User login
With over 50,000 completed hysteroscopic sterilization procedures worldwide, and 5 years of data, what do we know so far about this innovation? It is now almost 4 years since the FDA approved Essure (Conceptus; San Carlos, Calif), the first hysteroscopic sterilization method available for use in the United States. Two other systems are in the works: Adiana (Adiana; Redwood City, Calif) has completed its Phase III clinical trial and Ovion (American Medical Systems; Minnetonka, Minn) is just beginning its clinical trial this year.
Comparison of the devices
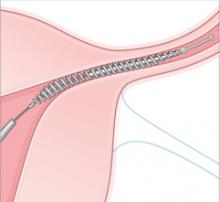
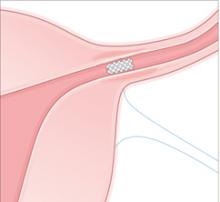
Essure is a disposable delivery system with polyethylene (PET) fibers wound in and around a stainless steel inner coil. An outer coil of nitinol, a superelastic titanium/nickel alloy, is deployed to anchor the device across the uterotubal junction. Wound down, the micro-insert is 0.8 mm in diameter. Once released, the coil expands to 1.5 to 2.0 mm to hold the inner coil and PET fibers in place at the uterine cornua.
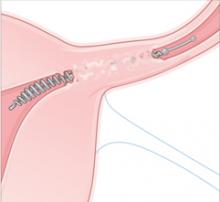
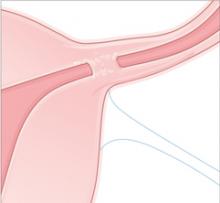
Over a period of about 3 months, the PET fibers elicit tissue ingrowth and proximal tubal occlusion. Women must use additional contraception during this time. Documentation of occlusion by a hysterosalpingogram about 3 months after device placement is required before patients may rely on the device and stop birth control.
Adiana (not yet available in the United States) uses a combination of controlled epithelial destruction and insertion of a porous biomatrix to induce vascularized tissue ingrowth. A catheter placed through the operating channel of a small hysteroscope delivers low-power bipolar electrosurgical energy to the tubal orifice (average less than 1 watt to the endosalpinx). A pushrod then delivers a small porous matrix of material into the tubal lumen. Ingrowth of healthy, vascularized tissue occurs over approximately 3 months, to occlude the tubes.
Retention of the matrix and tubal occlusion are documented by both transvaginal ultrasonography and hysterosalpingogram before patients may discontinue additional contraception.
Accessing the tubes
One of the greatest hurdles for occluding the fallopian tubes hysteroscopically is access to the tubes. Both the Adiana clinical trial (not yet published) and the post-market analysis of Essure (not yet published) have demonstrated excellent bilateral placement rates.
Technique is not hard to learn. Both types of devices are inserted through the operating channel of a small hysteroscope. Initial concerns about the ability to access the tubal ostia do not appear to be an issue—at least for those early-adopter physicians performing the procedures. Both clinical trials included gynecologists who were not experienced in operative hysteroscopy. These studies found that cannulation of the tubes is a technique that is easy to learn and rapidly accomplished in most circumstances.
Bilateral placement rates were similar for both devices in the pivotal trials: Adiana 95% (612/655); Essure 90% (464/518).
What are the contraindications?
Approximately 10% of patients have factors that preclude bilateral device placement:
Anatomic factors
- Blocked or stenotic tubes
- Intrauterine adhesions
- Visual field obstructed by polyps, fibroids, or shaggy endometrium
- Lateral tubes
Device or procedure failures due to
- Tubal spasm
- Patient pain/intolerance
- Device malfunction
A second procedure (after correcting the initial problems) will be successful in many women who have what appears to be a technical glitch.
Clinical outcomes, so far
Ultimately, of course, the success of these procedures and the benefits to our patients will be determined by the placement rates in the real world and the ability of a majority of women to rely on the devices for permanent sterilization.
What can we say so far?
Bilateral placement rates
Although the bilateral placement rates for Essure in the pivotal trial were 88% with one attempt, increasing to 92% of all patients enrolled with a second attempt, the data from the postmarket study is even more promising. After FDA approval in November 2002, the manufacturer trained gynecologists in the procedure, and then monitored clinical outcomes in the initial cases performed by these surgeons once they had completed their training and mandatory proctored cases (data not yet published).
Physicians who participated in the clinical trial were excluded from this analysis. The bilateral device placement rate for these women treated by novice users is over 94%. Adiana’s Phase III trial data demonstrate a similar bilateral success rate. It appears that despite the misgivings of many ObGyns, these systems are easy to learn.
I have incorporated the following tips and tricks into my office practice with great results. Patients are thrilled with their experience and leave ready to recruit their friends for the procedure.
- Pretreatment with a nonsteroidal anti-inflammatory drug to block prostaglandin release and uterotubal spasm
- Scheduling the procedure for the early follicular phase of the menstrual cycle to minimize shaggy endometrium, or
- Suppressing the endometrium with progestins from the first day of menses until the scheduled procedure
- Use of warm fluid for uterine distension to reduce spasm
- Placement of topical lidocaine gel into the uterus 10 to 15 minutes prior to the procedure
- Use of a pressure bag to assure adequate uterine distension
Patient safety
Few complications have been reported with either technique. There were the expected rare vasovagal reactions, as well as 2 cases of hypervolemia with Essure and 1 case of hyponatremia in the Adiana trial. Both of these situations should be avoidable with proper monitoring and limiting distension fluids to isotonic solutions. All patients recovered fully. There were no problems with persistent pain or changes in menstrual patterns at 1 year in the Essure trial.
