination in time; (2) differentiate the diagnosis based on the Gd pattern; (3) predict short-term disease activity; and (4) characterize activity in the setting of progression. When monitoring pwMS, the use of Gd may be useful in the first year of follow-up, particularly if in the setting of low potency medications or for patients for whom the detection of one or more active lesions would lead to a change in disease-modifying agents. Gd also should be used to first, confirm a clinical exacerbation (if needed); second, further characterize a lesion suggestive of progressive multifocal encephalopathy or monitor this disease over time; and third, monitor lesion burden change in patients with large confluent lesions, the count of which otherwise may be difficult.
MRI During Pregnancy and Lactation
The consensus recommendations state that Gd contrast–enhanced MRI is not absolutely contraindicated during pregnancy, although its use should be limited to strictly necessary situations, particularly those involving differential diagnosis, such as cerebral venous thrombosis or monitoring of possibly enlarging lesion burden. The use of Gd is not contraindicated during lactation, as only a small proportion (< 0.4%) passes into the breast milk, leading to an exposure to < 1% of the permitted Gd dose for neonates.12,13
Harmonizing MRI Reports
The consensus recommendations propose reporting the exact lesion count on T2-weighted (T2-w) images when lesions are < 20, or specifying if the number of T2 lesions is between 20 and 50, between 50 and 100, or uncountable, eg, confluent large lesions. Similarly, for the spinal cord, the consensus recommendations propose reporting the exact lesion count on T2-w images when lesions are < 10, or otherwise report that > 10 lesions are seen.
The VHA workgroup proposed reporting a mild, moderate, or severe T2-lesion burden for a T2-lesion count < 20, between 20 and 50, and > 50, respectively. For follow-up MRIs, notation should be made if there is any change in lesion number, indicating the number of new lesions whenever possible. At each timepoint, the presence of active lesions on postcontrast images should be accurately defined.
Dissemination and Implementation
To implement and disseminate these proposed recommendations within the VHA, a workgroup of neurologists and radiologists was formed in late 2020. A review and discussion of the importance of each of the proposed MRI protocols for veterans with MS was held along with possible modifications to balance the intent of meeting standards of care with resources of individual US Department of Veterans Affairs (VA) medical centers and veterans’ needs. The final protocol recommendations were agreed on by group consensus.
In general, this VHA workgroup felt that the current adopted MRI protocols in several VA medical centers (based on previously proposed recommendations) were similar to the ones newly proposed and that implementing changes to meet the 2021 criteria would not be a major challenge.14,15 Possible regional and nonregional barriers were discussed. The result of these discussions led to a modified version of what could be considered more stringent guidelines to accommodate medical centers that had fewer imaging resources. This modified protocol offers a viable alternative that allows for minimizing heterogeneities while recognizing the capabilities of the available scanner fleet and meeting the needs of specific centers or veterans. Finally, the workgroup recognized a fundamental obstacle toward this harmonization process in the heterogeneity in vendors and scanner field strength, factors that have previously limited implementation.
The guidelines and proposed changes were then presented to the VA National Radiology Program Office, examined, and discussed for consensus. No changes were felt to be needed, and the recommendation to implement these guidelines in MS regional programs, whenever possible, was deemed appropriate.
At this time, a focused communication plan has been implemented to diffuse the use of this protocol at MS regional programs in the MSCoE network. We will work iteratively with individual sites to practically apply the guidelines, learn about challenges, and work through them to optimize local implementation.
Conclusions
Standardized MRI protocols are fundamental for the care of veterans with MS. Mitigating interscan variabilities should be recognized as a priority by scientific and clinical expert committees. Several guidelines have been developed over the years to standardize MRI acquisition protocols and interpretations, while updating the same to the latest discoveries.4,5,8,14,15 The VHA has been historically committed to these international efforts, with the goal to excel in the care of veterans with MS by providing access to state-of-the-art technologies. To this end, the initial Consortium of MS Centers MRI protocol was implemented in several MSCoE VA Regional Program sites a decade ago.14 Efforts continue to update protocol recommendations as needed and to promote their dissemination across the VHA enterprise.
