User login
CASE Healthy woman with hot flashes inquires about HT
A 54-year-old healthy woman with a history of hypothyroidism taking thyroid replacement medication comes in for her annual visit. Her last menstrual period was over 2 years ago and she reports severe hot flashes. They have greatly affected her quality of life and she must take frequent breaks at work. She wakes up frequently at night due to night sweats, which is impacting her sleep and, subsequently, her energy level. She has noted increased vaginal dryness so has been abstaining from sexual intercourse due to the discomfort. She has an intact uterus. Her family history is significant for heart disease, diagnosed in her mother at age 75.
On physical examination, she is normotensive and well-appearing. Her body mass index (BMI) is 21 kg/m2. Labs obtained prior to her visit show normal renal and liver function. Her high-density lipid (HDL) level is 55 mg/dL, her low-density lipid (LDL) level is 80 mg/dL, and her triglyceride level is 100 mg/dL; HbA1c is 5.5 mmol/mol.
She is interested in learning more about menopausal hormone therapy (HT) and whether or not she would be a candidate.
What information do you need to know to counsel and manage this patient?
Menopausal HT prescribing practices have changed over the last few decades as a better understanding of the risks and benefits of treatment have emerged. Prior to 2002, HT was commonly used for treatment of symptoms associated with menopause and was thought to have beneficial effects for chronic disease prevention.1-4 After data from the Women’s Health Initiative (WHI) was released, concerns arose around the effect of HT on cardiovascular health and risk of breast cancer. As a result, HT prescriptions fell precipitously after around 2002.5 Since then, postintervention analysis and cumulative 18-year follow-up of WHI data, along with results from subsequent randomized controlled trials, including the Kronos Early Estrogen Prevention Study (KEEPS) and the Early Versus Late Intervention Trial with Estradiol (ELITE), have demonstrated a favorable safety profile for healthy women starting HT early in menopause (less than age 60, or within 10 years from their final menstrual period).5-11
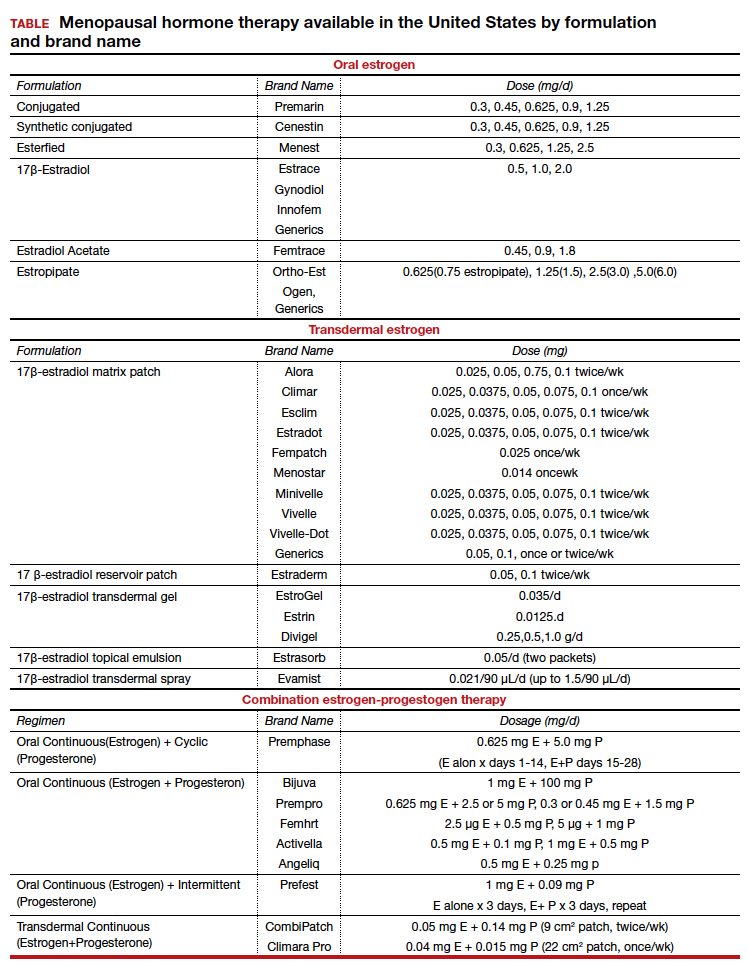
There are many types, formulations, and routes of HT, and the effects and risks differ for each (TABLE). For example, oral estrogen therapy, such as conjugated equine estrogens, portend a higher risk of adverse effects compared with transdermal formulations. Topical and transdermal estrogens bypass first-pass hepatic metabolism and thus are associated with a lower risk of venous thromboembolism (VTE) compared with oral formulations.12-14 A progestogen such as micronized progesterone is used in postmenopausal women with a uterus to protect the endometrium from unopposed estrogen therapy (ET). While it comes in oral and transdermal forms, the oral formulation is most widely used and studied in the United States; transdermal forms do not provide adequate endometrial protection and should not be used in combination therapy.15,16
Risks and benefits
Cardiovascular risk
Over time, the benefits and risks of HT use in menopausal patients have been further elucidated and defined, although they remain complex and dependent on patient clinical characteristics. HT remains the most effective treatment for vasomotor symptoms (VMS) and the genitourinary syndrome of menopause.17,18 In 2002, concerns for increased cardiovascular disease (CVD) and breast cancer risk resulted in early cessation of the WHI trial. Since that time the risk of CVD in postmenopausal women taking HT has been found to be more nuanced. In fact, updates in the literature have shown that HT results in a reduction of coronary heart disease if started in healthy women younger than age 60 years within 10 years of menopause.7,9-11 With this updated information, the North American Menopause society (NAMS), American College of Obstetricians and Gynecologists and the Endocrine Society have published guidelines supporting the initiation of HT for symptomatic healthy women: under the age of 60, within 10 years of menopause, and without contraindications. After age 60 years and further from menopause, the benefits and risks become less known.18-20
Risk stratification allows for more comprehensive counseling in use of HT for treatment of bothersome VMS. From a cardiovascular health standpoint, calculating an atherosclerotic CVD (ASCVD) risk score helps to evaluate appropriateness of HT prescribing:
- For those with low 10-year CVD risk (<5%), either oral or transdermal HT is appropriate.
- For those with moderate 10-year CVD risk (5%-10%), transdermal HT is recommended over oral HT.
- For those with high 10-year CVD risk (>10%), HT is not recommended.19,21
Breast cancer risk
Follow up since the initial WHI publication have shown that breast cancer risk is largely dependent on the formulation and route of HT used. Oral estrogen combined with a progestogen has been shown to increase the risk of invasive breast cancer, though very rarely.22 To put it into context, the absolute risk of breast cancer based on follow-up studies from WHI showed less than 1 additional case per 1,000 person years of use; less risk than associated with drinking 2 glasses of wine per day and similar to that of obesity and/or sedentary lifestyle.23,24 Studies have shown estrogen treatment alone for postmenopausal women does not appear to increase the risk of breast cancer. In fact, follow-up data from WHI showed a nonsignificant reduction in breast cancer risk for those taking ET alone.25
Breast cancer risk stratification is helpful when determining appropriateness of HT in postmenopausal women. Generally, if using risk stratification models for breast cancer (ie, Gail Risk model or international breast cancer intervention study [IBIS] tool), a patient who is average to moderate risk, HT can be offered with appropriate counseling. By contrast, a patient who is high risk should have a more detailed discussion about their risk (surveillance and risk-reducing treatments), and they may consider nonhormonal options for treatment of VMS. Women with a history of breast cancer should not be prescribed systemic HT.
