User login
SAN ANTONIO – It’s still too soon to know whether neurostimulation will be the therapeutic advance that treatment-refractory epilepsy patients have been waiting for, but the possibility that it might be has the epilepsy community buzzing.
For approximately 30% of epilepsy patients, seizures cannot be controlled with antiepileptic drugs or surgery. New long-term safety and efficacy data for vagus nerve simulation (VNS) and the results of recent pivotal trials of two approaches to direct brain stimulation offer beacons of hope to these patients, Dr. Gregory K. Bergey said in a plenary session on neurostimulation at the annual meeting of the American Epilepsy Society.
"One of the frustrating things for those of us treating patients with epilepsy has been the fact that, although a number of new antiepileptic drugs have been developed over the past 10-15 years and most are better tolerated and have better pharmacokinetic profiles than earlier drugs, the number of patients with seizures that don’t respond to medical therapy has not been significantly reduced," said Dr. Bergey, director of the Johns Hopkins Epilepsy Center, Baltimore.
"So we’re stepping back and saying, ‘Is there some other way we can treat these patients?’ That has been the impetus for looking at neurostimulation, which has been around for well over a decade, and what we’re seeing is exciting."
Although the 40%-50% response rates observed in direct brain stimulation trials do not appear to be overwhelming, "this is just the beginning," Dr. Bergey stressed in an interview. "As opposed to a drug trial, where you go up to a certain dose and it either works or it doesn’t work, in the case of neurostimulation we don’t know the optimal stimulus parameters, and I think that’s what you’re going to begin to see over the next several years," he said.
"There’s going to be a lot of investigation into neurostimulation of the brain structures to try to figure out who are the best candidates and what the best stimulus parameters are. It’s easy to say we’re stimulating the brain, but do we stimulate 100 times per second, 50 times per second, 25 times per second, and what should the stimulus intensities be?"
Vagus Nerve Stimulation. Currently, Cyberonics’ VNS Therapy System is the only Food and Drug Administration–approved form of neurostimulation for the treatment of epilepsy. The technology was approved in 1997 for the treatment of medically refractory partial-onset seizures in patients 12 years or older. It consists of a stimulator that sends electric impulses to the left vagus nerve in the neck via a lead wire that is implanted under the skin. Studies since 1997 have indicated efficacy in generalized seizure disorders and children as well, according to Dr. Elinor Ben-Menachem, professor of neurology and epilepsy at the Institute for Clinical Neurosciences and Physiology, Göteborg (Sweden) University.
To date, more than 60,000 patients worldwide have been treated with VNS, and studies suggest that approximately 50% of patients who undergo the procedure experience a long-term decrease in mean seizure frequency of 50% or more. But fewer than 10% become seizure-free, Dr. Ben-Menachem said during the neurostimulation plenary presentation.
"[VNS] has a long history now, and what we know is that it does not cure or affect seizures immediately. We actually don’t notice a change in seizure activity until about 18 months or 2 years after starting."
For example, a recent long-term follow-up study of VNS patients in the Czech Republic showed that at 1 year post implantation, 44.4% of patients achieved more than 50% seizure reduction. The percentage of patients who reached that level of seizure reduction then increased from 58.7% at 2 years after implant to 64.4% at 5 years. At the 5-year mark, 15.5% of the patients had achieved a minimum 90% seizure reduction, and 5.5% were seizure free (Seizure 2009;18: 269-74).
The mechanism of action of VNS remains uncertain, but a number of possibilities have been suggested, including arousal of the reticular formation; stimulation of locus coeruleus and noradrenaline pathways; changes in a neurotransmitter, amino acid, or neuropeptide; or indirect thalamus stimulation, according to Dr. Ben-Menachem. "It’s also possible that there is long-term learning through synaptic structural changes," she said. "The more I work with this, the more I think it is a learning paradigm. It’s like learning to play the piano. You can’t just sit down and play, you have to redo and redo until the brain is trained."
In a recent study of 144 patients who had undergone VNS implantation, 10 patients were seizure free for more than 1 year post implantation, 89 patients experienced seizure improvement, and no changes were observed in 45 patients. "Stepwise multivariate analysis showed that unilateral interictal epileptiform discharges [IEDs], cortical dysgenesis, and younger age at implantation were independent predictors of seizure freedom in the long-term follow-up," they wrote (Seizure 2010;19: 264-8).
