User login
Total hip arthroplasty (THA) effectively provides adequate pain relief and favorable outcomes in patients with hip osteoarthritis (OA). However, leg-length discrepancy (LLD) is still a significant cause of morbidity,1 including nerve damage,2,3 low back pain,2,4,5 and abnormal gait.2,6,7 Although most of the LLD values reported in the literature fall under the acceptable threshold of 10 mm,8 some patients report dissatisfaction,9 leading to litigation against orthopedic surgeons.2 However, lower extremity lengthening is sometimes needed to achieve adequate hip joint stability and prevent dislocations.2,10
Several methods have been developed to help surgeons estimate the change in leg length during surgery in an attempt to improve clinical outcomes. Use of guide pins as a reference on the pelvis decreased LLD and improved outcomes in some published studies.11,12 Preoperative templating of implant size, cup position, and level of femoral neck cut is very important in helping minimize clinically significant LLD after THA.2,13,14 Computer-assisted THA has also been introduced to try to improve component positioning, restoration of hip center of rotation, and minimizing of LLD.15-17 However, cost and increased operative time have prevented widespread adoption of computer-assisted surgery in THA.
Proponents of different surgical approaches have argued about the superiority of one approach over another. The posterior approach is the gold standard in THA because it is safe, easy to perform, and, if needed, extensile.11 However, exact determination of the intraoperative 3-dimensional (3-D) orientation of the pelvis, and subsequently of LLD, is challenging when the patient lies in the lateral position. The anterior approach has gained in popularity because of its advantages in accelerating postoperative rehabilitation and decreasing hospital length of stay.18 Placing the patient supine is advantageous because it allows leveling of the pelvis and estimation of LLD (by comparing the positions of the lower extremities).19 The anterior approach also allows for radiographic measurements on the operating table.19,20 However, this approach has a high learning curve21 and is not extensile.21 To date, no study has shown superiority of the anterior approach over either the conventional posterior approach or the robot-assisted posterior approach in minimizing LLD after THA.
We conducted a study to compare LLD in patients who underwent THA performed with a robot-assisted posterior approach (RTHA), a fluoroscopy-guided anterior approach (ATHA), or a conventional posterior approach (PTHA). We hypothesized that, compared with PTHA, both RTHA and ATHA would result in reduced LLD.
Materials and Methods
We reviewed all RTHAs, ATHAs, and PTHAs performed by Dr. Domb between September 2008 and December 2012. Study inclusion criteria were a diagnosis of hip OA and the availability of postoperative supine anteroposterior pelvis radiographs. Exclusion criteria were a diagnosis other than hip OA, missing or improper postoperative radiographs (radiographs with rotated or tilted pelvis),22 and radiographs on which at least one of the lesser trochanters was difficult to define. Of the 155 cases included in the study, 67 were RTHAs, 29 were ATHAs, and 59 were PTHAs.
All patients scheduled for THA underwent preoperative planning; plain radiographs were used to determine component size and position, level of neck cut, and amount of leg lengthening or shortening needed. In all RTHA cases, computed tomography of the involved hip was performed before surgery. The MAKO system (MAKO Surgical Corporation, Davie, Florida) was used to develop a patient-specific 3-D model of the pelvis and proximal femur, and this model was used to guide THA execution. The system was then used to detect patient-specific landmarks during surgery, to register the femur and the acetabulum, and to help determine the position of the pelvis and proximal femur during surgery. This system, which uses a haptic robotic arm that guides acetabular reaming and cup placement, provides feedback regarding cup placement, stem version, leg length, and global offset. Pelvic tilt and rotation were accounted for by the MAKO software, and all provided measurements were made on the coronal (functional) plane of the body, as described by Murray.23 ATHA was performed with the patient in the supine position on a Hana table (Mizuho OSI, Union City, California) with fluoroscopic guidance. PTHA was performed in the conventional way, with the patient in the lateral position.
Radiographic measurements of LLD were made with TraumaCad software (Build 2.2.535.0; Voyant Health, Petah-Tikva, Israel). The accuracy of this software has been studied and reported in the literature.24-26 Radiographs were calibrated using the known size of each femoral head as a marker. The reference on the pelvis was the interobturator line (line tangent to inferior border of obturator foramina), and the reference on the femurs was the most superior and medial aspect of each lesser trochanter. Two lines were drawn, each perpendicular to the interobturator line, starting from the previously defined reference point on each lesser trochanter. The difference in length between these 2 lines was recorded as the LLD. Values were recorded relative to the operative extremity. For example, if the operative extremity was longer than the nonoperative extremity, the LLD was given a positive value.
To eliminate bias and increase measurement accuracy, the study had each of 2 observers collect the LLD data twice, 2 months apart. These observers were blinded to each other’s results and to the type of surgery performed. (Neither observer was Dr. Domb, the senior surgeon.) IBM SPSS Statistics software (Version 20; IBM, Armonk, New York) was used for statistical analysis. Each patient’s 4 measurements were averaged into a single number for LLD, and the absolute LLD values were used in all statistical analyses. Means, standard deviations (SDs), and 95% confidence intervals (CIs) were calculated for LLD in each of the 3 groups. Pearson correlation coefficient was used to determine interobserver and intraobserver reliability. One-way analysis of variance (ANOVA) was used to compare group means for age, body mass index (BMI), and LLD. In each group, number of outliers was determined with outliers set at LLDs of more than 3 mm and more than 5 mm. Fischer exact test was used to compare number of outliers in each group. P < .05 was considered statistically significant.
Results
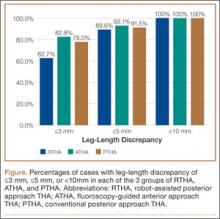
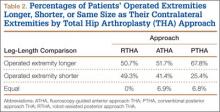
Table 1 lists the demographic data, including age, sex, and BMI, and compares the means. There were strong interobserver and intraobserver correlations for all LLD measurements (r > 0.9; P < .001). Mean (SD) LLD was 2.7 (1.8) mm (95% CI, 2.3-3.2) in the RTHA group, 1.8 (1.6) mm (95% CI, 1.2-2.4) in the ATHA group, and 1.9 (1.6) mm (95% CI, 1.5-2.4) in the PTHA group (P = .01). When LLD of more than 3 mm was set as an outlier, percentage of outliers was 37.3% (RTHA), 17.2% (ATHA), and 22% (PTHA) (P = .06-.78). When LLD of more than 5 mm was set as an outlier, percentage of outliers was 10.4% (RTHA), 6.9% (ATHA), and 8.5% (PTHA) (P = .72 to >.99). No patient in any group had LLD of 10 mm or more (Figure). Table 2 lists percentages of patients’ operated extremities that were longer, shorter, or the same size as their contralateral extremities. Six (9.0%) of the 67 RTHA patients, 4 (13.8%) of the 29 ATHA patients, and 3 (5.1%) of the 59 PTHA patients had a contralateral THA.
