User login
The transition to menopause begins with ovarian fluctuation and hormonal changes, often beginning before significant changes in menstruation. Reproductive aging with loss of follicular activity progresses over a wide age range (42 to 58 years) with an average onset at approximately age 47, ranging from 4 to 8 years. Although most women have heard about menopause, defined as 12 months after the last period, they often lack understanding about perimenopause or that the menopausal transition usually begins 5 years before menopause.1
Perimenopause, defined as early and late menopause transition stages, may be viewed as a window of potential vulnerability for women who develop or have worsening menstrual-related mood disorders. Over time, hormonal fluctuations often lead to menstrual cycle irregularity (either shorter or longer). Changes occurring during perimenopause may be confusing as it may not be clear whether symptoms are related to menopause, aging, or stress. Often not recognized or treated adequately, perimenopausal symptoms may be challenging to navigate for both women and clinicians.
The perimenopausal process is often even more confusing for women with early menopause—whether due to bilateral oophorectomy, chemotherapy or radiation therapy, genetics, or an autoimmune process—because of lack of recognition that an early menopausal transition is occurring or what solutions are available for symptoms. While there is support in the workplace for women during pregnancy and breastfeeding, there remains little support or recognition for the oft challenging perimenopausal transition leading to menopause.
Perimenopause: Common symptoms and treatments
Symptoms may be related to either estrogen level deficiency or excess during perimenopause, and these level changes may even occur within the same cycle.
Cyclic breast tenderness may develop, worsened by caffeine or high salt intake (which can be potentially improved, although without clinical trial evidence, with decreased caffeine or a trial of evening primrose oil or vitamin E).
Changes in menstrual flow and frequency of menses are typical. Flow may be lighter or heavier, longer or shorter, and there may be cycle variability, missed menses, or midcycle spotting.2 Bleeding may be heavy, with or without cramping. In addition to imaging with vaginal ultrasonography or hysteroscopy to identify structural issues, symptoms may be managed with nonsteroidal anti-inflammatory drugs (NSAIDs), hormonal therapy (HT) with short hormone-free interval contraceptives, oral progestogens, or progestin intrauterine systems. Newer medical treatments include antifibrinolytic drugs and selective progesterone-receptor modulators. Uterine ablation to decrease or stop bleeding is effective if there are no structural abnormalities, such as fibroids or polyps or the presence of adenomyosis, where glands will regrow into the endometrium after ablation. Endometrial biopsy is indicated for persistent abnormal uterine bleeding or those with risk factors such as chronic anovulation.
Worsening headaches or menstrual migraines may be triggered by hormonal changes, which may respond to NSAIDs; dihydroergotamine; triptans; the combination of aspirin, acetaminophen, and caffeine; or estrogen the week before menses. For women taking oral contraceptives (OCPs), adding estradiol the week before menses, or using the OCP continuously, may decrease headache frequency. These short-term prophylactic strategies during the perimenstrual time are often effective. If not, preventive therapy is available for women with frequent, severe headaches.
Mood complaints and poor sleep are independently associated with menstrual irregularity, and can lead to fatigue or anxiety, worsening premenstrual syndrome, or depressive moods. Sleep is disrupted premenstrually for up to one-third of women, and sleep disruption is particularly prevalent in those with premenstrual mood disorders and worsens during perimenopause.3
Reproductive hormones act on the neurotransmitter systems in the brain involved in mood regulation and emotion. The fluctuating hormones occurring during perimenopause may exacerbate pre-existing menstrual-related mood disorders. A subset of women experience depressive moods due to perimenopausal elevations in ovarian hormones.4 Others may exhibit increased mood sensitivity with the ovarian hormone withdrawal accompanying late menopause transition and early postmenopausal phase.5 There is significant comorbidity between premenstrual mood disorder (PMDD) and postpartum depression.6 During perimenopause and early menopause, clinicians should ask about prior hormonally-related depression (puberty, postpartum) and recognize that current or past premenstrual syndrome may worsen into a more severe premenstrual dysphoric disorder. Evidence-based treatments for PMDD include selective serotonin reuptake inhibitors (SSRIs); either taken continuously or only during the luteal phase; drospirenone-containing oral contraceptives, often with shorter pill-free intervals; GnRH analogues with or without hormone add-back; and cognitive behavioral therapy.7 For women whose perimenopausal moods improve with HT or develop worsened mood sensitivity with ovarian hormone withdrawal, clinicians should recognize that mood may worsen when treatment is ceased.5
Continue to: Menopausal symptoms...
Menopausal symptoms
Vasomotor symptoms (VMS), hot flashes, or night sweats occur in up to 75% of women as they develop more menstrual irregularity and move closer to their final period and menopause.
Hot flashes are transient episodes of flushing with the sensation of warmth (up to intense heat) on the upper body and face or head, often associated with sweating, chills or flushing, an increase in heart rate, and lowered blood pressure. Hot flashes can sometimes be preceded by an intense feeling of dread, followed by rapid heat dissipation. The etiology of hot flashes is still not clear, but the neurokinin receptors are involved. They are related to small fluctuations in core body temperature superimposed on a narrow thermoneutral zone in symptomatic women. Hot flashes are triggered when core body temperature rises above the upper (sweating) threshold. Shivering occurs if the core body temperature falls below the lower threshold. Sleep may be disrupted, with less rapid eye movement (REM) sleep, and associated with throwing covers on and off or changing sheets or nightclothes. On average, hot flashes last 7.2 years,8 and they are more bothersome if night sweats interfere with sleep or disrupt performance during the day.
In the Stages of Reproductive Aging Workshop (STRAW + 10), women reported VMS within 1-3 years after the menopausal transition.8 Four trajectories of hot flashes were identified in the Study of Women’s Health Across the Nation (SWAN) trial,9 including low levels throughout the menopause transition, early onset, late onset, and a group which had frequent hot flashes, starting early and lasting longer. Serum estrogen levels were not predictive of hot flash frequency or severity.
Hot flashes have been associated with low levels of exercise, cigarette smoking, high follicle-stimulating hormone levels and low estradiol levels, increasing body mass index, ethnicity (with hot flashes more common among Black and Hispanic women), low socioeconomic status, prior PMDD, anxiety, perceived stress, and depression.8 Women with a history of premenstrual syndrome, stress, sexual dysfunction, physical inactivity, or hot flashes are more vulnerable to depressive symptoms during perimenopause and early menopause.5
Depression may co-occur or overlap with menopause symptoms. Diagnosis involves menopausal stage, co-occurring psychiatric and menopause symptoms, psychosocial stressors, and a validated screening tool such as PQ9. Treatments for perimenopausal depression, such as antidepressants, psychotherapy, or cognitive behavioral therapy, are recommended first line for perimenopausal depression. Estrogen therapy has not been approved to treat perimenopausal depression but appears to have antidepressant effects in perimenopausal women, particularly those with bothersome vasomotor symptoms.5
Anxiety can worsen during menopause, and may respond to calming apps, meditation, cognitive behavioral therapy, hypnosis, yoga or tai chi, HT, or antianxiety medications.
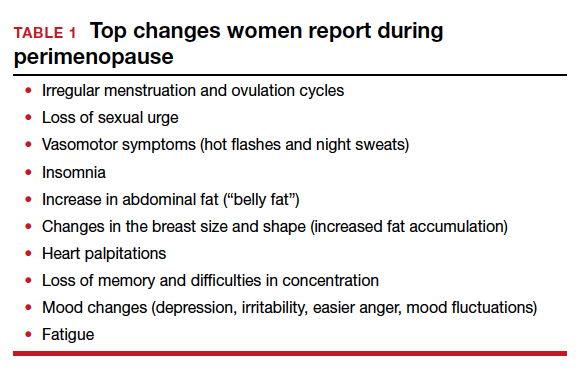
Weight gain around the abdomen (ie, belly fat) is a common complaint during the menopausal transition, despite women reporting not changing their eating or exercise patterns. Increasing exercise or bursts of higher intensity, decreasing portion sizes or limiting carbohydrates and alcohol may help.
Memory and concentration problems, described as brain fog, tend to be more of an issue in perimenopause and level out after menopause. Counsel midlife women that these changes are not due to dementia but are related to normal aging, hormonal changes, mood, stress, or other life circumstances. Identifying and addressing sleep issues and mood disorders may help mitigate brain fog, as can advising women to avoid excess caffeine, alcohol, nicotine, and eating before bed. Improvements in memory, cognition, and health have been found with the Mediterranean diet, regular exercise, avoiding multitasking, and engaging in mentally stimulating activities.
Sleeping concerns in peri- and postmenopausal women include sleeping less and more frequent insomnia. Women are more likely to use prescription sleeping aids during these times of their lives. The data from SWAN8 show that the menopausal transition is related to self-reported difficulty sleeping, independent of age. Sleep latency interval is increased while REM sleep decreases. Night sweats can trigger awakenings in the first half of the night. The perceived decline in sleep quality also may be attributed to general aging effects, nocturnal urination, sleep-related disorders such as sleep apnea or restless legs, or chronic pain, stress, or depression.10 Suggestions for management include sleep apps, cognitive behavioral therapy, low-dose antidepressant therapy, addressing sleep routines, and HT. Hypnotics should be avoided.
