User login
The Evolution of Insulin Therapy in Diabetes Mellitus
The modern management of diabetes mellitus (DM) began with the discovery of insulin by Banting and Best in 1921 (see The Evolution of Insulin Therapy in Diabetes Mellitus in this supplement). Since that time, numerous additional classes of glucose-lowering agents have been introduced for the treatment of type 2 DM (T2DM). These medications primarily act by addressing 2 of the key defects of T2DM, insulin resistance and pancreatic β-cell dysfunction. T2DM is a progressive disease process that requires continued adjustment of therapy to maintain treatment goals. Most patients with T2DM will require insulin therapy at some point in their lives.
Role of Insulin in Type 2 Diabetes Mellitus Management
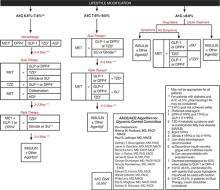
Consensus guidelines developed by the American Association of Clinical Endocrinologists/American College of Endocrinology (AACE/ACE) recommend initiating insulin when oral therapy fails to achieve glycemic control, A1C > 9.0% in treatment-naïve patients, or if the patient is symptomatic with glucose toxicity (polyuria, polydipsia, and weight loss) (FIGURE 1).1
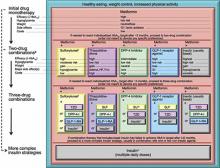
Similar consensus guidelines developed by the American Diabetes Association/European Association for the Study of Diabetes (ADA/EASD) advise the initiation of glucose-lowering therapy for most patients with T2DM with the combination of lifestyle modifications, diet, and metformin (FIGURE 2).2 For patients who do not achieve or maintain glycemic control over 3 months, or thereabouts, with metformin, a second oral agent should be added. Alternatives include a glucagon-like peptide-1 receptor (GLP-1R) agonist or basal insulin. Insulin should be strongly considered as initial therapy for a patient with significant symptoms of hyperglycemia and/or plasma glucose >300-350 mg/dL or A1C ≥10.0%.
The major role of insulin in the management of patients with T2DM stems from several important attributes. First, insulin is the only treatment that works in patients with advanced β-cell deficiency. It acts directly on tissues to regulate glucose homeostasis, unlike other glucose-lowering agents that require the presence of sufficient endogenous insulin to exert their effects as insulin sensitizers, secretagogues, incretin mimetics, amylin analogs, and other factors. This also means that the mechanism of action of insulin is complementary to those of other glucose-lowering agents. Second, there is less of a ceiling effect with insulin. That is, increasing the dose of insulin results in a progressive lowering of blood glucose in the majority of patients, with the major limitation being the risk for hypoglycemia. Third, the glucose-lowering efficacy of insulin is durable, unlike that of other glucose-lowering agents that depend on endogenous insulin secretion for continued effectiveness. Fourth, insulin improves the lipid profile, particularly triglyceride levels.2-5 Fifth, regarding the long-term safety and tolerability of insulin, it is well established that weight gain, likely mediated via reduction of glycosuria, and hypoglycemia are typically the most concerning adverse events encountered. Allergic reactions, which were a more common complication of animal-sourced insulins, are infrequent with the insulin analogs.6-17 Finally, the availability of insulin in different formulations allows for targeting fasting plasma glucose or postprandial glucose, and individualization of therapy (see The Evolution of Insulin Therapy in Diabetes Mellitus in this supplement.)
While both the AACE/ACE and ADA/EASD consensus guidelines provide treatment “algorithms,” both make it clear that these are suggested approaches suitable for the population with T2DM (FIGURE 1, FIGURE 2). The specific treatment approach must be individualized based on patient-specific factors such as age, comorbid conditions, and tolerance of hypoglycemia.
FIGURE 1
Role of insulin in the management of patients with type 2 diabetes mellitus according to the AACE/ACE1
AACE, American Association of Clinical Endocrinologists; ACE, American College of Endocrinology; AGI, α-glucosidase inhibitor; DPP4, dipeptidyl-peptidase-4 inhibitor; FPG, fasting plasma glucose; GLP-1, glucagon–like peptide-1 agonist; MET, metformin; PPG, postprandial glucose; SU, sulfonylurea; TZD, thiazolidinedione.
Reprinted from American Association of Clinical Endocrinologists. AACE/ACE Diabetes Algorithm for Glycemic Control. Available at https://www.aace.com/sites/default/files/GlycemicControlAlgorithmPPT.pdf. Accessed April 4, 2012, with permission from the American Association of Clinical Endocrinologists.
FIGURE 2
Role of insulin in the management of patients with type 2 diabetes mellitus according to the ADA/EASD2
Moving from the top to the bottom of the figure, potential sequences of antihyperglycemic therapy. In most patients, begin with lifestyle changes; metformin monotherapy is added at, or soon after, diagnosis (unless there are explicit contraindications). If the HbA1c target is not achieved after ~3 months, consider 1 of the 5 treatment options combined with metformin: an SU, TZD, DPP-4-i, GLP-1-RA, or basal insulin. (The order in the chart is determined by historical introduction and route of administration and is not meant to denote any specific preference.) Choice is based on patient and drug characteristics, with the over-riding goal of improving glycemic control while minimizing side effects. Shared decision making with the patient may help in the selection of therapeutic options. The figure displays drugs commonly used both in the United States and/or Europe. Rapid-acting secretagogues (meglitinides) may be used in place of SUs. Other drugs not shown (α-glucosidase inhibitors, colesevelam, dopamine agonists, pramlintide) may be used where available in selected patients but have modest efficacy and/or limiting side effects. In patients intolerant of, or with contraindications for, metformin, select initial drug from other classes depicted and proceed accordingly. In this circumstance, while published trials are generally lacking, it is reasonable to consider 3-drug combinations other than metformin. Insulin is likely to be more effective than most other agents as a third-line therapy, especially when HbA1c is very high (eg, ≥ 9.0%). The therapeutic regimen should include some basal insulin before moving to more complex insulin strategies. Dashed arrow line on the left-hand side of the figure denotes the option of a more rapid progression from a 2-drug combination directly to multiple daily insulin doses, in those patients with severe hyperglycemia (eg, HbA1c, ≥ 10.0–12.0%).
DPP-4, dipeptidyl peptidase-4; DPP-4-i, DPP-4 inhibitor; Fx’s, bone fractures; GI, gastrointestinal; GLP-1, glucagon-like peptide 1; GLP-1-RA, GLP-1 receptor agonist; HbA1c, hemoglobin A1c; HF, heart failure; NPH, neutral protamine Hagedorn; SU, sulfonylurea; TZD, thiazolidinedione.
aConsider beginning at this stage in patients with very high HbA1c (eg, ≥ 9%); bConsider rapid-acting, non-SU secretagogues (meglitinides) in patients with irregular meal schedules or who develop late postprandial hypoglycemia on SUs; cUsually a basal insulin (NPH, glargine, detemir) in combination with noninsulin agents; dCertain noninsulin agents may be continued with insulin. Consider beginning at this stage if patient presents with severe hyperglycemia (≥ 16.7–19.4 mmol/L [≥ 300–350 mg/dL]; HbA1c≥ 10.0–12.0%) with or without catabolic features (weight loss, ketosis, etc).
Diabetes Care by American Diabetes Association. Copyright 2012. Reproduced with permission of AMERICAN DIABETES ASSOCIATION in the format Journal via Copyright Clearance Center.
Individualizing Therapy
The importance of individualizing therapy in a way that allows patients with T2DM to effectively self-manage their disease cannot be overstated. A study involving 1381 patients with T2DM cared for by 42 primary care physicians was conducted to estimate the magnitude of effect that physicians have on glycemic control.18 Hierarchical linear modeling showed that physician-related factors were associated with a statistically significant but modest variability in A1C change (2%) for the entire patient group. On the face of it, this finding might be discouraging. Further analysis showed, however, that for patients whose A1C did improve, physician-related factors accounted for 5% of the overall change in A1C (P = .005). On the other hand, physician-related factors had no impact on patients whose A1C did not improve or worsened. These results support the role that physicians play in affecting patient outcomes. The results also make it clear that without a physician’s influence, a patient’s glycemic outcomes may be difficult to change. The question is: How best can a physician influence patient outcomes?
A 2011 survey of patients with DM, general practitioners, and DM specialists reported that clinicians tended to underestimate patients’ perceived seriousness of the disease, while overestimating patients’ level of distress. In addition, physicians had difficulty identifying which DM-related complications concerned patients most and the information and support patients needed to feel more at ease with DM. Patients placed greater importance on having easy access to their physicians rather than more time with them. But most importantly, the survey investigators concluded that patients generally wished for greater involvement in decision making and being provided more information.19 These findings suggest that patients understand that T2DM is a largely self-managed, chronic disease, and want a collaborative relationship with their physician.
Patient Barriers to Insulin Therapy
Numerous factors have been identified as impeding patients’ willingness to initiate insulin therapy (TABLE 1).20-24 Barriers often vary from patient to patient and, in fact, may change over time in an individual patient. It is crucial, therefore, to identify the root reasons for a patient’s apprehension with insulin when talking about options for intensifying treatment. Once insulin has been initiated, the patient should be asked about continuing or new concerns regarding insulin therapy (and DM management in general), including adherence.
TABLE 1
Barriers to insulin therapy identified by patients20-24
| Lack of understanding of serious nature of type 2 diabetes mellitus |
| Fear of addiction to insulin |
| Fear of hypoglycemia |
| Concern about weight gain |
| Repeated experiences of failing to achieve satisfactory glycemic control |
| Perception that quality of previous treatment was low |
| Needle phobia |
| Treatment complexity |
| Concern of social stigmatization |
| Perceived failure and low self-efficacy |
| Belief of becoming more ill |
| Out-of-pocket cost |
| Perceived negative impact on quality of life |
| Comorbidities such as poor eyesight, arthritis, forgetfulness |
A recent, international survey of 1400 patients with insulin-naïve T2DM reported that 3 negative beliefs about insulin were prominent: (1) feeling that the disease was worsening; (2) fear of injection; and (3) a feeling of personal failure.20 Certain patient comorbidities, such as poor eyesight, arthritis, and forgetfulness, might also serve as barriers to self-management of DM with insulin. Additional comorbidities may contribute as indirect barriers, such as the need for polypharmacy, which may make the initiation of additional treatments such as insulin logistically or financially difficult.
It is possible that the discussion about initiating insulin may uncover patient concerns about T2DM in general. The Diabetes Attitudes, Wishes, and Needs (DAWN) study reported that psychosocial issues were the major source of difficulty in patient self-management (TABLE 2).25 In fact, 85% of people who reported a high level of distress at the time of diagnosis of T2DM continued to experience psychological distress at a mean follow-up of 15 years.
TABLE 2
Patients experiencing various aspects of diabetes-related distress25
| Diabetes-related distress | Respondents who agree (%) |
|---|---|
| I feel stressed because of my diabetes. | 32.7 |
| I feel burned out because of my diabetes. | 18.1 |
| I feel that diabetes is preventing me from doing what I want to do. | 35.9 |
| I am constantly afraid of my diabetes getting worse. | 43.8 |
| I worry about not being able to carry out my family responsibilities in the future. | 30.1 |
| My diabetes causes me worries about my financial future. | 25.8 |
| My family and friends put too much pressure on me about my diabetes. | 14.7 |
| The community I live in is intolerant of diabetes. | 13.6 |
| Diabetes Care by American Diabetes Association. Copyright 2012. Reproduced with permission of AMERICAN DIABETES ASSOCIATION in the format Journal via Copyright Clearance Center. | |
Addressing psychosocial issues and other barriers is crucial in the discussion of self-management because those with more negative feelings about starting insulin are most unwilling to start insulin.20 One factor that may contribute to these negative feelings is repeated experiences of failing to achieve satisfactory glycemic control with oral glucose-lowering agents.23 Conversely, those who have experienced improved glycemic control with intensification of prior glucose-lowering therapy may be more accepting of initiating insulin therapy.23,26 These findings are a reminder of the importance of a treat-to-target approach to management, in which the target glycemic goal, generally A1C < 7.0%, is achieved within 2 to 3 months of diagnosis and maintained at that level through intensification of therapy as needed.
Addressing psychosocial issues can be a challenge in today’s busy primary care practice due to limited time and lack of training in the management of such issues. However, implementation of various strategies has been reported to facilitate and, in some cases, shorten a patient’s visit. For example, one small study reported that visits were shorter if the physician acknowledged and responded positively to a patient’s stated or implied concerns (17.6 minutes vs 20.1 minutes).27 Missing or ignoring the patient’s concerns often led the patient to bring up the same concern one or more additional times resulting in a longer office visit. These results underscore the importance of asking patients to identify their concerns or questions at the beginning of the office visit. The patient can fill out a questionnaire in the waiting room or be encouraged to write down and prioritize their questions and concerns specific to the visit. If the patient identifies more concerns or questions than can be reasonably addressed in one visit, there should be agreement to address the most pressing ones during the current visit and the remaining concerns and questions during the next visit. This “agenda-setting” approach has been reported to offer several advantages.28 From the patient’s perspective, the quality of the physician-patient interaction was much improved, in part because physicians took time to explain points in a way that was easy to understand. Advantages to the physician with an agenda-setting approach included “feeling more in control,” “less stressed by simply knowing what was on the patient’s mind,” “feeling less rushed,” and “enjoying patient encounters more.” Contrary to the study cited above, physicians found that patients’ visits often were longer, especially those of older patients. One physician, however, noted that the visit “takes more time now, but saves time later.” As noted in this study, additional time spent with the patient can lead to improved job satisfaction for the physician.29
The agenda-setting approach requires that the physician ask the patient to list his or her concerns and questions, and then actively listen to the patient. Once the agenda for the visit is established, employing the “ask, listen, empathize” communication style can lead to effective physician-patient communication and problem-solving. Using this approach, the physician asks questions to gain a clear understanding of the patient’s concerns and then uses active listening with little, if any, interruption.30,31 Since the goal is to solve problems with rather than for the patient, active listening without offering opinions, judgements, or advice while offering empathy is essential. Through reflection and discussion, the physician can help the patient to identify his or her issues and acceptable solutions.
The importance of good communication between physician and patient cannot be overstated. Additional communication skills to keep in mind are: (1) speak slowly using nonmedical language; (2) limit the amount of information and repeat it; (3) draw pictures and/or use visual aids; and (4) ask the patient to repeat instructions and key concepts. In addition to enhancing patients’ understanding, visual images may be particularly beneficial in keeping patients motivated to improve self-management, including adherence to therapy. For example, it may be helpful to graphically track the patient’s glycemic progress. This can be done by establishing an actionable A1C goal (generally < 7.0%) and a time frame to achieve the goal (eg, 2 to 3 months).32 A graph can be constructed beginning with the patient’s current, preinsulin A1C level, with updates at each visit. In addition to motivating the patient and reinforcing adherence, the graph can also be used to demonstrate when further treatment intensification is needed. Additional general strategies that can be employed when considering the initiation of insulin are shown in TABLE 3. Implementation of strategies such as these by family physicians provides patient outcomes comparable to those implemented by endocrinologists or diabetes specialists.33
TABLE 3
General strategies for initiating insulin therapy
| Invite the patient to take an active role in treatment decisions. |
| Remind the patient that type 2 diabetes is primarily self-managed. |
| Discuss the progressive nature of β-cell dysfunction in type 2 diabetes. |
| Emphasize the physiologic role of insulin to maintain glucose homeostasis. |
| Discuss that insulin will help to achieve glycemic control and minimize the risk for long-term complications. |
| Discuss that treatment will be modified as needed to maintain glycemic control and to best meet their needs, capabilities, and interest. |
| Utilize insulin pen devices whenever possible. |
| Emphasize the importance of lifestyle management. |
| Ask if hearing other patients talk of their experiences with insulin therapy would be helpful; consider a group office visit. |
| Discuss and provide the patient with an individualized, written action plan that includes insulin dosing, self-monitoring of blood glucose, and signs/symptoms of hypoglycemia and other adverse events with appropriate action(s) to take. |
| Simplify diabetes (and comorbidities) treatment whenever possible. |
The remainder of this article uses case studies to further explore various patient barriers to insulin therapy and strategies for addressing them with the patient. While other therapies may be appropriate in the case studies below as recommended by current guidelines, these case studies will focus on insulin. In addition, dosing strategies for initiating and intensifying insulin therapy are discussed. Changes to the treatment plan to adjust for comorbidities, such as hypertension and dyslipidemia, or for smoking cessation or aspirin therapy, are not addressed in these cases, but are crucial components of comprehensive management.
CASE STUDY 1
RF is a 49-year-old female insurance analyst diagnosed with T2DM 6 years ago. Initial therapy with lifestyle modifications and metformin has since been intensified. Glimepiride was added, then pioglitazone was added 1.5 years ago when the A1C had risen to 7.5%. There is no evidence of cardiovascular disease. She reports bothersome lower extremity edema and an 8-pound weight increase since starting pioglitazone treatment. RF states that she takes her medications every day, although she acknowledges that she sometimes forgets on Sundays.
Clinical Impression
After taking her history, performing a physical examination, and reviewing her laboratory and self-monitored blood glucose (SMBG) data, her physician concludes that her treatment plan needs to be changed (TABLE 4, TABLE 5).
TABLE 4
Case study 1: Chart notes
| Physical examination | Laboratory tests | Lifestyle habits | Current therapy | |
|---|---|---|---|---|
| Glucose-lowering | Other | |||
| BP: 126/80 mm Hg Weight: 176 lb (79.2 kg) BMI: 27 kg/m2 Eyes: no retinopathy Neurology: intact Skin: intact | SCr: 1.4 mg/dL Albuminuria: negative A1C: 8.2% Cholesterol: Total: 204 mg/dL LDL: 134 mg/dL HDL: 36 mg/dL | Exercise: Walks 2 miles 3-4 d/wk Nutrition: eats 3-4 meals/d | Metformin 1000 mg BID Glimepiride 8 mg QD Pioglitazone 45 mg QD | Lisinopril 30 mg QD Simvastatin 40 mg QD ASA 80 mg QD |
| ASA, acetylsalicylic acid; BMI, body mass index; BP, blood pressure; HDL, high-density lipoprotein; LDL, low-density lipoprotein; SCr, serum creatinine. | ||||
TABLE 5
Case study 1: Self-monitored blood glucose (mg/dL) over the previous 2 weeks
| Day | Fasting | 2 h Post-breakfast | 2 h Post-lunch | 2 h Post-dinner |
|---|---|---|---|---|
| Wednesday | 205 | |||
| Thursday | 158 | |||
| Friday | 179 | |||
| Saturday | 201 | 162 | ||
| Sunday | ||||
| Monday | 166 | |||
| Tuesday | 189 | |||
| Wednesday | ||||
| Thursday | 153 | 221 | ||
| Friday | 150 | |||
| Saturday | 199 | 186 | 213 | |
| Sunday | ||||
| Monday | 181 | |||
| Tuesday | 167 |
Treatment Plan
- Initiate basal insulin once daily in the evening.
- Continue glimepiride, but reduce pioglitazone to 15 mg once daily (or discontinue if cost is a concern).
- Ask RF to monitor fasting blood glucose and self-adjust insulin doses as appropriate.
Barriers
While discussing the need to change the treatment plan and the physician’s suggestion that RF begin basal insulin, RF asks her physician for another few months on her current regimen stating that she will try harder to take her medications on Sundays. She also voices concern that insulin treatment requires injections and that she is concerned about what her coworkers and friends might think. The physician confirms that these concerns are understandable; he also confirms that RF is fearful of needles. The following are possible responses that RF’s physician could use to address these concerns.
Patient’s concern: Perceived failure/low self-efficacy
Physician responses:
- We all forget to do things from time to time, but overall I think you have done a great job taking your medications.
- As we have talked about before, with T2DM there is a progressive loss of insulin production over time regardless of what you do. That is why we added glimepiride and then pioglita-zone and that is why we need to make a change now and put you back in control of your diabetes. It is likely that further changes will be needed and we can discuss and agree on them together.
Patient’s concern: Social stigmatization
Physician responses:
- We can begin by having you administer insulin once daily in the evening in the privacy of your home.
- The insulin can be administered with a device that looks like a pen. It is small and can be carried in your purse; it does not need to be refrigerated once opened. If the time comes that you will need to administer a dose of insulin during the day, you can easily administer the insulin discretely in a public restroom or your work area.
- The use of insulin is more common than it was even a few years ago. In fact, about 5 million people in the United States use insulin to replace what is missing, control blood sugars, and decrease the risk for diabetes complications.34
Patient’s concern: Fear of needles
Physician’s responses:
- Insulin can be injected using a pen device with short, ultrathin needles that makes most of the injections painless. I would like you to see how simple and painless the injection can be by using this sample pen here in the office.
- Many patients are concerned about giving themselves an injection at first, but they quickly become comfortable doing so.
Dosing
Treatment with basal insulin can be initiated using one of several approaches. Using the treat-to-target approach, basal insulin 10 U once daily is initiated.35 The starting dose should be reduced to 6 U if the initial pre-breakfast or pre-dinner blood glucose is < 126 mg/dL or the patient’s body mass index (BMI) is < 26 kg/m2.36 Alternatively, the ADA/EASD recommends starting with 0.2 U/kg, which may be more practical in very overweight or obese patients.2 Titration of the basal insulin dose can be accomplished using one of the following physician-directed or patient-driven treat-to-target titration algorithms (TABLE 6).35,37,38 The insulin dose should be titrated based on the pre-breakfast fasting blood glucose level.