Discussion
Our study results showed that RTHA, ATHA, and PTHA were equally effective in minimizing LLD. There was a statistically significant difference in mean LLD among the 3 groups studied. The RTHA group had the largest mean (SD) LLD: 2.7 (1.8) mm. However, statistically significant differences do not always indicate clinical significance.27 Therefore, comparison of the 3 groups’ means is not enough for drawing significant conclusions. The more important point to consider is the number of cases of LLD of 10 mm or more—a discrepancy that would be perceptible to patients and thus become a source of dissatisfaction with painless THA.28 Patients perceive LLD when shortening exceeds 10 mm and lengthening exceeds 6 mm,29 or when LLD is more than 10 mm.16,19,20 Despite significant differences in means, all our cases came in under the 10-mm threshold. When the threshold was decreased to 5 mm (and to 3 mm), there was no statistically significant difference among the groups in the number of cases above the threshold.
LLD remains a source of significant post-THA comorbidity and patient dissatisfaction.1-7,19 Despite surgeons’ efforts to minimize LLD, some patients can detect even a subtle LLD after surgery.1,8,29 Most LLD values reported in the literature fall under the 10-mm threshold.16,19,20 In some cases, however, postoperative LLD is more than 1 cm, enough to prompt litigation against orthopedic surgeons.2 Surgeons have tried to improve LLD with use of multiple techniques, including use of intraoperative measuring devices,30 patient positioning during surgery,20 use of computer-assisted surgery,19 and use of intraoperative fluoroscopy.20
Proponents of computer-assisted THA have argued that this technique improves accuracy in placing the acetabular cup in the safe zone,31 minimizes LLD, and restores femoral offset.32,33 Manzotti and colleagues16 reported on 48 cases of computer-assisted THA matched to 48 cases of conventional THA using the posterior approach. Mean (SD) LLD was 5.06 (2.99) mm in the computer-assisted group and 7.64 (4.36) mm in the conventional group; there was a statistically significant difference in favor of the computer-assisted group (P = .04). However, 5 patients in the computer-assisted group and 13 in the conventional group had LLD of more than 10 mm, and the difference was statistically significant.16 Moreover, the study population was heterogeneous, with 12 patients in both groups having developmental dysplasia as a primary diagnosis.16 All the cases in our study had a diagnosis of OA, and no case had LLD of 10 mm or more.
Several advantages have been proposed for the anterior approach. The supine position (with direct comparison of leg lengths) and the use of fluoroscopy have been described as advantageous in minimizing LLD.20,21 In their study of 494 primary THAs performed with the anterior approach, Matta and colleagues20 reported mean (SD) postoperative LLD of 3 (2) mm (range, 0-26 mm) and concluded that the anterior approach was effective in restoring leg lengths and ensuring proper cup placement while not increasing the dislocation rate. However, they did not compare this approach with others or with computer-assisted THA with respect to LLD.
In another study, Nam and colleagues19 compared LLD after THA performed with 3 different approaches (anterior, conventional posterior, posterior-navigated) and found no statistically significant difference in LLD among the groups. However, LLD was more than 10 mm in 2.2% of anterior cases, 4.4% of conventional posterior cases, and 4.4% of posterior-navigated cases. When 5 mm was used as a cutoff, percentage of patients who were outliers was 31.1% (anterior), 20% (conventional posterior), and 23.3% (navigated-posterior). Our data showed superior results in using 5 mm as a cutoff, with percentage of outliers of 6.9% with ATHA, 8.5% with PTHA, and 10.4% with RTHA. However, Nam and colleagues19 used a larger patient cohort and different techniques for measuring LLD on anteroposterior pelvis radiographs.
The most likely reason that the groups in our study were comparable in terms of LLD accuracy and lack of outliers over the 10-mm cutoff was Dr. Domb’s high accuracy in minimizing LLD using each of the 3 techniques. For ATHA, mean (SD) LLD was 1.8 (1.6) mm (no LLD of ≥10 mm), better than the 3 (2) mm (0.9% with LLD of >10 mm) reported by Matta and colleagues20 and the 3.8 (3.9) mm (2.2% with LLD of >10 mm) reported by Nam and colleagues.19 For PTHA, mean (SD) LLD was 1.9 (1.6) mm (no LLD of ≥10 mm), comparable to some of the best results reported in the literature—for example, the 1 mm (3% with LLD of >10 mm) reported by Woolson and colleagues.34 For RTHA, mean (SD) LLD was 2.7 (1.8) mm (no LLD of ≥10 mm), superior to the 3.9 (2.7) mm (4.4% with LLD of >10 mm) reported by Nam and colleagues19 for posterior-navigated THA and the 5.06 (2.99) mm (10.4% with LLD of >10 mm) reported by Manzotti and colleagues16 for computer-assisted THA.
This study had several notable strengths. All patients had a diagnosis of hip OA and were operated on by a single surgeon. Radiographs were calibrated using the size of the implanted femoral head. Radiographic data were measured using the same technique in all cases and were collected twice by 2 observers (not the senior surgeon) to decrease bias and determine interobserver and intraobserver reliability. In addition, surgeon experience might have played an important role in minimizing LLD regardless of technique and approach used for THA.
Study limitations were different number of cases in each group, lack of matching, lack of clinical follow-up, and lack of long-term assessment of clinical outcomes and complications.
Conclusion
As performed by an experienced surgeon, RTHA, ATHA, and PTHA did not differ in obtaining minimal LLD. All 3 groups had a low frequency of outliers, using thresholds of 3 mm and 5 mm, and no patient in any group had LLD of 10 mm or more. All 3 techniques are effective in achieving accuracy in LLD.
1. Maloney WJ, Keeney JA. Leg length discrepancy after total hip arthroplasty. J Arthroplasty. 2004;19(4 suppl 1):108-110.
2. Clark CR, Huddleston HD, Schoch EP 3rd, Thomas BJ. Leg-length discrepancy after total hip arthroplasty. J Am Acad Orthop Surg. 2006;14(1):38-45.
3. Edwards BN, Tullos HS, Noble PC. Contributory factors and etiology of sciatic nerve palsy in total hip arthroplasty. Clin Orthop. 1987;(218):136-141.
4. Giles LG, Taylor JR. Low-back pain associated with leg length inequality. Spine. 1981;6(5):510-521.
5. Parvizi J, Sharkey PF, Bissett GA, Rothman RH, Hozack WJ. Surgical treatment of limb-length discrepancy following total hip arthroplasty. J Bone Joint Surg Am. 2003;85(12):2310-2317.
6. Edeen J, Sharkey PF, Alexander AH. Clinical significance of leg-length inequality after total hip arthroplasty. Am J Orthop. 1995;24(4):347-351.
7. Gurney B, Mermier C, Robergs R, Gibson A, Rivero D. Effects of limb-length discrepancy on gait economy and lower-extremity muscle activity in older adults. J Bone Joint Surg Am. 2001;83(6):907-915.
8. O’Brien S, Kernohan G, Fitzpatrick C, Hill J, Beverland D. Perception of imposed leg length inequality in normal subjects. Hip Int. 2010;20(4):505-511.
9. Hofmann AA, Skrzynski MC. Leg-length inequality and nerve palsy in total hip arthroplasty: a lawyer awaits! Orthopedics. 2000;23(9):943-944.
