User login
The septate uterus is not only the most common Müllerian anomaly, it is the uterine malformation associated with the poorest reproductive outcomes, including recurrent pregnancy loss, preterm labor, malpresentation, and probably infertility. Although many patients with a uterine septum are asymptomatic and conceive and deliver without any difficulty, those who do have poor outcomes can benefit from transection of the septum.
The simplicity of hysteroscopic septoplasty, with its low rates of intraoperative complications and postoperative sequelae, provides experienced gynecologic surgeons with the opportunity to remarkably improve reproductive and obstetric outcomes for their patients with this anomaly.
The procedure has proven to be safe and effective for women with a history of recurrent miscarriage and other poor reproductive outcomes. Although a causal relationship between the septate uterus and infertility remains unproven, encouraging findings from numerous retrospective and observational studies are supporting the procedure’s use in patients with unexplained primary infertility as well.
Incidence and Effects
Müllerian anomalies are an embryologic phenomenon of the female reproductive tract. The anomalies are the result of a defect in the elongation, fusion, canalization, or septal reabsorption of the Müllerian/paramesonephric ducts.
Normally, at approximately 9 weeks’ gestation, these steps occur without incident and result in the creation of a single unified uterine cavity. In some cases, however, incomplete or failed reabsorption of the intervening partition of Müllerian products results in a persistent fibromuscular uterine septum. The extent of the septum varies; usually, it partially affects the uterine cavity rather than completely dividing it.
The septate uterus, the most common type of Müllerian anomaly, has been estimated to occur in 3%-7% of the general population. Its clinical sequelae include increased rates of spontaneous abortion, preterm delivery, intrauterine growth restriction, and malpresentation, compared with rates in women without a septum, as well as a higher rate of cesarean delivery.
Estimates of pregnancy rates in patients with a septate uterus have ranged from 5% to 40%, and miscarriage rates of 70%-90% have been reported. Thus, live birth rates in this population are poor.
Although uterine septa are closely related to recurrent miscarriage, the effect of the septate uterus on fertility is controversial. Some experts have proposed that the septate uterus may at least contribute to otherwise unexplained infertility by adversely affecting implantation. The endometrium overlying the septum may be different from the neighboring endometrium within the uterine cavity, it is believed, although this relationship is not yet directly correlated with primary infertility.
Dr. Luigi Fedele and his colleagues in Milan reported ultrastructural changes in biopsy sites from the septum, compared with the sites in the lateral uterine wall, using scanning electron microcopy. These histological factors included reductions in the number of glandular ostia, an irregular distribution of glandular ostia, incomplete ciliogenesis, and reductions in the ciliated cell ratio. The authors further concluded that septal tissue had decreased sensitivity to steroid hormones (Fertil. Steril. 1996;65:750-2).
Indications for Surgery
Currently, indications for surgical correction of a uterine septum include pelvic pain, endometriosis, an obstructing phenomenon, recurrent miscarriage, and history of preterm delivery.
Infertility is a controversial indication for surgery, as its association with the septate uterus has not been demonstrated by randomized studies. Several observational studies, however, have shown promising results with postoperative pregnancy rates of 25%-70% in patients with primary infertility, and there is consequently a movement to expand the use of hysteroscopic septoplasty to this subset of patients.
In one systematic review of 18 studies (including one retrospective cohort study of 64 women conducted by the review’s authors), the overall pregnancy rate after hysteroscopic septoplasty was 60% and the overall live birth rate was 45% (Reprod. Biol. Endocrinol. 2010;8:52-60).
A more recently published prospective study of women with unexplained primary infertility yielded remarkable results. Of 88 patients who underwent septoplasty, 48% conceived within a mean time to conception of approximately 7 months. Nearly 80% of these women conceived spontaneously, and more than 80% had live births. Approximately 71% of the deliveries were vaginal.
The only identifiable factor associated with reproductive failure in this study was a uterine septum. Patients had unexplained primary infertility for at least 48 months, and the study excluded patients with any history of miscarriage, abortion, or other factors that could contribute to infertility, such as endometriosis (Eur. J. Obstet. Gynecol. Reprod. Biol. 2011;155:54-7).