Sexuality issues are common complaints during the menopausal transition. Cross-sectional data reported from a longitudinal, population-based Australian cohort of women aged 45 to 55 years, found a decrease in sexual responsivity, sexual frequency, libido, vaginal dyspareunia, and more partner problems.11 Low libido may be related to relationship issues, dyspareunia with vaginal narrowing, loss of lubrication, levator spasm, stress, anxiety, exhaustion or mood disorder, lowered hormone levels, excess alcohol intake, underlying health concerns, or a side effect of medications for depression or pain. There is no direct correlation between testosterone levels and libido.
When HT at menopause may be helpful
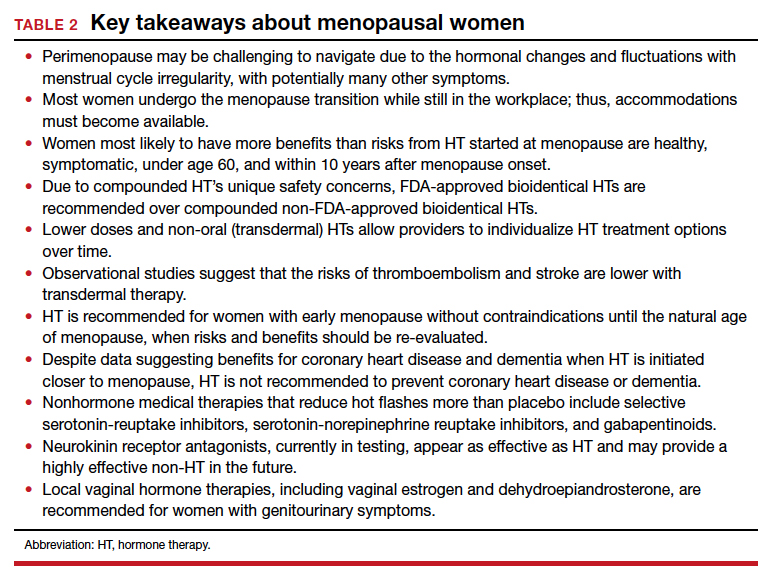
For healthy symptomatic women without contraindications who are younger than age 60, or within 10 years of menopause onset, the benefits of initiating HT most likely outweigh the risks to relieve bothersome hot flashes and night sweats.12-17 For older women, or for those further from menopause, the greater absolute risks of coronary heart disease, stroke, venous thromboembolism, and dementia, in general, outweigh the potential benefits.12-17 Extended durations of HT have less safety and efficacy data and should be considered primarily for those with persistent menopausal symptoms, with periodic re-evaluation.13,14 For bothersome genitourinary syndrome of menopause symptoms that do not respond to vaginal moisturizers or lubricants, low-dose vaginal HTs are encouraged.13-17
Continue to: Early-onset menopause...
Early-onset menopause
According to observational studies,18 early menopause is associated with a higher risk of osteoporosis, coronary heart disease, cognitive changes, vaginal dryness, loss of libido, and mood changes. Studies have shown that women with early menopause who take HT, without contraindications, to the average age of menopause (age 52) decrease the health risks of early menopause (bone loss, heart disease, mood, and cognition changes).13,14,18
Women with early menopause, whether spontaneous or following bilateral oophorectomy or cancer treatment, should be counseled to get adequate calcium (dietary recommended over supplementation) and vitamin D intake, eat a healthy diet, and exercise regularly. Evaluation should include risk for bone loss, heart disease, mood changes, and vaginal changes.
Extended use of HT
Up to 8% of women have hot flashes for 20 years or more after menopause.19 The decision to continue or to stop HT is not always clear for women:
- with persistent hot flashes after a trial period of HT discontinuation
- with bone loss that cannot be treated with bone-specific medications
- who request continuation for quality of life.
Extended use of HT should include an ongoing assessment of its risks and benefits, periodic trials off of HT, and documentation of rationale and informed discussions about continuing. Lower doses and transdermal therapies appear safer, as does micronized progesterone instead of more potent synthetic progestins.13-17
Genitourinary syndrome of menopause
Once women are further into menopause, they may notice vaginal dryness, vulvar itching or burning, bothersome vaginal discharge, or urinary urgency or frequency. The development of painful intercourse frequently occurs, a combination of the loss of estrogen with thinning of the vaginal mucosa, a loss of the acidic vaginal milieu with less elasticity, and spasm of the levator muscles. Some women develop urinary tract infections after intercourse or have more frequent reoccurrences. First-line therapy is often vaginal moisturizers and lubricants. Vaginal therapies (estradiol, conjugated estrogen, or dehydroepiandrosterone) or oral selective estrogen-receptor modulators (SERMs; ospemifene) improve vaginal dryness and dyspareunia.13,14 Pelvic therapy has also proved valuable for incontinence, pelvic floor dysfunction, and levator spasms.20
Where are there gaps in clinician knowledge?
Studies on emotional health, mood, and sleep need to incorporate measures of menstrual timing into data collection and analyses. Does the sleep disruption occurring premenstrually during perimenopause disproportionately contribute to a woman’s vulnerability to depressive disorders? The risk of clinically significant depressive symptoms increases 1.5- to 2.9-fold in the menopause transition.5 Research into premenstrual dysphoria during the menopause transition may identify different trajectories in the timing of symptoms related to either cycle itself or the ovarian hormone fluctuations or both.21 Gamma-aminobutyric acid (GABA)-modulating drugs, such as sepranolone, which blocks allopregnanolone’s actions at the GABAA receptor, may allow treatment of menstrual-related mood disorders without the need for hormonal interventions.21
Despite extended observational trial data, more data are needed to inform us about the long-term risks and benefits of using menopausal HT, particularly when initiated at menopause and to help address the timing of HT discontinuation. Furthermore, there are many unanswered questions. For instance:
- How much safer are lower dose and transdermal therapies?
- Do untreated hot flashes increase the risk of cardiovascular disease or dementia?
- Will newer non-HT options, such as the neurokinin receptor antagonists that are in testing but are not yet available, lower cardiovascular or dementia risks?
- What will be the risks and benefits for the newer estrogen in testing (estetrol, or E4), considered a natural estrogen and which appears to have lower thrombotic risks?
- What will be the role of intravaginal energy-based therapies, such as vaginal laser or radiofrequency devices?
- How do we address diverse populations and the effects of menopause on race, gender, culture, prior trauma, and socioeconomic status?
Lack of recognition of menopausal symptoms, particularly in the workplace
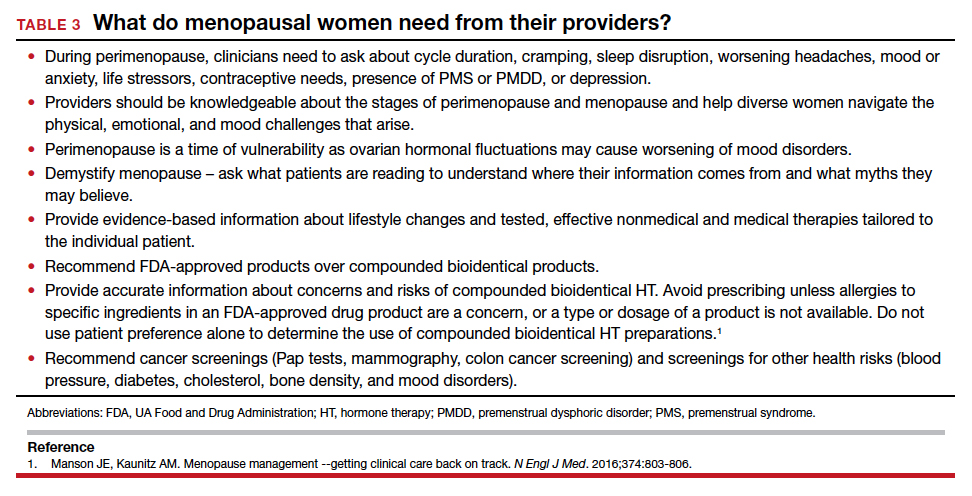
Clinicians need to understand the varied physical and emotional symptoms that may occur with hormonal changes as women traverse perimenopause and early menopause. We need to recognize that the lack of discussion about women’s health during this time may make women feel ashamed and fearful of bringing up their symptoms due to fear of being dismissed or stigmatized.22 Women may not seek help until a crisis at home or work occurs, as they may fear that admitting symptoms or a need for help or time away from work will threaten how they are viewed at work or affect their chances of promotion. Although there are economic costs around menopause for appointments, tests, therapies, and missed time at work, not addressing menopausal health leads to poorer performance, workplace absences, and additional medical costs.22
Conclusion
Menopause occurs naturally as a part of a woman’s life cycle. However, women need assistance navigating perimenopausal hormonal fluctuations and decisions about HT once in menopause. Increased awareness and education about perimenopause and menopause will allow compassionate, individualized, informed care, including lifestyle changes, behavioral or complementary strategies, or medical therapies, hormonal or nonhormonal.27 As a medical society, we need to challenge the stigma associated with aging and menopause and educate ourselves and our patients to help women navigate this challenging time. ●
Myth 1: All hot flashes are the same
The truth: Seventy-five percent of women will have hot flashes, but only 25% are severe enough to cause women to seek treatment. Duration varies with identified patterns, including starting early or late, being mild or starting early, and going late. Ethnicity affects the duration of hot flashes, with longer durations seen in Black and Hispanic women. About 15% of women have had hot flashes for more than 15 or 20 years.1,2
Myth 2: There is no help for hot flashes
The truth: For some women, lifestyle changes are helpful, such as dressing in layers, turning down the thermostat at night, avoiding hot beverages or alcohol, or using technology (Femtech) for cooling devices. Over-the-counter products that are available, but are not clearly proven to help more than placebo, include soy (which may be estrogenic), black cohosh supplements, and nutritional supplements. Cognitive behavioral therapy, hypnosis, weight loss, or mindfulness may help.3 Nonhormone medications such as low-dose antidepressants or gabapentin have shown benefit. Newer treatments in testing, including neurokinin receptor antagonists, appear to work quickly and as effectively as HT. When initiating HT, healthy women with bothersome hot flashes under age 60 or within 10 years of menopause are the best candidates for HT; many lower doses and oral and non-oral therapies are available.