10. Miyamoto RG, Kaplan KM, Levine BR, Egol KA, Zuckerman JD. Surgical management of hip fractures: an evidence-based review of the literature. I: femoral neck fractures. J Am Acad Orthop Surg. 2008;16(10):596-607.
11. Ranawat CS, Rao RR, Rodriguez JA, Bhende HS. Correction of limb-length inequality during total hip arthroplasty. J Arthroplasty. 2001;16(6):715-720.
12. McGee HM, Scott JH. A simple method of obtaining equal leg length in total hip arthroplasty. Clin Orthop. 1985;(194):269-270.
13. Della Valle AG, Padgett DE, Salvati EA. Preoperative planning for primary total hip arthroplasty. J Am Acad Orthop Surg. 2005;13(7):455-462.
14. Gonzalez Della Valle A, Slullitel G, Piccaluga F, Salvati EA. The precision and usefulness of preoperative planning for cemented and hybrid primary total hip arthroplasty. J Arthroplasty. 2005;20(1):51-58.
15. Confalonieri N, Manzotti A, Montironi F, Pullen C. Leg length discrepancy, dislocation rate, and offset in total hip replacement using a short modular stem: navigation vs conventional freehand. Orthopedics. 2008;31(10 suppl 1).
16. Manzotti A, Cerveri P, De Momi E, Pullen C, Confalonieri N. Does computer-assisted surgery benefit leg length restoration in total hip replacement? Navigation versus conventional freehand. Int Orthop. 2011;35(1):19-24.
17. Nishio S, Fukunishi S, Fukui T, Fujihara Y, Yoshiya S. Adjustment of leg length using imageless navigation THA software without a femoral tracker. J Orthop Sci. 2011;16(2):171-176.
18. Martin CT, Pugely AJ, Gao Y, Clark CR. A comparison of hospital length of stay and short-term morbidity between the anterior and the posterior approaches to total hip arthroplasty. J Arthroplasty. 2013;28(5):849-854.
19. Nam D, Sculco PK, Abdel MP, Alexiades MM, Figgie MP, Mayman DJ. Leg-length inequalities following THA based on surgical technique. Orthopedics. 2013;36(4):e395-e400.
20. Matta JM, Shahrdar C, Ferguson T. Single-incision anterior approach for total hip arthroplasty on an orthopaedic table. Clin Orthop. 2005;(441):115-124.
21. Yi C, Agudelo JF, Dayton MR, Morgan SJ. Early complications of anterior supine intermuscular total hip arthroplasty. Orthopedics. 2013;36(3):e276-e281.
22. Siebenrock KA, Kalbermatten DF, Ganz R. Effect of pelvic tilt on acetabular retroversion: a study of pelves from cadavers. Clin Orthop. 2003;(407):241-248.
23. Murray DW. The definition and measurement of acetabular orientation. J Bone Joint Surg Br. 1993;75(2):228-232.
24. Kumar PG, Kirmani SJ, Humberg H, Kavarthapu V, Li P. Reproducibility and accuracy of templating uncemented THA with digital radiographic and digital TraumaCad templating software. Orthopedics. 2009;32(11):815.
25. Steinberg EL, Shasha N, Menahem A, Dekel S. Preoperative planning of total hip replacement using the TraumaCad system. Arch Orthop Trauma Surg. 2010;130(12):1429-1432.
26. Westacott DJ, McArthur J, King RJ, Foguet P. Assessment of cup orientation in hip resurfacing: a comparison of TraumaCad and computed tomography. J Orthop Surg Res. 2013;8:8.
27. Copay AG, Subach BR, Glassman SD, Polly DW Jr, Schuler TC. Understanding the minimum clinically important difference: a review of concepts and methods. Spine J. 2007;7(5):541-546.
28. Abraham WD, Dimon JH 3rd. Leg length discrepancy in total hip arthroplasty. Orthop Clin North Am. 1992;23(2):201-209.
29. Konyves A, Bannister GC. The importance of leg length discrepancy after total hip arthroplasty. J Bone Joint Surg Br. 2005;87(2):155-157.
30. Matsuda K, Nakamura S, Matsushita T. A simple method to minimize limb-length discrepancy after hip arthroplasty. Acta Orthop. 2006;77(3):375-379.
31. Haaker RG, Tiedjen K, Ottersbach A, Rubenthaler F, Stockheim M, Stiehl JB. Comparison of conventional versus computer-navigated acetabular component insertion. J Arthroplasty. 2007;22(2):151-159.
32. Renkawitz T, Schuster T, Herold T, et al. Measuring leg length and offset with an imageless navigation system during total hip arthroplasty: is it really accurate? Int J Med Robot. 2009;5(2):192-197.
33. Nakamura N, Sugano N, Nishii T, Kakimoto A, Miki H. A comparison between robotic-assisted and manual implantation of cementless total hip arthroplasty. Clin Orthop. 2010;468(4):1072-1081.
34. Woolson ST, Hartford JM, Sawyer A. Results of a method of leg-length equalization for patients undergoing primary total hip replacement. J Arthroplasty. 1999;14(2):159-164.
Total hip arthroplasty (THA) effectively provides adequate pain relief and favorable outcomes in patients with hip osteoarthritis (OA). However, leg-length discrepancy (LLD) is still a significant cause of morbidity,1 including nerve damage,2,3 low back pain,2,4,5 and abnormal gait.2,6,7 Although most of the LLD values reported in the literature fall under the acceptable threshold of 10 mm,8 some patients report dissatisfaction,9 leading to litigation against orthopedic surgeons.2 However, lower extremity lengthening is sometimes needed to achieve adequate hip joint stability and prevent dislocations.2,10
Several methods have been developed to help surgeons estimate the change in leg length during surgery in an attempt to improve clinical outcomes. Use of guide pins as a reference on the pelvis decreased LLD and improved outcomes in some published studies.11,12 Preoperative templating of implant size, cup position, and level of femoral neck cut is very important in helping minimize clinically significant LLD after THA.2,13,14 Computer-assisted THA has also been introduced to try to improve component positioning, restoration of hip center of rotation, and minimizing of LLD.15-17 However, cost and increased operative time have prevented widespread adoption of computer-assisted surgery in THA.
Proponents of different surgical approaches have argued about the superiority of one approach over another. The posterior approach is the gold standard in THA because it is safe, easy to perform, and, if needed, extensile.11 However, exact determination of the intraoperative 3-dimensional (3-D) orientation of the pelvis, and subsequently of LLD, is challenging when the patient lies in the lateral position. The anterior approach has gained in popularity because of its advantages in accelerating postoperative rehabilitation and decreasing hospital length of stay.18 Placing the patient supine is advantageous because it allows leveling of the pelvis and estimation of LLD (by comparing the positions of the lower extremities).19 The anterior approach also allows for radiographic measurements on the operating table.19,20 However, this approach has a high learning curve21 and is not extensile.21 To date, no study has shown superiority of the anterior approach over either the conventional posterior approach or the robot-assisted posterior approach in minimizing LLD after THA.
We conducted a study to compare LLD in patients who underwent THA performed with a robot-assisted posterior approach (RTHA), a fluoroscopy-guided anterior approach (ATHA), or a conventional posterior approach (PTHA). We hypothesized that, compared with PTHA, both RTHA and ATHA would result in reduced LLD.
