User login
SAN DIEGO – A Massachusetts General Hospital neuroimaging algorithm that is used to help in the selection of appropriate treatment for patients with severe ischemic strokes caused by anterior circulation occlusions led to significant improvements in mortality and outcomes and a decrease in the number of stroke interventions after it was implemented at the Cleveland Clinic, according to Dr. Ramon Gilberto Gonzalez.
With a few exceptions, the algorithm does not use perfusion imaging, either by MRI or CT, in the assessment of anterior circulation occlusion (ACO) patients for intravenous tissue plasminogen activator (TPA) or endovascular therapy.
"One of the challenges in imaging stroke is that it is very heterogeneous. You don’t know what is going to show up in your emergency department. It is important to formulate an imaging program that can optimize all the information you get from all patients, whether they need intra-arterial therapy or just watchful waiting. A system is needed that is efficient for all patients, and that is a challenge," Dr. Gonzalez said at the annual meeting of the American Society of Neuroradiology. He is lead author of the paper describing the algorithm and director of the neuroradiology division at Massachusetts General Hospital (MGH), Boston.
The algorithm was developed from both experience and evidence, Dr. Gonzalez explained. Individual neuroradiology and neurology faculty from MGH presented the best evidence from the literature and clinical experience regarding imaging methods and the National Institutes of Health Stroke Scale (NIHSS). Other faculty and fellows who heard the presentations met to weigh the evidence and make recommendations. The methods were rated on such metrics as sensitivity and specificity, value for patient care, usability in the acute setting, work flow, repeatability, reliability, and clinical efficacy.
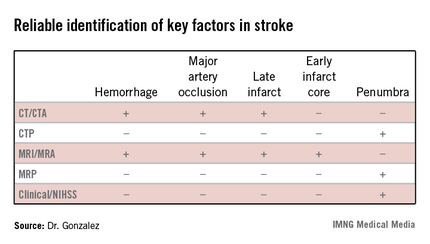
The algorithm reflected how well different imaging methods provided information on key factors in stroke. "In addition to time and hemorrhage, we must consider how much of the brain has already died [the core] and how much of the brain is likely to die if you do not do anything to help" the penumbra. Each imaging method has benefits and weaknesses for different stroke parameters, Dr. Gonzalez said. (See table.) CT/CT angiography (CTA) and MRI/MR angiography (MRA) were found to be useful for demonstrating hemorrhage, major artery occlusion, and late infarct, while only MRI/MRA helped visualize the early infarct core.
In the algorithm, all patients presenting with a stroke syndrome (possible hemorrhage or large infarct) receive a neurological exam, including the NIHSS. "The single most important parameter is the neurological exam," according to Dr. Gonzalez, which he said can determine an overall size of the penumbra and core combined. "The neurologic exam is just as good as CTP [CT perfusion] or MRP [MRI perfusion]."
According to the stroke imaging algorithm (J. Neurointervent. Surg. 2013;5:i7-12), the first imaging study should be a noncontrast CT (NCCT), followed by a CTA if a proximal occlusion is accessible and the patient is eligible for MRI. If the NCCT does not demonstrate a hemorrhage or large hypodensity, and the patient is within the time window, TPA is prepared while the CTA is performed, and the infusion is started. If the patient has a distal internal carotid artery and/or proximal middle cerebral artery occlusion, he will undergo diffusion-weighted imaging (DWI). Patients with DWI lesions less than 70 mL in volume are sent for intra-arterial therapy as long as they meet clinical and medical criteria. "DWI is really the only method we have to determine the core volume with sufficient precision to be able to make good clinical decisions," Dr. Gonzalez said.
Perfusion CT and perfusion MR are not part of the recommended imaging workup, with some exceptions. "For many years, radiologists thought you could substitute DWI with CT perfusion. We came to the conclusion that you cannot," Dr. Gonzalez said. The reviewing panel found that there was no or poor evidence that CTP could be used for early estimation of the infarct core or penumbra, and had no proven role in selecting ACO patients for IV thrombolysis or endovascular therapy. Similarly, they found the evidence indicated that MRP had no proven role in selecting ACO patients for endovascular therapy. Perfusion imaging may be appropriate if patients cannot be scanned by an MRI or are not otherwise eligible for intra-arterial therapy, or if perfusion data are needed for another reason.
After adoption of the algorithm, the number of CT perfusion exams performed at MGH on stroke patients dropped from 40-50 per month to about 10 per month. No discernible effects on the rate of good outcomes (P = .67) or median modified Rankin Scale score (P = .85) were found.
A little more than a year of testing at the Cleveland Clinic showed that the algorithm resulted in a significant reduction in mortality and improvement of modified Rankin Scale scores independent of the treatment received. Interestingly, the number of interventions for stroke decreased by 40% as patient selection through imaging targeted patients more likely to benefit and excluded those not suitable for intra-arterial therapy (Stroke 2012;43:A161).
