User login
Participants in randomized controlled trials of biologics for inflammatory bowel disease do not adequately represent real-world patients because of stringent inclusion criteria for the trials, reported Dr. Christina Ha and her colleagues in the September issue of Clinical Gastroenterology and Hepatology.
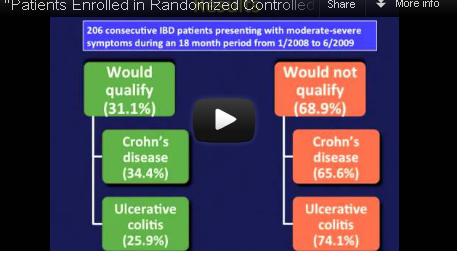
Indeed, among 125 Crohn’s disease (CD) patients seen in routine clinical practice at a tertiary care center, only 43 – just 34% – would have qualified for enrollment in at least one of seven randomized controlled trials (RCTs) for biologics, calling into question the generalizability of these trials’ results. The corresponding percentage for ulcerative colitis (UC) patients was only 25%.
Video Source: American Gastroenterological Association
Dr. Ha, of the division of gastroenterology at Johns Hopkins University, Baltimore, and her colleagues reviewed the medical records of 206 patients with moderate to severe CD or UC presenting to the Mount Sinai Medical Center in New York for adjustment of therapy during 2008-2009.
The researchers then looked at seven randomized controlled trials of biologics in CD as well as two trials of biologics in UC, and applied their inclusion and exclusion criteria to her real-world study population (Clin. Gastroenterol. Hepatol. 2012 September [doi: 10.1016/j.cgh.2012.02.004]).
Among the Crohn’s patients, "trial eligibility for the RCTs for infliximab, adalimumab, certolizumab pegol, and natalizumab ranged from 8% for the SONIC [Study of Biologic and Immunomodulator Naïve Patients in Crohn’s Disease] trial to 27% for the CHARM [Crohn’s Trial of the Fully Human Antibody Adalimumab for Remission Maintenance] and PRECISE [Pegylated Antibody Fragment Evaluation in Crohn’s Disease] trials," wrote the investigators.
Similarly, among the 81 UC patients, only 21 (25%) would have qualified for enrollment into the ACT (Active Ulcerative Colitis Trial) 1 or 2 trials.
Among the Crohn’s patients, the most common reasons for ineligibility were symptomatic strictures or abscesses (n = 51, 62.2%), recent exposure or prior nonresponse to anti–tumor necrosis factor drugs (n = 42, 51.2%), use of high-dose steroids (n = 15, 18.3%), and comorbid cardiovascular or pulmonary disease and malignancies (n = 21, 25.6%).
"The most common reason for trial ineligibility for UC was current rectal therapy usage (n = 34, 56.7%)," wrote the authors. Other reasons included steroid naivety (n = 27, 45.0%); new diagnoses of UC (n = 10, 16.7%); or need for colectomy due to age, comorbidity, or concomitant dysplasia found during colonoscopy (n = 9, 15.0%).
Finally, the authors assessed the outcomes of patients who would not have qualified for the biologics trials but initiated biologic therapy nevertheless. In the CD cohort, "ultimately, almost 50% of these ‘trial ineligible’ patients underwent surgery, either as a primary therapy or due to inadequate response to biologics or immunomodulators," wrote the authors. In the UC group, roughly one-quarter of would-be ineligible patients underwent colectomy at 4-12 weeks.
The authors conceded that if patients had been studied longitudinally, "with the inclusion and exclusion criteria applied at the time patients were first being considered for immunomodulators or biologics or at the time of diagnosis, a larger percentage of patients may have been eligible for trial participation."
Nevertheless, Dr. Ha and her colleagues concluded, "pragmatic trials of the major IBD therapeutics would not only serve to validate RCT findings, but provide additional insight regarding medication safety across a broader patient population."
Several of the authors disclosed financial relationships with pharmaceutical companies, including the makers of biologics. The authors stated that this study received no grant support.
Participants in randomized controlled trials of biologics for inflammatory bowel disease do not adequately represent real-world patients because of stringent inclusion criteria for the trials, reported Dr. Christina Ha and her colleagues in the September issue of Clinical Gastroenterology and Hepatology.
Indeed, among 125 Crohn’s disease (CD) patients seen in routine clinical practice at a tertiary care center, only 43 – just 34% – would have qualified for enrollment in at least one of seven randomized controlled trials (RCTs) for biologics, calling into question the generalizability of these trials’ results. The corresponding percentage for ulcerative colitis (UC) patients was only 25%.
Video Source: American Gastroenterological Association
Dr. Ha, of the division of gastroenterology at Johns Hopkins University, Baltimore, and her colleagues reviewed the medical records of 206 patients with moderate to severe CD or UC presenting to the Mount Sinai Medical Center in New York for adjustment of therapy during 2008-2009.
The researchers then looked at seven randomized controlled trials of biologics in CD as well as two trials of biologics in UC, and applied their inclusion and exclusion criteria to her real-world study population (Clin. Gastroenterol. Hepatol. 2012 September [doi: 10.1016/j.cgh.2012.02.004]).
Among the Crohn’s patients, "trial eligibility for the RCTs for infliximab, adalimumab, certolizumab pegol, and natalizumab ranged from 8% for the SONIC [Study of Biologic and Immunomodulator Naïve Patients in Crohn’s Disease] trial to 27% for the CHARM [Crohn’s Trial of the Fully Human Antibody Adalimumab for Remission Maintenance] and PRECISE [Pegylated Antibody Fragment Evaluation in Crohn’s Disease] trials," wrote the investigators.
