User login
ATLANTA – The implantation of nitinol coils that grab and compress diseased lung tissue, thereby allowing for better functioning of healthy tissue, significantly improved quality of life, exercise capacity, and pulmonary lung function in a randomized controlled trial of patients with severe emphysema and hyperinflation.
Specifically, use of the investigational self-actuating, implantable devices in the RESET (Randomized Controlled Trial of RePneu Endobronchial Coils for the Treatment of Severe Emphysema with Hyperinflation) study was associated with a significantly improved mean St. George’s Respiratory Questionnaire score at 90 days after the final treatment in 23 patients who received active treatment, compared with 24 controls who received best medical care.
After adjustment for baseline variables, the between-group difference in the scores was 8.35 points in favor of the treatment group, Dr. Zaid Zoumot reported at the annual meeting of the American College of Chest Physicians.
The lung-volume reduction coils also were associated with significant improvements in mean 6-minute walk distance (mean between-group difference of 63.5 m in favor of the treatment group) and forced expiratory volume in 1 second (FEV1, mean between-group difference of 12% in favor of the treatment group), said Dr. Zoumot of Royal Brompton and Harefield Hospital Trust, London.
The between-group difference in change in mean residual volume did not reach statistical significance, despite a 0.64-L reduction in the treatment group compared with the control group, he noted.
RESET participants were adults with severe emphysema and hyperinflation with significant dyspnea and gas trapping who were screened at three participating centers in the United Kingdom. Those randomized to the treatment group initially underwent implantation of the coils in one lung, with treatment of the contralateral lung after 1 month if appropriate.
Treatment was generally safe and well tolerated; three patients in the treatment group had pneumothoraces, which were picked up on chest x-ray routinely performed 1 hour following the procedure and treated successfully, Dr. Zoumot said. No differences in adverse effects occurred between the groups after the first month of follow-up, including in exacerbations of chronic obstructive pulmonary disorder, he added.
"The safety profile was definitely acceptable, and in fact, the procedures were a lot safer than other endobronchial lung-volume reduction devices at this same stage of development, and certainly a lot safer than lung-volume reduction surgery, which has a quite high morbidity and mortality rate," he said.
The findings are encouraging given the limited therapeutic options for patients with severe emphysema with gas trapping and hyperinflation – particularly those with heterogeneous disease, he said.
Drug therapy is typically of little benefit in these patients, and although lung-volume reduction surgery and endobronchial valve treatment can be helpful in some patients, their use is precluded in many patients, including those with heterogeneous disease in the absence of collateral ventilation, he explained.
The RePneu lung-volume reduction coils, however, provide a minimally invasive mechanical approach to lung-volume reduction that is effective in both homogeneous and heterogeneous emphysema, with benefits unaffected by collateral ventilation, he said.
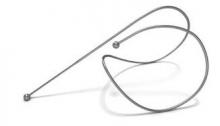
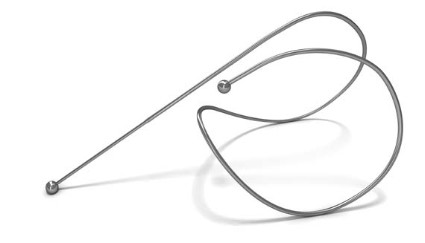
The coils, which are made entirely of nitinol – a highly biocompatible "shape memory" material used in numerous implantable devices – are deployed to the lung bronchoscopically using a proprietary delivery system. Initially, the coils are encased in a sheath to allow delivery in a straight configuration, but once they are in place they return to their original coil configuration, gathering and compressing the diseased tissue as they recoil.
The goal is to implant 10 coils per lobe in a fanlike distribution, Dr. Zoumot said. The procedure, which took about 45 minutes on average in this study, is typically performed under conscious sedation, he added.
Patients in the current study will be followed until 12 months after their final treatment, with results reported at both 6 and 12 months. A larger, multicenter randomized controlled trial with longer follow-up is also set to begin recruiting, Dr. Zoumot said.
