User login
Over the past several decades, a large body of evidence has emerged demonstrating the adverse impact of depressive disorder on heart disease. This evidence confirms the early suspicion of observant clinicians that psychological factors play a significant role in the genesis and course of heart disease, as well as confirming the ancient belief in a mind-body connection in general and a connection between human moods and the heart in particular. Given the high prevalence of these two disorders, we need a better understanding of the impact of depressive disorder on heart disease, the proposed underlying pathophysiologic mechanisms, and the effects of treating depression in relation to risk reduction in patients with heart disease.
In this article, I will focus on (1) reviewing the results of meta-analyses examining the association of depression with cardiac diseases, (2) discussing the relationship between depression and mental stress–induced myocardial ischemia, (3) reviewing the available studies of the treatment of depression in patients with cardiac disease, and (4) discussing future directions for research in this area.
ASSOCIATION OF DEPRESSION WITH PROGRESSION OF CARDIAC DISEASES
As a disease of the brain, depression is common. The lifetime prevalence of major depressive disorder, a significant form of depression, is 16.2%.1 The point prevalence of depression in medically ill patients is much higher, ranging from 20% to 50%, and the prevalence of milder depression is even more common. Despite this substantial prevalence, depression (especially in its milder forms) is rarely recognized. It often occurs insidiously, confusing its sufferer into believing that it is part of his or her character rather than an illness.
An invisible killer
The adverse effects of depression manifest in many aspects of life—from relationships to job performance to compliance with medical treatments—and can be so severe as to render the condition an “invisible killer.” The first evidence of this emerged in the medical literature in 1937 when Malzberg2 reported that patients with melancholia had a significantly higher death rate than the general population and that cardiac death occurred in more than 40% of those patients. Although it took another several decades for the field to accelerate, ample data have now been gathered to prove an unshakable association between depression and progression of cardiac diseases. Instead of reviewing results of each study, I will present the results of several meta-analyses.
Prognosis of post–myocardial infarction patients with depression
In a meta-analysis published in 2004, van Melle et al3 examined data derived from the MEDLINE, EMBASE, and PsycINFO databases between 1975 and 2003 on the prognostic association of post–myocardial infarction (MI) depression with mortality and cardiovascular events. Twenty-two studies met the selection criteria (post-MI status with measurement of depression and up to 2 years of follow-up); these studies included a total 6,367 post-MI patients and had an average follow-up of 13.7 months. The analysis revealed that post-MI depression was associated with each of the following:
- All-cause mortality (fixed-effects odds ratio [OR] = 2.38; 95% confidence interval [CI], 1.76 to 3.22; P < .00001)
- Cardiac mortality (fixed-effects OR = 2.59; 95% CI, 1.77 to 3.77; P < .00001)
- Occurrence of cardiovascular events (random-effects OR = 1.95; 95% CI, 1.33 to 2.85; P = .0006).
Prognosis of depressed patients with ischemic heart disease
In another 2004 meta-analysis, Barth et al4 examined the association of depression with mortality among patients with other forms of ischemic heart disease (IHD) (ie, beyond just MI) using data derived from English- and German-language databases (MEDLINE, PsycINFO, and PSYNDEX) from 1980 to 2003. A total of 11,905 patients from 20 cohorts were included. Although depression assessment was heterogeneous among the studies included, the unfavorable impact of depression on mortality among IHD patients was consistently observed regardless of whether the depression was self-reported or detected by psychiatric professionals. The risk of dying in the first 2 years after initial assessment was more than two times higher in patients with high depressive symptoms than in those with low depressive symptoms (OR = 2.24; 95% CI, 1.37 to 3.60). This negative prognostic impact remained over the long term and after adjustment for other risk factors (hazard ratio [HR] = 1.76; 95% CI, 1.27 to 2.43). Although clinical depression had no significant effect on mortality within the first 6 months after initial assessment (OR = 2.07; 95% CI, 0.82 to 5.26), after 2 years it was associated with a greater than twofold higher risk of death (OR = 2.61; 95% CI, 1.53 to 4.47).4
Prognosis of depressed patients with heart failure
Several studies over the past decade, including one from my research group,5 have prospectively examined the impact of depression on outcomes in patients with heart failure (HF). Rutledge et al6 used meta-analysis to summarize the findings of eight independent cohort studies that tracked the association between depression and mortality or cardiac events in a total of 1,845 patients with HF; follow-up ranged from 6 months to more than 4 years. They found that those patients who were depressed had higher rates of death and secondary events (relative risk [RR] = 2.1; 95% CI, 1.7 to 2.6) compared with their nondepressed counterparts, as well as trends toward increased health care use and higher rates of hospitalization and emergency room visitation.
Development of ischemic heart disease in depressed patients
To assess depression’s role as a potential predictor of IHD development, Rugulies7 reviewed data from MEDLINE (1966 to 2000) and PsycINFO (1887 to 2000), selecting 11 cohort studies based on assessment of patients by standardized psychometric scale (clinical depression or depressed symptoms) and “hard” events (fatal/nonfatal MI, coronary death, or cardiac death). Among the 36,549 individuals in these studies, the overall RR for development of IHD in depressed subjects (as compared with nondepressed subjects) was 1.64 (95% CI, 1.29 to 2.08; P < .001). Sensitivity analysis revealed that clinical depression was a stronger predictor of IHD (RR = 2.69; 95% CI, 1.63 to 4.43; P < .001) than depressive symptoms were (RR = 1.49; 95% CI = 1.16 to 1.92; P = .02).
In summary, individuals with depressive disorder, even mild forms, are more likely to develop IHD than are individuals without depression. The increased likelihood of developing IHD is independent of conventional risk factors. Therefore, depression is a primary risk factor for IHD. Depression is also a secondary risk factor, independent of conventional risk factors, for significantly worse prognosis in patients with MI, other forms of IHD, and HF. Depression’s adverse effect on HF prognosis is independent of the baseline impairment in cardiac function and of the ischemic etiology of HF.
DEPRESSION AND MENTAL STRESS–INDUCED MYOCARDIAL ISCHEMIA
Of the numerous proposed pathophysiologic mechanisms explaining the adverse impact of depression on cardiac diseases, I would like to emphasize the clinical and research significance of mental stress–induced myocardial ischemia (MSIMI).
Myocardial ischemia is an important measure of the clinical manifestation of IHD. Ambulatory electrocardiographic monitoring yielded the insight that myocardial ischemia occurs frequently and transiently during daily living; it usually occurs in the context of a lower heart rate, is asymptomatic or silent, does not necessarily involve high-intensity physical activity, and commonly occurs in conjunction with increased negative emotions.8,9
Over the past 2 to 3 decades, several laboratories have consistently demonstrated that mental stress testing elicits myocardial ischemia in patients with documented IHD.8,10,11 The prevalence of MSIMI, defined by wall motion abnormality and/or significantly reduced ejection fraction, is comparable to that of exercise-induced myocardial ischemia in the laboratory setting.12
Differences from exercise-induced ischemia
MSIMI differs from exercise-induced ischemia in several notable ways. It occurs silently most of the time and rarely results in ischemic electrocardiographic changes. Mental stress induces greater frequency and severity of left ventricular dysfunction. Furthermore, mental stress testing causes a greater diastolic blood pressure response but a modest increase in heart rate, whereas exercise testing elicits a smaller elevation in diastolic blood pressure but a several-fold increase in heart rate.
A key mechanism: Transient coronary vasoconstriction
One of the underlying mechanisms by which mental stress induces myocardial ischemia in susceptible patients is transient coronary vasoconstriction. Yueng et al13 used an intracoronary Doppler catheter to assess the change in coronary blood flow during mental stress testing and endothelium-dependent vasodilation in a group of patients with IHD. Coronary artery responses varied from 38% constriction to 29% dilation, with changes in coronary blood flow ranging from a decrease of 48% to an increase of 42%. Interestingly, although it has been proposed that mental stress triggers release of catecholamines that induce coronary vasoconstriction, the direction and magnitude of the change were not predicted by changes in heart rate, blood pressure, or plasma norepinephrine level. The change in coronary perfusion was correlated, however, with the response to acetylcholine infusion.13
Dakak et al14 showed that while the coronary microcirculation dilated during mental stress testing in individuals without IHD, it failed to dilate during such testing in IHD patients, a response that is likely mediated by alpha-adrenergic receptor activation. Furthermore, systemic vascular resistance has been found to increase significantly during mental stress and to be positively correlated with increases in plasma epinephrine.15 In contrast, systemic vascular resistance was reduced significantly during exercise testing, and there was no relationship between the exercise-induced hemodynamic change and the plasma epinephrine level.15 Compared with exercise-induced ischemia, epinephrine-induced ischemia (which may occur during emotional distress) is marked by smaller increases in heart rate and rate-pressure product and by a marked increase in contractility.16
MSIMI predicts cardiac events
From a prognostic standpoint, MSIMI consistently predicts an increase in future adverse cardiac events.11,17–19 In a sample of 132 IHD patients with a recent positive exercise test,11 MSIMI was associated with an increase in cardiac events during 5-year follow-up (OR = 2.8; 95% CI, 1.0 to 7.7; P < .05) independent of patients’ age, history of prior MI, or baseline cardiac function. In contrast, exercise-induced ischemia was not predictive for adverse cardiac events (OR = 1.5; 95% CI, 0.6 to 3.9; P = .39) in this same sample.