Expulsion of the devices was associated with proximal positioning of the devices in all cases (3%). Patients had no symptoms, and most were able to have a second procedure with excellent placement and retention. Expulsions were identified at the postprocedure scout film or hysterosalpingogram.
Tubal perforation was noted in 0.9% of the patients. Predisposing factors were preexisting tubal occlusion or hydrosalpinx.
Perforations were asymptomatic, as well. Laparoscopic evaluation in 3 cases demonstrated no adhesions or reactions to the tiny perforation sites.
Delivery. An outer coil of nitinol, a superelastic titanium/nickel alloy is deployed to anchor the device across the uterotubal junction. Once released, the coil expands to 1.5 to 2.0 mm to hold the inner coil and PET fibers in place at the uterine cornua.
Occlusion. Over about 3 months, the PET fibers elicit tissue ingrowth and proximal tubal occlusion. Women must use additional contraception during this time. Documentation of occlusion by a hysterosalpingogram is required before patients may discontinue additional contraception.
Images: Rich Larocco
Anesthesia. Because hysteroscopic sterilization procedures may be performed without general anesthesia and by design avoid the need to access the abdominal cavity, they should be inherently safer for patients than the other available surgical sterilization methods. In the Essure and Adiana trials,1 more than 50% of the patients underwent their procedures under local anesthesia with no additional intravenous sedation. The others had local anesthesia with IV sedation. Only 1 patient (in the Essure trial) underwent general anesthesia.
Patient satisfaction and tolerance of the procedure were excellent; 88% of patients described their experience as good to excellent. Only 4% rated their procedure pain as severe. At discharge (approximately 40 minutes after conclusion of the procedure), 79% of patients had no pain and required no pain medication.
What are the potential complications?
The rare but devastating complications associated with laparoscopic sterilization should be avoidable with the hysteroscopic approach—at least for the 90% of patients for whom access to both tubes is feasible.
Ideal candidates. Hysteroscopic access seems to be the ideal approach for occlusion of the fallopian tubes in patients with medical conditions that may increase the risk of abdominal access, or for whom general anesthesia imposes added risk.
Problem conditions. The following clinical conditions and comorbidities all present significant challenges for the laparoscopic surgeon:
- Cardiac disease
- Thrombophilias
- Immune suppression
- Renal transplant
- Morbid obesity
- Previous abdominal surgery, especially bowel procedures
Delivery. A catheter placed through the operating channel of a small hysteroscope delivers low-power bipolar electrosurgical energy to the tubal orifice. A pushrod then delivers a small porous matrix of material into the tubal lumen.
Occlusion. Ingrowth of healthy, vascularized tissue occurs over approximately 3 months, to occlude the tubes. Retention of the matrix and tubal occlusion are documented by both transvaginal ultrasonography and hysterosalpingogram before patients may discontinue additional contraception.
Images: Rich Larocco
How many pregnancies?
There have been no pregnancies in Phase II and III trials of Essure, thus far. In July 2003, Cooper et al1 reported no pregnancies in 7,532 woman-months of use.
Conceptus is aware of 64 pregnancies among more than 50,000 procedures performed worldwide. None appeared to have occurred with proper demonstration of bilateral tubal occlusion after device placement. Most appear to be luteal phase pregnancies present at the time of the sterilization procedure, or failure of either the patient or the physician to assure tubal obstruction prior to stopping additional birth control methods.
There are no documented ectopic pregnancies, although 1 of the 64 reported cases may have been a very early tubal gestation. The patient was treated with methotrexate without firm documentation of the location of the pregnancy.
There are 2 pregnancies among the 605 patients with bilateral Adiana devices (6,860 woman-months of use as of September 2005). One resulted from an error in interpreting the hysterosalpingogram. The other did occur with a properly placed device and occlusion demonstrated on hysterosalpingogram.
It appears that hysteroscopic sterilization with Essure will have acceptable and preventable failure rates (longer term data and postmarket analysis are not yet available for the Adiana device). The calculated 5-year success rate is more than 99% for Essure; this compares favorably with all other surgical sterilization methods.
Do benefits outweigh costs?
The overall cost of hysteroscopic sterilization methods compares favorably with laparoscopic approaches. The expense of the disposable equipment is recouped by avoiding the costs of general anesthesia, and operating room and facility charges.
Advantages of the office setting
Payment for physicians is slightly more than the reimbursement for laparoscopic sterilization performed in a facility.
The real benefit to ObGyns, however, is in moving the procedure into the office environment. This allows us great flexibility in scheduling, and avoids the “down time” required for traveling to a facility, waiting for operating room turnover, anesthesia, and paperwork.
Benefit to healthcare systems. Researchers in closed healthcare systems have analyzed the expenses associated with Essure compared with laparoscopic tubal sterilization. When all costs associated with hysteroscopic sterilization are considered, including the need for additional procedures (when the tubes are not accessible or the procedure fails) and the 3-month hysterosalpingography, there remained a significant savings to the healthcare system for these procedures, compared with laparoscopic techniques.2
Ask women who are currently using contraceptive steroids about their menstrual cycles before they started hormonal birth control. Remind women who had menorrhagia or irregular cycles that no method of sterilization will manage their cycles.
