User login
orlando – A digital device that patients swallow every time they take a medication may be an answer to improving compliance and disease control. An aim of this digital health system is to encourage patient engagement and provider interaction. Despite available and efficacious medicines, only slightly more than half of patients with type 2 diabetes mellitus have glycated hemoglobin (HbA1c), hypertension, or LDL cholesterol under good control. One study showed only 17% had all three under control (Endocr Pract. 2016;22:689-98).
“Important in this are the facts of medication nonadherence by the patient, poor patient engagement frequently in their care, as well as from a clinician perspective, therapeutic inertia,” said Dr. Juan Frias at the annual meeting of the American Association of Clinical Endocrinologists. Approximately half of patients with chronic diseases do not take their medications as prescribed (Circulation. 2013;128:29-41).
To improve treatment adherence, diagnose the reasons for a patient not reaching therapeutic goals, address those issues with patient education or counseling or changes in medication, and engage with the patient to reinforce adherence goals. A digital system called Proteus Discover has been designed to assist patients and clinicians with all these tasks.
Proteus uses ingestible sensor-detectable medications. Each medication and dose of medication has a unique marker. A compounding pharmacy co-encapsulates a tiny detectable chip about the size of a grain of sand with a medication as prescribed by a physician. The patient wears a Band-Aid sized skin patch sensor that transmits to a mobile device that uploads to a secure cloud server. The sensor can tell if a medication has been taken and also serves as a pedometer and activity gauge.
The information is available to the patient, who can gain insight into medication taking, activity, and rest, along with other health parameters that are entered into the system. Entering the system through a provider portal and with the patient’s permission, the clinician can see patient behavior patterns outside of the clinic, including medication adherence, which helps to determine the best treatment for that patient. The system provides a report to the clinician that can be a point of discussion when the patient visits the clinic.
Trial demonstrates better medication adherence and goal attainment
Dr. Frias, CEO and principal investigator of National Research Institute in Los Angeles, described a 12 week multicenter cluster-randomized pilot study involving 90 patients with uncontrolled hypertension and type 2 diabetes to investigate the effect of Proteus on blood pressure, HbA1c, and LDL cholesterol reduction. Other goals of the trial were to promote medication adherence and physical activity and alert providers to a need to make more medical decisions.
Cluster randomization meant that each of the 16 trial sites randomized patients to one of three treatment arms within that site. The arms were: Proteus Discover for 4 or 12 weeks or usual care. Patients had to have systolic blood pressure of 140 mm Hg or greater, have failed therapy with 2 or more antihypertensive agents, and have HbA1c at 7% or greater on metformin or a sulfonylurea. Two-thirds of the subjects also had dyslipidemia and were treated with statins. Subjects were excluded if they had a history of acute or chronic dermatitis, had a skin allergy or sensitivity to adhesive medical tape, or had secondary causes for uncontrolled hypertension or type 2 diabetes.
Usual care consisted of all standard interventions, including medication titrations, patient education, and lifestyle coaching. All subjects took medications for 12 weeks and were followed for 12 weeks after enrollment. Available medications were various doses of lisinopril, losartan, hydrochlorothiazide, amlodipine, atorvastatin, metformin, and glipizide.
All the arms were fairly well balanced as to age (58-62 years), ethnicity, employment, education, and income. About one quarter had incomes in the $20,000-$40,000 range, and about half had incomes of $20,000 or less. Body mass index was 32 kg/m2 in the Proteus and usual care arms. Total cholesterol was similar for the Proteus and usual care groups at 173-177 mg/dL, but LDL was slightly higher in the Proteus arms (103 vs. 95 mg/dL).
Better adherence and outcomes for patients using Proteus
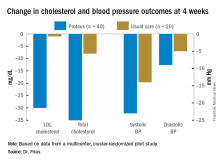
Dr. Frias presented 4-week results for blood pressure and cholesterol reduction. The 4- and 12-week Proteus arms were combined for this analysis, since up to 4 weeks they received the same intervention. Blood pressure, LDL cholesterol, and total cholesterol were all significantly reduced in the Proteus group, compared with the usual care group. At week 4, 83% of the subjects in the Proteus group had reached a blood pressure goal of less than 140/90 mm Hg vs. 33% in the usual care arm (difference of 50%; 95% confidence interval, 24%, 76%). Across all 11 medications/doses, the adherence was between 80% and 89% for every one.
The system spurred clinicians to make more treatment decisions for their patients. Providers made more changes to treatment for the Proteus patients, compared with the usual care providers (50% vs. 36%), gave more adherence counseling (28% vs. 0%), and provided more patient education (42% vs. 9%). Patients using the Proteus Discover system expressed high levels of satisfaction and acceptance of the technology (83%-100%), including ease of use in one’s daily routine, learning to use it, motivation to manage one’s health, better discussions with providers, understanding their care plan, seeing how they take their medications, and applying and wearing the sensor patch.