Myth 3: Compounded bioidentical hormones made by a compounding pharmacy are safer and more effective than FDA-approved ones
The truth: Compounded bioidentical hormones are touted as safer or more effective, but there is no good evidence to back up those claims. Whether US Food and Drug Administration (FDA)-approved or compounded, hormones come from the same precursors and have potential risks. With custom compounded HT, there is additional concern about precisely what is in the compounded product, whether levels are similar batch to batch, and the degree of absorption of progesterone, which is better absorbed oral.4-6 FDA-approved bioidentical HTs have been tested for safety, proven to contain consistent, effective levels of hormones, and are monitored by the FDA. For menopausal symptoms, FDA-approved therapies are available as estradiol (oral, patch, spray, gel, lotion, and vaginal ring) and progesterone (as an oral compound or combined with estradiol). Pellets made of compounded hormones have shown higher serum levels and more adverse events.5,7
Myth 4: Menopause causes weight gain
The truth is that fluctuating and declining hormones and the slowing of metabolism affect weight. Weight gain is not inevitable, just harder to prevent. Many women gain an average of 5 lb (2.27 kg) at midlife, which is mainly related to aging and lifestyle and not to menopause or HT. However, menopause may be related to body composition and fat distribution changes. Counsel women to decrease portion sizes, limit carbs, and increase exercise intensity, including strength training. The goal is 30 minutes of moderate aerobic activity per day, all at once or through smaller time increments, to improve their energy, mood, and sleep.
References
1. The NAMS 2017 HT Position Statement Advisory Panel. The 2017 HT position statement of The North American Menopause Society. Menopause. 2017;24:728-753.
2. Pinkerton JV. HT for postmenopausal women. N Engl J Med. 2020;382:446-455.
3. Paramsothy P. Duration of the menopausal transition is longer in women with young age at onset: the multiethnic Study of Women’s Health Across the Nation. Menopause. 2017;24:142-149.
4. Kingsberg SA, Schaffir J, Faught BM, et al. Female sexual health: barriers to optimal outcomes and a roadmap for improved patient-clinician communications. J Womens Health (Larchmt). 2019;28:432-443.
5. Eisenlohr-Moul TA, Kaiser G, Weise C, et al. Are there temporal subtypes of premenstrual dysphoric disorder? Using group-based trajectory modeling to identify individual differences in symptom change. Psychol Med. 2020;50:964-972.
6. Seibel M, Seibel S. Working through Menopause: The Impact on Women, Businesses and the Bottom Line. Bookbaby. March 8, 2022.
7. Kingsberg SA, Schaffir J, Faught BM, et al. Female sexual health: barriers to optimal outcomes and a roadmap for improved patient-clinician communications. J Womens Health (Larchmt). 2019;28:432-443.
- Paramsothy P. Duration of the menopausal transition is longer in women with young age at onset: the multiethnic Study of Women’s Health Across the Nation. Menopause. 2017;24:142–149.
- Harlow SD, Gass M, Hall JE, et al. STRAW 10 Collaborative Group. Executive summary of the Stages of Reproductive Aging Workshop + 10: addressing the unfinished agenda of staging reproductive aging. Menopause. 2012;19:387-95.
- Meers JM, Nowakowski S. Sleep, premenstrual mood disorder, and women’s health. Curr Opin Psychol. 2020;34:43-49.
- Sander B, Gordon JL. Premenstrual mood symptoms in the perimenopause. Curr Psychiatry Rep. 2021;23:73.
- Maki PM, Kornstein SG, Joffe H, et al. Guidelines for the evaluation and treatment of perimenopausal depression: summary and recommendations. J Women’s Health. 2019;28:117–134.
- Cao S, Jones M, Tooth L, et al. History of premenstrual syndrome and development of postpartum depression: a systematic review and meta-analysis. J Psychiatr Res. 2020;121:82–90.
- Rapkin AJ, Korotkaya Y, Taylor KC. Contraception counseling for women with premenstrual dysphoric disorder (PMDD): current perspectives. Open Access J Contracept. 2019;10:27–39.
- Avis NE, Crawford SL, Greendale G, et al; Study of Women's Health Across the Nation. Duration of menopausal vasomotor symptoms over the menopause transition. JAMA Intern Med. 2015;175:531.
- Tepper PG, Brooks MM, Randolph JF Jr, et al. Characterizing the trajectories of vasomotor symptoms across the menopausal transition. Menopause. 2016;23:1067-1074.
- Kravitz HM, Ganz PA, Bromberger J, et al. Sleep difficulty in women at midlife: a community survey of sleep and the menopausal transition. Menopause. 2003;10:19-28.
- Dennerstein L, Dudley EC, Hopper JL, et al. A prospective population-based study of menopausal symptoms. Obstet Gynecol. 2000;96:351-358.
- Manson JE, Chlebowski RT, Stefanick ML, et al. Menopausal HT and health outcomes during the intervention and extended poststopping phases of the Women’s Health Initiative randomized trials. JAMA. 2013;310:1353-1368.
- The NAMS 2017 HT Position Statement Advisory Panel. T he 2017 HT position statement of The North American Menopause Society. Menopause. 2017;24:728-753.
- Pinkerton JV. HT for postmenopausal women. N Engl J Med. 2020;382:446-455.
- Stuenkel CA, Davis SR, Gompel A, et al. Treatment of symptoms of the menopause: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2015;100:39754011.
- Manson JE, Kaunitz AM. Menopause management—getting clinical care back on track. N Engl J Med. 2016;374:803–806.
- American College of Obstetricians and Gynecologists. Practice Bulletin No. 141: Management of menopausal symptoms. Obstet Gynecol. 2014;123:202-216.
- Shuster LT, Rhodes DJ, Gostout BS, et al. Premature menopause or early menopause: long-term health consequences. Maturitas. 2010;65:161-166.
- Zeleke BM, Davis SR, Fradkin P, et al. Vasomotor symptoms and urogenital atrophy in older women: a systematic review. Climacteric. 2015;18:112-120.
- Kingsberg SA, Schaffir J, Faught BM, et al. Female sexual health: barriers to optimal outcomes and a roadmap for improved patient-clinician communications. J Womens Health (Larchmt). 2019;28:432-443.
- Eisenlohr-Moul TA, Kaiser G, Weise C, et al. Are there temporal subtypes of pre- menstrual dysphoric disorder? Using group-based trajectory modeling to identify individual differences in symptom change. Psychol Med. 2020;50: 964-972.
- Seibel M, Seibel S. Working through Menopause: The Impact on Women, Businesses and the Bottom Line. Bookbaby. March 8, 2022.
- Jackson LM, Parker RM, Mattison DR, eds. The Clinical Utility of Compounded Bioidentical HT: A Review of Safety, Effectiveness, and Use. Washington, DC: National Academies Press; 2020.
- Pinkerton JV. Concerns about safety and efficacy of compounded bioidentical HT. Menopause. 2021;28:847-849.
- Liu JH, Pinkerton JV. Prescription therapies. In: CJ Crandall, ed. Menopause Practice: A Clinician’s Guide, 6th ed. Pepper Pike, OH: The North American Menopause Society; 2019: 277-309.
- Jiang X, Bossert A, Parthasarathy KN, et al. Safety assessment of compounded non-FDA-approved hormonal therapy versus FDA-approved hormonal therapy in treating postmenopausal women. Menopause. 2021;28:867-874.
- Aninye IO, Laitner MH, Chinnappan S; Society for Women’s Health Research Menopause Working Group. Menopause preparedness: perspectives for patient, provider, and policymaker consideration. Menopause. 2021;28:1186-1191.
The transition to menopause begins with ovarian fluctuation and hormonal changes, often beginning before significant changes in menstruation. Reproductive aging with loss of follicular activity progresses over a wide age range (42 to 58 years) with an average onset at approximately age 47, ranging from 4 to 8 years. Although most women have heard about menopause, defined as 12 months after the last period, they often lack understanding about perimenopause or that the menopausal transition usually begins 5 years before menopause.1
Perimenopause, defined as early and late menopause transition stages, may be viewed as a window of potential vulnerability for women who develop or have worsening menstrual-related mood disorders. Over time, hormonal fluctuations often lead to menstrual cycle irregularity (either shorter or longer). Changes occurring during perimenopause may be confusing as it may not be clear whether symptoms are related to menopause, aging, or stress. Often not recognized or treated adequately, perimenopausal symptoms may be challenging to navigate for both women and clinicians.
