User login
A relatively new diagnostic tool, the Symptom Intensity Scale, is an easy, quick way to assess both regional pain and fatigue in a patient. It can be used to establish the diagnosis of fibromyalgia syndrome and measure its severity in daily clinical practice without the need to count tender points. It can also be used to detect fibromyalgia as a comorbidity in other clinical illnesses; by uncovering fibromyalgia, the questionnaire serves as a surrogate measure of depression, anxiety, other serious personality disorders, previous or ongoing abuse, and, when fatigue is the dominant symptom, a consideration of obstructive sleep apnea—all part of the pathoetiology of fibromyalgia in that individual.
This manuscript reviews previous criteria and definitions by which fibromyalgia syndrome was recognized, describes how the new questionnaire was developed, and discusses its implications. It is not meant as a review of the pathogenesis or treatment of fibromyalgia or when to send the patient to the rheumatologist. Each of those topics requires lengthy and complex discussions, which are beyond the scope of this paper.
A COMMON, MULTIFACTORIAL DISEASE
The pathoetiology of fibromyalgia syndrome is rooted in disordered sleep, increased stress, and abnormal neurosensory processing, with secondary endocrine and autonomic dysfunction in those who are genetically predisposed.1–4 Because fibromyalgia is multifactorial, it is best understood from the perspective of an inclusive biopsychosocial model rather than a limited biomedical model.5 Its characteristic signs and symptoms are best understood as emanating from a physiologic state, called central sensitization syndrome, in which the nervous system overresponds to stimuli.1,3 This anomalous state of heightened nervous system response is not confined to the peripheral nervous system, but is also present in the autonomic and central nervous systems.3,4
Fibromyalgia syndrome is common, affecting 0.5% to 5% of the general population,6 and is either the second or third most common diagnosis in a rheumatology practice. Importantly for internists, a diagnosis of fibromyalgia syndrome should be made in 10% to 15% of primary care patients.7 The high prevalence alone demands diagnostic recognition.
KNOWN IN HISTORY AND LITERATURE
Although the designation fibromyalgia syndrome is new, the illness has been with us for as long as we’ve been us. In fact, the word rheumatology may have its origin in fibromyalgia syndrome. Galen (about 180 ad) blamed the symptoms of diffuse pain on the “rheuma,” which has been interpreted as “a great fluxion which races [from the center?] to various parts of the body, and goes from one to another.”8 (Is this the origin of blood-letting as a treatment for diseases?) In 1592, the French physician Guillaume de Baillou introduced the term rheumatism to describe both muscle and joint pain.9
Literature also knows fibromyalgia syndrome. Hans Christian Andersen described a supersensitive princess for whom a pea beneath many mattresses was sufficient to ruin her sleep. In The Fall of the House of Usher, Edgar Allan Poe described Roderick Usher as having an “acute bodily illness and mental disorder that oppressed him.” Usher would wear garments of only soft texture because rough cloth was painful. Light hurt his eyes, forcing him to keep the curtains drawn. Although he had previously played and enjoyed violin music, he could no longer tolerate the sound of the violin. In fact, he suffered such hyperacusis that he could hear his sister moving in her grave many floors below. Other stories by Poe such as Rats in the Wall and The Tell-Tale Heart give more evidence that he was well acquainted with the symptoms of central sensitization syndrome.
HOW THE DEFINITION HAS EVOLVED
To recognize fibromyalgia we need an accurate definition, which has evolved over the years. If we don’t know where we’ve been, it is difficult to understand where we are now or how we got here.
Gowers,10 in 1904, was the first to describe diffuse pain as “fibrositis.” He believed that the pain was due to proliferation or inflammation (or both) of subcutaneous and fibrous tissue, a histopathology that has not been satisfactorily demonstrated to this date. Unfortunately for our purposes, his paper was a descriptive essay that made no attempt at codification. In fact, attempts to clinically define and classify fibromyalgia syndrome have been relatively recent.
