User login
Clinical pharmacy specialist interventions after patient consultation resulted in statistically significant increases in HbA1c levels in patients at risk for hypoglycemia who relaxed their therapy.
Intensive glycemic lowering for the treatment for type 2 diabetes mellitus (T2DM) has been shown to decrease microvascular and macrovascular outcomes in the UK Prospective Diabetes Study (UKPDS) without any risk of increased harm.1,2 Over the past decade, evidence has shown that the outcomes and risk do not hold true in an older population with additional comorbidities and longer duration of DM. Both the Action to Control Cardiovascular Risk in Diabetes (ACCORD) and Veterans Affairs Diabetes Trial (VADT) trials showed no decreased incidence of macrovascular or microvascular complications of DM with intensive glucose lowering but an additional risk of hypoglycemia and even death.2-4
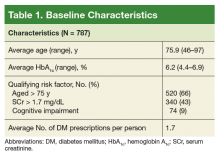
Patient-specific risk factors, such as age, impaired renal function, and cognitive impairment, have been shown to lead to an increased risk of hypoglycemia independent of hemoglobin A1c (HbA1c). Dementia and cognitive impairment are associated with a 2.42 and 1.72 times greater risk of hypoglycemia, respectively, compared with a patient without dementia or cognitive impairment.5 A post-hoc analysis of the ACCORD trial that analyzed the risk of hypoglycemia in subgroup populations showed an increased risk of hypoglycemia in those with a serum creatinine (SCr) level > 1.3 mg/dL (hazard ratio, 1.66, P < .01) and increasing age. Risk of hypoglycemia was highest in those aged ≥ 75 years but increased by 3% for every subsequent year (P < .01).6 These risk factors should be addressed and considered in individual patients with DM to safely guide therapy.
The evidence from these landmark trials has led to increased HbA1c goals for specific patient populations in the most recent 2017 VA/DoD Clinical Practice Guideline (CPG) for the Management of Type 2 Diabetes Mellitus in Primary Care.7 The majority of patients with T2DM now qualify for HBA1c goals > 7.0%. According to the 2017 VA/DoD CPG, younger patients with the absence of a major comorbidity and life expectancy of > 10 to 15 years with mild or absent microvascular complications is the only group of patients who should be treated to an A1c goal of 6.0 to 7.0%.7 The use of shared decision making and patient education to set glycemic goals based on “patient capabilities, needs, goals, prior treatment experience, and preferences” also should be used to increase patient education and satisfaction.7
In December 2014, the VA introduced the Hypoglycemia Safety Initiative (HSI). The goal of the HSI is to “enable veterans living with diabetes to work more closely with their VA clinicians to personalize health care goals and improve self-management of the disease.”8 This goal also aligns with the US Department of Health and Human Services National Action Plan for Adverse Drug Event Prevention. One of 3 initial targets of this plan includes DM agents and the prevention of hypoglycemia.9
To combat the risk of hypoglycemia and potentially negative outcomes, as part of the HSI, the VA is implementing a clinical reminder within the Computerized Patient Record System (CPRS) that will prompt the primary care team to screen select patients at risk for hypoglycemia. The purpose of this project was to identify patients at high risk of hypoglycemia, individualize HbA1c goals, and consider de-escalation in therapy, using shared decision making.
Methods
This quality improvement project, conducted at the Fayetteville VA Medical Center (FVAMC), consisted of outpatient services provided at 2 health care centers and 6 community-based outpatient clinics. The project was exempt from institutional review board approval as the protocol met national VA criteria as a quality assurance project.
Patients were identified using the HSI Corporate Data Warehouse (CDR) reports. Once patients were identified, a list was distributed to the appropriate clinical pharmacy specialist (CPS), according to patient aligned care teams (PACTs). The CPS contacted the patient via telephone or in person to conduct hypoglycemia screening. Patients on a sulfonylurea or insulin and an HbA1c < 7% plus 1 risk factor for hypoglycemia (aged ≥ 75 years, serum creatinine[SCr] ≥1.7 mg/dL, diagnosis of cognitive impairment, or prescribed a cholinesterase inhibitor) were included. These risk factors were chosen to align with the future clinical reminder, which is based on an increased risk of hypoglycemia seen in these patient populations.
Patients were included if they were receiving antidiabetic medications through the FVAMC or outside of the VA and/or prescribed by a non-VA provider. Medications and doses prescribed by a non-VA provider were verified with the patient verbally during the initial interview. Once contacted by the CPS, any patients who no longer met inclusion criteria were excluded.
