User login
CASE Insurer denies drug coverage
Ms. X, age 65, has a 35-year history of bipolar I disorder (BD I) characterized by psychotic mania and severe suicidal depression. For the past year, her symptoms have been well controlled with aripiprazole, 5 mg/d; trazodone, 50 mg at bedtime; and citalopram, 20 mg/d. Because her health insurance has changed, Ms. X asks to be switched to an alternative antipsychotic because the new provider denied coverage of aripiprazole.
While taking aripiprazole, Ms. X did not report any extrapyramidal side effects, including tardive dyskinesia. Her Abnormal Involuntary Movement Scale (AIMS) score is 4. No significant abnormal movements were noted on examination during previous medication management sessions.
We decide to replace aripiprazole with quetiapine, 50 mg/d. At a 2-week follow-up visit, Ms. X is noted to have euphoric mood and reduced need to sleep, flight of ideas, increased talkativeness, and paranoia. We also notice that she has significant tongue rolling and lip smacking, which she says started 10 days after changing from aripiprazole to quetiapine. Her AIMS score is 17.
What could be causing Ms. X’s tongue rolling and lip smacking?
a) an irreversible syndrome usually starting after 1 or 2 years of continuous exposure to antipsychotics
b) a self-limited condition expected to resolve completely within 12 weeks
c) an acute manifestation of an antipsychotic that can respond to an anticholinergic agent
d) none of the above
The authors’ observations
Tardive dyskinesia (TD) refers to at least moderate abnormal involuntary movements in ≥1 areas of the body or at least mild movements in ≥2 areas of the body, developing after ≥3 months of cumulative exposure (continuous or discontinuous) to dopamine D2 receptor-blocking agents.1 AIMS is a 14-item, clinician-administered questionnaire designed to evaluate such movements and track their severity over time. The first 10 items are rated on 5-point scale (0 = none; 1 = minimal; 2 = mild; 3 = moderate; 4 = severe), with items 1 to 4 assessing orofacial movements, 5 to 7 assessing extremity and truncal movements, and 8 to 10 assessing overall severity, impairment, and subjective distress. Items 11 to 13 assess dental status because lack of teeth can result in oral movements mimicking TDs. The last item assesses whether these movements disappear during sleep.
HISTORY Poor response
Ms. X was given a diagnosis of BD I at age 30; she first started taking antipsychotics 10 years later. Previous psychotropic trials included lamotrigine, divalproex sodium, risperidone, and ziprasidone, which were ineffective or poorly tolerated. Her medical history includes obstructive sleep apnea, narcolepsy, type 2 diabetes mellitus, hypertension, dyslipidemia, fibromyalgia, gastroesophageal reflux disease, and hypothyroidism. She takes metformin, omeprazole, pravastatin, carvedilol, insulin, levothyroxine, methylphenidate (for hypersomnia), and enalapril.
What is the next best step in management?
a) discontinue quetiapine
b) replace quetiapine with clozapine
c) increase quetiapine to target manic symptoms and reassess in a few weeks
d) continue quetiapine and treat abnormal movements with benztropine
TREATMENT Increase dosage
We increase quetiapine to 150 mg/d to target Ms. X’s manic symptoms. She is scheduled for a follow-up visit in 4 weeks but is instructed to return to the clinic earlier if her manic symptoms do not improve. At the 4-week follow-up visit, Ms. X does not have any abnormal movements and her manic symptoms have resolved. Her AIMS score is 4. Her husband reports that her abnormal movements resolved 4 days after increasing quetiapine to 150 mg/d.
The authors’ observations
Second-generation antipsychotics are known to have a lower risk of extrapyramidal adverse reactions compared with older first-generation antipsychotics.2,3 TD differs from other extrapyramidal symptoms (EPS) because of its delayed onset. Risk factors for TD include:
• female sex
• age >50
• history of brain damage
• long-term antipsychotic use
• diagnosis of a mood disorder.
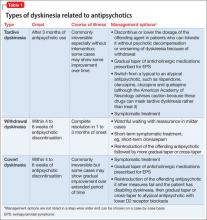
Gardos et al4 described 2 other forms of delayed dyskinesias related to antipsychotic use but resulting from antipsychotic discontinuation: withdrawal dyskinesia and covert dyskinesia. Evidence for these types of antipsychotic discontinuation syndromes mostly is anecdotal.5,6Table 1 highlights 3 different types of dyskinesias and their management.
Withdrawal dyskinesia has been described as a syndrome resembling TD that appears after discontinuation or dosage reduction of an antipsychotic in a patient who does not have an earlier TD diagnosis.7 The prevalence of withdrawal dyskinesia among patients undergoing antipsychotic discontinuation is approximately 30%.8 Cases of withdrawal dyskinesia are self-limited and resolve in 1 to 3 months.9,10 We believe that Ms. X’s movement disorder was withdrawal dyskinesia from aripiprazole because her symptoms started 10 days after the drug was discontinued, and was self-limited and reversible.
Similar to TD, withdrawal dyskinesia can present in different forms:
• tongue protrusion movements
• facial grimacing
• ticks
• chorea
• tremors
• athetosis
• involuntary vocalizations
• abnormal movements of hands and legs
• “dyspnea” due to involvement of respiratory musculature.5,11
There may be a sex difference in duration of withdrawal dyskinesias, because symptoms persist longer in females.9
Although covert dyskinesia also develops after discontinuation or dosage reduction of a dopamine-blocking agent, the symptoms usually are permanent, and could require reintroducing the antipsychotic or management with evidence-based treatments for TD, such as tetrabenazine or amantadine.6,12
What is the cause of Ms. X’s abnormal involuntary movements?
a) quetiapine-induced D2 receptor hypersensitivity
b) aripiprazole-induced cholinergic overactivity
c) quetiapine-induced cholinergic overactivity
d) aripiprazole-induced D2 receptor hypersensitivity
The authors’ observations
Pathophysiology of this condition is unknown but different theories have been proposed. D2 receptor up-regulation and hypersensitivity to compensate for chronic D2 receptor blockade by antipsychotics is a commonly cited theory.7,13 Discontinuation of an antipsychotic can make this D2 receptor up-regulation and hypersensitivity manifest as withdrawal dyskinesia by creating a temporary hyperdopaminergic state in basal ganglia. Other theories implicate decrease of γ-aminobutyric acid (GABA) in the globus pallidus (GP) and substantia nigra (SN) regions of the brain, and oxidative damage to GABAergic interneurons in GP and SN from excess production of catecholamines in response to chronic dopamine blockade.14
It has been proposed that patients with withdrawal dyskinesia might be in an early phase of D2 receptor modulation that, if continued because of use of the antipsychotic implicated in withdrawal dyskinesia, can lead to development of TD.4,7,8 A feature of withdrawal dyskinesia that differentiates it from TD is that it usually remits spontaneously within several weeks to a few months.4,7 Because of this characteristic, Schultz et al8 propose that, if withdrawal dyskinesia is identified early in treatment, it may be possible to prevent development of persistent TD.