TABLE 6
Physician-directed or patient-driven treat-to-target titration algorithms
| Riddle et al35 | Davies et al37 | Meneghini et al38 | ||||
|---|---|---|---|---|---|---|
| Start with 10 U/d bedtime basal insulin and adjust weekly | Start with 10* U/d bedtime basal insulin and adjust weekly (physician-directed) Or Start with a dose numerically equivalent to the highest FPG (in millimoles/L)† over the previous 7 days and adjust every 3 days (patient-managed) | Start with basal insulin once daily and adjust every 3 days | ||||
| Mean of self-monitored FPG values from preceding 2 days | Change in insulin dose (U/d)# | Mean of self-monitored FPG values from preceding 3 days | Change in insulin dose (U/d) (physician-directed) | Change in insulin dose (U/d) (patient-managed) | Mean of self-monitored FPG values from preceding 3 days | Change in insulin dose (U/d) |
| ≥180 mg/dL 140-180 mg/dL 120-140 mg/dL 100-120 mg/dL | +8 +6 +4 +2 | ≥180 mg/dL (≥10 mmol/L) 140-179 mg/dL (7.8-9.9 mmol/L) 120-139 mg/dL (6.7 – 7.7 mmol/L) 100-119 mg/dL (5.5-6.6 mmol/L) | +6 to +8 +4 +2 0 to +2 | +2 +2 +2 0 to +2 | >110 mg/dL 80-110 mg/dL <80 mg/dL | +3 0 -3 |
| FPG, fasting plasma glucose. *In insulin-naive patients. †For example, if the highest FPG over the previous 7 days was 7 mmol/L, start with 7 U.#Small insulin dose decreases (2-4 U/d per adjustment) were allowed if severe hypoglycemia (requiring assistance) or plasma-referenced glucose < 56 mg/dL was documented in the preceding week. Reproduced with permission. Meneghini LF, et al. J Fam Pract. 2011;60(9 Suppl 1):S21-S28. Quadrant HealthCom Inc. Copyright 2011. | ||||||
Follow-Up Visit
RF begins basal insulin 10 U in the evening and is given simple instructions for insulin dose titration based on fasting plasma glucose results. At her follow-up visit, RF reports that she has increased her basal insulin to 18 U administered once daily. Review of her SMBG results show that her blood glucose levels throughout the day have improved, but are still not at goal. RF’s physician commends her on the progress she has made. RF and her physician agree that she should continue to increase her basal insulin dose. Eight months after beginning basal insulin, RF is administering 28 U (0.35 U/kg) of basal insulin in the evening. Review of her SMBG results over the previous 2 weeks show that her blood glucose rises during the day and is highest after dinner; her current A1C is 7.2%.
Treatment Plan
- Discuss dietary and lifestyle complements to insulin therapy such as:
- Use SMBG to identify foods that raise her blood glucose.
CASE STUDY 2
LW is a 64-year-old male with longstanding hypertension diagnosed with T2DM 8 years ago for which he was treated initially with lifestyle management and metformin. He has since been treated with other oral agents as add-on therapy; glipizide was discontinued due to hypoglycemia when he skips meals (usually lunch); pioglitazone was discontinued after the patient expressed concerns about the risk for bladder cancer he heard on television. He has mild retinopathy and mild loss of vibration sensation in the feet; there is no evidence of cardiovascular disease. He was diagnosed with osteoarthritis 3 years ago.
Clinical Impression
After taking his history, performing a physical examination, and reviewing his laboratory and SMBG data, his physician concludes that his treatment plan needs to be changed (FIGURE 3, TABLE 7, TABLE 8).
TABLE 7
Case study 2: Chart notes
| Physical examination | Laboratory tests | Lifestyle habits | Current therapy | |
|---|---|---|---|---|
| Glucose-lowering | Other | |||
| BP: 124/76 mm Hg Weight: 204 lb (92.7 kg) BMI: 31 kg/m2 Eyes: mild retinopathy Neurology: occasional tingling on bottom of right foot Skin: intact | SCr: 1.9 mg/dL eGFR: 51 mL/min Albuminuria: negative A1C: 8.1% Cholesterol: Total: 218 mg/dL LDL: 118 mg/dL HDL: 55 mg/dL Triglyceride: 204 mg/dL | Exercise: takes dog on occasional walk but otherwise sedentary Nutrition: eats 4 meals/d | Metformin 1000 mg BID Acarbose 50 mg TID Sitagliptin 100 mg QD | Lisinopril/HCTZ 20/25 mg QD Amlodipine 10 mg QD Acetaminophen extended-release 650 mg TID ASA 80 mg QD |
| ASA, acetylsalicylic acid; BMI, body mass index; BP, blood pressure; eGFR, estimated glomerular filtration rate; HCTZ, hydrochlorothiazide; HDL, high-density lipoprotein; LDL, low-density lipoprotein; SCr, serum creatinine. | ||||
TABLE 8
Case study 2: Self-monitored blood glucose (mg/dL) over the previous 2 weeks
| Day | Fasting | 2 h Post-breakfast | 2 h Post-lunch | 2 h Post-dinner |
|---|---|---|---|---|
| Tuesday | 135 | |||
| Wednesday | ||||
| Thursday | 196 | |||
| Friday | 152 | 174 | ||
| Saturday | ||||
| Sunday | 208 | |||
| Monday | 142 | 193 | ||
| Tuesday | ||||
| Wednesday | 130 | 156 | ||
| Thursday | ||||
| Friday | ||||
| Saturday | ||||
| Sunday | 151 | |||
| Monday |
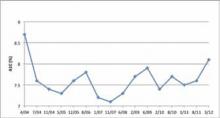
FIGURE 3
Case study 2: A1C levels for April 2004 to March 2012
Treatment Plan
- Discontinue metformin since LW’s serum creatinine is > 1.5 mg/dL.
- Initiate either basal insulin once daily in the evening or premix insulin at dinner.
- Ask LW to monitor his blood glucose and self-adjust insulin doses as appropriate.
- Stress the importance of exercise and proper nutrition; gain agreement on short-term goals for exercise and nutrition.
Barriers
LW’s physician recommends that his treatment plan be changed and insulin therapy initiated. LW quickly responds that previous changes to his treatment regimen have not resulted in his achieving an A1C < 7.0%. He also doubts that he can use a syringe to draw up the correct dose and then self-administer due to his arthritis. The following are possible responses his physician could use to address these concerns.
Patient concern: Repeated experience of failing to achieve glycemic control, ie, A1C < 7.0%
Physician responses:
- While achieving an A1C < 7.0% is a realistic goal that reduces the risks for vascular complications of diabetes, any reduction of A1C will be of benefit.
- I would like to work with you to implement a new plan that we both believe will enable you to improve your diabetes control and ideally achieve an A1C < 7.0%.
Patient concern: Self-administering due to arthritis
Physician responses:
- Instead of using a syringe and vial to draw up and administer insulin, I would like you to use an insulin pen device. As you can see, it is easy to handle and you can easily select the correct dose.
- If you choose to start on premix insulin, the pen device contains both types of insulin together in one dose.
Dosing
Treatment with basal insulin once daily in the evening can be initiated and titrated based on pre-breakfast blood glucose as in Case Study 1. Alternatively, treatment with premix insulin can be initiated at a dose of 12 U administered within 15 minutes of dinner initiation. The premix dose can be titrated using the algorithm employed in the 1-2-3 Study based on pre-breakfast blood glucose (TABLE 9).39 After 16 weeks, 41% of patients in the 1-2-3 Study achieved an A1C < 7.0% from a baseline A1C of 8.6%.
TABLE 9
1-2-3 Study algorithm39
| Pre-breakfast SMBG (mg/dL) | Adjustment of pre-dinner dose (U) |
|---|---|
| <80 | -3 |
| 80-110 | No change |
| 111-140 | +3 |
| 141-180 | +6 |
| > 180 | +9 |
| SMBG, self-monitored blood glucose. | |
Follow-Up Visit
LW began basal insulin 10 U in the evening. Over the next 5.5 months, he titrated his dose such that his current dose is 46 U (0.50 U/kg) in the evening. His current A1C is 7.3%. Review of his SMBG shows consistently high 2-hour post-lunch blood glucose levels. Although further increasing his basal insulin dose is an option, in most of the treat-to-target studies, the daily dose of basal insulin given once daily averaged between 0.4 and 0.6 U/kg.35,37,40,41 LW and his physician agree that adding rapid-acting insulin at lunch is the best option. The starting dose of rapid-acting bolus insulin is 4 to 6 U administered prior to the largest meal of the day or, as in this case, prior to the meal with the largest postprandial blood glucose excursion.42,43 Alternatively, the dose of rapid-acting insulin could be calculated as 10% of the total daily dose of basal insulin, which in this case is 5 U (10% x 46 U). The dose of basal insulin would be reduced by 5 U if the rapid-acting insulin is given at dinner in order to reduce the risk for nocturnal hypoglycemia. The dose of the bolus insulin can be titrated using the ExtraSTEP algorithm (TABLE 10).42 Alternatively, the SimpleSTEP algorithm can be used which does not require a 2-hour postprandial glucose measurement.42
TABLE 10
Algorithms for adjusting insulin aspart42
| ExtraSTEP algorithm | SimpleSTEP algorithm | |||
|---|---|---|---|---|
| 2-h Post-meal PG level (mg/dL) | Insulin aspart adjustment (U) | Pre-meal BG (mg/dL) | Bedtime BG (mg/dL) | Insulin aspart adjustment (U) |
| <72* | -2 | <72* | <72* | -2 |
| 72-144 | 0 | 72-108 | 72-144 | 0 |
| 145-180 | +2 | 109-162 | 145-180 | +2 |
| >180 | +4 | >162 | >180 | +4 |
| BG, blood glucose; PG, plasma glucose. *One or more PG values <72 mg/dL without obvious explanation. Reproduced with permission. Meneghini LF, et al. J Fam Pract. 2011;60(9 Suppl 1):S21-S28. Quadrant HealthCom Inc. Copyright 2011. | ||||
Plan
- Begin rapid-acting insulin 5 U at lunch.
- Continue basal insulin at 46 U in the evening.
- Ask LW to continue to titrate basal insulin based on the pre-breakfast blood glucose level and the lunch time bolus insulin dose based on the 2-hour post-lunch SMBG (ExtraSTEP); alternatively, adjust based on the pre-dinner blood glucose level (SimpleSTEP).
CASE STUDY 3
MB is a 46-year-old male who had not consulted a physician since having a physical examination 6 years ago. He presented 2 weeks ago with frequent urination (7-8 times/day) and feeling tired; he also noted losing 5 pounds (2.25 kg) over the preceding 3.5 months despite no changes in his diet. MB is a regional salesperson with an erratic schedule. During the week, he eats lunch and most dinners in a restaurant. On the weekend, he goes to a local bar with his friends. He does light yard work, but does not exercise regularly. He is a current smoker with a 36 pack-year history. Urinalysis shows ketonuria and microalbuminuria. His A1C reported back today is 10.8%, confirming a diagnosis of uncontrolled and symptomatic DM.
Clinical Impression
After taking his history, performing a physical examination, and reviewing his laboratory data, MB’s physician confirms a diagnosis of DM (TABLE 11). While it is likely that MB has T2DM, his physician wants to rule out type 1 DM and latent autoimmune diabetes of the adult (LADA), so he orders tests for antibodies (GAD, IA-2, ICA). The antibody testing is negative, making T2DM the most likely diagnosis.
TABLE 11
Case study 3: Chart notes
| Physical examination | Laboratory tests | Lifestyle habits | Current therapy | |
|---|---|---|---|---|
| Glucose-lowering | Other | |||
| BP: 142/88 mm Hg Weight: 176 lb (79.2 kg) BMI: 27 kg/m2 Eyes: no retinopathy Neurology: intact Skin: intact | SCr: 1.4 mg/dL Microalbumin:creatinine ratio: 140 mg/g creatinine Ketonuria: 1+ A1C: 10.8% Cholesterol: Total: 210 mg/dL LDL: 146 mg/dL HDL: 30 mg/dL | Exercise: light yard work, no regular exercise Nutrition: 3 meals/d, eats most meals in a restaurant (lunch M-F; dinner 3-4 nights/wk) | None | None |
| BMI, body mass index; BP, blood pressure; HDL, high-density lipoprotein; LDL, low-density lipoprotein; SCr, serum creatinine. | ||||
Treatment Plan
- Initiate basal-bolus therapy with fixed bolus doses of rapid-acting insulin at each meal (prandial insulin).
- Ask MB to monitor blood glucose before meals and at bedtime.
- Provide MB with a supplemental scale to correct hyperglycemia before meals.
- Stress the importance of exercise and proper nutrition; gain agreement for short-term goals for exercise and nutrition; refer for diabetes and nutrition education if available.
- Discuss the importance of smoking cessation; develop a plan.
- Consider metformin and other non-insulin therapies when A1C is under control.
Barriers
MB is surprised that he has T2DM and is clearly anxious at receiving the diagnosis. He expresses concern about starting insulin because his uncle died within a year of starting insulin. MB also recalls that his uncle was always giving himself shots and monitoring his blood glucose level. He wants to know whether there is a simpler treatment option if he agrees to start insulin treatment. He also wants to know whether he will have to remain on insulin for the rest of his life. The following are possible responses his physician could use to address these concerns.
Patient concern: Fear of death
Physician responses:
- Uncontrolled high blood sugars over a long period of time can cause serious complications, such as kidney and heart disease that can result in death. That is why it is important that we work together to gain control of your blood sugar levels over the next few months and then modify your treatment as needed to maintain control.
- Unfortunately for many patients in the past, treatment with insulin was not used until it was too late and people already had serious complications from DM. This is likely the case for your uncle.
Patient concern: Treatment complexity
Physician responses:
- Right now we have to control your blood glucose rapidly so your pancreas can regain some function and your body can better respond to insulin.
- I will also provide you with step-by-step written instructions you can follow that describe how to start insulin and how to monitor your blood glucose.
- We will communicate as often as you need to adjust your insulin doses over the next few weeks; when you feel comfortable, I can even show you how to adjust your insulin dose before a meal to correct a high blood sugar.
- We can try this treatment for 3 months and then reevaluate your response, how you feel, and whether you want to continue to modify your treatment plan to keep your blood sugars controlled.
Patient concern: Lack of understanding that T2DM is a serious disease
Physician responses:
- Please understand that T2DM is a serious disease that increases your risk for heart disease, stroke, blindness, and other diseases. Unfortunately, since diabetes does not cause bad symptoms until it is actually too late, many patients do not make the effort to properly control their diabetes. By working together, we can reduce the risk for these complications and do some screening tests to detect any complications before they become irreversible.
Dosing
There are several approaches to determining the initial doses of basal and prandial (bolus) insulin. One approach is to estimate the total daily dose (TDD) of insulin by multiplying the patient’s weight in kilograms by 0.5 U/kg/d.44 Half of the TDD is given as basal insulin replacement; the other half is divided into 3 fixed preprandial doses of rapid-acting insulin. When the patient is ready to take on more complex management, the supplemental dose for bolus insulin can be calculated using a correction factor. If the bolus insulin is a rapid-acting insulin analog, 1800 is divided by the TDD of insulin; 1500 is used for a short-acting human insulin. This correction factor is an estimate of the fall in blood glucose per unit of bolus insulin. In our patient, the TDD would be: 80 kg x 0.5 U/kg/d or 40 U/d of insulin. Thus, 1 U of insulin should lower the blood glucose by about 45 mg/dL (1800/40 U = 45 mg/dL). For every 45 mg/dL above the pre-meal target, the patient would add 1 U of rapid-acting insulin to correct the hyperglycemia over the next 4 to 5 hours. The basal and prandial insulin doses would be titrated on a periodic basis (perhaps every 1 to 2 weeks) until the daytime levels of blood glucose are on target. The fasting (pre-breakfast) blood glucose would be used to adjust the basal insulin dose, while the pre-lunch, pre-dinner, and bedtime blood glucose results would be used to adjust the pre-breakfast, pre-lunch, and pre-dinner prandial (rapid-acting) insulin doses, respectively.
An alternative approach to initiating basal-bolus therapy is the PREFER algorithm.45 Here, the basal insulin dose is 10 U initially. The bolus doses are administered in a 3:1:2 ratio, so if the total of the 3 bolus doses is 12 U/d, the initial bolus doses would be 6 (breakfast), 2 (lunch), and 4 (dinner) U. The mean basal (once-daily) and bolus insulin doses observed in PREFER are shown in TABLE 12 and TABLE 13.
TABLE 12
Case study 3: Calculating initial basal-bolus insulin doses
| Algorithm | Calculations | Patient MB |
|---|---|---|
| Meneghini44 | TDD = (total body weight [kg]) (0.5 U/kg/d) Basal insulin dose* = (50%) (TDD) Bolus insulin dose† = (10%-20%) (TDD) | TDD = (0.5 U/kg/d)(80kg) = 40 U/d |
| Basal = (50%) (40 U/d) = 20 U/d | ||
| Bolus = (10%-20%) (40 U/d) = 4 to 8 U/meal | ||
| CF = 1800/40 U/d = 45 mg/dL per 1 unit | ||
| PREFER45 | Basal insulin dose* = 10 U (14 U if BMI > 32 kg/m2) Bolus insulin dose† = ratio of 3:1:2 (breakfast:lunch:dinner) Note: At week 26, the bolus insulin doses were divided into the 3 daily meals in approximately a 1:1:1 ratio | |
| BMI, body mass index; CF, correction factor; TDD, total daily dose of insulin. *Once daily; †Three meals per day. | ||
TABLE 13
Titrating the basal insulin dose using the PREFER algorithm45
| Pre-breakfast blood glucose (mg/dL) | Basal insulin dose adjustment (U) |
|---|---|
| < 56 | -4 |
| 56-72 | -2 |
| 73-125 | No change |
| 126-140 | +2 |
| 141-160 | +4 |
| 161-180 | +6 |
| 181-200 | +8 |
| > 200 | +10 |
Follow-up Visit
MB begins with basal insulin 20 U in the evening and bolus insulin at doses of 7 U before each meal. Over the next several months, MB has titrated his insulin doses; his current doses are: 32 U (basal), 11 U (bolus-breakfast), 7 U (bolus-lunch), and 10 U (bolus-dinner). He experienced 1 episode of mild hypoglycemia (SMBG, 50 mg/dL) one afternoon following a particularly active morning (TABLE 14). His current A1C is 7.4%. MB’s physician congratulates him on the progress he has made in dramatically lowering his blood glucose level—and his risk for diabetes-related complications. While MB appreciates his physician’s support and admits that he does not feel tired and generally feels better, which is likely due to resolution of glucotoxicity, he is not happy that he has gained 5.5 pounds (2.5 kg).46 He also finds the timing and administration of bolus insulin difficult.
TABLE 14
Case study 3: Self-monitored blood glucose (mg/dL) over the previous 2 weeks
| Day | Fasting | 2 h Post-breakfast | 2 h Post-lunch | 2 h Post-dinner |
|---|---|---|---|---|
| Wednesday | ||||
| Thursday | 168 | |||
| Friday | 106 | 166 | 174 | |
| Saturday | 88 | |||
| Sunday | 195 | |||
| Monday | 134 | |||
| Tuesday | 172 | |||
| Wednesday | 130 | 156 | ||
| Thursday | 112 | 168 | ||
| Friday | 92 | 164 | ||
| Saturday | 50 | 149 | 159 | 176 |
| Sunday | 94 | 174 | 210 | |
| Monday | 176 | 184 | ||
| Tuesday | 117 | 169 |
Plan
- Continue basal insulin once-daily in the evening.
- Add metformin 500 mg BID and increase to 1000 mg BID as tolerated.
- Consider weaning down the bolus insulin doses and substituting them with a GLP-1R agonist, dipeptidyl peptidase-4 inhibitor, or short-acting secretagogue. If so, continue rapid-acting insulin during transition. [Note: the following combinations are not currently approved by the US FDA: exenatide twice-daily and prandial insulin; exenatide once-weekly and insulin; liraglutide and prandial insulin; linagliptin and insulin.]
CASE STUDY 4
KW is a 62-year-old female diagnosed with T2DM 12 years ago. Treatment with lifestyle management and metformin initially provided glycemic control. Glimepiride was subsequently added and eventually the patient was started on basal insulin. The current dose of basal insulin is 60 U in the evening. Five months ago her A1C was found to be 7.9% and more recently 8.3%. She drinks alcohol occasionally and smokes. KW works as an executive secretary and has a consistent meal and activity schedule.
Clinical Impression
Following completion of the history, physical examination, and review of her laboratory data, KW’s physician concludes that her insulin regimen should be intensified (TABLE 15, TABLE 16).