Hench11 proposed the first clinical definition in 1976, and it probably did more harm than good. His criteria were two: pain, and no physiologic explanation. The diagnosis was therefore made by ruling out everything else rather than by ruling it in by clinical criteria. Consequently, the diagnosing physician had to investigate the symptom or symptoms by ordering potentially limitless testing, which all had to be normal before the diagnosis could be entertained. I continue to see this phenomenon today as new patients with classic fibromyalgia syndrome arrive carrying reports of normal magnetic imaging of the entire body and serologic testing—a “connective tissue disease workup.”
Counting tender points
Smythe (1979)12 was the first to define and classify fibromyalgia syndrome as a rule-in diagnosis. Smythe’s criteria included tender points in at least 12 of 14 anatomic locations using 4 kg of pressure. In practice, the pressure is approximate—the nail bed blanches in a normotensive examiner with a force of 4 kg. He also described four necessary signs and symptoms: diffuse pain of at least 3 months’ duration, disturbed sleep, skin-roll tenderness at the upper trapezius border, and normal results on laboratory tests. He and Moldofsky13 also found a relationship between disordered slow-wave sleep and the symptoms of fibromyalgia syndrome.
Yunus et al (1981)14 compared signs and symptoms in 50 patients with fibromyalgia syndrome and 50 healthy controls to develop criteria for the disease. Of the resulting criteria, two were mandatory: diffuse pain of at least 3 months’ duration and lack of other obvious causes. The definition also required tenderness in at least 5 of 40 tender points and outlined 10 minor criteria.
Signs and factors that modulate fibromyalgia syndrome and that were derived from these minor criteria are still clinically important today. Factors that aggravate pain include cold or humid weather, fatigue, sedentary state, anxiety, and overactivity. Relieving factors include a hot shower, physical activity, warm dry weather, and massage.
The American College of Rheumatology (1990). Understanding that at any one time approximately 15% of the general population experiences widespread pain,15 a committee of the American College of Rheumatology (ACR) set out to differentiate patients with fibromyalgia syndrome from those with less severe widespread pain. The committee compared signs and symptoms in 293 patients deemed by experts to have fibromyalgia syndrome and 265 control patients matched for age, sex, and concomitant rheumatic disorders. 16
The symptom of widespread pain of at least 3 months’ duration and tenderness in at least 11 of 18 points became the ACR’s diagnostic criteria and provided a sensitivity of 88% and a specificity of 81% compared with the experts’ opinion as the gold standard test.
Low specificity is one of the recognized problems with the ACR criteria: 19% of patients with at least 11 tender points did not have fibromyalgia syndrome. In addition, tender points don’t correlate well with some measures of illness activity, such as the Fibromyalgia Impact Questionnaire.17
Is the tender-point count a good measure?
The best argument for continuing to count tender points as part of the clinical evaluation is that it is a measure of severity. Higher numbers of tender points indicate greater psychological distress and greater severity and frequency of other, closely related fibromyalgia symptoms.18,19 Nearly everyone in the general population has at least a few tender points.16 In fibromyalgia syndrome, the tender-point count is a good status surrogate, a measure of the state of the illness.
But should a state/status measure be used as an illness trait and a criterion for diagnosis? I believe not. Consider, as an analogy, the use of the erythrocyte sedimentation rate in patients with rheumatoid arthritis. An elevated sedimentation rate may indicate increased systemic inflammation, but it is a measure of the status of rheumatoid arthritis, not a trait of this disease. This is why I believe that most rheumatologists would disagree with using some value of the erythrocyte sedimentation rate as a criterion for the diagnosis of rheumatoid arthritis and, by analogy, the tender-point count as a criterion for the diagnosis of fibromyalgia syndrome. Also, the number of tender points, a surrogate for diffuse pain, does not fully capture the essence of the illness, in which accompanying fatigue is often severe and nearly always present.20
A CONTEMPORARY DEFINITION AND ITS VALIDATION
As the concept of fibromyalgia syndrome evolved, a movement away from tender points took hold.21
The Manchester criteria22 used a pain diagram to establish the diagnosis, in which the patient indicated the areas of pain on a simple drawing, obviating the need for tender points. It showed good agreement with the ACR criteria, and in fact identified patients with more severe symptoms.