Diagnosis
Many imaging modalities have been used in the diagnosis of a uterine septum, including hysterosalpingogram (HSG), 2D and 3D ultrasound, saline infusion sonohysterography, and MRI. Müllerian anomalies may be paired with anomalies of the urinary tract, although the correlation with uterine septa is present less frequently than with other uterine anomalies. Nevertheless, evaluation of the urinary tract should be performed as part of the diagnostic work-up in patients with anomalies and thus may influence the diagnostic approach.
An HSG can elucidate the contour of the endometrial cavity and any communications including tubal patency. HSG is not universally considered a reliable diagnostic strategy, however, as the serosal surface of the uterus is not examined.
By contrast, as Dr. Fedele has demonstrated, sensitivity and specificity may be up to 100% with the use of 3D sonography. Ultrasound also can provide an assessment of other pelvic structures, such as ovaries and kidneys, that may contribute to a patient’s symptoms or be associated with Müllerian anomalies.
MRI is another noninvasive diagnostic strategy for characterizing Müllerian anomalies. It is both sensitive and specific, and is considered a valuable strategy as it allows for thorough evaluation of both the internal and external contour of the uterus.
Although advancements in MRI and ultrasound have improved diagnostic accuracy, concurrent laparoscopy at the time of hysteroscopic treatment remains the standard in confirming the diagnostic impressions formed by initial imaging. At our institution, we employ a combination of in-office 2D and 3D ultrasound, as well as saline infusion sonohysterography, and then confirm our findings laparoscopically at the time of surgery.
Treatment, Complication Risk
Uterine septa classically were treated with abdominal surgery, but advances in operative hysteroscopy have led to equally efficacious treatment with the advantage of decreased morbidity.
Potential intraoperative complications of hysteroscopic transection of uterine septa include bleeding, distention media overload, and perforation, with an associated risk of damage to nearby structures. Generally, septa are avascular, making the risk of both hemorrhage and distention media overload quite minimal. These complications are on the order of less than 1%, and typically the procedure is performed without incident on an outpatient basis.
Delayed complications of hysteroscopic septoplasty include the formation of intrauterine adhesions and the risk of uterine rupture with subsequent pregnancies. Although case reports of uterine rupture after hysteroscopic septoplasty exist, rupture is a very rare event because the integrity of the myometrium is generally preserved.
Adhesion formation, however, has been reported in up to approximately 7% of cases following hysteroscopic transection of uterine septum, according to a review published last year (Semin. Reprod. Med. 2011;29:83-94). Adhesion formation can further compromise the gestational performance of these patients.
Operative Technique
In preparation for operative hysteroscopy, many surgeons recommend priming the endometrial lining to provide optimal visualization. Progestins, oral contraceptive pills (OCs), or a GnRH analog can be used for this purpose. Alternatively, the procedure may be coordinated or timed with the patient’s early proliferative phase.
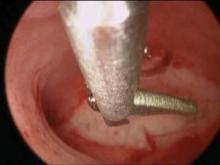
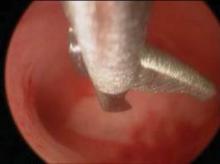
Hysteroscopic treatment involves incising the uterine septum and allowing the fibromuscular tissue to retract to the level of the surrounding endometrium. Surgeons have used microscissors, electrosurgery, or even fiberoptic laser energy, although this latter technique is currently less common given its expense, manipulation difficulties, and requirement for specialized training.
Although energy-based techniques provide hemostasis and may offer greater ease to a procedure involving a thick septum, use of these techniques increases the risk of postoperative intrauterine adhesion formation from endometrial and myometrial thermal damage. The use of microscissors, an energy-free technique, avoids thermal damage to the surrounding tissue and the subsequent increased risk of intrauterine adhesive disease.
A 12- or 25-degree lens provides accurate and continuous orientation of the ostia and instruments. A 5-7 Fr semi-rigid scissors offer sturdiness and maneuverability.
The surgical technique involves the incision of the septum equidistant between the anterior and posterior walls and traveling toward the fundus without inciting trauma to the myometrium of the fundus. Specifically, the septum is incised with both ostia in the visual field and midway between the anterior and posterior wall of the uterus. The incision is extended cephalad to the level of the uterine fundus.
