User login
Vaccines are used to prevent bacterial infections, such as pneumonia, and viral diseases, such as influenza.1 Recently, they are being used to prevent cancers. For example, the human papilloma virus vaccine prevents infection with the virus that is associated with cervical cancer.2 Now there is another purpose for vaccines: They can be used therapeutically. Vaccines can be given even after a person manifests a tumor; they can cause the tumor to shrink or disappear. Therapeutic vaccines hold the potential to effect profound changes in the treatment of several cancers.
Biologics
Biologics, a recently developed class of weapons, can be but aren’t always relatively specific to cancer cells. Biologic agents include inhibitors and monoclonal antibodies. Inhibitors target specific functions, such as angiogenesis. However, inhibitors are not always selective for cancer cells. Angiogenesis inhibitors block formation of blood vessels. Like cytotoxic chemotherapy agents, they block the formation of blood vessels in normal cells as well as cancer cells. Cancer cells, however, generally grow more rapidly than do normal host cells, so they need more blood vessels faster to nourish their cells.3
Some inhibitors are more specific. An inhibitor molecule can be directed against a specific transcript that does not occur in normal cells. In normal cells, the breakpoint cluster region (BCR) gene on chromosome 22 directs synthesis of its protein product, whereas the abelson murine leukemia viral oncogene homolog 1 (ABL1) gene on chromosome 9 specifies another protein. However, in chronic myelogenous leukemia, the ends of those 2 chromosomes translocate, and the BCR/ABL1 transcript is a hybrid of RNA derived from the original chromosome and that of the newly attached, translocated chromosome 9. This hybrid RNA directs synthesis of a fusion protein, a tyrosine kinase; the fusion protein is not produced in normal host cells. Thus imatinib, an inhibitor directed at the fusion protein, inhibits the cancer cells specifically.4
Monoclonal Antibodies
Monoclonal antibodies exploit the host’s immune system to destroy cancer cells. Like the inhibitors, monoclonal antibodies can be directed against a specific functional protein, which is not necessarily specific to tumor cells, or against a protein or family of proteins unique to the cancer cells. For example, trastuzumab is a monoclonal antibody directed against the HER2/neu oncogene, which is amplified in some breast cancers.5 The host’s immune system recognizes the antibodyantigen complex and signals its macrophages to destroy the complex, and the cancer cell dies.
Vaccines also exploit the immune system. One way to immunize against a virus, is to introduce a live or killed virus or a part of a virus—usually a part of a viral protein—into the recipient. The immune system recognizes the foreign virus and makes antibodies. When the immune system is challenged by exposure to the pathogenic virus, the antiviral antibodies recognize and bind to it. The host’s macrophages then engulf the antigenantibody complex and destroy it.6 This type of vaccine prevents infection.
Viral Vaccines
Few vaccines prevent specific cancers. Immunization against the hepatitis B virus, for example, confers immunity to a virus whose infection is a major risk factor for hepatocellular carcinoma.7 Thus it is not truly an anticancer vaccine but rather an antiviral vaccine. Similarly, the HPV
vaccine does not prevent cervical cancer but confers immunity
to a virus that causes cervical cancer.2
Using vaccines to prevent cancer in general is now becoming feasible. Cancer vaccines in use or in development at this time are of 2 general types: those derived from a single tumor and are designed to elicit immunity to that particular tumor in that particular individual host; and those designed to provoke an immune response to that particular type of tumor in any host. The latter type can be mass produced, whereas vaccines of the first type are restricted to the host of origin.
Autologous Vaccines
At the time of biopsy or surgical excision, tumors are mechanically and enzymatically dissociated into single cells, then grown in tissue culture. When they are cultured in suspension with agitation, the cancer stem cells (CSCs) form spheroids, and other cell types do not. The CSCs are the progenitor cells of the specific tumor, much like bone marrow stem cells are the progenitors of the myeloid, erythroid, and megakaryocyte (platelet) cell lineages of human blood. After the CSCs are expanded, the spheroids are harvested, and their RNA is extracted. The messenger RNAs, which specify the proteins made by the CSCs, are converted to complementary DNAs, which are then amplified using the polymerase chain reaction.8
Meanwhile, the patient’s peripheral blood mononuclear cells (PBMCs) are harvested from the blood by leukapheresis. The PBMCs are then enriched for monocytes by immunologic depletion of B and T cells. The monocytes are cultured in the presence of interleukin-4 and granulocytemacrophage-colony-stimulating factor (GM-CSF), and they become immature dendritic cells after 5 days. These immature dendritic cells are transfected with the amplified DNA from the CSC tumor spheres and grown for 2 more days in a medium supplemented with interleukin-1b (IL-1b), interleukin-6 (IL-6), tumor necrosis factor-α, and prostaglandin E2, then tested for markers characteristic of dendritic cells and frozen.
Aliquots of these transfected dendritic cells are injected into the patient at intervals over several months. Dendritic cells, which are the most efficient cells at eliciting an immune response, present the tumor CSC antigens to the patient’s immune system, which develops antibodies to the antigens presented on the dendritic cells and proceeds to destroy the tumor CSCs remaining in the patient’s tumor. Without its CSCs, the tumor is unable to revitalize itself, the rest of the tumor cells eventually die, and the tumor shrinks. The patient is monitored for immune response, and the tumor is regularly imaged. This vaccine is an example of therapeutic use: It can cause tumor regression.8
Sipuleucil-T is another example of an autologous vaccine used for therapeutic purposes. The patient’s antigenpresenting cells (APCs) are harvested by leukopheresis. They are then cultured in the presence of the protein made by fusing prostatic antigen phosphatase (PAP) with the GM-CSF to form the fusion protein PAP-GM-CSF. These modified APCs are then reinjected into the same host. Sipuleucil-T is now FDA approved for the treatment of metastatic castrate-resistant prostate cancer (mCRPC) and has been shown to extend life by approximately 4 months.9
Universal Vaccine for a Specific Tumor
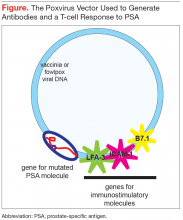
Cancer vaccines can also be prepared in a more conventional manner, analogous to the more commonly used vaccines, and given to all patients with that specific tumor. PROSTVAC is another vaccine for mCRPC as well as for earlier-stage prostate cancer. The DNA specifying prostate-specific antigen (PSA), the secreted protein expressed by the tumor, is mutated very slightly and inserted into a vaccinia virus (Figure). Also inserted into the vaccinia virus are DNAs encoding 3 proteins that
stimulate the patient’s immune system: B7.1, intercellular adhesion molecule-1 (ICAM-1), and leukocyte functionassociated antigen-3 (LFA-3).