Continue to: Additional HT benefits...
Additional HT benefits
The benefits of HT in postmenopausal women include improved bone health and reduction of fractures; reduction of risk for type 2 diabetes mellitus (T2DM); improvement of insulin sensitivity; improvement of lipid profiles with increased HDL and decreased LDL levels; and reduction of colon cancer risk.25 For women aged younger than 60 years who start HT within 10 years of their last menstrual period, HT has been shown to cause a reduction in all-cause mortality. Important risks to counsel patients on when starting HT include the low risk of stroke and venous thromboembolism (VTE) when using oral formulations.26
CASE Resolved
Her ASCVD risk score, based on her history, estimates her 10-year CVD risk to be low (<5%). Thus, from a cardiovascular standpoint, either oral or transdermal HT would be an appropriate option. Her IBIS 10-year score is 1.5%, placing her in a low-risk category for breast cancer based on her personal and family history. Given that she is less than 60 years of age and within 10 years of menopause, along with her low-risk stratification for CVD and breast cancer, she would be an appropriate patient to begin combined HT with an estrogen plus an oral progesterone, such as an estradiol patch 0.0375 mg twice weekly, along with oral micronized progesterone 100 mg nightly. The dose could be increased over time based on symptoms and tolerability of the treatment.
ALTERNATE CASE 1 The patient has additional risk factors
Consider the patient case with the following additions to her history: the patient has a BMI of 34 kg/m2, a history of well-controlled hypertension while taking amlodipine 5 mg, and an ASCVD risk score of 7.5%. She reports severe VMS that are greatly impacting her quality of life. How would your recommendations or counseling change?
Focus on healthy lifestyle
Obesity and hypertension, both common chronic conditions, pose additional risks to be accounted for when counseling on and approaching HT prescribing. Her alternate ASCVD risk score places her at moderate risk for CVD within 10 years, based on guidelines as discussed above. It would still be appropriate to offer her combined HT after a shared decision-making discussion that includes a focus on healthy lifestyle habits.
Consider transdermal HT in obese women
Longitudinal studies have found that weight gain is more a consequence of aging, regardless of menopausal status. Fat distribution and body composition changes are a menopause-related phenomenon driven by estrogen deficiency. HT has been shown to preserve lean body mass and reduce visceral adiposity, resulting in favorable effects of body composition. Still, obesity results in increased risk of CVD, VTE, and certain hormone-sensitive cancers.27 When considering HT in obese patients, a transdermal estrogen route is preferred to reduce risks.
For women with hypertension, prescribe transdermal HT
Overall, studies have found that HT has a neutral effect on blood pressure.25 When considering formulation of HT, micronized progesterone, dydrogesterone, and drospirenone seem to be most neutral and possibly even beneficial on blood pressure compared with synthetic progestins.26 Oral estrogen is associated with increased vasoconstriction and/or increased sodium retention with resultant worsened regulation of blood pressure in women with hypertension, so transdermal estrogen is preferred for women with hypertension.26 Hypertension is a component of the ASCVD risk score; factoring this into a patient’s clinical picture is important when discussing appropriateness of HT prescribing. To minimize risks, the transdermal route of estrogen is preferred for those with hypertension.
Continue to: ALTERNATE CASE 1 Resolved...
ALTERNATE CASE 1 Resolved
She has a moderate ASCVD risk score, is obese, and has a history of hypertension. Through shared decision making, you ultimately start her on transdermal estrogen and micronized progesterone to treat her quality-of-life-impacting VMS, a formulation that is most likely to mitigate the possible risks in her clinical case. You see her back in the clinic every 3-6 months to monitor her blood pressure.
ALTERNATE CASE 2 The patient has a high risk for breast cancer
The patient reveals further her significant family history of breast cancer in her maternal grandmother and mother, both diagnosed in their 50s. You calculate her risk of breast cancer with a model that incorporates family history. Her Tyrer Cuzick-IBIS 10-year risk score is >5% and lifetime risk is >20%, putting her at high risk for breast cancer. Since she has a uterus and would need concomitant progesterone therapy, her risk for breast cancer is higher than if she was taking ET alone. Ultimately, together you and the patient decide to trial nonhormonal options for her VMS.
What are nonhormonal options for treatment of VMS?
While HT remains the most effective treatment for VMS, there are multiple nonhormonal treatments for women who are either at too high a risk for HT or who favor other options, which are outlined in the NAMS 2015 nonhormonal management position statement.27 Cognitive behavioral therapy (CBT) has been shown to decrease bother related to VMS but not frequency. Clinical hypnosis has been shown to reduce hot flash frequency and improve sleep. Paroxetine salt (7.5 mg/day) remains the only FDA nonhormonal-approved medication for treatment of moderate to severe vasomotor symptoms. Off label use of other selective serotonin reuptake inhibitors (SSRIs) and selective norepinephrine reuptake inhibitors have been shown in studies to reduce VMS including paroxetine at slightly higher doses (10 mg/day–20 mg/day), citalopram (10 mg/day–20 mg/day), escitalopram (10 mg/day–20 mg/day), venlafaxine (37.5 mg/day–150 mg/day), and desvenlafaxine (50 mg/day–100 mg/day). Other treatments that could be considered include off-label use of gabapentin (900 mg/day–2,400 mg/day), oxybutynin (2.5–5 mg twice daily) or clonidine (0.1 mg/day–1 mg/day divided in doses) since they all have data demonstrating they are beneficial at reducing VMS.
Nonhormonal options that may be helpful but are recommended with caution due to lack of data include weight loss, mindfulness-based stress reduction, s-equol derivatives of soy isoflavones and a stellate ganglion block. Further evidence and studies are needed for the aforementioned options.27
ALTERNATE CASE 2 Resolved
She may consider any of the nonhormonal options discussed. If she meets with a medical breast specialist to discuss her elevated risk of breast cancer and considers starting risk-reducing medications, particularly tamoxifen, you will want to avoid medications that have significant CPY 2D6 inhibition, such as paroxetine and fluoxetine. Safer choices would include venlafaxine, escitalopram, or citalopram.
The bottom line
In summary, the benefits and risks of HT in the treatment of VMS remain nuanced. For healthy women younger than 60 years of age and within 10 years from their last menstrual period, the benefits of HT largely outweigh the risks. Shared decision making, along with individualized and appropriate risk stratification specific for women, can guide appropriateness of HT prescribing. For those women who cannot take HT or choose not to, there are many nonhormonal options that will help manage their bothersome VMS. ●
- Carr BR, Wilson JD. Disorders of the ovary and female reproductive tract. In: Isselbacher KJ, Braunwald E, Wilson JD, eds. Harrisons’ Principles of Internal Medicine, 13th ed. New York, NY: McGraw-Hill; 1994:2016-2017.