The CPS used a national VA hypoglycemia screening note template to ask the patient about frequency and severity of hypoglycemia. Hypoglycemia was defined as a self-monitored blood glucose < 70 mg/dL with or without symptoms. An additional definition consisted of typical hypoglycemia symptoms as reported by the patient even if self-monitored blood glucose was not obtained while exhibiting symptoms. Using shared decision making between the CPS and veteran, antidiabetic therapy was either relaxed or continued. Relaxing therapy was defined as decreasing doses or discontinuation of antidiabetic medications that are known for potentiating hypoglycemia (ie, sulfonylurea and insulin).
The CPS had autonomy in deciding how much to lower dose(s) or when to discontinue medication(s). Additional counseling in proper medication administration, including appropriate timing of medication administration, also could have been the sole intervention needed for a given patient who experienced hypoglycemia. Counseling would have been considered continuation of therapy. For example, if a patient was experiencing hypoglycemia while taking a sulfonylurea twice daily, the CPS would provide counseling on proper timing of medication administration 20 to 30 minutes before morning and evening meals rather than the patient’s perceived administration of twice daily without regard to meals. Even in patients who met inclusion criteria but who did not experience any hypoglycemia symptoms, the CPS and patient could use shared decision making with emphasis on appropriate HbA1c goals to determine whether relaxation in therapy was appropriate.
Data Collection
Baseline demographics, including prespecified risk factors for hypoglycemia, were collected. Data were imported into the HSI CDW from the national VA hypoglycemia screening note template completed by the CPS. From the data CDW, frequency and severity of hypoglycemia were recorded. The CPS interventions were also quantified; HbA1c data were obtained in patients in whom therapy was relaxed 3- to 6-months postintervention.
Statistical Analysis
Descriptive statistics (mean, range) were used for analyzing results. A t test with a 1-tailed distribution was used to analyze the change in HbA1c after CPS intervention (α = .05).
Results
On August 17, 2017, 839 patients were identified across all FVAMC facilities from the HSI data CDW. Patients were contacted through February 16, 2018. A total of 52 patients were excluded as they no longer met inclusion criteria or were deceased at time of review.
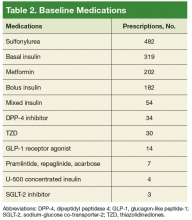
The most commonly prescribed antidiabetic prescription was a sulfonylurea (482 prescriptions) followed by basal insulin (319 prescriptions; Table 2).
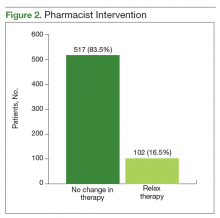
The CPS used shared decision making to relax antidiabetic therapy in 102 (16.5%) of the total number of patients contacted (Figure 2). Lab orders were entered for the patient to obtain an HbA1c in 3 to 6 months in those in whom therapy was relaxed.
Discussion
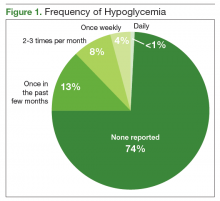
The primary objective of this project was to identify patients at risk for hypoglycemia. Approximately 1 in 4 patients reported any incidence of hypoglycemia, which shows that the prespecified inclusion criteria was an appropriate guide for hypoglycemia screening. The episodes of hypoglycemia were typically infrequent, occurring only once every few months. This could have contributed to a lower rate of therapy changes compared with the rate of hypoglycemia. Overall, hypoglycemia was not severe; 83% of patients did not report any symptoms of faintness. Pharmacists were able to intervene and relax therapy in 102 patients to try to prevent episodes of hypoglycemia and negative outcomes. These interventions led to a statistically significant increase in average HbA1c in these patients. Throughout these encounters with the CPS and patient, there were also innumerable outcomes secondary to the use of shared decision making. Regardless of medication changes, there was increased patient education concerning hypoglycemia treatment, medication administration times, and HbA1c goals.
This project’s strengths included the large sample size, appropriate inclusion criteria that identified patients at risk for hypoglycemia, and the use of shared decision making. It was also beneficial to obtain HbA1c levels after a relaxation in therapy for objective outcomes. The increase in HbA1c levels showed a statistically significant gain, which led to more patients having an HbA1c closer to a CPG-recommended goal range, given their risk factors for hypoglycemia. This pharmacy initiative fostered increased communication between providers and CPS within the PACT team. The pharmacist was not consulted by the provider for management of these patients with DM, so therapy relaxation was documented in CPRS and was addressed at the patient’s next primary care appointment. Some changes also required discussion with the primary care provider prior to relaxation in therapy. By initiating these discussions with providers, opportunities arose for additional education on appropriate HbA1c goals and why therapy should be relaxed in select patient populations.