Similarly, among the 81 UC patients, only 21 (25%) would have qualified for enrollment into the ACT (Active Ulcerative Colitis Trial) 1 or 2 trials.
Among the Crohn’s patients, the most common reasons for ineligibility were symptomatic strictures or abscesses (n = 51, 62.2%), recent exposure or prior nonresponse to anti–tumor necrosis factor drugs (n = 42, 51.2%), use of high-dose steroids (n = 15, 18.3%), and comorbid cardiovascular or pulmonary disease and malignancies (n = 21, 25.6%).
"The most common reason for trial ineligibility for UC was current rectal therapy usage (n = 34, 56.7%)," wrote the authors. Other reasons included steroid naivety (n = 27, 45.0%); new diagnoses of UC (n = 10, 16.7%); or need for colectomy due to age, comorbidity, or concomitant dysplasia found during colonoscopy (n = 9, 15.0%).
Finally, the authors assessed the outcomes of patients who would not have qualified for the biologics trials but initiated biologic therapy nevertheless. In the CD cohort, "ultimately, almost 50% of these ‘trial ineligible’ patients underwent surgery, either as a primary therapy or due to inadequate response to biologics or immunomodulators," wrote the authors. In the UC group, roughly one-quarter of would-be ineligible patients underwent colectomy at 4-12 weeks.
The authors conceded that if patients had been studied longitudinally, "with the inclusion and exclusion criteria applied at the time patients were first being considered for immunomodulators or biologics or at the time of diagnosis, a larger percentage of patients may have been eligible for trial participation."
Nevertheless, Dr. Ha and her colleagues concluded, "pragmatic trials of the major IBD therapeutics would not only serve to validate RCT findings, but provide additional insight regarding medication safety across a broader patient population."
Several of the authors disclosed financial relationships with pharmaceutical companies, including the makers of biologics. The authors stated that this study received no grant support.
Participants in randomized controlled trials of biologics for inflammatory bowel disease do not adequately represent real-world patients because of stringent inclusion criteria for the trials, reported Dr. Christina Ha and her colleagues in the September issue of Clinical Gastroenterology and Hepatology.
Indeed, among 125 Crohn’s disease (CD) patients seen in routine clinical practice at a tertiary care center, only 43 – just 34% – would have qualified for enrollment in at least one of seven randomized controlled trials (RCTs) for biologics, calling into question the generalizability of these trials’ results. The corresponding percentage for ulcerative colitis (UC) patients was only 25%.
Video Source: American Gastroenterological Association
Dr. Ha, of the division of gastroenterology at Johns Hopkins University, Baltimore, and her colleagues reviewed the medical records of 206 patients with moderate to severe CD or UC presenting to the Mount Sinai Medical Center in New York for adjustment of therapy during 2008-2009.
The researchers then looked at seven randomized controlled trials of biologics in CD as well as two trials of biologics in UC, and applied their inclusion and exclusion criteria to her real-world study population (Clin. Gastroenterol. Hepatol. 2012 September [doi: 10.1016/j.cgh.2012.02.004]).
Among the Crohn’s patients, "trial eligibility for the RCTs for infliximab, adalimumab, certolizumab pegol, and natalizumab ranged from 8% for the SONIC [Study of Biologic and Immunomodulator Naïve Patients in Crohn’s Disease] trial to 27% for the CHARM [Crohn’s Trial of the Fully Human Antibody Adalimumab for Remission Maintenance] and PRECISE [Pegylated Antibody Fragment Evaluation in Crohn’s Disease] trials," wrote the investigators.
Similarly, among the 81 UC patients, only 21 (25%) would have qualified for enrollment into the ACT (Active Ulcerative Colitis Trial) 1 or 2 trials.
Among the Crohn’s patients, the most common reasons for ineligibility were symptomatic strictures or abscesses (n = 51, 62.2%), recent exposure or prior nonresponse to anti–tumor necrosis factor drugs (n = 42, 51.2%), use of high-dose steroids (n = 15, 18.3%), and comorbid cardiovascular or pulmonary disease and malignancies (n = 21, 25.6%).
"The most common reason for trial ineligibility for UC was current rectal therapy usage (n = 34, 56.7%)," wrote the authors. Other reasons included steroid naivety (n = 27, 45.0%); new diagnoses of UC (n = 10, 16.7%); or need for colectomy due to age, comorbidity, or concomitant dysplasia found during colonoscopy (n = 9, 15.0%).
Finally, the authors assessed the outcomes of patients who would not have qualified for the biologics trials but initiated biologic therapy nevertheless. In the CD cohort, "ultimately, almost 50% of these ‘trial ineligible’ patients underwent surgery, either as a primary therapy or due to inadequate response to biologics or immunomodulators," wrote the authors. In the UC group, roughly one-quarter of would-be ineligible patients underwent colectomy at 4-12 weeks.
The authors conceded that if patients had been studied longitudinally, "with the inclusion and exclusion criteria applied at the time patients were first being considered for immunomodulators or biologics or at the time of diagnosis, a larger percentage of patients may have been eligible for trial participation."
Nevertheless, Dr. Ha and her colleagues concluded, "pragmatic trials of the major IBD therapeutics would not only serve to validate RCT findings, but provide additional insight regarding medication safety across a broader patient population."
Several of the authors disclosed financial relationships with pharmaceutical companies, including the makers of biologics. The authors stated that this study received no grant support.
FROM CLINICAL GASTROENTEROLOGY AND HEPATOLOGY