This recombinant viral vector is propagated in tissue culture, purified, and administered to the patient subcutaneously. The virus replicates for a short period in the host, expressing the 3 immunostimulatory molecules and the mutated PSA molecule protein product, which differs by 1 amino acid from that expressed by the patient’s tumor. The vaccinia virus and the mutant protein are recognized as foreign by the patient’s immune system, whereas the 3 immunostimulatory molecules are not, as they are the same as the host’s immunostimulatory molecules.
Subsequent boosts, administered over about 5 months, are with a fowlpox virus vector (which does not replicate in the patient) containing the same 4 genes that had been inserted into the vaccinia virus. This strategy enhances the immune response to the mutant tumor product and ensures that the host is not generating a response to the vaccinia virus. The mutant tumor product is sufficiently similar to the patient’s native tumor product that the immune system mounts a response to both proteins. The T cells generated in response to the mutant protein bind to both PSA and mutated PSA molecule proteins, and cells harboring these proteins are destroyed.10
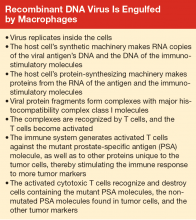
Whereas the T-cell response is important for the activity of the PROSTVAC vaccine, and there is no evidence for formation of anti-PSA antibodies, other vaccines exploit both B- and T-cell responses.10,11 After a virus commandeers the host cell’s replication, transcription, and translation machinery, the macrophage breaks down the viral protein product (the mutated or immunogenic molecule) into small fragments. These fragments bind to major histocompatibility complex (MHC) class II molecules, which are produced by the macrophages. These complexes of antigen (virus) fragments with MHC class II molecules are transported to the surface of the macrophage, where the antigen is presented to lymphocytes. A receptor on a B lymphocyte adheres to the viral antigen and thus becomes an activated B cell, which divides and produces many copies. The B lymphocytes mature into plasma cells and release antibodies that adhere to the virus. A helper T-cell receptor can adhere to the antigen-MHC class II complex displayed on the B cell and activate the lymphocyte, causing the release of more and different cytokines. The cytokines stimulate the activated B cell to divide, producing functionally mature antibodies that recognize and attach to the virus. Macrophages can then engulf and destroy the virus, or the antibody-linked viruses can be excreted in the urine or stool.12
When viruses (eg, the recombinant vaccinia virus of the PROSTVAC vaccine) infect cells and replicate, fragments of viral proteins become attached to MHC class I molecules (see Box). These complexes attach to the cell surface and are presented to cytotoxic T cells. The activated cytotoxic T cells divide and destroy cells harboring the virus or viral antigens.12
Some of the B lymphocytes become memory B cells. Similarly, some of the T lymphocytes become memory T cells. These memory B cells respond and replicate rapidly on re-exposure to the virus or viral proteins to produce antigen-specific antibodies.12
When a macrophage engulfs and breaks down a tumor cell harboring a PSA molecule, other tumor-specific protein fragments are released. These tumor-specific fragments then serve as antigens and elicit an immune response, generating even more antitumor antibodies and T-cell interactions. Thus the tumor regresses. Importantly, this type of vaccine can be mass produced.
Vaccines With Chemotherapy
Another approach to the design of a tumor-specific vaccine is based on tumor-associated peptides (TUMAPs). These TUMAPs are highly overexpressed in tumors relative to a number of normal, healthy tissues. Nine highly immunogenic TUMAPs from 80 specimens of the same tumor type, identified by mass spectrometry, gene expression profiling, literature-based functional assessment, bioinformatics, and human T-cell assays, are used to prepare IMA901, a multipeptide vaccine against the specific tumor, renal cell carcinoma. The pool of selected peptides, composed of 9 to 16 amino acids, is then administered to patients following a dose of the immunomodulator GM-CSF for a total of as many as 17 injections. Low-dose cyclophosphamide administered prior to the first vaccination downregulates regulator T cells, enhancing immune response and resulting in prolonged survival.13 This type of vaccine can also be mass produced.
Conclusions
The vaccines that are currently in use or in clinical trials are being used in patients with advanced disease. As such, they are therapeutic rather than prophylactic. It is inevitable that more vaccines will earn FDA approval. Eventually, they will be used in earlier-stage disease. It is conceivable that some can be used prophylactically when a malignancy first becomes detectable, especially as more sensitive detection methods are developed. Vaccine delivery systems are likely to change as researchers investigate and adopt matrix materials that increase efficacy.
Cancer vaccines hold the potential for greater selectivity in treatment of malignancies. These vaccines may enable us to use cytotoxic chemotherapy agents less often or perhaps more effectively when used in conjunction with vaccines. Vaccines that can be mass produced could conceivably decrease the financial burden of cancer treatment as well as the human cost of malignant diseases and their therapy and care requirements.
Solid-tumor and hematologic malignancy vaccines are coming into wider use as the science is better understood and more methods of generating immune responses are explored. A personalized cancer vaccine, sipuleucel-T, is FDA approved for clinical use against mCRPC.9 A poxvirus-based vaccine, PROSTVAC, also against mCRPC, is in phase 3 clinical trials.10,11 The multipeptide renal cell carcinoma vaccine IMA901 is also in phase 3 clinical trials.13 A personalized vaccine against glioblastoma multiforme CSCs has extended progression-free survival in its initial pretrial study.8,14 Additional glioblastoma, melanoma, and other vaccines are in clinical trials and under development.