Most of the adverse events associated with VNS therapy, such as hoarseness and cough, tend to be mild and are stimulation related, Dr. Ben-Menachem explained. "Typically, they occur only during stimulation and they generally diminish over time on their own, or they may be diminished or eliminated by adjustment of the parameter settings."
Programmed Deep Brain Stimulation. The programmed deep brain stimulation device manufactured by Medtronic, one of the two emerging neurostimulation treatments for intractable epilepsy that is under FDA review, demonstrated efficacy in a pivotal trial that involved stimulation in the anterior thalamus. This site has connections with the temporal lobe, which is a common site for the origin of partial seizures, Dr. Bergey explained.
The device, which is already approved for Parkinson's disease, comprises two leads* that are implanted bilaterally into the target structure with a pulse generator placed below the clavicle. Each lead contains four electrodes. The device delivers stimuli at scheduled intervals "to hopefully modulate and reduce the number of seizures the patient is having," he said.
In the Medtronic-funded Stimulation of the Anterior Nucleus of the Thalamus for Epilepsy (SANTE) study, 110 patients with medically refractory partial seizures were implanted with the device and randomized to intermittent bilateral stimulation (1 minute on/5 minutes off) or no-stimulation for a 3-month blinded stage, followed by unblinded stimulation for all of the patients (Epilepsia 2010;51:899-908). At the end of the blinded period, patients who received stimulation experienced a median seizure reduction of 40.4%, compared with 14.5% of patients with the stimulator off, reported study coauthor Dr. Vincenta Salanova of Indiana University, Indianapolis.
In the open-label follow-up, 56% of all the patients had greater than 50% seizure reduction at 2 years, and there was a median 68% reduction in seizures among the 42 patients for whom 3-year data were available. Over the course of the study, "14 [12.7%] of the patients were seizure free for at least 6 months," she reported in a press briefing at the meeting.
Although the mechanism of action is not fully understood, Dr. Salanova said that "the thalamus has connections between the limbic system and the frontal lobe, so it’s possible that high-frequency stimulation may prevent the propagation of seizures."
Five deaths occurred in the study population, but none were attributed to lead implantation or stimulation, Dr. Salanova stressed. There were no symptomatic or clinically significant hemorrhages associated with implantation, but 4.5% of patients experienced asymptomatic intracranial hemorrhages – detected via neuroimaging – that were not clinically significant. Additionally, two patients experienced seizures that were linked to the stimulus, which were resolved by lowering the voltage.
Direct stimulation of the hippocampus may also offer seizure relief in some patients, according to Dr. Richard Wennberg of the University of Toronto. "The hippocampus is clinically recognized as a region of high epileptogenicity, and animal studies have demonstrated antiepileptic properties of electrical fields applied to the region," he said in a presentation during the neurostimulation plenary session, noting that the goal of direct hippocampal stimulation is to prevent seizure generation and spread from the temporal limbic region.
To date, the experimental procedure has been evaluated in small series and has shown some efficacy, Dr. Wennberg said. For example, in a recent study designed to assess the effect of continuous electrical stimulation of the hippocampus bilaterally, two patients with seizures from both mesial temporal lobes who were not candidates for surgical resection were implanted bilaterally with two four-contact electrodes along the hippocampal axis. After randomization to either stimulation on or off conditions for 3-month intervals, seizure frequency decreased by 33% during stimulation, and stayed and remained lower by 25% for the 3 months after stimulation was turned off, after which the seizure frequency returned to baseline, the authors reported. Although seizure frequency was reduced both during and for a period after bilateral hippocampal stimulation, "the overall impact in this study is not as robust as has been previously reported," the authors stated (Epilepsia 2010;51:304-7).
Responsive Neurostimulation. Another direct brain neurostimulating technology under FDA review is the Responsive Neurostimulator System (RNS) by NeuroPace. The system detects and aborts spontaneously occurring abnormal discharges* to prevent seizures, explained Dr. Lawrence J. Hirsch of Columbia University in New York. "It is designed to respond within seconds to abnormal activity in the brain by delivering a series of up to five stimuli to terminate the abnormal discharge."
The RNS device is implanted in a recess of the skull, and is connected to up to two four-contact electrodes that are placed within the brain or on the brain surface, depending on where the seizures begin. The device collects and stores seizure information, which the patient subsequently downloads to a laptop using a wand. Physicians can access the stored electrocorticograms via a secure Web page through which they can adjust detection and stimulation parameters specific to the individual patient, Dr. Hirsch said during the neurostimulation plenary session.