Materials and Methods
We reviewed all RTHAs, ATHAs, and PTHAs performed by Dr. Domb between September 2008 and December 2012. Study inclusion criteria were a diagnosis of hip OA and the availability of postoperative supine anteroposterior pelvis radiographs. Exclusion criteria were a diagnosis other than hip OA, missing or improper postoperative radiographs (radiographs with rotated or tilted pelvis),22 and radiographs on which at least one of the lesser trochanters was difficult to define. Of the 155 cases included in the study, 67 were RTHAs, 29 were ATHAs, and 59 were PTHAs.
All patients scheduled for THA underwent preoperative planning; plain radiographs were used to determine component size and position, level of neck cut, and amount of leg lengthening or shortening needed. In all RTHA cases, computed tomography of the involved hip was performed before surgery. The MAKO system (MAKO Surgical Corporation, Davie, Florida) was used to develop a patient-specific 3-D model of the pelvis and proximal femur, and this model was used to guide THA execution. The system was then used to detect patient-specific landmarks during surgery, to register the femur and the acetabulum, and to help determine the position of the pelvis and proximal femur during surgery. This system, which uses a haptic robotic arm that guides acetabular reaming and cup placement, provides feedback regarding cup placement, stem version, leg length, and global offset. Pelvic tilt and rotation were accounted for by the MAKO software, and all provided measurements were made on the coronal (functional) plane of the body, as described by Murray.23 ATHA was performed with the patient in the supine position on a Hana table (Mizuho OSI, Union City, California) with fluoroscopic guidance. PTHA was performed in the conventional way, with the patient in the lateral position.
Radiographic measurements of LLD were made with TraumaCad software (Build 2.2.535.0; Voyant Health, Petah-Tikva, Israel). The accuracy of this software has been studied and reported in the literature.24-26 Radiographs were calibrated using the known size of each femoral head as a marker. The reference on the pelvis was the interobturator line (line tangent to inferior border of obturator foramina), and the reference on the femurs was the most superior and medial aspect of each lesser trochanter. Two lines were drawn, each perpendicular to the interobturator line, starting from the previously defined reference point on each lesser trochanter. The difference in length between these 2 lines was recorded as the LLD. Values were recorded relative to the operative extremity. For example, if the operative extremity was longer than the nonoperative extremity, the LLD was given a positive value.
To eliminate bias and increase measurement accuracy, the study had each of 2 observers collect the LLD data twice, 2 months apart. These observers were blinded to each other’s results and to the type of surgery performed. (Neither observer was Dr. Domb, the senior surgeon.) IBM SPSS Statistics software (Version 20; IBM, Armonk, New York) was used for statistical analysis. Each patient’s 4 measurements were averaged into a single number for LLD, and the absolute LLD values were used in all statistical analyses. Means, standard deviations (SDs), and 95% confidence intervals (CIs) were calculated for LLD in each of the 3 groups. Pearson correlation coefficient was used to determine interobserver and intraobserver reliability. One-way analysis of variance (ANOVA) was used to compare group means for age, body mass index (BMI), and LLD. In each group, number of outliers was determined with outliers set at LLDs of more than 3 mm and more than 5 mm. Fischer exact test was used to compare number of outliers in each group. P < .05 was considered statistically significant.
Results
Table 1 lists the demographic data, including age, sex, and BMI, and compares the means. There were strong interobserver and intraobserver correlations for all LLD measurements (r > 0.9; P < .001). Mean (SD) LLD was 2.7 (1.8) mm (95% CI, 2.3-3.2) in the RTHA group, 1.8 (1.6) mm (95% CI, 1.2-2.4) in the ATHA group, and 1.9 (1.6) mm (95% CI, 1.5-2.4) in the PTHA group (P = .01). When LLD of more than 3 mm was set as an outlier, percentage of outliers was 37.3% (RTHA), 17.2% (ATHA), and 22% (PTHA) (P = .06-.78). When LLD of more than 5 mm was set as an outlier, percentage of outliers was 10.4% (RTHA), 6.9% (ATHA), and 8.5% (PTHA) (P = .72 to >.99). No patient in any group had LLD of 10 mm or more (Figure). Table 2 lists percentages of patients’ operated extremities that were longer, shorter, or the same size as their contralateral extremities. Six (9.0%) of the 67 RTHA patients, 4 (13.8%) of the 29 ATHA patients, and 3 (5.1%) of the 59 PTHA patients had a contralateral THA.
Discussion
Our study results showed that RTHA, ATHA, and PTHA were equally effective in minimizing LLD. There was a statistically significant difference in mean LLD among the 3 groups studied. The RTHA group had the largest mean (SD) LLD: 2.7 (1.8) mm. However, statistically significant differences do not always indicate clinical significance.27 Therefore, comparison of the 3 groups’ means is not enough for drawing significant conclusions. The more important point to consider is the number of cases of LLD of 10 mm or more—a discrepancy that would be perceptible to patients and thus become a source of dissatisfaction with painless THA.28 Patients perceive LLD when shortening exceeds 10 mm and lengthening exceeds 6 mm,29 or when LLD is more than 10 mm.16,19,20 Despite significant differences in means, all our cases came in under the 10-mm threshold. When the threshold was decreased to 5 mm (and to 3 mm), there was no statistically significant difference among the groups in the number of cases above the threshold.
LLD remains a source of significant post-THA comorbidity and patient dissatisfaction.1-7,19 Despite surgeons’ efforts to minimize LLD, some patients can detect even a subtle LLD after surgery.1,8,29 Most LLD values reported in the literature fall under the 10-mm threshold.16,19,20 In some cases, however, postoperative LLD is more than 1 cm, enough to prompt litigation against orthopedic surgeons.2 Surgeons have tried to improve LLD with use of multiple techniques, including use of intraoperative measuring devices,30 patient positioning during surgery,20 use of computer-assisted surgery,19 and use of intraoperative fluoroscopy.20
Proponents of computer-assisted THA have argued that this technique improves accuracy in placing the acetabular cup in the safe zone,31 minimizes LLD, and restores femoral offset.32,33 Manzotti and colleagues16 reported on 48 cases of computer-assisted THA matched to 48 cases of conventional THA using the posterior approach. Mean (SD) LLD was 5.06 (2.99) mm in the computer-assisted group and 7.64 (4.36) mm in the conventional group; there was a statistically significant difference in favor of the computer-assisted group (P = .04). However, 5 patients in the computer-assisted group and 13 in the conventional group had LLD of more than 10 mm, and the difference was statistically significant.16 Moreover, the study population was heterogeneous, with 12 patients in both groups having developmental dysplasia as a primary diagnosis.16 All the cases in our study had a diagnosis of OA, and no case had LLD of 10 mm or more.
Several advantages have been proposed for the anterior approach. The supine position (with direct comparison of leg lengths) and the use of fluoroscopy have been described as advantageous in minimizing LLD.20,21 In their study of 494 primary THAs performed with the anterior approach, Matta and colleagues20 reported mean (SD) postoperative LLD of 3 (2) mm (range, 0-26 mm) and concluded that the anterior approach was effective in restoring leg lengths and ensuring proper cup placement while not increasing the dislocation rate. However, they did not compare this approach with others or with computer-assisted THA with respect to LLD.
