Cardiovascular diseases are some of the most common conditions found in the geriatric population. Ocular manifestations of systemic cardiovascular conditions often are the initial presentation of the systemic disease. Identifying these findings help reveal the underlying disease and prevent more serious visual and systemic complications or even death.
Hypoperfusion retinopathy can occur as an early manifestation of carotid occlusive disease. It results from poor arterial perfusion pressure secondary to significant or complete carotid artery blockage resulting in retinal changes. Atherosclerotic disease is generally the main culprit. Early manifestations can be seen as midperipheral retinal hemorrhages, dilated often nontortuous veins, and retinal neovascularization. Untreated or advanced cases of carotid occlusive disease can lead to a more serious ocular ischemic syndrome, which encompasses a panocular ischemia and can result in severe vision loss and neovascular glaucoma. Restoration of arterial perfusion pressure is the main goal for managing this condition.
Case Report
A 71-year-old white male was referred by his primary care physician (PCP) to the eye clinic for a routine comprehensive eye exam. The patient reported that his current progressive lenses, prescribed 2 years prior, were not strong enough at both distance and near, and that his eyes often felt dry. The symptoms were gradual in onset since his prior exam with no reported flashes, floaters, loss of vision, headaches, or ocular irritations.
The patient’s medical history was significant for morbid obesity, hypertension, borderline diabetes mellitus, and obstructive sleep apnea. His ocular history included recurrent conjunctivitis. At the time of the visit, the patient’s medications included 81 mg aspirin, 10 mg benazepril, 1,000 mg fish oil, 80 mg simvastatin, and use of a continuous positive airway pressure machine.
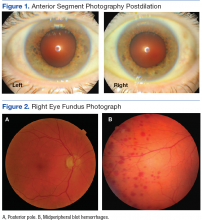
Best-corrected Snellen visual acuity was stable to his last eye exam at 20/25+2 right eye and 20/25-1 left eye with a manifest refraction of +2.25-0.75 × 077, and +2.75-1.25 × 096 in the right and left eye, respectively. Pupils were equally round and reactive to light with no afferent pupillary defect. Extraocular motility and finger counting fields were unremarkable. Anterior segment evaluation revealed lax bilateral upper lid apposition and mild cataracts in both eyes but were otherwise unremarkable (Figure 1). Dilated fundus examination revealed extensive hemorrhaging in the midperipheral retina of the right eye only (Figure 2). The left eye retina showed no abnormalities.
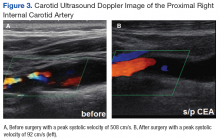
At this point the patient declined any additional symptoms, including eye pain, headache, transient vision loss, jaw claudication, and stroke signs. A complete blood count and hemoglobin A1c (HbA1c) was ordered, and all findings were unremarkable with no evidence of blood dyscrasia and with a HbA1c of 6.0. A carotid ultrasound (CUS) was also performed and revealed severe narrowing of the proximal section of the right internal carotid artery (ICA) with a trickle flow (Figure 3). The peak systolic velocity (PSV) at this level was 508 cm/s. There also was severe narrowing and turbulent flow in both the mid and distal portions of the right ICA. The patient was sent for a vascular evaluation 2 days following the CUS.
Based on the ocular findings and CUS results, the diagnosis of hypoperfusion retinopathy secondary to carotid occlusive disease was made. Because the patient was asymptomatic with no additional ocular sequelae, he was scheduled for an eye clinic follow-up in 2 months. The electrocardiogram, chest X-ray, and exercise stress test results were negative for acute cardiopulmonary disease, ischemia, or arrhythmias. A computed tomography angiography was performed and confirmed a high-grade lesion of the right ICA of > 95%. The vascular surgeon reported an 11% risk of stroke within 5 years and a 1% risk of stroke with surgery. Based on these results the patient underwent a right carotid endarterectomy (CEA) 2 weeks later. A follow-up CUS was performed 1 month post-CEA and revealed no abnormal fluid or significant plaque with a PSV of 92 cm/s (prior to surgery PSV was 508 cm/s) (Figure 3).
The patient returned to the eye clinic 1 month after the CEA. Gonioscopy revealed no neovascularization of the iris or angle and the dilated eye exam showed resolution of the midperipheral blot hemorrhages in his right eye with no evidence of retinal neovascularization.
Discussion
Hypoperfusion retinopathy is characterized by posterior retinal changes secondary to chronic ocular ischemia from decreased arterial perfusion related to significant or complete carotid artery stenosis.1-5 Early literature referred to this condition as venous stasis retinopathy; however, this term is misleading as the condition results from a reduction in arterial perfusion pressure and the term describes venous outflow obstruction.6 The terms carotid ischemic retinopathy, ischemic oculopathy, and hypotensive retinopathy also have been used interchangeably when describing hypoperfusion retinopathy.6