Safety was excellent. Twenty-seven adverse effects occurred in the Proteus arms, none of them serious, and about half were attributed to the device, mostly self-limited rashes. Seven adverse effects were attributed to the medications, mostly gastrointestinal side effects. There were four adverse effects in the usual care group, two of them serious.
Overall, compared with about 50% typical medication adherence, Proteus users had 84% adherence, which was associated with better blood pressure and LDL cholesterol control, compared with usual care.
Session moderator Dr. David Lieb, associate professor of medicine at Eastern Virginia Medical School in Norfolk, said the Proteus system may be very helpful for patients who are taking multiple medications to prompt them when to take them. “I could easily see somebody who has this system in place where they’re also able to measure their blood pressure and get that information to their provider and for their provider to make changes in their medicine dose,” he said.
This research study of 90 patients was quite manageable, but “what about when you have 300 people on this therapy and all those data are starting to come in? Who’s going to manage those data... and look at it all?” Dr. Lieb wondered. Patients may have to be taught to understand the data and make changes on their own to their medication behavior, exercise, and other factors within their control.
Half the patients in the study made $20,000 or less a year. “If you could help underserved patients with their compliance and all those other things... that would be fantastic. It’s a huge area of need,” he said. People will need internet access to upload their data to the cloud server.
Another question is how the data can interface with the various electronic health records in use and generate reports.
Proteus Discover is approved by the U.S. Food and Drug Administration and is available now.
orlando – A digital device that patients swallow every time they take a medication may be an answer to improving compliance and disease control. An aim of this digital health system is to encourage patient engagement and provider interaction. Despite available and efficacious medicines, only slightly more than half of patients with type 2 diabetes mellitus have glycated hemoglobin (HbA1c), hypertension, or LDL cholesterol under good control. One study showed only 17% had all three under control (Endocr Pract. 2016;22:689-98).
“Important in this are the facts of medication nonadherence by the patient, poor patient engagement frequently in their care, as well as from a clinician perspective, therapeutic inertia,” said Dr. Juan Frias at the annual meeting of the American Association of Clinical Endocrinologists. Approximately half of patients with chronic diseases do not take their medications as prescribed (Circulation. 2013;128:29-41).
To improve treatment adherence, diagnose the reasons for a patient not reaching therapeutic goals, address those issues with patient education or counseling or changes in medication, and engage with the patient to reinforce adherence goals. A digital system called Proteus Discover has been designed to assist patients and clinicians with all these tasks.
Proteus uses ingestible sensor-detectable medications. Each medication and dose of medication has a unique marker. A compounding pharmacy co-encapsulates a tiny detectable chip about the size of a grain of sand with a medication as prescribed by a physician. The patient wears a Band-Aid sized skin patch sensor that transmits to a mobile device that uploads to a secure cloud server. The sensor can tell if a medication has been taken and also serves as a pedometer and activity gauge.
The information is available to the patient, who can gain insight into medication taking, activity, and rest, along with other health parameters that are entered into the system. Entering the system through a provider portal and with the patient’s permission, the clinician can see patient behavior patterns outside of the clinic, including medication adherence, which helps to determine the best treatment for that patient. The system provides a report to the clinician that can be a point of discussion when the patient visits the clinic.
Trial demonstrates better medication adherence and goal attainment
Dr. Frias, CEO and principal investigator of National Research Institute in Los Angeles, described a 12 week multicenter cluster-randomized pilot study involving 90 patients with uncontrolled hypertension and type 2 diabetes to investigate the effect of Proteus on blood pressure, HbA1c, and LDL cholesterol reduction. Other goals of the trial were to promote medication adherence and physical activity and alert providers to a need to make more medical decisions.
Cluster randomization meant that each of the 16 trial sites randomized patients to one of three treatment arms within that site. The arms were: Proteus Discover for 4 or 12 weeks or usual care. Patients had to have systolic blood pressure of 140 mm Hg or greater, have failed therapy with 2 or more antihypertensive agents, and have HbA1c at 7% or greater on metformin or a sulfonylurea. Two-thirds of the subjects also had dyslipidemia and were treated with statins. Subjects were excluded if they had a history of acute or chronic dermatitis, had a skin allergy or sensitivity to adhesive medical tape, or had secondary causes for uncontrolled hypertension or type 2 diabetes.
Usual care consisted of all standard interventions, including medication titrations, patient education, and lifestyle coaching. All subjects took medications for 12 weeks and were followed for 12 weeks after enrollment. Available medications were various doses of lisinopril, losartan, hydrochlorothiazide, amlodipine, atorvastatin, metformin, and glipizide.