Look carefully for dyskinetic movements in patients who have recently discontinued or decreased the dosage of their antipsychotic. Non-compliance and partial compliance are common problems among patients taking an antipsychotic.15 Therefore, careful watchfulness for withdrawal dyskinesias at all times can be beneficial. Inquiring about recent history of these dyskinesias in such patients is probably more useful than an exam because the dyskinesias may not be evident on exam when these patients show up for their follow-up visit, because of their self-limited nature.8
Treatment options
If a patient is noted to have a withdrawal-emergent dyskinesia, a clinician has options to prevent TD, including:
• decreasing the dosage of the antipsychotic
• switching from a typical antipsychotic to an atypical antipsychotic
• switching from one atypical to another with lesser affinity for striatal D2 receptor, such as clozapine or quetiapine.16,17
In addition, researchers are investigating the use of vitamin B6, Ginkgo biloba, amantadine, levetiracetam, melatonin, tetrabenazine, zonisamide, branched chain amino acids, clonazepam, and vitamin E as treatment alternatives for TD.
Tetrabenazine acts by blocking vesicular monoamine transporter type 2, thereby inhibiting release of monoamines, including dopamine into synaptic cleft area in basal ganglia.18 Clonazepam’s benefit for TD relates to its facilitation of GABAergic neurotransmission, because reduced GABAergic transmission in GP and SN has been associ ated with hyperkinetic movements, including TD.14Ginkgo biloba and melatonin exert their beneficial effects in TD through their antioxidant function.14
The agents listed in Table 219 could be used on a short-term basis for symptomatic treatment of withdrawal dyskinesias.1,18,20
Withdrawal dyskinesia has been reported with aripiprazole discontinuation and is thought to be related to aripiprazole’s strong affinity for D2 receptors.21 Aripiprazole at dosages of 15 to 30 mg/d can occupy more than 80% of the striatal D2 dopamine receptors. The dosage of ≥30 mg/d can lead to receptor occupancy of >90%.22 Studies have shown that EPS correlate with D2 receptor occupancy in steady-state conditions, and occupancy exceeding 80% results in these symptoms.22
Compared with aripiprazole, quetiapine has weak affinity for D2 receptors (Table 3), making it an unlikely culprit if dyskinesia emerges within 2 weeks of initation.22 We believe that, in Ms. X’s case, quetiapine might have masked the severity of aripiprazole withdrawal dyskinesia by causing some degree of D2 receptor blockade. It may have decreased the duration of withdrawal dyskinesia by the same effect on D2 receptors. It may have lasted longer if aripiprazole was not replaced by another antipsychotic. This is particularly evident because dyskinesia improved quickly when quetiapine was titrated to 150 mg/d. The higher quetiapine dosage of 150 mg/d is closer to 5 mg/d of aripiprazole in terms of D2 receptor occupancy and affinity. However, quetiapine is weaker than aripiprazole in terms of D2 receptor occupancy at all dosages, and therefore less likely to cause EPS.16
Summing up
Withdrawal dyskinesia in the absence of a history of TD is a common symptom of antipsychotic discontinuation or dosage reduction after long-term use of an antipsychotic. It is more commonly seen with antipsychotics with high D2 receptor occupancy, and has been hypothesized to be related to D2 receptor supersensitivity to ambient dopamine, resulting as a compensatory response to chronic D2 blockade by this class of medication.
Evidence suggests that reversible withdrawal dyskinesia could represent a prodrome to irreversible TD. Therefore, keeping a watchful eye for these movements during the exam, along with specific inquiry about withdrawal dyskinesias while taking a history at every follow-up visit, is important because doing so can:
• inform the clinician about partial compliance or noncompliance to these medications, which could lead to treatment failure
• help prevent development of irreversible TD syndrome.
Ms. X’s case reminds clinicians (1) to be aware of this unexpected side effect occurring even with second-generation antipsychotics and (2) that they should consider EPS in patients while they are discontinuing their drugs. Furthermore, it is important for clinical and medicolegal reasons to inform our patients that different forms of dyskinesias can be potential side effects of antipsychotics.
Bottom Line
Dyskinesias can result from withdrawal of both typical and atypical antipsychotics, and usually are self-limited. Withdrawal dyskinesia may represent a prodrome to tardive dyskinesia; early recognition may aid in preventing development of persistent tardive dyskinesia.
Related Resources
• Abnormal Involuntary Movement Scale. http://www.cqaimh.org/pdf/toolaims.pdf.
• Goldberg JF, Ernst CL. Managing the side effects of psychotropic medications. Arlington, VA: American Psychiatric Publishing, Inc; 2012.
• Tarsay D. Tardive dyskinesia: prevention and treatment. http:// www.uptodate.com/contents/tardive-dyskinesia-prevention-and-treatment?topicKey=NEURO%2F4908&elapsedTimeMs=3 &view=print&displayedView=full#.
Drug Brand Names
Amantadine • Symmetrel
Aripiprazole • Abilify
Benztropine • Cogentin
Carvedilol • Coreg
Citalopram • Celexa
Clonazepam • Klonopin
Clozapine • Clozaril
Divalproex sodium • Depakote
Donepezil • Aricept
Enalapril • Vasotec
Haloperidol • Haldol
Lamotrigine • Lamictal
Levetiracetam • Keppra
Levothyroxine • Levoxyl, Synthroid
Metformin • Glucophage
Methylphenidate • Ritalin
Olanzapine • Zyprexa
Omeprazole • Prilosec
Pravastatin • Pravachol
Quetiapine • Seroquel
Risperidone • Risperdal
Tetrabenazine • Xenazine
Trazodone • Desyrel, Oleptro
Ziprasidone • Geodon
Zonisamide • Zonegran
Disclosures
The authors report no financial relationships with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Bhidayasiri R1, Fahn S, Weiner WJ, et al; American Academy of Neurology. Evidence-based guideline: treatment of tardive syndromes: report of the Guideline Development Subcommittee of the American Academy of Neurology. Neurology. 2013;81(5):463-469.