If a broad septum limits visualization of both ostia, sequential thinning incisions are made along each side of the septum from the apex to the fundus. These incisions create a wedge that can ultimately be transected. The end point may be subtle, but continuous movement from one ostium to the other with the hysteroscope, and a uniform appearance of the uterine fundus, should be achieved. Bleeding may be an additional indication that the septum has been completely transected as this indicates myometrial disruption.
A complete septum is considered a rare phenomenon and may best be surgically treated only by providers with advanced hysteroscopic experience. In this case, visualization of the ectocervix must first be maximized. The initiation of the transection can be performed using a handheld instrument such as scissors, blade, or electrosurgical pencil and then continued hysteroscopically in a manner similar to transection of a partial septum.
Concomitant laparoscopic visualization can confirm prior findings revealed by imaging, provide assurance that the bowel is not adherent to the peritoneal surface of the uterus, and reveal proximity of the hysteroscopic instruments to the uterine serosal surface, thus decreasing risk of perforation during the procedure. Additionally, blanching of the uterine serosa or visualization of the hysteroscopic light can illustrate proximity to the serosal surface.
Coexisting pelvic pathology also can be diagnosed and treated at the time of the hysteroscopic procedure, as in the case of endometriosis.
Postop Care, Follow-Up
Postoperative care and follow-up must include strategies for preventing intrauterine adhesion formation and for confirming success of the procedure.
Multiple methods, from the placement of an intrauterine device or catheter to estrogen supplementation, have been proposed to minimize or prevent intrauterine adhesion formation following hysteroscopic septoplasty.
The IUD initially used for the purpose of separating endometrial surfaces over the operative site during healing was the Lippe’s loop – an inert device consisting of a thin polyethylene wire bent into a series of "S" shapes. It was removed from the U.S. market in the 1980s and is no longer widely available. The two currently approved IUDs – copper-based or progestin-containing – both raise concerns postoperatively, as copper can cause inflammation and progestins cause thinning of the endometrium lining.
For these reasons and in the setting of poor supportive data, IUDs are no longer used following hysteroscopic septoplasty.
Intrauterine catheters or stents are sometimes used following septoplasty under the same philosophy espoused by the IUD proponents – that adhesion formation can be prevented by the physical separation of endometrial surfaces during healing.
The surgeon should be extremely mindful of intrauterine pressure when a stent is placed, as the theoretical risk of uterine rupture exists if the myometrium is significantly disrupted during the procedure. Stents include a pediatric Foley balloon or specific intrauterine devices sold commercially.
Supplemental estrogen may be used to stimulate endometrial proliferation and, therefore, promote healing over the operative site. Multiple regimens exist and are sometimes paired with progestins. No standard regimen is reported in the literature and, thus, this choice depends on one’s familiarity and comfort.
Dr. Megan Daw is an AAGL/SRS fellow in minimally invasive gynecologic surgery at Advocate Lutheran General Hospital in Park Ridge, Ill. She matriculated at the University of North Carolina at Chapel Hill, then completed her residency in obstetrics and gynecology at the University of California at San Francisco in June 2010. Dr. Daw said that she has no disclosures to report.
The septate uterus is not only the most common Müllerian anomaly, it is the uterine malformation associated with the poorest reproductive outcomes, including recurrent pregnancy loss, preterm labor, malpresentation, and probably infertility. Although many patients with a uterine septum are asymptomatic and conceive and deliver without any difficulty, those who do have poor outcomes can benefit from transection of the septum.
The simplicity of hysteroscopic septoplasty, with its low rates of intraoperative complications and postoperative sequelae, provides experienced gynecologic surgeons with the opportunity to remarkably improve reproductive and obstetric outcomes for their patients with this anomaly.
The procedure has proven to be safe and effective for women with a history of recurrent miscarriage and other poor reproductive outcomes. Although a causal relationship between the septate uterus and infertility remains unproven, encouraging findings from numerous retrospective and observational studies are supporting the procedure’s use in patients with unexplained primary infertility as well.
Incidence and Effects
Müllerian anomalies are an embryologic phenomenon of the female reproductive tract. The anomalies are the result of a defect in the elongation, fusion, canalization, or septal reabsorption of the Müllerian/paramesonephric ducts.