TABLE 15
Case study 4: Chart notes
| Physical examination | Laboratory tests | Lifestyle habits | Current therapy | |
|---|---|---|---|---|
| Glucose-lowering | Other | |||
| BP: 126/78 mm Hg Weight: 176 lb (79.2 kg) BMI: 32 kg/m2 Eyes: no retinopathy Neurology: intact Skin: intact | SCr: 1.0 mg/dL Albuminuria: negative A1C: 8.3% Cholesterol Total: 172 mg/dL LDL: 96 mg/dL HDL: 46 mg/dL Triglycerides: 138 mg/dL | Exercise: sedentary Nutrition: 3 meals/d with large dinner | Metformin 1000 mg BID Basal insulin 60 U in the evening | ASA 80 mg QD Pravastatin 40 mg qHS |
| BMI, body mass index; BP, blood pressure; HDL, high-density lipoprotein; LDL, low-density lipoprotein; SCr, serum creatinine. | ||||
TABLE 16
Case study 4: Self-monitored blood glucose (mg/dL) over the previous 2 weeks
| Day | Fasting | 2 h Post-breakfast | 2 h Post-lunch | 2 h Post-dinner |
|---|---|---|---|---|
| Friday | ||||
| Saturday | 156 | 244 | ||
| Sunday | 253 | |||
| Monday | ||||
| Tuesday | ||||
| Wednesday | 148 | 227 | ||
| Thursday | ||||
| Friday | ||||
| Saturday | 179 | |||
| Sunday | 160 | |||
| Monday | ||||
| Tuesday | ||||
| Wednesday | ||||
| Thursday |
Plan
- Discontinue basal insulin.
- Begin premix insulin twice daily before breakfast and dinner.
- Ask KW to monitor blood glucose two times daily and, if appropriate, teach her how to self-adjust insulin doses.
- Stress the importance of exercise and proper nutrition; gain agreement on short-term goals for exercise and nutrition.
- Discuss the importance of smoking cessation; develop a plan.
Barriers
The physician discusses with KW that her consistent meal and activity schedule would make switching to premix insulin twice daily a good choice. KW is generally in agreement with the change, but wonders whether hypoglycemia might be more likely. She also asks if she might gain more weight in addition to the 3 pounds (1.35 kg) she has gained since starting basal insulin.
Patient concern: Hypoglycemia
Physician responses:
- Hypoglycemia remains a concern, and is more frequently seen with premix than with basal insulin; however, as long as you remain consistent with your meal and activity schedule, the risk for bad hypoglycemia is low.
- We should review your written action plan so that you are sure what signs or symptoms of a low blood sugar might occur and what you should do to treat them.
Patient concern: Weight gain
Physician responses:
- It is possible that you might gain a few additional pounds. You can avoid this by increasing your physical activity, and importantly, continue healthy eating. We should schedule a time for you to meet again with a dietician who can discuss options that might work for you.
Dosing
There are different approaches for converting from basal insulin to twice-daily premix insulin. One approach is to determine the TDD of basal insulin, and give half at breakfast and the other half at dinner as premix insulin.39 Since KW is taking 60 U of basal in the evening, she should take 30 U at breakfast and 30 U at dinner. Dose titration is according to the 1-2-3 Study algorithm shown in case study 2.
Another approach is to administer biphasic insulin aspart 70/30 0.2 U/kg before breakfast and 0.1 U/kg before dinner as was done in the PREFER study (TABLE 13).45 Subsequent dosing can be determined based on the PREFER algorithm below. Of note is that at study end, premix insulin doses were equally divided between breakfast and dinner. Breakfast and dinner doses are titrated based on blood glucose levels before dinner and breakfast, respectively. In the PREFER study, the use of premix insulin provided comparable A1C reduction as basal-bolus therapy (basal once daily + bolus TID) in insulin-naïve patients. However, patients previously treated with basal insulin such as KW experienced greater A1C reductions with basal-bolus insulin than with premix insulin.
1. Rodbard HW, Jellinger PS, Davidson JA, et al. Statement by an American Association of Clinical Endocrinologists/American College of Endocrinology consensus panel on type 2 diabetes mellitus: an algorithm for glycemic control [published correction appears in Endocr Pract. 2009;15(7):768-770]. Endocr Pract. 2009;15(6):540-559.
2. Inzucchi SE, Bergenstal RM, Buse JB, et al. Management of hyperglycemia in type 2 diabetes: a patient-centered approach: position statement of the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD) [published online ahead of print April 19, 2012]. Diabetes Care. doi:10.2337/dc12-0413.
3. Lalić NM, Micić D, Antić S, et al. Effect of biphasic insulin aspart on glucose and lipid control in patients with type 2 diabetes mellitus. Expert Opin Pharmacother. 2007;8(17):2895-2901.
4. Reynolds LR, Kingsley FJ, Karounos DG, Tannock LR. Differential effects of rosiglitazone and insulin glargine on inflammatory markers, glycemic control, and lipids in type 2 diabetes. Diabetes Res Clin Pract. 2007;77(2):180-187.
5. Rosak C, Jung R, Hofmann U. Insulin glargine maintains equivalent glycemic control and better lipometabolic control than NPH insulin in type 1 diabetes patients who missed a meal. Horm Metab Res. 2008;40(8):544-548.
6. Ampudia-Blasco FJ, Girbes J, Carmena R. A case of lipoatrophy with insulin glargine: long-acting insulin analogs are not exempt from this complication. Diabetes Care. 2005;28(12):2983.-
7. Griffin ME, Feder A, Tamborlane WV. Lipoatrophy associated with lispro insulin in insulin pump therapy: an old complication, a new cause? Diabetes Care. 2001;24(1):174.-
8. Fineberg SE, Huang J, Brunelle R, Gulliya KS, Anderson JH, Jr. Effect of long-term exposure to insulin lispro on the induction of antibody response in patients with type 1 or type 2 diabetes. Diabetes Care. 2003;26(1):89-96.
9. Moyes V, Driver R, Croom A, Mirakian R, Chowdhury TA. Insulin allergy in a patient with type 2 diabetes successfully treated with continuous subcutaneous insulin infusion. Diabet Med. 2006;23(2):204-206.
10. Ghosh S, McCann V, Bartle L, Collier A, Malik I. Allergy to insulin detemir. Diabet Med. 2007;24(11):1307.-
11. Blumer I. Severe, delayed insulin detemir injection site reaction. Diabet Med. 2008;25(8):1008.-
12. Pérez E, González R, Martínez J, Iglesias J, Matheu V. Detemir insulin-induced anaphylaxis. Ann Allergy Asthma Immunol. 2009;102(2):174-175.
13. Mollar-Puchades MA, Villanueva IL. Insulin glulisine in the treatment of allergy to rapid acting insulin and its rapid acting analogs. Diabetes Res Clin Pract. 2009;83(1):e21-e22.
14. Kawasaki F, Kamei S, Tatsumi F, et al. Gallbladder edema in type 1 diabetic patient due to delayed-type insulin allergy. Intern Med. 2009;48(17):1545-1549.
15. Wang C, Ding ZY, Shu SQ, et al. Severe insulin allergy after percutaneous transluminal coronary angioplasty. Clin Ther. 2009;31(3):569-574.
16. Ozaki N, Oiso Y. Immunologic tolerance to the insulin analogue glulisine. Diabetes Care. 2010;33(3):e39.-
17. Koroscil T, Kagzi Y, Zacharias D. Failure of multiple therapies in the treatment of a type 1 diabetic patient with insulin allergy: a case report. Endocr Pract. 2011;17(1):91-94.
18. Tuerk PW, Mueller M, Egede LE. Estimating physician effects on glycemic control in the treatment of diabetes: methods, effects sizes, and implications for treatment policy. Diabetes Care. 2008;31(5):869-873.
19. Hajos TR, Polonsky WH, Twisk JW, Dain MP, Snoek FJ. Do physicians understand type 2 diabetes patients’ perceptions of seriousness; the emotional impact and needs for care improvement? A cross-national survey. Patient Educ Couns. 2011;85(2):258-263.
20. Polonsky WH, Hajos TR, Dain MP, Snoek FJ. Are patients with type 2 diabetes reluctant to start insulin therapy? An examination of the scope and underpinnings of psychological insulin resistance in a large, international population. Curr Med Res Opin. 2011;27(6):1169-1174.
21. Nam S, Chesla C, Stotts NA, Kroon L, Janson SL. Factors associated with psychological insulin resistance in individuals with type 2 diabetes. Diabetes Care. 2010;33(8):1747-1749.
22. Nakar S, Yitzhaki G, Rosenberg R, Vinker S. Transition to insulin in type 2 diabetes: family physicians’ misconception of patients’ fears contributes to existing barriers. J Diabetes Complications. 2007;21(4):220-226.
23. Snoek FJ. Breaking the barriers to optimal glycaemic control—what physicians need to know from patients’ perspectives. Int J Clin Pract Suppl. 2002;(129):80-84.
24. Korytkowski M. When oral agents fail: practical barriers to starting insulin. Int J Obes Relat Metab Disord. 2002;26(Suppl 3):S18-S24.
25. Skovlund SE, Peyrot M. The Diabetes Attitudes, Wishes and Needs (DAWN) program: a new approach to improving outcomes of diabetes care. Diabetes Spectrum. 2005;18(3):136-142.
26. Jenkins N, Hallowell N, Farmer AJ, Holman RR, Lawton J. Initiating insulin as part of the Treating To Target in Type 2 Diabetes (4-T) trial: an interview study of patients’ and health professionals’ experiences. Diabetes Care. 2010;33(10):2178-2180.
27. Levinson W, Gorawara-Bhat R, Lamb J. A study of patient clues and physician responses in primary care and surgical settings. JAMA. 2000;284(8):1021-1027.
28. Rodriguez HP, Anastario MP, Frankel RM, et al. Can teaching agenda-setting skills to physicians improve clinical interaction quality? A controlled intervention. BMC Med Educ. 2008;8:3.-
29. Solomon J. How strategies for managing patient visit time affect physician job satisfaction: a qualitative analysis. J Gen Intern Med. 2008;23(6):775-780.
30. Funnell MM, Anderson RM. Empowerment and self-management of diabetes. Clin Diabetes. 2004;22(3):123-127.
31. Funnell MM, Anderson RM. Are patients or outcomes more important? Rev Endocrinol. 2008;2(8):49-51.
32. Shaefer CF. Clinical inertia: overcoming a major barrier to diabetes management. Insulin. 2006;1(2):61-64.
33. Harris S, Yale JF, Dempsey E, Gerstein H. Can family physicians help patients initiate basal insulin therapy successfully?: randomized trial of patient-titrated insulin glargine compared with standard oral therapy: lessons for family practice from the Canadian INSIGHT trial. Can Fam Physician. 2008;54(4):550-558.
34. Centers for Disease Control and Prevention. National diabetes fact sheet national estimates and general information on diabetes and prediabetes in the United States, 2011. Atlanta, GA: U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, 2011. http://www.cdc.gov/diabetes/pubs/pdf/ndfs_2011.pdf. Accessed February 10, 2011.
35. Riddle MC, Rosenstock J, Gerich J. Insulin Glargine 4002 Study Investigators. The treat-to-target trial: randomized addition of glargine or human NPH insulin to oral therapy of type 2 diabetic patients. Diabetes Care. 2003;26(11):3080-3086.
36. Hermansen K, Davies M, Derezinski T, Martinez Ravn G, Clauson P, Home P. A 26-week, randomized, parallel, treat-to-target trial comparing insulin detemir with NPH insulin as add-on therapy to oral glucose-lowering drugs in insulin-naive people with type 2 diabetes [published correction appears in Diabetes Care. 2007;30(4):1035]. Diabetes Care. 2006;29(6):1269-1274.
37. Davies M, Storms F, Shutler S, Bianchi-Biscay M, Gomis R. ATLANTUS Study Group. Improvement of glycemic control in subjects with poorly controlled type 2 diabetes: comparison of two treatment algorithms using insulin glargine. Diabetes Care. 2005;28(6):1282-1288.
38. Meneghini L, Koenen C, Weng W, Selam JL. The usage of a simplified self-titration dosing guideline (303 Algorithm) for insulin detemir in patients with type 2 diabetes—results of the randomized, controlled PREDICTIVE 303 study. Diabetes Obes Metab. 2007;9(6):902-913.
39. Garber AJ, Wahlen J, Wahl T, et al. Attainment of glycaemic goals in type 2 diabetes with once-, twice-, or thrice-daily dosing with biphasic insulin aspart 70/30 (The 1-2-3 study). Diabetes Obes Metab. 2006;8(1):58-66.
40. Philis-Tsimikas A, Charpentier G, Clauson P, Ravn GM, Roberts VL, Thorsteinsson B. Comparison of once-daily insulin detemir with NPH insulin added to a regimen of oral antidiabetic drugs in poorly controlled type 2 diabetes [published correction appears in Clin Ther. 2006;28(11):1967]. Clin Ther. 2006;28(10):1569-1581.
41. Blonde L, Merilainen M, Karwe V, Raskin P. TITRATE Study Group. Patient-directed titration for achieving glycaemic goals using a once-daily basal insulin analogue: an assessment of two different fasting plasma glucose targets—the TITRATE study. Diabetes Obes Metab. 2009;11(6):623-631.
42. Meneghini L, Mersebach H, Kumar S, Svendsen AL, Hermansen K. Comparison of 2 intensification regimens with rapid-acting insulin aspart in type 2 diabetes mellitus inadequately controlled by once-daily insulin detemir and oral antidiabetes drugs: the step-wise randomized study. Endocr Pract. 2011;17(5):727-736.
43. Lankisch MR, Ferlinz KC, Leahy JL, Scherbaum WA. Orals Plus Apidra and LANTUS (OPAL) study group. Introducing a simplified approach to insulin therapy in type 2 diabetes: a comparison of two single-dose regimens of insulin glulisine plus insulin glargine and oral antidiabetic drugs [published correction appears in Diabetes Obes Metab. 2010;12(5):461]. Diabetes Obes Metab 2008;10(12):1178-1185.
44. Meneghini L. Why and how to use insulin therapy earlier in the management of type 2 diabetes. South Med J. 2007;100(2):164-174.
45. Liebl A, Prager R, Binz K, Kaiser M, Bergenstal R, Gallwitz B. PREFER Study Group. Comparison of insulin analogue regimens in people with type 2 diabetes mellitus in the PREFER Study: a randomized controlled trial. Diabetes Obes Metab. 2009;11(1):45-52.
46. Braun A, Sämann A, Kubiak T, et al. Effects of metabolic control, patient education and initiation of insulin therapy on the quality of life of patients with type 2 diabetes mellitus. Patient Educ Couns. 2008;73(1):50-59.
The Evolution of Insulin Therapy in Diabetes Mellitus
The modern management of diabetes mellitus (DM) began with the discovery of insulin by Banting and Best in 1921 (see The Evolution of Insulin Therapy in Diabetes Mellitus in this supplement). Since that time, numerous additional classes of glucose-lowering agents have been introduced for the treatment of type 2 DM (T2DM). These medications primarily act by addressing 2 of the key defects of T2DM, insulin resistance and pancreatic β-cell dysfunction. T2DM is a progressive disease process that requires continued adjustment of therapy to maintain treatment goals. Most patients with T2DM will require insulin therapy at some point in their lives.
Role of Insulin in Type 2 Diabetes Mellitus Management
Consensus guidelines developed by the American Association of Clinical Endocrinologists/American College of Endocrinology (AACE/ACE) recommend initiating insulin when oral therapy fails to achieve glycemic control, A1C > 9.0% in treatment-naïve patients, or if the patient is symptomatic with glucose toxicity (polyuria, polydipsia, and weight loss) (FIGURE 1).1
Similar consensus guidelines developed by the American Diabetes Association/European Association for the Study of Diabetes (ADA/EASD) advise the initiation of glucose-lowering therapy for most patients with T2DM with the combination of lifestyle modifications, diet, and metformin (FIGURE 2).2 For patients who do not achieve or maintain glycemic control over 3 months, or thereabouts, with metformin, a second oral agent should be added. Alternatives include a glucagon-like peptide-1 receptor (GLP-1R) agonist or basal insulin. Insulin should be strongly considered as initial therapy for a patient with significant symptoms of hyperglycemia and/or plasma glucose >300-350 mg/dL or A1C ≥10.0%.
The major role of insulin in the management of patients with T2DM stems from several important attributes. First, insulin is the only treatment that works in patients with advanced β-cell deficiency. It acts directly on tissues to regulate glucose homeostasis, unlike other glucose-lowering agents that require the presence of sufficient endogenous insulin to exert their effects as insulin sensitizers, secretagogues, incretin mimetics, amylin analogs, and other factors. This also means that the mechanism of action of insulin is complementary to those of other glucose-lowering agents. Second, there is less of a ceiling effect with insulin. That is, increasing the dose of insulin results in a progressive lowering of blood glucose in the majority of patients, with the major limitation being the risk for hypoglycemia. Third, the glucose-lowering efficacy of insulin is durable, unlike that of other glucose-lowering agents that depend on endogenous insulin secretion for continued effectiveness. Fourth, insulin improves the lipid profile, particularly triglyceride levels.2-5 Fifth, regarding the long-term safety and tolerability of insulin, it is well established that weight gain, likely mediated via reduction of glycosuria, and hypoglycemia are typically the most concerning adverse events encountered. Allergic reactions, which were a more common complication of animal-sourced insulins, are infrequent with the insulin analogs.6-17 Finally, the availability of insulin in different formulations allows for targeting fasting plasma glucose or postprandial glucose, and individualization of therapy (see The Evolution of Insulin Therapy in Diabetes Mellitus in this supplement.)
While both the AACE/ACE and ADA/EASD consensus guidelines provide treatment “algorithms,” both make it clear that these are suggested approaches suitable for the population with T2DM (FIGURE 1, FIGURE 2). The specific treatment approach must be individualized based on patient-specific factors such as age, comorbid conditions, and tolerance of hypoglycemia.
FIGURE 1
Role of insulin in the management of patients with type 2 diabetes mellitus according to the AACE/ACE1
AACE, American Association of Clinical Endocrinologists; ACE, American College of Endocrinology; AGI, α-glucosidase inhibitor; DPP4, dipeptidyl-peptidase-4 inhibitor; FPG, fasting plasma glucose; GLP-1, glucagon–like peptide-1 agonist; MET, metformin; PPG, postprandial glucose; SU, sulfonylurea; TZD, thiazolidinedione.
Reprinted from American Association of Clinical Endocrinologists. AACE/ACE Diabetes Algorithm for Glycemic Control. Available at https://www.aace.com/sites/default/files/GlycemicControlAlgorithmPPT.pdf. Accessed April 4, 2012, with permission from the American Association of Clinical Endocrinologists.
FIGURE 2
Role of insulin in the management of patients with type 2 diabetes mellitus according to the ADA/EASD2
Moving from the top to the bottom of the figure, potential sequences of antihyperglycemic therapy. In most patients, begin with lifestyle changes; metformin monotherapy is added at, or soon after, diagnosis (unless there are explicit contraindications). If the HbA1c target is not achieved after ~3 months, consider 1 of the 5 treatment options combined with metformin: an SU, TZD, DPP-4-i, GLP-1-RA, or basal insulin. (The order in the chart is determined by historical introduction and route of administration and is not meant to denote any specific preference.) Choice is based on patient and drug characteristics, with the over-riding goal of improving glycemic control while minimizing side effects. Shared decision making with the patient may help in the selection of therapeutic options. The figure displays drugs commonly used both in the United States and/or Europe. Rapid-acting secretagogues (meglitinides) may be used in place of SUs. Other drugs not shown (α-glucosidase inhibitors, colesevelam, dopamine agonists, pramlintide) may be used where available in selected patients but have modest efficacy and/or limiting side effects. In patients intolerant of, or with contraindications for, metformin, select initial drug from other classes depicted and proceed accordingly. In this circumstance, while published trials are generally lacking, it is reasonable to consider 3-drug combinations other than metformin. Insulin is likely to be more effective than most other agents as a third-line therapy, especially when HbA1c is very high (eg, ≥ 9.0%). The therapeutic regimen should include some basal insulin before moving to more complex insulin strategies. Dashed arrow line on the left-hand side of the figure denotes the option of a more rapid progression from a 2-drug combination directly to multiple daily insulin doses, in those patients with severe hyperglycemia (eg, HbA1c, ≥ 10.0–12.0%).
DPP-4, dipeptidyl peptidase-4; DPP-4-i, DPP-4 inhibitor; Fx’s, bone fractures; GI, gastrointestinal; GLP-1, glucagon-like peptide 1; GLP-1-RA, GLP-1 receptor agonist; HbA1c, hemoglobin A1c; HF, heart failure; NPH, neutral protamine Hagedorn; SU, sulfonylurea; TZD, thiazolidinedione.
aConsider beginning at this stage in patients with very high HbA1c (eg, ≥ 9%); bConsider rapid-acting, non-SU secretagogues (meglitinides) in patients with irregular meal schedules or who develop late postprandial hypoglycemia on SUs; cUsually a basal insulin (NPH, glargine, detemir) in combination with noninsulin agents; dCertain noninsulin agents may be continued with insulin. Consider beginning at this stage if patient presents with severe hyperglycemia (≥ 16.7–19.4 mmol/L [≥ 300–350 mg/dL]; HbA1c≥ 10.0–12.0%) with or without catabolic features (weight loss, ketosis, etc).
