Angiotensin-converting enzyme (ACE) inhibitors are commonly used medications in the treatment of hypertension in the ambulatory care setting. Serum sodium concentrations are not usually affected in the majority of patients treated with ACE inhibitors. Nonetheless, hyponatremia, defined as serum sodium level < 135 mEq/L, has been reported in patients taking ACE inhibitors.1,2 The authors report a case of hyponatremia attributed to the use of lisinopril.
Case Presentation
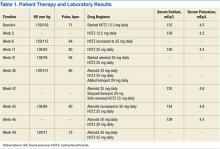
In 2012, a 49-year-old man with a past medical history significant for polysubstance abuse, alcohol use, and hypertension was referred to the pharmacy clinic by his primary care physician (PCP) for management of hypertension. At the PCP visit, the patient’s blood pressure (BP) was above the goal of < 140/90 mm Hg and hydrochlorothiazide (HCTZ) 12.5 mg by mouth daily monotherapy was initiated. At the follow-up pharmacy appointment 6 weeks later, his BP remained uncontrolled, and HCTZ was increased to 25 mg daily. Of note, at that time the patient reported drinking about 6 beers per week. His electrolytes—serum sodium, potassium, chloride, carbon dioxide (CO2), blood urea nitrogen (BUN), and serum creatinine (SCr)—were all within normal limits and stable to previous baseline results after taking HCTZ for about 2 weeks.
The patient returned to the pharmacy clinic at week 11, and his BP was controlled (136/83 mm Hg) on HCTZ 25 mg daily. His electrolytes, BUN, and SCr continued to be stable. The patient requested another appointment 8 weeks later to continue to monitor his BP.
The patient did not return to the pharmacy clinic until week 31, when he reported that he was told his BP was very high when attempting to donate plasma. The patient reported drinking a “6 pack of beer per day” at that visit. Two BP readings were taken in the clinic. The first of systolic blood pressure (SBP) was 144 mm Hg and the second was below the patient’s goal (< 140 mm Hg). His pulse (94 bpm) was also noted to be higher than baseline range (66-84 bpm). Atenolol 25 mg daily by mouth was added to the patient’s regimen of HCTZ 25 mg daily.
The patient returned at week 38, and his BP of 149/101 mm Hg was elevated above goal range. Lisinopril was added to HCTZ 25 mg in a combination formulation of lisinopril/HCTZ 20 mg/25 mg by mouth daily. The patient reported drinking 4 beers on days he worked (5 days per week) and 6 beers on days he was off (2 days per week). A repeated electrolyte, BUN, and SCr panel 1 month later (week 42) revealed a drop in the patient’s sodium level from 136 mEq/L (baseline) to 130 mEq/L (Table 1). Other measured electrolytes remained within normal limits with the exception of a slight decrease in serum chloride to 93 mEq/L (Table 2). No symptoms of hyponatremia were noted.
The patient was instructed to cut the lisinopril/HCTZ tablet in half and take it daily, then repeat blood work in 5 days. The patient’s repeated laboratory work noted an increase in sodium level to 134 mEq/L. All other measured electrolytes, including serum chloride, were within normal limits. During his follow-up visit, the patient reported stopping lisinopril/HCTZ altogether and resuming HCTZ 25 mg daily for the 5 days prior, in lieu of taking the reduced lisinopril/HCTZ dose as instructed. The patient continued to report drinking 6 beers daily.
Medication was changed to HCTZ 25 mg daily, with lisinopril discontinued, and atenolol increased to 50 mg daily. At week 46, the patient repeated electrolytes, BUN, and SCr laboratory work while on HCTZ 25 mg and atenolol 50 mg daily, and the serum sodium level increased to 139 mEq/L. After the laboratory work at week 49, he noted a reduction in alcohol to 4 beers daily at the pharmacy appointment. The patient’s BP was controlled to below the < 140/90 mm Hg goal. Medications were not changed. He was instructed to follow up with his PCP and return to the pharmacy clinic as needed for BP control.
Of note, serum magnesium levels are not included in the standard electrolyte panel and must be ordered separately. Additionally, serum magnesium levels are not monitored routinely with thiazide and ACE inhibitor therapy. In this case, serum magnesium levels were not drawn at baseline or in subsequent laboratory monitoring.
Discussion
This case demonstrates a potential link between administration of lisinopril and the development of hyponatremia. Adverse effects (AEs) of ACE inhibitors frequently include elevation in SCr, hyperkalemia, and/or a dry cough. Hyponatremia, although not commonly associated with ACE inhibitors, has been reported in the literature.1,2