The perimenopausal process is often even more confusing for women with early menopause—whether due to bilateral oophorectomy, chemotherapy or radiation therapy, genetics, or an autoimmune process—because of lack of recognition that an early menopausal transition is occurring or what solutions are available for symptoms. While there is support in the workplace for women during pregnancy and breastfeeding, there remains little support or recognition for the oft challenging perimenopausal transition leading to menopause.
Perimenopause: Common symptoms and treatments
Symptoms may be related to either estrogen level deficiency or excess during perimenopause, and these level changes may even occur within the same cycle.
Cyclic breast tenderness may develop, worsened by caffeine or high salt intake (which can be potentially improved, although without clinical trial evidence, with decreased caffeine or a trial of evening primrose oil or vitamin E).
Changes in menstrual flow and frequency of menses are typical. Flow may be lighter or heavier, longer or shorter, and there may be cycle variability, missed menses, or midcycle spotting.2 Bleeding may be heavy, with or without cramping. In addition to imaging with vaginal ultrasonography or hysteroscopy to identify structural issues, symptoms may be managed with nonsteroidal anti-inflammatory drugs (NSAIDs), hormonal therapy (HT) with short hormone-free interval contraceptives, oral progestogens, or progestin intrauterine systems. Newer medical treatments include antifibrinolytic drugs and selective progesterone-receptor modulators. Uterine ablation to decrease or stop bleeding is effective if there are no structural abnormalities, such as fibroids or polyps or the presence of adenomyosis, where glands will regrow into the endometrium after ablation. Endometrial biopsy is indicated for persistent abnormal uterine bleeding or those with risk factors such as chronic anovulation.
Worsening headaches or menstrual migraines may be triggered by hormonal changes, which may respond to NSAIDs; dihydroergotamine; triptans; the combination of aspirin, acetaminophen, and caffeine; or estrogen the week before menses. For women taking oral contraceptives (OCPs), adding estradiol the week before menses, or using the OCP continuously, may decrease headache frequency. These short-term prophylactic strategies during the perimenstrual time are often effective. If not, preventive therapy is available for women with frequent, severe headaches.
Mood complaints and poor sleep are independently associated with menstrual irregularity, and can lead to fatigue or anxiety, worsening premenstrual syndrome, or depressive moods. Sleep is disrupted premenstrually for up to one-third of women, and sleep disruption is particularly prevalent in those with premenstrual mood disorders and worsens during perimenopause.3
Reproductive hormones act on the neurotransmitter systems in the brain involved in mood regulation and emotion. The fluctuating hormones occurring during perimenopause may exacerbate pre-existing menstrual-related mood disorders. A subset of women experience depressive moods due to perimenopausal elevations in ovarian hormones.4 Others may exhibit increased mood sensitivity with the ovarian hormone withdrawal accompanying late menopause transition and early postmenopausal phase.5 There is significant comorbidity between premenstrual mood disorder (PMDD) and postpartum depression.6 During perimenopause and early menopause, clinicians should ask about prior hormonally-related depression (puberty, postpartum) and recognize that current or past premenstrual syndrome may worsen into a more severe premenstrual dysphoric disorder. Evidence-based treatments for PMDD include selective serotonin reuptake inhibitors (SSRIs); either taken continuously or only during the luteal phase; drospirenone-containing oral contraceptives, often with shorter pill-free intervals; GnRH analogues with or without hormone add-back; and cognitive behavioral therapy.7 For women whose perimenopausal moods improve with HT or develop worsened mood sensitivity with ovarian hormone withdrawal, clinicians should recognize that mood may worsen when treatment is ceased.5

Continue to: Menopausal symptoms...
Menopausal symptoms
Vasomotor symptoms (VMS), hot flashes, or night sweats occur in up to 75% of women as they develop more menstrual irregularity and move closer to their final period and menopause.
Hot flashes are transient episodes of flushing with the sensation of warmth (up to intense heat) on the upper body and face or head, often associated with sweating, chills or flushing, an increase in heart rate, and lowered blood pressure. Hot flashes can sometimes be preceded by an intense feeling of dread, followed by rapid heat dissipation. The etiology of hot flashes is still not clear, but the neurokinin receptors are involved. They are related to small fluctuations in core body temperature superimposed on a narrow thermoneutral zone in symptomatic women. Hot flashes are triggered when core body temperature rises above the upper (sweating) threshold. Shivering occurs if the core body temperature falls below the lower threshold. Sleep may be disrupted, with less rapid eye movement (REM) sleep, and associated with throwing covers on and off or changing sheets or nightclothes. On average, hot flashes last 7.2 years,8 and they are more bothersome if night sweats interfere with sleep or disrupt performance during the day.
In the Stages of Reproductive Aging Workshop (STRAW + 10), women reported VMS within 1-3 years after the menopausal transition.8 Four trajectories of hot flashes were identified in the Study of Women’s Health Across the Nation (SWAN) trial,9 including low levels throughout the menopause transition, early onset, late onset, and a group which had frequent hot flashes, starting early and lasting longer. Serum estrogen levels were not predictive of hot flash frequency or severity.
Hot flashes have been associated with low levels of exercise, cigarette smoking, high follicle-stimulating hormone levels and low estradiol levels, increasing body mass index, ethnicity (with hot flashes more common among Black and Hispanic women), low socioeconomic status, prior PMDD, anxiety, perceived stress, and depression.8 Women with a history of premenstrual syndrome, stress, sexual dysfunction, physical inactivity, or hot flashes are more vulnerable to depressive symptoms during perimenopause and early menopause.5
Depression may co-occur or overlap with menopause symptoms. Diagnosis involves menopausal stage, co-occurring psychiatric and menopause symptoms, psychosocial stressors, and a validated screening tool such as PQ9. Treatments for perimenopausal depression, such as antidepressants, psychotherapy, or cognitive behavioral therapy, are recommended first line for perimenopausal depression. Estrogen therapy has not been approved to treat perimenopausal depression but appears to have antidepressant effects in perimenopausal women, particularly those with bothersome vasomotor symptoms.5
Anxiety can worsen during menopause, and may respond to calming apps, meditation, cognitive behavioral therapy, hypnosis, yoga or tai chi, HT, or antianxiety medications.
Weight gain around the abdomen (ie, belly fat) is a common complaint during the menopausal transition, despite women reporting not changing their eating or exercise patterns. Increasing exercise or bursts of higher intensity, decreasing portion sizes or limiting carbohydrates and alcohol may help.
Memory and concentration problems, described as brain fog, tend to be more of an issue in perimenopause and level out after menopause. Counsel midlife women that these changes are not due to dementia but are related to normal aging, hormonal changes, mood, stress, or other life circumstances. Identifying and addressing sleep issues and mood disorders may help mitigate brain fog, as can advising women to avoid excess caffeine, alcohol, nicotine, and eating before bed. Improvements in memory, cognition, and health have been found with the Mediterranean diet, regular exercise, avoiding multitasking, and engaging in mentally stimulating activities.
Sleeping concerns in peri- and postmenopausal women include sleeping less and more frequent insomnia. Women are more likely to use prescription sleeping aids during these times of their lives. The data from SWAN8 show that the menopausal transition is related to self-reported difficulty sleeping, independent of age. Sleep latency interval is increased while REM sleep decreases. Night sweats can trigger awakenings in the first half of the night. The perceived decline in sleep quality also may be attributed to general aging effects, nocturnal urination, sleep-related disorders such as sleep apnea or restless legs, or chronic pain, stress, or depression.10 Suggestions for management include sleep apps, cognitive behavioral therapy, low-dose antidepressant therapy, addressing sleep routines, and HT. Hypnotics should be avoided.
Sexuality issues are common complaints during the menopausal transition. Cross-sectional data reported from a longitudinal, population-based Australian cohort of women aged 45 to 55 years, found a decrease in sexual responsivity, sexual frequency, libido, vaginal dyspareunia, and more partner problems.11 Low libido may be related to relationship issues, dyspareunia with vaginal narrowing, loss of lubrication, levator spasm, stress, anxiety, exhaustion or mood disorder, lowered hormone levels, excess alcohol intake, underlying health concerns, or a side effect of medications for depression or pain. There is no direct correlation between testosterone levels and libido.
When HT at menopause may be helpful
For healthy symptomatic women without contraindications who are younger than age 60, or within 10 years of menopause onset, the benefits of initiating HT most likely outweigh the risks to relieve bothersome hot flashes and night sweats.12-17 For older women, or for those further from menopause, the greater absolute risks of coronary heart disease, stroke, venous thromboembolism, and dementia, in general, outweigh the potential benefits.12-17 Extended durations of HT have less safety and efficacy data and should be considered primarily for those with persistent menopausal symptoms, with periodic re-evaluation.13,14 For bothersome genitourinary syndrome of menopause symptoms that do not respond to vaginal moisturizers or lubricants, low-dose vaginal HTs are encouraged.13-17

Continue to: Early-onset menopause...