2. Dolder CR, Jeste DV. Incidence of tardive dyskinesia with typical versus atypical antipsychotics in very high risk patients. Biol Psychiatry. 2003;53(12):1142-1145.
3. Correll CU, Leucht S, Kane JM. Lower risk for tardive dyskinesia associated with second-generation antipsychotics: a systematic review of 1-year studies. Am J Psychiatry. 2004;161(3):414-425.
4. Gardos G, Cole JO, Tarsy D. Withdrawal syndromes associated with antipsychotic drugs. Am J Psychiatry. 1978;135(11):1321-1324.
5. Salomon C, Hamilton B. Antipsychotic discontinuation syndromes: a narrative review of the evidence and its integration into Australian mental health nursing textbooks. Int J Ment Health Nurs. 2014;23(1):69-78.
6. Moseley CN, Simpson-Khanna HA, Catalano G, et al. Covert dyskinesia associated with aripiprazole: a case report and review of the literature. Clin Neuropharmacol. 2013;36(4):128-130.
7. Anand VS, Dewan MJ. Withdrawal-emergent dyskinesia in a patient on risperidone undergoing dosage reduction. Ann Clin Psychiatry. 1996;8(3):179-182.
8. Schultz SK, Miller DD, Arndt S, et al. Withdrawal-emergent dyskinesia in patients with schizophrenia during antipsychotic discontinuation. Biol Psychiatry. 1995;38(11):713-719.
9. Degkwitz R, Bauer MP, Gruber M, et al. Time relationship between the appearance of persisting extrapyramidal hyperkineses and psychotic recurrences following sudden interruption of prolonged neuroleptic therapy of chronic schizophrenic patients [in German]. Arzneimittelforschung. 1970;20(7):890-893.
10. Sethi KD. Tardive dyskinesias. In: Adler CH, Ahlskog JE, eds. Parkinson’s disease and movement disorders: diagnosis and treatment guidelines for the practicing physician. New York, NY: Humana Press; 2000:331-338.
11. Diagnostic and statistical manual of mental disorders, fifth edition. Washington, DC: American Psychiatric Association; 2013.
12. Horváth K, Aschermann Z, Komoly S, et al. Treatment of tardive syndromes [in Hungarian]. Psychiatr Hung. 2014;29(2):214-224.
13. Samaha AN, Seeman P, Stewart J, et al. “Breakthrough” dopamine supersensitivity during ongoing antipsychotic treatment leads to treatment failure over time. J Neurosci. 2007;27(11):2979-2986.
14. Thelma B, Srivastava V, Tiwari AK. Genetic underpinnings of tardive dyskinesia: passing the baton to pharmacogenetics. Pharmacogenomics. 2008;9(9):1285-1306.
15. Keith SJ, Kane JM. Partial compliance and patient consequences in schizophrenia: our patients can do better. J Clin Psychiatry. 2003;64(11):1308-1315.
16. Lieberman JA, Saltz BL, Johns CA, et al. The effects of clozapine on tardive dyskinesia. Br J Psychiatry. 1991;158:503-510.
17. Farah A. Atypicality of atypical antipsychotics. Prim Care Companion J Clin Psychiatry. 2005;7(6):268-274.
18. Rana AQ, Chaudry ZM, Blanchet PJ. New and emerging treatments for symptomatic tardive dyskinesia. Drug Des Devel Ther. 2013;7:1329-1340.
19. Shekelle PG, Woolf SH, Eccles M, et al. Developing clinical guidelines. West J Med. 1999;170(6):348-351.
20. Cloud LJ, Zutshi D, Factor SA. Tardive dyskinesia: therapeutic options for an increasingly common disorder. Neurotherapeutics. 2014;11(1):166-176.
21. Urbano M, Spiegel D, Rai A. Atypical antipsychotic withdrawal dyskinesia in 4 patients with mood disorders. J Clin Psychopharmacol. 2007;27(6):705-707.
22. Pani L, Pira L, Marchese G. Antipsychotic efficacy: relationship to optimal D2-receptor occupancy. Eur Psychiatry. 2007;22(5):267-275.
CASE Insurer denies drug coverage
Ms. X, age 65, has a 35-year history of bipolar I disorder (BD I) characterized by psychotic mania and severe suicidal depression. For the past year, her symptoms have been well controlled with aripiprazole, 5 mg/d; trazodone, 50 mg at bedtime; and citalopram, 20 mg/d. Because her health insurance has changed, Ms. X asks to be switched to an alternative antipsychotic because the new provider denied coverage of aripiprazole.
While taking aripiprazole, Ms. X did not report any extrapyramidal side effects, including tardive dyskinesia. Her Abnormal Involuntary Movement Scale (AIMS) score is 4. No significant abnormal movements were noted on examination during previous medication management sessions.
We decide to replace aripiprazole with quetiapine, 50 mg/d. At a 2-week follow-up visit, Ms. X is noted to have euphoric mood and reduced need to sleep, flight of ideas, increased talkativeness, and paranoia. We also notice that she has significant tongue rolling and lip smacking, which she says started 10 days after changing from aripiprazole to quetiapine. Her AIMS score is 17.
What could be causing Ms. X’s tongue rolling and lip smacking?
a) an irreversible syndrome usually starting after 1 or 2 years of continuous exposure to antipsychotics
b) a self-limited condition expected to resolve completely within 12 weeks
c) an acute manifestation of an antipsychotic that can respond to an anticholinergic agent
d) none of the above
The authors’ observations
Tardive dyskinesia (TD) refers to at least moderate abnormal involuntary movements in ≥1 areas of the body or at least mild movements in ≥2 areas of the body, developing after ≥3 months of cumulative exposure (continuous or discontinuous) to dopamine D2 receptor-blocking agents.1 AIMS is a 14-item, clinician-administered questionnaire designed to evaluate such movements and track their severity over time. The first 10 items are rated on 5-point scale (0 = none; 1 = minimal; 2 = mild; 3 = moderate; 4 = severe), with items 1 to 4 assessing orofacial movements, 5 to 7 assessing extremity and truncal movements, and 8 to 10 assessing overall severity, impairment, and subjective distress. Items 11 to 13 assess dental status because lack of teeth can result in oral movements mimicking TDs. The last item assesses whether these movements disappear during sleep.