Normally, at approximately 9 weeks’ gestation, these steps occur without incident and result in the creation of a single unified uterine cavity. In some cases, however, incomplete or failed reabsorption of the intervening partition of Müllerian products results in a persistent fibromuscular uterine septum. The extent of the septum varies; usually, it partially affects the uterine cavity rather than completely dividing it.
The septate uterus, the most common type of Müllerian anomaly, has been estimated to occur in 3%-7% of the general population. Its clinical sequelae include increased rates of spontaneous abortion, preterm delivery, intrauterine growth restriction, and malpresentation, compared with rates in women without a septum, as well as a higher rate of cesarean delivery.
Estimates of pregnancy rates in patients with a septate uterus have ranged from 5% to 40%, and miscarriage rates of 70%-90% have been reported. Thus, live birth rates in this population are poor.
Although uterine septa are closely related to recurrent miscarriage, the effect of the septate uterus on fertility is controversial. Some experts have proposed that the septate uterus may at least contribute to otherwise unexplained infertility by adversely affecting implantation. The endometrium overlying the septum may be different from the neighboring endometrium within the uterine cavity, it is believed, although this relationship is not yet directly correlated with primary infertility.
Dr. Luigi Fedele and his colleagues in Milan reported ultrastructural changes in biopsy sites from the septum, compared with the sites in the lateral uterine wall, using scanning electron microcopy. These histological factors included reductions in the number of glandular ostia, an irregular distribution of glandular ostia, incomplete ciliogenesis, and reductions in the ciliated cell ratio. The authors further concluded that septal tissue had decreased sensitivity to steroid hormones (Fertil. Steril. 1996;65:750-2).
Indications for Surgery
Currently, indications for surgical correction of a uterine septum include pelvic pain, endometriosis, an obstructing phenomenon, recurrent miscarriage, and history of preterm delivery.
Infertility is a controversial indication for surgery, as its association with the septate uterus has not been demonstrated by randomized studies. Several observational studies, however, have shown promising results with postoperative pregnancy rates of 25%-70% in patients with primary infertility, and there is consequently a movement to expand the use of hysteroscopic septoplasty to this subset of patients.
In one systematic review of 18 studies (including one retrospective cohort study of 64 women conducted by the review’s authors), the overall pregnancy rate after hysteroscopic septoplasty was 60% and the overall live birth rate was 45% (Reprod. Biol. Endocrinol. 2010;8:52-60).
A more recently published prospective study of women with unexplained primary infertility yielded remarkable results. Of 88 patients who underwent septoplasty, 48% conceived within a mean time to conception of approximately 7 months. Nearly 80% of these women conceived spontaneously, and more than 80% had live births. Approximately 71% of the deliveries were vaginal.
The only identifiable factor associated with reproductive failure in this study was a uterine septum. Patients had unexplained primary infertility for at least 48 months, and the study excluded patients with any history of miscarriage, abortion, or other factors that could contribute to infertility, such as endometriosis (Eur. J. Obstet. Gynecol. Reprod. Biol. 2011;155:54-7).
Diagnosis
Many imaging modalities have been used in the diagnosis of a uterine septum, including hysterosalpingogram (HSG), 2D and 3D ultrasound, saline infusion sonohysterography, and MRI. Müllerian anomalies may be paired with anomalies of the urinary tract, although the correlation with uterine septa is present less frequently than with other uterine anomalies. Nevertheless, evaluation of the urinary tract should be performed as part of the diagnostic work-up in patients with anomalies and thus may influence the diagnostic approach.
An HSG can elucidate the contour of the endometrial cavity and any communications including tubal patency. HSG is not universally considered a reliable diagnostic strategy, however, as the serosal surface of the uterus is not examined.
By contrast, as Dr. Fedele has demonstrated, sensitivity and specificity may be up to 100% with the use of 3D sonography. Ultrasound also can provide an assessment of other pelvic structures, such as ovaries and kidneys, that may contribute to a patient’s symptoms or be associated with Müllerian anomalies.
MRI is another noninvasive diagnostic strategy for characterizing Müllerian anomalies. It is both sensitive and specific, and is considered a valuable strategy as it allows for thorough evaluation of both the internal and external contour of the uterus.
Although advancements in MRI and ultrasound have improved diagnostic accuracy, concurrent laparoscopy at the time of hysteroscopic treatment remains the standard in confirming the diagnostic impressions formed by initial imaging. At our institution, we employ a combination of in-office 2D and 3D ultrasound, as well as saline infusion sonohysterography, and then confirm our findings laparoscopically at the time of surgery.