Depression correlates with MSIMI occurrence
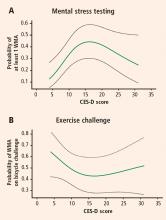
The mean CES-D score was 8.2 (SD = 7.4; range, 0 to 47) and the median score was 7. Logistic regression models using restricted cubic splines revealed a curvilinear relation between CES-D scores and the probability of ischemia triggered by mental stress testing and exercise testing. For patients with CES-D scores less than or equal to 19 (81.5% of the study population), a 5-point increment in the CES-D score was associated with a roughly twofold increase in the likelihood of MSIMI (Figure 1A). For patients with CES-D scores greater than 19, the relation between scores and ischemia during mental stress tended to be inverse (Figure 1A), but these patients represented a small portion of the study sample (18.5%). In contrast, depression was not related to the occurrence of exercise-induced ischemia (Figure 1B). This finding strongly indicates that MSIMI may be a significant mechanism by which depression increases the risk of mortality and morbidity in patients with IHD. A few patients in this study had severe depressive symptoms (CES-D scores > 19), which makes interpretation of the result very difficult. Because only 18.5% of the patients had CES-D scores greater than 19, this pattern of results needs to be confirmed in a sample with a greater representation of these more severely depressed patients.20
INSIGHTS FROM STUDIES OF DEPRESSION THERAPY IN CARDIAC PATIENTS
Among antidepressants, selective serotonin reuptake inhibitors (SSRIs) have been uniformly demonstrated to be effective in improving depressive symptoms and relatively safe for cardiac patients.21–24 Not surprisingly, tricyclic antidepressants have been found to cause more cardiac problems.21 Mirtazapine, a central nervous system alpha-2 antagonist, failed to improve depressive symptoms in depressed post-MI patients in the Myocardial Infarction and Depression Intervention Trial (MIND-IT),25,26 but because the results from this study have been presented only in abstract form, more details will be necessary to gain insight into explanations for this failure.
Although psychotherapy has been found to be quite effective among depressed patients without other medical illnesses, its effectiveness among patients with cardiac disease has not been impressive to date (Table 1).
No evidence of prognostic benefit from psychotherapy
Results from evaluations of psychotherapeutic interventions on cardiac prognosis have been rather disappointing (Table 1). The Enhancing Recovery In Coronary Heart Disease Patients (ENRICHD) study,27 which involved randomization of 2,481 post-MI patients with depression and/or low perceived social support to usual care or cognitive behavior therapy, failed to show an impact of cognitive behavior therapy on the combined end point of death or nonfatal MI. Similarly, the Montreal Heart Attack Readjustment Trial (M-HART)28 failed to demonstrate a benefit from home-based psychosocial nursing intervention on cardiac prognosis in IHD patients. These studies suggested that psychotherapeutic intervention might have differing or even opposite effects on the two genders.
Potential prognostic benefit from antidepressant therapy
In theory, adequate treatment of depression could affect dysregulated physiologic factors as well as dysregulated psychosocial factors, thereby leading to improved cardiac outcomes. There is physiologic evidence to support beneficial pleiotropic effects of antidepressant medications in IHD, such as reduced platelet activity29–31 and improvement in low heart rate variability32–34 with both sertraline and paroxetine.
The MIND-IT study evaluated mirtazapine for post-MI depression using a randomized placebo-controlled design.25 However, this trial failed to find a significant treatment effect for either depression or cardiac outcomes.26 These results may have been related to a lack of statistical power, as only 209 treated patients were compared with 122 patients receiving usual care. This trial also raises the question whether any nontricyclic antidepressant (other than SSRIs) might have beneficial effects on cardiovascular outcomes, or whether such an effect might be limited to SSRIs alone.
Provocative results emerged from the Sertraline Antidepressant Heart Attack Randomized Trial (SADHART),23 a randomized, double-blind, placebo-controlled investigation of the safety and efficacy of sertraline for major depressive disorder among 369 patients with recent MI or unstable angina. Patients receiving sertraline had fewer cardiac events (death, MI, stroke, worsened angina, or onset of HF) compared with patients taking placebo. The relative risk ratio for having at least one cardiac event was 0.77 with sertraline therapy, but this reduction in risk was not statistically significant (95% CI, 0.51 to 1.16). Although these findings suggest that sertraline may improve cardiac outcomes, the study was not adequately powered to detect differences on this measure. Power calculations indicate that in order to confirm a 20% reduction in relative risk in a randomized trial, a sample of at least 4,000 depressed patients with acute coronary syndrome would be required.23 Based on the cost of SADHART, the estimated expense to complete such a study is approximately $200 million.
The SADHART-CHF trial is a randomized, double-blind, placebo-controlled study examining sertraline’s efficacy for major depressive disorder among patients with HF, as well as its effects on mortality and cardiac outcomes. This trial is in its last year of enrollment, and results will be forthcoming in 2008.
FUTURE DIRECTIONS
These recent insights into depression’s impact on cardiac disease give rise to several new questions to consider:
- Expand research to patients with depressive symptoms? To date, investigations into treatment effects have focused only on patients with cardiac disease who have major depressive disorder. However, depressive symptoms as reported on self-administered questionnaires consistently have been shown to be a risk for poor cardiac outcomes. Should we expand our interventional studies to patients with self-reported depressive symptoms?
- How thoroughly to test for differences among antidepressants? Three of the six SSRIs have been studied among depressed cardiac patients. Based on the available findings, can we assume that all SSRIs have the same efficacy and safety profiles and are similarly cardiovascularly protective? Should every antidepressant or SSRI be tested? Should head-to-head comparison studies be conducted? Tricyclic antidepressants are cardiotoxic, and central nervous system alpha-2 antagonists like mirtazapine may not be effective, but what about other types of antidepressants for depressed cardiac patients?
- Is there a role for studying surrogate end points? Studies examining the effects of an intervention on mortality and/or morbidity can be very expensive. As research budgets tighten, can we instead test the effects of depression therapy on some surrogate end points?
Our laboratory has been funded by the National Heart, Lung, and Blood Institute to compare the effects of escitalopram with those of placebo on MSIMI in patients with stable IHD and a score of 5 or greater on the Beck Depression Inventory. This study, the Responses of Myocardial Ischemia to Escitalopram Treatment (REMIT) trial, will provide SSRI therapy to patients with a broad spectrum of depressive symptoms (not just major depressive disorder), assess the ischemic activity induced by mental stress testing as its primary end point, and explore the effects on other hypothesized mechanisms of depression that adversely affect cardiac diseases (platelet aggregation, inflammatory biomarkers, etc). Stay tuned for the results in the near future.
- Kessler RC, Berglund P, Demler O, et al. The epidemiology of major depressive disorder: results from the National Comorbidity Survey Replication (NCS-R). JAMA 2003; 289:3095–3105.
- Malzberg B. Mortality among patients with involutional melancholia. Am J Psychiatry 1937; 93:1231–1238.
- van Melle JP, de Jonge P, Spijkerman TA, et al. Prognostic association of depression following myocardial infarction with mortality and cardiovascular events: a meta-analysis. Psychosom Med 2004; 66:814–822.
- Barth J, Schumacher M, Herrmann-Lingen C. Depression as a risk factor for mortality in patients with coronary heart disease: a meta-analysis. Psychosom Med 2004; 66:802–813.
- Jiang W, Alexander J, Christopher E, et al. Relationship of depression to increased risk of mortality and rehospitalization in patients with congestive heart failure. Arch Intern Med 2001; 161:1849–1856.
- Rutledge T, Reis VA, Linke SE, Greenberg BH, Mills PJ. Depression in heart failure: a meta-analytic review of prevalence, intervention effects, and associations with clinical outcomes. J Am Coll Cardiol 2006; 48:1527–1537.
- Rugulies R. Depression as a predictor for coronary heart disease: a review and meta-analysis. Am J Prev Med 2002; 23:51–61.
- Deanfield JE, Shea M, Ribiero P, et al. Transient ST-segment depression as a marker of myocardial ischemia during daily life. Am J Cardiol 1984; 54:1195–1200.