This commentary is part of the continuous effort of the MSCoE to align with contemporary guidelines, apply the highest scientific standards, and achieve consistent outcomes for veterans with MS. For more important details of the clinical scenarios when additional/optional sequences or scans can be acquired, we advise the reader to refer to the 2021 MAGNIMS-CMSC-NAIMS Consensus Recommendations on the Use of MRI in Patients With Multiple Sclerosis.8
1. Wallin MT, Culpepper WJ, Campbell JD, et al. The prevalence of MS in the United States: A population-based estimate using health claims data. Neurology. 2019;92(10):e1029-e1040. doi:10.1212/WNL.0000000000007035
2. Nelson LM, Wallin MT, Marrie RA, et al. A new way to estimate neurologic disease prevalence in the United States: Illustrated with MS. Neurology. 2019;92(10):469-480. doi:10.1212/WNL.0000000000007044
3. Culpepper WJ, Wallin MT, Magder LS, et al. VHA Multiple Sclerosis Surveillance Registry and its similarities to other contemporary multiple sclerosis cohorts. J Rehabil Res Dev. 2015;52(3):263-272. doi:10.1682/JRRD.2014.07.0172
4. Wattjes MP, Rovira À, Miller D, et al. Evidence-based guidelines: MAGNIMS consensus guidelines on the use of MRI in multiple sclerosis--establishing disease prognosis and monitoring patients. Nat Rev Neurol. 2015;11(10):597-606. doi:10.1038/nrneurol.2015.157
5. Rovira À, Wattjes MP, Tintoré M, et al. Evidence-based guidelines: MAGNIMS consensus guidelines on the use of MRI in multiple sclerosis-clinical implementation in the diagnostic process. Nat Rev Neurol. 2015;11(8):471-482. doi:10.1038/nrneurol.2015.106
6. Thompson AJ, Banwell BL, Barkhof F, et al. Diagnosis of multiple sclerosis: 2017 revisions of the McDonald criteria. Lancet Neurol. 2018;17(2):162-173. doi:10.1016/S1474-4422(17)30470-2
7. Reich DS, Lucchinetti CF, Calabresi PA. Multiple sclerosis. N Engl J Med. 2018;378(2):169-180. doi:10.1056/NEJMra1401483
8. Wattjes MP, Ciccarelli O, Reich DS, et al. 2021 MAGNIMS-CMSC-NAIMS consensus recommendations on the use of MRI in patients with multiple sclerosis. Lancet Neurol. 2021;20(8):653-670. doi:10.1016/S1474-4422(21)00095-8
9. Saslow L, Li DKB, Halper J, et al. An International Standardized Magnetic Resonance Imaging Protocol for Diagnosis and Follow-up of Patients with Multiple Sclerosis: Advocacy, Dissemination, and Implementation Strategies. Int J MS Care. 2020;22(5):226-232. doi:10.7224/1537-2073.2020-094
10. Cameron MH, Haselkorn JK, Wallin MT. The Multiple Sclerosis Centers of Excellence: a model of excellence in the VA. Fed Pract. 2020;37(suppl 1):S6-S10.
11. Grossman RI, Gonzalez-Scarano F, Atlas SW, Galetta S, Silberberg DH. Multiple sclerosis: gadolinium enhancement in MR imaging. Radiology. 1986;161(3):721-725. doi:10.1148/radiology.161.3.3786722
12. European Society of Urogenital Radiology. ESUR guidelines on contrast agent, 10.0. March 2018. Accessed March 11, 2022. https://www.esur.org/fileadmin/content/2019/ESUR_Guidelines_10.0_Final_Version.pdf
13. Sundgren PC, Leander P. Is administration of gadolinium-based contrast media to pregnant women and small children justified?. J Magn Reson Imaging. 2011;34(4):750-757. doi:10.1002/jmri.22413
14. Simon JH, Li D, Traboulsee A, et al. Standardized MR imaging protocol for multiple sclerosis: Consortium of MS Centers consensus guidelines. AJNR Am J Neuroradiol. 2006;27(2):455-461.