Diabetes Care by American Diabetes Association. Copyright 2012. Reproduced with permission of AMERICAN DIABETES ASSOCIATION in the format Journal via Copyright Clearance Center.
Individualizing Therapy
The importance of individualizing therapy in a way that allows patients with T2DM to effectively self-manage their disease cannot be overstated. A study involving 1381 patients with T2DM cared for by 42 primary care physicians was conducted to estimate the magnitude of effect that physicians have on glycemic control.18 Hierarchical linear modeling showed that physician-related factors were associated with a statistically significant but modest variability in A1C change (2%) for the entire patient group. On the face of it, this finding might be discouraging. Further analysis showed, however, that for patients whose A1C did improve, physician-related factors accounted for 5% of the overall change in A1C (P = .005). On the other hand, physician-related factors had no impact on patients whose A1C did not improve or worsened. These results support the role that physicians play in affecting patient outcomes. The results also make it clear that without a physician’s influence, a patient’s glycemic outcomes may be difficult to change. The question is: How best can a physician influence patient outcomes?
A 2011 survey of patients with DM, general practitioners, and DM specialists reported that clinicians tended to underestimate patients’ perceived seriousness of the disease, while overestimating patients’ level of distress. In addition, physicians had difficulty identifying which DM-related complications concerned patients most and the information and support patients needed to feel more at ease with DM. Patients placed greater importance on having easy access to their physicians rather than more time with them. But most importantly, the survey investigators concluded that patients generally wished for greater involvement in decision making and being provided more information.19 These findings suggest that patients understand that T2DM is a largely self-managed, chronic disease, and want a collaborative relationship with their physician.
Patient Barriers to Insulin Therapy
Numerous factors have been identified as impeding patients’ willingness to initiate insulin therapy (TABLE 1).20-24 Barriers often vary from patient to patient and, in fact, may change over time in an individual patient. It is crucial, therefore, to identify the root reasons for a patient’s apprehension with insulin when talking about options for intensifying treatment. Once insulin has been initiated, the patient should be asked about continuing or new concerns regarding insulin therapy (and DM management in general), including adherence.
TABLE 1
Barriers to insulin therapy identified by patients20-24
| Lack of understanding of serious nature of type 2 diabetes mellitus |
| Fear of addiction to insulin |
| Fear of hypoglycemia |
| Concern about weight gain |
| Repeated experiences of failing to achieve satisfactory glycemic control |
| Perception that quality of previous treatment was low |
| Needle phobia |
| Treatment complexity |
| Concern of social stigmatization |
| Perceived failure and low self-efficacy |
| Belief of becoming more ill |
| Out-of-pocket cost |
| Perceived negative impact on quality of life |
| Comorbidities such as poor eyesight, arthritis, forgetfulness |
A recent, international survey of 1400 patients with insulin-naïve T2DM reported that 3 negative beliefs about insulin were prominent: (1) feeling that the disease was worsening; (2) fear of injection; and (3) a feeling of personal failure.20 Certain patient comorbidities, such as poor eyesight, arthritis, and forgetfulness, might also serve as barriers to self-management of DM with insulin. Additional comorbidities may contribute as indirect barriers, such as the need for polypharmacy, which may make the initiation of additional treatments such as insulin logistically or financially difficult.
It is possible that the discussion about initiating insulin may uncover patient concerns about T2DM in general. The Diabetes Attitudes, Wishes, and Needs (DAWN) study reported that psychosocial issues were the major source of difficulty in patient self-management (TABLE 2).25 In fact, 85% of people who reported a high level of distress at the time of diagnosis of T2DM continued to experience psychological distress at a mean follow-up of 15 years.
TABLE 2
Patients experiencing various aspects of diabetes-related distress25
| Diabetes-related distress | Respondents who agree (%) |
|---|---|
| I feel stressed because of my diabetes. | 32.7 |
| I feel burned out because of my diabetes. | 18.1 |
| I feel that diabetes is preventing me from doing what I want to do. | 35.9 |
| I am constantly afraid of my diabetes getting worse. | 43.8 |
| I worry about not being able to carry out my family responsibilities in the future. | 30.1 |
| My diabetes causes me worries about my financial future. | 25.8 |
| My family and friends put too much pressure on me about my diabetes. | 14.7 |
| The community I live in is intolerant of diabetes. | 13.6 |
| Diabetes Care by American Diabetes Association. Copyright 2012. Reproduced with permission of AMERICAN DIABETES ASSOCIATION in the format Journal via Copyright Clearance Center. | |
Addressing psychosocial issues and other barriers is crucial in the discussion of self-management because those with more negative feelings about starting insulin are most unwilling to start insulin.20 One factor that may contribute to these negative feelings is repeated experiences of failing to achieve satisfactory glycemic control with oral glucose-lowering agents.23 Conversely, those who have experienced improved glycemic control with intensification of prior glucose-lowering therapy may be more accepting of initiating insulin therapy.23,26 These findings are a reminder of the importance of a treat-to-target approach to management, in which the target glycemic goal, generally A1C < 7.0%, is achieved within 2 to 3 months of diagnosis and maintained at that level through intensification of therapy as needed.
Addressing psychosocial issues can be a challenge in today’s busy primary care practice due to limited time and lack of training in the management of such issues. However, implementation of various strategies has been reported to facilitate and, in some cases, shorten a patient’s visit. For example, one small study reported that visits were shorter if the physician acknowledged and responded positively to a patient’s stated or implied concerns (17.6 minutes vs 20.1 minutes).27 Missing or ignoring the patient’s concerns often led the patient to bring up the same concern one or more additional times resulting in a longer office visit. These results underscore the importance of asking patients to identify their concerns or questions at the beginning of the office visit. The patient can fill out a questionnaire in the waiting room or be encouraged to write down and prioritize their questions and concerns specific to the visit. If the patient identifies more concerns or questions than can be reasonably addressed in one visit, there should be agreement to address the most pressing ones during the current visit and the remaining concerns and questions during the next visit. This “agenda-setting” approach has been reported to offer several advantages.28 From the patient’s perspective, the quality of the physician-patient interaction was much improved, in part because physicians took time to explain points in a way that was easy to understand. Advantages to the physician with an agenda-setting approach included “feeling more in control,” “less stressed by simply knowing what was on the patient’s mind,” “feeling less rushed,” and “enjoying patient encounters more.” Contrary to the study cited above, physicians found that patients’ visits often were longer, especially those of older patients. One physician, however, noted that the visit “takes more time now, but saves time later.” As noted in this study, additional time spent with the patient can lead to improved job satisfaction for the physician.29
The agenda-setting approach requires that the physician ask the patient to list his or her concerns and questions, and then actively listen to the patient. Once the agenda for the visit is established, employing the “ask, listen, empathize” communication style can lead to effective physician-patient communication and problem-solving. Using this approach, the physician asks questions to gain a clear understanding of the patient’s concerns and then uses active listening with little, if any, interruption.30,31 Since the goal is to solve problems with rather than for the patient, active listening without offering opinions, judgements, or advice while offering empathy is essential. Through reflection and discussion, the physician can help the patient to identify his or her issues and acceptable solutions.
The importance of good communication between physician and patient cannot be overstated. Additional communication skills to keep in mind are: (1) speak slowly using nonmedical language; (2) limit the amount of information and repeat it; (3) draw pictures and/or use visual aids; and (4) ask the patient to repeat instructions and key concepts. In addition to enhancing patients’ understanding, visual images may be particularly beneficial in keeping patients motivated to improve self-management, including adherence to therapy. For example, it may be helpful to graphically track the patient’s glycemic progress. This can be done by establishing an actionable A1C goal (generally < 7.0%) and a time frame to achieve the goal (eg, 2 to 3 months).32 A graph can be constructed beginning with the patient’s current, preinsulin A1C level, with updates at each visit. In addition to motivating the patient and reinforcing adherence, the graph can also be used to demonstrate when further treatment intensification is needed. Additional general strategies that can be employed when considering the initiation of insulin are shown in TABLE 3. Implementation of strategies such as these by family physicians provides patient outcomes comparable to those implemented by endocrinologists or diabetes specialists.33
TABLE 3
General strategies for initiating insulin therapy
| Invite the patient to take an active role in treatment decisions. |
| Remind the patient that type 2 diabetes is primarily self-managed. |
| Discuss the progressive nature of β-cell dysfunction in type 2 diabetes. |
| Emphasize the physiologic role of insulin to maintain glucose homeostasis. |
| Discuss that insulin will help to achieve glycemic control and minimize the risk for long-term complications. |
| Discuss that treatment will be modified as needed to maintain glycemic control and to best meet their needs, capabilities, and interest. |
| Utilize insulin pen devices whenever possible. |
| Emphasize the importance of lifestyle management. |
| Ask if hearing other patients talk of their experiences with insulin therapy would be helpful; consider a group office visit. |
| Discuss and provide the patient with an individualized, written action plan that includes insulin dosing, self-monitoring of blood glucose, and signs/symptoms of hypoglycemia and other adverse events with appropriate action(s) to take. |
| Simplify diabetes (and comorbidities) treatment whenever possible. |
The remainder of this article uses case studies to further explore various patient barriers to insulin therapy and strategies for addressing them with the patient. While other therapies may be appropriate in the case studies below as recommended by current guidelines, these case studies will focus on insulin. In addition, dosing strategies for initiating and intensifying insulin therapy are discussed. Changes to the treatment plan to adjust for comorbidities, such as hypertension and dyslipidemia, or for smoking cessation or aspirin therapy, are not addressed in these cases, but are crucial components of comprehensive management.
CASE STUDY 1
RF is a 49-year-old female insurance analyst diagnosed with T2DM 6 years ago. Initial therapy with lifestyle modifications and metformin has since been intensified. Glimepiride was added, then pioglitazone was added 1.5 years ago when the A1C had risen to 7.5%. There is no evidence of cardiovascular disease. She reports bothersome lower extremity edema and an 8-pound weight increase since starting pioglitazone treatment. RF states that she takes her medications every day, although she acknowledges that she sometimes forgets on Sundays.
Clinical Impression
After taking her history, performing a physical examination, and reviewing her laboratory and self-monitored blood glucose (SMBG) data, her physician concludes that her treatment plan needs to be changed (TABLE 4, TABLE 5).
TABLE 4
Case study 1: Chart notes
| Physical examination | Laboratory tests | Lifestyle habits | Current therapy | |
|---|---|---|---|---|
| Glucose-lowering | Other | |||
| BP: 126/80 mm Hg Weight: 176 lb (79.2 kg) BMI: 27 kg/m2 Eyes: no retinopathy Neurology: intact Skin: intact | SCr: 1.4 mg/dL Albuminuria: negative A1C: 8.2% Cholesterol: Total: 204 mg/dL LDL: 134 mg/dL HDL: 36 mg/dL | Exercise: Walks 2 miles 3-4 d/wk Nutrition: eats 3-4 meals/d | Metformin 1000 mg BID Glimepiride 8 mg QD Pioglitazone 45 mg QD | Lisinopril 30 mg QD Simvastatin 40 mg QD ASA 80 mg QD |
| ASA, acetylsalicylic acid; BMI, body mass index; BP, blood pressure; HDL, high-density lipoprotein; LDL, low-density lipoprotein; SCr, serum creatinine. | ||||
TABLE 5
Case study 1: Self-monitored blood glucose (mg/dL) over the previous 2 weeks
| Day | Fasting | 2 h Post-breakfast | 2 h Post-lunch | 2 h Post-dinner |
|---|---|---|---|---|
| Wednesday | 205 | |||
| Thursday | 158 | |||
| Friday | 179 | |||
| Saturday | 201 | 162 | ||
| Sunday | ||||
| Monday | 166 | |||
| Tuesday | 189 | |||
| Wednesday | ||||
| Thursday | 153 | 221 | ||
| Friday | 150 | |||
| Saturday | 199 | 186 | 213 | |
| Sunday | ||||
| Monday | 181 | |||
| Tuesday | 167 |
Treatment Plan
- Initiate basal insulin once daily in the evening.
- Continue glimepiride, but reduce pioglitazone to 15 mg once daily (or discontinue if cost is a concern).
- Ask RF to monitor fasting blood glucose and self-adjust insulin doses as appropriate.
Barriers
While discussing the need to change the treatment plan and the physician’s suggestion that RF begin basal insulin, RF asks her physician for another few months on her current regimen stating that she will try harder to take her medications on Sundays. She also voices concern that insulin treatment requires injections and that she is concerned about what her coworkers and friends might think. The physician confirms that these concerns are understandable; he also confirms that RF is fearful of needles. The following are possible responses that RF’s physician could use to address these concerns.
Patient’s concern: Perceived failure/low self-efficacy
Physician responses:
- We all forget to do things from time to time, but overall I think you have done a great job taking your medications.
- As we have talked about before, with T2DM there is a progressive loss of insulin production over time regardless of what you do. That is why we added glimepiride and then pioglita-zone and that is why we need to make a change now and put you back in control of your diabetes. It is likely that further changes will be needed and we can discuss and agree on them together.
Patient’s concern: Social stigmatization
Physician responses:
- We can begin by having you administer insulin once daily in the evening in the privacy of your home.
- The insulin can be administered with a device that looks like a pen. It is small and can be carried in your purse; it does not need to be refrigerated once opened. If the time comes that you will need to administer a dose of insulin during the day, you can easily administer the insulin discretely in a public restroom or your work area.
- The use of insulin is more common than it was even a few years ago. In fact, about 5 million people in the United States use insulin to replace what is missing, control blood sugars, and decrease the risk for diabetes complications.34
Patient’s concern: Fear of needles
Physician’s responses:
- Insulin can be injected using a pen device with short, ultrathin needles that makes most of the injections painless. I would like you to see how simple and painless the injection can be by using this sample pen here in the office.
- Many patients are concerned about giving themselves an injection at first, but they quickly become comfortable doing so.
Dosing
Treatment with basal insulin can be initiated using one of several approaches. Using the treat-to-target approach, basal insulin 10 U once daily is initiated.35 The starting dose should be reduced to 6 U if the initial pre-breakfast or pre-dinner blood glucose is < 126 mg/dL or the patient’s body mass index (BMI) is < 26 kg/m2.36 Alternatively, the ADA/EASD recommends starting with 0.2 U/kg, which may be more practical in very overweight or obese patients.2 Titration of the basal insulin dose can be accomplished using one of the following physician-directed or patient-driven treat-to-target titration algorithms (TABLE 6).35,37,38 The insulin dose should be titrated based on the pre-breakfast fasting blood glucose level.
TABLE 6
Physician-directed or patient-driven treat-to-target titration algorithms
| Riddle et al35 | Davies et al37 | Meneghini et al38 | ||||
|---|---|---|---|---|---|---|
| Start with 10 U/d bedtime basal insulin and adjust weekly | Start with 10* U/d bedtime basal insulin and adjust weekly (physician-directed) Or Start with a dose numerically equivalent to the highest FPG (in millimoles/L)† over the previous 7 days and adjust every 3 days (patient-managed) | Start with basal insulin once daily and adjust every 3 days | ||||
| Mean of self-monitored FPG values from preceding 2 days | Change in insulin dose (U/d)# | Mean of self-monitored FPG values from preceding 3 days | Change in insulin dose (U/d) (physician-directed) | Change in insulin dose (U/d) (patient-managed) | Mean of self-monitored FPG values from preceding 3 days | Change in insulin dose (U/d) |
| ≥180 mg/dL 140-180 mg/dL 120-140 mg/dL 100-120 mg/dL | +8 +6 +4 +2 | ≥180 mg/dL (≥10 mmol/L) 140-179 mg/dL (7.8-9.9 mmol/L) 120-139 mg/dL (6.7 – 7.7 mmol/L) 100-119 mg/dL (5.5-6.6 mmol/L) | +6 to +8 +4 +2 0 to +2 | +2 +2 +2 0 to +2 | >110 mg/dL 80-110 mg/dL <80 mg/dL | +3 0 -3 |
| FPG, fasting plasma glucose. *In insulin-naive patients. †For example, if the highest FPG over the previous 7 days was 7 mmol/L, start with 7 U.#Small insulin dose decreases (2-4 U/d per adjustment) were allowed if severe hypoglycemia (requiring assistance) or plasma-referenced glucose < 56 mg/dL was documented in the preceding week. Reproduced with permission. Meneghini LF, et al. J Fam Pract. 2011;60(9 Suppl 1):S21-S28. Quadrant HealthCom Inc. Copyright 2011. | ||||||
Follow-Up Visit
RF begins basal insulin 10 U in the evening and is given simple instructions for insulin dose titration based on fasting plasma glucose results. At her follow-up visit, RF reports that she has increased her basal insulin to 18 U administered once daily. Review of her SMBG results show that her blood glucose levels throughout the day have improved, but are still not at goal. RF’s physician commends her on the progress she has made. RF and her physician agree that she should continue to increase her basal insulin dose. Eight months after beginning basal insulin, RF is administering 28 U (0.35 U/kg) of basal insulin in the evening. Review of her SMBG results over the previous 2 weeks show that her blood glucose rises during the day and is highest after dinner; her current A1C is 7.2%.
Treatment Plan
- Discuss dietary and lifestyle complements to insulin therapy such as:
- Use SMBG to identify foods that raise her blood glucose.
CASE STUDY 2
LW is a 64-year-old male with longstanding hypertension diagnosed with T2DM 8 years ago for which he was treated initially with lifestyle management and metformin. He has since been treated with other oral agents as add-on therapy; glipizide was discontinued due to hypoglycemia when he skips meals (usually lunch); pioglitazone was discontinued after the patient expressed concerns about the risk for bladder cancer he heard on television. He has mild retinopathy and mild loss of vibration sensation in the feet; there is no evidence of cardiovascular disease. He was diagnosed with osteoarthritis 3 years ago.
Clinical Impression
After taking his history, performing a physical examination, and reviewing his laboratory and SMBG data, his physician concludes that his treatment plan needs to be changed (FIGURE 3, TABLE 7, TABLE 8).
TABLE 7
Case study 2: Chart notes
| Physical examination | Laboratory tests | Lifestyle habits | Current therapy | |
|---|---|---|---|---|
| Glucose-lowering | Other | |||
| BP: 124/76 mm Hg Weight: 204 lb (92.7 kg) BMI: 31 kg/m2 Eyes: mild retinopathy Neurology: occasional tingling on bottom of right foot Skin: intact | SCr: 1.9 mg/dL eGFR: 51 mL/min Albuminuria: negative A1C: 8.1% Cholesterol: Total: 218 mg/dL LDL: 118 mg/dL HDL: 55 mg/dL Triglyceride: 204 mg/dL | Exercise: takes dog on occasional walk but otherwise sedentary Nutrition: eats 4 meals/d | Metformin 1000 mg BID Acarbose 50 mg TID Sitagliptin 100 mg QD | Lisinopril/HCTZ 20/25 mg QD Amlodipine 10 mg QD Acetaminophen extended-release 650 mg TID ASA 80 mg QD |
| ASA, acetylsalicylic acid; BMI, body mass index; BP, blood pressure; eGFR, estimated glomerular filtration rate; HCTZ, hydrochlorothiazide; HDL, high-density lipoprotein; LDL, low-density lipoprotein; SCr, serum creatinine. | ||||
TABLE 8
Case study 2: Self-monitored blood glucose (mg/dL) over the previous 2 weeks
| Day | Fasting | 2 h Post-breakfast | 2 h Post-lunch | 2 h Post-dinner |
|---|---|---|---|---|
| Tuesday | 135 | |||
| Wednesday | ||||
| Thursday | 196 | |||
| Friday | 152 | 174 | ||
| Saturday | ||||
| Sunday | 208 | |||
| Monday | 142 | 193 | ||
| Tuesday | ||||
| Wednesday | 130 | 156 | ||
| Thursday | ||||
| Friday | ||||
| Saturday | ||||
| Sunday | 151 | |||
| Monday |
FIGURE 3
Case study 2: A1C levels for April 2004 to March 2012
Treatment Plan
- Discontinue metformin since LW’s serum creatinine is > 1.5 mg/dL.
- Initiate either basal insulin once daily in the evening or premix insulin at dinner.
- Ask LW to monitor his blood glucose and self-adjust insulin doses as appropriate.
- Stress the importance of exercise and proper nutrition; gain agreement on short-term goals for exercise and nutrition.
Barriers
LW’s physician recommends that his treatment plan be changed and insulin therapy initiated. LW quickly responds that previous changes to his treatment regimen have not resulted in his achieving an A1C < 7.0%. He also doubts that he can use a syringe to draw up the correct dose and then self-administer due to his arthritis. The following are possible responses his physician could use to address these concerns.
Patient concern: Repeated experience of failing to achieve glycemic control, ie, A1C < 7.0%
Physician responses:
- While achieving an A1C < 7.0% is a realistic goal that reduces the risks for vascular complications of diabetes, any reduction of A1C will be of benefit.
- I would like to work with you to implement a new plan that we both believe will enable you to improve your diabetes control and ideally achieve an A1C < 7.0%.
Patient concern: Self-administering due to arthritis
Physician responses:
- Instead of using a syringe and vial to draw up and administer insulin, I would like you to use an insulin pen device. As you can see, it is easy to handle and you can easily select the correct dose.
- If you choose to start on premix insulin, the pen device contains both types of insulin together in one dose.