Early-onset menopause
According to observational studies,18 early menopause is associated with a higher risk of osteoporosis, coronary heart disease, cognitive changes, vaginal dryness, loss of libido, and mood changes. Studies have shown that women with early menopause who take HT, without contraindications, to the average age of menopause (age 52) decrease the health risks of early menopause (bone loss, heart disease, mood, and cognition changes).13,14,18
Women with early menopause, whether spontaneous or following bilateral oophorectomy or cancer treatment, should be counseled to get adequate calcium (dietary recommended over supplementation) and vitamin D intake, eat a healthy diet, and exercise regularly. Evaluation should include risk for bone loss, heart disease, mood changes, and vaginal changes.
Extended use of HT
Up to 8% of women have hot flashes for 20 years or more after menopause.19 The decision to continue or to stop HT is not always clear for women:
- with persistent hot flashes after a trial period of HT discontinuation
- with bone loss that cannot be treated with bone-specific medications
- who request continuation for quality of life.
Extended use of HT should include an ongoing assessment of its risks and benefits, periodic trials off of HT, and documentation of rationale and informed discussions about continuing. Lower doses and transdermal therapies appear safer, as does micronized progesterone instead of more potent synthetic progestins.13-17
Genitourinary syndrome of menopause
Once women are further into menopause, they may notice vaginal dryness, vulvar itching or burning, bothersome vaginal discharge, or urinary urgency or frequency. The development of painful intercourse frequently occurs, a combination of the loss of estrogen with thinning of the vaginal mucosa, a loss of the acidic vaginal milieu with less elasticity, and spasm of the levator muscles. Some women develop urinary tract infections after intercourse or have more frequent reoccurrences. First-line therapy is often vaginal moisturizers and lubricants. Vaginal therapies (estradiol, conjugated estrogen, or dehydroepiandrosterone) or oral selective estrogen-receptor modulators (SERMs; ospemifene) improve vaginal dryness and dyspareunia.13,14 Pelvic therapy has also proved valuable for incontinence, pelvic floor dysfunction, and levator spasms.20
Where are there gaps in clinician knowledge?
Studies on emotional health, mood, and sleep need to incorporate measures of menstrual timing into data collection and analyses. Does the sleep disruption occurring premenstrually during perimenopause disproportionately contribute to a woman’s vulnerability to depressive disorders? The risk of clinically significant depressive symptoms increases 1.5- to 2.9-fold in the menopause transition.5 Research into premenstrual dysphoria during the menopause transition may identify different trajectories in the timing of symptoms related to either cycle itself or the ovarian hormone fluctuations or both.21 Gamma-aminobutyric acid (GABA)-modulating drugs, such as sepranolone, which blocks allopregnanolone’s actions at the GABAA receptor, may allow treatment of menstrual-related mood disorders without the need for hormonal interventions.21
Despite extended observational trial data, more data are needed to inform us about the long-term risks and benefits of using menopausal HT, particularly when initiated at menopause and to help address the timing of HT discontinuation. Furthermore, there are many unanswered questions. For instance:
- How much safer are lower dose and transdermal therapies?
- Do untreated hot flashes increase the risk of cardiovascular disease or dementia?
- Will newer non-HT options, such as the neurokinin receptor antagonists that are in testing but are not yet available, lower cardiovascular or dementia risks?
- What will be the risks and benefits for the newer estrogen in testing (estetrol, or E4), considered a natural estrogen and which appears to have lower thrombotic risks?
- What will be the role of intravaginal energy-based therapies, such as vaginal laser or radiofrequency devices?
- How do we address diverse populations and the effects of menopause on race, gender, culture, prior trauma, and socioeconomic status?
Lack of recognition of menopausal symptoms, particularly in the workplace
Clinicians need to understand the varied physical and emotional symptoms that may occur with hormonal changes as women traverse perimenopause and early menopause. We need to recognize that the lack of discussion about women’s health during this time may make women feel ashamed and fearful of bringing up their symptoms due to fear of being dismissed or stigmatized.22 Women may not seek help until a crisis at home or work occurs, as they may fear that admitting symptoms or a need for help or time away from work will threaten how they are viewed at work or affect their chances of promotion. Although there are economic costs around menopause for appointments, tests, therapies, and missed time at work, not addressing menopausal health leads to poorer performance, workplace absences, and additional medical costs.22

Conclusion
Menopause occurs naturally as a part of a woman’s life cycle. However, women need assistance navigating perimenopausal hormonal fluctuations and decisions about HT once in menopause. Increased awareness and education about perimenopause and menopause will allow compassionate, individualized, informed care, including lifestyle changes, behavioral or complementary strategies, or medical therapies, hormonal or nonhormonal.27 As a medical society, we need to challenge the stigma associated with aging and menopause and educate ourselves and our patients to help women navigate this challenging time. ●
Myth 1: All hot flashes are the same
The truth: Seventy-five percent of women will have hot flashes, but only 25% are severe enough to cause women to seek treatment. Duration varies with identified patterns, including starting early or late, being mild or starting early, and going late. Ethnicity affects the duration of hot flashes, with longer durations seen in Black and Hispanic women. About 15% of women have had hot flashes for more than 15 or 20 years.1,2
Myth 2: There is no help for hot flashes
The truth: For some women, lifestyle changes are helpful, such as dressing in layers, turning down the thermostat at night, avoiding hot beverages or alcohol, or using technology (Femtech) for cooling devices. Over-the-counter products that are available, but are not clearly proven to help more than placebo, include soy (which may be estrogenic), black cohosh supplements, and nutritional supplements. Cognitive behavioral therapy, hypnosis, weight loss, or mindfulness may help.3 Nonhormone medications such as low-dose antidepressants or gabapentin have shown benefit. Newer treatments in testing, including neurokinin receptor antagonists, appear to work quickly and as effectively as HT. When initiating HT, healthy women with bothersome hot flashes under age 60 or within 10 years of menopause are the best candidates for HT; many lower doses and oral and non-oral therapies are available.
Myth 3: Compounded bioidentical hormones made by a compounding pharmacy are safer and more effective than FDA-approved ones
The truth: Compounded bioidentical hormones are touted as safer or more effective, but there is no good evidence to back up those claims. Whether US Food and Drug Administration (FDA)-approved or compounded, hormones come from the same precursors and have potential risks. With custom compounded HT, there is additional concern about precisely what is in the compounded product, whether levels are similar batch to batch, and the degree of absorption of progesterone, which is better absorbed oral.4-6 FDA-approved bioidentical HTs have been tested for safety, proven to contain consistent, effective levels of hormones, and are monitored by the FDA. For menopausal symptoms, FDA-approved therapies are available as estradiol (oral, patch, spray, gel, lotion, and vaginal ring) and progesterone (as an oral compound or combined with estradiol). Pellets made of compounded hormones have shown higher serum levels and more adverse events.5,7
Myth 4: Menopause causes weight gain
The truth is that fluctuating and declining hormones and the slowing of metabolism affect weight. Weight gain is not inevitable, just harder to prevent. Many women gain an average of 5 lb (2.27 kg) at midlife, which is mainly related to aging and lifestyle and not to menopause or HT. However, menopause may be related to body composition and fat distribution changes. Counsel women to decrease portion sizes, limit carbs, and increase exercise intensity, including strength training. The goal is 30 minutes of moderate aerobic activity per day, all at once or through smaller time increments, to improve their energy, mood, and sleep.
References
1. The NAMS 2017 HT Position Statement Advisory Panel. The 2017 HT position statement of The North American Menopause Society. Menopause. 2017;24:728-753.
2. Pinkerton JV. HT for postmenopausal women. N Engl J Med. 2020;382:446-455.
3. Paramsothy P. Duration of the menopausal transition is longer in women with young age at onset: the multiethnic Study of Women’s Health Across the Nation. Menopause. 2017;24:142-149.
4. Kingsberg SA, Schaffir J, Faught BM, et al. Female sexual health: barriers to optimal outcomes and a roadmap for improved patient-clinician communications. J Womens Health (Larchmt). 2019;28:432-443.
5. Eisenlohr-Moul TA, Kaiser G, Weise C, et al. Are there temporal subtypes of premenstrual dysphoric disorder? Using group-based trajectory modeling to identify individual differences in symptom change. Psychol Med. 2020;50:964-972.
6. Seibel M, Seibel S. Working through Menopause: The Impact on Women, Businesses and the Bottom Line. Bookbaby. March 8, 2022.
7. Kingsberg SA, Schaffir J, Faught BM, et al. Female sexual health: barriers to optimal outcomes and a roadmap for improved patient-clinician communications. J Womens Health (Larchmt). 2019;28:432-443.
The transition to menopause begins with ovarian fluctuation and hormonal changes, often beginning before significant changes in menstruation. Reproductive aging with loss of follicular activity progresses over a wide age range (42 to 58 years) with an average onset at approximately age 47, ranging from 4 to 8 years. Although most women have heard about menopause, defined as 12 months after the last period, they often lack understanding about perimenopause or that the menopausal transition usually begins 5 years before menopause.1
Perimenopause, defined as early and late menopause transition stages, may be viewed as a window of potential vulnerability for women who develop or have worsening menstrual-related mood disorders. Over time, hormonal fluctuations often lead to menstrual cycle irregularity (either shorter or longer). Changes occurring during perimenopause may be confusing as it may not be clear whether symptoms are related to menopause, aging, or stress. Often not recognized or treated adequately, perimenopausal symptoms may be challenging to navigate for both women and clinicians.