Limitations
Some limitations to this project were the use of telephone encounters and interpharmacist variability. Patients who were contacted via telephone by a pharmacist who was unknown to them were more hesitant to make changes. Patients managed for DM by non-VA providers or patients receiving medications at a non-VA pharmacy were also reluctant to implement changes. Education was the major intervention for these patients. Pharmacists were instructed to use their clinical judgment in addition to shared decision making with the patient when relaxing therapy. There was no protocol for medication changes. Although interpharmacist variability is identified as a weakness, it could be considered more representative of daily practice.
Additionally, despite a statistically significant increase in HbA1c, which would presumably lead to fewer episodes of hypoglycemia, patients were not contacted again after the intervention to inquire whether hypoglycemia had decreased. Studies targeted at the impact of less frequent hypoglycemia events, including fewer emergency department visits, hospital admissions, or primary care walk-in appointments, would improve the clinical significance of these data. As the HSI is implemented nationally within the VA, more data will be available to better evaluate the applicability of this clinical reminder. Locally, the criteria for the clinical reminder has proved to capture a significant number of patients experiencing hypoglycemia. Using national data will also help to guide the frequency of screening needed in this population.
Conclusion
The implementation of the HSI led to increased provider and patient awareness of hypoglycemia. The CPS interventions have resulted in statistically significant increases in HbA1c levels, which would seemingly decrease the patient’s risk of adverse outcomes as shown in the ACCORD and VADT trials.
1. UK Prospective Diabetes Study (UKPDS) Group. Effect of intensive blood-glucose control with metformin on complications in overweight patients with type 2 diabetes (UKPDS 34). UK Prospective Diabetes Study (UKPDS) Group. Lancet. 1998;352(9131):854-865.
2. Kirkman MS, Mahmud H, Korytkowski MT. Intensive blood glucose control and vascular outcomes in patients with type 2 diabetes mellitus. Endocrinol Metab Clin North Am. 2018;47(1):81-96.
3. Action to Control Cardiovascular Risk in Diabetes Study Group, Gerstein HC, Miller ME, et al. Effects of intensive glucose lowering in type 2 diabetes. N Engl J Med. 2008;358(24):2545-2559.
4. Duckworth W, Abraira C, Moritz T, et al; VADT Investigators. Glucose control and vascular complications in veterans with type 2 diabetes. N Engl J Med. 2009;360(2):129-139.
5. Feil DG, Rajan M, Soroka O, Tseng CL, Miller DR, Pogach LM. Risk of hypoglycemia in older veterans with dementia and cognitive impairment: implications for practice and policy. J Am Geriatr Soc. 2011;59(12):2263-2272.
6. Miller ME, Bonds DE, Gerstein HC, et al; ACCORD Investigators. The effects of baseline characteristics, glycaemia treatment approach, and glycated haemoglobin concentration on the risk of severe hypoglycaemia: post hoc epidemiological analysis of the ACCORD study. BMJ. 2010;340:b5444.
7. US Department of Veterans Affairs, Department of Defense. VA/DoD Clinical Practice Guideline for the Management of Type 2 Diabetes Mellitus in Primary Care. Version 5.0. https://www.healthquality.va.gov/guidelines/CD/diabetes/VADoDD MCPGFinal508.pdf. Published 2017. Accessed September 28, 2018.
8. US Department of Veterans Affairs. VA implements national hypoglycemic safety initiative. https://www.qualityandsafety.va.gov/docs/HSI-Clinician-PressRelease2014.pdf. Published December 10, 2014. Accessed September 28, 2018.
9. US Department of Health and Human Services, Office of Disease Prevention and Health Promotion. National Action Plan for Adverse Drug Event Prevention. https://health.gov/hcq/pdfs/ADE-Action-Plan-508c.pdf. Published 2014. Accessed September 28, 2018.
Clinical pharmacy specialist interventions after patient consultation resulted in statistically significant increases in HbA1c levels in patients at risk for hypoglycemia who relaxed their therapy.
Clinical pharmacy specialist interventions after patient consultation resulted in statistically significant increases in HbA1c levels in patients at risk for hypoglycemia who relaxed their therapy.
Intensive glycemic lowering for the treatment for type 2 diabetes mellitus (T2DM) has been shown to decrease microvascular and macrovascular outcomes in the UK Prospective Diabetes Study (UKPDS) without any risk of increased harm.1,2 Over the past decade, evidence has shown that the outcomes and risk do not hold true in an older population with additional comorbidities and longer duration of DM. Both the Action to Control Cardiovascular Risk in Diabetes (ACCORD) and Veterans Affairs Diabetes Trial (VADT) trials showed no decreased incidence of macrovascular or microvascular complications of DM with intensive glucose lowering but an additional risk of hypoglycemia and even death.2-4
Patient-specific risk factors, such as age, impaired renal function, and cognitive impairment, have been shown to lead to an increased risk of hypoglycemia independent of hemoglobin A1c (HbA1c). Dementia and cognitive impairment are associated with a 2.42 and 1.72 times greater risk of hypoglycemia, respectively, compared with a patient without dementia or cognitive impairment.5 A post-hoc analysis of the ACCORD trial that analyzed the risk of hypoglycemia in subgroup populations showed an increased risk of hypoglycemia in those with a serum creatinine (SCr) level > 1.3 mg/dL (hazard ratio, 1.66, P < .01) and increasing age. Risk of hypoglycemia was highest in those aged ≥ 75 years but increased by 3% for every subsequent year (P < .01).6 These risk factors should be addressed and considered in individual patients with DM to safely guide therapy.