The addition of endometrial ablation to the hysteroscopic sterilization procedure is an option.3 However, only 1 of the global ablation technologies currently has FDA approval for concomitant treatment with Essure: Thermachoice (Ethicon Women’s Health and Urology; Somerville, NJ).
Alternatives to permanent sterilization
In counseling women about permanent sterilization, it is important to cover the alternatives, as well.
IUDs. We should also consider the levonorgestrel-containing intrauterine device (IUD), Mirena (Berlex; Montville, NJ), for patients with menorrhagia who desire long-term contraception. Studies have demonstrated excellent patient satisfaction with this system and reduction in menstrual blood loss equivalent to endometrial ablation.4 Although not a permanent solution, the IUD does provide superb contraception, failure rates are similar to sterilization, and management of menorrhagia is excellent, and the cost is less than 10% of a combined endometrial ablation and sterilization procedure. The ParaGard copper-containing IUD is a good choice for women with normal or light flow, but not for those with heavy cycles.
Systemic hormone methods. For women willing to consider systemic hormones, Depo-Provera or the Implanon implantable rod provide excellent long-term contraception.
Vasectomy. Remember that vasectomy remains an option; however, many women want the assurance that they are in control.
Coding and insurance
The difference in payment for hysteroscopic sterilization can be considerable, depending on the site. When performed anywhere other than your office, payment for use of the facility, medications, personnel, and equipment goes to the facility, whether an ambulatory surgery center or a hospital. When we do these procedures in our offices, our reimbursement reflects the fact that we are using our office space, exam table, equipment, supplies, and personnel.
When considering where to perform hysteroscopic sterilization, remember that no one is paying for our space, personnel, and equipment when we are not in the office. Therefore, there is a great advantage in getting our overhead reimbursed when we perform procedures in the office.
The total relative value units payable to the physician for the new CPT code 58565 (hysteroscopy, surgical, with bilateral fallopian tube cannulation to induce occlusion by placement of permanent implants), which is the code for all systems currently under study:
- 12.12 if performed in a facility
- 57.91 if performed in the office
Most payers cover hysteroscopic sterilization when the policy covers sterilization. Determination of coverage by Medicaid has been secured in at least 36 states.
Past the tipping point
It is time to begin to adopt this technology into routine gynecology practice, for the benefits it offers patients, and practicing surgeons, as well. The data are accumulating on the safety and effectiveness of hysteroscopic sterilization techniques—more than 50,000 procedures have been performed worldwide, and we have 5 years of data.
An apt analogy. Although it is true that we might initiate this approach in up to 10% of women who may ultimately require laparoscopy, there appears to be little downside to the attempt. I would suggest the analogy of attempting an endometrial biopsy in the office in lieu of a D&C under anesthesia for postmenopausal women.
True, we sometimes fail, but for the vast majority of patients, it is clearly beneficial to attempt the office procedure and avoid anesthesia. Similarly, by avoiding abdominal access and general anesthesia for sterilization, we are providing a safer and more pleasant procedure with rapid recovery for our patients. Those few who require a different approach will have invested little time, energy, effort, or risk if we learn to perform hysteroscopic sterilization in the office setting.
Dr. Levy is a consultant to Conceptus, Inc.
1. Cooper JM, Carignan CS, Cher D, Kerin JF. Microinsert nonincisional hysteroscopic sterilization. Obstet Gynecol. 2003;102:59-67.
2. Levie MD, Chudnoff SG. Office hysteroscopic sterilization compared with laparoscopic sterilization: a critical cost analysis. J Min Invasive Gynecol. 2005;12:318-322.
3. Valle RF, Valdez J, Wright TC, Kenney M. Concomitant Essure tubal sterilization and Thermachoice endometrial ablation: feasibility and safety. Fertil Steril. 2006;86:152-158.
4. Busfield RA, Farquhar CM, Sowter MC, et al. A randomized trial comparing the levonorgestrel intrauterine system and thermal balloon ablation for heavy menstrual bleeding. BJOG. 2006;113:257-263.
With over 50,000 completed hysteroscopic sterilization procedures worldwide, and 5 years of data, what do we know so far about this innovation? It is now almost 4 years since the FDA approved Essure (Conceptus; San Carlos, Calif), the first hysteroscopic sterilization method available for use in the United States. Two other systems are in the works: Adiana (Adiana; Redwood City, Calif) has completed its Phase III clinical trial and Ovion (American Medical Systems; Minnetonka, Minn) is just beginning its clinical trial this year.
Comparison of the devices
Essure is a disposable delivery system with polyethylene (PET) fibers wound in and around a stainless steel inner coil. An outer coil of nitinol, a superelastic titanium/nickel alloy, is deployed to anchor the device across the uterotubal junction. Wound down, the micro-insert is 0.8 mm in diameter. Once released, the coil expands to 1.5 to 2.0 mm to hold the inner coil and PET fibers in place at the uterine cornua.
Over a period of about 3 months, the PET fibers elicit tissue ingrowth and proximal tubal occlusion. Women must use additional contraception during this time. Documentation of occlusion by a hysterosalpingogram about 3 months after device placement is required before patients may rely on the device and stop birth control.