Treatment, Complication Risk
Uterine septa classically were treated with abdominal surgery, but advances in operative hysteroscopy have led to equally efficacious treatment with the advantage of decreased morbidity.
Potential intraoperative complications of hysteroscopic transection of uterine septa include bleeding, distention media overload, and perforation, with an associated risk of damage to nearby structures. Generally, septa are avascular, making the risk of both hemorrhage and distention media overload quite minimal. These complications are on the order of less than 1%, and typically the procedure is performed without incident on an outpatient basis.
Delayed complications of hysteroscopic septoplasty include the formation of intrauterine adhesions and the risk of uterine rupture with subsequent pregnancies. Although case reports of uterine rupture after hysteroscopic septoplasty exist, rupture is a very rare event because the integrity of the myometrium is generally preserved.
Adhesion formation, however, has been reported in up to approximately 7% of cases following hysteroscopic transection of uterine septum, according to a review published last year (Semin. Reprod. Med. 2011;29:83-94). Adhesion formation can further compromise the gestational performance of these patients.
Operative Technique
In preparation for operative hysteroscopy, many surgeons recommend priming the endometrial lining to provide optimal visualization. Progestins, oral contraceptive pills (OCs), or a GnRH analog can be used for this purpose. Alternatively, the procedure may be coordinated or timed with the patient’s early proliferative phase.
Hysteroscopic treatment involves incising the uterine septum and allowing the fibromuscular tissue to retract to the level of the surrounding endometrium. Surgeons have used microscissors, electrosurgery, or even fiberoptic laser energy, although this latter technique is currently less common given its expense, manipulation difficulties, and requirement for specialized training.
Although energy-based techniques provide hemostasis and may offer greater ease to a procedure involving a thick septum, use of these techniques increases the risk of postoperative intrauterine adhesion formation from endometrial and myometrial thermal damage. The use of microscissors, an energy-free technique, avoids thermal damage to the surrounding tissue and the subsequent increased risk of intrauterine adhesive disease.
A 12- or 25-degree lens provides accurate and continuous orientation of the ostia and instruments. A 5-7 Fr semi-rigid scissors offer sturdiness and maneuverability.
The surgical technique involves the incision of the septum equidistant between the anterior and posterior walls and traveling toward the fundus without inciting trauma to the myometrium of the fundus. Specifically, the septum is incised with both ostia in the visual field and midway between the anterior and posterior wall of the uterus. The incision is extended cephalad to the level of the uterine fundus.
If a broad septum limits visualization of both ostia, sequential thinning incisions are made along each side of the septum from the apex to the fundus. These incisions create a wedge that can ultimately be transected. The end point may be subtle, but continuous movement from one ostium to the other with the hysteroscope, and a uniform appearance of the uterine fundus, should be achieved. Bleeding may be an additional indication that the septum has been completely transected as this indicates myometrial disruption.
A complete septum is considered a rare phenomenon and may best be surgically treated only by providers with advanced hysteroscopic experience. In this case, visualization of the ectocervix must first be maximized. The initiation of the transection can be performed using a handheld instrument such as scissors, blade, or electrosurgical pencil and then continued hysteroscopically in a manner similar to transection of a partial septum.
Concomitant laparoscopic visualization can confirm prior findings revealed by imaging, provide assurance that the bowel is not adherent to the peritoneal surface of the uterus, and reveal proximity of the hysteroscopic instruments to the uterine serosal surface, thus decreasing risk of perforation during the procedure. Additionally, blanching of the uterine serosa or visualization of the hysteroscopic light can illustrate proximity to the serosal surface.
Coexisting pelvic pathology also can be diagnosed and treated at the time of the hysteroscopic procedure, as in the case of endometriosis.
Postop Care, Follow-Up
Postoperative care and follow-up must include strategies for preventing intrauterine adhesion formation and for confirming success of the procedure.
Multiple methods, from the placement of an intrauterine device or catheter to estrogen supplementation, have been proposed to minimize or prevent intrauterine adhesion formation following hysteroscopic septoplasty.