The evidence from these landmark trials has led to increased HbA1c goals for specific patient populations in the most recent 2017 VA/DoD Clinical Practice Guideline (CPG) for the Management of Type 2 Diabetes Mellitus in Primary Care.7 The majority of patients with T2DM now qualify for HBA1c goals > 7.0%. According to the 2017 VA/DoD CPG, younger patients with the absence of a major comorbidity and life expectancy of > 10 to 15 years with mild or absent microvascular complications is the only group of patients who should be treated to an A1c goal of 6.0 to 7.0%.7 The use of shared decision making and patient education to set glycemic goals based on “patient capabilities, needs, goals, prior treatment experience, and preferences” also should be used to increase patient education and satisfaction.7
In December 2014, the VA introduced the Hypoglycemia Safety Initiative (HSI). The goal of the HSI is to “enable veterans living with diabetes to work more closely with their VA clinicians to personalize health care goals and improve self-management of the disease.”8 This goal also aligns with the US Department of Health and Human Services National Action Plan for Adverse Drug Event Prevention. One of 3 initial targets of this plan includes DM agents and the prevention of hypoglycemia.9
To combat the risk of hypoglycemia and potentially negative outcomes, as part of the HSI, the VA is implementing a clinical reminder within the Computerized Patient Record System (CPRS) that will prompt the primary care team to screen select patients at risk for hypoglycemia. The purpose of this project was to identify patients at high risk of hypoglycemia, individualize HbA1c goals, and consider de-escalation in therapy, using shared decision making.
Methods
This quality improvement project, conducted at the Fayetteville VA Medical Center (FVAMC), consisted of outpatient services provided at 2 health care centers and 6 community-based outpatient clinics. The project was exempt from institutional review board approval as the protocol met national VA criteria as a quality assurance project.
Patients were identified using the HSI Corporate Data Warehouse (CDR) reports. Once patients were identified, a list was distributed to the appropriate clinical pharmacy specialist (CPS), according to patient aligned care teams (PACTs). The CPS contacted the patient via telephone or in person to conduct hypoglycemia screening. Patients on a sulfonylurea or insulin and an HbA1c < 7% plus 1 risk factor for hypoglycemia (aged ≥ 75 years, serum creatinine[SCr] ≥1.7 mg/dL, diagnosis of cognitive impairment, or prescribed a cholinesterase inhibitor) were included. These risk factors were chosen to align with the future clinical reminder, which is based on an increased risk of hypoglycemia seen in these patient populations.
Patients were included if they were receiving antidiabetic medications through the FVAMC or outside of the VA and/or prescribed by a non-VA provider. Medications and doses prescribed by a non-VA provider were verified with the patient verbally during the initial interview. Once contacted by the CPS, any patients who no longer met inclusion criteria were excluded.
The CPS used a national VA hypoglycemia screening note template to ask the patient about frequency and severity of hypoglycemia. Hypoglycemia was defined as a self-monitored blood glucose < 70 mg/dL with or without symptoms. An additional definition consisted of typical hypoglycemia symptoms as reported by the patient even if self-monitored blood glucose was not obtained while exhibiting symptoms. Using shared decision making between the CPS and veteran, antidiabetic therapy was either relaxed or continued. Relaxing therapy was defined as decreasing doses or discontinuation of antidiabetic medications that are known for potentiating hypoglycemia (ie, sulfonylurea and insulin).
The CPS had autonomy in deciding how much to lower dose(s) or when to discontinue medication(s). Additional counseling in proper medication administration, including appropriate timing of medication administration, also could have been the sole intervention needed for a given patient who experienced hypoglycemia. Counseling would have been considered continuation of therapy. For example, if a patient was experiencing hypoglycemia while taking a sulfonylurea twice daily, the CPS would provide counseling on proper timing of medication administration 20 to 30 minutes before morning and evening meals rather than the patient’s perceived administration of twice daily without regard to meals. Even in patients who met inclusion criteria but who did not experience any hypoglycemia symptoms, the CPS and patient could use shared decision making with emphasis on appropriate HbA1c goals to determine whether relaxation in therapy was appropriate.