The perimenopausal process is often even more confusing for women with early menopause—whether due to bilateral oophorectomy, chemotherapy or radiation therapy, genetics, or an autoimmune process—because of lack of recognition that an early menopausal transition is occurring or what solutions are available for symptoms. While there is support in the workplace for women during pregnancy and breastfeeding, there remains little support or recognition for the oft challenging perimenopausal transition leading to menopause.
Perimenopause: Common symptoms and treatments
Symptoms may be related to either estrogen level deficiency or excess during perimenopause, and these level changes may even occur within the same cycle.
Cyclic breast tenderness may develop, worsened by caffeine or high salt intake (which can be potentially improved, although without clinical trial evidence, with decreased caffeine or a trial of evening primrose oil or vitamin E).
Changes in menstrual flow and frequency of menses are typical. Flow may be lighter or heavier, longer or shorter, and there may be cycle variability, missed menses, or midcycle spotting.2 Bleeding may be heavy, with or without cramping. In addition to imaging with vaginal ultrasonography or hysteroscopy to identify structural issues, symptoms may be managed with nonsteroidal anti-inflammatory drugs (NSAIDs), hormonal therapy (HT) with short hormone-free interval contraceptives, oral progestogens, or progestin intrauterine systems. Newer medical treatments include antifibrinolytic drugs and selective progesterone-receptor modulators. Uterine ablation to decrease or stop bleeding is effective if there are no structural abnormalities, such as fibroids or polyps or the presence of adenomyosis, where glands will regrow into the endometrium after ablation. Endometrial biopsy is indicated for persistent abnormal uterine bleeding or those with risk factors such as chronic anovulation.
Worsening headaches or menstrual migraines may be triggered by hormonal changes, which may respond to NSAIDs; dihydroergotamine; triptans; the combination of aspirin, acetaminophen, and caffeine; or estrogen the week before menses. For women taking oral contraceptives (OCPs), adding estradiol the week before menses, or using the OCP continuously, may decrease headache frequency. These short-term prophylactic strategies during the perimenstrual time are often effective. If not, preventive therapy is available for women with frequent, severe headaches.
Mood complaints and poor sleep are independently associated with menstrual irregularity, and can lead to fatigue or anxiety, worsening premenstrual syndrome, or depressive moods. Sleep is disrupted premenstrually for up to one-third of women, and sleep disruption is particularly prevalent in those with premenstrual mood disorders and worsens during perimenopause.3
Reproductive hormones act on the neurotransmitter systems in the brain involved in mood regulation and emotion. The fluctuating hormones occurring during perimenopause may exacerbate pre-existing menstrual-related mood disorders. A subset of women experience depressive moods due to perimenopausal elevations in ovarian hormones.4 Others may exhibit increased mood sensitivity with the ovarian hormone withdrawal accompanying late menopause transition and early postmenopausal phase.5 There is significant comorbidity between premenstrual mood disorder (PMDD) and postpartum depression.6 During perimenopause and early menopause, clinicians should ask about prior hormonally-related depression (puberty, postpartum) and recognize that current or past premenstrual syndrome may worsen into a more severe premenstrual dysphoric disorder. Evidence-based treatments for PMDD include selective serotonin reuptake inhibitors (SSRIs); either taken continuously or only during the luteal phase; drospirenone-containing oral contraceptives, often with shorter pill-free intervals; GnRH analogues with or without hormone add-back; and cognitive behavioral therapy.7 For women whose perimenopausal moods improve with HT or develop worsened mood sensitivity with ovarian hormone withdrawal, clinicians should recognize that mood may worsen when treatment is ceased.5

Continue to: Menopausal symptoms...
Menopausal symptoms
Vasomotor symptoms (VMS), hot flashes, or night sweats occur in up to 75% of women as they develop more menstrual irregularity and move closer to their final period and menopause.
Hot flashes are transient episodes of flushing with the sensation of warmth (up to intense heat) on the upper body and face or head, often associated with sweating, chills or flushing, an increase in heart rate, and lowered blood pressure. Hot flashes can sometimes be preceded by an intense feeling of dread, followed by rapid heat dissipation. The etiology of hot flashes is still not clear, but the neurokinin receptors are involved. They are related to small fluctuations in core body temperature superimposed on a narrow thermoneutral zone in symptomatic women. Hot flashes are triggered when core body temperature rises above the upper (sweating) threshold. Shivering occurs if the core body temperature falls below the lower threshold. Sleep may be disrupted, with less rapid eye movement (REM) sleep, and associated with throwing covers on and off or changing sheets or nightclothes. On average, hot flashes last 7.2 years,8 and they are more bothersome if night sweats interfere with sleep or disrupt performance during the day.
In the Stages of Reproductive Aging Workshop (STRAW + 10), women reported VMS within 1-3 years after the menopausal transition.8 Four trajectories of hot flashes were identified in the Study of Women’s Health Across the Nation (SWAN) trial,9 including low levels throughout the menopause transition, early onset, late onset, and a group which had frequent hot flashes, starting early and lasting longer. Serum estrogen levels were not predictive of hot flash frequency or severity.
Hot flashes have been associated with low levels of exercise, cigarette smoking, high follicle-stimulating hormone levels and low estradiol levels, increasing body mass index, ethnicity (with hot flashes more common among Black and Hispanic women), low socioeconomic status, prior PMDD, anxiety, perceived stress, and depression.8 Women with a history of premenstrual syndrome, stress, sexual dysfunction, physical inactivity, or hot flashes are more vulnerable to depressive symptoms during perimenopause and early menopause.5
Depression may co-occur or overlap with menopause symptoms. Diagnosis involves menopausal stage, co-occurring psychiatric and menopause symptoms, psychosocial stressors, and a validated screening tool such as PQ9. Treatments for perimenopausal depression, such as antidepressants, psychotherapy, or cognitive behavioral therapy, are recommended first line for perimenopausal depression. Estrogen therapy has not been approved to treat perimenopausal depression but appears to have antidepressant effects in perimenopausal women, particularly those with bothersome vasomotor symptoms.5
Anxiety can worsen during menopause, and may respond to calming apps, meditation, cognitive behavioral therapy, hypnosis, yoga or tai chi, HT, or antianxiety medications.
Weight gain around the abdomen (ie, belly fat) is a common complaint during the menopausal transition, despite women reporting not changing their eating or exercise patterns. Increasing exercise or bursts of higher intensity, decreasing portion sizes or limiting carbohydrates and alcohol may help.
Memory and concentration problems, described as brain fog, tend to be more of an issue in perimenopause and level out after menopause. Counsel midlife women that these changes are not due to dementia but are related to normal aging, hormonal changes, mood, stress, or other life circumstances. Identifying and addressing sleep issues and mood disorders may help mitigate brain fog, as can advising women to avoid excess caffeine, alcohol, nicotine, and eating before bed. Improvements in memory, cognition, and health have been found with the Mediterranean diet, regular exercise, avoiding multitasking, and engaging in mentally stimulating activities.
Sleeping concerns in peri- and postmenopausal women include sleeping less and more frequent insomnia. Women are more likely to use prescription sleeping aids during these times of their lives. The data from SWAN8 show that the menopausal transition is related to self-reported difficulty sleeping, independent of age. Sleep latency interval is increased while REM sleep decreases. Night sweats can trigger awakenings in the first half of the night. The perceived decline in sleep quality also may be attributed to general aging effects, nocturnal urination, sleep-related disorders such as sleep apnea or restless legs, or chronic pain, stress, or depression.10 Suggestions for management include sleep apps, cognitive behavioral therapy, low-dose antidepressant therapy, addressing sleep routines, and HT. Hypnotics should be avoided.
Sexuality issues are common complaints during the menopausal transition. Cross-sectional data reported from a longitudinal, population-based Australian cohort of women aged 45 to 55 years, found a decrease in sexual responsivity, sexual frequency, libido, vaginal dyspareunia, and more partner problems.11 Low libido may be related to relationship issues, dyspareunia with vaginal narrowing, loss of lubrication, levator spasm, stress, anxiety, exhaustion or mood disorder, lowered hormone levels, excess alcohol intake, underlying health concerns, or a side effect of medications for depression or pain. There is no direct correlation between testosterone levels and libido.
When HT at menopause may be helpful
For healthy symptomatic women without contraindications who are younger than age 60, or within 10 years of menopause onset, the benefits of initiating HT most likely outweigh the risks to relieve bothersome hot flashes and night sweats.12-17 For older women, or for those further from menopause, the greater absolute risks of coronary heart disease, stroke, venous thromboembolism, and dementia, in general, outweigh the potential benefits.12-17 Extended durations of HT have less safety and efficacy data and should be considered primarily for those with persistent menopausal symptoms, with periodic re-evaluation.13,14 For bothersome genitourinary syndrome of menopause symptoms that do not respond to vaginal moisturizers or lubricants, low-dose vaginal HTs are encouraged.13-17

Continue to: Early-onset menopause...