- Davidson MH, Maki KC, Marx P, et al. Effects of continuous estrogen and estrogen-progestin replacement regimens on cardiovascular risk markers in postmenopausel women. Arch Intern Med. 2000;160:3315-3325. doi: 10.1001/archinte.160.21.3315.
- Grodstein F, Manson JE, Colditz GA, et al. A prospective, observational study of postmenopausal hormone therapy and primary prevention of cardiovascular disease. Ann Intern Med. 2000;133:933-941. doi: 10.7326/0003-4819-133-12-200012190-00008.
- Grady D, Rubin SM, Petitti DB, et al. Hormone therapy to prevent disease and prolong life in postmenopausal women. Ann Intern Med. 1992;117:1016-1037. doi: 10.7326/0003-4819-117-12-1016.
- Rossouw JE, Manson JE, Kaunitz AM, et al. Lessons learned from the Women’s Health Initiative trials of menopausal hormone therapy. Obstet Gynecol. 2013;121:172-176. doi: 10.1097/aog.0b013e31827a08c8.
- Manson JE, Hsia J, Johnson KC, et al. Estrogen plus progestin and the risk of coronary heart disease. NEJM. 2003;349:523-534. doi: 10.1056/NEJMoa030808.
- Manson JE, Chlebowski RT, Stefanick ML, et al. Menopausal hormone therapy and health outcomes during the intervention and extended poststopping phases of the Women’s Health Initiative randomized trials. JAMA. 2013;310:1353-1368. doi: 10.1001/jama.2013.278040.
- Santen RJ, Allred DC, Ardoin SP, et al. Postmenopausal hormone therapy: an Endocrine Society scientific statement. J Clin Endocrinol Metab. 2010;95:S1-S66. doi: 10.1210/jc.2009-2509.
- Manson JE, Aragaki AK, Rossouw JE, et al. Menopausal hormone therapy and long-term all-cause and cause-specific mortality: the Women’s Health Initiative randomized trials. JAMA. 2017;318:927-938. doi: 10.1001/jama.2017.11217.
- Hodis HN, Mack WJ, Henderson VW, et al. Vacular effects of early versus late postmenopausal treatment with estradiol. NEJM. 2016;374:1221-1231. doi: 10.1056/NEJMoa1505241.
- Taylor HS, Tal A, Pal L, et al. Effects of oral vs transdermal estrogen therapy on sexual function in early postmenopause: ancillary study of the Kronos Early Estrogen Prevention Study (KEEPS). JAMA Intern Med. 2017;177:1471-1479. doi: 10.1001/jamainternmed.2017.3877.
- Liu JH, Pinkerton JV. Prescription therapies. In: CJ Crandall, ed. Menopause Practice: A Clinician’s Guide, 6th ed. Pepper Pike, OH: The North American Menopause Society; 2019:277-309.
- Files J, Kling JM. Transdermal delivery of bioidentical estrogen in menopausal hormone therapy: a clinical review. Expert Opin Drug Deliv. 2020;17:543-549. doi: 10.1080/17425247.2020.1700949.
- Canonico M, Carcaillon L, Plu-Bureau G, et al. Postmenopausal hormone therapy and risk of stroke: impact of the route of estrogen administration and type of progestogen. Stroke. 2016;47:1734-1741. doi: 10.1161/STROKEAHA.116.013052.
- Hitchcok CL, Prior JC. Oral micronized progesterone for vasomotor symptoms—a placebo-controlled randomized trial in healthy post-menopausal women. Menopause. 2001;8:10-16.
- Effects of hormone replacement therapy on endometrial histology in postmenopausal women. The Postmenopausal Estrogen/Progestin Interventions (PEPI) Trial. The writing Group for the PEPI Trial. JAMA. 1996;275:370-375. doi: 10.1001/jama.1996.03530290040035.
- Pinkerton JV. Hormone therapy for postmenopausal women. N Engl J Med. 2020;382:446-55. doi:10.1056/NEJMcp1714787.
- The 2022 hormone therapy position statement of The North American Menopause Society. Menopause. 2022;29:767-794. doi:10.1097/GME.00000000000000002028.
- Stuenkel CA, Davis SR, Gompel A, et al. Treatment of symptoms of the menopause: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2015;100:3975-4011. doi: 10.1210/jc.2015-2236.
- American College of Obstetricians and Gynecologists. Practice Bulletin No. 141: Management of Menopausal Symptoms. Obstet Gynecol. 2014;123:202-216. doi: 10.1097/01.AOG.0000441353.20693.78.
- Manson JE. Current recommendations: what is the clinician to do? Fertil Steril. 2014;101:916. World Cancer Research Fund/American Institute for Cancer Research. Continuous Update Project Expert Report 2018. Alcoholic drinks and the risk of cancer. https://www.wcrf.org/sites/default/files/Alcoholic-Drinks.pdf. 2018.
- Anderson GL, Chlebowski RT, Aragaki AK, et al. Conjugated equine oestrogen and breast cancer incidence and mortality in postmenopausal women with hysterectomy: Extended follow-up of Women’s Health Initiative randomized placebo-controlled trial. Lancet Oncol. 2012;5:476-486. doi: 10.1016/S1470-2045(12)70075-X.
- World Cancer Research Fund/American Institute for Cancer Research. Continuous Update Project Expert Report 2018. Diet, nutrition, physical activity and breast cancer. www.aicr.org/continuous-update-project/breast-cancer.html. 2018.
- Anderson GL, Chlebowski RT, Aragaki AK, et al. Conjugated equine oestrogen and breast cancer incidence and mortality in postmenopausal women with hysterectomy: Extended follow-up of the Women’s Health Initiative randomized placebo-controlled trial. Lancet Oncol. 2012;5:476-486. doi: 10.1016/S1470-2045(12)70075-X.
- Mehta J, Kling JM, Manson JE. Risks, benefits and treatment modalities of menopausal hormone therapy: current concepts. Front Endocrinol (Laussane). 2021;12:564781. doi: 10.3389/fendo.2021.564781.
- Kapoor E, Kling JM, Lobo AS, et al. Menopausal hormone therapy in women with chronic medical conditions. Best Pract Res Clin Endocrinol Metab. 2021:35;101578. doi: 10.1016/j.beem.2021.101578.
- NAMS position statement advisory panel. Nonhormonal management of menopause-associated vasomotor symptoms: 2015 position statement of The North American Menopause Society. Menopause. 2015:22:1155-72. doi: 10.1097/GME.0000000000000546.
CASE Healthy woman with hot flashes inquires about HT
A 54-year-old healthy woman with a history of hypothyroidism taking thyroid replacement medication comes in for her annual visit. Her last menstrual period was over 2 years ago and she reports severe hot flashes. They have greatly affected her quality of life and she must take frequent breaks at work. She wakes up frequently at night due to night sweats, which is impacting her sleep and, subsequently, her energy level. She has noted increased vaginal dryness so has been abstaining from sexual intercourse due to the discomfort. She has an intact uterus. Her family history is significant for heart disease, diagnosed in her mother at age 75.
On physical examination, she is normotensive and well-appearing. Her body mass index (BMI) is 21 kg/m2. Labs obtained prior to her visit show normal renal and liver function. Her high-density lipid (HDL) level is 55 mg/dL, her low-density lipid (LDL) level is 80 mg/dL, and her triglyceride level is 100 mg/dL; HbA1c is 5.5 mmol/mol.