The IUD initially used for the purpose of separating endometrial surfaces over the operative site during healing was the Lippe’s loop – an inert device consisting of a thin polyethylene wire bent into a series of "S" shapes. It was removed from the U.S. market in the 1980s and is no longer widely available. The two currently approved IUDs – copper-based or progestin-containing – both raise concerns postoperatively, as copper can cause inflammation and progestins cause thinning of the endometrium lining.
For these reasons and in the setting of poor supportive data, IUDs are no longer used following hysteroscopic septoplasty.
Intrauterine catheters or stents are sometimes used following septoplasty under the same philosophy espoused by the IUD proponents – that adhesion formation can be prevented by the physical separation of endometrial surfaces during healing.
The surgeon should be extremely mindful of intrauterine pressure when a stent is placed, as the theoretical risk of uterine rupture exists if the myometrium is significantly disrupted during the procedure. Stents include a pediatric Foley balloon or specific intrauterine devices sold commercially.
Supplemental estrogen may be used to stimulate endometrial proliferation and, therefore, promote healing over the operative site. Multiple regimens exist and are sometimes paired with progestins. No standard regimen is reported in the literature and, thus, this choice depends on one’s familiarity and comfort.
Dr. Megan Daw is an AAGL/SRS fellow in minimally invasive gynecologic surgery at Advocate Lutheran General Hospital in Park Ridge, Ill. She matriculated at the University of North Carolina at Chapel Hill, then completed her residency in obstetrics and gynecology at the University of California at San Francisco in June 2010. Dr. Daw said that she has no disclosures to report.
The septate uterus is not only the most common Müllerian anomaly, it is the uterine malformation associated with the poorest reproductive outcomes, including recurrent pregnancy loss, preterm labor, malpresentation, and probably infertility. Although many patients with a uterine septum are asymptomatic and conceive and deliver without any difficulty, those who do have poor outcomes can benefit from transection of the septum.
The simplicity of hysteroscopic septoplasty, with its low rates of intraoperative complications and postoperative sequelae, provides experienced gynecologic surgeons with the opportunity to remarkably improve reproductive and obstetric outcomes for their patients with this anomaly.
The procedure has proven to be safe and effective for women with a history of recurrent miscarriage and other poor reproductive outcomes. Although a causal relationship between the septate uterus and infertility remains unproven, encouraging findings from numerous retrospective and observational studies are supporting the procedure’s use in patients with unexplained primary infertility as well.
Incidence and Effects
Müllerian anomalies are an embryologic phenomenon of the female reproductive tract. The anomalies are the result of a defect in the elongation, fusion, canalization, or septal reabsorption of the Müllerian/paramesonephric ducts.
Normally, at approximately 9 weeks’ gestation, these steps occur without incident and result in the creation of a single unified uterine cavity. In some cases, however, incomplete or failed reabsorption of the intervening partition of Müllerian products results in a persistent fibromuscular uterine septum. The extent of the septum varies; usually, it partially affects the uterine cavity rather than completely dividing it.
The septate uterus, the most common type of Müllerian anomaly, has been estimated to occur in 3%-7% of the general population. Its clinical sequelae include increased rates of spontaneous abortion, preterm delivery, intrauterine growth restriction, and malpresentation, compared with rates in women without a septum, as well as a higher rate of cesarean delivery.
Estimates of pregnancy rates in patients with a septate uterus have ranged from 5% to 40%, and miscarriage rates of 70%-90% have been reported. Thus, live birth rates in this population are poor.
Although uterine septa are closely related to recurrent miscarriage, the effect of the septate uterus on fertility is controversial. Some experts have proposed that the septate uterus may at least contribute to otherwise unexplained infertility by adversely affecting implantation. The endometrium overlying the septum may be different from the neighboring endometrium within the uterine cavity, it is believed, although this relationship is not yet directly correlated with primary infertility.
Dr. Luigi Fedele and his colleagues in Milan reported ultrastructural changes in biopsy sites from the septum, compared with the sites in the lateral uterine wall, using scanning electron microcopy. These histological factors included reductions in the number of glandular ostia, an irregular distribution of glandular ostia, incomplete ciliogenesis, and reductions in the ciliated cell ratio. The authors further concluded that septal tissue had decreased sensitivity to steroid hormones (Fertil. Steril. 1996;65:750-2).