In the pivotal clinical trial of the RNS system, 191 patients with medically intractable, partial-onset seizures localized to one or two foci received the cranial implant. During a blinded period, patients received active or sham stimulation, followed by an open-label phase in which all the patients received active stimulation. During the entire blinded evaluation period, active stimulation was associated with a mean 37.9% reduction in seizure frequency, compared with a mean 17% reduction during the sham activation, Dr. Hirsch said.
"In the final month of the blinded period – month 4 to 5 – the respective reduction in seizure frequency was 42% and 9%." During the last 3 months of the open-label period, "47% of the patients had a greater than 50% seizure reduction," he said. "And at 4 years post implant, more than 50% of the patients had at least a 50% reduction in seizure frequency."
A subset analysis showed that neither prior surgery nor the number of seizure foci had an effect on treatment response, Dr. Hirsch noted. "It also showed that [RNS] is possibly more effective with medial temporal onset."
With respect to adverse events, implant site infections were reported in 5% of the patients, and led to explantation in 2%. The combined rate of status epilepticus reported in all trials of the device (256 patients) was 3.5%, and included episodes occurring between 5 months and 5 years post implant. Intracranial hemorrhage was reported in 4% of the patients, and included only one patient with neurological sequelae, which was chronic headache, he said.
The chronic, intracranial EEG recordings provided by the RNS technology have other potentially valuable uses, including seizure prediction/warning; seizure awareness and counting as a way to assess treatment efficacy; identification of circadian, catamenial, and other ictal and interictal patterns; and the lateralization of bitemporal seizures, Dr. Hirsch said.
Dr. Bergey disclosed financial relationships with Pfizer, UCB, and Eli Lilly. Dr. Ben-Menachem disclosed financial relationships with UCB, Eisai, Janssen, Cilag, Cyberonics, Lundbeck, and Sunovion. Dr. Wennberg disclosed a financial relationship with Medtronic. Dr. Hirsch reported having no financial disclosures.
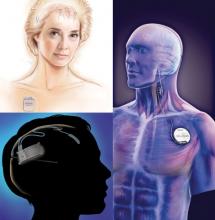
Medtronic's DBS system (top left) stimulates the anterior nucleus of the thalamus, whereas NeuroPace's RNS device (bottom left) responds to abnormal activity in targeted areas and Cyberonics' VNS Therapy System (right) periodically stimulates the left vagus nerve. (Photo Credit: top left: (c) Medtronic Inc., bottom left: (c) NeuroPace Inc., right: (c) Cyberonics Inc.)
* CORRECTION, 12/16/2010: The original version of this article misstated the function of the Responsive Neurostimulator System (RNS) by NeuroPace Inc. The system detects and aborts spontaneously occurring abnormal discharges. Also, the programmed deep brain stimulation device manufactured by Medtronic uses two leads that are placed bilaterally into the anterior thalamus. Each lead contains four electrodes. This version has been updated.
SAN ANTONIO – It’s still too soon to know whether neurostimulation will be the therapeutic advance that treatment-refractory epilepsy patients have been waiting for, but the possibility that it might be has the epilepsy community buzzing.
For approximately 30% of epilepsy patients, seizures cannot be controlled with antiepileptic drugs or surgery. New long-term safety and efficacy data for vagus nerve simulation (VNS) and the results of recent pivotal trials of two approaches to direct brain stimulation offer beacons of hope to these patients, Dr. Gregory K. Bergey said in a plenary session on neurostimulation at the annual meeting of the American Epilepsy Society.
"One of the frustrating things for those of us treating patients with epilepsy has been the fact that, although a number of new antiepileptic drugs have been developed over the past 10-15 years and most are better tolerated and have better pharmacokinetic profiles than earlier drugs, the number of patients with seizures that don’t respond to medical therapy has not been significantly reduced," said Dr. Bergey, director of the Johns Hopkins Epilepsy Center, Baltimore.
"So we’re stepping back and saying, ‘Is there some other way we can treat these patients?’ That has been the impetus for looking at neurostimulation, which has been around for well over a decade, and what we’re seeing is exciting."
Although the 40%-50% response rates observed in direct brain stimulation trials do not appear to be overwhelming, "this is just the beginning," Dr. Bergey stressed in an interview. "As opposed to a drug trial, where you go up to a certain dose and it either works or it doesn’t work, in the case of neurostimulation we don’t know the optimal stimulus parameters, and I think that’s what you’re going to begin to see over the next several years," he said.