This study was funded by PneumRx, the maker of the RePneu coils, and the study sites. Dr. Zoumot reported receiving grant funding and payment for travel expenses from PneumRx.
ATLANTA – The implantation of nitinol coils that grab and compress diseased lung tissue, thereby allowing for better functioning of healthy tissue, significantly improved quality of life, exercise capacity, and pulmonary lung function in a randomized controlled trial of patients with severe emphysema and hyperinflation.
Specifically, use of the investigational self-actuating, implantable devices in the RESET (Randomized Controlled Trial of RePneu Endobronchial Coils for the Treatment of Severe Emphysema with Hyperinflation) study was associated with a significantly improved mean St. George’s Respiratory Questionnaire score at 90 days after the final treatment in 23 patients who received active treatment, compared with 24 controls who received best medical care.
After adjustment for baseline variables, the between-group difference in the scores was 8.35 points in favor of the treatment group, Dr. Zaid Zoumot reported at the annual meeting of the American College of Chest Physicians.
The lung-volume reduction coils also were associated with significant improvements in mean 6-minute walk distance (mean between-group difference of 63.5 m in favor of the treatment group) and forced expiratory volume in 1 second (FEV1, mean between-group difference of 12% in favor of the treatment group), said Dr. Zoumot of Royal Brompton and Harefield Hospital Trust, London.
The between-group difference in change in mean residual volume did not reach statistical significance, despite a 0.64-L reduction in the treatment group compared with the control group, he noted.
RESET participants were adults with severe emphysema and hyperinflation with significant dyspnea and gas trapping who were screened at three participating centers in the United Kingdom. Those randomized to the treatment group initially underwent implantation of the coils in one lung, with treatment of the contralateral lung after 1 month if appropriate.
Treatment was generally safe and well tolerated; three patients in the treatment group had pneumothoraces, which were picked up on chest x-ray routinely performed 1 hour following the procedure and treated successfully, Dr. Zoumot said. No differences in adverse effects occurred between the groups after the first month of follow-up, including in exacerbations of chronic obstructive pulmonary disorder, he added.
"The safety profile was definitely acceptable, and in fact, the procedures were a lot safer than other endobronchial lung-volume reduction devices at this same stage of development, and certainly a lot safer than lung-volume reduction surgery, which has a quite high morbidity and mortality rate," he said.
The findings are encouraging given the limited therapeutic options for patients with severe emphysema with gas trapping and hyperinflation – particularly those with heterogeneous disease, he said.
Drug therapy is typically of little benefit in these patients, and although lung-volume reduction surgery and endobronchial valve treatment can be helpful in some patients, their use is precluded in many patients, including those with heterogeneous disease in the absence of collateral ventilation, he explained.
The RePneu lung-volume reduction coils, however, provide a minimally invasive mechanical approach to lung-volume reduction that is effective in both homogeneous and heterogeneous emphysema, with benefits unaffected by collateral ventilation, he said.
The coils, which are made entirely of nitinol – a highly biocompatible "shape memory" material used in numerous implantable devices – are deployed to the lung bronchoscopically using a proprietary delivery system. Initially, the coils are encased in a sheath to allow delivery in a straight configuration, but once they are in place they return to their original coil configuration, gathering and compressing the diseased tissue as they recoil.
The goal is to implant 10 coils per lobe in a fanlike distribution, Dr. Zoumot said. The procedure, which took about 45 minutes on average in this study, is typically performed under conscious sedation, he added.
Patients in the current study will be followed until 12 months after their final treatment, with results reported at both 6 and 12 months. A larger, multicenter randomized controlled trial with longer follow-up is also set to begin recruiting, Dr. Zoumot said.
This study was funded by PneumRx, the maker of the RePneu coils, and the study sites. Dr. Zoumot reported receiving grant funding and payment for travel expenses from PneumRx.
ATLANTA – The implantation of nitinol coils that grab and compress diseased lung tissue, thereby allowing for better functioning of healthy tissue, significantly improved quality of life, exercise capacity, and pulmonary lung function in a randomized controlled trial of patients with severe emphysema and hyperinflation.