HISTORY Poor response
Ms. X was given a diagnosis of BD I at age 30; she first started taking antipsychotics 10 years later. Previous psychotropic trials included lamotrigine, divalproex sodium, risperidone, and ziprasidone, which were ineffective or poorly tolerated. Her medical history includes obstructive sleep apnea, narcolepsy, type 2 diabetes mellitus, hypertension, dyslipidemia, fibromyalgia, gastroesophageal reflux disease, and hypothyroidism. She takes metformin, omeprazole, pravastatin, carvedilol, insulin, levothyroxine, methylphenidate (for hypersomnia), and enalapril.
What is the next best step in management?
a) discontinue quetiapine
b) replace quetiapine with clozapine
c) increase quetiapine to target manic symptoms and reassess in a few weeks
d) continue quetiapine and treat abnormal movements with benztropine
TREATMENT Increase dosage
We increase quetiapine to 150 mg/d to target Ms. X’s manic symptoms. She is scheduled for a follow-up visit in 4 weeks but is instructed to return to the clinic earlier if her manic symptoms do not improve. At the 4-week follow-up visit, Ms. X does not have any abnormal movements and her manic symptoms have resolved. Her AIMS score is 4. Her husband reports that her abnormal movements resolved 4 days after increasing quetiapine to 150 mg/d.
The authors’ observations
Second-generation antipsychotics are known to have a lower risk of extrapyramidal adverse reactions compared with older first-generation antipsychotics.2,3 TD differs from other extrapyramidal symptoms (EPS) because of its delayed onset. Risk factors for TD include:
• female sex
• age >50
• history of brain damage
• long-term antipsychotic use
• diagnosis of a mood disorder.
Gardos et al4 described 2 other forms of delayed dyskinesias related to antipsychotic use but resulting from antipsychotic discontinuation: withdrawal dyskinesia and covert dyskinesia. Evidence for these types of antipsychotic discontinuation syndromes mostly is anecdotal.5,6Table 1 highlights 3 different types of dyskinesias and their management.
Withdrawal dyskinesia has been described as a syndrome resembling TD that appears after discontinuation or dosage reduction of an antipsychotic in a patient who does not have an earlier TD diagnosis.7 The prevalence of withdrawal dyskinesia among patients undergoing antipsychotic discontinuation is approximately 30%.8 Cases of withdrawal dyskinesia are self-limited and resolve in 1 to 3 months.9,10 We believe that Ms. X’s movement disorder was withdrawal dyskinesia from aripiprazole because her symptoms started 10 days after the drug was discontinued, and was self-limited and reversible.
Similar to TD, withdrawal dyskinesia can present in different forms:
• tongue protrusion movements
• facial grimacing
• ticks
• chorea
• tremors
• athetosis
• involuntary vocalizations
• abnormal movements of hands and legs
• “dyspnea” due to involvement of respiratory musculature.5,11
There may be a sex difference in duration of withdrawal dyskinesias, because symptoms persist longer in females.9
Although covert dyskinesia also develops after discontinuation or dosage reduction of a dopamine-blocking agent, the symptoms usually are permanent, and could require reintroducing the antipsychotic or management with evidence-based treatments for TD, such as tetrabenazine or amantadine.6,12
What is the cause of Ms. X’s abnormal involuntary movements?
a) quetiapine-induced D2 receptor hypersensitivity
b) aripiprazole-induced cholinergic overactivity
c) quetiapine-induced cholinergic overactivity
d) aripiprazole-induced D2 receptor hypersensitivity
The authors’ observations
Pathophysiology of this condition is unknown but different theories have been proposed. D2 receptor up-regulation and hypersensitivity to compensate for chronic D2 receptor blockade by antipsychotics is a commonly cited theory.7,13 Discontinuation of an antipsychotic can make this D2 receptor up-regulation and hypersensitivity manifest as withdrawal dyskinesia by creating a temporary hyperdopaminergic state in basal ganglia. Other theories implicate decrease of γ-aminobutyric acid (GABA) in the globus pallidus (GP) and substantia nigra (SN) regions of the brain, and oxidative damage to GABAergic interneurons in GP and SN from excess production of catecholamines in response to chronic dopamine blockade.14
It has been proposed that patients with withdrawal dyskinesia might be in an early phase of D2 receptor modulation that, if continued because of use of the antipsychotic implicated in withdrawal dyskinesia, can lead to development of TD.4,7,8 A feature of withdrawal dyskinesia that differentiates it from TD is that it usually remits spontaneously within several weeks to a few months.4,7 Because of this characteristic, Schultz et al8 propose that, if withdrawal dyskinesia is identified early in treatment, it may be possible to prevent development of persistent TD.
Look carefully for dyskinetic movements in patients who have recently discontinued or decreased the dosage of their antipsychotic. Non-compliance and partial compliance are common problems among patients taking an antipsychotic.15 Therefore, careful watchfulness for withdrawal dyskinesias at all times can be beneficial. Inquiring about recent history of these dyskinesias in such patients is probably more useful than an exam because the dyskinesias may not be evident on exam when these patients show up for their follow-up visit, because of their self-limited nature.8
Treatment options
If a patient is noted to have a withdrawal-emergent dyskinesia, a clinician has options to prevent TD, including:
• decreasing the dosage of the antipsychotic
• switching from a typical antipsychotic to an atypical antipsychotic
• switching from one atypical to another with lesser affinity for striatal D2 receptor, such as clozapine or quetiapine.16,17
In addition, researchers are investigating the use of vitamin B6, Ginkgo biloba, amantadine, levetiracetam, melatonin, tetrabenazine, zonisamide, branched chain amino acids, clonazepam, and vitamin E as treatment alternatives for TD.