In another study, Nam and colleagues19 compared LLD after THA performed with 3 different approaches (anterior, conventional posterior, posterior-navigated) and found no statistically significant difference in LLD among the groups. However, LLD was more than 10 mm in 2.2% of anterior cases, 4.4% of conventional posterior cases, and 4.4% of posterior-navigated cases. When 5 mm was used as a cutoff, percentage of patients who were outliers was 31.1% (anterior), 20% (conventional posterior), and 23.3% (navigated-posterior). Our data showed superior results in using 5 mm as a cutoff, with percentage of outliers of 6.9% with ATHA, 8.5% with PTHA, and 10.4% with RTHA. However, Nam and colleagues19 used a larger patient cohort and different techniques for measuring LLD on anteroposterior pelvis radiographs.
The most likely reason that the groups in our study were comparable in terms of LLD accuracy and lack of outliers over the 10-mm cutoff was Dr. Domb’s high accuracy in minimizing LLD using each of the 3 techniques. For ATHA, mean (SD) LLD was 1.8 (1.6) mm (no LLD of ≥10 mm), better than the 3 (2) mm (0.9% with LLD of >10 mm) reported by Matta and colleagues20 and the 3.8 (3.9) mm (2.2% with LLD of >10 mm) reported by Nam and colleagues.19 For PTHA, mean (SD) LLD was 1.9 (1.6) mm (no LLD of ≥10 mm), comparable to some of the best results reported in the literature—for example, the 1 mm (3% with LLD of >10 mm) reported by Woolson and colleagues.34 For RTHA, mean (SD) LLD was 2.7 (1.8) mm (no LLD of ≥10 mm), superior to the 3.9 (2.7) mm (4.4% with LLD of >10 mm) reported by Nam and colleagues19 for posterior-navigated THA and the 5.06 (2.99) mm (10.4% with LLD of >10 mm) reported by Manzotti and colleagues16 for computer-assisted THA.
This study had several notable strengths. All patients had a diagnosis of hip OA and were operated on by a single surgeon. Radiographs were calibrated using the size of the implanted femoral head. Radiographic data were measured using the same technique in all cases and were collected twice by 2 observers (not the senior surgeon) to decrease bias and determine interobserver and intraobserver reliability. In addition, surgeon experience might have played an important role in minimizing LLD regardless of technique and approach used for THA.
Study limitations were different number of cases in each group, lack of matching, lack of clinical follow-up, and lack of long-term assessment of clinical outcomes and complications.
Conclusion
As performed by an experienced surgeon, RTHA, ATHA, and PTHA did not differ in obtaining minimal LLD. All 3 groups had a low frequency of outliers, using thresholds of 3 mm and 5 mm, and no patient in any group had LLD of 10 mm or more. All 3 techniques are effective in achieving accuracy in LLD.
Total hip arthroplasty (THA) effectively provides adequate pain relief and favorable outcomes in patients with hip osteoarthritis (OA). However, leg-length discrepancy (LLD) is still a significant cause of morbidity,1 including nerve damage,2,3 low back pain,2,4,5 and abnormal gait.2,6,7 Although most of the LLD values reported in the literature fall under the acceptable threshold of 10 mm,8 some patients report dissatisfaction,9 leading to litigation against orthopedic surgeons.2 However, lower extremity lengthening is sometimes needed to achieve adequate hip joint stability and prevent dislocations.2,10
Several methods have been developed to help surgeons estimate the change in leg length during surgery in an attempt to improve clinical outcomes. Use of guide pins as a reference on the pelvis decreased LLD and improved outcomes in some published studies.11,12 Preoperative templating of implant size, cup position, and level of femoral neck cut is very important in helping minimize clinically significant LLD after THA.2,13,14 Computer-assisted THA has also been introduced to try to improve component positioning, restoration of hip center of rotation, and minimizing of LLD.15-17 However, cost and increased operative time have prevented widespread adoption of computer-assisted surgery in THA.
Proponents of different surgical approaches have argued about the superiority of one approach over another. The posterior approach is the gold standard in THA because it is safe, easy to perform, and, if needed, extensile.11 However, exact determination of the intraoperative 3-dimensional (3-D) orientation of the pelvis, and subsequently of LLD, is challenging when the patient lies in the lateral position. The anterior approach has gained in popularity because of its advantages in accelerating postoperative rehabilitation and decreasing hospital length of stay.18 Placing the patient supine is advantageous because it allows leveling of the pelvis and estimation of LLD (by comparing the positions of the lower extremities).19 The anterior approach also allows for radiographic measurements on the operating table.19,20 However, this approach has a high learning curve21 and is not extensile.21 To date, no study has shown superiority of the anterior approach over either the conventional posterior approach or the robot-assisted posterior approach in minimizing LLD after THA.
We conducted a study to compare LLD in patients who underwent THA performed with a robot-assisted posterior approach (RTHA), a fluoroscopy-guided anterior approach (ATHA), or a conventional posterior approach (PTHA). We hypothesized that, compared with PTHA, both RTHA and ATHA would result in reduced LLD.
Materials and Methods
We reviewed all RTHAs, ATHAs, and PTHAs performed by Dr. Domb between September 2008 and December 2012. Study inclusion criteria were a diagnosis of hip OA and the availability of postoperative supine anteroposterior pelvis radiographs. Exclusion criteria were a diagnosis other than hip OA, missing or improper postoperative radiographs (radiographs with rotated or tilted pelvis),22 and radiographs on which at least one of the lesser trochanters was difficult to define. Of the 155 cases included in the study, 67 were RTHAs, 29 were ATHAs, and 59 were PTHAs.
All patients scheduled for THA underwent preoperative planning; plain radiographs were used to determine component size and position, level of neck cut, and amount of leg lengthening or shortening needed. In all RTHA cases, computed tomography of the involved hip was performed before surgery. The MAKO system (MAKO Surgical Corporation, Davie, Florida) was used to develop a patient-specific 3-D model of the pelvis and proximal femur, and this model was used to guide THA execution. The system was then used to detect patient-specific landmarks during surgery, to register the femur and the acetabulum, and to help determine the position of the pelvis and proximal femur during surgery. This system, which uses a haptic robotic arm that guides acetabular reaming and cup placement, provides feedback regarding cup placement, stem version, leg length, and global offset. Pelvic tilt and rotation were accounted for by the MAKO software, and all provided measurements were made on the coronal (functional) plane of the body, as described by Murray.23 ATHA was performed with the patient in the supine position on a Hana table (Mizuho OSI, Union City, California) with fluoroscopic guidance. PTHA was performed in the conventional way, with the patient in the lateral position.
Radiographic measurements of LLD were made with TraumaCad software (Build 2.2.535.0; Voyant Health, Petah-Tikva, Israel). The accuracy of this software has been studied and reported in the literature.24-26 Radiographs were calibrated using the known size of each femoral head as a marker. The reference on the pelvis was the interobturator line (line tangent to inferior border of obturator foramina), and the reference on the femurs was the most superior and medial aspect of each lesser trochanter. Two lines were drawn, each perpendicular to the interobturator line, starting from the previously defined reference point on each lesser trochanter. The difference in length between these 2 lines was recorded as the LLD. Values were recorded relative to the operative extremity. For example, if the operative extremity was longer than the nonoperative extremity, the LLD was given a positive value.