She is interested in learning more about menopausal hormone therapy (HT) and whether or not she would be a candidate.
What information do you need to know to counsel and manage this patient?
Menopausal HT prescribing practices have changed over the last few decades as a better understanding of the risks and benefits of treatment have emerged. Prior to 2002, HT was commonly used for treatment of symptoms associated with menopause and was thought to have beneficial effects for chronic disease prevention.1-4 After data from the Women’s Health Initiative (WHI) was released, concerns arose around the effect of HT on cardiovascular health and risk of breast cancer. As a result, HT prescriptions fell precipitously after around 2002.5 Since then, postintervention analysis and cumulative 18-year follow-up of WHI data, along with results from subsequent randomized controlled trials, including the Kronos Early Estrogen Prevention Study (KEEPS) and the Early Versus Late Intervention Trial with Estradiol (ELITE), have demonstrated a favorable safety profile for healthy women starting HT early in menopause (less than age 60, or within 10 years from their final menstrual period).5-11
There are many types, formulations, and routes of HT, and the effects and risks differ for each (TABLE). For example, oral estrogen therapy, such as conjugated equine estrogens, portend a higher risk of adverse effects compared with transdermal formulations. Topical and transdermal estrogens bypass first-pass hepatic metabolism and thus are associated with a lower risk of venous thromboembolism (VTE) compared with oral formulations.12-14 A progestogen such as micronized progesterone is used in postmenopausal women with a uterus to protect the endometrium from unopposed estrogen therapy (ET). While it comes in oral and transdermal forms, the oral formulation is most widely used and studied in the United States; transdermal forms do not provide adequate endometrial protection and should not be used in combination therapy.15,16

Risks and benefits
Cardiovascular risk
Over time, the benefits and risks of HT use in menopausal patients have been further elucidated and defined, although they remain complex and dependent on patient clinical characteristics. HT remains the most effective treatment for vasomotor symptoms (VMS) and the genitourinary syndrome of menopause.17,18 In 2002, concerns for increased cardiovascular disease (CVD) and breast cancer risk resulted in early cessation of the WHI trial. Since that time the risk of CVD in postmenopausal women taking HT has been found to be more nuanced. In fact, updates in the literature have shown that HT results in a reduction of coronary heart disease if started in healthy women younger than age 60 years within 10 years of menopause.7,9-11 With this updated information, the North American Menopause society (NAMS), American College of Obstetricians and Gynecologists and the Endocrine Society have published guidelines supporting the initiation of HT for symptomatic healthy women: under the age of 60, within 10 years of menopause, and without contraindications. After age 60 years and further from menopause, the benefits and risks become less known.18-20
Risk stratification allows for more comprehensive counseling in use of HT for treatment of bothersome VMS. From a cardiovascular health standpoint, calculating an atherosclerotic CVD (ASCVD) risk score helps to evaluate appropriateness of HT prescribing:
- For those with low 10-year CVD risk (<5%), either oral or transdermal HT is appropriate.
- For those with moderate 10-year CVD risk (5%-10%), transdermal HT is recommended over oral HT.
- For those with high 10-year CVD risk (>10%), HT is not recommended.19,21
Breast cancer risk
Follow up since the initial WHI publication have shown that breast cancer risk is largely dependent on the formulation and route of HT used. Oral estrogen combined with a progestogen has been shown to increase the risk of invasive breast cancer, though very rarely.22 To put it into context, the absolute risk of breast cancer based on follow-up studies from WHI showed less than 1 additional case per 1,000 person years of use; less risk than associated with drinking 2 glasses of wine per day and similar to that of obesity and/or sedentary lifestyle.23,24 Studies have shown estrogen treatment alone for postmenopausal women does not appear to increase the risk of breast cancer. In fact, follow-up data from WHI showed a nonsignificant reduction in breast cancer risk for those taking ET alone.25
Breast cancer risk stratification is helpful when determining appropriateness of HT in postmenopausal women. Generally, if using risk stratification models for breast cancer (ie, Gail Risk model or international breast cancer intervention study [IBIS] tool), a patient who is average to moderate risk, HT can be offered with appropriate counseling. By contrast, a patient who is high risk should have a more detailed discussion about their risk (surveillance and risk-reducing treatments), and they may consider nonhormonal options for treatment of VMS. Women with a history of breast cancer should not be prescribed systemic HT.
Continue to: Additional HT benefits...
Additional HT benefits
The benefits of HT in postmenopausal women include improved bone health and reduction of fractures; reduction of risk for type 2 diabetes mellitus (T2DM); improvement of insulin sensitivity; improvement of lipid profiles with increased HDL and decreased LDL levels; and reduction of colon cancer risk.25 For women aged younger than 60 years who start HT within 10 years of their last menstrual period, HT has been shown to cause a reduction in all-cause mortality. Important risks to counsel patients on when starting HT include the low risk of stroke and venous thromboembolism (VTE) when using oral formulations.26
CASE Resolved
Her ASCVD risk score, based on her history, estimates her 10-year CVD risk to be low (<5%). Thus, from a cardiovascular standpoint, either oral or transdermal HT would be an appropriate option. Her IBIS 10-year score is 1.5%, placing her in a low-risk category for breast cancer based on her personal and family history. Given that she is less than 60 years of age and within 10 years of menopause, along with her low-risk stratification for CVD and breast cancer, she would be an appropriate patient to begin combined HT with an estrogen plus an oral progesterone, such as an estradiol patch 0.0375 mg twice weekly, along with oral micronized progesterone 100 mg nightly. The dose could be increased over time based on symptoms and tolerability of the treatment.
ALTERNATE CASE 1 The patient has additional risk factors
Consider the patient case with the following additions to her history: the patient has a BMI of 34 kg/m2, a history of well-controlled hypertension while taking amlodipine 5 mg, and an ASCVD risk score of 7.5%. She reports severe VMS that are greatly impacting her quality of life. How would your recommendations or counseling change?
Focus on healthy lifestyle
Obesity and hypertension, both common chronic conditions, pose additional risks to be accounted for when counseling on and approaching HT prescribing. Her alternate ASCVD risk score places her at moderate risk for CVD within 10 years, based on guidelines as discussed above. It would still be appropriate to offer her combined HT after a shared decision-making discussion that includes a focus on healthy lifestyle habits.
Consider transdermal HT in obese women
Longitudinal studies have found that weight gain is more a consequence of aging, regardless of menopausal status. Fat distribution and body composition changes are a menopause-related phenomenon driven by estrogen deficiency. HT has been shown to preserve lean body mass and reduce visceral adiposity, resulting in favorable effects of body composition. Still, obesity results in increased risk of CVD, VTE, and certain hormone-sensitive cancers.27 When considering HT in obese patients, a transdermal estrogen route is preferred to reduce risks.
For women with hypertension, prescribe transdermal HT
Overall, studies have found that HT has a neutral effect on blood pressure.25 When considering formulation of HT, micronized progesterone, dydrogesterone, and drospirenone seem to be most neutral and possibly even beneficial on blood pressure compared with synthetic progestins.26 Oral estrogen is associated with increased vasoconstriction and/or increased sodium retention with resultant worsened regulation of blood pressure in women with hypertension, so transdermal estrogen is preferred for women with hypertension.26 Hypertension is a component of the ASCVD risk score; factoring this into a patient’s clinical picture is important when discussing appropriateness of HT prescribing. To minimize risks, the transdermal route of estrogen is preferred for those with hypertension.