Data Collection
Baseline demographics, including prespecified risk factors for hypoglycemia, were collected. Data were imported into the HSI CDW from the national VA hypoglycemia screening note template completed by the CPS. From the data CDW, frequency and severity of hypoglycemia were recorded. The CPS interventions were also quantified; HbA1c data were obtained in patients in whom therapy was relaxed 3- to 6-months postintervention.
Statistical Analysis
Descriptive statistics (mean, range) were used for analyzing results. A t test with a 1-tailed distribution was used to analyze the change in HbA1c after CPS intervention (α = .05).
Results
On August 17, 2017, 839 patients were identified across all FVAMC facilities from the HSI data CDW. Patients were contacted through February 16, 2018. A total of 52 patients were excluded as they no longer met inclusion criteria or were deceased at time of review.
The most commonly prescribed antidiabetic prescription was a sulfonylurea (482 prescriptions) followed by basal insulin (319 prescriptions; Table 2).
The CPS used shared decision making to relax antidiabetic therapy in 102 (16.5%) of the total number of patients contacted (Figure 2). Lab orders were entered for the patient to obtain an HbA1c in 3 to 6 months in those in whom therapy was relaxed.
Discussion
The primary objective of this project was to identify patients at risk for hypoglycemia. Approximately 1 in 4 patients reported any incidence of hypoglycemia, which shows that the prespecified inclusion criteria was an appropriate guide for hypoglycemia screening. The episodes of hypoglycemia were typically infrequent, occurring only once every few months. This could have contributed to a lower rate of therapy changes compared with the rate of hypoglycemia. Overall, hypoglycemia was not severe; 83% of patients did not report any symptoms of faintness. Pharmacists were able to intervene and relax therapy in 102 patients to try to prevent episodes of hypoglycemia and negative outcomes. These interventions led to a statistically significant increase in average HbA1c in these patients. Throughout these encounters with the CPS and patient, there were also innumerable outcomes secondary to the use of shared decision making. Regardless of medication changes, there was increased patient education concerning hypoglycemia treatment, medication administration times, and HbA1c goals.
This project’s strengths included the large sample size, appropriate inclusion criteria that identified patients at risk for hypoglycemia, and the use of shared decision making. It was also beneficial to obtain HbA1c levels after a relaxation in therapy for objective outcomes. The increase in HbA1c levels showed a statistically significant gain, which led to more patients having an HbA1c closer to a CPG-recommended goal range, given their risk factors for hypoglycemia. This pharmacy initiative fostered increased communication between providers and CPS within the PACT team. The pharmacist was not consulted by the provider for management of these patients with DM, so therapy relaxation was documented in CPRS and was addressed at the patient’s next primary care appointment. Some changes also required discussion with the primary care provider prior to relaxation in therapy. By initiating these discussions with providers, opportunities arose for additional education on appropriate HbA1c goals and why therapy should be relaxed in select patient populations.
Limitations
Some limitations to this project were the use of telephone encounters and interpharmacist variability. Patients who were contacted via telephone by a pharmacist who was unknown to them were more hesitant to make changes. Patients managed for DM by non-VA providers or patients receiving medications at a non-VA pharmacy were also reluctant to implement changes. Education was the major intervention for these patients. Pharmacists were instructed to use their clinical judgment in addition to shared decision making with the patient when relaxing therapy. There was no protocol for medication changes. Although interpharmacist variability is identified as a weakness, it could be considered more representative of daily practice.
Additionally, despite a statistically significant increase in HbA1c, which would presumably lead to fewer episodes of hypoglycemia, patients were not contacted again after the intervention to inquire whether hypoglycemia had decreased. Studies targeted at the impact of less frequent hypoglycemia events, including fewer emergency department visits, hospital admissions, or primary care walk-in appointments, would improve the clinical significance of these data. As the HSI is implemented nationally within the VA, more data will be available to better evaluate the applicability of this clinical reminder. Locally, the criteria for the clinical reminder has proved to capture a significant number of patients experiencing hypoglycemia. Using national data will also help to guide the frequency of screening needed in this population.
Conclusion
The implementation of the HSI led to increased provider and patient awareness of hypoglycemia. The CPS interventions have resulted in statistically significant increases in HbA1c levels, which would seemingly decrease the patient’s risk of adverse outcomes as shown in the ACCORD and VADT trials.