Author disclosures
The author reports no actual or potential conflicts of interest with regard to this article.
Disclaimer
The opinions expressed herein are those of the author and do not necessarily reflect those of Federal Practitioner, Frontline Medical Communications Inc., the U.S. Government, or any of its agencies. This article may discuss unlabeled or investigational use of certain drugs. Please review the complete prescribing information for specific drugs or drug combinations—including indications, contraindications, warnings, and adverse effects—before administering pharmacologic therapy to patients.
Click here to continue reading.
1. U.S. Department of Health and Human Services, National Institutes of Health, National Institute of Allergy and Infectious Diseases. Understanding vaccines: what they are, how they work. National Institute of Allergy and Infectious Diseases Website. http://www.niaid.nih.gov/topics/vaccines/documents/undvacc.pdf. Published January 2008. Accessed March 24, 2015. .
2. Hagensee ME, Yaegashi N, Galloway GA. Self-assembly of human papilloma virus type 1 capsids by expression of the L1 protein alone or by coexpression of the L1 and L2 capsid proteins. J Virol. 1993;67(1):315-322.
3. American Cancer Society. Anti-angiogenesis treatment. American Cancer Society Website. http://www.cancer.org/acs/groups/cid/documents/webcontent/002988-pdf.pdf. Revised March 10, 2009. Accessed March 24, 2015.
4. Gambacorti-Passerini CB, Gunby RH, Piazza R, Galietta A, Rostagno R, Scapozza L. Molecular mechanisms of resistance to imatinib in Philadelphia-chromosomepositive leukaemias. Lancet Oncol. 2003;4(2):75-85.
5. Hudis CA. Trastuzumab—mechanism of action and use in clinical practice. N Engl J Med. 2007;357(1):39-51.
6. NPI reference guide on vaccines and vaccine safety: How vaccines work. PATH Website. http://www.path.org/vaccineresources/files/How_Vaccines_Work.pdf. Accessed March 24, 2015.
7. Beasley RP, Hwang LY, Lin CC, Chien CS. Hepatocellular carcinoma and hepatitis B virus: a prospective study of 22 707 men in Taiwan. Lancet. 1981;318(8256):1129-1133.
8. Vik-Mo EO, Nyakas M, Mikkelsen BV, et al. Therapeutic vaccination against autologous cancer stem cells with mRNA-transfected dendritic cells in patients with glioblastoma. Cancer Immunol Immunother. 2013;62(9):1499-1509.
9. Kantoff PW, Higano CS, Shore ND, et al; IMPACT Study Investigators. Sipuleucel-T immunotherapy for castration-resistant prostate cancer. N Engl J Med. 2010;363(5):411-422.
10. Gulley JL, Madan RA, Tsang KY, et al. Immune impact induced by PROSTVAC (PSA-TRICOM), a therapeutic vaccine for prostate cancer. Cancer Immunol Res. 2014;2(2):133-141.
11. Campbell CT, Gulley JL, Oyelaran O, Hodge JW, Schlom J, Gildersleeve JC. Humoral response to a viral glycan correlates with survival on PROSTVAC VF. Proc Natl Acad Sci USA. 2014;111(17):E1749-E1758.
12. Public Health England. Immunity and how vaccines work: The green book, chapter 1. GOV.UK Website. https://www.gov.uk/government/publications/immunity-and-how-vaccines-work-the-green-book-chapter-1. Published March 19, 2013. Accessed March 24, 2015.
13. Walter S, Weinschenk T, Stenzl A, et al. Multipeptide immune response to cancer vaccine IMA901 after single-dose cyclophosphamide associates with longer patient survival. Nat Med. 2012;18(8):1254-1261.
14. Ottenhausen M, Bodhinayake I, Banu M, Kesavabhotla K, Ray A, Boockvar JA. Industry progress report on neuro-oncology: biotech update 2013. J Neurooncol. 2013;115(2):311-316.
Vaccines are used to prevent bacterial infections, such as pneumonia, and viral diseases, such as influenza.1 Recently, they are being used to prevent cancers. For example, the human papilloma virus vaccine prevents infection with the virus that is associated with cervical cancer.2 Now there is another purpose for vaccines: They can be used therapeutically. Vaccines can be given even after a person manifests a tumor; they can cause the tumor to shrink or disappear. Therapeutic vaccines hold the potential to effect profound changes in the treatment of several cancers.
Biologics
Biologics, a recently developed class of weapons, can be but aren’t always relatively specific to cancer cells. Biologic agents include inhibitors and monoclonal antibodies. Inhibitors target specific functions, such as angiogenesis. However, inhibitors are not always selective for cancer cells. Angiogenesis inhibitors block formation of blood vessels. Like cytotoxic chemotherapy agents, they block the formation of blood vessels in normal cells as well as cancer cells. Cancer cells, however, generally grow more rapidly than do normal host cells, so they need more blood vessels faster to nourish their cells.3
Some inhibitors are more specific. An inhibitor molecule can be directed against a specific transcript that does not occur in normal cells. In normal cells, the breakpoint cluster region (BCR) gene on chromosome 22 directs synthesis of its protein product, whereas the abelson murine leukemia viral oncogene homolog 1 (ABL1) gene on chromosome 9 specifies another protein. However, in chronic myelogenous leukemia, the ends of those 2 chromosomes translocate, and the BCR/ABL1 transcript is a hybrid of RNA derived from the original chromosome and that of the newly attached, translocated chromosome 9. This hybrid RNA directs synthesis of a fusion protein, a tyrosine kinase; the fusion protein is not produced in normal host cells. Thus imatinib, an inhibitor directed at the fusion protein, inhibits the cancer cells specifically.4
Monoclonal Antibodies
Monoclonal antibodies exploit the host’s immune system to destroy cancer cells. Like the inhibitors, monoclonal antibodies can be directed against a specific functional protein, which is not necessarily specific to tumor cells, or against a protein or family of proteins unique to the cancer cells. For example, trastuzumab is a monoclonal antibody directed against the HER2/neu oncogene, which is amplified in some breast cancers.5 The host’s immune system recognizes the antibodyantigen complex and signals its macrophages to destroy the complex, and the cancer cell dies.