- Gullette ECD, Blumenthal JA, Babyak M, et al. Effects of mental stress on myocardial ischemia during daily life. JAMA 1997; 277:1521–1526.
- Rozanski A, Bairey CN, Krantz DS, et al. Mental stress and the induction of myocardial ischemia in patients with ischemic heart disease. N Engl J Med 1988; 318:1005–1011.
- Jiang W, Babyak M, Krantz DS, et al. Mental stress–induced myocardial ischemia and cardiac events. JAMA 1996; 275:1651–1656.
- Blumenthal JA, Jiang W, Waugh RA, et al. Mental stress-induced ischemia in the laboratory and ambulatory ischemia during daily life. Association and hemodynamic features. Circulation 1995; 92:2102–2108.
- Yeung AC, Vekshtein VI, Krantz DS, et al. The effect of atherosclerosis on the vasomotor response of coronary arteries to mental stress. N Engl J Med 1991; 325:1551–1556.
- Dakak N, Quyyumi AA, Eisenhofer G, Goldstein DS, Cannon RO. Sympathetically mediated effects of mental stress on the cardiac microcirculation of patients with coronary artery disease Am J Cardiol 1995; 76:125–130.
- Goldberg AD, Becker LC, Bonsall R, et al. Ischemic, hemodynamic, and neurohormonal responses to mental and exercise stress. Experience from the Psychophysiological Investigations of Myocardial Ischemia Study (PIMI). Circulation 1996; 94:2402–2409.
- Sung BH, Wilson MF, Robinson C, et al. Mechanisms of myocardial ischemia induced by epinephrine: comparison with exercise-induced ischemia. Psychosom Med 1988; 4:381–393.
- Specchia G, Falcone C, Traversi E, et al. Mental stress as a provocative test in patients with various clinical syndromes of coronary heart disease. Circulation 1991; 83(Suppl 4):II108–II114.
- Krantz DS, Santiago HT, Kop WJ, Bairey Merz CN, Rozanski A, Gottdiener JS. Prognostic value of mental stress testing in coronary artery disease. Am J Cardiol 1999; 84:1292–1297.
- Sheps DS, McMahon RP, Becker L, et al. Mental stress-induced ischemia and all-cause mortality in patients with coronary artery eisease: results from the Psychopysiological Investigations of Myocardial Ischemia Study. Circulation 2002; 105:1780–1784.
- Jiang W, Babyak MA, Rozanski A, et al. Depression and increased myocardial ischemic activity in patients with ischemic heart disease. Am Heart J 2003; 146:55–61.
- Roose SP, Laghrissi-Thode F, Kennedy JS, et al. Comparison of paroxetine and nortriptyline in depressed patients with ischemic heart disease. JAMA 1998; 279:287–291.
- Nelson JC, Kennedy JS, Pollock BG, et al. Treatment of major depression with nortriptyline and paroxetine in patients with ischemic heart disease. Am J Psychiatry 1999; 156:1024–1028.
- Glassman AH, O’Connor CM, Califf RM, et al. Sertraline Antidepressant Heart Attack Randomized Trial (SADHART) Group. Sertraline treatment of major depression in patients with acute MI or unstable angina. JAMA 2002; 288:701–709.
- Lespérance F, Frasure-Smith N, Koszycki D, et al. Effects of citalopram and interpersonal psychotherapy on depression in patients with coronary artery disease: the Candaian Cardiac Randomized Evaluation of Antidepressant and Psychotherapy Efficacy (CREATE) trial. JAMA 2007; 297:367–379.
- van den Brink RH, Van Melle JP, Honig A, et al. Treatment of depression after myocardial infarction and the effects on cardiac prognosis and quality of life: rationale and outline of the Myocardial INfarction and Depression-Intervention Trial (MIND-IT). Am Heart J 2002; 144:219–225.
- De Jonge P, Hong A, Schene AH, et al. Effects of antidepressive therapy for the treatment of depression following myocardial infarction: results from the Myocardial Infarction and Depression Intervention Trial (MIND-IT) [abstract]. Psychosom Med 2006; 68:A-7.
- Berkman LF, Blumenthal J, Burg M, et al; ENRICHD investigators. Effects of treating depression and low perceived social support on clinical events after myocardial infarction: the Enhancing Recovery in Coronary Heart Disease Patients (ENRICHD) Randomized Trial. JAMA 2003; 289:3106–3116.
- Frasure-Smith N, Lespérance F, Prince RH, et al. Randomised trial of home-based psychosocial nursing intervention for patients recovering from myocardial infarction. Lancet 1997; 350:473–479.
- Pollock BG, Laghrissi-Thode F, Wagner WR. Evaluation of platelet activation in depressed patients with ischemic heart disease after paroxetine or nortriptyline treatment. J Clin Psychopharmacol 2000; 20:137–140.
- Musselman DL, Marzec UM, Manatunga A, et al. Platelet reactivity in depressed patients treated with paroxetine: preliminary findings. Arch Gen Psychiatry 2000; 57:875–882.
- Serebruany VL, Glassman AH, Malinin AI, et al. Platelet/endothelial biomarkers in depressed patients treated with the selective serotonin reuptake inhibitor sertraline after acute coronary events: the Sertraline AntiDepressant Heart Attack Randomized Trial (SADHART) Platelet Substudy. Circulation 2003; 108:939–944.
- Yeragani VK, Pesce V, Jayaraman A, et al. Major depression with ischemic heart disease: effects of paroxetine and nortriptyline on long-term heart rate variability measures. Biol Psychiatry 2002; 52:418–429.
- Yeragani VK, Roose S, Mallavarapu M, et al. Major depression with ischemic heart disease: effects of paroxetine and nortriptyline on measures of nonlinearity and chaos of heart rate. Neuropsychobiology 2002; 46:125–135.
- Rechlin T. The effects of psychopharmacological therapy on heart rate variation. Nervenarzt 1995; 66:678–685.
Over the past several decades, a large body of evidence has emerged demonstrating the adverse impact of depressive disorder on heart disease. This evidence confirms the early suspicion of observant clinicians that psychological factors play a significant role in the genesis and course of heart disease, as well as confirming the ancient belief in a mind-body connection in general and a connection between human moods and the heart in particular. Given the high prevalence of these two disorders, we need a better understanding of the impact of depressive disorder on heart disease, the proposed underlying pathophysiologic mechanisms, and the effects of treating depression in relation to risk reduction in patients with heart disease.
In this article, I will focus on (1) reviewing the results of meta-analyses examining the association of depression with cardiac diseases, (2) discussing the relationship between depression and mental stress–induced myocardial ischemia, (3) reviewing the available studies of the treatment of depression in patients with cardiac disease, and (4) discussing future directions for research in this area.
ASSOCIATION OF DEPRESSION WITH PROGRESSION OF CARDIAC DISEASES
As a disease of the brain, depression is common. The lifetime prevalence of major depressive disorder, a significant form of depression, is 16.2%.1 The point prevalence of depression in medically ill patients is much higher, ranging from 20% to 50%, and the prevalence of milder depression is even more common. Despite this substantial prevalence, depression (especially in its milder forms) is rarely recognized. It often occurs insidiously, confusing its sufferer into believing that it is part of his or her character rather than an illness.
An invisible killer
The adverse effects of depression manifest in many aspects of life—from relationships to job performance to compliance with medical treatments—and can be so severe as to render the condition an “invisible killer.” The first evidence of this emerged in the medical literature in 1937 when Malzberg2 reported that patients with melancholia had a significantly higher death rate than the general population and that cardiac death occurred in more than 40% of those patients. Although it took another several decades for the field to accelerate, ample data have now been gathered to prove an unshakable association between depression and progression of cardiac diseases. Instead of reviewing results of each study, I will present the results of several meta-analyses.
Prognosis of post–myocardial infarction patients with depression
In a meta-analysis published in 2004, van Melle et al3 examined data derived from the MEDLINE, EMBASE, and PsycINFO databases between 1975 and 2003 on the prognostic association of post–myocardial infarction (MI) depression with mortality and cardiovascular events. Twenty-two studies met the selection criteria (post-MI status with measurement of depression and up to 2 years of follow-up); these studies included a total 6,367 post-MI patients and had an average follow-up of 13.7 months. The analysis revealed that post-MI depression was associated with each of the following:
- All-cause mortality (fixed-effects odds ratio [OR] = 2.38; 95% confidence interval [CI], 1.76 to 3.22; P < .00001)
- Cardiac mortality (fixed-effects OR = 2.59; 95% CI, 1.77 to 3.77; P < .00001)
- Occurrence of cardiovascular events (random-effects OR = 1.95; 95% CI, 1.33 to 2.85; P = .0006).