Intensive glycemic lowering for the treatment for type 2 diabetes mellitus (T2DM) has been shown to decrease microvascular and macrovascular outcomes in the UK Prospective Diabetes Study (UKPDS) without any risk of increased harm.1,2 Over the past decade, evidence has shown that the outcomes and risk do not hold true in an older population with additional comorbidities and longer duration of DM. Both the Action to Control Cardiovascular Risk in Diabetes (ACCORD) and Veterans Affairs Diabetes Trial (VADT) trials showed no decreased incidence of macrovascular or microvascular complications of DM with intensive glucose lowering but an additional risk of hypoglycemia and even death.2-4
Patient-specific risk factors, such as age, impaired renal function, and cognitive impairment, have been shown to lead to an increased risk of hypoglycemia independent of hemoglobin A1c (HbA1c). Dementia and cognitive impairment are associated with a 2.42 and 1.72 times greater risk of hypoglycemia, respectively, compared with a patient without dementia or cognitive impairment.5 A post-hoc analysis of the ACCORD trial that analyzed the risk of hypoglycemia in subgroup populations showed an increased risk of hypoglycemia in those with a serum creatinine (SCr) level > 1.3 mg/dL (hazard ratio, 1.66, P < .01) and increasing age. Risk of hypoglycemia was highest in those aged ≥ 75 years but increased by 3% for every subsequent year (P < .01).6 These risk factors should be addressed and considered in individual patients with DM to safely guide therapy.
The evidence from these landmark trials has led to increased HbA1c goals for specific patient populations in the most recent 2017 VA/DoD Clinical Practice Guideline (CPG) for the Management of Type 2 Diabetes Mellitus in Primary Care.7 The majority of patients with T2DM now qualify for HBA1c goals > 7.0%. According to the 2017 VA/DoD CPG, younger patients with the absence of a major comorbidity and life expectancy of > 10 to 15 years with mild or absent microvascular complications is the only group of patients who should be treated to an A1c goal of 6.0 to 7.0%.7 The use of shared decision making and patient education to set glycemic goals based on “patient capabilities, needs, goals, prior treatment experience, and preferences” also should be used to increase patient education and satisfaction.7
In December 2014, the VA introduced the Hypoglycemia Safety Initiative (HSI). The goal of the HSI is to “enable veterans living with diabetes to work more closely with their VA clinicians to personalize health care goals and improve self-management of the disease.”8 This goal also aligns with the US Department of Health and Human Services National Action Plan for Adverse Drug Event Prevention. One of 3 initial targets of this plan includes DM agents and the prevention of hypoglycemia.9
To combat the risk of hypoglycemia and potentially negative outcomes, as part of the HSI, the VA is implementing a clinical reminder within the Computerized Patient Record System (CPRS) that will prompt the primary care team to screen select patients at risk for hypoglycemia. The purpose of this project was to identify patients at high risk of hypoglycemia, individualize HbA1c goals, and consider de-escalation in therapy, using shared decision making.
Methods
This quality improvement project, conducted at the Fayetteville VA Medical Center (FVAMC), consisted of outpatient services provided at 2 health care centers and 6 community-based outpatient clinics. The project was exempt from institutional review board approval as the protocol met national VA criteria as a quality assurance project.
Patients were identified using the HSI Corporate Data Warehouse (CDR) reports. Once patients were identified, a list was distributed to the appropriate clinical pharmacy specialist (CPS), according to patient aligned care teams (PACTs). The CPS contacted the patient via telephone or in person to conduct hypoglycemia screening. Patients on a sulfonylurea or insulin and an HbA1c < 7% plus 1 risk factor for hypoglycemia (aged ≥ 75 years, serum creatinine[SCr] ≥1.7 mg/dL, diagnosis of cognitive impairment, or prescribed a cholinesterase inhibitor) were included. These risk factors were chosen to align with the future clinical reminder, which is based on an increased risk of hypoglycemia seen in these patient populations.
Patients were included if they were receiving antidiabetic medications through the FVAMC or outside of the VA and/or prescribed by a non-VA provider. Medications and doses prescribed by a non-VA provider were verified with the patient verbally during the initial interview. Once contacted by the CPS, any patients who no longer met inclusion criteria were excluded.
The CPS used a national VA hypoglycemia screening note template to ask the patient about frequency and severity of hypoglycemia. Hypoglycemia was defined as a self-monitored blood glucose < 70 mg/dL with or without symptoms. An additional definition consisted of typical hypoglycemia symptoms as reported by the patient even if self-monitored blood glucose was not obtained while exhibiting symptoms. Using shared decision making between the CPS and veteran, antidiabetic therapy was either relaxed or continued. Relaxing therapy was defined as decreasing doses or discontinuation of antidiabetic medications that are known for potentiating hypoglycemia (ie, sulfonylurea and insulin).