Specifically, use of the investigational self-actuating, implantable devices in the RESET (Randomized Controlled Trial of RePneu Endobronchial Coils for the Treatment of Severe Emphysema with Hyperinflation) study was associated with a significantly improved mean St. George’s Respiratory Questionnaire score at 90 days after the final treatment in 23 patients who received active treatment, compared with 24 controls who received best medical care.
After adjustment for baseline variables, the between-group difference in the scores was 8.35 points in favor of the treatment group, Dr. Zaid Zoumot reported at the annual meeting of the American College of Chest Physicians.
The lung-volume reduction coils also were associated with significant improvements in mean 6-minute walk distance (mean between-group difference of 63.5 m in favor of the treatment group) and forced expiratory volume in 1 second (FEV1, mean between-group difference of 12% in favor of the treatment group), said Dr. Zoumot of Royal Brompton and Harefield Hospital Trust, London.
The between-group difference in change in mean residual volume did not reach statistical significance, despite a 0.64-L reduction in the treatment group compared with the control group, he noted.
RESET participants were adults with severe emphysema and hyperinflation with significant dyspnea and gas trapping who were screened at three participating centers in the United Kingdom. Those randomized to the treatment group initially underwent implantation of the coils in one lung, with treatment of the contralateral lung after 1 month if appropriate.
Treatment was generally safe and well tolerated; three patients in the treatment group had pneumothoraces, which were picked up on chest x-ray routinely performed 1 hour following the procedure and treated successfully, Dr. Zoumot said. No differences in adverse effects occurred between the groups after the first month of follow-up, including in exacerbations of chronic obstructive pulmonary disorder, he added.
"The safety profile was definitely acceptable, and in fact, the procedures were a lot safer than other endobronchial lung-volume reduction devices at this same stage of development, and certainly a lot safer than lung-volume reduction surgery, which has a quite high morbidity and mortality rate," he said.
The findings are encouraging given the limited therapeutic options for patients with severe emphysema with gas trapping and hyperinflation – particularly those with heterogeneous disease, he said.
Drug therapy is typically of little benefit in these patients, and although lung-volume reduction surgery and endobronchial valve treatment can be helpful in some patients, their use is precluded in many patients, including those with heterogeneous disease in the absence of collateral ventilation, he explained.
The RePneu lung-volume reduction coils, however, provide a minimally invasive mechanical approach to lung-volume reduction that is effective in both homogeneous and heterogeneous emphysema, with benefits unaffected by collateral ventilation, he said.
The coils, which are made entirely of nitinol – a highly biocompatible "shape memory" material used in numerous implantable devices – are deployed to the lung bronchoscopically using a proprietary delivery system. Initially, the coils are encased in a sheath to allow delivery in a straight configuration, but once they are in place they return to their original coil configuration, gathering and compressing the diseased tissue as they recoil.
The goal is to implant 10 coils per lobe in a fanlike distribution, Dr. Zoumot said. The procedure, which took about 45 minutes on average in this study, is typically performed under conscious sedation, he added.
Patients in the current study will be followed until 12 months after their final treatment, with results reported at both 6 and 12 months. A larger, multicenter randomized controlled trial with longer follow-up is also set to begin recruiting, Dr. Zoumot said.
This study was funded by PneumRx, the maker of the RePneu coils, and the study sites. Dr. Zoumot reported receiving grant funding and payment for travel expenses from PneumRx.
FROM THE ANNUAL MEETING OF THE AMERICAN COLLEGE OF CHEST PHYSICIANS
Major Finding: Implantation of lung-volume reduction coils was associated with a mean between-group difference of 63.5 m in 6-minute walk distance and a mean between-group difference of 12% in FEV1 in favor of the treatment group.
Data Source: This was a randomized controlled trial (RESET) of 23 patients who received active treatment and 24 controls who received best medical care.
Disclosures: This study was funded by PneumRx, the maker of the RePneu coils, and the study sites. Dr. Zoumot reported receiving grant funding and payment for travel expenses from PneumRx.