Dosing
Treatment with basal insulin once daily in the evening can be initiated and titrated based on pre-breakfast blood glucose as in Case Study 1. Alternatively, treatment with premix insulin can be initiated at a dose of 12 U administered within 15 minutes of dinner initiation. The premix dose can be titrated using the algorithm employed in the 1-2-3 Study based on pre-breakfast blood glucose (TABLE 9).39 After 16 weeks, 41% of patients in the 1-2-3 Study achieved an A1C < 7.0% from a baseline A1C of 8.6%.
TABLE 9
1-2-3 Study algorithm39
| Pre-breakfast SMBG (mg/dL) | Adjustment of pre-dinner dose (U) |
|---|---|
| <80 | -3 |
| 80-110 | No change |
| 111-140 | +3 |
| 141-180 | +6 |
| > 180 | +9 |
| SMBG, self-monitored blood glucose. | |
Follow-Up Visit
LW began basal insulin 10 U in the evening. Over the next 5.5 months, he titrated his dose such that his current dose is 46 U (0.50 U/kg) in the evening. His current A1C is 7.3%. Review of his SMBG shows consistently high 2-hour post-lunch blood glucose levels. Although further increasing his basal insulin dose is an option, in most of the treat-to-target studies, the daily dose of basal insulin given once daily averaged between 0.4 and 0.6 U/kg.35,37,40,41 LW and his physician agree that adding rapid-acting insulin at lunch is the best option. The starting dose of rapid-acting bolus insulin is 4 to 6 U administered prior to the largest meal of the day or, as in this case, prior to the meal with the largest postprandial blood glucose excursion.42,43 Alternatively, the dose of rapid-acting insulin could be calculated as 10% of the total daily dose of basal insulin, which in this case is 5 U (10% x 46 U). The dose of basal insulin would be reduced by 5 U if the rapid-acting insulin is given at dinner in order to reduce the risk for nocturnal hypoglycemia. The dose of the bolus insulin can be titrated using the ExtraSTEP algorithm (TABLE 10).42 Alternatively, the SimpleSTEP algorithm can be used which does not require a 2-hour postprandial glucose measurement.42
TABLE 10
Algorithms for adjusting insulin aspart42
| ExtraSTEP algorithm | SimpleSTEP algorithm | |||
|---|---|---|---|---|
| 2-h Post-meal PG level (mg/dL) | Insulin aspart adjustment (U) | Pre-meal BG (mg/dL) | Bedtime BG (mg/dL) | Insulin aspart adjustment (U) |
| <72* | -2 | <72* | <72* | -2 |
| 72-144 | 0 | 72-108 | 72-144 | 0 |
| 145-180 | +2 | 109-162 | 145-180 | +2 |
| >180 | +4 | >162 | >180 | +4 |
| BG, blood glucose; PG, plasma glucose. *One or more PG values <72 mg/dL without obvious explanation. Reproduced with permission. Meneghini LF, et al. J Fam Pract. 2011;60(9 Suppl 1):S21-S28. Quadrant HealthCom Inc. Copyright 2011. | ||||
Plan
- Begin rapid-acting insulin 5 U at lunch.
- Continue basal insulin at 46 U in the evening.
- Ask LW to continue to titrate basal insulin based on the pre-breakfast blood glucose level and the lunch time bolus insulin dose based on the 2-hour post-lunch SMBG (ExtraSTEP); alternatively, adjust based on the pre-dinner blood glucose level (SimpleSTEP).
CASE STUDY 3
MB is a 46-year-old male who had not consulted a physician since having a physical examination 6 years ago. He presented 2 weeks ago with frequent urination (7-8 times/day) and feeling tired; he also noted losing 5 pounds (2.25 kg) over the preceding 3.5 months despite no changes in his diet. MB is a regional salesperson with an erratic schedule. During the week, he eats lunch and most dinners in a restaurant. On the weekend, he goes to a local bar with his friends. He does light yard work, but does not exercise regularly. He is a current smoker with a 36 pack-year history. Urinalysis shows ketonuria and microalbuminuria. His A1C reported back today is 10.8%, confirming a diagnosis of uncontrolled and symptomatic DM.
Clinical Impression
After taking his history, performing a physical examination, and reviewing his laboratory data, MB’s physician confirms a diagnosis of DM (TABLE 11). While it is likely that MB has T2DM, his physician wants to rule out type 1 DM and latent autoimmune diabetes of the adult (LADA), so he orders tests for antibodies (GAD, IA-2, ICA). The antibody testing is negative, making T2DM the most likely diagnosis.
TABLE 11
Case study 3: Chart notes
| Physical examination | Laboratory tests | Lifestyle habits | Current therapy | |
|---|---|---|---|---|
| Glucose-lowering | Other | |||
| BP: 142/88 mm Hg Weight: 176 lb (79.2 kg) BMI: 27 kg/m2 Eyes: no retinopathy Neurology: intact Skin: intact | SCr: 1.4 mg/dL Microalbumin:creatinine ratio: 140 mg/g creatinine Ketonuria: 1+ A1C: 10.8% Cholesterol: Total: 210 mg/dL LDL: 146 mg/dL HDL: 30 mg/dL | Exercise: light yard work, no regular exercise Nutrition: 3 meals/d, eats most meals in a restaurant (lunch M-F; dinner 3-4 nights/wk) | None | None |
| BMI, body mass index; BP, blood pressure; HDL, high-density lipoprotein; LDL, low-density lipoprotein; SCr, serum creatinine. | ||||
Treatment Plan
- Initiate basal-bolus therapy with fixed bolus doses of rapid-acting insulin at each meal (prandial insulin).
- Ask MB to monitor blood glucose before meals and at bedtime.
- Provide MB with a supplemental scale to correct hyperglycemia before meals.
- Stress the importance of exercise and proper nutrition; gain agreement for short-term goals for exercise and nutrition; refer for diabetes and nutrition education if available.
- Discuss the importance of smoking cessation; develop a plan.
- Consider metformin and other non-insulin therapies when A1C is under control.
Barriers
MB is surprised that he has T2DM and is clearly anxious at receiving the diagnosis. He expresses concern about starting insulin because his uncle died within a year of starting insulin. MB also recalls that his uncle was always giving himself shots and monitoring his blood glucose level. He wants to know whether there is a simpler treatment option if he agrees to start insulin treatment. He also wants to know whether he will have to remain on insulin for the rest of his life. The following are possible responses his physician could use to address these concerns.
Patient concern: Fear of death
Physician responses:
- Uncontrolled high blood sugars over a long period of time can cause serious complications, such as kidney and heart disease that can result in death. That is why it is important that we work together to gain control of your blood sugar levels over the next few months and then modify your treatment as needed to maintain control.
- Unfortunately for many patients in the past, treatment with insulin was not used until it was too late and people already had serious complications from DM. This is likely the case for your uncle.
Patient concern: Treatment complexity
Physician responses:
- Right now we have to control your blood glucose rapidly so your pancreas can regain some function and your body can better respond to insulin.
- I will also provide you with step-by-step written instructions you can follow that describe how to start insulin and how to monitor your blood glucose.
- We will communicate as often as you need to adjust your insulin doses over the next few weeks; when you feel comfortable, I can even show you how to adjust your insulin dose before a meal to correct a high blood sugar.
- We can try this treatment for 3 months and then reevaluate your response, how you feel, and whether you want to continue to modify your treatment plan to keep your blood sugars controlled.
Patient concern: Lack of understanding that T2DM is a serious disease
Physician responses:
- Please understand that T2DM is a serious disease that increases your risk for heart disease, stroke, blindness, and other diseases. Unfortunately, since diabetes does not cause bad symptoms until it is actually too late, many patients do not make the effort to properly control their diabetes. By working together, we can reduce the risk for these complications and do some screening tests to detect any complications before they become irreversible.
Dosing
There are several approaches to determining the initial doses of basal and prandial (bolus) insulin. One approach is to estimate the total daily dose (TDD) of insulin by multiplying the patient’s weight in kilograms by 0.5 U/kg/d.44 Half of the TDD is given as basal insulin replacement; the other half is divided into 3 fixed preprandial doses of rapid-acting insulin. When the patient is ready to take on more complex management, the supplemental dose for bolus insulin can be calculated using a correction factor. If the bolus insulin is a rapid-acting insulin analog, 1800 is divided by the TDD of insulin; 1500 is used for a short-acting human insulin. This correction factor is an estimate of the fall in blood glucose per unit of bolus insulin. In our patient, the TDD would be: 80 kg x 0.5 U/kg/d or 40 U/d of insulin. Thus, 1 U of insulin should lower the blood glucose by about 45 mg/dL (1800/40 U = 45 mg/dL). For every 45 mg/dL above the pre-meal target, the patient would add 1 U of rapid-acting insulin to correct the hyperglycemia over the next 4 to 5 hours. The basal and prandial insulin doses would be titrated on a periodic basis (perhaps every 1 to 2 weeks) until the daytime levels of blood glucose are on target. The fasting (pre-breakfast) blood glucose would be used to adjust the basal insulin dose, while the pre-lunch, pre-dinner, and bedtime blood glucose results would be used to adjust the pre-breakfast, pre-lunch, and pre-dinner prandial (rapid-acting) insulin doses, respectively.
An alternative approach to initiating basal-bolus therapy is the PREFER algorithm.45 Here, the basal insulin dose is 10 U initially. The bolus doses are administered in a 3:1:2 ratio, so if the total of the 3 bolus doses is 12 U/d, the initial bolus doses would be 6 (breakfast), 2 (lunch), and 4 (dinner) U. The mean basal (once-daily) and bolus insulin doses observed in PREFER are shown in TABLE 12 and TABLE 13.
TABLE 12
Case study 3: Calculating initial basal-bolus insulin doses
| Algorithm | Calculations | Patient MB |
|---|---|---|
| Meneghini44 | TDD = (total body weight [kg]) (0.5 U/kg/d) Basal insulin dose* = (50%) (TDD) Bolus insulin dose† = (10%-20%) (TDD) | TDD = (0.5 U/kg/d)(80kg) = 40 U/d |
| Basal = (50%) (40 U/d) = 20 U/d | ||
| Bolus = (10%-20%) (40 U/d) = 4 to 8 U/meal | ||
| CF = 1800/40 U/d = 45 mg/dL per 1 unit | ||
| PREFER45 | Basal insulin dose* = 10 U (14 U if BMI > 32 kg/m2) Bolus insulin dose† = ratio of 3:1:2 (breakfast:lunch:dinner) Note: At week 26, the bolus insulin doses were divided into the 3 daily meals in approximately a 1:1:1 ratio | |
| BMI, body mass index; CF, correction factor; TDD, total daily dose of insulin. *Once daily; †Three meals per day. | ||
TABLE 13
Titrating the basal insulin dose using the PREFER algorithm45
| Pre-breakfast blood glucose (mg/dL) | Basal insulin dose adjustment (U) |
|---|---|
| < 56 | -4 |
| 56-72 | -2 |
| 73-125 | No change |
| 126-140 | +2 |
| 141-160 | +4 |
| 161-180 | +6 |
| 181-200 | +8 |
| > 200 | +10 |
Follow-up Visit
MB begins with basal insulin 20 U in the evening and bolus insulin at doses of 7 U before each meal. Over the next several months, MB has titrated his insulin doses; his current doses are: 32 U (basal), 11 U (bolus-breakfast), 7 U (bolus-lunch), and 10 U (bolus-dinner). He experienced 1 episode of mild hypoglycemia (SMBG, 50 mg/dL) one afternoon following a particularly active morning (TABLE 14). His current A1C is 7.4%. MB’s physician congratulates him on the progress he has made in dramatically lowering his blood glucose level—and his risk for diabetes-related complications. While MB appreciates his physician’s support and admits that he does not feel tired and generally feels better, which is likely due to resolution of glucotoxicity, he is not happy that he has gained 5.5 pounds (2.5 kg).46 He also finds the timing and administration of bolus insulin difficult.
TABLE 14
Case study 3: Self-monitored blood glucose (mg/dL) over the previous 2 weeks
| Day | Fasting | 2 h Post-breakfast | 2 h Post-lunch | 2 h Post-dinner |
|---|---|---|---|---|
| Wednesday | ||||
| Thursday | 168 | |||
| Friday | 106 | 166 | 174 | |
| Saturday | 88 | |||
| Sunday | 195 | |||
| Monday | 134 | |||
| Tuesday | 172 | |||
| Wednesday | 130 | 156 | ||
| Thursday | 112 | 168 | ||
| Friday | 92 | 164 | ||
| Saturday | 50 | 149 | 159 | 176 |
| Sunday | 94 | 174 | 210 | |
| Monday | 176 | 184 | ||
| Tuesday | 117 | 169 |
Plan
- Continue basal insulin once-daily in the evening.
- Add metformin 500 mg BID and increase to 1000 mg BID as tolerated.
- Consider weaning down the bolus insulin doses and substituting them with a GLP-1R agonist, dipeptidyl peptidase-4 inhibitor, or short-acting secretagogue. If so, continue rapid-acting insulin during transition. [Note: the following combinations are not currently approved by the US FDA: exenatide twice-daily and prandial insulin; exenatide once-weekly and insulin; liraglutide and prandial insulin; linagliptin and insulin.]
CASE STUDY 4
KW is a 62-year-old female diagnosed with T2DM 12 years ago. Treatment with lifestyle management and metformin initially provided glycemic control. Glimepiride was subsequently added and eventually the patient was started on basal insulin. The current dose of basal insulin is 60 U in the evening. Five months ago her A1C was found to be 7.9% and more recently 8.3%. She drinks alcohol occasionally and smokes. KW works as an executive secretary and has a consistent meal and activity schedule.
Clinical Impression
Following completion of the history, physical examination, and review of her laboratory data, KW’s physician concludes that her insulin regimen should be intensified (TABLE 15, TABLE 16).
TABLE 15
Case study 4: Chart notes
| Physical examination | Laboratory tests | Lifestyle habits | Current therapy | |
|---|---|---|---|---|
| Glucose-lowering | Other | |||
| BP: 126/78 mm Hg Weight: 176 lb (79.2 kg) BMI: 32 kg/m2 Eyes: no retinopathy Neurology: intact Skin: intact | SCr: 1.0 mg/dL Albuminuria: negative A1C: 8.3% Cholesterol Total: 172 mg/dL LDL: 96 mg/dL HDL: 46 mg/dL Triglycerides: 138 mg/dL | Exercise: sedentary Nutrition: 3 meals/d with large dinner | Metformin 1000 mg BID Basal insulin 60 U in the evening | ASA 80 mg QD Pravastatin 40 mg qHS |
| BMI, body mass index; BP, blood pressure; HDL, high-density lipoprotein; LDL, low-density lipoprotein; SCr, serum creatinine. | ||||
TABLE 16
Case study 4: Self-monitored blood glucose (mg/dL) over the previous 2 weeks
| Day | Fasting | 2 h Post-breakfast | 2 h Post-lunch | 2 h Post-dinner |
|---|---|---|---|---|
| Friday | ||||
| Saturday | 156 | 244 | ||
| Sunday | 253 | |||
| Monday | ||||
| Tuesday | ||||
| Wednesday | 148 | 227 | ||
| Thursday | ||||
| Friday | ||||
| Saturday | 179 | |||
| Sunday | 160 | |||
| Monday | ||||
| Tuesday | ||||
| Wednesday | ||||
| Thursday |
Plan
- Discontinue basal insulin.
- Begin premix insulin twice daily before breakfast and dinner.
- Ask KW to monitor blood glucose two times daily and, if appropriate, teach her how to self-adjust insulin doses.
- Stress the importance of exercise and proper nutrition; gain agreement on short-term goals for exercise and nutrition.
- Discuss the importance of smoking cessation; develop a plan.
Barriers
The physician discusses with KW that her consistent meal and activity schedule would make switching to premix insulin twice daily a good choice. KW is generally in agreement with the change, but wonders whether hypoglycemia might be more likely. She also asks if she might gain more weight in addition to the 3 pounds (1.35 kg) she has gained since starting basal insulin.
Patient concern: Hypoglycemia
Physician responses:
- Hypoglycemia remains a concern, and is more frequently seen with premix than with basal insulin; however, as long as you remain consistent with your meal and activity schedule, the risk for bad hypoglycemia is low.
- We should review your written action plan so that you are sure what signs or symptoms of a low blood sugar might occur and what you should do to treat them.
Patient concern: Weight gain
Physician responses:
- It is possible that you might gain a few additional pounds. You can avoid this by increasing your physical activity, and importantly, continue healthy eating. We should schedule a time for you to meet again with a dietician who can discuss options that might work for you.
Dosing
There are different approaches for converting from basal insulin to twice-daily premix insulin. One approach is to determine the TDD of basal insulin, and give half at breakfast and the other half at dinner as premix insulin.39 Since KW is taking 60 U of basal in the evening, she should take 30 U at breakfast and 30 U at dinner. Dose titration is according to the 1-2-3 Study algorithm shown in case study 2.
Another approach is to administer biphasic insulin aspart 70/30 0.2 U/kg before breakfast and 0.1 U/kg before dinner as was done in the PREFER study (TABLE 13).45 Subsequent dosing can be determined based on the PREFER algorithm below. Of note is that at study end, premix insulin doses were equally divided between breakfast and dinner. Breakfast and dinner doses are titrated based on blood glucose levels before dinner and breakfast, respectively. In the PREFER study, the use of premix insulin provided comparable A1C reduction as basal-bolus therapy (basal once daily + bolus TID) in insulin-naïve patients. However, patients previously treated with basal insulin such as KW experienced greater A1C reductions with basal-bolus insulin than with premix insulin.
The Evolution of Insulin Therapy in Diabetes Mellitus
The modern management of diabetes mellitus (DM) began with the discovery of insulin by Banting and Best in 1921 (see The Evolution of Insulin Therapy in Diabetes Mellitus in this supplement). Since that time, numerous additional classes of glucose-lowering agents have been introduced for the treatment of type 2 DM (T2DM). These medications primarily act by addressing 2 of the key defects of T2DM, insulin resistance and pancreatic β-cell dysfunction. T2DM is a progressive disease process that requires continued adjustment of therapy to maintain treatment goals. Most patients with T2DM will require insulin therapy at some point in their lives.
Role of Insulin in Type 2 Diabetes Mellitus Management
Consensus guidelines developed by the American Association of Clinical Endocrinologists/American College of Endocrinology (AACE/ACE) recommend initiating insulin when oral therapy fails to achieve glycemic control, A1C > 9.0% in treatment-naïve patients, or if the patient is symptomatic with glucose toxicity (polyuria, polydipsia, and weight loss) (FIGURE 1).1
Similar consensus guidelines developed by the American Diabetes Association/European Association for the Study of Diabetes (ADA/EASD) advise the initiation of glucose-lowering therapy for most patients with T2DM with the combination of lifestyle modifications, diet, and metformin (FIGURE 2).2 For patients who do not achieve or maintain glycemic control over 3 months, or thereabouts, with metformin, a second oral agent should be added. Alternatives include a glucagon-like peptide-1 receptor (GLP-1R) agonist or basal insulin. Insulin should be strongly considered as initial therapy for a patient with significant symptoms of hyperglycemia and/or plasma glucose >300-350 mg/dL or A1C ≥10.0%.
The major role of insulin in the management of patients with T2DM stems from several important attributes. First, insulin is the only treatment that works in patients with advanced β-cell deficiency. It acts directly on tissues to regulate glucose homeostasis, unlike other glucose-lowering agents that require the presence of sufficient endogenous insulin to exert their effects as insulin sensitizers, secretagogues, incretin mimetics, amylin analogs, and other factors. This also means that the mechanism of action of insulin is complementary to those of other glucose-lowering agents. Second, there is less of a ceiling effect with insulin. That is, increasing the dose of insulin results in a progressive lowering of blood glucose in the majority of patients, with the major limitation being the risk for hypoglycemia. Third, the glucose-lowering efficacy of insulin is durable, unlike that of other glucose-lowering agents that depend on endogenous insulin secretion for continued effectiveness. Fourth, insulin improves the lipid profile, particularly triglyceride levels.2-5 Fifth, regarding the long-term safety and tolerability of insulin, it is well established that weight gain, likely mediated via reduction of glycosuria, and hypoglycemia are typically the most concerning adverse events encountered. Allergic reactions, which were a more common complication of animal-sourced insulins, are infrequent with the insulin analogs.6-17 Finally, the availability of insulin in different formulations allows for targeting fasting plasma glucose or postprandial glucose, and individualization of therapy (see The Evolution of Insulin Therapy in Diabetes Mellitus in this supplement.)
While both the AACE/ACE and ADA/EASD consensus guidelines provide treatment “algorithms,” both make it clear that these are suggested approaches suitable for the population with T2DM (FIGURE 1, FIGURE 2). The specific treatment approach must be individualized based on patient-specific factors such as age, comorbid conditions, and tolerance of hypoglycemia.
FIGURE 1
Role of insulin in the management of patients with type 2 diabetes mellitus according to the AACE/ACE1
AACE, American Association of Clinical Endocrinologists; ACE, American College of Endocrinology; AGI, α-glucosidase inhibitor; DPP4, dipeptidyl-peptidase-4 inhibitor; FPG, fasting plasma glucose; GLP-1, glucagon–like peptide-1 agonist; MET, metformin; PPG, postprandial glucose; SU, sulfonylurea; TZD, thiazolidinedione.