"There’s going to be a lot of investigation into neurostimulation of the brain structures to try to figure out who are the best candidates and what the best stimulus parameters are. It’s easy to say we’re stimulating the brain, but do we stimulate 100 times per second, 50 times per second, 25 times per second, and what should the stimulus intensities be?"
Vagus Nerve Stimulation. Currently, Cyberonics’ VNS Therapy System is the only Food and Drug Administration–approved form of neurostimulation for the treatment of epilepsy. The technology was approved in 1997 for the treatment of medically refractory partial-onset seizures in patients 12 years or older. It consists of a stimulator that sends electric impulses to the left vagus nerve in the neck via a lead wire that is implanted under the skin. Studies since 1997 have indicated efficacy in generalized seizure disorders and children as well, according to Dr. Elinor Ben-Menachem, professor of neurology and epilepsy at the Institute for Clinical Neurosciences and Physiology, Göteborg (Sweden) University.
To date, more than 60,000 patients worldwide have been treated with VNS, and studies suggest that approximately 50% of patients who undergo the procedure experience a long-term decrease in mean seizure frequency of 50% or more. But fewer than 10% become seizure-free, Dr. Ben-Menachem said during the neurostimulation plenary presentation.
"[VNS] has a long history now, and what we know is that it does not cure or affect seizures immediately. We actually don’t notice a change in seizure activity until about 18 months or 2 years after starting."
For example, a recent long-term follow-up study of VNS patients in the Czech Republic showed that at 1 year post implantation, 44.4% of patients achieved more than 50% seizure reduction. The percentage of patients who reached that level of seizure reduction then increased from 58.7% at 2 years after implant to 64.4% at 5 years. At the 5-year mark, 15.5% of the patients had achieved a minimum 90% seizure reduction, and 5.5% were seizure free (Seizure 2009;18: 269-74).
The mechanism of action of VNS remains uncertain, but a number of possibilities have been suggested, including arousal of the reticular formation; stimulation of locus coeruleus and noradrenaline pathways; changes in a neurotransmitter, amino acid, or neuropeptide; or indirect thalamus stimulation, according to Dr. Ben-Menachem. "It’s also possible that there is long-term learning through synaptic structural changes," she said. "The more I work with this, the more I think it is a learning paradigm. It’s like learning to play the piano. You can’t just sit down and play, you have to redo and redo until the brain is trained."
In a recent study of 144 patients who had undergone VNS implantation, 10 patients were seizure free for more than 1 year post implantation, 89 patients experienced seizure improvement, and no changes were observed in 45 patients. "Stepwise multivariate analysis showed that unilateral interictal epileptiform discharges [IEDs], cortical dysgenesis, and younger age at implantation were independent predictors of seizure freedom in the long-term follow-up," they wrote (Seizure 2010;19: 264-8).
Most of the adverse events associated with VNS therapy, such as hoarseness and cough, tend to be mild and are stimulation related, Dr. Ben-Menachem explained. "Typically, they occur only during stimulation and they generally diminish over time on their own, or they may be diminished or eliminated by adjustment of the parameter settings."
Programmed Deep Brain Stimulation. The programmed deep brain stimulation device manufactured by Medtronic, one of the two emerging neurostimulation treatments for intractable epilepsy that is under FDA review, demonstrated efficacy in a pivotal trial that involved stimulation in the anterior thalamus. This site has connections with the temporal lobe, which is a common site for the origin of partial seizures, Dr. Bergey explained.
The device, which is already approved for Parkinson's disease, comprises two leads* that are implanted bilaterally into the target structure with a pulse generator placed below the clavicle. Each lead contains four electrodes. The device delivers stimuli at scheduled intervals "to hopefully modulate and reduce the number of seizures the patient is having," he said.
In the Medtronic-funded Stimulation of the Anterior Nucleus of the Thalamus for Epilepsy (SANTE) study, 110 patients with medically refractory partial seizures were implanted with the device and randomized to intermittent bilateral stimulation (1 minute on/5 minutes off) or no-stimulation for a 3-month blinded stage, followed by unblinded stimulation for all of the patients (Epilepsia 2010;51:899-908). At the end of the blinded period, patients who received stimulation experienced a median seizure reduction of 40.4%, compared with 14.5% of patients with the stimulator off, reported study coauthor Dr. Vincenta Salanova of Indiana University, Indianapolis.