Early-onset menopause
According to observational studies,18 early menopause is associated with a higher risk of osteoporosis, coronary heart disease, cognitive changes, vaginal dryness, loss of libido, and mood changes. Studies have shown that women with early menopause who take HT, without contraindications, to the average age of menopause (age 52) decrease the health risks of early menopause (bone loss, heart disease, mood, and cognition changes).13,14,18
Women with early menopause, whether spontaneous or following bilateral oophorectomy or cancer treatment, should be counseled to get adequate calcium (dietary recommended over supplementation) and vitamin D intake, eat a healthy diet, and exercise regularly. Evaluation should include risk for bone loss, heart disease, mood changes, and vaginal changes.
Extended use of HT
Up to 8% of women have hot flashes for 20 years or more after menopause.19 The decision to continue or to stop HT is not always clear for women:
- with persistent hot flashes after a trial period of HT discontinuation
- with bone loss that cannot be treated with bone-specific medications
- who request continuation for quality of life.
Extended use of HT should include an ongoing assessment of its risks and benefits, periodic trials off of HT, and documentation of rationale and informed discussions about continuing. Lower doses and transdermal therapies appear safer, as does micronized progesterone instead of more potent synthetic progestins.13-17
Genitourinary syndrome of menopause
Once women are further into menopause, they may notice vaginal dryness, vulvar itching or burning, bothersome vaginal discharge, or urinary urgency or frequency. The development of painful intercourse frequently occurs, a combination of the loss of estrogen with thinning of the vaginal mucosa, a loss of the acidic vaginal milieu with less elasticity, and spasm of the levator muscles. Some women develop urinary tract infections after intercourse or have more frequent reoccurrences. First-line therapy is often vaginal moisturizers and lubricants. Vaginal therapies (estradiol, conjugated estrogen, or dehydroepiandrosterone) or oral selective estrogen-receptor modulators (SERMs; ospemifene) improve vaginal dryness and dyspareunia.13,14 Pelvic therapy has also proved valuable for incontinence, pelvic floor dysfunction, and levator spasms.20
Where are there gaps in clinician knowledge?
Studies on emotional health, mood, and sleep need to incorporate measures of menstrual timing into data collection and analyses. Does the sleep disruption occurring premenstrually during perimenopause disproportionately contribute to a woman’s vulnerability to depressive disorders? The risk of clinically significant depressive symptoms increases 1.5- to 2.9-fold in the menopause transition.5 Research into premenstrual dysphoria during the menopause transition may identify different trajectories in the timing of symptoms related to either cycle itself or the ovarian hormone fluctuations or both.21 Gamma-aminobutyric acid (GABA)-modulating drugs, such as sepranolone, which blocks allopregnanolone’s actions at the GABAA receptor, may allow treatment of menstrual-related mood disorders without the need for hormonal interventions.21
Despite extended observational trial data, more data are needed to inform us about the long-term risks and benefits of using menopausal HT, particularly when initiated at menopause and to help address the timing of HT discontinuation. Furthermore, there are many unanswered questions. For instance:
- How much safer are lower dose and transdermal therapies?
- Do untreated hot flashes increase the risk of cardiovascular disease or dementia?
- Will newer non-HT options, such as the neurokinin receptor antagonists that are in testing but are not yet available, lower cardiovascular or dementia risks?
- What will be the risks and benefits for the newer estrogen in testing (estetrol, or E4), considered a natural estrogen and which appears to have lower thrombotic risks?
- What will be the role of intravaginal energy-based therapies, such as vaginal laser or radiofrequency devices?
- How do we address diverse populations and the effects of menopause on race, gender, culture, prior trauma, and socioeconomic status?
Lack of recognition of menopausal symptoms, particularly in the workplace
Clinicians need to understand the varied physical and emotional symptoms that may occur with hormonal changes as women traverse perimenopause and early menopause. We need to recognize that the lack of discussion about women’s health during this time may make women feel ashamed and fearful of bringing up their symptoms due to fear of being dismissed or stigmatized.22 Women may not seek help until a crisis at home or work occurs, as they may fear that admitting symptoms or a need for help or time away from work will threaten how they are viewed at work or affect their chances of promotion. Although there are economic costs around menopause for appointments, tests, therapies, and missed time at work, not addressing menopausal health leads to poorer performance, workplace absences, and additional medical costs.22

Conclusion
Menopause occurs naturally as a part of a woman’s life cycle. However, women need assistance navigating perimenopausal hormonal fluctuations and decisions about HT once in menopause. Increased awareness and education about perimenopause and menopause will allow compassionate, individualized, informed care, including lifestyle changes, behavioral or complementary strategies, or medical therapies, hormonal or nonhormonal.27 As a medical society, we need to challenge the stigma associated with aging and menopause and educate ourselves and our patients to help women navigate this challenging time. ●
Myth 1: All hot flashes are the same
The truth: Seventy-five percent of women will have hot flashes, but only 25% are severe enough to cause women to seek treatment. Duration varies with identified patterns, including starting early or late, being mild or starting early, and going late. Ethnicity affects the duration of hot flashes, with longer durations seen in Black and Hispanic women. About 15% of women have had hot flashes for more than 15 or 20 years.1,2
Myth 2: There is no help for hot flashes
The truth: For some women, lifestyle changes are helpful, such as dressing in layers, turning down the thermostat at night, avoiding hot beverages or alcohol, or using technology (Femtech) for cooling devices. Over-the-counter products that are available, but are not clearly proven to help more than placebo, include soy (which may be estrogenic), black cohosh supplements, and nutritional supplements. Cognitive behavioral therapy, hypnosis, weight loss, or mindfulness may help.3 Nonhormone medications such as low-dose antidepressants or gabapentin have shown benefit. Newer treatments in testing, including neurokinin receptor antagonists, appear to work quickly and as effectively as HT. When initiating HT, healthy women with bothersome hot flashes under age 60 or within 10 years of menopause are the best candidates for HT; many lower doses and oral and non-oral therapies are available.
Myth 3: Compounded bioidentical hormones made by a compounding pharmacy are safer and more effective than FDA-approved ones
The truth: Compounded bioidentical hormones are touted as safer or more effective, but there is no good evidence to back up those claims. Whether US Food and Drug Administration (FDA)-approved or compounded, hormones come from the same precursors and have potential risks. With custom compounded HT, there is additional concern about precisely what is in the compounded product, whether levels are similar batch to batch, and the degree of absorption of progesterone, which is better absorbed oral.4-6 FDA-approved bioidentical HTs have been tested for safety, proven to contain consistent, effective levels of hormones, and are monitored by the FDA. For menopausal symptoms, FDA-approved therapies are available as estradiol (oral, patch, spray, gel, lotion, and vaginal ring) and progesterone (as an oral compound or combined with estradiol). Pellets made of compounded hormones have shown higher serum levels and more adverse events.5,7
Myth 4: Menopause causes weight gain
The truth is that fluctuating and declining hormones and the slowing of metabolism affect weight. Weight gain is not inevitable, just harder to prevent. Many women gain an average of 5 lb (2.27 kg) at midlife, which is mainly related to aging and lifestyle and not to menopause or HT. However, menopause may be related to body composition and fat distribution changes. Counsel women to decrease portion sizes, limit carbs, and increase exercise intensity, including strength training. The goal is 30 minutes of moderate aerobic activity per day, all at once or through smaller time increments, to improve their energy, mood, and sleep.
References
1. The NAMS 2017 HT Position Statement Advisory Panel. The 2017 HT position statement of The North American Menopause Society. Menopause. 2017;24:728-753.
2. Pinkerton JV. HT for postmenopausal women. N Engl J Med. 2020;382:446-455.
3. Paramsothy P. Duration of the menopausal transition is longer in women with young age at onset: the multiethnic Study of Women’s Health Across the Nation. Menopause. 2017;24:142-149.
4. Kingsberg SA, Schaffir J, Faught BM, et al. Female sexual health: barriers to optimal outcomes and a roadmap for improved patient-clinician communications. J Womens Health (Larchmt). 2019;28:432-443.
5. Eisenlohr-Moul TA, Kaiser G, Weise C, et al. Are there temporal subtypes of premenstrual dysphoric disorder? Using group-based trajectory modeling to identify individual differences in symptom change. Psychol Med. 2020;50:964-972.
6. Seibel M, Seibel S. Working through Menopause: The Impact on Women, Businesses and the Bottom Line. Bookbaby. March 8, 2022.
7. Kingsberg SA, Schaffir J, Faught BM, et al. Female sexual health: barriers to optimal outcomes and a roadmap for improved patient-clinician communications. J Womens Health (Larchmt). 2019;28:432-443.
- Paramsothy P. Duration of the menopausal transition is longer in women with young age at onset: the multiethnic Study of Women’s Health Across the Nation. Menopause. 2017;24:142–149.
- Harlow SD, Gass M, Hall JE, et al. STRAW 10 Collaborative Group. Executive summary of the Stages of Reproductive Aging Workshop + 10: addressing the unfinished agenda of staging reproductive aging. Menopause. 2012;19:387-95.
- Meers JM, Nowakowski S. Sleep, premenstrual mood disorder, and women’s health. Curr Opin Psychol. 2020;34:43-49.