Reprinted from American Association of Clinical Endocrinologists. AACE/ACE Diabetes Algorithm for Glycemic Control. Available at https://www.aace.com/sites/default/files/GlycemicControlAlgorithmPPT.pdf. Accessed April 4, 2012, with permission from the American Association of Clinical Endocrinologists.
FIGURE 2
Role of insulin in the management of patients with type 2 diabetes mellitus according to the ADA/EASD2
Moving from the top to the bottom of the figure, potential sequences of antihyperglycemic therapy. In most patients, begin with lifestyle changes; metformin monotherapy is added at, or soon after, diagnosis (unless there are explicit contraindications). If the HbA1c target is not achieved after ~3 months, consider 1 of the 5 treatment options combined with metformin: an SU, TZD, DPP-4-i, GLP-1-RA, or basal insulin. (The order in the chart is determined by historical introduction and route of administration and is not meant to denote any specific preference.) Choice is based on patient and drug characteristics, with the over-riding goal of improving glycemic control while minimizing side effects. Shared decision making with the patient may help in the selection of therapeutic options. The figure displays drugs commonly used both in the United States and/or Europe. Rapid-acting secretagogues (meglitinides) may be used in place of SUs. Other drugs not shown (α-glucosidase inhibitors, colesevelam, dopamine agonists, pramlintide) may be used where available in selected patients but have modest efficacy and/or limiting side effects. In patients intolerant of, or with contraindications for, metformin, select initial drug from other classes depicted and proceed accordingly. In this circumstance, while published trials are generally lacking, it is reasonable to consider 3-drug combinations other than metformin. Insulin is likely to be more effective than most other agents as a third-line therapy, especially when HbA1c is very high (eg, ≥ 9.0%). The therapeutic regimen should include some basal insulin before moving to more complex insulin strategies. Dashed arrow line on the left-hand side of the figure denotes the option of a more rapid progression from a 2-drug combination directly to multiple daily insulin doses, in those patients with severe hyperglycemia (eg, HbA1c, ≥ 10.0–12.0%).
DPP-4, dipeptidyl peptidase-4; DPP-4-i, DPP-4 inhibitor; Fx’s, bone fractures; GI, gastrointestinal; GLP-1, glucagon-like peptide 1; GLP-1-RA, GLP-1 receptor agonist; HbA1c, hemoglobin A1c; HF, heart failure; NPH, neutral protamine Hagedorn; SU, sulfonylurea; TZD, thiazolidinedione.
aConsider beginning at this stage in patients with very high HbA1c (eg, ≥ 9%); bConsider rapid-acting, non-SU secretagogues (meglitinides) in patients with irregular meal schedules or who develop late postprandial hypoglycemia on SUs; cUsually a basal insulin (NPH, glargine, detemir) in combination with noninsulin agents; dCertain noninsulin agents may be continued with insulin. Consider beginning at this stage if patient presents with severe hyperglycemia (≥ 16.7–19.4 mmol/L [≥ 300–350 mg/dL]; HbA1c≥ 10.0–12.0%) with or without catabolic features (weight loss, ketosis, etc).
Diabetes Care by American Diabetes Association. Copyright 2012. Reproduced with permission of AMERICAN DIABETES ASSOCIATION in the format Journal via Copyright Clearance Center.
Individualizing Therapy
The importance of individualizing therapy in a way that allows patients with T2DM to effectively self-manage their disease cannot be overstated. A study involving 1381 patients with T2DM cared for by 42 primary care physicians was conducted to estimate the magnitude of effect that physicians have on glycemic control.18 Hierarchical linear modeling showed that physician-related factors were associated with a statistically significant but modest variability in A1C change (2%) for the entire patient group. On the face of it, this finding might be discouraging. Further analysis showed, however, that for patients whose A1C did improve, physician-related factors accounted for 5% of the overall change in A1C (P = .005). On the other hand, physician-related factors had no impact on patients whose A1C did not improve or worsened. These results support the role that physicians play in affecting patient outcomes. The results also make it clear that without a physician’s influence, a patient’s glycemic outcomes may be difficult to change. The question is: How best can a physician influence patient outcomes?
A 2011 survey of patients with DM, general practitioners, and DM specialists reported that clinicians tended to underestimate patients’ perceived seriousness of the disease, while overestimating patients’ level of distress. In addition, physicians had difficulty identifying which DM-related complications concerned patients most and the information and support patients needed to feel more at ease with DM. Patients placed greater importance on having easy access to their physicians rather than more time with them. But most importantly, the survey investigators concluded that patients generally wished for greater involvement in decision making and being provided more information.19 These findings suggest that patients understand that T2DM is a largely self-managed, chronic disease, and want a collaborative relationship with their physician.
Patient Barriers to Insulin Therapy
Numerous factors have been identified as impeding patients’ willingness to initiate insulin therapy (TABLE 1).20-24 Barriers often vary from patient to patient and, in fact, may change over time in an individual patient. It is crucial, therefore, to identify the root reasons for a patient’s apprehension with insulin when talking about options for intensifying treatment. Once insulin has been initiated, the patient should be asked about continuing or new concerns regarding insulin therapy (and DM management in general), including adherence.
TABLE 1
Barriers to insulin therapy identified by patients20-24
| Lack of understanding of serious nature of type 2 diabetes mellitus |
| Fear of addiction to insulin |
| Fear of hypoglycemia |
| Concern about weight gain |
| Repeated experiences of failing to achieve satisfactory glycemic control |
| Perception that quality of previous treatment was low |
| Needle phobia |
| Treatment complexity |
| Concern of social stigmatization |
| Perceived failure and low self-efficacy |
| Belief of becoming more ill |
| Out-of-pocket cost |
| Perceived negative impact on quality of life |
| Comorbidities such as poor eyesight, arthritis, forgetfulness |
A recent, international survey of 1400 patients with insulin-naïve T2DM reported that 3 negative beliefs about insulin were prominent: (1) feeling that the disease was worsening; (2) fear of injection; and (3) a feeling of personal failure.20 Certain patient comorbidities, such as poor eyesight, arthritis, and forgetfulness, might also serve as barriers to self-management of DM with insulin. Additional comorbidities may contribute as indirect barriers, such as the need for polypharmacy, which may make the initiation of additional treatments such as insulin logistically or financially difficult.
It is possible that the discussion about initiating insulin may uncover patient concerns about T2DM in general. The Diabetes Attitudes, Wishes, and Needs (DAWN) study reported that psychosocial issues were the major source of difficulty in patient self-management (TABLE 2).25 In fact, 85% of people who reported a high level of distress at the time of diagnosis of T2DM continued to experience psychological distress at a mean follow-up of 15 years.
TABLE 2
Patients experiencing various aspects of diabetes-related distress25
| Diabetes-related distress | Respondents who agree (%) |
|---|---|
| I feel stressed because of my diabetes. | 32.7 |
| I feel burned out because of my diabetes. | 18.1 |
| I feel that diabetes is preventing me from doing what I want to do. | 35.9 |
| I am constantly afraid of my diabetes getting worse. | 43.8 |
| I worry about not being able to carry out my family responsibilities in the future. | 30.1 |
| My diabetes causes me worries about my financial future. | 25.8 |
| My family and friends put too much pressure on me about my diabetes. | 14.7 |
| The community I live in is intolerant of diabetes. | 13.6 |
| Diabetes Care by American Diabetes Association. Copyright 2012. Reproduced with permission of AMERICAN DIABETES ASSOCIATION in the format Journal via Copyright Clearance Center. | |
Addressing psychosocial issues and other barriers is crucial in the discussion of self-management because those with more negative feelings about starting insulin are most unwilling to start insulin.20 One factor that may contribute to these negative feelings is repeated experiences of failing to achieve satisfactory glycemic control with oral glucose-lowering agents.23 Conversely, those who have experienced improved glycemic control with intensification of prior glucose-lowering therapy may be more accepting of initiating insulin therapy.23,26 These findings are a reminder of the importance of a treat-to-target approach to management, in which the target glycemic goal, generally A1C < 7.0%, is achieved within 2 to 3 months of diagnosis and maintained at that level through intensification of therapy as needed.
Addressing psychosocial issues can be a challenge in today’s busy primary care practice due to limited time and lack of training in the management of such issues. However, implementation of various strategies has been reported to facilitate and, in some cases, shorten a patient’s visit. For example, one small study reported that visits were shorter if the physician acknowledged and responded positively to a patient’s stated or implied concerns (17.6 minutes vs 20.1 minutes).27 Missing or ignoring the patient’s concerns often led the patient to bring up the same concern one or more additional times resulting in a longer office visit. These results underscore the importance of asking patients to identify their concerns or questions at the beginning of the office visit. The patient can fill out a questionnaire in the waiting room or be encouraged to write down and prioritize their questions and concerns specific to the visit. If the patient identifies more concerns or questions than can be reasonably addressed in one visit, there should be agreement to address the most pressing ones during the current visit and the remaining concerns and questions during the next visit. This “agenda-setting” approach has been reported to offer several advantages.28 From the patient’s perspective, the quality of the physician-patient interaction was much improved, in part because physicians took time to explain points in a way that was easy to understand. Advantages to the physician with an agenda-setting approach included “feeling more in control,” “less stressed by simply knowing what was on the patient’s mind,” “feeling less rushed,” and “enjoying patient encounters more.” Contrary to the study cited above, physicians found that patients’ visits often were longer, especially those of older patients. One physician, however, noted that the visit “takes more time now, but saves time later.” As noted in this study, additional time spent with the patient can lead to improved job satisfaction for the physician.29
The agenda-setting approach requires that the physician ask the patient to list his or her concerns and questions, and then actively listen to the patient. Once the agenda for the visit is established, employing the “ask, listen, empathize” communication style can lead to effective physician-patient communication and problem-solving. Using this approach, the physician asks questions to gain a clear understanding of the patient’s concerns and then uses active listening with little, if any, interruption.30,31 Since the goal is to solve problems with rather than for the patient, active listening without offering opinions, judgements, or advice while offering empathy is essential. Through reflection and discussion, the physician can help the patient to identify his or her issues and acceptable solutions.
The importance of good communication between physician and patient cannot be overstated. Additional communication skills to keep in mind are: (1) speak slowly using nonmedical language; (2) limit the amount of information and repeat it; (3) draw pictures and/or use visual aids; and (4) ask the patient to repeat instructions and key concepts. In addition to enhancing patients’ understanding, visual images may be particularly beneficial in keeping patients motivated to improve self-management, including adherence to therapy. For example, it may be helpful to graphically track the patient’s glycemic progress. This can be done by establishing an actionable A1C goal (generally < 7.0%) and a time frame to achieve the goal (eg, 2 to 3 months).32 A graph can be constructed beginning with the patient’s current, preinsulin A1C level, with updates at each visit. In addition to motivating the patient and reinforcing adherence, the graph can also be used to demonstrate when further treatment intensification is needed. Additional general strategies that can be employed when considering the initiation of insulin are shown in TABLE 3. Implementation of strategies such as these by family physicians provides patient outcomes comparable to those implemented by endocrinologists or diabetes specialists.33
TABLE 3
General strategies for initiating insulin therapy
| Invite the patient to take an active role in treatment decisions. |
| Remind the patient that type 2 diabetes is primarily self-managed. |
| Discuss the progressive nature of β-cell dysfunction in type 2 diabetes. |
| Emphasize the physiologic role of insulin to maintain glucose homeostasis. |
| Discuss that insulin will help to achieve glycemic control and minimize the risk for long-term complications. |
| Discuss that treatment will be modified as needed to maintain glycemic control and to best meet their needs, capabilities, and interest. |
| Utilize insulin pen devices whenever possible. |
| Emphasize the importance of lifestyle management. |
| Ask if hearing other patients talk of their experiences with insulin therapy would be helpful; consider a group office visit. |
| Discuss and provide the patient with an individualized, written action plan that includes insulin dosing, self-monitoring of blood glucose, and signs/symptoms of hypoglycemia and other adverse events with appropriate action(s) to take. |
| Simplify diabetes (and comorbidities) treatment whenever possible. |
The remainder of this article uses case studies to further explore various patient barriers to insulin therapy and strategies for addressing them with the patient. While other therapies may be appropriate in the case studies below as recommended by current guidelines, these case studies will focus on insulin. In addition, dosing strategies for initiating and intensifying insulin therapy are discussed. Changes to the treatment plan to adjust for comorbidities, such as hypertension and dyslipidemia, or for smoking cessation or aspirin therapy, are not addressed in these cases, but are crucial components of comprehensive management.
CASE STUDY 1
RF is a 49-year-old female insurance analyst diagnosed with T2DM 6 years ago. Initial therapy with lifestyle modifications and metformin has since been intensified. Glimepiride was added, then pioglitazone was added 1.5 years ago when the A1C had risen to 7.5%. There is no evidence of cardiovascular disease. She reports bothersome lower extremity edema and an 8-pound weight increase since starting pioglitazone treatment. RF states that she takes her medications every day, although she acknowledges that she sometimes forgets on Sundays.
Clinical Impression
After taking her history, performing a physical examination, and reviewing her laboratory and self-monitored blood glucose (SMBG) data, her physician concludes that her treatment plan needs to be changed (TABLE 4, TABLE 5).
TABLE 4
Case study 1: Chart notes
| Physical examination | Laboratory tests | Lifestyle habits | Current therapy | |
|---|---|---|---|---|
| Glucose-lowering | Other | |||
| BP: 126/80 mm Hg Weight: 176 lb (79.2 kg) BMI: 27 kg/m2 Eyes: no retinopathy Neurology: intact Skin: intact | SCr: 1.4 mg/dL Albuminuria: negative A1C: 8.2% Cholesterol: Total: 204 mg/dL LDL: 134 mg/dL HDL: 36 mg/dL | Exercise: Walks 2 miles 3-4 d/wk Nutrition: eats 3-4 meals/d | Metformin 1000 mg BID Glimepiride 8 mg QD Pioglitazone 45 mg QD | Lisinopril 30 mg QD Simvastatin 40 mg QD ASA 80 mg QD |
| ASA, acetylsalicylic acid; BMI, body mass index; BP, blood pressure; HDL, high-density lipoprotein; LDL, low-density lipoprotein; SCr, serum creatinine. | ||||
TABLE 5
Case study 1: Self-monitored blood glucose (mg/dL) over the previous 2 weeks
| Day | Fasting | 2 h Post-breakfast | 2 h Post-lunch | 2 h Post-dinner |
|---|---|---|---|---|
| Wednesday | 205 | |||
| Thursday | 158 | |||
| Friday | 179 | |||
| Saturday | 201 | 162 | ||
| Sunday | ||||
| Monday | 166 | |||
| Tuesday | 189 | |||
| Wednesday | ||||
| Thursday | 153 | 221 | ||
| Friday | 150 | |||
| Saturday | 199 | 186 | 213 | |
| Sunday | ||||
| Monday | 181 | |||
| Tuesday | 167 |
Treatment Plan
- Initiate basal insulin once daily in the evening.
- Continue glimepiride, but reduce pioglitazone to 15 mg once daily (or discontinue if cost is a concern).
- Ask RF to monitor fasting blood glucose and self-adjust insulin doses as appropriate.
Barriers
While discussing the need to change the treatment plan and the physician’s suggestion that RF begin basal insulin, RF asks her physician for another few months on her current regimen stating that she will try harder to take her medications on Sundays. She also voices concern that insulin treatment requires injections and that she is concerned about what her coworkers and friends might think. The physician confirms that these concerns are understandable; he also confirms that RF is fearful of needles. The following are possible responses that RF’s physician could use to address these concerns.
Patient’s concern: Perceived failure/low self-efficacy
Physician responses:
- We all forget to do things from time to time, but overall I think you have done a great job taking your medications.
- As we have talked about before, with T2DM there is a progressive loss of insulin production over time regardless of what you do. That is why we added glimepiride and then pioglita-zone and that is why we need to make a change now and put you back in control of your diabetes. It is likely that further changes will be needed and we can discuss and agree on them together.
Patient’s concern: Social stigmatization
Physician responses:
- We can begin by having you administer insulin once daily in the evening in the privacy of your home.
- The insulin can be administered with a device that looks like a pen. It is small and can be carried in your purse; it does not need to be refrigerated once opened. If the time comes that you will need to administer a dose of insulin during the day, you can easily administer the insulin discretely in a public restroom or your work area.
- The use of insulin is more common than it was even a few years ago. In fact, about 5 million people in the United States use insulin to replace what is missing, control blood sugars, and decrease the risk for diabetes complications.34
Patient’s concern: Fear of needles
Physician’s responses:
- Insulin can be injected using a pen device with short, ultrathin needles that makes most of the injections painless. I would like you to see how simple and painless the injection can be by using this sample pen here in the office.
- Many patients are concerned about giving themselves an injection at first, but they quickly become comfortable doing so.
Dosing
Treatment with basal insulin can be initiated using one of several approaches. Using the treat-to-target approach, basal insulin 10 U once daily is initiated.35 The starting dose should be reduced to 6 U if the initial pre-breakfast or pre-dinner blood glucose is < 126 mg/dL or the patient’s body mass index (BMI) is < 26 kg/m2.36 Alternatively, the ADA/EASD recommends starting with 0.2 U/kg, which may be more practical in very overweight or obese patients.2 Titration of the basal insulin dose can be accomplished using one of the following physician-directed or patient-driven treat-to-target titration algorithms (TABLE 6).35,37,38 The insulin dose should be titrated based on the pre-breakfast fasting blood glucose level.
TABLE 6
Physician-directed or patient-driven treat-to-target titration algorithms
| Riddle et al35 | Davies et al37 | Meneghini et al38 | ||||
|---|---|---|---|---|---|---|
| Start with 10 U/d bedtime basal insulin and adjust weekly | Start with 10* U/d bedtime basal insulin and adjust weekly (physician-directed) Or Start with a dose numerically equivalent to the highest FPG (in millimoles/L)† over the previous 7 days and adjust every 3 days (patient-managed) | Start with basal insulin once daily and adjust every 3 days | ||||
| Mean of self-monitored FPG values from preceding 2 days | Change in insulin dose (U/d)# | Mean of self-monitored FPG values from preceding 3 days | Change in insulin dose (U/d) (physician-directed) | Change in insulin dose (U/d) (patient-managed) | Mean of self-monitored FPG values from preceding 3 days | Change in insulin dose (U/d) |
| ≥180 mg/dL 140-180 mg/dL 120-140 mg/dL 100-120 mg/dL | +8 +6 +4 +2 | ≥180 mg/dL (≥10 mmol/L) 140-179 mg/dL (7.8-9.9 mmol/L) 120-139 mg/dL (6.7 – 7.7 mmol/L) 100-119 mg/dL (5.5-6.6 mmol/L) | +6 to +8 +4 +2 0 to +2 | +2 +2 +2 0 to +2 | >110 mg/dL 80-110 mg/dL <80 mg/dL | +3 0 -3 |
| FPG, fasting plasma glucose. *In insulin-naive patients. †For example, if the highest FPG over the previous 7 days was 7 mmol/L, start with 7 U.#Small insulin dose decreases (2-4 U/d per adjustment) were allowed if severe hypoglycemia (requiring assistance) or plasma-referenced glucose < 56 mg/dL was documented in the preceding week. Reproduced with permission. Meneghini LF, et al. J Fam Pract. 2011;60(9 Suppl 1):S21-S28. Quadrant HealthCom Inc. Copyright 2011. | ||||||
Follow-Up Visit
RF begins basal insulin 10 U in the evening and is given simple instructions for insulin dose titration based on fasting plasma glucose results. At her follow-up visit, RF reports that she has increased her basal insulin to 18 U administered once daily. Review of her SMBG results show that her blood glucose levels throughout the day have improved, but are still not at goal. RF’s physician commends her on the progress she has made. RF and her physician agree that she should continue to increase her basal insulin dose. Eight months after beginning basal insulin, RF is administering 28 U (0.35 U/kg) of basal insulin in the evening. Review of her SMBG results over the previous 2 weeks show that her blood glucose rises during the day and is highest after dinner; her current A1C is 7.2%.
Treatment Plan
- Discuss dietary and lifestyle complements to insulin therapy such as:
- Use SMBG to identify foods that raise her blood glucose.
CASE STUDY 2
LW is a 64-year-old male with longstanding hypertension diagnosed with T2DM 8 years ago for which he was treated initially with lifestyle management and metformin. He has since been treated with other oral agents as add-on therapy; glipizide was discontinued due to hypoglycemia when he skips meals (usually lunch); pioglitazone was discontinued after the patient expressed concerns about the risk for bladder cancer he heard on television. He has mild retinopathy and mild loss of vibration sensation in the feet; there is no evidence of cardiovascular disease. He was diagnosed with osteoarthritis 3 years ago.
Clinical Impression
After taking his history, performing a physical examination, and reviewing his laboratory and SMBG data, his physician concludes that his treatment plan needs to be changed (FIGURE 3, TABLE 7, TABLE 8).