Tetrabenazine acts by blocking vesicular monoamine transporter type 2, thereby inhibiting release of monoamines, including dopamine into synaptic cleft area in basal ganglia.18 Clonazepam’s benefit for TD relates to its facilitation of GABAergic neurotransmission, because reduced GABAergic transmission in GP and SN has been associ ated with hyperkinetic movements, including TD.14Ginkgo biloba and melatonin exert their beneficial effects in TD through their antioxidant function.14
The agents listed in Table 219 could be used on a short-term basis for symptomatic treatment of withdrawal dyskinesias.1,18,20
Withdrawal dyskinesia has been reported with aripiprazole discontinuation and is thought to be related to aripiprazole’s strong affinity for D2 receptors.21 Aripiprazole at dosages of 15 to 30 mg/d can occupy more than 80% of the striatal D2 dopamine receptors. The dosage of ≥30 mg/d can lead to receptor occupancy of >90%.22 Studies have shown that EPS correlate with D2 receptor occupancy in steady-state conditions, and occupancy exceeding 80% results in these symptoms.22
Compared with aripiprazole, quetiapine has weak affinity for D2 receptors (Table 3), making it an unlikely culprit if dyskinesia emerges within 2 weeks of initation.22 We believe that, in Ms. X’s case, quetiapine might have masked the severity of aripiprazole withdrawal dyskinesia by causing some degree of D2 receptor blockade. It may have decreased the duration of withdrawal dyskinesia by the same effect on D2 receptors. It may have lasted longer if aripiprazole was not replaced by another antipsychotic. This is particularly evident because dyskinesia improved quickly when quetiapine was titrated to 150 mg/d. The higher quetiapine dosage of 150 mg/d is closer to 5 mg/d of aripiprazole in terms of D2 receptor occupancy and affinity. However, quetiapine is weaker than aripiprazole in terms of D2 receptor occupancy at all dosages, and therefore less likely to cause EPS.16
Summing up
Withdrawal dyskinesia in the absence of a history of TD is a common symptom of antipsychotic discontinuation or dosage reduction after long-term use of an antipsychotic. It is more commonly seen with antipsychotics with high D2 receptor occupancy, and has been hypothesized to be related to D2 receptor supersensitivity to ambient dopamine, resulting as a compensatory response to chronic D2 blockade by this class of medication.
Evidence suggests that reversible withdrawal dyskinesia could represent a prodrome to irreversible TD. Therefore, keeping a watchful eye for these movements during the exam, along with specific inquiry about withdrawal dyskinesias while taking a history at every follow-up visit, is important because doing so can:
• inform the clinician about partial compliance or noncompliance to these medications, which could lead to treatment failure
• help prevent development of irreversible TD syndrome.
Ms. X’s case reminds clinicians (1) to be aware of this unexpected side effect occurring even with second-generation antipsychotics and (2) that they should consider EPS in patients while they are discontinuing their drugs. Furthermore, it is important for clinical and medicolegal reasons to inform our patients that different forms of dyskinesias can be potential side effects of antipsychotics.
Bottom Line
Dyskinesias can result from withdrawal of both typical and atypical antipsychotics, and usually are self-limited. Withdrawal dyskinesia may represent a prodrome to tardive dyskinesia; early recognition may aid in preventing development of persistent tardive dyskinesia.
Related Resources
• Abnormal Involuntary Movement Scale. http://www.cqaimh.org/pdf/toolaims.pdf.
• Goldberg JF, Ernst CL. Managing the side effects of psychotropic medications. Arlington, VA: American Psychiatric Publishing, Inc; 2012.
• Tarsay D. Tardive dyskinesia: prevention and treatment. http:// www.uptodate.com/contents/tardive-dyskinesia-prevention-and-treatment?topicKey=NEURO%2F4908&elapsedTimeMs=3 &view=print&displayedView=full#.
Drug Brand Names
Amantadine • Symmetrel
Aripiprazole • Abilify
Benztropine • Cogentin
Carvedilol • Coreg
Citalopram • Celexa
Clonazepam • Klonopin
Clozapine • Clozaril
Divalproex sodium • Depakote
Donepezil • Aricept
Enalapril • Vasotec
Haloperidol • Haldol
Lamotrigine • Lamictal
Levetiracetam • Keppra
Levothyroxine • Levoxyl, Synthroid
Metformin • Glucophage
Methylphenidate • Ritalin
Olanzapine • Zyprexa
Omeprazole • Prilosec
Pravastatin • Pravachol
Quetiapine • Seroquel
Risperidone • Risperdal
Tetrabenazine • Xenazine
Trazodone • Desyrel, Oleptro
Ziprasidone • Geodon
Zonisamide • Zonegran
Disclosures
The authors report no financial relationships with any company whose products are mentioned in this article or with manufacturers of competing products.
CASE Insurer denies drug coverage
Ms. X, age 65, has a 35-year history of bipolar I disorder (BD I) characterized by psychotic mania and severe suicidal depression. For the past year, her symptoms have been well controlled with aripiprazole, 5 mg/d; trazodone, 50 mg at bedtime; and citalopram, 20 mg/d. Because her health insurance has changed, Ms. X asks to be switched to an alternative antipsychotic because the new provider denied coverage of aripiprazole.
While taking aripiprazole, Ms. X did not report any extrapyramidal side effects, including tardive dyskinesia. Her Abnormal Involuntary Movement Scale (AIMS) score is 4. No significant abnormal movements were noted on examination during previous medication management sessions.
We decide to replace aripiprazole with quetiapine, 50 mg/d. At a 2-week follow-up visit, Ms. X is noted to have euphoric mood and reduced need to sleep, flight of ideas, increased talkativeness, and paranoia. We also notice that she has significant tongue rolling and lip smacking, which she says started 10 days after changing from aripiprazole to quetiapine. Her AIMS score is 17.
What could be causing Ms. X’s tongue rolling and lip smacking?
a) an irreversible syndrome usually starting after 1 or 2 years of continuous exposure to antipsychotics
b) a self-limited condition expected to resolve completely within 12 weeks
c) an acute manifestation of an antipsychotic that can respond to an anticholinergic agent
d) none of the above
The authors’ observations
Tardive dyskinesia (TD) refers to at least moderate abnormal involuntary movements in ≥1 areas of the body or at least mild movements in ≥2 areas of the body, developing after ≥3 months of cumulative exposure (continuous or discontinuous) to dopamine D2 receptor-blocking agents.1 AIMS is a 14-item, clinician-administered questionnaire designed to evaluate such movements and track their severity over time. The first 10 items are rated on 5-point scale (0 = none; 1 = minimal; 2 = mild; 3 = moderate; 4 = severe), with items 1 to 4 assessing orofacial movements, 5 to 7 assessing extremity and truncal movements, and 8 to 10 assessing overall severity, impairment, and subjective distress. Items 11 to 13 assess dental status because lack of teeth can result in oral movements mimicking TDs. The last item assesses whether these movements disappear during sleep.