Adiana (not yet available in the United States) uses a combination of controlled epithelial destruction and insertion of a porous biomatrix to induce vascularized tissue ingrowth. A catheter placed through the operating channel of a small hysteroscope delivers low-power bipolar electrosurgical energy to the tubal orifice (average less than 1 watt to the endosalpinx). A pushrod then delivers a small porous matrix of material into the tubal lumen. Ingrowth of healthy, vascularized tissue occurs over approximately 3 months, to occlude the tubes.
Retention of the matrix and tubal occlusion are documented by both transvaginal ultrasonography and hysterosalpingogram before patients may discontinue additional contraception.
Accessing the tubes
One of the greatest hurdles for occluding the fallopian tubes hysteroscopically is access to the tubes. Both the Adiana clinical trial (not yet published) and the post-market analysis of Essure (not yet published) have demonstrated excellent bilateral placement rates.
Technique is not hard to learn. Both types of devices are inserted through the operating channel of a small hysteroscope. Initial concerns about the ability to access the tubal ostia do not appear to be an issue—at least for those early-adopter physicians performing the procedures. Both clinical trials included gynecologists who were not experienced in operative hysteroscopy. These studies found that cannulation of the tubes is a technique that is easy to learn and rapidly accomplished in most circumstances.
Bilateral placement rates were similar for both devices in the pivotal trials: Adiana 95% (612/655); Essure 90% (464/518).
What are the contraindications?
Approximately 10% of patients have factors that preclude bilateral device placement:
Anatomic factors
- Blocked or stenotic tubes
- Intrauterine adhesions
- Visual field obstructed by polyps, fibroids, or shaggy endometrium
- Lateral tubes
Device or procedure failures due to
- Tubal spasm
- Patient pain/intolerance
- Device malfunction
A second procedure (after correcting the initial problems) will be successful in many women who have what appears to be a technical glitch.
Clinical outcomes, so far
Ultimately, of course, the success of these procedures and the benefits to our patients will be determined by the placement rates in the real world and the ability of a majority of women to rely on the devices for permanent sterilization.
What can we say so far?
Bilateral placement rates
Although the bilateral placement rates for Essure in the pivotal trial were 88% with one attempt, increasing to 92% of all patients enrolled with a second attempt, the data from the postmarket study is even more promising. After FDA approval in November 2002, the manufacturer trained gynecologists in the procedure, and then monitored clinical outcomes in the initial cases performed by these surgeons once they had completed their training and mandatory proctored cases (data not yet published).
Physicians who participated in the clinical trial were excluded from this analysis. The bilateral device placement rate for these women treated by novice users is over 94%. Adiana’s Phase III trial data demonstrate a similar bilateral success rate. It appears that despite the misgivings of many ObGyns, these systems are easy to learn.
I have incorporated the following tips and tricks into my office practice with great results. Patients are thrilled with their experience and leave ready to recruit their friends for the procedure.
- Pretreatment with a nonsteroidal anti-inflammatory drug to block prostaglandin release and uterotubal spasm
- Scheduling the procedure for the early follicular phase of the menstrual cycle to minimize shaggy endometrium, or
- Suppressing the endometrium with progestins from the first day of menses until the scheduled procedure
- Use of warm fluid for uterine distension to reduce spasm
- Placement of topical lidocaine gel into the uterus 10 to 15 minutes prior to the procedure
- Use of a pressure bag to assure adequate uterine distension
Patient safety
Few complications have been reported with either technique. There were the expected rare vasovagal reactions, as well as 2 cases of hypervolemia with Essure and 1 case of hyponatremia in the Adiana trial. Both of these situations should be avoidable with proper monitoring and limiting distension fluids to isotonic solutions. All patients recovered fully. There were no problems with persistent pain or changes in menstrual patterns at 1 year in the Essure trial.
Expulsion of the devices was associated with proximal positioning of the devices in all cases (3%). Patients had no symptoms, and most were able to have a second procedure with excellent placement and retention. Expulsions were identified at the postprocedure scout film or hysterosalpingogram.
Tubal perforation was noted in 0.9% of the patients. Predisposing factors were preexisting tubal occlusion or hydrosalpinx.
Perforations were asymptomatic, as well. Laparoscopic evaluation in 3 cases demonstrated no adhesions or reactions to the tiny perforation sites.
Delivery. An outer coil of nitinol, a superelastic titanium/nickel alloy is deployed to anchor the device across the uterotubal junction. Once released, the coil expands to 1.5 to 2.0 mm to hold the inner coil and PET fibers in place at the uterine cornua.
Occlusion. Over about 3 months, the PET fibers elicit tissue ingrowth and proximal tubal occlusion. Women must use additional contraception during this time. Documentation of occlusion by a hysterosalpingogram is required before patients may discontinue additional contraception.
Images: Rich Larocco
Anesthesia. Because hysteroscopic sterilization procedures may be performed without general anesthesia and by design avoid the need to access the abdominal cavity, they should be inherently safer for patients than the other available surgical sterilization methods. In the Essure and Adiana trials,1 more than 50% of the patients underwent their procedures under local anesthesia with no additional intravenous sedation. The others had local anesthesia with IV sedation. Only 1 patient (in the Essure trial) underwent general anesthesia.
Patient satisfaction and tolerance of the procedure were excellent; 88% of patients described their experience as good to excellent. Only 4% rated their procedure pain as severe. At discharge (approximately 40 minutes after conclusion of the procedure), 79% of patients had no pain and required no pain medication.
What are the potential complications?