Dr. Gonzalez reported having no relevant financial disclosures.
SAN DIEGO – A Massachusetts General Hospital neuroimaging algorithm that is used to help in the selection of appropriate treatment for patients with severe ischemic strokes caused by anterior circulation occlusions led to significant improvements in mortality and outcomes and a decrease in the number of stroke interventions after it was implemented at the Cleveland Clinic, according to Dr. Ramon Gilberto Gonzalez.
With a few exceptions, the algorithm does not use perfusion imaging, either by MRI or CT, in the assessment of anterior circulation occlusion (ACO) patients for intravenous tissue plasminogen activator (TPA) or endovascular therapy.

"One of the challenges in imaging stroke is that it is very heterogeneous. You don’t know what is going to show up in your emergency department. It is important to formulate an imaging program that can optimize all the information you get from all patients, whether they need intra-arterial therapy or just watchful waiting. A system is needed that is efficient for all patients, and that is a challenge," Dr. Gonzalez said at the annual meeting of the American Society of Neuroradiology. He is lead author of the paper describing the algorithm and director of the neuroradiology division at Massachusetts General Hospital (MGH), Boston.
The algorithm was developed from both experience and evidence, Dr. Gonzalez explained. Individual neuroradiology and neurology faculty from MGH presented the best evidence from the literature and clinical experience regarding imaging methods and the National Institutes of Health Stroke Scale (NIHSS). Other faculty and fellows who heard the presentations met to weigh the evidence and make recommendations. The methods were rated on such metrics as sensitivity and specificity, value for patient care, usability in the acute setting, work flow, repeatability, reliability, and clinical efficacy.
The algorithm reflected how well different imaging methods provided information on key factors in stroke. "In addition to time and hemorrhage, we must consider how much of the brain has already died [the core] and how much of the brain is likely to die if you do not do anything to help" the penumbra. Each imaging method has benefits and weaknesses for different stroke parameters, Dr. Gonzalez said. (See table.) CT/CT angiography (CTA) and MRI/MR angiography (MRA) were found to be useful for demonstrating hemorrhage, major artery occlusion, and late infarct, while only MRI/MRA helped visualize the early infarct core.
In the algorithm, all patients presenting with a stroke syndrome (possible hemorrhage or large infarct) receive a neurological exam, including the NIHSS. "The single most important parameter is the neurological exam," according to Dr. Gonzalez, which he said can determine an overall size of the penumbra and core combined. "The neurologic exam is just as good as CTP [CT perfusion] or MRP [MRI perfusion]."
According to the stroke imaging algorithm (J. Neurointervent. Surg. 2013;5:i7-12), the first imaging study should be a noncontrast CT (NCCT), followed by a CTA if a proximal occlusion is accessible and the patient is eligible for MRI. If the NCCT does not demonstrate a hemorrhage or large hypodensity, and the patient is within the time window, TPA is prepared while the CTA is performed, and the infusion is started. If the patient has a distal internal carotid artery and/or proximal middle cerebral artery occlusion, he will undergo diffusion-weighted imaging (DWI). Patients with DWI lesions less than 70 mL in volume are sent for intra-arterial therapy as long as they meet clinical and medical criteria. "DWI is really the only method we have to determine the core volume with sufficient precision to be able to make good clinical decisions," Dr. Gonzalez said.
Perfusion CT and perfusion MR are not part of the recommended imaging workup, with some exceptions. "For many years, radiologists thought you could substitute DWI with CT perfusion. We came to the conclusion that you cannot," Dr. Gonzalez said. The reviewing panel found that there was no or poor evidence that CTP could be used for early estimation of the infarct core or penumbra, and had no proven role in selecting ACO patients for IV thrombolysis or endovascular therapy. Similarly, they found the evidence indicated that MRP had no proven role in selecting ACO patients for endovascular therapy. Perfusion imaging may be appropriate if patients cannot be scanned by an MRI or are not otherwise eligible for intra-arterial therapy, or if perfusion data are needed for another reason.
After adoption of the algorithm, the number of CT perfusion exams performed at MGH on stroke patients dropped from 40-50 per month to about 10 per month. No discernible effects on the rate of good outcomes (P = .67) or median modified Rankin Scale score (P = .85) were found.
A little more than a year of testing at the Cleveland Clinic showed that the algorithm resulted in a significant reduction in mortality and improvement of modified Rankin Scale scores independent of the treatment received. Interestingly, the number of interventions for stroke decreased by 40% as patient selection through imaging targeted patients more likely to benefit and excluded those not suitable for intra-arterial therapy (Stroke 2012;43:A161).