Prognosis of depressed patients with ischemic heart disease
In another 2004 meta-analysis, Barth et al4 examined the association of depression with mortality among patients with other forms of ischemic heart disease (IHD) (ie, beyond just MI) using data derived from English- and German-language databases (MEDLINE, PsycINFO, and PSYNDEX) from 1980 to 2003. A total of 11,905 patients from 20 cohorts were included. Although depression assessment was heterogeneous among the studies included, the unfavorable impact of depression on mortality among IHD patients was consistently observed regardless of whether the depression was self-reported or detected by psychiatric professionals. The risk of dying in the first 2 years after initial assessment was more than two times higher in patients with high depressive symptoms than in those with low depressive symptoms (OR = 2.24; 95% CI, 1.37 to 3.60). This negative prognostic impact remained over the long term and after adjustment for other risk factors (hazard ratio [HR] = 1.76; 95% CI, 1.27 to 2.43). Although clinical depression had no significant effect on mortality within the first 6 months after initial assessment (OR = 2.07; 95% CI, 0.82 to 5.26), after 2 years it was associated with a greater than twofold higher risk of death (OR = 2.61; 95% CI, 1.53 to 4.47).4
Prognosis of depressed patients with heart failure
Several studies over the past decade, including one from my research group,5 have prospectively examined the impact of depression on outcomes in patients with heart failure (HF). Rutledge et al6 used meta-analysis to summarize the findings of eight independent cohort studies that tracked the association between depression and mortality or cardiac events in a total of 1,845 patients with HF; follow-up ranged from 6 months to more than 4 years. They found that those patients who were depressed had higher rates of death and secondary events (relative risk [RR] = 2.1; 95% CI, 1.7 to 2.6) compared with their nondepressed counterparts, as well as trends toward increased health care use and higher rates of hospitalization and emergency room visitation.
Development of ischemic heart disease in depressed patients
To assess depression’s role as a potential predictor of IHD development, Rugulies7 reviewed data from MEDLINE (1966 to 2000) and PsycINFO (1887 to 2000), selecting 11 cohort studies based on assessment of patients by standardized psychometric scale (clinical depression or depressed symptoms) and “hard” events (fatal/nonfatal MI, coronary death, or cardiac death). Among the 36,549 individuals in these studies, the overall RR for development of IHD in depressed subjects (as compared with nondepressed subjects) was 1.64 (95% CI, 1.29 to 2.08; P < .001). Sensitivity analysis revealed that clinical depression was a stronger predictor of IHD (RR = 2.69; 95% CI, 1.63 to 4.43; P < .001) than depressive symptoms were (RR = 1.49; 95% CI = 1.16 to 1.92; P = .02).
In summary, individuals with depressive disorder, even mild forms, are more likely to develop IHD than are individuals without depression. The increased likelihood of developing IHD is independent of conventional risk factors. Therefore, depression is a primary risk factor for IHD. Depression is also a secondary risk factor, independent of conventional risk factors, for significantly worse prognosis in patients with MI, other forms of IHD, and HF. Depression’s adverse effect on HF prognosis is independent of the baseline impairment in cardiac function and of the ischemic etiology of HF.
DEPRESSION AND MENTAL STRESS–INDUCED MYOCARDIAL ISCHEMIA
Of the numerous proposed pathophysiologic mechanisms explaining the adverse impact of depression on cardiac diseases, I would like to emphasize the clinical and research significance of mental stress–induced myocardial ischemia (MSIMI).
Myocardial ischemia is an important measure of the clinical manifestation of IHD. Ambulatory electrocardiographic monitoring yielded the insight that myocardial ischemia occurs frequently and transiently during daily living; it usually occurs in the context of a lower heart rate, is asymptomatic or silent, does not necessarily involve high-intensity physical activity, and commonly occurs in conjunction with increased negative emotions.8,9
Over the past 2 to 3 decades, several laboratories have consistently demonstrated that mental stress testing elicits myocardial ischemia in patients with documented IHD.8,10,11 The prevalence of MSIMI, defined by wall motion abnormality and/or significantly reduced ejection fraction, is comparable to that of exercise-induced myocardial ischemia in the laboratory setting.12
Differences from exercise-induced ischemia
MSIMI differs from exercise-induced ischemia in several notable ways. It occurs silently most of the time and rarely results in ischemic electrocardiographic changes. Mental stress induces greater frequency and severity of left ventricular dysfunction. Furthermore, mental stress testing causes a greater diastolic blood pressure response but a modest increase in heart rate, whereas exercise testing elicits a smaller elevation in diastolic blood pressure but a several-fold increase in heart rate.
A key mechanism: Transient coronary vasoconstriction
One of the underlying mechanisms by which mental stress induces myocardial ischemia in susceptible patients is transient coronary vasoconstriction. Yueng et al13 used an intracoronary Doppler catheter to assess the change in coronary blood flow during mental stress testing and endothelium-dependent vasodilation in a group of patients with IHD. Coronary artery responses varied from 38% constriction to 29% dilation, with changes in coronary blood flow ranging from a decrease of 48% to an increase of 42%. Interestingly, although it has been proposed that mental stress triggers release of catecholamines that induce coronary vasoconstriction, the direction and magnitude of the change were not predicted by changes in heart rate, blood pressure, or plasma norepinephrine level. The change in coronary perfusion was correlated, however, with the response to acetylcholine infusion.13
Dakak et al14 showed that while the coronary microcirculation dilated during mental stress testing in individuals without IHD, it failed to dilate during such testing in IHD patients, a response that is likely mediated by alpha-adrenergic receptor activation. Furthermore, systemic vascular resistance has been found to increase significantly during mental stress and to be positively correlated with increases in plasma epinephrine.15 In contrast, systemic vascular resistance was reduced significantly during exercise testing, and there was no relationship between the exercise-induced hemodynamic change and the plasma epinephrine level.15 Compared with exercise-induced ischemia, epinephrine-induced ischemia (which may occur during emotional distress) is marked by smaller increases in heart rate and rate-pressure product and by a marked increase in contractility.16
MSIMI predicts cardiac events
From a prognostic standpoint, MSIMI consistently predicts an increase in future adverse cardiac events.11,17–19 In a sample of 132 IHD patients with a recent positive exercise test,11 MSIMI was associated with an increase in cardiac events during 5-year follow-up (OR = 2.8; 95% CI, 1.0 to 7.7; P < .05) independent of patients’ age, history of prior MI, or baseline cardiac function. In contrast, exercise-induced ischemia was not predictive for adverse cardiac events (OR = 1.5; 95% CI, 0.6 to 3.9; P = .39) in this same sample.
Depression correlates with MSIMI occurrence
The mean CES-D score was 8.2 (SD = 7.4; range, 0 to 47) and the median score was 7. Logistic regression models using restricted cubic splines revealed a curvilinear relation between CES-D scores and the probability of ischemia triggered by mental stress testing and exercise testing. For patients with CES-D scores less than or equal to 19 (81.5% of the study population), a 5-point increment in the CES-D score was associated with a roughly twofold increase in the likelihood of MSIMI (Figure 1A). For patients with CES-D scores greater than 19, the relation between scores and ischemia during mental stress tended to be inverse (Figure 1A), but these patients represented a small portion of the study sample (18.5%). In contrast, depression was not related to the occurrence of exercise-induced ischemia (Figure 1B). This finding strongly indicates that MSIMI may be a significant mechanism by which depression increases the risk of mortality and morbidity in patients with IHD. A few patients in this study had severe depressive symptoms (CES-D scores > 19), which makes interpretation of the result very difficult. Because only 18.5% of the patients had CES-D scores greater than 19, this pattern of results needs to be confirmed in a sample with a greater representation of these more severely depressed patients.20
INSIGHTS FROM STUDIES OF DEPRESSION THERAPY IN CARDIAC PATIENTS
Among antidepressants, selective serotonin reuptake inhibitors (SSRIs) have been uniformly demonstrated to be effective in improving depressive symptoms and relatively safe for cardiac patients.21–24 Not surprisingly, tricyclic antidepressants have been found to cause more cardiac problems.21 Mirtazapine, a central nervous system alpha-2 antagonist, failed to improve depressive symptoms in depressed post-MI patients in the Myocardial Infarction and Depression Intervention Trial (MIND-IT),25,26 but because the results from this study have been presented only in abstract form, more details will be necessary to gain insight into explanations for this failure.
Although psychotherapy has been found to be quite effective among depressed patients without other medical illnesses, its effectiveness among patients with cardiac disease has not been impressive to date (Table 1).