In the open-label follow-up, 56% of all the patients had greater than 50% seizure reduction at 2 years, and there was a median 68% reduction in seizures among the 42 patients for whom 3-year data were available. Over the course of the study, "14 [12.7%] of the patients were seizure free for at least 6 months," she reported in a press briefing at the meeting.
Although the mechanism of action is not fully understood, Dr. Salanova said that "the thalamus has connections between the limbic system and the frontal lobe, so it’s possible that high-frequency stimulation may prevent the propagation of seizures."
Five deaths occurred in the study population, but none were attributed to lead implantation or stimulation, Dr. Salanova stressed. There were no symptomatic or clinically significant hemorrhages associated with implantation, but 4.5% of patients experienced asymptomatic intracranial hemorrhages – detected via neuroimaging – that were not clinically significant. Additionally, two patients experienced seizures that were linked to the stimulus, which were resolved by lowering the voltage.
Direct stimulation of the hippocampus may also offer seizure relief in some patients, according to Dr. Richard Wennberg of the University of Toronto. "The hippocampus is clinically recognized as a region of high epileptogenicity, and animal studies have demonstrated antiepileptic properties of electrical fields applied to the region," he said in a presentation during the neurostimulation plenary session, noting that the goal of direct hippocampal stimulation is to prevent seizure generation and spread from the temporal limbic region.
To date, the experimental procedure has been evaluated in small series and has shown some efficacy, Dr. Wennberg said. For example, in a recent study designed to assess the effect of continuous electrical stimulation of the hippocampus bilaterally, two patients with seizures from both mesial temporal lobes who were not candidates for surgical resection were implanted bilaterally with two four-contact electrodes along the hippocampal axis. After randomization to either stimulation on or off conditions for 3-month intervals, seizure frequency decreased by 33% during stimulation, and stayed and remained lower by 25% for the 3 months after stimulation was turned off, after which the seizure frequency returned to baseline, the authors reported. Although seizure frequency was reduced both during and for a period after bilateral hippocampal stimulation, "the overall impact in this study is not as robust as has been previously reported," the authors stated (Epilepsia 2010;51:304-7).
Responsive Neurostimulation. Another direct brain neurostimulating technology under FDA review is the Responsive Neurostimulator System (RNS) by NeuroPace. The system detects and aborts spontaneously occurring abnormal discharges* to prevent seizures, explained Dr. Lawrence J. Hirsch of Columbia University in New York. "It is designed to respond within seconds to abnormal activity in the brain by delivering a series of up to five stimuli to terminate the abnormal discharge."
The RNS device is implanted in a recess of the skull, and is connected to up to two four-contact electrodes that are placed within the brain or on the brain surface, depending on where the seizures begin. The device collects and stores seizure information, which the patient subsequently downloads to a laptop using a wand. Physicians can access the stored electrocorticograms via a secure Web page through which they can adjust detection and stimulation parameters specific to the individual patient, Dr. Hirsch said during the neurostimulation plenary session.
In the pivotal clinical trial of the RNS system, 191 patients with medically intractable, partial-onset seizures localized to one or two foci received the cranial implant. During a blinded period, patients received active or sham stimulation, followed by an open-label phase in which all the patients received active stimulation. During the entire blinded evaluation period, active stimulation was associated with a mean 37.9% reduction in seizure frequency, compared with a mean 17% reduction during the sham activation, Dr. Hirsch said.
"In the final month of the blinded period – month 4 to 5 – the respective reduction in seizure frequency was 42% and 9%." During the last 3 months of the open-label period, "47% of the patients had a greater than 50% seizure reduction," he said. "And at 4 years post implant, more than 50% of the patients had at least a 50% reduction in seizure frequency."
A subset analysis showed that neither prior surgery nor the number of seizure foci had an effect on treatment response, Dr. Hirsch noted. "It also showed that [RNS] is possibly more effective with medial temporal onset."
With respect to adverse events, implant site infections were reported in 5% of the patients, and led to explantation in 2%. The combined rate of status epilepticus reported in all trials of the device (256 patients) was 3.5%, and included episodes occurring between 5 months and 5 years post implant. Intracranial hemorrhage was reported in 4% of the patients, and included only one patient with neurological sequelae, which was chronic headache, he said.