Vaccines also exploit the immune system. One way to immunize against a virus, is to introduce a live or killed virus or a part of a virus—usually a part of a viral protein—into the recipient. The immune system recognizes the foreign virus and makes antibodies. When the immune system is challenged by exposure to the pathogenic virus, the antiviral antibodies recognize and bind to it. The host’s macrophages then engulf the antigenantibody complex and destroy it.6 This type of vaccine prevents infection.
Viral Vaccines
Few vaccines prevent specific cancers. Immunization against the hepatitis B virus, for example, confers immunity to a virus whose infection is a major risk factor for hepatocellular carcinoma.7 Thus it is not truly an anticancer vaccine but rather an antiviral vaccine. Similarly, the HPV
vaccine does not prevent cervical cancer but confers immunity
to a virus that causes cervical cancer.2
Using vaccines to prevent cancer in general is now becoming feasible. Cancer vaccines in use or in development at this time are of 2 general types: those derived from a single tumor and are designed to elicit immunity to that particular tumor in that particular individual host; and those designed to provoke an immune response to that particular type of tumor in any host. The latter type can be mass produced, whereas vaccines of the first type are restricted to the host of origin.
Autologous Vaccines
At the time of biopsy or surgical excision, tumors are mechanically and enzymatically dissociated into single cells, then grown in tissue culture. When they are cultured in suspension with agitation, the cancer stem cells (CSCs) form spheroids, and other cell types do not. The CSCs are the progenitor cells of the specific tumor, much like bone marrow stem cells are the progenitors of the myeloid, erythroid, and megakaryocyte (platelet) cell lineages of human blood. After the CSCs are expanded, the spheroids are harvested, and their RNA is extracted. The messenger RNAs, which specify the proteins made by the CSCs, are converted to complementary DNAs, which are then amplified using the polymerase chain reaction.8
Meanwhile, the patient’s peripheral blood mononuclear cells (PBMCs) are harvested from the blood by leukapheresis. The PBMCs are then enriched for monocytes by immunologic depletion of B and T cells. The monocytes are cultured in the presence of interleukin-4 and granulocytemacrophage-colony-stimulating factor (GM-CSF), and they become immature dendritic cells after 5 days. These immature dendritic cells are transfected with the amplified DNA from the CSC tumor spheres and grown for 2 more days in a medium supplemented with interleukin-1b (IL-1b), interleukin-6 (IL-6), tumor necrosis factor-α, and prostaglandin E2, then tested for markers characteristic of dendritic cells and frozen.
Aliquots of these transfected dendritic cells are injected into the patient at intervals over several months. Dendritic cells, which are the most efficient cells at eliciting an immune response, present the tumor CSC antigens to the patient’s immune system, which develops antibodies to the antigens presented on the dendritic cells and proceeds to destroy the tumor CSCs remaining in the patient’s tumor. Without its CSCs, the tumor is unable to revitalize itself, the rest of the tumor cells eventually die, and the tumor shrinks. The patient is monitored for immune response, and the tumor is regularly imaged. This vaccine is an example of therapeutic use: It can cause tumor regression.8
Sipuleucil-T is another example of an autologous vaccine used for therapeutic purposes. The patient’s antigenpresenting cells (APCs) are harvested by leukopheresis. They are then cultured in the presence of the protein made by fusing prostatic antigen phosphatase (PAP) with the GM-CSF to form the fusion protein PAP-GM-CSF. These modified APCs are then reinjected into the same host. Sipuleucil-T is now FDA approved for the treatment of metastatic castrate-resistant prostate cancer (mCRPC) and has been shown to extend life by approximately 4 months.9
Universal Vaccine for a Specific Tumor
Cancer vaccines can also be prepared in a more conventional manner, analogous to the more commonly used vaccines, and given to all patients with that specific tumor. PROSTVAC is another vaccine for mCRPC as well as for earlier-stage prostate cancer. The DNA specifying prostate-specific antigen (PSA), the secreted protein expressed by the tumor, is mutated very slightly and inserted into a vaccinia virus (Figure). Also inserted into the vaccinia virus are DNAs encoding 3 proteins that
stimulate the patient’s immune system: B7.1, intercellular adhesion molecule-1 (ICAM-1), and leukocyte functionassociated antigen-3 (LFA-3).
This recombinant viral vector is propagated in tissue culture, purified, and administered to the patient subcutaneously. The virus replicates for a short period in the host, expressing the 3 immunostimulatory molecules and the mutated PSA molecule protein product, which differs by 1 amino acid from that expressed by the patient’s tumor. The vaccinia virus and the mutant protein are recognized as foreign by the patient’s immune system, whereas the 3 immunostimulatory molecules are not, as they are the same as the host’s immunostimulatory molecules.