No evidence of prognostic benefit from psychotherapy
Results from evaluations of psychotherapeutic interventions on cardiac prognosis have been rather disappointing (Table 1). The Enhancing Recovery In Coronary Heart Disease Patients (ENRICHD) study,27 which involved randomization of 2,481 post-MI patients with depression and/or low perceived social support to usual care or cognitive behavior therapy, failed to show an impact of cognitive behavior therapy on the combined end point of death or nonfatal MI. Similarly, the Montreal Heart Attack Readjustment Trial (M-HART)28 failed to demonstrate a benefit from home-based psychosocial nursing intervention on cardiac prognosis in IHD patients. These studies suggested that psychotherapeutic intervention might have differing or even opposite effects on the two genders.
Potential prognostic benefit from antidepressant therapy
In theory, adequate treatment of depression could affect dysregulated physiologic factors as well as dysregulated psychosocial factors, thereby leading to improved cardiac outcomes. There is physiologic evidence to support beneficial pleiotropic effects of antidepressant medications in IHD, such as reduced platelet activity29–31 and improvement in low heart rate variability32–34 with both sertraline and paroxetine.
The MIND-IT study evaluated mirtazapine for post-MI depression using a randomized placebo-controlled design.25 However, this trial failed to find a significant treatment effect for either depression or cardiac outcomes.26 These results may have been related to a lack of statistical power, as only 209 treated patients were compared with 122 patients receiving usual care. This trial also raises the question whether any nontricyclic antidepressant (other than SSRIs) might have beneficial effects on cardiovascular outcomes, or whether such an effect might be limited to SSRIs alone.
Provocative results emerged from the Sertraline Antidepressant Heart Attack Randomized Trial (SADHART),23 a randomized, double-blind, placebo-controlled investigation of the safety and efficacy of sertraline for major depressive disorder among 369 patients with recent MI or unstable angina. Patients receiving sertraline had fewer cardiac events (death, MI, stroke, worsened angina, or onset of HF) compared with patients taking placebo. The relative risk ratio for having at least one cardiac event was 0.77 with sertraline therapy, but this reduction in risk was not statistically significant (95% CI, 0.51 to 1.16). Although these findings suggest that sertraline may improve cardiac outcomes, the study was not adequately powered to detect differences on this measure. Power calculations indicate that in order to confirm a 20% reduction in relative risk in a randomized trial, a sample of at least 4,000 depressed patients with acute coronary syndrome would be required.23 Based on the cost of SADHART, the estimated expense to complete such a study is approximately $200 million.
The SADHART-CHF trial is a randomized, double-blind, placebo-controlled study examining sertraline’s efficacy for major depressive disorder among patients with HF, as well as its effects on mortality and cardiac outcomes. This trial is in its last year of enrollment, and results will be forthcoming in 2008.
FUTURE DIRECTIONS
These recent insights into depression’s impact on cardiac disease give rise to several new questions to consider:
- Expand research to patients with depressive symptoms? To date, investigations into treatment effects have focused only on patients with cardiac disease who have major depressive disorder. However, depressive symptoms as reported on self-administered questionnaires consistently have been shown to be a risk for poor cardiac outcomes. Should we expand our interventional studies to patients with self-reported depressive symptoms?
- How thoroughly to test for differences among antidepressants? Three of the six SSRIs have been studied among depressed cardiac patients. Based on the available findings, can we assume that all SSRIs have the same efficacy and safety profiles and are similarly cardiovascularly protective? Should every antidepressant or SSRI be tested? Should head-to-head comparison studies be conducted? Tricyclic antidepressants are cardiotoxic, and central nervous system alpha-2 antagonists like mirtazapine may not be effective, but what about other types of antidepressants for depressed cardiac patients?
- Is there a role for studying surrogate end points? Studies examining the effects of an intervention on mortality and/or morbidity can be very expensive. As research budgets tighten, can we instead test the effects of depression therapy on some surrogate end points?
Our laboratory has been funded by the National Heart, Lung, and Blood Institute to compare the effects of escitalopram with those of placebo on MSIMI in patients with stable IHD and a score of 5 or greater on the Beck Depression Inventory. This study, the Responses of Myocardial Ischemia to Escitalopram Treatment (REMIT) trial, will provide SSRI therapy to patients with a broad spectrum of depressive symptoms (not just major depressive disorder), assess the ischemic activity induced by mental stress testing as its primary end point, and explore the effects on other hypothesized mechanisms of depression that adversely affect cardiac diseases (platelet aggregation, inflammatory biomarkers, etc). Stay tuned for the results in the near future.
Over the past several decades, a large body of evidence has emerged demonstrating the adverse impact of depressive disorder on heart disease. This evidence confirms the early suspicion of observant clinicians that psychological factors play a significant role in the genesis and course of heart disease, as well as confirming the ancient belief in a mind-body connection in general and a connection between human moods and the heart in particular. Given the high prevalence of these two disorders, we need a better understanding of the impact of depressive disorder on heart disease, the proposed underlying pathophysiologic mechanisms, and the effects of treating depression in relation to risk reduction in patients with heart disease.
In this article, I will focus on (1) reviewing the results of meta-analyses examining the association of depression with cardiac diseases, (2) discussing the relationship between depression and mental stress–induced myocardial ischemia, (3) reviewing the available studies of the treatment of depression in patients with cardiac disease, and (4) discussing future directions for research in this area.
ASSOCIATION OF DEPRESSION WITH PROGRESSION OF CARDIAC DISEASES
As a disease of the brain, depression is common. The lifetime prevalence of major depressive disorder, a significant form of depression, is 16.2%.1 The point prevalence of depression in medically ill patients is much higher, ranging from 20% to 50%, and the prevalence of milder depression is even more common. Despite this substantial prevalence, depression (especially in its milder forms) is rarely recognized. It often occurs insidiously, confusing its sufferer into believing that it is part of his or her character rather than an illness.
An invisible killer
The adverse effects of depression manifest in many aspects of life—from relationships to job performance to compliance with medical treatments—and can be so severe as to render the condition an “invisible killer.” The first evidence of this emerged in the medical literature in 1937 when Malzberg2 reported that patients with melancholia had a significantly higher death rate than the general population and that cardiac death occurred in more than 40% of those patients. Although it took another several decades for the field to accelerate, ample data have now been gathered to prove an unshakable association between depression and progression of cardiac diseases. Instead of reviewing results of each study, I will present the results of several meta-analyses.
Prognosis of post–myocardial infarction patients with depression
In a meta-analysis published in 2004, van Melle et al3 examined data derived from the MEDLINE, EMBASE, and PsycINFO databases between 1975 and 2003 on the prognostic association of post–myocardial infarction (MI) depression with mortality and cardiovascular events. Twenty-two studies met the selection criteria (post-MI status with measurement of depression and up to 2 years of follow-up); these studies included a total 6,367 post-MI patients and had an average follow-up of 13.7 months. The analysis revealed that post-MI depression was associated with each of the following:
- All-cause mortality (fixed-effects odds ratio [OR] = 2.38; 95% confidence interval [CI], 1.76 to 3.22; P < .00001)
- Cardiac mortality (fixed-effects OR = 2.59; 95% CI, 1.77 to 3.77; P < .00001)
- Occurrence of cardiovascular events (random-effects OR = 1.95; 95% CI, 1.33 to 2.85; P = .0006).
Prognosis of depressed patients with ischemic heart disease
In another 2004 meta-analysis, Barth et al4 examined the association of depression with mortality among patients with other forms of ischemic heart disease (IHD) (ie, beyond just MI) using data derived from English- and German-language databases (MEDLINE, PsycINFO, and PSYNDEX) from 1980 to 2003. A total of 11,905 patients from 20 cohorts were included. Although depression assessment was heterogeneous among the studies included, the unfavorable impact of depression on mortality among IHD patients was consistently observed regardless of whether the depression was self-reported or detected by psychiatric professionals. The risk of dying in the first 2 years after initial assessment was more than two times higher in patients with high depressive symptoms than in those with low depressive symptoms (OR = 2.24; 95% CI, 1.37 to 3.60). This negative prognostic impact remained over the long term and after adjustment for other risk factors (hazard ratio [HR] = 1.76; 95% CI, 1.27 to 2.43). Although clinical depression had no significant effect on mortality within the first 6 months after initial assessment (OR = 2.07; 95% CI, 0.82 to 5.26), after 2 years it was associated with a greater than twofold higher risk of death (OR = 2.61; 95% CI, 1.53 to 4.47).4
Prognosis of depressed patients with heart failure
Several studies over the past decade, including one from my research group,5 have prospectively examined the impact of depression on outcomes in patients with heart failure (HF). Rutledge et al6 used meta-analysis to summarize the findings of eight independent cohort studies that tracked the association between depression and mortality or cardiac events in a total of 1,845 patients with HF; follow-up ranged from 6 months to more than 4 years. They found that those patients who were depressed had higher rates of death and secondary events (relative risk [RR] = 2.1; 95% CI, 1.7 to 2.6) compared with their nondepressed counterparts, as well as trends toward increased health care use and higher rates of hospitalization and emergency room visitation.