To eliminate bias and increase measurement accuracy, the study had each of 2 observers collect the LLD data twice, 2 months apart. These observers were blinded to each other’s results and to the type of surgery performed. (Neither observer was Dr. Domb, the senior surgeon.) IBM SPSS Statistics software (Version 20; IBM, Armonk, New York) was used for statistical analysis. Each patient’s 4 measurements were averaged into a single number for LLD, and the absolute LLD values were used in all statistical analyses. Means, standard deviations (SDs), and 95% confidence intervals (CIs) were calculated for LLD in each of the 3 groups. Pearson correlation coefficient was used to determine interobserver and intraobserver reliability. One-way analysis of variance (ANOVA) was used to compare group means for age, body mass index (BMI), and LLD. In each group, number of outliers was determined with outliers set at LLDs of more than 3 mm and more than 5 mm. Fischer exact test was used to compare number of outliers in each group. P < .05 was considered statistically significant.
Results
Table 1 lists the demographic data, including age, sex, and BMI, and compares the means. There were strong interobserver and intraobserver correlations for all LLD measurements (r > 0.9; P < .001). Mean (SD) LLD was 2.7 (1.8) mm (95% CI, 2.3-3.2) in the RTHA group, 1.8 (1.6) mm (95% CI, 1.2-2.4) in the ATHA group, and 1.9 (1.6) mm (95% CI, 1.5-2.4) in the PTHA group (P = .01). When LLD of more than 3 mm was set as an outlier, percentage of outliers was 37.3% (RTHA), 17.2% (ATHA), and 22% (PTHA) (P = .06-.78). When LLD of more than 5 mm was set as an outlier, percentage of outliers was 10.4% (RTHA), 6.9% (ATHA), and 8.5% (PTHA) (P = .72 to >.99). No patient in any group had LLD of 10 mm or more (Figure). Table 2 lists percentages of patients’ operated extremities that were longer, shorter, or the same size as their contralateral extremities. Six (9.0%) of the 67 RTHA patients, 4 (13.8%) of the 29 ATHA patients, and 3 (5.1%) of the 59 PTHA patients had a contralateral THA.
Discussion
Our study results showed that RTHA, ATHA, and PTHA were equally effective in minimizing LLD. There was a statistically significant difference in mean LLD among the 3 groups studied. The RTHA group had the largest mean (SD) LLD: 2.7 (1.8) mm. However, statistically significant differences do not always indicate clinical significance.27 Therefore, comparison of the 3 groups’ means is not enough for drawing significant conclusions. The more important point to consider is the number of cases of LLD of 10 mm or more—a discrepancy that would be perceptible to patients and thus become a source of dissatisfaction with painless THA.28 Patients perceive LLD when shortening exceeds 10 mm and lengthening exceeds 6 mm,29 or when LLD is more than 10 mm.16,19,20 Despite significant differences in means, all our cases came in under the 10-mm threshold. When the threshold was decreased to 5 mm (and to 3 mm), there was no statistically significant difference among the groups in the number of cases above the threshold.
LLD remains a source of significant post-THA comorbidity and patient dissatisfaction.1-7,19 Despite surgeons’ efforts to minimize LLD, some patients can detect even a subtle LLD after surgery.1,8,29 Most LLD values reported in the literature fall under the 10-mm threshold.16,19,20 In some cases, however, postoperative LLD is more than 1 cm, enough to prompt litigation against orthopedic surgeons.2 Surgeons have tried to improve LLD with use of multiple techniques, including use of intraoperative measuring devices,30 patient positioning during surgery,20 use of computer-assisted surgery,19 and use of intraoperative fluoroscopy.20
Proponents of computer-assisted THA have argued that this technique improves accuracy in placing the acetabular cup in the safe zone,31 minimizes LLD, and restores femoral offset.32,33 Manzotti and colleagues16 reported on 48 cases of computer-assisted THA matched to 48 cases of conventional THA using the posterior approach. Mean (SD) LLD was 5.06 (2.99) mm in the computer-assisted group and 7.64 (4.36) mm in the conventional group; there was a statistically significant difference in favor of the computer-assisted group (P = .04). However, 5 patients in the computer-assisted group and 13 in the conventional group had LLD of more than 10 mm, and the difference was statistically significant.16 Moreover, the study population was heterogeneous, with 12 patients in both groups having developmental dysplasia as a primary diagnosis.16 All the cases in our study had a diagnosis of OA, and no case had LLD of 10 mm or more.
Several advantages have been proposed for the anterior approach. The supine position (with direct comparison of leg lengths) and the use of fluoroscopy have been described as advantageous in minimizing LLD.20,21 In their study of 494 primary THAs performed with the anterior approach, Matta and colleagues20 reported mean (SD) postoperative LLD of 3 (2) mm (range, 0-26 mm) and concluded that the anterior approach was effective in restoring leg lengths and ensuring proper cup placement while not increasing the dislocation rate. However, they did not compare this approach with others or with computer-assisted THA with respect to LLD.
In another study, Nam and colleagues19 compared LLD after THA performed with 3 different approaches (anterior, conventional posterior, posterior-navigated) and found no statistically significant difference in LLD among the groups. However, LLD was more than 10 mm in 2.2% of anterior cases, 4.4% of conventional posterior cases, and 4.4% of posterior-navigated cases. When 5 mm was used as a cutoff, percentage of patients who were outliers was 31.1% (anterior), 20% (conventional posterior), and 23.3% (navigated-posterior). Our data showed superior results in using 5 mm as a cutoff, with percentage of outliers of 6.9% with ATHA, 8.5% with PTHA, and 10.4% with RTHA. However, Nam and colleagues19 used a larger patient cohort and different techniques for measuring LLD on anteroposterior pelvis radiographs.
The most likely reason that the groups in our study were comparable in terms of LLD accuracy and lack of outliers over the 10-mm cutoff was Dr. Domb’s high accuracy in minimizing LLD using each of the 3 techniques. For ATHA, mean (SD) LLD was 1.8 (1.6) mm (no LLD of ≥10 mm), better than the 3 (2) mm (0.9% with LLD of >10 mm) reported by Matta and colleagues20 and the 3.8 (3.9) mm (2.2% with LLD of >10 mm) reported by Nam and colleagues.19 For PTHA, mean (SD) LLD was 1.9 (1.6) mm (no LLD of ≥10 mm), comparable to some of the best results reported in the literature—for example, the 1 mm (3% with LLD of >10 mm) reported by Woolson and colleagues.34 For RTHA, mean (SD) LLD was 2.7 (1.8) mm (no LLD of ≥10 mm), superior to the 3.9 (2.7) mm (4.4% with LLD of >10 mm) reported by Nam and colleagues19 for posterior-navigated THA and the 5.06 (2.99) mm (10.4% with LLD of >10 mm) reported by Manzotti and colleagues16 for computer-assisted THA.