Indications for Surgery
Currently, indications for surgical correction of a uterine septum include pelvic pain, endometriosis, an obstructing phenomenon, recurrent miscarriage, and history of preterm delivery.
Infertility is a controversial indication for surgery, as its association with the septate uterus has not been demonstrated by randomized studies. Several observational studies, however, have shown promising results with postoperative pregnancy rates of 25%-70% in patients with primary infertility, and there is consequently a movement to expand the use of hysteroscopic septoplasty to this subset of patients.
In one systematic review of 18 studies (including one retrospective cohort study of 64 women conducted by the review’s authors), the overall pregnancy rate after hysteroscopic septoplasty was 60% and the overall live birth rate was 45% (Reprod. Biol. Endocrinol. 2010;8:52-60).
A more recently published prospective study of women with unexplained primary infertility yielded remarkable results. Of 88 patients who underwent septoplasty, 48% conceived within a mean time to conception of approximately 7 months. Nearly 80% of these women conceived spontaneously, and more than 80% had live births. Approximately 71% of the deliveries were vaginal.
The only identifiable factor associated with reproductive failure in this study was a uterine septum. Patients had unexplained primary infertility for at least 48 months, and the study excluded patients with any history of miscarriage, abortion, or other factors that could contribute to infertility, such as endometriosis (Eur. J. Obstet. Gynecol. Reprod. Biol. 2011;155:54-7).
Diagnosis
Many imaging modalities have been used in the diagnosis of a uterine septum, including hysterosalpingogram (HSG), 2D and 3D ultrasound, saline infusion sonohysterography, and MRI. Müllerian anomalies may be paired with anomalies of the urinary tract, although the correlation with uterine septa is present less frequently than with other uterine anomalies. Nevertheless, evaluation of the urinary tract should be performed as part of the diagnostic work-up in patients with anomalies and thus may influence the diagnostic approach.
An HSG can elucidate the contour of the endometrial cavity and any communications including tubal patency. HSG is not universally considered a reliable diagnostic strategy, however, as the serosal surface of the uterus is not examined.
By contrast, as Dr. Fedele has demonstrated, sensitivity and specificity may be up to 100% with the use of 3D sonography. Ultrasound also can provide an assessment of other pelvic structures, such as ovaries and kidneys, that may contribute to a patient’s symptoms or be associated with Müllerian anomalies.
MRI is another noninvasive diagnostic strategy for characterizing Müllerian anomalies. It is both sensitive and specific, and is considered a valuable strategy as it allows for thorough evaluation of both the internal and external contour of the uterus.
Although advancements in MRI and ultrasound have improved diagnostic accuracy, concurrent laparoscopy at the time of hysteroscopic treatment remains the standard in confirming the diagnostic impressions formed by initial imaging. At our institution, we employ a combination of in-office 2D and 3D ultrasound, as well as saline infusion sonohysterography, and then confirm our findings laparoscopically at the time of surgery.
Treatment, Complication Risk
Uterine septa classically were treated with abdominal surgery, but advances in operative hysteroscopy have led to equally efficacious treatment with the advantage of decreased morbidity.
Potential intraoperative complications of hysteroscopic transection of uterine septa include bleeding, distention media overload, and perforation, with an associated risk of damage to nearby structures. Generally, septa are avascular, making the risk of both hemorrhage and distention media overload quite minimal. These complications are on the order of less than 1%, and typically the procedure is performed without incident on an outpatient basis.
Delayed complications of hysteroscopic septoplasty include the formation of intrauterine adhesions and the risk of uterine rupture with subsequent pregnancies. Although case reports of uterine rupture after hysteroscopic septoplasty exist, rupture is a very rare event because the integrity of the myometrium is generally preserved.
Adhesion formation, however, has been reported in up to approximately 7% of cases following hysteroscopic transection of uterine septum, according to a review published last year (Semin. Reprod. Med. 2011;29:83-94). Adhesion formation can further compromise the gestational performance of these patients.
Operative Technique
In preparation for operative hysteroscopy, many surgeons recommend priming the endometrial lining to provide optimal visualization. Progestins, oral contraceptive pills (OCs), or a GnRH analog can be used for this purpose. Alternatively, the procedure may be coordinated or timed with the patient’s early proliferative phase.