Continue to: ALTERNATE CASE 1 Resolved...
ALTERNATE CASE 1 Resolved
She has a moderate ASCVD risk score, is obese, and has a history of hypertension. Through shared decision making, you ultimately start her on transdermal estrogen and micronized progesterone to treat her quality-of-life-impacting VMS, a formulation that is most likely to mitigate the possible risks in her clinical case. You see her back in the clinic every 3-6 months to monitor her blood pressure.
ALTERNATE CASE 2 The patient has a high risk for breast cancer
The patient reveals further her significant family history of breast cancer in her maternal grandmother and mother, both diagnosed in their 50s. You calculate her risk of breast cancer with a model that incorporates family history. Her Tyrer Cuzick-IBIS 10-year risk score is >5% and lifetime risk is >20%, putting her at high risk for breast cancer. Since she has a uterus and would need concomitant progesterone therapy, her risk for breast cancer is higher than if she was taking ET alone. Ultimately, together you and the patient decide to trial nonhormonal options for her VMS.
What are nonhormonal options for treatment of VMS?
While HT remains the most effective treatment for VMS, there are multiple nonhormonal treatments for women who are either at too high a risk for HT or who favor other options, which are outlined in the NAMS 2015 nonhormonal management position statement.27 Cognitive behavioral therapy (CBT) has been shown to decrease bother related to VMS but not frequency. Clinical hypnosis has been shown to reduce hot flash frequency and improve sleep. Paroxetine salt (7.5 mg/day) remains the only FDA nonhormonal-approved medication for treatment of moderate to severe vasomotor symptoms. Off label use of other selective serotonin reuptake inhibitors (SSRIs) and selective norepinephrine reuptake inhibitors have been shown in studies to reduce VMS including paroxetine at slightly higher doses (10 mg/day–20 mg/day), citalopram (10 mg/day–20 mg/day), escitalopram (10 mg/day–20 mg/day), venlafaxine (37.5 mg/day–150 mg/day), and desvenlafaxine (50 mg/day–100 mg/day). Other treatments that could be considered include off-label use of gabapentin (900 mg/day–2,400 mg/day), oxybutynin (2.5–5 mg twice daily) or clonidine (0.1 mg/day–1 mg/day divided in doses) since they all have data demonstrating they are beneficial at reducing VMS.
Nonhormonal options that may be helpful but are recommended with caution due to lack of data include weight loss, mindfulness-based stress reduction, s-equol derivatives of soy isoflavones and a stellate ganglion block. Further evidence and studies are needed for the aforementioned options.27
ALTERNATE CASE 2 Resolved
She may consider any of the nonhormonal options discussed. If she meets with a medical breast specialist to discuss her elevated risk of breast cancer and considers starting risk-reducing medications, particularly tamoxifen, you will want to avoid medications that have significant CPY 2D6 inhibition, such as paroxetine and fluoxetine. Safer choices would include venlafaxine, escitalopram, or citalopram.
The bottom line
In summary, the benefits and risks of HT in the treatment of VMS remain nuanced. For healthy women younger than 60 years of age and within 10 years from their last menstrual period, the benefits of HT largely outweigh the risks. Shared decision making, along with individualized and appropriate risk stratification specific for women, can guide appropriateness of HT prescribing. For those women who cannot take HT or choose not to, there are many nonhormonal options that will help manage their bothersome VMS. ●
CASE Healthy woman with hot flashes inquires about HT
A 54-year-old healthy woman with a history of hypothyroidism taking thyroid replacement medication comes in for her annual visit. Her last menstrual period was over 2 years ago and she reports severe hot flashes. They have greatly affected her quality of life and she must take frequent breaks at work. She wakes up frequently at night due to night sweats, which is impacting her sleep and, subsequently, her energy level. She has noted increased vaginal dryness so has been abstaining from sexual intercourse due to the discomfort. She has an intact uterus. Her family history is significant for heart disease, diagnosed in her mother at age 75.
On physical examination, she is normotensive and well-appearing. Her body mass index (BMI) is 21 kg/m2. Labs obtained prior to her visit show normal renal and liver function. Her high-density lipid (HDL) level is 55 mg/dL, her low-density lipid (LDL) level is 80 mg/dL, and her triglyceride level is 100 mg/dL; HbA1c is 5.5 mmol/mol.
She is interested in learning more about menopausal hormone therapy (HT) and whether or not she would be a candidate.
What information do you need to know to counsel and manage this patient?
Menopausal HT prescribing practices have changed over the last few decades as a better understanding of the risks and benefits of treatment have emerged. Prior to 2002, HT was commonly used for treatment of symptoms associated with menopause and was thought to have beneficial effects for chronic disease prevention.1-4 After data from the Women’s Health Initiative (WHI) was released, concerns arose around the effect of HT on cardiovascular health and risk of breast cancer. As a result, HT prescriptions fell precipitously after around 2002.5 Since then, postintervention analysis and cumulative 18-year follow-up of WHI data, along with results from subsequent randomized controlled trials, including the Kronos Early Estrogen Prevention Study (KEEPS) and the Early Versus Late Intervention Trial with Estradiol (ELITE), have demonstrated a favorable safety profile for healthy women starting HT early in menopause (less than age 60, or within 10 years from their final menstrual period).5-11
There are many types, formulations, and routes of HT, and the effects and risks differ for each (TABLE). For example, oral estrogen therapy, such as conjugated equine estrogens, portend a higher risk of adverse effects compared with transdermal formulations. Topical and transdermal estrogens bypass first-pass hepatic metabolism and thus are associated with a lower risk of venous thromboembolism (VTE) compared with oral formulations.12-14 A progestogen such as micronized progesterone is used in postmenopausal women with a uterus to protect the endometrium from unopposed estrogen therapy (ET). While it comes in oral and transdermal forms, the oral formulation is most widely used and studied in the United States; transdermal forms do not provide adequate endometrial protection and should not be used in combination therapy.15,16

Risks and benefits
Cardiovascular risk
Over time, the benefits and risks of HT use in menopausal patients have been further elucidated and defined, although they remain complex and dependent on patient clinical characteristics. HT remains the most effective treatment for vasomotor symptoms (VMS) and the genitourinary syndrome of menopause.17,18 In 2002, concerns for increased cardiovascular disease (CVD) and breast cancer risk resulted in early cessation of the WHI trial. Since that time the risk of CVD in postmenopausal women taking HT has been found to be more nuanced. In fact, updates in the literature have shown that HT results in a reduction of coronary heart disease if started in healthy women younger than age 60 years within 10 years of menopause.7,9-11 With this updated information, the North American Menopause society (NAMS), American College of Obstetricians and Gynecologists and the Endocrine Society have published guidelines supporting the initiation of HT for symptomatic healthy women: under the age of 60, within 10 years of menopause, and without contraindications. After age 60 years and further from menopause, the benefits and risks become less known.18-20
Risk stratification allows for more comprehensive counseling in use of HT for treatment of bothersome VMS. From a cardiovascular health standpoint, calculating an atherosclerotic CVD (ASCVD) risk score helps to evaluate appropriateness of HT prescribing:
- For those with low 10-year CVD risk (<5%), either oral or transdermal HT is appropriate.