The rare but devastating complications associated with laparoscopic sterilization should be avoidable with the hysteroscopic approach—at least for the 90% of patients for whom access to both tubes is feasible.
Ideal candidates. Hysteroscopic access seems to be the ideal approach for occlusion of the fallopian tubes in patients with medical conditions that may increase the risk of abdominal access, or for whom general anesthesia imposes added risk.
Problem conditions. The following clinical conditions and comorbidities all present significant challenges for the laparoscopic surgeon:
- Cardiac disease
- Thrombophilias
- Immune suppression
- Renal transplant
- Morbid obesity
- Previous abdominal surgery, especially bowel procedures
Delivery. A catheter placed through the operating channel of a small hysteroscope delivers low-power bipolar electrosurgical energy to the tubal orifice. A pushrod then delivers a small porous matrix of material into the tubal lumen.
Occlusion. Ingrowth of healthy, vascularized tissue occurs over approximately 3 months, to occlude the tubes. Retention of the matrix and tubal occlusion are documented by both transvaginal ultrasonography and hysterosalpingogram before patients may discontinue additional contraception.
Images: Rich Larocco
How many pregnancies?
There have been no pregnancies in Phase II and III trials of Essure, thus far. In July 2003, Cooper et al1 reported no pregnancies in 7,532 woman-months of use.
Conceptus is aware of 64 pregnancies among more than 50,000 procedures performed worldwide. None appeared to have occurred with proper demonstration of bilateral tubal occlusion after device placement. Most appear to be luteal phase pregnancies present at the time of the sterilization procedure, or failure of either the patient or the physician to assure tubal obstruction prior to stopping additional birth control methods.
There are no documented ectopic pregnancies, although 1 of the 64 reported cases may have been a very early tubal gestation. The patient was treated with methotrexate without firm documentation of the location of the pregnancy.
There are 2 pregnancies among the 605 patients with bilateral Adiana devices (6,860 woman-months of use as of September 2005). One resulted from an error in interpreting the hysterosalpingogram. The other did occur with a properly placed device and occlusion demonstrated on hysterosalpingogram.
It appears that hysteroscopic sterilization with Essure will have acceptable and preventable failure rates (longer term data and postmarket analysis are not yet available for the Adiana device). The calculated 5-year success rate is more than 99% for Essure; this compares favorably with all other surgical sterilization methods.
Do benefits outweigh costs?
The overall cost of hysteroscopic sterilization methods compares favorably with laparoscopic approaches. The expense of the disposable equipment is recouped by avoiding the costs of general anesthesia, and operating room and facility charges.
Advantages of the office setting
Payment for physicians is slightly more than the reimbursement for laparoscopic sterilization performed in a facility.
The real benefit to ObGyns, however, is in moving the procedure into the office environment. This allows us great flexibility in scheduling, and avoids the “down time” required for traveling to a facility, waiting for operating room turnover, anesthesia, and paperwork.
Benefit to healthcare systems. Researchers in closed healthcare systems have analyzed the expenses associated with Essure compared with laparoscopic tubal sterilization. When all costs associated with hysteroscopic sterilization are considered, including the need for additional procedures (when the tubes are not accessible or the procedure fails) and the 3-month hysterosalpingography, there remained a significant savings to the healthcare system for these procedures, compared with laparoscopic techniques.2
Ask women who are currently using contraceptive steroids about their menstrual cycles before they started hormonal birth control. Remind women who had menorrhagia or irregular cycles that no method of sterilization will manage their cycles.
The addition of endometrial ablation to the hysteroscopic sterilization procedure is an option.3 However, only 1 of the global ablation technologies currently has FDA approval for concomitant treatment with Essure: Thermachoice (Ethicon Women’s Health and Urology; Somerville, NJ).
Alternatives to permanent sterilization
In counseling women about permanent sterilization, it is important to cover the alternatives, as well.
IUDs. We should also consider the levonorgestrel-containing intrauterine device (IUD), Mirena (Berlex; Montville, NJ), for patients with menorrhagia who desire long-term contraception. Studies have demonstrated excellent patient satisfaction with this system and reduction in menstrual blood loss equivalent to endometrial ablation.4 Although not a permanent solution, the IUD does provide superb contraception, failure rates are similar to sterilization, and management of menorrhagia is excellent, and the cost is less than 10% of a combined endometrial ablation and sterilization procedure. The ParaGard copper-containing IUD is a good choice for women with normal or light flow, but not for those with heavy cycles.
Systemic hormone methods. For women willing to consider systemic hormones, Depo-Provera or the Implanon implantable rod provide excellent long-term contraception.
Vasectomy. Remember that vasectomy remains an option; however, many women want the assurance that they are in control.
Coding and insurance
The difference in payment for hysteroscopic sterilization can be considerable, depending on the site. When performed anywhere other than your office, payment for use of the facility, medications, personnel, and equipment goes to the facility, whether an ambulatory surgery center or a hospital. When we do these procedures in our offices, our reimbursement reflects the fact that we are using our office space, exam table, equipment, supplies, and personnel.
When considering where to perform hysteroscopic sterilization, remember that no one is paying for our space, personnel, and equipment when we are not in the office. Therefore, there is a great advantage in getting our overhead reimbursed when we perform procedures in the office.
The total relative value units payable to the physician for the new CPT code 58565 (hysteroscopy, surgical, with bilateral fallopian tube cannulation to induce occlusion by placement of permanent implants), which is the code for all systems currently under study:
- 12.12 if performed in a facility
- 57.91 if performed in the office
Most payers cover hysteroscopic sterilization when the policy covers sterilization. Determination of coverage by Medicaid has been secured in at least 36 states.