HISTORY Poor response
Ms. X was given a diagnosis of BD I at age 30; she first started taking antipsychotics 10 years later. Previous psychotropic trials included lamotrigine, divalproex sodium, risperidone, and ziprasidone, which were ineffective or poorly tolerated. Her medical history includes obstructive sleep apnea, narcolepsy, type 2 diabetes mellitus, hypertension, dyslipidemia, fibromyalgia, gastroesophageal reflux disease, and hypothyroidism. She takes metformin, omeprazole, pravastatin, carvedilol, insulin, levothyroxine, methylphenidate (for hypersomnia), and enalapril.
What is the next best step in management?
a) discontinue quetiapine
b) replace quetiapine with clozapine
c) increase quetiapine to target manic symptoms and reassess in a few weeks
d) continue quetiapine and treat abnormal movements with benztropine
TREATMENT Increase dosage
We increase quetiapine to 150 mg/d to target Ms. X’s manic symptoms. She is scheduled for a follow-up visit in 4 weeks but is instructed to return to the clinic earlier if her manic symptoms do not improve. At the 4-week follow-up visit, Ms. X does not have any abnormal movements and her manic symptoms have resolved. Her AIMS score is 4. Her husband reports that her abnormal movements resolved 4 days after increasing quetiapine to 150 mg/d.
The authors’ observations
Second-generation antipsychotics are known to have a lower risk of extrapyramidal adverse reactions compared with older first-generation antipsychotics.2,3 TD differs from other extrapyramidal symptoms (EPS) because of its delayed onset. Risk factors for TD include:
• female sex
• age >50
• history of brain damage
• long-term antipsychotic use
• diagnosis of a mood disorder.
Gardos et al4 described 2 other forms of delayed dyskinesias related to antipsychotic use but resulting from antipsychotic discontinuation: withdrawal dyskinesia and covert dyskinesia. Evidence for these types of antipsychotic discontinuation syndromes mostly is anecdotal.5,6Table 1 highlights 3 different types of dyskinesias and their management.
Withdrawal dyskinesia has been described as a syndrome resembling TD that appears after discontinuation or dosage reduction of an antipsychotic in a patient who does not have an earlier TD diagnosis.7 The prevalence of withdrawal dyskinesia among patients undergoing antipsychotic discontinuation is approximately 30%.8 Cases of withdrawal dyskinesia are self-limited and resolve in 1 to 3 months.9,10 We believe that Ms. X’s movement disorder was withdrawal dyskinesia from aripiprazole because her symptoms started 10 days after the drug was discontinued, and was self-limited and reversible.
Similar to TD, withdrawal dyskinesia can present in different forms:
• tongue protrusion movements
• facial grimacing
• ticks
• chorea
• tremors
• athetosis
• involuntary vocalizations
• abnormal movements of hands and legs
• “dyspnea” due to involvement of respiratory musculature.5,11
There may be a sex difference in duration of withdrawal dyskinesias, because symptoms persist longer in females.9
Although covert dyskinesia also develops after discontinuation or dosage reduction of a dopamine-blocking agent, the symptoms usually are permanent, and could require reintroducing the antipsychotic or management with evidence-based treatments for TD, such as tetrabenazine or amantadine.6,12
What is the cause of Ms. X’s abnormal involuntary movements?
a) quetiapine-induced D2 receptor hypersensitivity
b) aripiprazole-induced cholinergic overactivity
c) quetiapine-induced cholinergic overactivity
d) aripiprazole-induced D2 receptor hypersensitivity
The authors’ observations
Pathophysiology of this condition is unknown but different theories have been proposed. D2 receptor up-regulation and hypersensitivity to compensate for chronic D2 receptor blockade by antipsychotics is a commonly cited theory.7,13 Discontinuation of an antipsychotic can make this D2 receptor up-regulation and hypersensitivity manifest as withdrawal dyskinesia by creating a temporary hyperdopaminergic state in basal ganglia. Other theories implicate decrease of γ-aminobutyric acid (GABA) in the globus pallidus (GP) and substantia nigra (SN) regions of the brain, and oxidative damage to GABAergic interneurons in GP and SN from excess production of catecholamines in response to chronic dopamine blockade.14
It has been proposed that patients with withdrawal dyskinesia might be in an early phase of D2 receptor modulation that, if continued because of use of the antipsychotic implicated in withdrawal dyskinesia, can lead to development of TD.4,7,8 A feature of withdrawal dyskinesia that differentiates it from TD is that it usually remits spontaneously within several weeks to a few months.4,7 Because of this characteristic, Schultz et al8 propose that, if withdrawal dyskinesia is identified early in treatment, it may be possible to prevent development of persistent TD.
Look carefully for dyskinetic movements in patients who have recently discontinued or decreased the dosage of their antipsychotic. Non-compliance and partial compliance are common problems among patients taking an antipsychotic.15 Therefore, careful watchfulness for withdrawal dyskinesias at all times can be beneficial. Inquiring about recent history of these dyskinesias in such patients is probably more useful than an exam because the dyskinesias may not be evident on exam when these patients show up for their follow-up visit, because of their self-limited nature.8
Treatment options
If a patient is noted to have a withdrawal-emergent dyskinesia, a clinician has options to prevent TD, including:
• decreasing the dosage of the antipsychotic
• switching from a typical antipsychotic to an atypical antipsychotic
• switching from one atypical to another with lesser affinity for striatal D2 receptor, such as clozapine or quetiapine.16,17
In addition, researchers are investigating the use of vitamin B6, Ginkgo biloba, amantadine, levetiracetam, melatonin, tetrabenazine, zonisamide, branched chain amino acids, clonazepam, and vitamin E as treatment alternatives for TD.