- For those with moderate 10-year CVD risk (5%-10%), transdermal HT is recommended over oral HT.
- For those with high 10-year CVD risk (>10%), HT is not recommended.19,21
Breast cancer risk
Follow up since the initial WHI publication have shown that breast cancer risk is largely dependent on the formulation and route of HT used. Oral estrogen combined with a progestogen has been shown to increase the risk of invasive breast cancer, though very rarely.22 To put it into context, the absolute risk of breast cancer based on follow-up studies from WHI showed less than 1 additional case per 1,000 person years of use; less risk than associated with drinking 2 glasses of wine per day and similar to that of obesity and/or sedentary lifestyle.23,24 Studies have shown estrogen treatment alone for postmenopausal women does not appear to increase the risk of breast cancer. In fact, follow-up data from WHI showed a nonsignificant reduction in breast cancer risk for those taking ET alone.25
Breast cancer risk stratification is helpful when determining appropriateness of HT in postmenopausal women. Generally, if using risk stratification models for breast cancer (ie, Gail Risk model or international breast cancer intervention study [IBIS] tool), a patient who is average to moderate risk, HT can be offered with appropriate counseling. By contrast, a patient who is high risk should have a more detailed discussion about their risk (surveillance and risk-reducing treatments), and they may consider nonhormonal options for treatment of VMS. Women with a history of breast cancer should not be prescribed systemic HT.
Continue to: Additional HT benefits...
Additional HT benefits
The benefits of HT in postmenopausal women include improved bone health and reduction of fractures; reduction of risk for type 2 diabetes mellitus (T2DM); improvement of insulin sensitivity; improvement of lipid profiles with increased HDL and decreased LDL levels; and reduction of colon cancer risk.25 For women aged younger than 60 years who start HT within 10 years of their last menstrual period, HT has been shown to cause a reduction in all-cause mortality. Important risks to counsel patients on when starting HT include the low risk of stroke and venous thromboembolism (VTE) when using oral formulations.26
CASE Resolved
Her ASCVD risk score, based on her history, estimates her 10-year CVD risk to be low (<5%). Thus, from a cardiovascular standpoint, either oral or transdermal HT would be an appropriate option. Her IBIS 10-year score is 1.5%, placing her in a low-risk category for breast cancer based on her personal and family history. Given that she is less than 60 years of age and within 10 years of menopause, along with her low-risk stratification for CVD and breast cancer, she would be an appropriate patient to begin combined HT with an estrogen plus an oral progesterone, such as an estradiol patch 0.0375 mg twice weekly, along with oral micronized progesterone 100 mg nightly. The dose could be increased over time based on symptoms and tolerability of the treatment.
ALTERNATE CASE 1 The patient has additional risk factors
Consider the patient case with the following additions to her history: the patient has a BMI of 34 kg/m2, a history of well-controlled hypertension while taking amlodipine 5 mg, and an ASCVD risk score of 7.5%. She reports severe VMS that are greatly impacting her quality of life. How would your recommendations or counseling change?
Focus on healthy lifestyle
Obesity and hypertension, both common chronic conditions, pose additional risks to be accounted for when counseling on and approaching HT prescribing. Her alternate ASCVD risk score places her at moderate risk for CVD within 10 years, based on guidelines as discussed above. It would still be appropriate to offer her combined HT after a shared decision-making discussion that includes a focus on healthy lifestyle habits.
Consider transdermal HT in obese women
Longitudinal studies have found that weight gain is more a consequence of aging, regardless of menopausal status. Fat distribution and body composition changes are a menopause-related phenomenon driven by estrogen deficiency. HT has been shown to preserve lean body mass and reduce visceral adiposity, resulting in favorable effects of body composition. Still, obesity results in increased risk of CVD, VTE, and certain hormone-sensitive cancers.27 When considering HT in obese patients, a transdermal estrogen route is preferred to reduce risks.
For women with hypertension, prescribe transdermal HT
Overall, studies have found that HT has a neutral effect on blood pressure.25 When considering formulation of HT, micronized progesterone, dydrogesterone, and drospirenone seem to be most neutral and possibly even beneficial on blood pressure compared with synthetic progestins.26 Oral estrogen is associated with increased vasoconstriction and/or increased sodium retention with resultant worsened regulation of blood pressure in women with hypertension, so transdermal estrogen is preferred for women with hypertension.26 Hypertension is a component of the ASCVD risk score; factoring this into a patient’s clinical picture is important when discussing appropriateness of HT prescribing. To minimize risks, the transdermal route of estrogen is preferred for those with hypertension.
Continue to: ALTERNATE CASE 1 Resolved...
ALTERNATE CASE 1 Resolved
She has a moderate ASCVD risk score, is obese, and has a history of hypertension. Through shared decision making, you ultimately start her on transdermal estrogen and micronized progesterone to treat her quality-of-life-impacting VMS, a formulation that is most likely to mitigate the possible risks in her clinical case. You see her back in the clinic every 3-6 months to monitor her blood pressure.
ALTERNATE CASE 2 The patient has a high risk for breast cancer
The patient reveals further her significant family history of breast cancer in her maternal grandmother and mother, both diagnosed in their 50s. You calculate her risk of breast cancer with a model that incorporates family history. Her Tyrer Cuzick-IBIS 10-year risk score is >5% and lifetime risk is >20%, putting her at high risk for breast cancer. Since she has a uterus and would need concomitant progesterone therapy, her risk for breast cancer is higher than if she was taking ET alone. Ultimately, together you and the patient decide to trial nonhormonal options for her VMS.
What are nonhormonal options for treatment of VMS?
While HT remains the most effective treatment for VMS, there are multiple nonhormonal treatments for women who are either at too high a risk for HT or who favor other options, which are outlined in the NAMS 2015 nonhormonal management position statement.27 Cognitive behavioral therapy (CBT) has been shown to decrease bother related to VMS but not frequency. Clinical hypnosis has been shown to reduce hot flash frequency and improve sleep. Paroxetine salt (7.5 mg/day) remains the only FDA nonhormonal-approved medication for treatment of moderate to severe vasomotor symptoms. Off label use of other selective serotonin reuptake inhibitors (SSRIs) and selective norepinephrine reuptake inhibitors have been shown in studies to reduce VMS including paroxetine at slightly higher doses (10 mg/day–20 mg/day), citalopram (10 mg/day–20 mg/day), escitalopram (10 mg/day–20 mg/day), venlafaxine (37.5 mg/day–150 mg/day), and desvenlafaxine (50 mg/day–100 mg/day). Other treatments that could be considered include off-label use of gabapentin (900 mg/day–2,400 mg/day), oxybutynin (2.5–5 mg twice daily) or clonidine (0.1 mg/day–1 mg/day divided in doses) since they all have data demonstrating they are beneficial at reducing VMS.