Subsequent boosts, administered over about 5 months, are with a fowlpox virus vector (which does not replicate in the patient) containing the same 4 genes that had been inserted into the vaccinia virus. This strategy enhances the immune response to the mutant tumor product and ensures that the host is not generating a response to the vaccinia virus. The mutant tumor product is sufficiently similar to the patient’s native tumor product that the immune system mounts a response to both proteins. The T cells generated in response to the mutant protein bind to both PSA and mutated PSA molecule proteins, and cells harboring these proteins are destroyed.10
Whereas the T-cell response is important for the activity of the PROSTVAC vaccine, and there is no evidence for formation of anti-PSA antibodies, other vaccines exploit both B- and T-cell responses.10,11 After a virus commandeers the host cell’s replication, transcription, and translation machinery, the macrophage breaks down the viral protein product (the mutated or immunogenic molecule) into small fragments. These fragments bind to major histocompatibility complex (MHC) class II molecules, which are produced by the macrophages. These complexes of antigen (virus) fragments with MHC class II molecules are transported to the surface of the macrophage, where the antigen is presented to lymphocytes. A receptor on a B lymphocyte adheres to the viral antigen and thus becomes an activated B cell, which divides and produces many copies. The B lymphocytes mature into plasma cells and release antibodies that adhere to the virus. A helper T-cell receptor can adhere to the antigen-MHC class II complex displayed on the B cell and activate the lymphocyte, causing the release of more and different cytokines. The cytokines stimulate the activated B cell to divide, producing functionally mature antibodies that recognize and attach to the virus. Macrophages can then engulf and destroy the virus, or the antibody-linked viruses can be excreted in the urine or stool.12
When viruses (eg, the recombinant vaccinia virus of the PROSTVAC vaccine) infect cells and replicate, fragments of viral proteins become attached to MHC class I molecules (see Box). These complexes attach to the cell surface and are presented to cytotoxic T cells. The activated cytotoxic T cells divide and destroy cells harboring the virus or viral antigens.12
Some of the B lymphocytes become memory B cells. Similarly, some of the T lymphocytes become memory T cells. These memory B cells respond and replicate rapidly on re-exposure to the virus or viral proteins to produce antigen-specific antibodies.12
When a macrophage engulfs and breaks down a tumor cell harboring a PSA molecule, other tumor-specific protein fragments are released. These tumor-specific fragments then serve as antigens and elicit an immune response, generating even more antitumor antibodies and T-cell interactions. Thus the tumor regresses. Importantly, this type of vaccine can be mass produced.
Vaccines With Chemotherapy
Another approach to the design of a tumor-specific vaccine is based on tumor-associated peptides (TUMAPs). These TUMAPs are highly overexpressed in tumors relative to a number of normal, healthy tissues. Nine highly immunogenic TUMAPs from 80 specimens of the same tumor type, identified by mass spectrometry, gene expression profiling, literature-based functional assessment, bioinformatics, and human T-cell assays, are used to prepare IMA901, a multipeptide vaccine against the specific tumor, renal cell carcinoma. The pool of selected peptides, composed of 9 to 16 amino acids, is then administered to patients following a dose of the immunomodulator GM-CSF for a total of as many as 17 injections. Low-dose cyclophosphamide administered prior to the first vaccination downregulates regulator T cells, enhancing immune response and resulting in prolonged survival.13 This type of vaccine can also be mass produced.
Conclusions
The vaccines that are currently in use or in clinical trials are being used in patients with advanced disease. As such, they are therapeutic rather than prophylactic. It is inevitable that more vaccines will earn FDA approval. Eventually, they will be used in earlier-stage disease. It is conceivable that some can be used prophylactically when a malignancy first becomes detectable, especially as more sensitive detection methods are developed. Vaccine delivery systems are likely to change as researchers investigate and adopt matrix materials that increase efficacy.
Cancer vaccines hold the potential for greater selectivity in treatment of malignancies. These vaccines may enable us to use cytotoxic chemotherapy agents less often or perhaps more effectively when used in conjunction with vaccines. Vaccines that can be mass produced could conceivably decrease the financial burden of cancer treatment as well as the human cost of malignant diseases and their therapy and care requirements.
Solid-tumor and hematologic malignancy vaccines are coming into wider use as the science is better understood and more methods of generating immune responses are explored. A personalized cancer vaccine, sipuleucel-T, is FDA approved for clinical use against mCRPC.9 A poxvirus-based vaccine, PROSTVAC, also against mCRPC, is in phase 3 clinical trials.10,11 The multipeptide renal cell carcinoma vaccine IMA901 is also in phase 3 clinical trials.13 A personalized vaccine against glioblastoma multiforme CSCs has extended progression-free survival in its initial pretrial study.8,14 Additional glioblastoma, melanoma, and other vaccines are in clinical trials and under development.
Author disclosures
The author reports no actual or potential conflicts of interest with regard to this article.
Disclaimer
The opinions expressed herein are those of the author and do not necessarily reflect those of Federal Practitioner, Frontline Medical Communications Inc., the U.S. Government, or any of its agencies. This article may discuss unlabeled or investigational use of certain drugs. Please review the complete prescribing information for specific drugs or drug combinations—including indications, contraindications, warnings, and adverse effects—before administering pharmacologic therapy to patients.
Click here to continue reading.
Vaccines are used to prevent bacterial infections, such as pneumonia, and viral diseases, such as influenza.1 Recently, they are being used to prevent cancers. For example, the human papilloma virus vaccine prevents infection with the virus that is associated with cervical cancer.2 Now there is another purpose for vaccines: They can be used therapeutically. Vaccines can be given even after a person manifests a tumor; they can cause the tumor to shrink or disappear. Therapeutic vaccines hold the potential to effect profound changes in the treatment of several cancers.