All the arms were fairly well balanced as to age (58-62 years), ethnicity, employment, education, and income. About one quarter had incomes in the $20,000-$40,000 range, and about half had incomes of $20,000 or less. Body mass index was 32 kg/m2 in the Proteus and usual care arms. Total cholesterol was similar for the Proteus and usual care groups at 173-177 mg/dL, but LDL was slightly higher in the Proteus arms (103 vs. 95 mg/dL).
Better adherence and outcomes for patients using Proteus
Dr. Frias presented 4-week results for blood pressure and cholesterol reduction. The 4- and 12-week Proteus arms were combined for this analysis, since up to 4 weeks they received the same intervention. Blood pressure, LDL cholesterol, and total cholesterol were all significantly reduced in the Proteus group, compared with the usual care group. At week 4, 83% of the subjects in the Proteus group had reached a blood pressure goal of less than 140/90 mm Hg vs. 33% in the usual care arm (difference of 50%; 95% confidence interval, 24%, 76%). Across all 11 medications/doses, the adherence was between 80% and 89% for every one.
The system spurred clinicians to make more treatment decisions for their patients. Providers made more changes to treatment for the Proteus patients, compared with the usual care providers (50% vs. 36%), gave more adherence counseling (28% vs. 0%), and provided more patient education (42% vs. 9%). Patients using the Proteus Discover system expressed high levels of satisfaction and acceptance of the technology (83%-100%), including ease of use in one’s daily routine, learning to use it, motivation to manage one’s health, better discussions with providers, understanding their care plan, seeing how they take their medications, and applying and wearing the sensor patch.
Safety was excellent. Twenty-seven adverse effects occurred in the Proteus arms, none of them serious, and about half were attributed to the device, mostly self-limited rashes. Seven adverse effects were attributed to the medications, mostly gastrointestinal side effects. There were four adverse effects in the usual care group, two of them serious.
Overall, compared with about 50% typical medication adherence, Proteus users had 84% adherence, which was associated with better blood pressure and LDL cholesterol control, compared with usual care.
Session moderator Dr. David Lieb, associate professor of medicine at Eastern Virginia Medical School in Norfolk, said the Proteus system may be very helpful for patients who are taking multiple medications to prompt them when to take them. “I could easily see somebody who has this system in place where they’re also able to measure their blood pressure and get that information to their provider and for their provider to make changes in their medicine dose,” he said.
This research study of 90 patients was quite manageable, but “what about when you have 300 people on this therapy and all those data are starting to come in? Who’s going to manage those data... and look at it all?” Dr. Lieb wondered. Patients may have to be taught to understand the data and make changes on their own to their medication behavior, exercise, and other factors within their control.
Half the patients in the study made $20,000 or less a year. “If you could help underserved patients with their compliance and all those other things... that would be fantastic. It’s a huge area of need,” he said. People will need internet access to upload their data to the cloud server.
Another question is how the data can interface with the various electronic health records in use and generate reports.
Proteus Discover is approved by the U.S. Food and Drug Administration and is available now.
orlando – A digital device that patients swallow every time they take a medication may be an answer to improving compliance and disease control. An aim of this digital health system is to encourage patient engagement and provider interaction. Despite available and efficacious medicines, only slightly more than half of patients with type 2 diabetes mellitus have glycated hemoglobin (HbA1c), hypertension, or LDL cholesterol under good control. One study showed only 17% had all three under control (Endocr Pract. 2016;22:689-98).
“Important in this are the facts of medication nonadherence by the patient, poor patient engagement frequently in their care, as well as from a clinician perspective, therapeutic inertia,” said Dr. Juan Frias at the annual meeting of the American Association of Clinical Endocrinologists. Approximately half of patients with chronic diseases do not take their medications as prescribed (Circulation. 2013;128:29-41).
To improve treatment adherence, diagnose the reasons for a patient not reaching therapeutic goals, address those issues with patient education or counseling or changes in medication, and engage with the patient to reinforce adherence goals. A digital system called Proteus Discover has been designed to assist patients and clinicians with all these tasks.
Proteus uses ingestible sensor-detectable medications. Each medication and dose of medication has a unique marker. A compounding pharmacy co-encapsulates a tiny detectable chip about the size of a grain of sand with a medication as prescribed by a physician. The patient wears a Band-Aid sized skin patch sensor that transmits to a mobile device that uploads to a secure cloud server. The sensor can tell if a medication has been taken and also serves as a pedometer and activity gauge.
The information is available to the patient, who can gain insight into medication taking, activity, and rest, along with other health parameters that are entered into the system. Entering the system through a provider portal and with the patient’s permission, the clinician can see patient behavior patterns outside of the clinic, including medication adherence, which helps to determine the best treatment for that patient. The system provides a report to the clinician that can be a point of discussion when the patient visits the clinic.