This study had several notable strengths. All patients had a diagnosis of hip OA and were operated on by a single surgeon. Radiographs were calibrated using the size of the implanted femoral head. Radiographic data were measured using the same technique in all cases and were collected twice by 2 observers (not the senior surgeon) to decrease bias and determine interobserver and intraobserver reliability. In addition, surgeon experience might have played an important role in minimizing LLD regardless of technique and approach used for THA.
Study limitations were different number of cases in each group, lack of matching, lack of clinical follow-up, and lack of long-term assessment of clinical outcomes and complications.
Conclusion
As performed by an experienced surgeon, RTHA, ATHA, and PTHA did not differ in obtaining minimal LLD. All 3 groups had a low frequency of outliers, using thresholds of 3 mm and 5 mm, and no patient in any group had LLD of 10 mm or more. All 3 techniques are effective in achieving accuracy in LLD.
1. Maloney WJ, Keeney JA. Leg length discrepancy after total hip arthroplasty. J Arthroplasty. 2004;19(4 suppl 1):108-110.
2. Clark CR, Huddleston HD, Schoch EP 3rd, Thomas BJ. Leg-length discrepancy after total hip arthroplasty. J Am Acad Orthop Surg. 2006;14(1):38-45.
3. Edwards BN, Tullos HS, Noble PC. Contributory factors and etiology of sciatic nerve palsy in total hip arthroplasty. Clin Orthop. 1987;(218):136-141.
4. Giles LG, Taylor JR. Low-back pain associated with leg length inequality. Spine. 1981;6(5):510-521.
5. Parvizi J, Sharkey PF, Bissett GA, Rothman RH, Hozack WJ. Surgical treatment of limb-length discrepancy following total hip arthroplasty. J Bone Joint Surg Am. 2003;85(12):2310-2317.
6. Edeen J, Sharkey PF, Alexander AH. Clinical significance of leg-length inequality after total hip arthroplasty. Am J Orthop. 1995;24(4):347-351.
7. Gurney B, Mermier C, Robergs R, Gibson A, Rivero D. Effects of limb-length discrepancy on gait economy and lower-extremity muscle activity in older adults. J Bone Joint Surg Am. 2001;83(6):907-915.
8. O’Brien S, Kernohan G, Fitzpatrick C, Hill J, Beverland D. Perception of imposed leg length inequality in normal subjects. Hip Int. 2010;20(4):505-511.
9. Hofmann AA, Skrzynski MC. Leg-length inequality and nerve palsy in total hip arthroplasty: a lawyer awaits! Orthopedics. 2000;23(9):943-944.
10. Miyamoto RG, Kaplan KM, Levine BR, Egol KA, Zuckerman JD. Surgical management of hip fractures: an evidence-based review of the literature. I: femoral neck fractures. J Am Acad Orthop Surg. 2008;16(10):596-607.
11. Ranawat CS, Rao RR, Rodriguez JA, Bhende HS. Correction of limb-length inequality during total hip arthroplasty. J Arthroplasty. 2001;16(6):715-720.
12. McGee HM, Scott JH. A simple method of obtaining equal leg length in total hip arthroplasty. Clin Orthop. 1985;(194):269-270.
13. Della Valle AG, Padgett DE, Salvati EA. Preoperative planning for primary total hip arthroplasty. J Am Acad Orthop Surg. 2005;13(7):455-462.
14. Gonzalez Della Valle A, Slullitel G, Piccaluga F, Salvati EA. The precision and usefulness of preoperative planning for cemented and hybrid primary total hip arthroplasty. J Arthroplasty. 2005;20(1):51-58.
15. Confalonieri N, Manzotti A, Montironi F, Pullen C. Leg length discrepancy, dislocation rate, and offset in total hip replacement using a short modular stem: navigation vs conventional freehand. Orthopedics. 2008;31(10 suppl 1).
16. Manzotti A, Cerveri P, De Momi E, Pullen C, Confalonieri N. Does computer-assisted surgery benefit leg length restoration in total hip replacement? Navigation versus conventional freehand. Int Orthop. 2011;35(1):19-24.
17. Nishio S, Fukunishi S, Fukui T, Fujihara Y, Yoshiya S. Adjustment of leg length using imageless navigation THA software without a femoral tracker. J Orthop Sci. 2011;16(2):171-176.
18. Martin CT, Pugely AJ, Gao Y, Clark CR. A comparison of hospital length of stay and short-term morbidity between the anterior and the posterior approaches to total hip arthroplasty. J Arthroplasty. 2013;28(5):849-854.
19. Nam D, Sculco PK, Abdel MP, Alexiades MM, Figgie MP, Mayman DJ. Leg-length inequalities following THA based on surgical technique. Orthopedics. 2013;36(4):e395-e400.
20. Matta JM, Shahrdar C, Ferguson T. Single-incision anterior approach for total hip arthroplasty on an orthopaedic table. Clin Orthop. 2005;(441):115-124.
21. Yi C, Agudelo JF, Dayton MR, Morgan SJ. Early complications of anterior supine intermuscular total hip arthroplasty. Orthopedics. 2013;36(3):e276-e281.
22. Siebenrock KA, Kalbermatten DF, Ganz R. Effect of pelvic tilt on acetabular retroversion: a study of pelves from cadavers. Clin Orthop. 2003;(407):241-248.
23. Murray DW. The definition and measurement of acetabular orientation. J Bone Joint Surg Br. 1993;75(2):228-232.
24. Kumar PG, Kirmani SJ, Humberg H, Kavarthapu V, Li P. Reproducibility and accuracy of templating uncemented THA with digital radiographic and digital TraumaCad templating software. Orthopedics. 2009;32(11):815.
25. Steinberg EL, Shasha N, Menahem A, Dekel S. Preoperative planning of total hip replacement using the TraumaCad system. Arch Orthop Trauma Surg. 2010;130(12):1429-1432.
26. Westacott DJ, McArthur J, King RJ, Foguet P. Assessment of cup orientation in hip resurfacing: a comparison of TraumaCad and computed tomography. J Orthop Surg Res. 2013;8:8.
27. Copay AG, Subach BR, Glassman SD, Polly DW Jr, Schuler TC. Understanding the minimum clinically important difference: a review of concepts and methods. Spine J. 2007;7(5):541-546.
28. Abraham WD, Dimon JH 3rd. Leg length discrepancy in total hip arthroplasty. Orthop Clin North Am. 1992;23(2):201-209.
29. Konyves A, Bannister GC. The importance of leg length discrepancy after total hip arthroplasty. J Bone Joint Surg Br. 2005;87(2):155-157.
30. Matsuda K, Nakamura S, Matsushita T. A simple method to minimize limb-length discrepancy after hip arthroplasty. Acta Orthop. 2006;77(3):375-379.
31. Haaker RG, Tiedjen K, Ottersbach A, Rubenthaler F, Stockheim M, Stiehl JB. Comparison of conventional versus computer-navigated acetabular component insertion. J Arthroplasty. 2007;22(2):151-159.
32. Renkawitz T, Schuster T, Herold T, et al. Measuring leg length and offset with an imageless navigation system during total hip arthroplasty: is it really accurate? Int J Med Robot. 2009;5(2):192-197.