Tetrabenazine acts by blocking vesicular monoamine transporter type 2, thereby inhibiting release of monoamines, including dopamine into synaptic cleft area in basal ganglia.18 Clonazepam’s benefit for TD relates to its facilitation of GABAergic neurotransmission, because reduced GABAergic transmission in GP and SN has been associ ated with hyperkinetic movements, including TD.14Ginkgo biloba and melatonin exert their beneficial effects in TD through their antioxidant function.14
The agents listed in Table 219 could be used on a short-term basis for symptomatic treatment of withdrawal dyskinesias.1,18,20
Withdrawal dyskinesia has been reported with aripiprazole discontinuation and is thought to be related to aripiprazole’s strong affinity for D2 receptors.21 Aripiprazole at dosages of 15 to 30 mg/d can occupy more than 80% of the striatal D2 dopamine receptors. The dosage of ≥30 mg/d can lead to receptor occupancy of >90%.22 Studies have shown that EPS correlate with D2 receptor occupancy in steady-state conditions, and occupancy exceeding 80% results in these symptoms.22
Compared with aripiprazole, quetiapine has weak affinity for D2 receptors (Table 3), making it an unlikely culprit if dyskinesia emerges within 2 weeks of initation.22 We believe that, in Ms. X’s case, quetiapine might have masked the severity of aripiprazole withdrawal dyskinesia by causing some degree of D2 receptor blockade. It may have decreased the duration of withdrawal dyskinesia by the same effect on D2 receptors. It may have lasted longer if aripiprazole was not replaced by another antipsychotic. This is particularly evident because dyskinesia improved quickly when quetiapine was titrated to 150 mg/d. The higher quetiapine dosage of 150 mg/d is closer to 5 mg/d of aripiprazole in terms of D2 receptor occupancy and affinity. However, quetiapine is weaker than aripiprazole in terms of D2 receptor occupancy at all dosages, and therefore less likely to cause EPS.16
Summing up
Withdrawal dyskinesia in the absence of a history of TD is a common symptom of antipsychotic discontinuation or dosage reduction after long-term use of an antipsychotic. It is more commonly seen with antipsychotics with high D2 receptor occupancy, and has been hypothesized to be related to D2 receptor supersensitivity to ambient dopamine, resulting as a compensatory response to chronic D2 blockade by this class of medication.
Evidence suggests that reversible withdrawal dyskinesia could represent a prodrome to irreversible TD. Therefore, keeping a watchful eye for these movements during the exam, along with specific inquiry about withdrawal dyskinesias while taking a history at every follow-up visit, is important because doing so can:
• inform the clinician about partial compliance or noncompliance to these medications, which could lead to treatment failure
• help prevent development of irreversible TD syndrome.
Ms. X’s case reminds clinicians (1) to be aware of this unexpected side effect occurring even with second-generation antipsychotics and (2) that they should consider EPS in patients while they are discontinuing their drugs. Furthermore, it is important for clinical and medicolegal reasons to inform our patients that different forms of dyskinesias can be potential side effects of antipsychotics.
Bottom Line
Dyskinesias can result from withdrawal of both typical and atypical antipsychotics, and usually are self-limited. Withdrawal dyskinesia may represent a prodrome to tardive dyskinesia; early recognition may aid in preventing development of persistent tardive dyskinesia.
Related Resources
• Abnormal Involuntary Movement Scale. http://www.cqaimh.org/pdf/toolaims.pdf.
• Goldberg JF, Ernst CL. Managing the side effects of psychotropic medications. Arlington, VA: American Psychiatric Publishing, Inc; 2012.
• Tarsay D. Tardive dyskinesia: prevention and treatment. http:// www.uptodate.com/contents/tardive-dyskinesia-prevention-and-treatment?topicKey=NEURO%2F4908&elapsedTimeMs=3 &view=print&displayedView=full#.
Drug Brand Names
Amantadine • Symmetrel
Aripiprazole • Abilify
Benztropine • Cogentin
Carvedilol • Coreg
Citalopram • Celexa
Clonazepam • Klonopin
Clozapine • Clozaril
Divalproex sodium • Depakote
Donepezil • Aricept
Enalapril • Vasotec
Haloperidol • Haldol
Lamotrigine • Lamictal
Levetiracetam • Keppra
Levothyroxine • Levoxyl, Synthroid
Metformin • Glucophage
Methylphenidate • Ritalin
Olanzapine • Zyprexa
Omeprazole • Prilosec
Pravastatin • Pravachol
Quetiapine • Seroquel
Risperidone • Risperdal
Tetrabenazine • Xenazine
Trazodone • Desyrel, Oleptro
Ziprasidone • Geodon
Zonisamide • Zonegran
Disclosures
The authors report no financial relationships with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Bhidayasiri R1, Fahn S, Weiner WJ, et al; American Academy of Neurology. Evidence-based guideline: treatment of tardive syndromes: report of the Guideline Development Subcommittee of the American Academy of Neurology. Neurology. 2013;81(5):463-469.
2. Dolder CR, Jeste DV. Incidence of tardive dyskinesia with typical versus atypical antipsychotics in very high risk patients. Biol Psychiatry. 2003;53(12):1142-1145.
3. Correll CU, Leucht S, Kane JM. Lower risk for tardive dyskinesia associated with second-generation antipsychotics: a systematic review of 1-year studies. Am J Psychiatry. 2004;161(3):414-425.
4. Gardos G, Cole JO, Tarsy D. Withdrawal syndromes associated with antipsychotic drugs. Am J Psychiatry. 1978;135(11):1321-1324.
5. Salomon C, Hamilton B. Antipsychotic discontinuation syndromes: a narrative review of the evidence and its integration into Australian mental health nursing textbooks. Int J Ment Health Nurs. 2014;23(1):69-78.
6. Moseley CN, Simpson-Khanna HA, Catalano G, et al. Covert dyskinesia associated with aripiprazole: a case report and review of the literature. Clin Neuropharmacol. 2013;36(4):128-130.
7. Anand VS, Dewan MJ. Withdrawal-emergent dyskinesia in a patient on risperidone undergoing dosage reduction. Ann Clin Psychiatry. 1996;8(3):179-182.
8. Schultz SK, Miller DD, Arndt S, et al. Withdrawal-emergent dyskinesia in patients with schizophrenia during antipsychotic discontinuation. Biol Psychiatry. 1995;38(11):713-719.
9. Degkwitz R, Bauer MP, Gruber M, et al. Time relationship between the appearance of persisting extrapyramidal hyperkineses and psychotic recurrences following sudden interruption of prolonged neuroleptic therapy of chronic schizophrenic patients [in German]. Arzneimittelforschung. 1970;20(7):890-893.
10. Sethi KD. Tardive dyskinesias. In: Adler CH, Ahlskog JE, eds. Parkinson’s disease and movement disorders: diagnosis and treatment guidelines for the practicing physician. New York, NY: Humana Press; 2000:331-338.