Past the tipping point
It is time to begin to adopt this technology into routine gynecology practice, for the benefits it offers patients, and practicing surgeons, as well. The data are accumulating on the safety and effectiveness of hysteroscopic sterilization techniques—more than 50,000 procedures have been performed worldwide, and we have 5 years of data.
An apt analogy. Although it is true that we might initiate this approach in up to 10% of women who may ultimately require laparoscopy, there appears to be little downside to the attempt. I would suggest the analogy of attempting an endometrial biopsy in the office in lieu of a D&C under anesthesia for postmenopausal women.
True, we sometimes fail, but for the vast majority of patients, it is clearly beneficial to attempt the office procedure and avoid anesthesia. Similarly, by avoiding abdominal access and general anesthesia for sterilization, we are providing a safer and more pleasant procedure with rapid recovery for our patients. Those few who require a different approach will have invested little time, energy, effort, or risk if we learn to perform hysteroscopic sterilization in the office setting.
Dr. Levy is a consultant to Conceptus, Inc.
With over 50,000 completed hysteroscopic sterilization procedures worldwide, and 5 years of data, what do we know so far about this innovation? It is now almost 4 years since the FDA approved Essure (Conceptus; San Carlos, Calif), the first hysteroscopic sterilization method available for use in the United States. Two other systems are in the works: Adiana (Adiana; Redwood City, Calif) has completed its Phase III clinical trial and Ovion (American Medical Systems; Minnetonka, Minn) is just beginning its clinical trial this year.
Comparison of the devices
Essure is a disposable delivery system with polyethylene (PET) fibers wound in and around a stainless steel inner coil. An outer coil of nitinol, a superelastic titanium/nickel alloy, is deployed to anchor the device across the uterotubal junction. Wound down, the micro-insert is 0.8 mm in diameter. Once released, the coil expands to 1.5 to 2.0 mm to hold the inner coil and PET fibers in place at the uterine cornua.
Over a period of about 3 months, the PET fibers elicit tissue ingrowth and proximal tubal occlusion. Women must use additional contraception during this time. Documentation of occlusion by a hysterosalpingogram about 3 months after device placement is required before patients may rely on the device and stop birth control.
Adiana (not yet available in the United States) uses a combination of controlled epithelial destruction and insertion of a porous biomatrix to induce vascularized tissue ingrowth. A catheter placed through the operating channel of a small hysteroscope delivers low-power bipolar electrosurgical energy to the tubal orifice (average less than 1 watt to the endosalpinx). A pushrod then delivers a small porous matrix of material into the tubal lumen. Ingrowth of healthy, vascularized tissue occurs over approximately 3 months, to occlude the tubes.
Retention of the matrix and tubal occlusion are documented by both transvaginal ultrasonography and hysterosalpingogram before patients may discontinue additional contraception.
Accessing the tubes
One of the greatest hurdles for occluding the fallopian tubes hysteroscopically is access to the tubes. Both the Adiana clinical trial (not yet published) and the post-market analysis of Essure (not yet published) have demonstrated excellent bilateral placement rates.
Technique is not hard to learn. Both types of devices are inserted through the operating channel of a small hysteroscope. Initial concerns about the ability to access the tubal ostia do not appear to be an issue—at least for those early-adopter physicians performing the procedures. Both clinical trials included gynecologists who were not experienced in operative hysteroscopy. These studies found that cannulation of the tubes is a technique that is easy to learn and rapidly accomplished in most circumstances.
Bilateral placement rates were similar for both devices in the pivotal trials: Adiana 95% (612/655); Essure 90% (464/518).
What are the contraindications?
Approximately 10% of patients have factors that preclude bilateral device placement:
Anatomic factors
- Blocked or stenotic tubes
- Intrauterine adhesions
- Visual field obstructed by polyps, fibroids, or shaggy endometrium
- Lateral tubes
Device or procedure failures due to
- Tubal spasm
- Patient pain/intolerance
- Device malfunction
A second procedure (after correcting the initial problems) will be successful in many women who have what appears to be a technical glitch.
Clinical outcomes, so far
Ultimately, of course, the success of these procedures and the benefits to our patients will be determined by the placement rates in the real world and the ability of a majority of women to rely on the devices for permanent sterilization.
What can we say so far?
Bilateral placement rates
Although the bilateral placement rates for Essure in the pivotal trial were 88% with one attempt, increasing to 92% of all patients enrolled with a second attempt, the data from the postmarket study is even more promising. After FDA approval in November 2002, the manufacturer trained gynecologists in the procedure, and then monitored clinical outcomes in the initial cases performed by these surgeons once they had completed their training and mandatory proctored cases (data not yet published).
Physicians who participated in the clinical trial were excluded from this analysis. The bilateral device placement rate for these women treated by novice users is over 94%. Adiana’s Phase III trial data demonstrate a similar bilateral success rate. It appears that despite the misgivings of many ObGyns, these systems are easy to learn.
I have incorporated the following tips and tricks into my office practice with great results. Patients are thrilled with their experience and leave ready to recruit their friends for the procedure.