The CPS had autonomy in deciding how much to lower dose(s) or when to discontinue medication(s). Additional counseling in proper medication administration, including appropriate timing of medication administration, also could have been the sole intervention needed for a given patient who experienced hypoglycemia. Counseling would have been considered continuation of therapy. For example, if a patient was experiencing hypoglycemia while taking a sulfonylurea twice daily, the CPS would provide counseling on proper timing of medication administration 20 to 30 minutes before morning and evening meals rather than the patient’s perceived administration of twice daily without regard to meals. Even in patients who met inclusion criteria but who did not experience any hypoglycemia symptoms, the CPS and patient could use shared decision making with emphasis on appropriate HbA1c goals to determine whether relaxation in therapy was appropriate.
Data Collection
Baseline demographics, including prespecified risk factors for hypoglycemia, were collected. Data were imported into the HSI CDW from the national VA hypoglycemia screening note template completed by the CPS. From the data CDW, frequency and severity of hypoglycemia were recorded. The CPS interventions were also quantified; HbA1c data were obtained in patients in whom therapy was relaxed 3- to 6-months postintervention.
Statistical Analysis
Descriptive statistics (mean, range) were used for analyzing results. A t test with a 1-tailed distribution was used to analyze the change in HbA1c after CPS intervention (α = .05).
Results
On August 17, 2017, 839 patients were identified across all FVAMC facilities from the HSI data CDW. Patients were contacted through February 16, 2018. A total of 52 patients were excluded as they no longer met inclusion criteria or were deceased at time of review.
The most commonly prescribed antidiabetic prescription was a sulfonylurea (482 prescriptions) followed by basal insulin (319 prescriptions; Table 2).
The CPS used shared decision making to relax antidiabetic therapy in 102 (16.5%) of the total number of patients contacted (Figure 2). Lab orders were entered for the patient to obtain an HbA1c in 3 to 6 months in those in whom therapy was relaxed.
Discussion
The primary objective of this project was to identify patients at risk for hypoglycemia. Approximately 1 in 4 patients reported any incidence of hypoglycemia, which shows that the prespecified inclusion criteria was an appropriate guide for hypoglycemia screening. The episodes of hypoglycemia were typically infrequent, occurring only once every few months. This could have contributed to a lower rate of therapy changes compared with the rate of hypoglycemia. Overall, hypoglycemia was not severe; 83% of patients did not report any symptoms of faintness. Pharmacists were able to intervene and relax therapy in 102 patients to try to prevent episodes of hypoglycemia and negative outcomes. These interventions led to a statistically significant increase in average HbA1c in these patients. Throughout these encounters with the CPS and patient, there were also innumerable outcomes secondary to the use of shared decision making. Regardless of medication changes, there was increased patient education concerning hypoglycemia treatment, medication administration times, and HbA1c goals.
This project’s strengths included the large sample size, appropriate inclusion criteria that identified patients at risk for hypoglycemia, and the use of shared decision making. It was also beneficial to obtain HbA1c levels after a relaxation in therapy for objective outcomes. The increase in HbA1c levels showed a statistically significant gain, which led to more patients having an HbA1c closer to a CPG-recommended goal range, given their risk factors for hypoglycemia. This pharmacy initiative fostered increased communication between providers and CPS within the PACT team. The pharmacist was not consulted by the provider for management of these patients with DM, so therapy relaxation was documented in CPRS and was addressed at the patient’s next primary care appointment. Some changes also required discussion with the primary care provider prior to relaxation in therapy. By initiating these discussions with providers, opportunities arose for additional education on appropriate HbA1c goals and why therapy should be relaxed in select patient populations.
Limitations
Some limitations to this project were the use of telephone encounters and interpharmacist variability. Patients who were contacted via telephone by a pharmacist who was unknown to them were more hesitant to make changes. Patients managed for DM by non-VA providers or patients receiving medications at a non-VA pharmacy were also reluctant to implement changes. Education was the major intervention for these patients. Pharmacists were instructed to use their clinical judgment in addition to shared decision making with the patient when relaxing therapy. There was no protocol for medication changes. Although interpharmacist variability is identified as a weakness, it could be considered more representative of daily practice.
Additionally, despite a statistically significant increase in HbA1c, which would presumably lead to fewer episodes of hypoglycemia, patients were not contacted again after the intervention to inquire whether hypoglycemia had decreased. Studies targeted at the impact of less frequent hypoglycemia events, including fewer emergency department visits, hospital admissions, or primary care walk-in appointments, would improve the clinical significance of these data. As the HSI is implemented nationally within the VA, more data will be available to better evaluate the applicability of this clinical reminder. Locally, the criteria for the clinical reminder has proved to capture a significant number of patients experiencing hypoglycemia. Using national data will also help to guide the frequency of screening needed in this population.
Conclusion
The implementation of the HSI led to increased provider and patient awareness of hypoglycemia. The CPS interventions have resulted in statistically significant increases in HbA1c levels, which would seemingly decrease the patient’s risk of adverse outcomes as shown in the ACCORD and VADT trials.