Nonhormonal options that may be helpful but are recommended with caution due to lack of data include weight loss, mindfulness-based stress reduction, s-equol derivatives of soy isoflavones and a stellate ganglion block. Further evidence and studies are needed for the aforementioned options.27
ALTERNATE CASE 2 Resolved
She may consider any of the nonhormonal options discussed. If she meets with a medical breast specialist to discuss her elevated risk of breast cancer and considers starting risk-reducing medications, particularly tamoxifen, you will want to avoid medications that have significant CPY 2D6 inhibition, such as paroxetine and fluoxetine. Safer choices would include venlafaxine, escitalopram, or citalopram.
The bottom line
In summary, the benefits and risks of HT in the treatment of VMS remain nuanced. For healthy women younger than 60 years of age and within 10 years from their last menstrual period, the benefits of HT largely outweigh the risks. Shared decision making, along with individualized and appropriate risk stratification specific for women, can guide appropriateness of HT prescribing. For those women who cannot take HT or choose not to, there are many nonhormonal options that will help manage their bothersome VMS. ●
- Carr BR, Wilson JD. Disorders of the ovary and female reproductive tract. In: Isselbacher KJ, Braunwald E, Wilson JD, eds. Harrisons’ Principles of Internal Medicine, 13th ed. New York, NY: McGraw-Hill; 1994:2016-2017.
- Davidson MH, Maki KC, Marx P, et al. Effects of continuous estrogen and estrogen-progestin replacement regimens on cardiovascular risk markers in postmenopausel women. Arch Intern Med. 2000;160:3315-3325. doi: 10.1001/archinte.160.21.3315.
- Grodstein F, Manson JE, Colditz GA, et al. A prospective, observational study of postmenopausal hormone therapy and primary prevention of cardiovascular disease. Ann Intern Med. 2000;133:933-941. doi: 10.7326/0003-4819-133-12-200012190-00008.
- Grady D, Rubin SM, Petitti DB, et al. Hormone therapy to prevent disease and prolong life in postmenopausal women. Ann Intern Med. 1992;117:1016-1037. doi: 10.7326/0003-4819-117-12-1016.
- Rossouw JE, Manson JE, Kaunitz AM, et al. Lessons learned from the Women’s Health Initiative trials of menopausal hormone therapy. Obstet Gynecol. 2013;121:172-176. doi: 10.1097/aog.0b013e31827a08c8.
- Manson JE, Hsia J, Johnson KC, et al. Estrogen plus progestin and the risk of coronary heart disease. NEJM. 2003;349:523-534. doi: 10.1056/NEJMoa030808.
- Manson JE, Chlebowski RT, Stefanick ML, et al. Menopausal hormone therapy and health outcomes during the intervention and extended poststopping phases of the Women’s Health Initiative randomized trials. JAMA. 2013;310:1353-1368. doi: 10.1001/jama.2013.278040.
- Santen RJ, Allred DC, Ardoin SP, et al. Postmenopausal hormone therapy: an Endocrine Society scientific statement. J Clin Endocrinol Metab. 2010;95:S1-S66. doi: 10.1210/jc.2009-2509.
- Manson JE, Aragaki AK, Rossouw JE, et al. Menopausal hormone therapy and long-term all-cause and cause-specific mortality: the Women’s Health Initiative randomized trials. JAMA. 2017;318:927-938. doi: 10.1001/jama.2017.11217.
- Hodis HN, Mack WJ, Henderson VW, et al. Vacular effects of early versus late postmenopausal treatment with estradiol. NEJM. 2016;374:1221-1231. doi: 10.1056/NEJMoa1505241.
- Taylor HS, Tal A, Pal L, et al. Effects of oral vs transdermal estrogen therapy on sexual function in early postmenopause: ancillary study of the Kronos Early Estrogen Prevention Study (KEEPS). JAMA Intern Med. 2017;177:1471-1479. doi: 10.1001/jamainternmed.2017.3877.
- Liu JH, Pinkerton JV. Prescription therapies. In: CJ Crandall, ed. Menopause Practice: A Clinician’s Guide, 6th ed. Pepper Pike, OH: The North American Menopause Society; 2019:277-309.
- Files J, Kling JM. Transdermal delivery of bioidentical estrogen in menopausal hormone therapy: a clinical review. Expert Opin Drug Deliv. 2020;17:543-549. doi: 10.1080/17425247.2020.1700949.
- Canonico M, Carcaillon L, Plu-Bureau G, et al. Postmenopausal hormone therapy and risk of stroke: impact of the route of estrogen administration and type of progestogen. Stroke. 2016;47:1734-1741. doi: 10.1161/STROKEAHA.116.013052.
- Hitchcok CL, Prior JC. Oral micronized progesterone for vasomotor symptoms—a placebo-controlled randomized trial in healthy post-menopausal women. Menopause. 2001;8:10-16.
- Effects of hormone replacement therapy on endometrial histology in postmenopausal women. The Postmenopausal Estrogen/Progestin Interventions (PEPI) Trial. The writing Group for the PEPI Trial. JAMA. 1996;275:370-375. doi: 10.1001/jama.1996.03530290040035.
- Pinkerton JV. Hormone therapy for postmenopausal women. N Engl J Med. 2020;382:446-55. doi:10.1056/NEJMcp1714787.
- The 2022 hormone therapy position statement of The North American Menopause Society. Menopause. 2022;29:767-794. doi:10.1097/GME.00000000000000002028.
- Stuenkel CA, Davis SR, Gompel A, et al. Treatment of symptoms of the menopause: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2015;100:3975-4011. doi: 10.1210/jc.2015-2236.
- American College of Obstetricians and Gynecologists. Practice Bulletin No. 141: Management of Menopausal Symptoms. Obstet Gynecol. 2014;123:202-216. doi: 10.1097/01.AOG.0000441353.20693.78.
- Manson JE. Current recommendations: what is the clinician to do? Fertil Steril. 2014;101:916. World Cancer Research Fund/American Institute for Cancer Research. Continuous Update Project Expert Report 2018. Alcoholic drinks and the risk of cancer. https://www.wcrf.org/sites/default/files/Alcoholic-Drinks.pdf. 2018.
- Anderson GL, Chlebowski RT, Aragaki AK, et al. Conjugated equine oestrogen and breast cancer incidence and mortality in postmenopausal women with hysterectomy: Extended follow-up of Women’s Health Initiative randomized placebo-controlled trial. Lancet Oncol. 2012;5:476-486. doi: 10.1016/S1470-2045(12)70075-X.
- World Cancer Research Fund/American Institute for Cancer Research. Continuous Update Project Expert Report 2018. Diet, nutrition, physical activity and breast cancer. www.aicr.org/continuous-update-project/breast-cancer.html. 2018.
- Anderson GL, Chlebowski RT, Aragaki AK, et al. Conjugated equine oestrogen and breast cancer incidence and mortality in postmenopausal women with hysterectomy: Extended follow-up of the Women’s Health Initiative randomized placebo-controlled trial. Lancet Oncol. 2012;5:476-486. doi: 10.1016/S1470-2045(12)70075-X.
- Mehta J, Kling JM, Manson JE. Risks, benefits and treatment modalities of menopausal hormone therapy: current concepts. Front Endocrinol (Laussane). 2021;12:564781. doi: 10.3389/fendo.2021.564781.