Development of ischemic heart disease in depressed patients
To assess depression’s role as a potential predictor of IHD development, Rugulies7 reviewed data from MEDLINE (1966 to 2000) and PsycINFO (1887 to 2000), selecting 11 cohort studies based on assessment of patients by standardized psychometric scale (clinical depression or depressed symptoms) and “hard” events (fatal/nonfatal MI, coronary death, or cardiac death). Among the 36,549 individuals in these studies, the overall RR for development of IHD in depressed subjects (as compared with nondepressed subjects) was 1.64 (95% CI, 1.29 to 2.08; P < .001). Sensitivity analysis revealed that clinical depression was a stronger predictor of IHD (RR = 2.69; 95% CI, 1.63 to 4.43; P < .001) than depressive symptoms were (RR = 1.49; 95% CI = 1.16 to 1.92; P = .02).
In summary, individuals with depressive disorder, even mild forms, are more likely to develop IHD than are individuals without depression. The increased likelihood of developing IHD is independent of conventional risk factors. Therefore, depression is a primary risk factor for IHD. Depression is also a secondary risk factor, independent of conventional risk factors, for significantly worse prognosis in patients with MI, other forms of IHD, and HF. Depression’s adverse effect on HF prognosis is independent of the baseline impairment in cardiac function and of the ischemic etiology of HF.
DEPRESSION AND MENTAL STRESS–INDUCED MYOCARDIAL ISCHEMIA
Of the numerous proposed pathophysiologic mechanisms explaining the adverse impact of depression on cardiac diseases, I would like to emphasize the clinical and research significance of mental stress–induced myocardial ischemia (MSIMI).
Myocardial ischemia is an important measure of the clinical manifestation of IHD. Ambulatory electrocardiographic monitoring yielded the insight that myocardial ischemia occurs frequently and transiently during daily living; it usually occurs in the context of a lower heart rate, is asymptomatic or silent, does not necessarily involve high-intensity physical activity, and commonly occurs in conjunction with increased negative emotions.8,9
Over the past 2 to 3 decades, several laboratories have consistently demonstrated that mental stress testing elicits myocardial ischemia in patients with documented IHD.8,10,11 The prevalence of MSIMI, defined by wall motion abnormality and/or significantly reduced ejection fraction, is comparable to that of exercise-induced myocardial ischemia in the laboratory setting.12
Differences from exercise-induced ischemia
MSIMI differs from exercise-induced ischemia in several notable ways. It occurs silently most of the time and rarely results in ischemic electrocardiographic changes. Mental stress induces greater frequency and severity of left ventricular dysfunction. Furthermore, mental stress testing causes a greater diastolic blood pressure response but a modest increase in heart rate, whereas exercise testing elicits a smaller elevation in diastolic blood pressure but a several-fold increase in heart rate.
A key mechanism: Transient coronary vasoconstriction
One of the underlying mechanisms by which mental stress induces myocardial ischemia in susceptible patients is transient coronary vasoconstriction. Yueng et al13 used an intracoronary Doppler catheter to assess the change in coronary blood flow during mental stress testing and endothelium-dependent vasodilation in a group of patients with IHD. Coronary artery responses varied from 38% constriction to 29% dilation, with changes in coronary blood flow ranging from a decrease of 48% to an increase of 42%. Interestingly, although it has been proposed that mental stress triggers release of catecholamines that induce coronary vasoconstriction, the direction and magnitude of the change were not predicted by changes in heart rate, blood pressure, or plasma norepinephrine level. The change in coronary perfusion was correlated, however, with the response to acetylcholine infusion.13
Dakak et al14 showed that while the coronary microcirculation dilated during mental stress testing in individuals without IHD, it failed to dilate during such testing in IHD patients, a response that is likely mediated by alpha-adrenergic receptor activation. Furthermore, systemic vascular resistance has been found to increase significantly during mental stress and to be positively correlated with increases in plasma epinephrine.15 In contrast, systemic vascular resistance was reduced significantly during exercise testing, and there was no relationship between the exercise-induced hemodynamic change and the plasma epinephrine level.15 Compared with exercise-induced ischemia, epinephrine-induced ischemia (which may occur during emotional distress) is marked by smaller increases in heart rate and rate-pressure product and by a marked increase in contractility.16
MSIMI predicts cardiac events
From a prognostic standpoint, MSIMI consistently predicts an increase in future adverse cardiac events.11,17–19 In a sample of 132 IHD patients with a recent positive exercise test,11 MSIMI was associated with an increase in cardiac events during 5-year follow-up (OR = 2.8; 95% CI, 1.0 to 7.7; P < .05) independent of patients’ age, history of prior MI, or baseline cardiac function. In contrast, exercise-induced ischemia was not predictive for adverse cardiac events (OR = 1.5; 95% CI, 0.6 to 3.9; P = .39) in this same sample.
Depression correlates with MSIMI occurrence
The mean CES-D score was 8.2 (SD = 7.4; range, 0 to 47) and the median score was 7. Logistic regression models using restricted cubic splines revealed a curvilinear relation between CES-D scores and the probability of ischemia triggered by mental stress testing and exercise testing. For patients with CES-D scores less than or equal to 19 (81.5% of the study population), a 5-point increment in the CES-D score was associated with a roughly twofold increase in the likelihood of MSIMI (Figure 1A). For patients with CES-D scores greater than 19, the relation between scores and ischemia during mental stress tended to be inverse (Figure 1A), but these patients represented a small portion of the study sample (18.5%). In contrast, depression was not related to the occurrence of exercise-induced ischemia (Figure 1B). This finding strongly indicates that MSIMI may be a significant mechanism by which depression increases the risk of mortality and morbidity in patients with IHD. A few patients in this study had severe depressive symptoms (CES-D scores > 19), which makes interpretation of the result very difficult. Because only 18.5% of the patients had CES-D scores greater than 19, this pattern of results needs to be confirmed in a sample with a greater representation of these more severely depressed patients.20
INSIGHTS FROM STUDIES OF DEPRESSION THERAPY IN CARDIAC PATIENTS
Among antidepressants, selective serotonin reuptake inhibitors (SSRIs) have been uniformly demonstrated to be effective in improving depressive symptoms and relatively safe for cardiac patients.21–24 Not surprisingly, tricyclic antidepressants have been found to cause more cardiac problems.21 Mirtazapine, a central nervous system alpha-2 antagonist, failed to improve depressive symptoms in depressed post-MI patients in the Myocardial Infarction and Depression Intervention Trial (MIND-IT),25,26 but because the results from this study have been presented only in abstract form, more details will be necessary to gain insight into explanations for this failure.
Although psychotherapy has been found to be quite effective among depressed patients without other medical illnesses, its effectiveness among patients with cardiac disease has not been impressive to date (Table 1).
No evidence of prognostic benefit from psychotherapy
Results from evaluations of psychotherapeutic interventions on cardiac prognosis have been rather disappointing (Table 1). The Enhancing Recovery In Coronary Heart Disease Patients (ENRICHD) study,27 which involved randomization of 2,481 post-MI patients with depression and/or low perceived social support to usual care or cognitive behavior therapy, failed to show an impact of cognitive behavior therapy on the combined end point of death or nonfatal MI. Similarly, the Montreal Heart Attack Readjustment Trial (M-HART)28 failed to demonstrate a benefit from home-based psychosocial nursing intervention on cardiac prognosis in IHD patients. These studies suggested that psychotherapeutic intervention might have differing or even opposite effects on the two genders.
Potential prognostic benefit from antidepressant therapy
In theory, adequate treatment of depression could affect dysregulated physiologic factors as well as dysregulated psychosocial factors, thereby leading to improved cardiac outcomes. There is physiologic evidence to support beneficial pleiotropic effects of antidepressant medications in IHD, such as reduced platelet activity29–31 and improvement in low heart rate variability32–34 with both sertraline and paroxetine.
The MIND-IT study evaluated mirtazapine for post-MI depression using a randomized placebo-controlled design.25 However, this trial failed to find a significant treatment effect for either depression or cardiac outcomes.26 These results may have been related to a lack of statistical power, as only 209 treated patients were compared with 122 patients receiving usual care. This trial also raises the question whether any nontricyclic antidepressant (other than SSRIs) might have beneficial effects on cardiovascular outcomes, or whether such an effect might be limited to SSRIs alone.