The chronic, intracranial EEG recordings provided by the RNS technology have other potentially valuable uses, including seizure prediction/warning; seizure awareness and counting as a way to assess treatment efficacy; identification of circadian, catamenial, and other ictal and interictal patterns; and the lateralization of bitemporal seizures, Dr. Hirsch said.
Dr. Bergey disclosed financial relationships with Pfizer, UCB, and Eli Lilly. Dr. Ben-Menachem disclosed financial relationships with UCB, Eisai, Janssen, Cilag, Cyberonics, Lundbeck, and Sunovion. Dr. Wennberg disclosed a financial relationship with Medtronic. Dr. Hirsch reported having no financial disclosures.
Medtronic's DBS system (top left) stimulates the anterior nucleus of the thalamus, whereas NeuroPace's RNS device (bottom left) responds to abnormal activity in targeted areas and Cyberonics' VNS Therapy System (right) periodically stimulates the left vagus nerve. (Photo Credit: top left: (c) Medtronic Inc., bottom left: (c) NeuroPace Inc., right: (c) Cyberonics Inc.)
* CORRECTION, 12/16/2010: The original version of this article misstated the function of the Responsive Neurostimulator System (RNS) by NeuroPace Inc. The system detects and aborts spontaneously occurring abnormal discharges. Also, the programmed deep brain stimulation device manufactured by Medtronic uses two leads that are placed bilaterally into the anterior thalamus. Each lead contains four electrodes. This version has been updated.
SAN ANTONIO – It’s still too soon to know whether neurostimulation will be the therapeutic advance that treatment-refractory epilepsy patients have been waiting for, but the possibility that it might be has the epilepsy community buzzing.
For approximately 30% of epilepsy patients, seizures cannot be controlled with antiepileptic drugs or surgery. New long-term safety and efficacy data for vagus nerve simulation (VNS) and the results of recent pivotal trials of two approaches to direct brain stimulation offer beacons of hope to these patients, Dr. Gregory K. Bergey said in a plenary session on neurostimulation at the annual meeting of the American Epilepsy Society.
"One of the frustrating things for those of us treating patients with epilepsy has been the fact that, although a number of new antiepileptic drugs have been developed over the past 10-15 years and most are better tolerated and have better pharmacokinetic profiles than earlier drugs, the number of patients with seizures that don’t respond to medical therapy has not been significantly reduced," said Dr. Bergey, director of the Johns Hopkins Epilepsy Center, Baltimore.
"So we’re stepping back and saying, ‘Is there some other way we can treat these patients?’ That has been the impetus for looking at neurostimulation, which has been around for well over a decade, and what we’re seeing is exciting."
Although the 40%-50% response rates observed in direct brain stimulation trials do not appear to be overwhelming, "this is just the beginning," Dr. Bergey stressed in an interview. "As opposed to a drug trial, where you go up to a certain dose and it either works or it doesn’t work, in the case of neurostimulation we don’t know the optimal stimulus parameters, and I think that’s what you’re going to begin to see over the next several years," he said.
"There’s going to be a lot of investigation into neurostimulation of the brain structures to try to figure out who are the best candidates and what the best stimulus parameters are. It’s easy to say we’re stimulating the brain, but do we stimulate 100 times per second, 50 times per second, 25 times per second, and what should the stimulus intensities be?"
Vagus Nerve Stimulation. Currently, Cyberonics’ VNS Therapy System is the only Food and Drug Administration–approved form of neurostimulation for the treatment of epilepsy. The technology was approved in 1997 for the treatment of medically refractory partial-onset seizures in patients 12 years or older. It consists of a stimulator that sends electric impulses to the left vagus nerve in the neck via a lead wire that is implanted under the skin. Studies since 1997 have indicated efficacy in generalized seizure disorders and children as well, according to Dr. Elinor Ben-Menachem, professor of neurology and epilepsy at the Institute for Clinical Neurosciences and Physiology, Göteborg (Sweden) University.
To date, more than 60,000 patients worldwide have been treated with VNS, and studies suggest that approximately 50% of patients who undergo the procedure experience a long-term decrease in mean seizure frequency of 50% or more. But fewer than 10% become seizure-free, Dr. Ben-Menachem said during the neurostimulation plenary presentation.
"[VNS] has a long history now, and what we know is that it does not cure or affect seizures immediately. We actually don’t notice a change in seizure activity until about 18 months or 2 years after starting."