Dr. Gonzalez reported having no relevant financial disclosures.
SAN DIEGO – A Massachusetts General Hospital neuroimaging algorithm that is used to help in the selection of appropriate treatment for patients with severe ischemic strokes caused by anterior circulation occlusions led to significant improvements in mortality and outcomes and a decrease in the number of stroke interventions after it was implemented at the Cleveland Clinic, according to Dr. Ramon Gilberto Gonzalez.
With a few exceptions, the algorithm does not use perfusion imaging, either by MRI or CT, in the assessment of anterior circulation occlusion (ACO) patients for intravenous tissue plasminogen activator (TPA) or endovascular therapy.

"One of the challenges in imaging stroke is that it is very heterogeneous. You don’t know what is going to show up in your emergency department. It is important to formulate an imaging program that can optimize all the information you get from all patients, whether they need intra-arterial therapy or just watchful waiting. A system is needed that is efficient for all patients, and that is a challenge," Dr. Gonzalez said at the annual meeting of the American Society of Neuroradiology. He is lead author of the paper describing the algorithm and director of the neuroradiology division at Massachusetts General Hospital (MGH), Boston.
The algorithm was developed from both experience and evidence, Dr. Gonzalez explained. Individual neuroradiology and neurology faculty from MGH presented the best evidence from the literature and clinical experience regarding imaging methods and the National Institutes of Health Stroke Scale (NIHSS). Other faculty and fellows who heard the presentations met to weigh the evidence and make recommendations. The methods were rated on such metrics as sensitivity and specificity, value for patient care, usability in the acute setting, work flow, repeatability, reliability, and clinical efficacy.
The algorithm reflected how well different imaging methods provided information on key factors in stroke. "In addition to time and hemorrhage, we must consider how much of the brain has already died [the core] and how much of the brain is likely to die if you do not do anything to help" the penumbra. Each imaging method has benefits and weaknesses for different stroke parameters, Dr. Gonzalez said. (See table.) CT/CT angiography (CTA) and MRI/MR angiography (MRA) were found to be useful for demonstrating hemorrhage, major artery occlusion, and late infarct, while only MRI/MRA helped visualize the early infarct core.
In the algorithm, all patients presenting with a stroke syndrome (possible hemorrhage or large infarct) receive a neurological exam, including the NIHSS. "The single most important parameter is the neurological exam," according to Dr. Gonzalez, which he said can determine an overall size of the penumbra and core combined. "The neurologic exam is just as good as CTP [CT perfusion] or MRP [MRI perfusion]."
According to the stroke imaging algorithm (J. Neurointervent. Surg. 2013;5:i7-12), the first imaging study should be a noncontrast CT (NCCT), followed by a CTA if a proximal occlusion is accessible and the patient is eligible for MRI. If the NCCT does not demonstrate a hemorrhage or large hypodensity, and the patient is within the time window, TPA is prepared while the CTA is performed, and the infusion is started. If the patient has a distal internal carotid artery and/or proximal middle cerebral artery occlusion, he will undergo diffusion-weighted imaging (DWI). Patients with DWI lesions less than 70 mL in volume are sent for intra-arterial therapy as long as they meet clinical and medical criteria. "DWI is really the only method we have to determine the core volume with sufficient precision to be able to make good clinical decisions," Dr. Gonzalez said.
Perfusion CT and perfusion MR are not part of the recommended imaging workup, with some exceptions. "For many years, radiologists thought you could substitute DWI with CT perfusion. We came to the conclusion that you cannot," Dr. Gonzalez said. The reviewing panel found that there was no or poor evidence that CTP could be used for early estimation of the infarct core or penumbra, and had no proven role in selecting ACO patients for IV thrombolysis or endovascular therapy. Similarly, they found the evidence indicated that MRP had no proven role in selecting ACO patients for endovascular therapy. Perfusion imaging may be appropriate if patients cannot be scanned by an MRI or are not otherwise eligible for intra-arterial therapy, or if perfusion data are needed for another reason.
After adoption of the algorithm, the number of CT perfusion exams performed at MGH on stroke patients dropped from 40-50 per month to about 10 per month. No discernible effects on the rate of good outcomes (P = .67) or median modified Rankin Scale score (P = .85) were found.
A little more than a year of testing at the Cleveland Clinic showed that the algorithm resulted in a significant reduction in mortality and improvement of modified Rankin Scale scores independent of the treatment received. Interestingly, the number of interventions for stroke decreased by 40% as patient selection through imaging targeted patients more likely to benefit and excluded those not suitable for intra-arterial therapy (Stroke 2012;43:A161).
Dr. Gonzalez reported having no relevant financial disclosures.
EXPERT ANALYSIS FROM THE ASNR ANNUAL MEETING