Hysteroscopic treatment involves incising the uterine septum and allowing the fibromuscular tissue to retract to the level of the surrounding endometrium. Surgeons have used microscissors, electrosurgery, or even fiberoptic laser energy, although this latter technique is currently less common given its expense, manipulation difficulties, and requirement for specialized training.
Although energy-based techniques provide hemostasis and may offer greater ease to a procedure involving a thick septum, use of these techniques increases the risk of postoperative intrauterine adhesion formation from endometrial and myometrial thermal damage. The use of microscissors, an energy-free technique, avoids thermal damage to the surrounding tissue and the subsequent increased risk of intrauterine adhesive disease.
A 12- or 25-degree lens provides accurate and continuous orientation of the ostia and instruments. A 5-7 Fr semi-rigid scissors offer sturdiness and maneuverability.
The surgical technique involves the incision of the septum equidistant between the anterior and posterior walls and traveling toward the fundus without inciting trauma to the myometrium of the fundus. Specifically, the septum is incised with both ostia in the visual field and midway between the anterior and posterior wall of the uterus. The incision is extended cephalad to the level of the uterine fundus.
If a broad septum limits visualization of both ostia, sequential thinning incisions are made along each side of the septum from the apex to the fundus. These incisions create a wedge that can ultimately be transected. The end point may be subtle, but continuous movement from one ostium to the other with the hysteroscope, and a uniform appearance of the uterine fundus, should be achieved. Bleeding may be an additional indication that the septum has been completely transected as this indicates myometrial disruption.
A complete septum is considered a rare phenomenon and may best be surgically treated only by providers with advanced hysteroscopic experience. In this case, visualization of the ectocervix must first be maximized. The initiation of the transection can be performed using a handheld instrument such as scissors, blade, or electrosurgical pencil and then continued hysteroscopically in a manner similar to transection of a partial septum.
Concomitant laparoscopic visualization can confirm prior findings revealed by imaging, provide assurance that the bowel is not adherent to the peritoneal surface of the uterus, and reveal proximity of the hysteroscopic instruments to the uterine serosal surface, thus decreasing risk of perforation during the procedure. Additionally, blanching of the uterine serosa or visualization of the hysteroscopic light can illustrate proximity to the serosal surface.
Coexisting pelvic pathology also can be diagnosed and treated at the time of the hysteroscopic procedure, as in the case of endometriosis.
Postop Care, Follow-Up
Postoperative care and follow-up must include strategies for preventing intrauterine adhesion formation and for confirming success of the procedure.
Multiple methods, from the placement of an intrauterine device or catheter to estrogen supplementation, have been proposed to minimize or prevent intrauterine adhesion formation following hysteroscopic septoplasty.
The IUD initially used for the purpose of separating endometrial surfaces over the operative site during healing was the Lippe’s loop – an inert device consisting of a thin polyethylene wire bent into a series of "S" shapes. It was removed from the U.S. market in the 1980s and is no longer widely available. The two currently approved IUDs – copper-based or progestin-containing – both raise concerns postoperatively, as copper can cause inflammation and progestins cause thinning of the endometrium lining.
For these reasons and in the setting of poor supportive data, IUDs are no longer used following hysteroscopic septoplasty.
Intrauterine catheters or stents are sometimes used following septoplasty under the same philosophy espoused by the IUD proponents – that adhesion formation can be prevented by the physical separation of endometrial surfaces during healing.
The surgeon should be extremely mindful of intrauterine pressure when a stent is placed, as the theoretical risk of uterine rupture exists if the myometrium is significantly disrupted during the procedure. Stents include a pediatric Foley balloon or specific intrauterine devices sold commercially.
Supplemental estrogen may be used to stimulate endometrial proliferation and, therefore, promote healing over the operative site. Multiple regimens exist and are sometimes paired with progestins. No standard regimen is reported in the literature and, thus, this choice depends on one’s familiarity and comfort.
Dr. Megan Daw is an AAGL/SRS fellow in minimally invasive gynecologic surgery at Advocate Lutheran General Hospital in Park Ridge, Ill. She matriculated at the University of North Carolina at Chapel Hill, then completed her residency in obstetrics and gynecology at the University of California at San Francisco in June 2010. Dr. Daw said that she has no disclosures to report.