- Pretreatment with a nonsteroidal anti-inflammatory drug to block prostaglandin release and uterotubal spasm
- Scheduling the procedure for the early follicular phase of the menstrual cycle to minimize shaggy endometrium, or
- Suppressing the endometrium with progestins from the first day of menses until the scheduled procedure
- Use of warm fluid for uterine distension to reduce spasm
- Placement of topical lidocaine gel into the uterus 10 to 15 minutes prior to the procedure
- Use of a pressure bag to assure adequate uterine distension
Patient safety
Few complications have been reported with either technique. There were the expected rare vasovagal reactions, as well as 2 cases of hypervolemia with Essure and 1 case of hyponatremia in the Adiana trial. Both of these situations should be avoidable with proper monitoring and limiting distension fluids to isotonic solutions. All patients recovered fully. There were no problems with persistent pain or changes in menstrual patterns at 1 year in the Essure trial.
Expulsion of the devices was associated with proximal positioning of the devices in all cases (3%). Patients had no symptoms, and most were able to have a second procedure with excellent placement and retention. Expulsions were identified at the postprocedure scout film or hysterosalpingogram.
Tubal perforation was noted in 0.9% of the patients. Predisposing factors were preexisting tubal occlusion or hydrosalpinx.
Perforations were asymptomatic, as well. Laparoscopic evaluation in 3 cases demonstrated no adhesions or reactions to the tiny perforation sites.
Delivery. An outer coil of nitinol, a superelastic titanium/nickel alloy is deployed to anchor the device across the uterotubal junction. Once released, the coil expands to 1.5 to 2.0 mm to hold the inner coil and PET fibers in place at the uterine cornua.
Occlusion. Over about 3 months, the PET fibers elicit tissue ingrowth and proximal tubal occlusion. Women must use additional contraception during this time. Documentation of occlusion by a hysterosalpingogram is required before patients may discontinue additional contraception.
Images: Rich Larocco
Anesthesia. Because hysteroscopic sterilization procedures may be performed without general anesthesia and by design avoid the need to access the abdominal cavity, they should be inherently safer for patients than the other available surgical sterilization methods. In the Essure and Adiana trials,1 more than 50% of the patients underwent their procedures under local anesthesia with no additional intravenous sedation. The others had local anesthesia with IV sedation. Only 1 patient (in the Essure trial) underwent general anesthesia.
Patient satisfaction and tolerance of the procedure were excellent; 88% of patients described their experience as good to excellent. Only 4% rated their procedure pain as severe. At discharge (approximately 40 minutes after conclusion of the procedure), 79% of patients had no pain and required no pain medication.
What are the potential complications?
The rare but devastating complications associated with laparoscopic sterilization should be avoidable with the hysteroscopic approach—at least for the 90% of patients for whom access to both tubes is feasible.
Ideal candidates. Hysteroscopic access seems to be the ideal approach for occlusion of the fallopian tubes in patients with medical conditions that may increase the risk of abdominal access, or for whom general anesthesia imposes added risk.
Problem conditions. The following clinical conditions and comorbidities all present significant challenges for the laparoscopic surgeon:
- Cardiac disease
- Thrombophilias
- Immune suppression
- Renal transplant
- Morbid obesity
- Previous abdominal surgery, especially bowel procedures
Delivery. A catheter placed through the operating channel of a small hysteroscope delivers low-power bipolar electrosurgical energy to the tubal orifice. A pushrod then delivers a small porous matrix of material into the tubal lumen.
Occlusion. Ingrowth of healthy, vascularized tissue occurs over approximately 3 months, to occlude the tubes. Retention of the matrix and tubal occlusion are documented by both transvaginal ultrasonography and hysterosalpingogram before patients may discontinue additional contraception.
Images: Rich Larocco
How many pregnancies?
There have been no pregnancies in Phase II and III trials of Essure, thus far. In July 2003, Cooper et al1 reported no pregnancies in 7,532 woman-months of use.
Conceptus is aware of 64 pregnancies among more than 50,000 procedures performed worldwide. None appeared to have occurred with proper demonstration of bilateral tubal occlusion after device placement. Most appear to be luteal phase pregnancies present at the time of the sterilization procedure, or failure of either the patient or the physician to assure tubal obstruction prior to stopping additional birth control methods.
There are no documented ectopic pregnancies, although 1 of the 64 reported cases may have been a very early tubal gestation. The patient was treated with methotrexate without firm documentation of the location of the pregnancy.
There are 2 pregnancies among the 605 patients with bilateral Adiana devices (6,860 woman-months of use as of September 2005). One resulted from an error in interpreting the hysterosalpingogram. The other did occur with a properly placed device and occlusion demonstrated on hysterosalpingogram.
It appears that hysteroscopic sterilization with Essure will have acceptable and preventable failure rates (longer term data and postmarket analysis are not yet available for the Adiana device). The calculated 5-year success rate is more than 99% for Essure; this compares favorably with all other surgical sterilization methods.
Do benefits outweigh costs?
The overall cost of hysteroscopic sterilization methods compares favorably with laparoscopic approaches. The expense of the disposable equipment is recouped by avoiding the costs of general anesthesia, and operating room and facility charges.
Advantages of the office setting
Payment for physicians is slightly more than the reimbursement for laparoscopic sterilization performed in a facility.
The real benefit to ObGyns, however, is in moving the procedure into the office environment. This allows us great flexibility in scheduling, and avoids the “down time” required for traveling to a facility, waiting for operating room turnover, anesthesia, and paperwork.