Provocative results emerged from the Sertraline Antidepressant Heart Attack Randomized Trial (SADHART),23 a randomized, double-blind, placebo-controlled investigation of the safety and efficacy of sertraline for major depressive disorder among 369 patients with recent MI or unstable angina. Patients receiving sertraline had fewer cardiac events (death, MI, stroke, worsened angina, or onset of HF) compared with patients taking placebo. The relative risk ratio for having at least one cardiac event was 0.77 with sertraline therapy, but this reduction in risk was not statistically significant (95% CI, 0.51 to 1.16). Although these findings suggest that sertraline may improve cardiac outcomes, the study was not adequately powered to detect differences on this measure. Power calculations indicate that in order to confirm a 20% reduction in relative risk in a randomized trial, a sample of at least 4,000 depressed patients with acute coronary syndrome would be required.23 Based on the cost of SADHART, the estimated expense to complete such a study is approximately $200 million.
The SADHART-CHF trial is a randomized, double-blind, placebo-controlled study examining sertraline’s efficacy for major depressive disorder among patients with HF, as well as its effects on mortality and cardiac outcomes. This trial is in its last year of enrollment, and results will be forthcoming in 2008.
FUTURE DIRECTIONS
These recent insights into depression’s impact on cardiac disease give rise to several new questions to consider:
- Expand research to patients with depressive symptoms? To date, investigations into treatment effects have focused only on patients with cardiac disease who have major depressive disorder. However, depressive symptoms as reported on self-administered questionnaires consistently have been shown to be a risk for poor cardiac outcomes. Should we expand our interventional studies to patients with self-reported depressive symptoms?
- How thoroughly to test for differences among antidepressants? Three of the six SSRIs have been studied among depressed cardiac patients. Based on the available findings, can we assume that all SSRIs have the same efficacy and safety profiles and are similarly cardiovascularly protective? Should every antidepressant or SSRI be tested? Should head-to-head comparison studies be conducted? Tricyclic antidepressants are cardiotoxic, and central nervous system alpha-2 antagonists like mirtazapine may not be effective, but what about other types of antidepressants for depressed cardiac patients?
- Is there a role for studying surrogate end points? Studies examining the effects of an intervention on mortality and/or morbidity can be very expensive. As research budgets tighten, can we instead test the effects of depression therapy on some surrogate end points?
Our laboratory has been funded by the National Heart, Lung, and Blood Institute to compare the effects of escitalopram with those of placebo on MSIMI in patients with stable IHD and a score of 5 or greater on the Beck Depression Inventory. This study, the Responses of Myocardial Ischemia to Escitalopram Treatment (REMIT) trial, will provide SSRI therapy to patients with a broad spectrum of depressive symptoms (not just major depressive disorder), assess the ischemic activity induced by mental stress testing as its primary end point, and explore the effects on other hypothesized mechanisms of depression that adversely affect cardiac diseases (platelet aggregation, inflammatory biomarkers, etc). Stay tuned for the results in the near future.
- Kessler RC, Berglund P, Demler O, et al. The epidemiology of major depressive disorder: results from the National Comorbidity Survey Replication (NCS-R). JAMA 2003; 289:3095–3105.
- Malzberg B. Mortality among patients with involutional melancholia. Am J Psychiatry 1937; 93:1231–1238.
- van Melle JP, de Jonge P, Spijkerman TA, et al. Prognostic association of depression following myocardial infarction with mortality and cardiovascular events: a meta-analysis. Psychosom Med 2004; 66:814–822.
- Barth J, Schumacher M, Herrmann-Lingen C. Depression as a risk factor for mortality in patients with coronary heart disease: a meta-analysis. Psychosom Med 2004; 66:802–813.
- Jiang W, Alexander J, Christopher E, et al. Relationship of depression to increased risk of mortality and rehospitalization in patients with congestive heart failure. Arch Intern Med 2001; 161:1849–1856.
- Rutledge T, Reis VA, Linke SE, Greenberg BH, Mills PJ. Depression in heart failure: a meta-analytic review of prevalence, intervention effects, and associations with clinical outcomes. J Am Coll Cardiol 2006; 48:1527–1537.
- Rugulies R. Depression as a predictor for coronary heart disease: a review and meta-analysis. Am J Prev Med 2002; 23:51–61.
- Deanfield JE, Shea M, Ribiero P, et al. Transient ST-segment depression as a marker of myocardial ischemia during daily life. Am J Cardiol 1984; 54:1195–1200.
- Gullette ECD, Blumenthal JA, Babyak M, et al. Effects of mental stress on myocardial ischemia during daily life. JAMA 1997; 277:1521–1526.
- Rozanski A, Bairey CN, Krantz DS, et al. Mental stress and the induction of myocardial ischemia in patients with ischemic heart disease. N Engl J Med 1988; 318:1005–1011.
- Jiang W, Babyak M, Krantz DS, et al. Mental stress–induced myocardial ischemia and cardiac events. JAMA 1996; 275:1651–1656.
- Blumenthal JA, Jiang W, Waugh RA, et al. Mental stress-induced ischemia in the laboratory and ambulatory ischemia during daily life. Association and hemodynamic features. Circulation 1995; 92:2102–2108.
- Yeung AC, Vekshtein VI, Krantz DS, et al. The effect of atherosclerosis on the vasomotor response of coronary arteries to mental stress. N Engl J Med 1991; 325:1551–1556.
- Dakak N, Quyyumi AA, Eisenhofer G, Goldstein DS, Cannon RO. Sympathetically mediated effects of mental stress on the cardiac microcirculation of patients with coronary artery disease Am J Cardiol 1995; 76:125–130.
- Goldberg AD, Becker LC, Bonsall R, et al. Ischemic, hemodynamic, and neurohormonal responses to mental and exercise stress. Experience from the Psychophysiological Investigations of Myocardial Ischemia Study (PIMI). Circulation 1996; 94:2402–2409.
- Sung BH, Wilson MF, Robinson C, et al. Mechanisms of myocardial ischemia induced by epinephrine: comparison with exercise-induced ischemia. Psychosom Med 1988; 4:381–393.
- Specchia G, Falcone C, Traversi E, et al. Mental stress as a provocative test in patients with various clinical syndromes of coronary heart disease. Circulation 1991; 83(Suppl 4):II108–II114.
- Krantz DS, Santiago HT, Kop WJ, Bairey Merz CN, Rozanski A, Gottdiener JS. Prognostic value of mental stress testing in coronary artery disease. Am J Cardiol 1999; 84:1292–1297.
- Sheps DS, McMahon RP, Becker L, et al. Mental stress-induced ischemia and all-cause mortality in patients with coronary artery eisease: results from the Psychopysiological Investigations of Myocardial Ischemia Study. Circulation 2002; 105:1780–1784.
- Jiang W, Babyak MA, Rozanski A, et al. Depression and increased myocardial ischemic activity in patients with ischemic heart disease. Am Heart J 2003; 146:55–61.
- Roose SP, Laghrissi-Thode F, Kennedy JS, et al. Comparison of paroxetine and nortriptyline in depressed patients with ischemic heart disease. JAMA 1998; 279:287–291.
- Nelson JC, Kennedy JS, Pollock BG, et al. Treatment of major depression with nortriptyline and paroxetine in patients with ischemic heart disease. Am J Psychiatry 1999; 156:1024–1028.
- Glassman AH, O’Connor CM, Califf RM, et al. Sertraline Antidepressant Heart Attack Randomized Trial (SADHART) Group. Sertraline treatment of major depression in patients with acute MI or unstable angina. JAMA 2002; 288:701–709.
- Lespérance F, Frasure-Smith N, Koszycki D, et al. Effects of citalopram and interpersonal psychotherapy on depression in patients with coronary artery disease: the Candaian Cardiac Randomized Evaluation of Antidepressant and Psychotherapy Efficacy (CREATE) trial. JAMA 2007; 297:367–379.
- van den Brink RH, Van Melle JP, Honig A, et al. Treatment of depression after myocardial infarction and the effects on cardiac prognosis and quality of life: rationale and outline of the Myocardial INfarction and Depression-Intervention Trial (MIND-IT). Am Heart J 2002; 144:219–225.
- De Jonge P, Hong A, Schene AH, et al. Effects of antidepressive therapy for the treatment of depression following myocardial infarction: results from the Myocardial Infarction and Depression Intervention Trial (MIND-IT) [abstract]. Psychosom Med 2006; 68:A-7.
- Berkman LF, Blumenthal J, Burg M, et al; ENRICHD investigators. Effects of treating depression and low perceived social support on clinical events after myocardial infarction: the Enhancing Recovery in Coronary Heart Disease Patients (ENRICHD) Randomized Trial. JAMA 2003; 289:3106–3116.
- Frasure-Smith N, Lespérance F, Prince RH, et al. Randomised trial of home-based psychosocial nursing intervention for patients recovering from myocardial infarction. Lancet 1997; 350:473–479.