11. Diagnostic and statistical manual of mental disorders, fifth edition. Washington, DC: American Psychiatric Association; 2013.
12. Horváth K, Aschermann Z, Komoly S, et al. Treatment of tardive syndromes [in Hungarian]. Psychiatr Hung. 2014;29(2):214-224.
13. Samaha AN, Seeman P, Stewart J, et al. “Breakthrough” dopamine supersensitivity during ongoing antipsychotic treatment leads to treatment failure over time. J Neurosci. 2007;27(11):2979-2986.
14. Thelma B, Srivastava V, Tiwari AK. Genetic underpinnings of tardive dyskinesia: passing the baton to pharmacogenetics. Pharmacogenomics. 2008;9(9):1285-1306.
15. Keith SJ, Kane JM. Partial compliance and patient consequences in schizophrenia: our patients can do better. J Clin Psychiatry. 2003;64(11):1308-1315.
16. Lieberman JA, Saltz BL, Johns CA, et al. The effects of clozapine on tardive dyskinesia. Br J Psychiatry. 1991;158:503-510.
17. Farah A. Atypicality of atypical antipsychotics. Prim Care Companion J Clin Psychiatry. 2005;7(6):268-274.
18. Rana AQ, Chaudry ZM, Blanchet PJ. New and emerging treatments for symptomatic tardive dyskinesia. Drug Des Devel Ther. 2013;7:1329-1340.
19. Shekelle PG, Woolf SH, Eccles M, et al. Developing clinical guidelines. West J Med. 1999;170(6):348-351.
20. Cloud LJ, Zutshi D, Factor SA. Tardive dyskinesia: therapeutic options for an increasingly common disorder. Neurotherapeutics. 2014;11(1):166-176.
21. Urbano M, Spiegel D, Rai A. Atypical antipsychotic withdrawal dyskinesia in 4 patients with mood disorders. J Clin Psychopharmacol. 2007;27(6):705-707.
22. Pani L, Pira L, Marchese G. Antipsychotic efficacy: relationship to optimal D2-receptor occupancy. Eur Psychiatry. 2007;22(5):267-275.
1. Bhidayasiri R1, Fahn S, Weiner WJ, et al; American Academy of Neurology. Evidence-based guideline: treatment of tardive syndromes: report of the Guideline Development Subcommittee of the American Academy of Neurology. Neurology. 2013;81(5):463-469.
2. Dolder CR, Jeste DV. Incidence of tardive dyskinesia with typical versus atypical antipsychotics in very high risk patients. Biol Psychiatry. 2003;53(12):1142-1145.
3. Correll CU, Leucht S, Kane JM. Lower risk for tardive dyskinesia associated with second-generation antipsychotics: a systematic review of 1-year studies. Am J Psychiatry. 2004;161(3):414-425.
4. Gardos G, Cole JO, Tarsy D. Withdrawal syndromes associated with antipsychotic drugs. Am J Psychiatry. 1978;135(11):1321-1324.
5. Salomon C, Hamilton B. Antipsychotic discontinuation syndromes: a narrative review of the evidence and its integration into Australian mental health nursing textbooks. Int J Ment Health Nurs. 2014;23(1):69-78.
6. Moseley CN, Simpson-Khanna HA, Catalano G, et al. Covert dyskinesia associated with aripiprazole: a case report and review of the literature. Clin Neuropharmacol. 2013;36(4):128-130.
7. Anand VS, Dewan MJ. Withdrawal-emergent dyskinesia in a patient on risperidone undergoing dosage reduction. Ann Clin Psychiatry. 1996;8(3):179-182.
8. Schultz SK, Miller DD, Arndt S, et al. Withdrawal-emergent dyskinesia in patients with schizophrenia during antipsychotic discontinuation. Biol Psychiatry. 1995;38(11):713-719.
9. Degkwitz R, Bauer MP, Gruber M, et al. Time relationship between the appearance of persisting extrapyramidal hyperkineses and psychotic recurrences following sudden interruption of prolonged neuroleptic therapy of chronic schizophrenic patients [in German]. Arzneimittelforschung. 1970;20(7):890-893.
10. Sethi KD. Tardive dyskinesias. In: Adler CH, Ahlskog JE, eds. Parkinson’s disease and movement disorders: diagnosis and treatment guidelines for the practicing physician. New York, NY: Humana Press; 2000:331-338.
11. Diagnostic and statistical manual of mental disorders, fifth edition. Washington, DC: American Psychiatric Association; 2013.
12. Horváth K, Aschermann Z, Komoly S, et al. Treatment of tardive syndromes [in Hungarian]. Psychiatr Hung. 2014;29(2):214-224.
13. Samaha AN, Seeman P, Stewart J, et al. “Breakthrough” dopamine supersensitivity during ongoing antipsychotic treatment leads to treatment failure over time. J Neurosci. 2007;27(11):2979-2986.
14. Thelma B, Srivastava V, Tiwari AK. Genetic underpinnings of tardive dyskinesia: passing the baton to pharmacogenetics. Pharmacogenomics. 2008;9(9):1285-1306.
15. Keith SJ, Kane JM. Partial compliance and patient consequences in schizophrenia: our patients can do better. J Clin Psychiatry. 2003;64(11):1308-1315.
16. Lieberman JA, Saltz BL, Johns CA, et al. The effects of clozapine on tardive dyskinesia. Br J Psychiatry. 1991;158:503-510.
17. Farah A. Atypicality of atypical antipsychotics. Prim Care Companion J Clin Psychiatry. 2005;7(6):268-274.
18. Rana AQ, Chaudry ZM, Blanchet PJ. New and emerging treatments for symptomatic tardive dyskinesia. Drug Des Devel Ther. 2013;7:1329-1340.
19. Shekelle PG, Woolf SH, Eccles M, et al. Developing clinical guidelines. West J Med. 1999;170(6):348-351.
20. Cloud LJ, Zutshi D, Factor SA. Tardive dyskinesia: therapeutic options for an increasingly common disorder. Neurotherapeutics. 2014;11(1):166-176.
21. Urbano M, Spiegel D, Rai A. Atypical antipsychotic withdrawal dyskinesia in 4 patients with mood disorders. J Clin Psychopharmacol. 2007;27(6):705-707.
22. Pani L, Pira L, Marchese G. Antipsychotic efficacy: relationship to optimal D2-receptor occupancy. Eur Psychiatry. 2007;22(5):267-275.