For example, a recent long-term follow-up study of VNS patients in the Czech Republic showed that at 1 year post implantation, 44.4% of patients achieved more than 50% seizure reduction. The percentage of patients who reached that level of seizure reduction then increased from 58.7% at 2 years after implant to 64.4% at 5 years. At the 5-year mark, 15.5% of the patients had achieved a minimum 90% seizure reduction, and 5.5% were seizure free (Seizure 2009;18: 269-74).
The mechanism of action of VNS remains uncertain, but a number of possibilities have been suggested, including arousal of the reticular formation; stimulation of locus coeruleus and noradrenaline pathways; changes in a neurotransmitter, amino acid, or neuropeptide; or indirect thalamus stimulation, according to Dr. Ben-Menachem. "It’s also possible that there is long-term learning through synaptic structural changes," she said. "The more I work with this, the more I think it is a learning paradigm. It’s like learning to play the piano. You can’t just sit down and play, you have to redo and redo until the brain is trained."
In a recent study of 144 patients who had undergone VNS implantation, 10 patients were seizure free for more than 1 year post implantation, 89 patients experienced seizure improvement, and no changes were observed in 45 patients. "Stepwise multivariate analysis showed that unilateral interictal epileptiform discharges [IEDs], cortical dysgenesis, and younger age at implantation were independent predictors of seizure freedom in the long-term follow-up," they wrote (Seizure 2010;19: 264-8).
Most of the adverse events associated with VNS therapy, such as hoarseness and cough, tend to be mild and are stimulation related, Dr. Ben-Menachem explained. "Typically, they occur only during stimulation and they generally diminish over time on their own, or they may be diminished or eliminated by adjustment of the parameter settings."
Programmed Deep Brain Stimulation. The programmed deep brain stimulation device manufactured by Medtronic, one of the two emerging neurostimulation treatments for intractable epilepsy that is under FDA review, demonstrated efficacy in a pivotal trial that involved stimulation in the anterior thalamus. This site has connections with the temporal lobe, which is a common site for the origin of partial seizures, Dr. Bergey explained.
The device, which is already approved for Parkinson's disease, comprises two leads* that are implanted bilaterally into the target structure with a pulse generator placed below the clavicle. Each lead contains four electrodes. The device delivers stimuli at scheduled intervals "to hopefully modulate and reduce the number of seizures the patient is having," he said.
In the Medtronic-funded Stimulation of the Anterior Nucleus of the Thalamus for Epilepsy (SANTE) study, 110 patients with medically refractory partial seizures were implanted with the device and randomized to intermittent bilateral stimulation (1 minute on/5 minutes off) or no-stimulation for a 3-month blinded stage, followed by unblinded stimulation for all of the patients (Epilepsia 2010;51:899-908). At the end of the blinded period, patients who received stimulation experienced a median seizure reduction of 40.4%, compared with 14.5% of patients with the stimulator off, reported study coauthor Dr. Vincenta Salanova of Indiana University, Indianapolis.
In the open-label follow-up, 56% of all the patients had greater than 50% seizure reduction at 2 years, and there was a median 68% reduction in seizures among the 42 patients for whom 3-year data were available. Over the course of the study, "14 [12.7%] of the patients were seizure free for at least 6 months," she reported in a press briefing at the meeting.
Although the mechanism of action is not fully understood, Dr. Salanova said that "the thalamus has connections between the limbic system and the frontal lobe, so it’s possible that high-frequency stimulation may prevent the propagation of seizures."
Five deaths occurred in the study population, but none were attributed to lead implantation or stimulation, Dr. Salanova stressed. There were no symptomatic or clinically significant hemorrhages associated with implantation, but 4.5% of patients experienced asymptomatic intracranial hemorrhages – detected via neuroimaging – that were not clinically significant. Additionally, two patients experienced seizures that were linked to the stimulus, which were resolved by lowering the voltage.
Direct stimulation of the hippocampus may also offer seizure relief in some patients, according to Dr. Richard Wennberg of the University of Toronto. "The hippocampus is clinically recognized as a region of high epileptogenicity, and animal studies have demonstrated antiepileptic properties of electrical fields applied to the region," he said in a presentation during the neurostimulation plenary session, noting that the goal of direct hippocampal stimulation is to prevent seizure generation and spread from the temporal limbic region.