Benefit to healthcare systems. Researchers in closed healthcare systems have analyzed the expenses associated with Essure compared with laparoscopic tubal sterilization. When all costs associated with hysteroscopic sterilization are considered, including the need for additional procedures (when the tubes are not accessible or the procedure fails) and the 3-month hysterosalpingography, there remained a significant savings to the healthcare system for these procedures, compared with laparoscopic techniques.2
Ask women who are currently using contraceptive steroids about their menstrual cycles before they started hormonal birth control. Remind women who had menorrhagia or irregular cycles that no method of sterilization will manage their cycles.
The addition of endometrial ablation to the hysteroscopic sterilization procedure is an option.3 However, only 1 of the global ablation technologies currently has FDA approval for concomitant treatment with Essure: Thermachoice (Ethicon Women’s Health and Urology; Somerville, NJ).
Alternatives to permanent sterilization
In counseling women about permanent sterilization, it is important to cover the alternatives, as well.
IUDs. We should also consider the levonorgestrel-containing intrauterine device (IUD), Mirena (Berlex; Montville, NJ), for patients with menorrhagia who desire long-term contraception. Studies have demonstrated excellent patient satisfaction with this system and reduction in menstrual blood loss equivalent to endometrial ablation.4 Although not a permanent solution, the IUD does provide superb contraception, failure rates are similar to sterilization, and management of menorrhagia is excellent, and the cost is less than 10% of a combined endometrial ablation and sterilization procedure. The ParaGard copper-containing IUD is a good choice for women with normal or light flow, but not for those with heavy cycles.
Systemic hormone methods. For women willing to consider systemic hormones, Depo-Provera or the Implanon implantable rod provide excellent long-term contraception.
Vasectomy. Remember that vasectomy remains an option; however, many women want the assurance that they are in control.
Coding and insurance
The difference in payment for hysteroscopic sterilization can be considerable, depending on the site. When performed anywhere other than your office, payment for use of the facility, medications, personnel, and equipment goes to the facility, whether an ambulatory surgery center or a hospital. When we do these procedures in our offices, our reimbursement reflects the fact that we are using our office space, exam table, equipment, supplies, and personnel.
When considering where to perform hysteroscopic sterilization, remember that no one is paying for our space, personnel, and equipment when we are not in the office. Therefore, there is a great advantage in getting our overhead reimbursed when we perform procedures in the office.
The total relative value units payable to the physician for the new CPT code 58565 (hysteroscopy, surgical, with bilateral fallopian tube cannulation to induce occlusion by placement of permanent implants), which is the code for all systems currently under study:
- 12.12 if performed in a facility
- 57.91 if performed in the office
Most payers cover hysteroscopic sterilization when the policy covers sterilization. Determination of coverage by Medicaid has been secured in at least 36 states.
Past the tipping point
It is time to begin to adopt this technology into routine gynecology practice, for the benefits it offers patients, and practicing surgeons, as well. The data are accumulating on the safety and effectiveness of hysteroscopic sterilization techniques—more than 50,000 procedures have been performed worldwide, and we have 5 years of data.
An apt analogy. Although it is true that we might initiate this approach in up to 10% of women who may ultimately require laparoscopy, there appears to be little downside to the attempt. I would suggest the analogy of attempting an endometrial biopsy in the office in lieu of a D&C under anesthesia for postmenopausal women.
True, we sometimes fail, but for the vast majority of patients, it is clearly beneficial to attempt the office procedure and avoid anesthesia. Similarly, by avoiding abdominal access and general anesthesia for sterilization, we are providing a safer and more pleasant procedure with rapid recovery for our patients. Those few who require a different approach will have invested little time, energy, effort, or risk if we learn to perform hysteroscopic sterilization in the office setting.
Dr. Levy is a consultant to Conceptus, Inc.
1. Cooper JM, Carignan CS, Cher D, Kerin JF. Microinsert nonincisional hysteroscopic sterilization. Obstet Gynecol. 2003;102:59-67.
2. Levie MD, Chudnoff SG. Office hysteroscopic sterilization compared with laparoscopic sterilization: a critical cost analysis. J Min Invasive Gynecol. 2005;12:318-322.
3. Valle RF, Valdez J, Wright TC, Kenney M. Concomitant Essure tubal sterilization and Thermachoice endometrial ablation: feasibility and safety. Fertil Steril. 2006;86:152-158.
4. Busfield RA, Farquhar CM, Sowter MC, et al. A randomized trial comparing the levonorgestrel intrauterine system and thermal balloon ablation for heavy menstrual bleeding. BJOG. 2006;113:257-263.
1. Cooper JM, Carignan CS, Cher D, Kerin JF. Microinsert nonincisional hysteroscopic sterilization. Obstet Gynecol. 2003;102:59-67.
2. Levie MD, Chudnoff SG. Office hysteroscopic sterilization compared with laparoscopic sterilization: a critical cost analysis. J Min Invasive Gynecol. 2005;12:318-322.
3. Valle RF, Valdez J, Wright TC, Kenney M. Concomitant Essure tubal sterilization and Thermachoice endometrial ablation: feasibility and safety. Fertil Steril. 2006;86:152-158.
4. Busfield RA, Farquhar CM, Sowter MC, et al. A randomized trial comparing the levonorgestrel intrauterine system and thermal balloon ablation for heavy menstrual bleeding. BJOG. 2006;113:257-263.
IN THIS ARTICLE