33. Nakamura N, Sugano N, Nishii T, Kakimoto A, Miki H. A comparison between robotic-assisted and manual implantation of cementless total hip arthroplasty. Clin Orthop. 2010;468(4):1072-1081.
34. Woolson ST, Hartford JM, Sawyer A. Results of a method of leg-length equalization for patients undergoing primary total hip replacement. J Arthroplasty. 1999;14(2):159-164.
1. Maloney WJ, Keeney JA. Leg length discrepancy after total hip arthroplasty. J Arthroplasty. 2004;19(4 suppl 1):108-110.
2. Clark CR, Huddleston HD, Schoch EP 3rd, Thomas BJ. Leg-length discrepancy after total hip arthroplasty. J Am Acad Orthop Surg. 2006;14(1):38-45.
3. Edwards BN, Tullos HS, Noble PC. Contributory factors and etiology of sciatic nerve palsy in total hip arthroplasty. Clin Orthop. 1987;(218):136-141.
4. Giles LG, Taylor JR. Low-back pain associated with leg length inequality. Spine. 1981;6(5):510-521.
5. Parvizi J, Sharkey PF, Bissett GA, Rothman RH, Hozack WJ. Surgical treatment of limb-length discrepancy following total hip arthroplasty. J Bone Joint Surg Am. 2003;85(12):2310-2317.
6. Edeen J, Sharkey PF, Alexander AH. Clinical significance of leg-length inequality after total hip arthroplasty. Am J Orthop. 1995;24(4):347-351.
7. Gurney B, Mermier C, Robergs R, Gibson A, Rivero D. Effects of limb-length discrepancy on gait economy and lower-extremity muscle activity in older adults. J Bone Joint Surg Am. 2001;83(6):907-915.
8. O’Brien S, Kernohan G, Fitzpatrick C, Hill J, Beverland D. Perception of imposed leg length inequality in normal subjects. Hip Int. 2010;20(4):505-511.
9. Hofmann AA, Skrzynski MC. Leg-length inequality and nerve palsy in total hip arthroplasty: a lawyer awaits! Orthopedics. 2000;23(9):943-944.
10. Miyamoto RG, Kaplan KM, Levine BR, Egol KA, Zuckerman JD. Surgical management of hip fractures: an evidence-based review of the literature. I: femoral neck fractures. J Am Acad Orthop Surg. 2008;16(10):596-607.
11. Ranawat CS, Rao RR, Rodriguez JA, Bhende HS. Correction of limb-length inequality during total hip arthroplasty. J Arthroplasty. 2001;16(6):715-720.
12. McGee HM, Scott JH. A simple method of obtaining equal leg length in total hip arthroplasty. Clin Orthop. 1985;(194):269-270.
13. Della Valle AG, Padgett DE, Salvati EA. Preoperative planning for primary total hip arthroplasty. J Am Acad Orthop Surg. 2005;13(7):455-462.
14. Gonzalez Della Valle A, Slullitel G, Piccaluga F, Salvati EA. The precision and usefulness of preoperative planning for cemented and hybrid primary total hip arthroplasty. J Arthroplasty. 2005;20(1):51-58.
15. Confalonieri N, Manzotti A, Montironi F, Pullen C. Leg length discrepancy, dislocation rate, and offset in total hip replacement using a short modular stem: navigation vs conventional freehand. Orthopedics. 2008;31(10 suppl 1).
16. Manzotti A, Cerveri P, De Momi E, Pullen C, Confalonieri N. Does computer-assisted surgery benefit leg length restoration in total hip replacement? Navigation versus conventional freehand. Int Orthop. 2011;35(1):19-24.
17. Nishio S, Fukunishi S, Fukui T, Fujihara Y, Yoshiya S. Adjustment of leg length using imageless navigation THA software without a femoral tracker. J Orthop Sci. 2011;16(2):171-176.
18. Martin CT, Pugely AJ, Gao Y, Clark CR. A comparison of hospital length of stay and short-term morbidity between the anterior and the posterior approaches to total hip arthroplasty. J Arthroplasty. 2013;28(5):849-854.
19. Nam D, Sculco PK, Abdel MP, Alexiades MM, Figgie MP, Mayman DJ. Leg-length inequalities following THA based on surgical technique. Orthopedics. 2013;36(4):e395-e400.
20. Matta JM, Shahrdar C, Ferguson T. Single-incision anterior approach for total hip arthroplasty on an orthopaedic table. Clin Orthop. 2005;(441):115-124.
21. Yi C, Agudelo JF, Dayton MR, Morgan SJ. Early complications of anterior supine intermuscular total hip arthroplasty. Orthopedics. 2013;36(3):e276-e281.
22. Siebenrock KA, Kalbermatten DF, Ganz R. Effect of pelvic tilt on acetabular retroversion: a study of pelves from cadavers. Clin Orthop. 2003;(407):241-248.
23. Murray DW. The definition and measurement of acetabular orientation. J Bone Joint Surg Br. 1993;75(2):228-232.
24. Kumar PG, Kirmani SJ, Humberg H, Kavarthapu V, Li P. Reproducibility and accuracy of templating uncemented THA with digital radiographic and digital TraumaCad templating software. Orthopedics. 2009;32(11):815.
25. Steinberg EL, Shasha N, Menahem A, Dekel S. Preoperative planning of total hip replacement using the TraumaCad system. Arch Orthop Trauma Surg. 2010;130(12):1429-1432.
26. Westacott DJ, McArthur J, King RJ, Foguet P. Assessment of cup orientation in hip resurfacing: a comparison of TraumaCad and computed tomography. J Orthop Surg Res. 2013;8:8.
27. Copay AG, Subach BR, Glassman SD, Polly DW Jr, Schuler TC. Understanding the minimum clinically important difference: a review of concepts and methods. Spine J. 2007;7(5):541-546.
28. Abraham WD, Dimon JH 3rd. Leg length discrepancy in total hip arthroplasty. Orthop Clin North Am. 1992;23(2):201-209.
29. Konyves A, Bannister GC. The importance of leg length discrepancy after total hip arthroplasty. J Bone Joint Surg Br. 2005;87(2):155-157.
30. Matsuda K, Nakamura S, Matsushita T. A simple method to minimize limb-length discrepancy after hip arthroplasty. Acta Orthop. 2006;77(3):375-379.
31. Haaker RG, Tiedjen K, Ottersbach A, Rubenthaler F, Stockheim M, Stiehl JB. Comparison of conventional versus computer-navigated acetabular component insertion. J Arthroplasty. 2007;22(2):151-159.
32. Renkawitz T, Schuster T, Herold T, et al. Measuring leg length and offset with an imageless navigation system during total hip arthroplasty: is it really accurate? Int J Med Robot. 2009;5(2):192-197.
33. Nakamura N, Sugano N, Nishii T, Kakimoto A, Miki H. A comparison between robotic-assisted and manual implantation of cementless total hip arthroplasty. Clin Orthop. 2010;468(4):1072-1081.
34. Woolson ST, Hartford JM, Sawyer A. Results of a method of leg-length equalization for patients undergoing primary total hip replacement. J Arthroplasty. 1999;14(2):159-164.