Biologics
Biologics, a recently developed class of weapons, can be but aren’t always relatively specific to cancer cells. Biologic agents include inhibitors and monoclonal antibodies. Inhibitors target specific functions, such as angiogenesis. However, inhibitors are not always selective for cancer cells. Angiogenesis inhibitors block formation of blood vessels. Like cytotoxic chemotherapy agents, they block the formation of blood vessels in normal cells as well as cancer cells. Cancer cells, however, generally grow more rapidly than do normal host cells, so they need more blood vessels faster to nourish their cells.3
Some inhibitors are more specific. An inhibitor molecule can be directed against a specific transcript that does not occur in normal cells. In normal cells, the breakpoint cluster region (BCR) gene on chromosome 22 directs synthesis of its protein product, whereas the abelson murine leukemia viral oncogene homolog 1 (ABL1) gene on chromosome 9 specifies another protein. However, in chronic myelogenous leukemia, the ends of those 2 chromosomes translocate, and the BCR/ABL1 transcript is a hybrid of RNA derived from the original chromosome and that of the newly attached, translocated chromosome 9. This hybrid RNA directs synthesis of a fusion protein, a tyrosine kinase; the fusion protein is not produced in normal host cells. Thus imatinib, an inhibitor directed at the fusion protein, inhibits the cancer cells specifically.4
Monoclonal Antibodies
Monoclonal antibodies exploit the host’s immune system to destroy cancer cells. Like the inhibitors, monoclonal antibodies can be directed against a specific functional protein, which is not necessarily specific to tumor cells, or against a protein or family of proteins unique to the cancer cells. For example, trastuzumab is a monoclonal antibody directed against the HER2/neu oncogene, which is amplified in some breast cancers.5 The host’s immune system recognizes the antibodyantigen complex and signals its macrophages to destroy the complex, and the cancer cell dies.
Vaccines also exploit the immune system. One way to immunize against a virus, is to introduce a live or killed virus or a part of a virus—usually a part of a viral protein—into the recipient. The immune system recognizes the foreign virus and makes antibodies. When the immune system is challenged by exposure to the pathogenic virus, the antiviral antibodies recognize and bind to it. The host’s macrophages then engulf the antigenantibody complex and destroy it.6 This type of vaccine prevents infection.
Viral Vaccines
Few vaccines prevent specific cancers. Immunization against the hepatitis B virus, for example, confers immunity to a virus whose infection is a major risk factor for hepatocellular carcinoma.7 Thus it is not truly an anticancer vaccine but rather an antiviral vaccine. Similarly, the HPV
vaccine does not prevent cervical cancer but confers immunity
to a virus that causes cervical cancer.2
Using vaccines to prevent cancer in general is now becoming feasible. Cancer vaccines in use or in development at this time are of 2 general types: those derived from a single tumor and are designed to elicit immunity to that particular tumor in that particular individual host; and those designed to provoke an immune response to that particular type of tumor in any host. The latter type can be mass produced, whereas vaccines of the first type are restricted to the host of origin.
Autologous Vaccines
At the time of biopsy or surgical excision, tumors are mechanically and enzymatically dissociated into single cells, then grown in tissue culture. When they are cultured in suspension with agitation, the cancer stem cells (CSCs) form spheroids, and other cell types do not. The CSCs are the progenitor cells of the specific tumor, much like bone marrow stem cells are the progenitors of the myeloid, erythroid, and megakaryocyte (platelet) cell lineages of human blood. After the CSCs are expanded, the spheroids are harvested, and their RNA is extracted. The messenger RNAs, which specify the proteins made by the CSCs, are converted to complementary DNAs, which are then amplified using the polymerase chain reaction.8
Meanwhile, the patient’s peripheral blood mononuclear cells (PBMCs) are harvested from the blood by leukapheresis. The PBMCs are then enriched for monocytes by immunologic depletion of B and T cells. The monocytes are cultured in the presence of interleukin-4 and granulocytemacrophage-colony-stimulating factor (GM-CSF), and they become immature dendritic cells after 5 days. These immature dendritic cells are transfected with the amplified DNA from the CSC tumor spheres and grown for 2 more days in a medium supplemented with interleukin-1b (IL-1b), interleukin-6 (IL-6), tumor necrosis factor-α, and prostaglandin E2, then tested for markers characteristic of dendritic cells and frozen.
Aliquots of these transfected dendritic cells are injected into the patient at intervals over several months. Dendritic cells, which are the most efficient cells at eliciting an immune response, present the tumor CSC antigens to the patient’s immune system, which develops antibodies to the antigens presented on the dendritic cells and proceeds to destroy the tumor CSCs remaining in the patient’s tumor. Without its CSCs, the tumor is unable to revitalize itself, the rest of the tumor cells eventually die, and the tumor shrinks. The patient is monitored for immune response, and the tumor is regularly imaged. This vaccine is an example of therapeutic use: It can cause tumor regression.8
Sipuleucil-T is another example of an autologous vaccine used for therapeutic purposes. The patient’s antigenpresenting cells (APCs) are harvested by leukopheresis. They are then cultured in the presence of the protein made by fusing prostatic antigen phosphatase (PAP) with the GM-CSF to form the fusion protein PAP-GM-CSF. These modified APCs are then reinjected into the same host. Sipuleucil-T is now FDA approved for the treatment of metastatic castrate-resistant prostate cancer (mCRPC) and has been shown to extend life by approximately 4 months.9
Universal Vaccine for a Specific Tumor
Cancer vaccines can also be prepared in a more conventional manner, analogous to the more commonly used vaccines, and given to all patients with that specific tumor. PROSTVAC is another vaccine for mCRPC as well as for earlier-stage prostate cancer. The DNA specifying prostate-specific antigen (PSA), the secreted protein expressed by the tumor, is mutated very slightly and inserted into a vaccinia virus (Figure). Also inserted into the vaccinia virus are DNAs encoding 3 proteins that
stimulate the patient’s immune system: B7.1, intercellular adhesion molecule-1 (ICAM-1), and leukocyte functionassociated antigen-3 (LFA-3).
This recombinant viral vector is propagated in tissue culture, purified, and administered to the patient subcutaneously. The virus replicates for a short period in the host, expressing the 3 immunostimulatory molecules and the mutated PSA molecule protein product, which differs by 1 amino acid from that expressed by the patient’s tumor. The vaccinia virus and the mutant protein are recognized as foreign by the patient’s immune system, whereas the 3 immunostimulatory molecules are not, as they are the same as the host’s immunostimulatory molecules.