TABLE 7
Case study 2: Chart notes
| Physical examination | Laboratory tests | Lifestyle habits | Current therapy | |
|---|---|---|---|---|
| Glucose-lowering | Other | |||
| BP: 124/76 mm Hg Weight: 204 lb (92.7 kg) BMI: 31 kg/m2 Eyes: mild retinopathy Neurology: occasional tingling on bottom of right foot Skin: intact | SCr: 1.9 mg/dL eGFR: 51 mL/min Albuminuria: negative A1C: 8.1% Cholesterol: Total: 218 mg/dL LDL: 118 mg/dL HDL: 55 mg/dL Triglyceride: 204 mg/dL | Exercise: takes dog on occasional walk but otherwise sedentary Nutrition: eats 4 meals/d | Metformin 1000 mg BID Acarbose 50 mg TID Sitagliptin 100 mg QD | Lisinopril/HCTZ 20/25 mg QD Amlodipine 10 mg QD Acetaminophen extended-release 650 mg TID ASA 80 mg QD |
| ASA, acetylsalicylic acid; BMI, body mass index; BP, blood pressure; eGFR, estimated glomerular filtration rate; HCTZ, hydrochlorothiazide; HDL, high-density lipoprotein; LDL, low-density lipoprotein; SCr, serum creatinine. | ||||
TABLE 8
Case study 2: Self-monitored blood glucose (mg/dL) over the previous 2 weeks
| Day | Fasting | 2 h Post-breakfast | 2 h Post-lunch | 2 h Post-dinner |
|---|---|---|---|---|
| Tuesday | 135 | |||
| Wednesday | ||||
| Thursday | 196 | |||
| Friday | 152 | 174 | ||
| Saturday | ||||
| Sunday | 208 | |||
| Monday | 142 | 193 | ||
| Tuesday | ||||
| Wednesday | 130 | 156 | ||
| Thursday | ||||
| Friday | ||||
| Saturday | ||||
| Sunday | 151 | |||
| Monday |
FIGURE 3
Case study 2: A1C levels for April 2004 to March 2012
Treatment Plan
- Discontinue metformin since LW’s serum creatinine is > 1.5 mg/dL.
- Initiate either basal insulin once daily in the evening or premix insulin at dinner.
- Ask LW to monitor his blood glucose and self-adjust insulin doses as appropriate.
- Stress the importance of exercise and proper nutrition; gain agreement on short-term goals for exercise and nutrition.
Barriers
LW’s physician recommends that his treatment plan be changed and insulin therapy initiated. LW quickly responds that previous changes to his treatment regimen have not resulted in his achieving an A1C < 7.0%. He also doubts that he can use a syringe to draw up the correct dose and then self-administer due to his arthritis. The following are possible responses his physician could use to address these concerns.
Patient concern: Repeated experience of failing to achieve glycemic control, ie, A1C < 7.0%
Physician responses:
- While achieving an A1C < 7.0% is a realistic goal that reduces the risks for vascular complications of diabetes, any reduction of A1C will be of benefit.
- I would like to work with you to implement a new plan that we both believe will enable you to improve your diabetes control and ideally achieve an A1C < 7.0%.
Patient concern: Self-administering due to arthritis
Physician responses:
- Instead of using a syringe and vial to draw up and administer insulin, I would like you to use an insulin pen device. As you can see, it is easy to handle and you can easily select the correct dose.
- If you choose to start on premix insulin, the pen device contains both types of insulin together in one dose.
Dosing
Treatment with basal insulin once daily in the evening can be initiated and titrated based on pre-breakfast blood glucose as in Case Study 1. Alternatively, treatment with premix insulin can be initiated at a dose of 12 U administered within 15 minutes of dinner initiation. The premix dose can be titrated using the algorithm employed in the 1-2-3 Study based on pre-breakfast blood glucose (TABLE 9).39 After 16 weeks, 41% of patients in the 1-2-3 Study achieved an A1C < 7.0% from a baseline A1C of 8.6%.
TABLE 9
1-2-3 Study algorithm39
| Pre-breakfast SMBG (mg/dL) | Adjustment of pre-dinner dose (U) |
|---|---|
| <80 | -3 |
| 80-110 | No change |
| 111-140 | +3 |
| 141-180 | +6 |
| > 180 | +9 |
| SMBG, self-monitored blood glucose. | |
Follow-Up Visit
LW began basal insulin 10 U in the evening. Over the next 5.5 months, he titrated his dose such that his current dose is 46 U (0.50 U/kg) in the evening. His current A1C is 7.3%. Review of his SMBG shows consistently high 2-hour post-lunch blood glucose levels. Although further increasing his basal insulin dose is an option, in most of the treat-to-target studies, the daily dose of basal insulin given once daily averaged between 0.4 and 0.6 U/kg.35,37,40,41 LW and his physician agree that adding rapid-acting insulin at lunch is the best option. The starting dose of rapid-acting bolus insulin is 4 to 6 U administered prior to the largest meal of the day or, as in this case, prior to the meal with the largest postprandial blood glucose excursion.42,43 Alternatively, the dose of rapid-acting insulin could be calculated as 10% of the total daily dose of basal insulin, which in this case is 5 U (10% x 46 U). The dose of basal insulin would be reduced by 5 U if the rapid-acting insulin is given at dinner in order to reduce the risk for nocturnal hypoglycemia. The dose of the bolus insulin can be titrated using the ExtraSTEP algorithm (TABLE 10).42 Alternatively, the SimpleSTEP algorithm can be used which does not require a 2-hour postprandial glucose measurement.42
TABLE 10
Algorithms for adjusting insulin aspart42
| ExtraSTEP algorithm | SimpleSTEP algorithm | |||
|---|---|---|---|---|
| 2-h Post-meal PG level (mg/dL) | Insulin aspart adjustment (U) | Pre-meal BG (mg/dL) | Bedtime BG (mg/dL) | Insulin aspart adjustment (U) |
| <72* | -2 | <72* | <72* | -2 |
| 72-144 | 0 | 72-108 | 72-144 | 0 |
| 145-180 | +2 | 109-162 | 145-180 | +2 |
| >180 | +4 | >162 | >180 | +4 |
| BG, blood glucose; PG, plasma glucose. *One or more PG values <72 mg/dL without obvious explanation. Reproduced with permission. Meneghini LF, et al. J Fam Pract. 2011;60(9 Suppl 1):S21-S28. Quadrant HealthCom Inc. Copyright 2011. | ||||
Plan
- Begin rapid-acting insulin 5 U at lunch.
- Continue basal insulin at 46 U in the evening.
- Ask LW to continue to titrate basal insulin based on the pre-breakfast blood glucose level and the lunch time bolus insulin dose based on the 2-hour post-lunch SMBG (ExtraSTEP); alternatively, adjust based on the pre-dinner blood glucose level (SimpleSTEP).
CASE STUDY 3
MB is a 46-year-old male who had not consulted a physician since having a physical examination 6 years ago. He presented 2 weeks ago with frequent urination (7-8 times/day) and feeling tired; he also noted losing 5 pounds (2.25 kg) over the preceding 3.5 months despite no changes in his diet. MB is a regional salesperson with an erratic schedule. During the week, he eats lunch and most dinners in a restaurant. On the weekend, he goes to a local bar with his friends. He does light yard work, but does not exercise regularly. He is a current smoker with a 36 pack-year history. Urinalysis shows ketonuria and microalbuminuria. His A1C reported back today is 10.8%, confirming a diagnosis of uncontrolled and symptomatic DM.
Clinical Impression
After taking his history, performing a physical examination, and reviewing his laboratory data, MB’s physician confirms a diagnosis of DM (TABLE 11). While it is likely that MB has T2DM, his physician wants to rule out type 1 DM and latent autoimmune diabetes of the adult (LADA), so he orders tests for antibodies (GAD, IA-2, ICA). The antibody testing is negative, making T2DM the most likely diagnosis.
TABLE 11
Case study 3: Chart notes
| Physical examination | Laboratory tests | Lifestyle habits | Current therapy | |
|---|---|---|---|---|
| Glucose-lowering | Other | |||
| BP: 142/88 mm Hg Weight: 176 lb (79.2 kg) BMI: 27 kg/m2 Eyes: no retinopathy Neurology: intact Skin: intact | SCr: 1.4 mg/dL Microalbumin:creatinine ratio: 140 mg/g creatinine Ketonuria: 1+ A1C: 10.8% Cholesterol: Total: 210 mg/dL LDL: 146 mg/dL HDL: 30 mg/dL | Exercise: light yard work, no regular exercise Nutrition: 3 meals/d, eats most meals in a restaurant (lunch M-F; dinner 3-4 nights/wk) | None | None |
| BMI, body mass index; BP, blood pressure; HDL, high-density lipoprotein; LDL, low-density lipoprotein; SCr, serum creatinine. | ||||
Treatment Plan
- Initiate basal-bolus therapy with fixed bolus doses of rapid-acting insulin at each meal (prandial insulin).
- Ask MB to monitor blood glucose before meals and at bedtime.
- Provide MB with a supplemental scale to correct hyperglycemia before meals.
- Stress the importance of exercise and proper nutrition; gain agreement for short-term goals for exercise and nutrition; refer for diabetes and nutrition education if available.
- Discuss the importance of smoking cessation; develop a plan.
- Consider metformin and other non-insulin therapies when A1C is under control.
Barriers
MB is surprised that he has T2DM and is clearly anxious at receiving the diagnosis. He expresses concern about starting insulin because his uncle died within a year of starting insulin. MB also recalls that his uncle was always giving himself shots and monitoring his blood glucose level. He wants to know whether there is a simpler treatment option if he agrees to start insulin treatment. He also wants to know whether he will have to remain on insulin for the rest of his life. The following are possible responses his physician could use to address these concerns.
Patient concern: Fear of death
Physician responses:
- Uncontrolled high blood sugars over a long period of time can cause serious complications, such as kidney and heart disease that can result in death. That is why it is important that we work together to gain control of your blood sugar levels over the next few months and then modify your treatment as needed to maintain control.
- Unfortunately for many patients in the past, treatment with insulin was not used until it was too late and people already had serious complications from DM. This is likely the case for your uncle.
Patient concern: Treatment complexity
Physician responses:
- Right now we have to control your blood glucose rapidly so your pancreas can regain some function and your body can better respond to insulin.
- I will also provide you with step-by-step written instructions you can follow that describe how to start insulin and how to monitor your blood glucose.
- We will communicate as often as you need to adjust your insulin doses over the next few weeks; when you feel comfortable, I can even show you how to adjust your insulin dose before a meal to correct a high blood sugar.
- We can try this treatment for 3 months and then reevaluate your response, how you feel, and whether you want to continue to modify your treatment plan to keep your blood sugars controlled.
Patient concern: Lack of understanding that T2DM is a serious disease
Physician responses:
- Please understand that T2DM is a serious disease that increases your risk for heart disease, stroke, blindness, and other diseases. Unfortunately, since diabetes does not cause bad symptoms until it is actually too late, many patients do not make the effort to properly control their diabetes. By working together, we can reduce the risk for these complications and do some screening tests to detect any complications before they become irreversible.
Dosing
There are several approaches to determining the initial doses of basal and prandial (bolus) insulin. One approach is to estimate the total daily dose (TDD) of insulin by multiplying the patient’s weight in kilograms by 0.5 U/kg/d.44 Half of the TDD is given as basal insulin replacement; the other half is divided into 3 fixed preprandial doses of rapid-acting insulin. When the patient is ready to take on more complex management, the supplemental dose for bolus insulin can be calculated using a correction factor. If the bolus insulin is a rapid-acting insulin analog, 1800 is divided by the TDD of insulin; 1500 is used for a short-acting human insulin. This correction factor is an estimate of the fall in blood glucose per unit of bolus insulin. In our patient, the TDD would be: 80 kg x 0.5 U/kg/d or 40 U/d of insulin. Thus, 1 U of insulin should lower the blood glucose by about 45 mg/dL (1800/40 U = 45 mg/dL). For every 45 mg/dL above the pre-meal target, the patient would add 1 U of rapid-acting insulin to correct the hyperglycemia over the next 4 to 5 hours. The basal and prandial insulin doses would be titrated on a periodic basis (perhaps every 1 to 2 weeks) until the daytime levels of blood glucose are on target. The fasting (pre-breakfast) blood glucose would be used to adjust the basal insulin dose, while the pre-lunch, pre-dinner, and bedtime blood glucose results would be used to adjust the pre-breakfast, pre-lunch, and pre-dinner prandial (rapid-acting) insulin doses, respectively.
An alternative approach to initiating basal-bolus therapy is the PREFER algorithm.45 Here, the basal insulin dose is 10 U initially. The bolus doses are administered in a 3:1:2 ratio, so if the total of the 3 bolus doses is 12 U/d, the initial bolus doses would be 6 (breakfast), 2 (lunch), and 4 (dinner) U. The mean basal (once-daily) and bolus insulin doses observed in PREFER are shown in TABLE 12 and TABLE 13.
TABLE 12
Case study 3: Calculating initial basal-bolus insulin doses
| Algorithm | Calculations | Patient MB |
|---|---|---|
| Meneghini44 | TDD = (total body weight [kg]) (0.5 U/kg/d) Basal insulin dose* = (50%) (TDD) Bolus insulin dose† = (10%-20%) (TDD) | TDD = (0.5 U/kg/d)(80kg) = 40 U/d |
| Basal = (50%) (40 U/d) = 20 U/d | ||
| Bolus = (10%-20%) (40 U/d) = 4 to 8 U/meal | ||
| CF = 1800/40 U/d = 45 mg/dL per 1 unit | ||
| PREFER45 | Basal insulin dose* = 10 U (14 U if BMI > 32 kg/m2) Bolus insulin dose† = ratio of 3:1:2 (breakfast:lunch:dinner) Note: At week 26, the bolus insulin doses were divided into the 3 daily meals in approximately a 1:1:1 ratio | |
| BMI, body mass index; CF, correction factor; TDD, total daily dose of insulin. *Once daily; †Three meals per day. | ||
TABLE 13
Titrating the basal insulin dose using the PREFER algorithm45
| Pre-breakfast blood glucose (mg/dL) | Basal insulin dose adjustment (U) |
|---|---|
| < 56 | -4 |
| 56-72 | -2 |
| 73-125 | No change |
| 126-140 | +2 |
| 141-160 | +4 |
| 161-180 | +6 |
| 181-200 | +8 |
| > 200 | +10 |
Follow-up Visit
MB begins with basal insulin 20 U in the evening and bolus insulin at doses of 7 U before each meal. Over the next several months, MB has titrated his insulin doses; his current doses are: 32 U (basal), 11 U (bolus-breakfast), 7 U (bolus-lunch), and 10 U (bolus-dinner). He experienced 1 episode of mild hypoglycemia (SMBG, 50 mg/dL) one afternoon following a particularly active morning (TABLE 14). His current A1C is 7.4%. MB’s physician congratulates him on the progress he has made in dramatically lowering his blood glucose level—and his risk for diabetes-related complications. While MB appreciates his physician’s support and admits that he does not feel tired and generally feels better, which is likely due to resolution of glucotoxicity, he is not happy that he has gained 5.5 pounds (2.5 kg).46 He also finds the timing and administration of bolus insulin difficult.
TABLE 14
Case study 3: Self-monitored blood glucose (mg/dL) over the previous 2 weeks
| Day | Fasting | 2 h Post-breakfast | 2 h Post-lunch | 2 h Post-dinner |
|---|---|---|---|---|
| Wednesday | ||||
| Thursday | 168 | |||
| Friday | 106 | 166 | 174 | |
| Saturday | 88 | |||
| Sunday | 195 | |||
| Monday | 134 | |||
| Tuesday | 172 | |||
| Wednesday | 130 | 156 | ||
| Thursday | 112 | 168 | ||
| Friday | 92 | 164 | ||
| Saturday | 50 | 149 | 159 | 176 |
| Sunday | 94 | 174 | 210 | |
| Monday | 176 | 184 | ||
| Tuesday | 117 | 169 |
Plan
- Continue basal insulin once-daily in the evening.
- Add metformin 500 mg BID and increase to 1000 mg BID as tolerated.
- Consider weaning down the bolus insulin doses and substituting them with a GLP-1R agonist, dipeptidyl peptidase-4 inhibitor, or short-acting secretagogue. If so, continue rapid-acting insulin during transition. [Note: the following combinations are not currently approved by the US FDA: exenatide twice-daily and prandial insulin; exenatide once-weekly and insulin; liraglutide and prandial insulin; linagliptin and insulin.]
CASE STUDY 4
KW is a 62-year-old female diagnosed with T2DM 12 years ago. Treatment with lifestyle management and metformin initially provided glycemic control. Glimepiride was subsequently added and eventually the patient was started on basal insulin. The current dose of basal insulin is 60 U in the evening. Five months ago her A1C was found to be 7.9% and more recently 8.3%. She drinks alcohol occasionally and smokes. KW works as an executive secretary and has a consistent meal and activity schedule.
Clinical Impression
Following completion of the history, physical examination, and review of her laboratory data, KW’s physician concludes that her insulin regimen should be intensified (TABLE 15, TABLE 16).
TABLE 15
Case study 4: Chart notes
| Physical examination | Laboratory tests | Lifestyle habits | Current therapy | |
|---|---|---|---|---|
| Glucose-lowering | Other | |||
| BP: 126/78 mm Hg Weight: 176 lb (79.2 kg) BMI: 32 kg/m2 Eyes: no retinopathy Neurology: intact Skin: intact | SCr: 1.0 mg/dL Albuminuria: negative A1C: 8.3% Cholesterol Total: 172 mg/dL LDL: 96 mg/dL HDL: 46 mg/dL Triglycerides: 138 mg/dL | Exercise: sedentary Nutrition: 3 meals/d with large dinner | Metformin 1000 mg BID Basal insulin 60 U in the evening | ASA 80 mg QD Pravastatin 40 mg qHS |
| BMI, body mass index; BP, blood pressure; HDL, high-density lipoprotein; LDL, low-density lipoprotein; SCr, serum creatinine. | ||||
TABLE 16
Case study 4: Self-monitored blood glucose (mg/dL) over the previous 2 weeks
| Day | Fasting | 2 h Post-breakfast | 2 h Post-lunch | 2 h Post-dinner |
|---|---|---|---|---|
| Friday | ||||
| Saturday | 156 | 244 | ||
| Sunday | 253 | |||
| Monday | ||||
| Tuesday | ||||
| Wednesday | 148 | 227 | ||
| Thursday | ||||
| Friday | ||||
| Saturday | 179 | |||
| Sunday | 160 | |||
| Monday | ||||
| Tuesday | ||||
| Wednesday | ||||
| Thursday |
Plan
- Discontinue basal insulin.
- Begin premix insulin twice daily before breakfast and dinner.
- Ask KW to monitor blood glucose two times daily and, if appropriate, teach her how to self-adjust insulin doses.
- Stress the importance of exercise and proper nutrition; gain agreement on short-term goals for exercise and nutrition.
- Discuss the importance of smoking cessation; develop a plan.
Barriers
The physician discusses with KW that her consistent meal and activity schedule would make switching to premix insulin twice daily a good choice. KW is generally in agreement with the change, but wonders whether hypoglycemia might be more likely. She also asks if she might gain more weight in addition to the 3 pounds (1.35 kg) she has gained since starting basal insulin.
Patient concern: Hypoglycemia
Physician responses:
- Hypoglycemia remains a concern, and is more frequently seen with premix than with basal insulin; however, as long as you remain consistent with your meal and activity schedule, the risk for bad hypoglycemia is low.
- We should review your written action plan so that you are sure what signs or symptoms of a low blood sugar might occur and what you should do to treat them.
Patient concern: Weight gain
Physician responses:
- It is possible that you might gain a few additional pounds. You can avoid this by increasing your physical activity, and importantly, continue healthy eating. We should schedule a time for you to meet again with a dietician who can discuss options that might work for you.
Dosing
There are different approaches for converting from basal insulin to twice-daily premix insulin. One approach is to determine the TDD of basal insulin, and give half at breakfast and the other half at dinner as premix insulin.39 Since KW is taking 60 U of basal in the evening, she should take 30 U at breakfast and 30 U at dinner. Dose titration is according to the 1-2-3 Study algorithm shown in case study 2.
Another approach is to administer biphasic insulin aspart 70/30 0.2 U/kg before breakfast and 0.1 U/kg before dinner as was done in the PREFER study (TABLE 13).45 Subsequent dosing can be determined based on the PREFER algorithm below. Of note is that at study end, premix insulin doses were equally divided between breakfast and dinner. Breakfast and dinner doses are titrated based on blood glucose levels before dinner and breakfast, respectively. In the PREFER study, the use of premix insulin provided comparable A1C reduction as basal-bolus therapy (basal once daily + bolus TID) in insulin-naïve patients. However, patients previously treated with basal insulin such as KW experienced greater A1C reductions with basal-bolus insulin than with premix insulin.
1. Rodbard HW, Jellinger PS, Davidson JA, et al. Statement by an American Association of Clinical Endocrinologists/American College of Endocrinology consensus panel on type 2 diabetes mellitus: an algorithm for glycemic control [published correction appears in Endocr Pract. 2009;15(7):768-770]. Endocr Pract. 2009;15(6):540-559.