To date, the experimental procedure has been evaluated in small series and has shown some efficacy, Dr. Wennberg said. For example, in a recent study designed to assess the effect of continuous electrical stimulation of the hippocampus bilaterally, two patients with seizures from both mesial temporal lobes who were not candidates for surgical resection were implanted bilaterally with two four-contact electrodes along the hippocampal axis. After randomization to either stimulation on or off conditions for 3-month intervals, seizure frequency decreased by 33% during stimulation, and stayed and remained lower by 25% for the 3 months after stimulation was turned off, after which the seizure frequency returned to baseline, the authors reported. Although seizure frequency was reduced both during and for a period after bilateral hippocampal stimulation, "the overall impact in this study is not as robust as has been previously reported," the authors stated (Epilepsia 2010;51:304-7).
Responsive Neurostimulation. Another direct brain neurostimulating technology under FDA review is the Responsive Neurostimulator System (RNS) by NeuroPace. The system detects and aborts spontaneously occurring abnormal discharges* to prevent seizures, explained Dr. Lawrence J. Hirsch of Columbia University in New York. "It is designed to respond within seconds to abnormal activity in the brain by delivering a series of up to five stimuli to terminate the abnormal discharge."
The RNS device is implanted in a recess of the skull, and is connected to up to two four-contact electrodes that are placed within the brain or on the brain surface, depending on where the seizures begin. The device collects and stores seizure information, which the patient subsequently downloads to a laptop using a wand. Physicians can access the stored electrocorticograms via a secure Web page through which they can adjust detection and stimulation parameters specific to the individual patient, Dr. Hirsch said during the neurostimulation plenary session.
In the pivotal clinical trial of the RNS system, 191 patients with medically intractable, partial-onset seizures localized to one or two foci received the cranial implant. During a blinded period, patients received active or sham stimulation, followed by an open-label phase in which all the patients received active stimulation. During the entire blinded evaluation period, active stimulation was associated with a mean 37.9% reduction in seizure frequency, compared with a mean 17% reduction during the sham activation, Dr. Hirsch said.
"In the final month of the blinded period – month 4 to 5 – the respective reduction in seizure frequency was 42% and 9%." During the last 3 months of the open-label period, "47% of the patients had a greater than 50% seizure reduction," he said. "And at 4 years post implant, more than 50% of the patients had at least a 50% reduction in seizure frequency."
A subset analysis showed that neither prior surgery nor the number of seizure foci had an effect on treatment response, Dr. Hirsch noted. "It also showed that [RNS] is possibly more effective with medial temporal onset."
With respect to adverse events, implant site infections were reported in 5% of the patients, and led to explantation in 2%. The combined rate of status epilepticus reported in all trials of the device (256 patients) was 3.5%, and included episodes occurring between 5 months and 5 years post implant. Intracranial hemorrhage was reported in 4% of the patients, and included only one patient with neurological sequelae, which was chronic headache, he said.
The chronic, intracranial EEG recordings provided by the RNS technology have other potentially valuable uses, including seizure prediction/warning; seizure awareness and counting as a way to assess treatment efficacy; identification of circadian, catamenial, and other ictal and interictal patterns; and the lateralization of bitemporal seizures, Dr. Hirsch said.
Dr. Bergey disclosed financial relationships with Pfizer, UCB, and Eli Lilly. Dr. Ben-Menachem disclosed financial relationships with UCB, Eisai, Janssen, Cilag, Cyberonics, Lundbeck, and Sunovion. Dr. Wennberg disclosed a financial relationship with Medtronic. Dr. Hirsch reported having no financial disclosures.
Medtronic's DBS system (top left) stimulates the anterior nucleus of the thalamus, whereas NeuroPace's RNS device (bottom left) responds to abnormal activity in targeted areas and Cyberonics' VNS Therapy System (right) periodically stimulates the left vagus nerve. (Photo Credit: top left: (c) Medtronic Inc., bottom left: (c) NeuroPace Inc., right: (c) Cyberonics Inc.)
* CORRECTION, 12/16/2010: The original version of this article misstated the function of the Responsive Neurostimulator System (RNS) by NeuroPace Inc. The system detects and aborts spontaneously occurring abnormal discharges. Also, the programmed deep brain stimulation device manufactured by Medtronic uses two leads that are placed bilaterally into the anterior thalamus. Each lead contains four electrodes. This version has been updated.
EXPERT ANALYSIS FROM THE ANNUAL MEETING OF THE AMERICAN EPILEPSY SOCIETY