Trial demonstrates better medication adherence and goal attainment
Dr. Frias, CEO and principal investigator of National Research Institute in Los Angeles, described a 12 week multicenter cluster-randomized pilot study involving 90 patients with uncontrolled hypertension and type 2 diabetes to investigate the effect of Proteus on blood pressure, HbA1c, and LDL cholesterol reduction. Other goals of the trial were to promote medication adherence and physical activity and alert providers to a need to make more medical decisions.
Cluster randomization meant that each of the 16 trial sites randomized patients to one of three treatment arms within that site. The arms were: Proteus Discover for 4 or 12 weeks or usual care. Patients had to have systolic blood pressure of 140 mm Hg or greater, have failed therapy with 2 or more antihypertensive agents, and have HbA1c at 7% or greater on metformin or a sulfonylurea. Two-thirds of the subjects also had dyslipidemia and were treated with statins. Subjects were excluded if they had a history of acute or chronic dermatitis, had a skin allergy or sensitivity to adhesive medical tape, or had secondary causes for uncontrolled hypertension or type 2 diabetes.
Usual care consisted of all standard interventions, including medication titrations, patient education, and lifestyle coaching. All subjects took medications for 12 weeks and were followed for 12 weeks after enrollment. Available medications were various doses of lisinopril, losartan, hydrochlorothiazide, amlodipine, atorvastatin, metformin, and glipizide.
All the arms were fairly well balanced as to age (58-62 years), ethnicity, employment, education, and income. About one quarter had incomes in the $20,000-$40,000 range, and about half had incomes of $20,000 or less. Body mass index was 32 kg/m2 in the Proteus and usual care arms. Total cholesterol was similar for the Proteus and usual care groups at 173-177 mg/dL, but LDL was slightly higher in the Proteus arms (103 vs. 95 mg/dL).
Better adherence and outcomes for patients using Proteus
Dr. Frias presented 4-week results for blood pressure and cholesterol reduction. The 4- and 12-week Proteus arms were combined for this analysis, since up to 4 weeks they received the same intervention. Blood pressure, LDL cholesterol, and total cholesterol were all significantly reduced in the Proteus group, compared with the usual care group. At week 4, 83% of the subjects in the Proteus group had reached a blood pressure goal of less than 140/90 mm Hg vs. 33% in the usual care arm (difference of 50%; 95% confidence interval, 24%, 76%). Across all 11 medications/doses, the adherence was between 80% and 89% for every one.
The system spurred clinicians to make more treatment decisions for their patients. Providers made more changes to treatment for the Proteus patients, compared with the usual care providers (50% vs. 36%), gave more adherence counseling (28% vs. 0%), and provided more patient education (42% vs. 9%). Patients using the Proteus Discover system expressed high levels of satisfaction and acceptance of the technology (83%-100%), including ease of use in one’s daily routine, learning to use it, motivation to manage one’s health, better discussions with providers, understanding their care plan, seeing how they take their medications, and applying and wearing the sensor patch.
Safety was excellent. Twenty-seven adverse effects occurred in the Proteus arms, none of them serious, and about half were attributed to the device, mostly self-limited rashes. Seven adverse effects were attributed to the medications, mostly gastrointestinal side effects. There were four adverse effects in the usual care group, two of them serious.
Overall, compared with about 50% typical medication adherence, Proteus users had 84% adherence, which was associated with better blood pressure and LDL cholesterol control, compared with usual care.
Session moderator Dr. David Lieb, associate professor of medicine at Eastern Virginia Medical School in Norfolk, said the Proteus system may be very helpful for patients who are taking multiple medications to prompt them when to take them. “I could easily see somebody who has this system in place where they’re also able to measure their blood pressure and get that information to their provider and for their provider to make changes in their medicine dose,” he said.
This research study of 90 patients was quite manageable, but “what about when you have 300 people on this therapy and all those data are starting to come in? Who’s going to manage those data... and look at it all?” Dr. Lieb wondered. Patients may have to be taught to understand the data and make changes on their own to their medication behavior, exercise, and other factors within their control.
Half the patients in the study made $20,000 or less a year. “If you could help underserved patients with their compliance and all those other things... that would be fantastic. It’s a huge area of need,” he said. People will need internet access to upload their data to the cloud server.
Another question is how the data can interface with the various electronic health records in use and generate reports.
Proteus Discover is approved by the U.S. Food and Drug Administration and is available now.
AT AACE 2016
Key clinical point: Feedback from a system of digital-enabled pills enhanced medication adherence.
Major finding: Patients using Proteus had 84% adherence and better risk control.
Data source: Randomized unblinded study of 90 patients.
Disclosures: Dr. Frias has study grants from AbbVie, Amgen, AstraZeneca, Boehringer Ingelheim, Bristol-Myers Squibb, Eli Lilly, Johnson & Johnson, Merck, Novo Nordisk, Pfizer, and Sanofi and has consulting relationships with Proteus Digital Health, Johnson & Johnson, AstraZeneca, CeQur, and Sanofi.