- Kapoor E, Kling JM, Lobo AS, et al. Menopausal hormone therapy in women with chronic medical conditions. Best Pract Res Clin Endocrinol Metab. 2021:35;101578. doi: 10.1016/j.beem.2021.101578.
- NAMS position statement advisory panel. Nonhormonal management of menopause-associated vasomotor symptoms: 2015 position statement of The North American Menopause Society. Menopause. 2015:22:1155-72. doi: 10.1097/GME.0000000000000546.
- Carr BR, Wilson JD. Disorders of the ovary and female reproductive tract. In: Isselbacher KJ, Braunwald E, Wilson JD, eds. Harrisons’ Principles of Internal Medicine, 13th ed. New York, NY: McGraw-Hill; 1994:2016-2017.
- Davidson MH, Maki KC, Marx P, et al. Effects of continuous estrogen and estrogen-progestin replacement regimens on cardiovascular risk markers in postmenopausel women. Arch Intern Med. 2000;160:3315-3325. doi: 10.1001/archinte.160.21.3315.
- Grodstein F, Manson JE, Colditz GA, et al. A prospective, observational study of postmenopausal hormone therapy and primary prevention of cardiovascular disease. Ann Intern Med. 2000;133:933-941. doi: 10.7326/0003-4819-133-12-200012190-00008.
- Grady D, Rubin SM, Petitti DB, et al. Hormone therapy to prevent disease and prolong life in postmenopausal women. Ann Intern Med. 1992;117:1016-1037. doi: 10.7326/0003-4819-117-12-1016.
- Rossouw JE, Manson JE, Kaunitz AM, et al. Lessons learned from the Women’s Health Initiative trials of menopausal hormone therapy. Obstet Gynecol. 2013;121:172-176. doi: 10.1097/aog.0b013e31827a08c8.
- Manson JE, Hsia J, Johnson KC, et al. Estrogen plus progestin and the risk of coronary heart disease. NEJM. 2003;349:523-534. doi: 10.1056/NEJMoa030808.
- Manson JE, Chlebowski RT, Stefanick ML, et al. Menopausal hormone therapy and health outcomes during the intervention and extended poststopping phases of the Women’s Health Initiative randomized trials. JAMA. 2013;310:1353-1368. doi: 10.1001/jama.2013.278040.
- Santen RJ, Allred DC, Ardoin SP, et al. Postmenopausal hormone therapy: an Endocrine Society scientific statement. J Clin Endocrinol Metab. 2010;95:S1-S66. doi: 10.1210/jc.2009-2509.
- Manson JE, Aragaki AK, Rossouw JE, et al. Menopausal hormone therapy and long-term all-cause and cause-specific mortality: the Women’s Health Initiative randomized trials. JAMA. 2017;318:927-938. doi: 10.1001/jama.2017.11217.
- Hodis HN, Mack WJ, Henderson VW, et al. Vacular effects of early versus late postmenopausal treatment with estradiol. NEJM. 2016;374:1221-1231. doi: 10.1056/NEJMoa1505241.
- Taylor HS, Tal A, Pal L, et al. Effects of oral vs transdermal estrogen therapy on sexual function in early postmenopause: ancillary study of the Kronos Early Estrogen Prevention Study (KEEPS). JAMA Intern Med. 2017;177:1471-1479. doi: 10.1001/jamainternmed.2017.3877.
- Liu JH, Pinkerton JV. Prescription therapies. In: CJ Crandall, ed. Menopause Practice: A Clinician’s Guide, 6th ed. Pepper Pike, OH: The North American Menopause Society; 2019:277-309.
- Files J, Kling JM. Transdermal delivery of bioidentical estrogen in menopausal hormone therapy: a clinical review. Expert Opin Drug Deliv. 2020;17:543-549. doi: 10.1080/17425247.2020.1700949.
- Canonico M, Carcaillon L, Plu-Bureau G, et al. Postmenopausal hormone therapy and risk of stroke: impact of the route of estrogen administration and type of progestogen. Stroke. 2016;47:1734-1741. doi: 10.1161/STROKEAHA.116.013052.
- Hitchcok CL, Prior JC. Oral micronized progesterone for vasomotor symptoms—a placebo-controlled randomized trial in healthy post-menopausal women. Menopause. 2001;8:10-16.
- Effects of hormone replacement therapy on endometrial histology in postmenopausal women. The Postmenopausal Estrogen/Progestin Interventions (PEPI) Trial. The writing Group for the PEPI Trial. JAMA. 1996;275:370-375. doi: 10.1001/jama.1996.03530290040035.
- Pinkerton JV. Hormone therapy for postmenopausal women. N Engl J Med. 2020;382:446-55. doi:10.1056/NEJMcp1714787.
- The 2022 hormone therapy position statement of The North American Menopause Society. Menopause. 2022;29:767-794. doi:10.1097/GME.00000000000000002028.
- Stuenkel CA, Davis SR, Gompel A, et al. Treatment of symptoms of the menopause: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2015;100:3975-4011. doi: 10.1210/jc.2015-2236.
- American College of Obstetricians and Gynecologists. Practice Bulletin No. 141: Management of Menopausal Symptoms. Obstet Gynecol. 2014;123:202-216. doi: 10.1097/01.AOG.0000441353.20693.78.
- Manson JE. Current recommendations: what is the clinician to do? Fertil Steril. 2014;101:916. World Cancer Research Fund/American Institute for Cancer Research. Continuous Update Project Expert Report 2018. Alcoholic drinks and the risk of cancer. https://www.wcrf.org/sites/default/files/Alcoholic-Drinks.pdf. 2018.
- Anderson GL, Chlebowski RT, Aragaki AK, et al. Conjugated equine oestrogen and breast cancer incidence and mortality in postmenopausal women with hysterectomy: Extended follow-up of Women’s Health Initiative randomized placebo-controlled trial. Lancet Oncol. 2012;5:476-486. doi: 10.1016/S1470-2045(12)70075-X.
- World Cancer Research Fund/American Institute for Cancer Research. Continuous Update Project Expert Report 2018. Diet, nutrition, physical activity and breast cancer. www.aicr.org/continuous-update-project/breast-cancer.html. 2018.
- Anderson GL, Chlebowski RT, Aragaki AK, et al. Conjugated equine oestrogen and breast cancer incidence and mortality in postmenopausal women with hysterectomy: Extended follow-up of the Women’s Health Initiative randomized placebo-controlled trial. Lancet Oncol. 2012;5:476-486. doi: 10.1016/S1470-2045(12)70075-X.
- Mehta J, Kling JM, Manson JE. Risks, benefits and treatment modalities of menopausal hormone therapy: current concepts. Front Endocrinol (Laussane). 2021;12:564781. doi: 10.3389/fendo.2021.564781.
- Kapoor E, Kling JM, Lobo AS, et al. Menopausal hormone therapy in women with chronic medical conditions. Best Pract Res Clin Endocrinol Metab. 2021:35;101578. doi: 10.1016/j.beem.2021.101578.
- NAMS position statement advisory panel. Nonhormonal management of menopause-associated vasomotor symptoms: 2015 position statement of The North American Menopause Society. Menopause. 2015:22:1155-72. doi: 10.1097/GME.0000000000000546.