1. UK Prospective Diabetes Study (UKPDS) Group. Effect of intensive blood-glucose control with metformin on complications in overweight patients with type 2 diabetes (UKPDS 34). UK Prospective Diabetes Study (UKPDS) Group. Lancet. 1998;352(9131):854-865.
2. Kirkman MS, Mahmud H, Korytkowski MT. Intensive blood glucose control and vascular outcomes in patients with type 2 diabetes mellitus. Endocrinol Metab Clin North Am. 2018;47(1):81-96.
3. Action to Control Cardiovascular Risk in Diabetes Study Group, Gerstein HC, Miller ME, et al. Effects of intensive glucose lowering in type 2 diabetes. N Engl J Med. 2008;358(24):2545-2559.
4. Duckworth W, Abraira C, Moritz T, et al; VADT Investigators. Glucose control and vascular complications in veterans with type 2 diabetes. N Engl J Med. 2009;360(2):129-139.
5. Feil DG, Rajan M, Soroka O, Tseng CL, Miller DR, Pogach LM. Risk of hypoglycemia in older veterans with dementia and cognitive impairment: implications for practice and policy. J Am Geriatr Soc. 2011;59(12):2263-2272.
6. Miller ME, Bonds DE, Gerstein HC, et al; ACCORD Investigators. The effects of baseline characteristics, glycaemia treatment approach, and glycated haemoglobin concentration on the risk of severe hypoglycaemia: post hoc epidemiological analysis of the ACCORD study. BMJ. 2010;340:b5444.
7. US Department of Veterans Affairs, Department of Defense. VA/DoD Clinical Practice Guideline for the Management of Type 2 Diabetes Mellitus in Primary Care. Version 5.0. https://www.healthquality.va.gov/guidelines/CD/diabetes/VADoDD MCPGFinal508.pdf. Published 2017. Accessed September 28, 2018.
8. US Department of Veterans Affairs. VA implements national hypoglycemic safety initiative. https://www.qualityandsafety.va.gov/docs/HSI-Clinician-PressRelease2014.pdf. Published December 10, 2014. Accessed September 28, 2018.
9. US Department of Health and Human Services, Office of Disease Prevention and Health Promotion. National Action Plan for Adverse Drug Event Prevention. https://health.gov/hcq/pdfs/ADE-Action-Plan-508c.pdf. Published 2014. Accessed September 28, 2018.
1. UK Prospective Diabetes Study (UKPDS) Group. Effect of intensive blood-glucose control with metformin on complications in overweight patients with type 2 diabetes (UKPDS 34). UK Prospective Diabetes Study (UKPDS) Group. Lancet. 1998;352(9131):854-865.
2. Kirkman MS, Mahmud H, Korytkowski MT. Intensive blood glucose control and vascular outcomes in patients with type 2 diabetes mellitus. Endocrinol Metab Clin North Am. 2018;47(1):81-96.
3. Action to Control Cardiovascular Risk in Diabetes Study Group, Gerstein HC, Miller ME, et al. Effects of intensive glucose lowering in type 2 diabetes. N Engl J Med. 2008;358(24):2545-2559.
4. Duckworth W, Abraira C, Moritz T, et al; VADT Investigators. Glucose control and vascular complications in veterans with type 2 diabetes. N Engl J Med. 2009;360(2):129-139.
5. Feil DG, Rajan M, Soroka O, Tseng CL, Miller DR, Pogach LM. Risk of hypoglycemia in older veterans with dementia and cognitive impairment: implications for practice and policy. J Am Geriatr Soc. 2011;59(12):2263-2272.
6. Miller ME, Bonds DE, Gerstein HC, et al; ACCORD Investigators. The effects of baseline characteristics, glycaemia treatment approach, and glycated haemoglobin concentration on the risk of severe hypoglycaemia: post hoc epidemiological analysis of the ACCORD study. BMJ. 2010;340:b5444.
7. US Department of Veterans Affairs, Department of Defense. VA/DoD Clinical Practice Guideline for the Management of Type 2 Diabetes Mellitus in Primary Care. Version 5.0. https://www.healthquality.va.gov/guidelines/CD/diabetes/VADoDD MCPGFinal508.pdf. Published 2017. Accessed September 28, 2018.
8. US Department of Veterans Affairs. VA implements national hypoglycemic safety initiative. https://www.qualityandsafety.va.gov/docs/HSI-Clinician-PressRelease2014.pdf. Published December 10, 2014. Accessed September 28, 2018.
9. US Department of Health and Human Services, Office of Disease Prevention and Health Promotion. National Action Plan for Adverse Drug Event Prevention. https://health.gov/hcq/pdfs/ADE-Action-Plan-508c.pdf. Published 2014. Accessed September 28, 2018.