2. Inzucchi SE, Bergenstal RM, Buse JB, et al. Management of hyperglycemia in type 2 diabetes: a patient-centered approach: position statement of the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD) [published online ahead of print April 19, 2012]. Diabetes Care. doi:10.2337/dc12-0413.
3. Lalić NM, Micić D, Antić S, et al. Effect of biphasic insulin aspart on glucose and lipid control in patients with type 2 diabetes mellitus. Expert Opin Pharmacother. 2007;8(17):2895-2901.
4. Reynolds LR, Kingsley FJ, Karounos DG, Tannock LR. Differential effects of rosiglitazone and insulin glargine on inflammatory markers, glycemic control, and lipids in type 2 diabetes. Diabetes Res Clin Pract. 2007;77(2):180-187.
5. Rosak C, Jung R, Hofmann U. Insulin glargine maintains equivalent glycemic control and better lipometabolic control than NPH insulin in type 1 diabetes patients who missed a meal. Horm Metab Res. 2008;40(8):544-548.
6. Ampudia-Blasco FJ, Girbes J, Carmena R. A case of lipoatrophy with insulin glargine: long-acting insulin analogs are not exempt from this complication. Diabetes Care. 2005;28(12):2983.-
7. Griffin ME, Feder A, Tamborlane WV. Lipoatrophy associated with lispro insulin in insulin pump therapy: an old complication, a new cause? Diabetes Care. 2001;24(1):174.-
8. Fineberg SE, Huang J, Brunelle R, Gulliya KS, Anderson JH, Jr. Effect of long-term exposure to insulin lispro on the induction of antibody response in patients with type 1 or type 2 diabetes. Diabetes Care. 2003;26(1):89-96.
9. Moyes V, Driver R, Croom A, Mirakian R, Chowdhury TA. Insulin allergy in a patient with type 2 diabetes successfully treated with continuous subcutaneous insulin infusion. Diabet Med. 2006;23(2):204-206.
10. Ghosh S, McCann V, Bartle L, Collier A, Malik I. Allergy to insulin detemir. Diabet Med. 2007;24(11):1307.-
11. Blumer I. Severe, delayed insulin detemir injection site reaction. Diabet Med. 2008;25(8):1008.-
12. Pérez E, González R, Martínez J, Iglesias J, Matheu V. Detemir insulin-induced anaphylaxis. Ann Allergy Asthma Immunol. 2009;102(2):174-175.
13. Mollar-Puchades MA, Villanueva IL. Insulin glulisine in the treatment of allergy to rapid acting insulin and its rapid acting analogs. Diabetes Res Clin Pract. 2009;83(1):e21-e22.
14. Kawasaki F, Kamei S, Tatsumi F, et al. Gallbladder edema in type 1 diabetic patient due to delayed-type insulin allergy. Intern Med. 2009;48(17):1545-1549.
15. Wang C, Ding ZY, Shu SQ, et al. Severe insulin allergy after percutaneous transluminal coronary angioplasty. Clin Ther. 2009;31(3):569-574.
16. Ozaki N, Oiso Y. Immunologic tolerance to the insulin analogue glulisine. Diabetes Care. 2010;33(3):e39.-
17. Koroscil T, Kagzi Y, Zacharias D. Failure of multiple therapies in the treatment of a type 1 diabetic patient with insulin allergy: a case report. Endocr Pract. 2011;17(1):91-94.
18. Tuerk PW, Mueller M, Egede LE. Estimating physician effects on glycemic control in the treatment of diabetes: methods, effects sizes, and implications for treatment policy. Diabetes Care. 2008;31(5):869-873.
19. Hajos TR, Polonsky WH, Twisk JW, Dain MP, Snoek FJ. Do physicians understand type 2 diabetes patients’ perceptions of seriousness; the emotional impact and needs for care improvement? A cross-national survey. Patient Educ Couns. 2011;85(2):258-263.
20. Polonsky WH, Hajos TR, Dain MP, Snoek FJ. Are patients with type 2 diabetes reluctant to start insulin therapy? An examination of the scope and underpinnings of psychological insulin resistance in a large, international population. Curr Med Res Opin. 2011;27(6):1169-1174.
21. Nam S, Chesla C, Stotts NA, Kroon L, Janson SL. Factors associated with psychological insulin resistance in individuals with type 2 diabetes. Diabetes Care. 2010;33(8):1747-1749.
22. Nakar S, Yitzhaki G, Rosenberg R, Vinker S. Transition to insulin in type 2 diabetes: family physicians’ misconception of patients’ fears contributes to existing barriers. J Diabetes Complications. 2007;21(4):220-226.
23. Snoek FJ. Breaking the barriers to optimal glycaemic control—what physicians need to know from patients’ perspectives. Int J Clin Pract Suppl. 2002;(129):80-84.
24. Korytkowski M. When oral agents fail: practical barriers to starting insulin. Int J Obes Relat Metab Disord. 2002;26(Suppl 3):S18-S24.
25. Skovlund SE, Peyrot M. The Diabetes Attitudes, Wishes and Needs (DAWN) program: a new approach to improving outcomes of diabetes care. Diabetes Spectrum. 2005;18(3):136-142.
26. Jenkins N, Hallowell N, Farmer AJ, Holman RR, Lawton J. Initiating insulin as part of the Treating To Target in Type 2 Diabetes (4-T) trial: an interview study of patients’ and health professionals’ experiences. Diabetes Care. 2010;33(10):2178-2180.
27. Levinson W, Gorawara-Bhat R, Lamb J. A study of patient clues and physician responses in primary care and surgical settings. JAMA. 2000;284(8):1021-1027.
28. Rodriguez HP, Anastario MP, Frankel RM, et al. Can teaching agenda-setting skills to physicians improve clinical interaction quality? A controlled intervention. BMC Med Educ. 2008;8:3.-
29. Solomon J. How strategies for managing patient visit time affect physician job satisfaction: a qualitative analysis. J Gen Intern Med. 2008;23(6):775-780.
30. Funnell MM, Anderson RM. Empowerment and self-management of diabetes. Clin Diabetes. 2004;22(3):123-127.
31. Funnell MM, Anderson RM. Are patients or outcomes more important? Rev Endocrinol. 2008;2(8):49-51.
32. Shaefer CF. Clinical inertia: overcoming a major barrier to diabetes management. Insulin. 2006;1(2):61-64.
33. Harris S, Yale JF, Dempsey E, Gerstein H. Can family physicians help patients initiate basal insulin therapy successfully?: randomized trial of patient-titrated insulin glargine compared with standard oral therapy: lessons for family practice from the Canadian INSIGHT trial. Can Fam Physician. 2008;54(4):550-558.
34. Centers for Disease Control and Prevention. National diabetes fact sheet national estimates and general information on diabetes and prediabetes in the United States, 2011. Atlanta, GA: U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, 2011. http://www.cdc.gov/diabetes/pubs/pdf/ndfs_2011.pdf. Accessed February 10, 2011.
35. Riddle MC, Rosenstock J, Gerich J. Insulin Glargine 4002 Study Investigators. The treat-to-target trial: randomized addition of glargine or human NPH insulin to oral therapy of type 2 diabetic patients. Diabetes Care. 2003;26(11):3080-3086.
36. Hermansen K, Davies M, Derezinski T, Martinez Ravn G, Clauson P, Home P. A 26-week, randomized, parallel, treat-to-target trial comparing insulin detemir with NPH insulin as add-on therapy to oral glucose-lowering drugs in insulin-naive people with type 2 diabetes [published correction appears in Diabetes Care. 2007;30(4):1035]. Diabetes Care. 2006;29(6):1269-1274.
37. Davies M, Storms F, Shutler S, Bianchi-Biscay M, Gomis R. ATLANTUS Study Group. Improvement of glycemic control in subjects with poorly controlled type 2 diabetes: comparison of two treatment algorithms using insulin glargine. Diabetes Care. 2005;28(6):1282-1288.
38. Meneghini L, Koenen C, Weng W, Selam JL. The usage of a simplified self-titration dosing guideline (303 Algorithm) for insulin detemir in patients with type 2 diabetes—results of the randomized, controlled PREDICTIVE 303 study. Diabetes Obes Metab. 2007;9(6):902-913.
39. Garber AJ, Wahlen J, Wahl T, et al. Attainment of glycaemic goals in type 2 diabetes with once-, twice-, or thrice-daily dosing with biphasic insulin aspart 70/30 (The 1-2-3 study). Diabetes Obes Metab. 2006;8(1):58-66.
40. Philis-Tsimikas A, Charpentier G, Clauson P, Ravn GM, Roberts VL, Thorsteinsson B. Comparison of once-daily insulin detemir with NPH insulin added to a regimen of oral antidiabetic drugs in poorly controlled type 2 diabetes [published correction appears in Clin Ther. 2006;28(11):1967]. Clin Ther. 2006;28(10):1569-1581.
41. Blonde L, Merilainen M, Karwe V, Raskin P. TITRATE Study Group. Patient-directed titration for achieving glycaemic goals using a once-daily basal insulin analogue: an assessment of two different fasting plasma glucose targets—the TITRATE study. Diabetes Obes Metab. 2009;11(6):623-631.
42. Meneghini L, Mersebach H, Kumar S, Svendsen AL, Hermansen K. Comparison of 2 intensification regimens with rapid-acting insulin aspart in type 2 diabetes mellitus inadequately controlled by once-daily insulin detemir and oral antidiabetes drugs: the step-wise randomized study. Endocr Pract. 2011;17(5):727-736.
43. Lankisch MR, Ferlinz KC, Leahy JL, Scherbaum WA. Orals Plus Apidra and LANTUS (OPAL) study group. Introducing a simplified approach to insulin therapy in type 2 diabetes: a comparison of two single-dose regimens of insulin glulisine plus insulin glargine and oral antidiabetic drugs [published correction appears in Diabetes Obes Metab. 2010;12(5):461]. Diabetes Obes Metab 2008;10(12):1178-1185.
44. Meneghini L. Why and how to use insulin therapy earlier in the management of type 2 diabetes. South Med J. 2007;100(2):164-174.
45. Liebl A, Prager R, Binz K, Kaiser M, Bergenstal R, Gallwitz B. PREFER Study Group. Comparison of insulin analogue regimens in people with type 2 diabetes mellitus in the PREFER Study: a randomized controlled trial. Diabetes Obes Metab. 2009;11(1):45-52.
46. Braun A, Sämann A, Kubiak T, et al. Effects of metabolic control, patient education and initiation of insulin therapy on the quality of life of patients with type 2 diabetes mellitus. Patient Educ Couns. 2008;73(1):50-59.
1. Rodbard HW, Jellinger PS, Davidson JA, et al. Statement by an American Association of Clinical Endocrinologists/American College of Endocrinology consensus panel on type 2 diabetes mellitus: an algorithm for glycemic control [published correction appears in Endocr Pract. 2009;15(7):768-770]. Endocr Pract. 2009;15(6):540-559.
2. Inzucchi SE, Bergenstal RM, Buse JB, et al. Management of hyperglycemia in type 2 diabetes: a patient-centered approach: position statement of the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD) [published online ahead of print April 19, 2012]. Diabetes Care. doi:10.2337/dc12-0413.
3. Lalić NM, Micić D, Antić S, et al. Effect of biphasic insulin aspart on glucose and lipid control in patients with type 2 diabetes mellitus. Expert Opin Pharmacother. 2007;8(17):2895-2901.
4. Reynolds LR, Kingsley FJ, Karounos DG, Tannock LR. Differential effects of rosiglitazone and insulin glargine on inflammatory markers, glycemic control, and lipids in type 2 diabetes. Diabetes Res Clin Pract. 2007;77(2):180-187.
5. Rosak C, Jung R, Hofmann U. Insulin glargine maintains equivalent glycemic control and better lipometabolic control than NPH insulin in type 1 diabetes patients who missed a meal. Horm Metab Res. 2008;40(8):544-548.
6. Ampudia-Blasco FJ, Girbes J, Carmena R. A case of lipoatrophy with insulin glargine: long-acting insulin analogs are not exempt from this complication. Diabetes Care. 2005;28(12):2983.-
7. Griffin ME, Feder A, Tamborlane WV. Lipoatrophy associated with lispro insulin in insulin pump therapy: an old complication, a new cause? Diabetes Care. 2001;24(1):174.-
8. Fineberg SE, Huang J, Brunelle R, Gulliya KS, Anderson JH, Jr. Effect of long-term exposure to insulin lispro on the induction of antibody response in patients with type 1 or type 2 diabetes. Diabetes Care. 2003;26(1):89-96.
9. Moyes V, Driver R, Croom A, Mirakian R, Chowdhury TA. Insulin allergy in a patient with type 2 diabetes successfully treated with continuous subcutaneous insulin infusion. Diabet Med. 2006;23(2):204-206.
10. Ghosh S, McCann V, Bartle L, Collier A, Malik I. Allergy to insulin detemir. Diabet Med. 2007;24(11):1307.-
11. Blumer I. Severe, delayed insulin detemir injection site reaction. Diabet Med. 2008;25(8):1008.-
12. Pérez E, González R, Martínez J, Iglesias J, Matheu V. Detemir insulin-induced anaphylaxis. Ann Allergy Asthma Immunol. 2009;102(2):174-175.
13. Mollar-Puchades MA, Villanueva IL. Insulin glulisine in the treatment of allergy to rapid acting insulin and its rapid acting analogs. Diabetes Res Clin Pract. 2009;83(1):e21-e22.
14. Kawasaki F, Kamei S, Tatsumi F, et al. Gallbladder edema in type 1 diabetic patient due to delayed-type insulin allergy. Intern Med. 2009;48(17):1545-1549.
15. Wang C, Ding ZY, Shu SQ, et al. Severe insulin allergy after percutaneous transluminal coronary angioplasty. Clin Ther. 2009;31(3):569-574.
16. Ozaki N, Oiso Y. Immunologic tolerance to the insulin analogue glulisine. Diabetes Care. 2010;33(3):e39.-
17. Koroscil T, Kagzi Y, Zacharias D. Failure of multiple therapies in the treatment of a type 1 diabetic patient with insulin allergy: a case report. Endocr Pract. 2011;17(1):91-94.
18. Tuerk PW, Mueller M, Egede LE. Estimating physician effects on glycemic control in the treatment of diabetes: methods, effects sizes, and implications for treatment policy. Diabetes Care. 2008;31(5):869-873.
19. Hajos TR, Polonsky WH, Twisk JW, Dain MP, Snoek FJ. Do physicians understand type 2 diabetes patients’ perceptions of seriousness; the emotional impact and needs for care improvement? A cross-national survey. Patient Educ Couns. 2011;85(2):258-263.
20. Polonsky WH, Hajos TR, Dain MP, Snoek FJ. Are patients with type 2 diabetes reluctant to start insulin therapy? An examination of the scope and underpinnings of psychological insulin resistance in a large, international population. Curr Med Res Opin. 2011;27(6):1169-1174.
21. Nam S, Chesla C, Stotts NA, Kroon L, Janson SL. Factors associated with psychological insulin resistance in individuals with type 2 diabetes. Diabetes Care. 2010;33(8):1747-1749.
22. Nakar S, Yitzhaki G, Rosenberg R, Vinker S. Transition to insulin in type 2 diabetes: family physicians’ misconception of patients’ fears contributes to existing barriers. J Diabetes Complications. 2007;21(4):220-226.
23. Snoek FJ. Breaking the barriers to optimal glycaemic control—what physicians need to know from patients’ perspectives. Int J Clin Pract Suppl. 2002;(129):80-84.
24. Korytkowski M. When oral agents fail: practical barriers to starting insulin. Int J Obes Relat Metab Disord. 2002;26(Suppl 3):S18-S24.
25. Skovlund SE, Peyrot M. The Diabetes Attitudes, Wishes and Needs (DAWN) program: a new approach to improving outcomes of diabetes care. Diabetes Spectrum. 2005;18(3):136-142.
26. Jenkins N, Hallowell N, Farmer AJ, Holman RR, Lawton J. Initiating insulin as part of the Treating To Target in Type 2 Diabetes (4-T) trial: an interview study of patients’ and health professionals’ experiences. Diabetes Care. 2010;33(10):2178-2180.
27. Levinson W, Gorawara-Bhat R, Lamb J. A study of patient clues and physician responses in primary care and surgical settings. JAMA. 2000;284(8):1021-1027.
28. Rodriguez HP, Anastario MP, Frankel RM, et al. Can teaching agenda-setting skills to physicians improve clinical interaction quality? A controlled intervention. BMC Med Educ. 2008;8:3.-
29. Solomon J. How strategies for managing patient visit time affect physician job satisfaction: a qualitative analysis. J Gen Intern Med. 2008;23(6):775-780.
30. Funnell MM, Anderson RM. Empowerment and self-management of diabetes. Clin Diabetes. 2004;22(3):123-127.
31. Funnell MM, Anderson RM. Are patients or outcomes more important? Rev Endocrinol. 2008;2(8):49-51.
32. Shaefer CF. Clinical inertia: overcoming a major barrier to diabetes management. Insulin. 2006;1(2):61-64.
33. Harris S, Yale JF, Dempsey E, Gerstein H. Can family physicians help patients initiate basal insulin therapy successfully?: randomized trial of patient-titrated insulin glargine compared with standard oral therapy: lessons for family practice from the Canadian INSIGHT trial. Can Fam Physician. 2008;54(4):550-558.
34. Centers for Disease Control and Prevention. National diabetes fact sheet national estimates and general information on diabetes and prediabetes in the United States, 2011. Atlanta, GA: U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, 2011. http://www.cdc.gov/diabetes/pubs/pdf/ndfs_2011.pdf. Accessed February 10, 2011.
35. Riddle MC, Rosenstock J, Gerich J. Insulin Glargine 4002 Study Investigators. The treat-to-target trial: randomized addition of glargine or human NPH insulin to oral therapy of type 2 diabetic patients. Diabetes Care. 2003;26(11):3080-3086.
36. Hermansen K, Davies M, Derezinski T, Martinez Ravn G, Clauson P, Home P. A 26-week, randomized, parallel, treat-to-target trial comparing insulin detemir with NPH insulin as add-on therapy to oral glucose-lowering drugs in insulin-naive people with type 2 diabetes [published correction appears in Diabetes Care. 2007;30(4):1035]. Diabetes Care. 2006;29(6):1269-1274.
37. Davies M, Storms F, Shutler S, Bianchi-Biscay M, Gomis R. ATLANTUS Study Group. Improvement of glycemic control in subjects with poorly controlled type 2 diabetes: comparison of two treatment algorithms using insulin glargine. Diabetes Care. 2005;28(6):1282-1288.
38. Meneghini L, Koenen C, Weng W, Selam JL. The usage of a simplified self-titration dosing guideline (303 Algorithm) for insulin detemir in patients with type 2 diabetes—results of the randomized, controlled PREDICTIVE 303 study. Diabetes Obes Metab. 2007;9(6):902-913.
39. Garber AJ, Wahlen J, Wahl T, et al. Attainment of glycaemic goals in type 2 diabetes with once-, twice-, or thrice-daily dosing with biphasic insulin aspart 70/30 (The 1-2-3 study). Diabetes Obes Metab. 2006;8(1):58-66.
40. Philis-Tsimikas A, Charpentier G, Clauson P, Ravn GM, Roberts VL, Thorsteinsson B. Comparison of once-daily insulin detemir with NPH insulin added to a regimen of oral antidiabetic drugs in poorly controlled type 2 diabetes [published correction appears in Clin Ther. 2006;28(11):1967]. Clin Ther. 2006;28(10):1569-1581.
41. Blonde L, Merilainen M, Karwe V, Raskin P. TITRATE Study Group. Patient-directed titration for achieving glycaemic goals using a once-daily basal insulin analogue: an assessment of two different fasting plasma glucose targets—the TITRATE study. Diabetes Obes Metab. 2009;11(6):623-631.
42. Meneghini L, Mersebach H, Kumar S, Svendsen AL, Hermansen K. Comparison of 2 intensification regimens with rapid-acting insulin aspart in type 2 diabetes mellitus inadequately controlled by once-daily insulin detemir and oral antidiabetes drugs: the step-wise randomized study. Endocr Pract. 2011;17(5):727-736.
43. Lankisch MR, Ferlinz KC, Leahy JL, Scherbaum WA. Orals Plus Apidra and LANTUS (OPAL) study group. Introducing a simplified approach to insulin therapy in type 2 diabetes: a comparison of two single-dose regimens of insulin glulisine plus insulin glargine and oral antidiabetic drugs [published correction appears in Diabetes Obes Metab. 2010;12(5):461]. Diabetes Obes Metab 2008;10(12):1178-1185.
44. Meneghini L. Why and how to use insulin therapy earlier in the management of type 2 diabetes. South Med J. 2007;100(2):164-174.
45. Liebl A, Prager R, Binz K, Kaiser M, Bergenstal R, Gallwitz B. PREFER Study Group. Comparison of insulin analogue regimens in people with type 2 diabetes mellitus in the PREFER Study: a randomized controlled trial. Diabetes Obes Metab. 2009;11(1):45-52.
46. Braun A, Sämann A, Kubiak T, et al. Effects of metabolic control, patient education and initiation of insulin therapy on the quality of life of patients with type 2 diabetes mellitus. Patient Educ Couns. 2008;73(1):50-59.