- Sander B, Gordon JL. Premenstrual mood symptoms in the perimenopause. Curr Psychiatry Rep. 2021;23:73.
- Maki PM, Kornstein SG, Joffe H, et al. Guidelines for the evaluation and treatment of perimenopausal depression: summary and recommendations. J Women’s Health. 2019;28:117–134.
- Cao S, Jones M, Tooth L, et al. History of premenstrual syndrome and development of postpartum depression: a systematic review and meta-analysis. J Psychiatr Res. 2020;121:82–90.
- Rapkin AJ, Korotkaya Y, Taylor KC. Contraception counseling for women with premenstrual dysphoric disorder (PMDD): current perspectives. Open Access J Contracept. 2019;10:27–39.
- Avis NE, Crawford SL, Greendale G, et al; Study of Women's Health Across the Nation. Duration of menopausal vasomotor symptoms over the menopause transition. JAMA Intern Med. 2015;175:531.
- Tepper PG, Brooks MM, Randolph JF Jr, et al. Characterizing the trajectories of vasomotor symptoms across the menopausal transition. Menopause. 2016;23:1067-1074.
- Kravitz HM, Ganz PA, Bromberger J, et al. Sleep difficulty in women at midlife: a community survey of sleep and the menopausal transition. Menopause. 2003;10:19-28.
- Dennerstein L, Dudley EC, Hopper JL, et al. A prospective population-based study of menopausal symptoms. Obstet Gynecol. 2000;96:351-358.
- Manson JE, Chlebowski RT, Stefanick ML, et al. Menopausal HT and health outcomes during the intervention and extended poststopping phases of the Women’s Health Initiative randomized trials. JAMA. 2013;310:1353-1368.
- The NAMS 2017 HT Position Statement Advisory Panel. T he 2017 HT position statement of The North American Menopause Society. Menopause. 2017;24:728-753.
- Pinkerton JV. HT for postmenopausal women. N Engl J Med. 2020;382:446-455.
- Stuenkel CA, Davis SR, Gompel A, et al. Treatment of symptoms of the menopause: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2015;100:39754011.
- Manson JE, Kaunitz AM. Menopause management—getting clinical care back on track. N Engl J Med. 2016;374:803–806.
- American College of Obstetricians and Gynecologists. Practice Bulletin No. 141: Management of menopausal symptoms. Obstet Gynecol. 2014;123:202-216.
- Shuster LT, Rhodes DJ, Gostout BS, et al. Premature menopause or early menopause: long-term health consequences. Maturitas. 2010;65:161-166.
- Zeleke BM, Davis SR, Fradkin P, et al. Vasomotor symptoms and urogenital atrophy in older women: a systematic review. Climacteric. 2015;18:112-120.
- Kingsberg SA, Schaffir J, Faught BM, et al. Female sexual health: barriers to optimal outcomes and a roadmap for improved patient-clinician communications. J Womens Health (Larchmt). 2019;28:432-443.
- Eisenlohr-Moul TA, Kaiser G, Weise C, et al. Are there temporal subtypes of pre- menstrual dysphoric disorder? Using group-based trajectory modeling to identify individual differences in symptom change. Psychol Med. 2020;50: 964-972.
- Seibel M, Seibel S. Working through Menopause: The Impact on Women, Businesses and the Bottom Line. Bookbaby. March 8, 2022.
- Jackson LM, Parker RM, Mattison DR, eds. The Clinical Utility of Compounded Bioidentical HT: A Review of Safety, Effectiveness, and Use. Washington, DC: National Academies Press; 2020.
- Pinkerton JV. Concerns about safety and efficacy of compounded bioidentical HT. Menopause. 2021;28:847-849.
- Liu JH, Pinkerton JV. Prescription therapies. In: CJ Crandall, ed. Menopause Practice: A Clinician’s Guide, 6th ed. Pepper Pike, OH: The North American Menopause Society; 2019: 277-309.
- Jiang X, Bossert A, Parthasarathy KN, et al. Safety assessment of compounded non-FDA-approved hormonal therapy versus FDA-approved hormonal therapy in treating postmenopausal women. Menopause. 2021;28:867-874.
- Aninye IO, Laitner MH, Chinnappan S; Society for Women’s Health Research Menopause Working Group. Menopause preparedness: perspectives for patient, provider, and policymaker consideration. Menopause. 2021;28:1186-1191.
- Paramsothy P. Duration of the menopausal transition is longer in women with young age at onset: the multiethnic Study of Women’s Health Across the Nation. Menopause. 2017;24:142–149.
- Harlow SD, Gass M, Hall JE, et al. STRAW 10 Collaborative Group. Executive summary of the Stages of Reproductive Aging Workshop + 10: addressing the unfinished agenda of staging reproductive aging. Menopause. 2012;19:387-95.
- Meers JM, Nowakowski S. Sleep, premenstrual mood disorder, and women’s health. Curr Opin Psychol. 2020;34:43-49.
- Sander B, Gordon JL. Premenstrual mood symptoms in the perimenopause. Curr Psychiatry Rep. 2021;23:73.
- Maki PM, Kornstein SG, Joffe H, et al. Guidelines for the evaluation and treatment of perimenopausal depression: summary and recommendations. J Women’s Health. 2019;28:117–134.
- Cao S, Jones M, Tooth L, et al. History of premenstrual syndrome and development of postpartum depression: a systematic review and meta-analysis. J Psychiatr Res. 2020;121:82–90.
- Rapkin AJ, Korotkaya Y, Taylor KC. Contraception counseling for women with premenstrual dysphoric disorder (PMDD): current perspectives. Open Access J Contracept. 2019;10:27–39.
- Avis NE, Crawford SL, Greendale G, et al; Study of Women's Health Across the Nation. Duration of menopausal vasomotor symptoms over the menopause transition. JAMA Intern Med. 2015;175:531.
- Tepper PG, Brooks MM, Randolph JF Jr, et al. Characterizing the trajectories of vasomotor symptoms across the menopausal transition. Menopause. 2016;23:1067-1074.
- Kravitz HM, Ganz PA, Bromberger J, et al. Sleep difficulty in women at midlife: a community survey of sleep and the menopausal transition. Menopause. 2003;10:19-28.
- Dennerstein L, Dudley EC, Hopper JL, et al. A prospective population-based study of menopausal symptoms. Obstet Gynecol. 2000;96:351-358.
- Manson JE, Chlebowski RT, Stefanick ML, et al. Menopausal HT and health outcomes during the intervention and extended poststopping phases of the Women’s Health Initiative randomized trials. JAMA. 2013;310:1353-1368.
- The NAMS 2017 HT Position Statement Advisory Panel. T he 2017 HT position statement of The North American Menopause Society. Menopause. 2017;24:728-753.
- Pinkerton JV. HT for postmenopausal women. N Engl J Med. 2020;382:446-455.
- Stuenkel CA, Davis SR, Gompel A, et al. Treatment of symptoms of the menopause: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2015;100:39754011.
- Manson JE, Kaunitz AM. Menopause management—getting clinical care back on track. N Engl J Med. 2016;374:803–806.
- American College of Obstetricians and Gynecologists. Practice Bulletin No. 141: Management of menopausal symptoms. Obstet Gynecol. 2014;123:202-216.
- Shuster LT, Rhodes DJ, Gostout BS, et al. Premature menopause or early menopause: long-term health consequences. Maturitas. 2010;65:161-166.
- Zeleke BM, Davis SR, Fradkin P, et al. Vasomotor symptoms and urogenital atrophy in older women: a systematic review. Climacteric. 2015;18:112-120.
- Kingsberg SA, Schaffir J, Faught BM, et al. Female sexual health: barriers to optimal outcomes and a roadmap for improved patient-clinician communications. J Womens Health (Larchmt). 2019;28:432-443.
- Eisenlohr-Moul TA, Kaiser G, Weise C, et al. Are there temporal subtypes of pre- menstrual dysphoric disorder? Using group-based trajectory modeling to identify individual differences in symptom change. Psychol Med. 2020;50: 964-972.
- Seibel M, Seibel S. Working through Menopause: The Impact on Women, Businesses and the Bottom Line. Bookbaby. March 8, 2022.
- Jackson LM, Parker RM, Mattison DR, eds. The Clinical Utility of Compounded Bioidentical HT: A Review of Safety, Effectiveness, and Use. Washington, DC: National Academies Press; 2020.
- Pinkerton JV. Concerns about safety and efficacy of compounded bioidentical HT. Menopause. 2021;28:847-849.
- Liu JH, Pinkerton JV. Prescription therapies. In: CJ Crandall, ed. Menopause Practice: A Clinician’s Guide, 6th ed. Pepper Pike, OH: The North American Menopause Society; 2019: 277-309.
- Jiang X, Bossert A, Parthasarathy KN, et al. Safety assessment of compounded non-FDA-approved hormonal therapy versus FDA-approved hormonal therapy in treating postmenopausal women. Menopause. 2021;28:867-874.
- Aninye IO, Laitner MH, Chinnappan S; Society for Women’s Health Research Menopause Working Group. Menopause preparedness: perspectives for patient, provider, and policymaker consideration. Menopause. 2021;28:1186-1191.