Subsequent boosts, administered over about 5 months, are with a fowlpox virus vector (which does not replicate in the patient) containing the same 4 genes that had been inserted into the vaccinia virus. This strategy enhances the immune response to the mutant tumor product and ensures that the host is not generating a response to the vaccinia virus. The mutant tumor product is sufficiently similar to the patient’s native tumor product that the immune system mounts a response to both proteins. The T cells generated in response to the mutant protein bind to both PSA and mutated PSA molecule proteins, and cells harboring these proteins are destroyed.10
Whereas the T-cell response is important for the activity of the PROSTVAC vaccine, and there is no evidence for formation of anti-PSA antibodies, other vaccines exploit both B- and T-cell responses.10,11 After a virus commandeers the host cell’s replication, transcription, and translation machinery, the macrophage breaks down the viral protein product (the mutated or immunogenic molecule) into small fragments. These fragments bind to major histocompatibility complex (MHC) class II molecules, which are produced by the macrophages. These complexes of antigen (virus) fragments with MHC class II molecules are transported to the surface of the macrophage, where the antigen is presented to lymphocytes. A receptor on a B lymphocyte adheres to the viral antigen and thus becomes an activated B cell, which divides and produces many copies. The B lymphocytes mature into plasma cells and release antibodies that adhere to the virus. A helper T-cell receptor can adhere to the antigen-MHC class II complex displayed on the B cell and activate the lymphocyte, causing the release of more and different cytokines. The cytokines stimulate the activated B cell to divide, producing functionally mature antibodies that recognize and attach to the virus. Macrophages can then engulf and destroy the virus, or the antibody-linked viruses can be excreted in the urine or stool.12
When viruses (eg, the recombinant vaccinia virus of the PROSTVAC vaccine) infect cells and replicate, fragments of viral proteins become attached to MHC class I molecules (see Box). These complexes attach to the cell surface and are presented to cytotoxic T cells. The activated cytotoxic T cells divide and destroy cells harboring the virus or viral antigens.12
Some of the B lymphocytes become memory B cells. Similarly, some of the T lymphocytes become memory T cells. These memory B cells respond and replicate rapidly on re-exposure to the virus or viral proteins to produce antigen-specific antibodies.12
When a macrophage engulfs and breaks down a tumor cell harboring a PSA molecule, other tumor-specific protein fragments are released. These tumor-specific fragments then serve as antigens and elicit an immune response, generating even more antitumor antibodies and T-cell interactions. Thus the tumor regresses. Importantly, this type of vaccine can be mass produced.
Vaccines With Chemotherapy
Another approach to the design of a tumor-specific vaccine is based on tumor-associated peptides (TUMAPs). These TUMAPs are highly overexpressed in tumors relative to a number of normal, healthy tissues. Nine highly immunogenic TUMAPs from 80 specimens of the same tumor type, identified by mass spectrometry, gene expression profiling, literature-based functional assessment, bioinformatics, and human T-cell assays, are used to prepare IMA901, a multipeptide vaccine against the specific tumor, renal cell carcinoma. The pool of selected peptides, composed of 9 to 16 amino acids, is then administered to patients following a dose of the immunomodulator GM-CSF for a total of as many as 17 injections. Low-dose cyclophosphamide administered prior to the first vaccination downregulates regulator T cells, enhancing immune response and resulting in prolonged survival.13 This type of vaccine can also be mass produced.
Conclusions
The vaccines that are currently in use or in clinical trials are being used in patients with advanced disease. As such, they are therapeutic rather than prophylactic. It is inevitable that more vaccines will earn FDA approval. Eventually, they will be used in earlier-stage disease. It is conceivable that some can be used prophylactically when a malignancy first becomes detectable, especially as more sensitive detection methods are developed. Vaccine delivery systems are likely to change as researchers investigate and adopt matrix materials that increase efficacy.
Cancer vaccines hold the potential for greater selectivity in treatment of malignancies. These vaccines may enable us to use cytotoxic chemotherapy agents less often or perhaps more effectively when used in conjunction with vaccines. Vaccines that can be mass produced could conceivably decrease the financial burden of cancer treatment as well as the human cost of malignant diseases and their therapy and care requirements.
Solid-tumor and hematologic malignancy vaccines are coming into wider use as the science is better understood and more methods of generating immune responses are explored. A personalized cancer vaccine, sipuleucel-T, is FDA approved for clinical use against mCRPC.9 A poxvirus-based vaccine, PROSTVAC, also against mCRPC, is in phase 3 clinical trials.10,11 The multipeptide renal cell carcinoma vaccine IMA901 is also in phase 3 clinical trials.13 A personalized vaccine against glioblastoma multiforme CSCs has extended progression-free survival in its initial pretrial study.8,14 Additional glioblastoma, melanoma, and other vaccines are in clinical trials and under development.
Author disclosures
The author reports no actual or potential conflicts of interest with regard to this article.
Disclaimer
The opinions expressed herein are those of the author and do not necessarily reflect those of Federal Practitioner, Frontline Medical Communications Inc., the U.S. Government, or any of its agencies. This article may discuss unlabeled or investigational use of certain drugs. Please review the complete prescribing information for specific drugs or drug combinations—including indications, contraindications, warnings, and adverse effects—before administering pharmacologic therapy to patients.
Click here to continue reading.
1. U.S. Department of Health and Human Services, National Institutes of Health, National Institute of Allergy and Infectious Diseases. Understanding vaccines: what they are, how they work. National Institute of Allergy and Infectious Diseases Website. http://www.niaid.nih.gov/topics/vaccines/documents/undvacc.pdf. Published January 2008. Accessed March 24, 2015. .
2. Hagensee ME, Yaegashi N, Galloway GA. Self-assembly of human papilloma virus type 1 capsids by expression of the L1 protein alone or by coexpression of the L1 and L2 capsid proteins. J Virol. 1993;67(1):315-322.
3. American Cancer Society. Anti-angiogenesis treatment. American Cancer Society Website. http://www.cancer.org/acs/groups/cid/documents/webcontent/002988-pdf.pdf. Revised March 10, 2009. Accessed March 24, 2015.