- Pollock BG, Laghrissi-Thode F, Wagner WR. Evaluation of platelet activation in depressed patients with ischemic heart disease after paroxetine or nortriptyline treatment. J Clin Psychopharmacol 2000; 20:137–140.
- Musselman DL, Marzec UM, Manatunga A, et al. Platelet reactivity in depressed patients treated with paroxetine: preliminary findings. Arch Gen Psychiatry 2000; 57:875–882.
- Serebruany VL, Glassman AH, Malinin AI, et al. Platelet/endothelial biomarkers in depressed patients treated with the selective serotonin reuptake inhibitor sertraline after acute coronary events: the Sertraline AntiDepressant Heart Attack Randomized Trial (SADHART) Platelet Substudy. Circulation 2003; 108:939–944.
- Yeragani VK, Pesce V, Jayaraman A, et al. Major depression with ischemic heart disease: effects of paroxetine and nortriptyline on long-term heart rate variability measures. Biol Psychiatry 2002; 52:418–429.
- Yeragani VK, Roose S, Mallavarapu M, et al. Major depression with ischemic heart disease: effects of paroxetine and nortriptyline on measures of nonlinearity and chaos of heart rate. Neuropsychobiology 2002; 46:125–135.
- Rechlin T. The effects of psychopharmacological therapy on heart rate variation. Nervenarzt 1995; 66:678–685.
- Kessler RC, Berglund P, Demler O, et al. The epidemiology of major depressive disorder: results from the National Comorbidity Survey Replication (NCS-R). JAMA 2003; 289:3095–3105.
- Malzberg B. Mortality among patients with involutional melancholia. Am J Psychiatry 1937; 93:1231–1238.
- van Melle JP, de Jonge P, Spijkerman TA, et al. Prognostic association of depression following myocardial infarction with mortality and cardiovascular events: a meta-analysis. Psychosom Med 2004; 66:814–822.
- Barth J, Schumacher M, Herrmann-Lingen C. Depression as a risk factor for mortality in patients with coronary heart disease: a meta-analysis. Psychosom Med 2004; 66:802–813.
- Jiang W, Alexander J, Christopher E, et al. Relationship of depression to increased risk of mortality and rehospitalization in patients with congestive heart failure. Arch Intern Med 2001; 161:1849–1856.
- Rutledge T, Reis VA, Linke SE, Greenberg BH, Mills PJ. Depression in heart failure: a meta-analytic review of prevalence, intervention effects, and associations with clinical outcomes. J Am Coll Cardiol 2006; 48:1527–1537.
- Rugulies R. Depression as a predictor for coronary heart disease: a review and meta-analysis. Am J Prev Med 2002; 23:51–61.
- Deanfield JE, Shea M, Ribiero P, et al. Transient ST-segment depression as a marker of myocardial ischemia during daily life. Am J Cardiol 1984; 54:1195–1200.
- Gullette ECD, Blumenthal JA, Babyak M, et al. Effects of mental stress on myocardial ischemia during daily life. JAMA 1997; 277:1521–1526.
- Rozanski A, Bairey CN, Krantz DS, et al. Mental stress and the induction of myocardial ischemia in patients with ischemic heart disease. N Engl J Med 1988; 318:1005–1011.
- Jiang W, Babyak M, Krantz DS, et al. Mental stress–induced myocardial ischemia and cardiac events. JAMA 1996; 275:1651–1656.
- Blumenthal JA, Jiang W, Waugh RA, et al. Mental stress-induced ischemia in the laboratory and ambulatory ischemia during daily life. Association and hemodynamic features. Circulation 1995; 92:2102–2108.
- Yeung AC, Vekshtein VI, Krantz DS, et al. The effect of atherosclerosis on the vasomotor response of coronary arteries to mental stress. N Engl J Med 1991; 325:1551–1556.
- Dakak N, Quyyumi AA, Eisenhofer G, Goldstein DS, Cannon RO. Sympathetically mediated effects of mental stress on the cardiac microcirculation of patients with coronary artery disease Am J Cardiol 1995; 76:125–130.
- Goldberg AD, Becker LC, Bonsall R, et al. Ischemic, hemodynamic, and neurohormonal responses to mental and exercise stress. Experience from the Psychophysiological Investigations of Myocardial Ischemia Study (PIMI). Circulation 1996; 94:2402–2409.
- Sung BH, Wilson MF, Robinson C, et al. Mechanisms of myocardial ischemia induced by epinephrine: comparison with exercise-induced ischemia. Psychosom Med 1988; 4:381–393.
- Specchia G, Falcone C, Traversi E, et al. Mental stress as a provocative test in patients with various clinical syndromes of coronary heart disease. Circulation 1991; 83(Suppl 4):II108–II114.
- Krantz DS, Santiago HT, Kop WJ, Bairey Merz CN, Rozanski A, Gottdiener JS. Prognostic value of mental stress testing in coronary artery disease. Am J Cardiol 1999; 84:1292–1297.
- Sheps DS, McMahon RP, Becker L, et al. Mental stress-induced ischemia and all-cause mortality in patients with coronary artery eisease: results from the Psychopysiological Investigations of Myocardial Ischemia Study. Circulation 2002; 105:1780–1784.
- Jiang W, Babyak MA, Rozanski A, et al. Depression and increased myocardial ischemic activity in patients with ischemic heart disease. Am Heart J 2003; 146:55–61.
- Roose SP, Laghrissi-Thode F, Kennedy JS, et al. Comparison of paroxetine and nortriptyline in depressed patients with ischemic heart disease. JAMA 1998; 279:287–291.
- Nelson JC, Kennedy JS, Pollock BG, et al. Treatment of major depression with nortriptyline and paroxetine in patients with ischemic heart disease. Am J Psychiatry 1999; 156:1024–1028.
- Glassman AH, O’Connor CM, Califf RM, et al. Sertraline Antidepressant Heart Attack Randomized Trial (SADHART) Group. Sertraline treatment of major depression in patients with acute MI or unstable angina. JAMA 2002; 288:701–709.
- Lespérance F, Frasure-Smith N, Koszycki D, et al. Effects of citalopram and interpersonal psychotherapy on depression in patients with coronary artery disease: the Candaian Cardiac Randomized Evaluation of Antidepressant and Psychotherapy Efficacy (CREATE) trial. JAMA 2007; 297:367–379.
- van den Brink RH, Van Melle JP, Honig A, et al. Treatment of depression after myocardial infarction and the effects on cardiac prognosis and quality of life: rationale and outline of the Myocardial INfarction and Depression-Intervention Trial (MIND-IT). Am Heart J 2002; 144:219–225.
- De Jonge P, Hong A, Schene AH, et al. Effects of antidepressive therapy for the treatment of depression following myocardial infarction: results from the Myocardial Infarction and Depression Intervention Trial (MIND-IT) [abstract]. Psychosom Med 2006; 68:A-7.
- Berkman LF, Blumenthal J, Burg M, et al; ENRICHD investigators. Effects of treating depression and low perceived social support on clinical events after myocardial infarction: the Enhancing Recovery in Coronary Heart Disease Patients (ENRICHD) Randomized Trial. JAMA 2003; 289:3106–3116.
- Frasure-Smith N, Lespérance F, Prince RH, et al. Randomised trial of home-based psychosocial nursing intervention for patients recovering from myocardial infarction. Lancet 1997; 350:473–479.
- Pollock BG, Laghrissi-Thode F, Wagner WR. Evaluation of platelet activation in depressed patients with ischemic heart disease after paroxetine or nortriptyline treatment. J Clin Psychopharmacol 2000; 20:137–140.
- Musselman DL, Marzec UM, Manatunga A, et al. Platelet reactivity in depressed patients treated with paroxetine: preliminary findings. Arch Gen Psychiatry 2000; 57:875–882.
- Serebruany VL, Glassman AH, Malinin AI, et al. Platelet/endothelial biomarkers in depressed patients treated with the selective serotonin reuptake inhibitor sertraline after acute coronary events: the Sertraline AntiDepressant Heart Attack Randomized Trial (SADHART) Platelet Substudy. Circulation 2003; 108:939–944.
- Yeragani VK, Pesce V, Jayaraman A, et al. Major depression with ischemic heart disease: effects of paroxetine and nortriptyline on long-term heart rate variability measures. Biol Psychiatry 2002; 52:418–429.
- Yeragani VK, Roose S, Mallavarapu M, et al. Major depression with ischemic heart disease: effects of paroxetine and nortriptyline on measures of nonlinearity and chaos of heart rate. Neuropsychobiology 2002; 46:125–135.
- Rechlin T. The effects of psychopharmacological therapy on heart rate variation. Nervenarzt 1995; 66:678–685.