15. Traboulsee A, Simon JH, Stone L, et al. Revised Recommendations of the Consortium of MS Centers Task Force for a Standardized MRI Protocol and Clinical Guidelines for the Diagnosis and Follow-Up of Multiple Sclerosis. AJNR Am J Neuroradiol. 2016;37(3):394-401. doi:10.3174/ajnr.A4539
1. Wallin MT, Culpepper WJ, Campbell JD, et al. The prevalence of MS in the United States: A population-based estimate using health claims data. Neurology. 2019;92(10):e1029-e1040. doi:10.1212/WNL.0000000000007035
2. Nelson LM, Wallin MT, Marrie RA, et al. A new way to estimate neurologic disease prevalence in the United States: Illustrated with MS. Neurology. 2019;92(10):469-480. doi:10.1212/WNL.0000000000007044
3. Culpepper WJ, Wallin MT, Magder LS, et al. VHA Multiple Sclerosis Surveillance Registry and its similarities to other contemporary multiple sclerosis cohorts. J Rehabil Res Dev. 2015;52(3):263-272. doi:10.1682/JRRD.2014.07.0172
4. Wattjes MP, Rovira À, Miller D, et al. Evidence-based guidelines: MAGNIMS consensus guidelines on the use of MRI in multiple sclerosis--establishing disease prognosis and monitoring patients. Nat Rev Neurol. 2015;11(10):597-606. doi:10.1038/nrneurol.2015.157
5. Rovira À, Wattjes MP, Tintoré M, et al. Evidence-based guidelines: MAGNIMS consensus guidelines on the use of MRI in multiple sclerosis-clinical implementation in the diagnostic process. Nat Rev Neurol. 2015;11(8):471-482. doi:10.1038/nrneurol.2015.106
6. Thompson AJ, Banwell BL, Barkhof F, et al. Diagnosis of multiple sclerosis: 2017 revisions of the McDonald criteria. Lancet Neurol. 2018;17(2):162-173. doi:10.1016/S1474-4422(17)30470-2
7. Reich DS, Lucchinetti CF, Calabresi PA. Multiple sclerosis. N Engl J Med. 2018;378(2):169-180. doi:10.1056/NEJMra1401483
8. Wattjes MP, Ciccarelli O, Reich DS, et al. 2021 MAGNIMS-CMSC-NAIMS consensus recommendations on the use of MRI in patients with multiple sclerosis. Lancet Neurol. 2021;20(8):653-670. doi:10.1016/S1474-4422(21)00095-8
9. Saslow L, Li DKB, Halper J, et al. An International Standardized Magnetic Resonance Imaging Protocol for Diagnosis and Follow-up of Patients with Multiple Sclerosis: Advocacy, Dissemination, and Implementation Strategies. Int J MS Care. 2020;22(5):226-232. doi:10.7224/1537-2073.2020-094
10. Cameron MH, Haselkorn JK, Wallin MT. The Multiple Sclerosis Centers of Excellence: a model of excellence in the VA. Fed Pract. 2020;37(suppl 1):S6-S10.
11. Grossman RI, Gonzalez-Scarano F, Atlas SW, Galetta S, Silberberg DH. Multiple sclerosis: gadolinium enhancement in MR imaging. Radiology. 1986;161(3):721-725. doi:10.1148/radiology.161.3.3786722
12. European Society of Urogenital Radiology. ESUR guidelines on contrast agent, 10.0. March 2018. Accessed March 11, 2022. https://www.esur.org/fileadmin/content/2019/ESUR_Guidelines_10.0_Final_Version.pdf
13. Sundgren PC, Leander P. Is administration of gadolinium-based contrast media to pregnant women and small children justified?. J Magn Reson Imaging. 2011;34(4):750-757. doi:10.1002/jmri.22413
14. Simon JH, Li D, Traboulsee A, et al. Standardized MR imaging protocol for multiple sclerosis: Consortium of MS Centers consensus guidelines. AJNR Am J Neuroradiol. 2006;27(2):455-461.
15. Traboulsee A, Simon JH, Stone L, et al. Revised Recommendations of the Consortium of MS Centers Task Force for a Standardized MRI Protocol and Clinical Guidelines for the Diagnosis and Follow-Up of Multiple Sclerosis. AJNR Am J Neuroradiol. 2016;37(3):394-401. doi:10.3174/ajnr.A4539