4. Gambacorti-Passerini CB, Gunby RH, Piazza R, Galietta A, Rostagno R, Scapozza L. Molecular mechanisms of resistance to imatinib in Philadelphia-chromosomepositive leukaemias. Lancet Oncol. 2003;4(2):75-85.
5. Hudis CA. Trastuzumab—mechanism of action and use in clinical practice. N Engl J Med. 2007;357(1):39-51.
6. NPI reference guide on vaccines and vaccine safety: How vaccines work. PATH Website. http://www.path.org/vaccineresources/files/How_Vaccines_Work.pdf. Accessed March 24, 2015.
7. Beasley RP, Hwang LY, Lin CC, Chien CS. Hepatocellular carcinoma and hepatitis B virus: a prospective study of 22 707 men in Taiwan. Lancet. 1981;318(8256):1129-1133.
8. Vik-Mo EO, Nyakas M, Mikkelsen BV, et al. Therapeutic vaccination against autologous cancer stem cells with mRNA-transfected dendritic cells in patients with glioblastoma. Cancer Immunol Immunother. 2013;62(9):1499-1509.
9. Kantoff PW, Higano CS, Shore ND, et al; IMPACT Study Investigators. Sipuleucel-T immunotherapy for castration-resistant prostate cancer. N Engl J Med. 2010;363(5):411-422.
10. Gulley JL, Madan RA, Tsang KY, et al. Immune impact induced by PROSTVAC (PSA-TRICOM), a therapeutic vaccine for prostate cancer. Cancer Immunol Res. 2014;2(2):133-141.
11. Campbell CT, Gulley JL, Oyelaran O, Hodge JW, Schlom J, Gildersleeve JC. Humoral response to a viral glycan correlates with survival on PROSTVAC VF. Proc Natl Acad Sci USA. 2014;111(17):E1749-E1758.
12. Public Health England. Immunity and how vaccines work: The green book, chapter 1. GOV.UK Website. https://www.gov.uk/government/publications/immunity-and-how-vaccines-work-the-green-book-chapter-1. Published March 19, 2013. Accessed March 24, 2015.
13. Walter S, Weinschenk T, Stenzl A, et al. Multipeptide immune response to cancer vaccine IMA901 after single-dose cyclophosphamide associates with longer patient survival. Nat Med. 2012;18(8):1254-1261.
14. Ottenhausen M, Bodhinayake I, Banu M, Kesavabhotla K, Ray A, Boockvar JA. Industry progress report on neuro-oncology: biotech update 2013. J Neurooncol. 2013;115(2):311-316.
1. U.S. Department of Health and Human Services, National Institutes of Health, National Institute of Allergy and Infectious Diseases. Understanding vaccines: what they are, how they work. National Institute of Allergy and Infectious Diseases Website. http://www.niaid.nih.gov/topics/vaccines/documents/undvacc.pdf. Published January 2008. Accessed March 24, 2015. .
2. Hagensee ME, Yaegashi N, Galloway GA. Self-assembly of human papilloma virus type 1 capsids by expression of the L1 protein alone or by coexpression of the L1 and L2 capsid proteins. J Virol. 1993;67(1):315-322.
3. American Cancer Society. Anti-angiogenesis treatment. American Cancer Society Website. http://www.cancer.org/acs/groups/cid/documents/webcontent/002988-pdf.pdf. Revised March 10, 2009. Accessed March 24, 2015.
4. Gambacorti-Passerini CB, Gunby RH, Piazza R, Galietta A, Rostagno R, Scapozza L. Molecular mechanisms of resistance to imatinib in Philadelphia-chromosomepositive leukaemias. Lancet Oncol. 2003;4(2):75-85.
5. Hudis CA. Trastuzumab—mechanism of action and use in clinical practice. N Engl J Med. 2007;357(1):39-51.
6. NPI reference guide on vaccines and vaccine safety: How vaccines work. PATH Website. http://www.path.org/vaccineresources/files/How_Vaccines_Work.pdf. Accessed March 24, 2015.
7. Beasley RP, Hwang LY, Lin CC, Chien CS. Hepatocellular carcinoma and hepatitis B virus: a prospective study of 22 707 men in Taiwan. Lancet. 1981;318(8256):1129-1133.
8. Vik-Mo EO, Nyakas M, Mikkelsen BV, et al. Therapeutic vaccination against autologous cancer stem cells with mRNA-transfected dendritic cells in patients with glioblastoma. Cancer Immunol Immunother. 2013;62(9):1499-1509.
9. Kantoff PW, Higano CS, Shore ND, et al; IMPACT Study Investigators. Sipuleucel-T immunotherapy for castration-resistant prostate cancer. N Engl J Med. 2010;363(5):411-422.
10. Gulley JL, Madan RA, Tsang KY, et al. Immune impact induced by PROSTVAC (PSA-TRICOM), a therapeutic vaccine for prostate cancer. Cancer Immunol Res. 2014;2(2):133-141.
11. Campbell CT, Gulley JL, Oyelaran O, Hodge JW, Schlom J, Gildersleeve JC. Humoral response to a viral glycan correlates with survival on PROSTVAC VF. Proc Natl Acad Sci USA. 2014;111(17):E1749-E1758.
12. Public Health England. Immunity and how vaccines work: The green book, chapter 1. GOV.UK Website. https://www.gov.uk/government/publications/immunity-and-how-vaccines-work-the-green-book-chapter-1. Published March 19, 2013. Accessed March 24, 2015.
13. Walter S, Weinschenk T, Stenzl A, et al. Multipeptide immune response to cancer vaccine IMA901 after single-dose cyclophosphamide associates with longer patient survival. Nat Med. 2012;18(8):1254-1261.
14. Ottenhausen M, Bodhinayake I, Banu M, Kesavabhotla K, Ray A, Boockvar JA. Industry progress report on neuro-oncology: biotech update 2013. J Neurooncol. 2013;115(2):311-316.