The London Fibromyalgia Epidemiology Study Screening Questionnaire,23 designed as an epidemiologic tool to estimate the prevalence of the syndrome, was the first test to specifically include both pain and fatigue.
White et al,24 in a very important subsequent study, showed that higher fatigue scores differentiated patients with widespread pain and only a few tender points (7–10) from those with more tender points. This report helped to set the stage for the Symptom Intensity Scale.
What the Symptom Intensity Scale measures
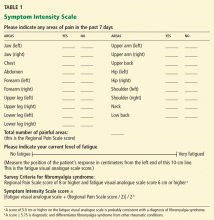
- The Regional Pain Scale score, which is the number of anatomic areas—out of a possible 19—in which the patient feels pain
- A fatigue visual analogue scale score, in which the patient makes a mark somewhere along a 10-cm line to indicate how tired he or she feels. Subsequently, the clinician measures the position of the mark from the left end of the line with a ruler.
How the Symptom Intensity Scale was developed
Wolfe (2003)25 mailed a survey to 12,799 patients who had rheumatoid arthritis, osteoarthritis, or fibromyalgia syndrome. The questionnaire asked respondents if they had pain in 38 articular and nonarticular anatomic regions and to complete a 10-cm fatigue visual analogue scale. He observed that pain in a subset of 19 primarily nonarticular sites differentiated fibromyalgia syndrome from the other two diseases. Calling the number of painful areas of these 19 sites the Regional Pain Scale, he analyzed this measure using Mokken analysis and Rasch analysis to ensure that the questionnaire was statistically valid.
Wolfe also showed that a score of at least 8 points on the Regional Pain Scale, combined with a score of at least 6 cm on the fatigue visual analogue scale, provided the best diagnostic precision consistent with a diagnosis of fibromyalgia syndrome. The combination of these two measures became known as the Survey Criteria.
Katz, Wolfe, and Michaud (2006)26 next compared the diagnostic precision of the Survey Criteria, the ACR criteria, and a physician’s clinical diagnosis. The clinicians made their clinical diagnosis by “considering the long-term patient-clinician experience [including] factors related to pain, tenderness, fatigue, sleep disturbance, comorbidity, and psychosocial variables,” or as I call it, the company fibromyalgia syndrome keeps (Table 2).7,14,16,20 The Survey Criteria (8 points or higher on the Regional Pain Scale plus 6 cm or higher on the fatigue visual analogue scale) showed a roughly 75% concordance among all three definitions in 206 patients with fibromyalgia syndrome. In a cohort with clinically diagnosed fibromyalgia syndrome, a Regional Pain Scale score of 8 or more had a sensitivity of 83.2%, a specificity of 87.6%, and a percent correct of 85.4%. The authors reported that a score of 6 cm or more on the fatigue visual analogue scale “was also at the optimum level” for diagnosing fibromyalgia, but they did not provide more information.
Wolfe and Rasker (2006),27 using these data, devised the Symptom Intensity Scale, the score of which is calculated as the fatigue visual analogue scale score plus half the Regional Pain Scale score, all divided by 2. The scale is therefore a continuous variable rather than a categorical one, and scores can range from 0 to 9.75.
The authors gave the questionnaire to 25,417 patients who had various rheumatic diseases and found that a score of 5.75 or higher differentiated fibromyalgia syndrome from other rheumatic diseases, identifying 95% of patients who would satisfy the Survey Criteria for fibromyalgia.
In addition, they found a linear relationship between the Symptom Intensity Scale score and key symptoms of fibromyalgia syndrome. Of even greater importance, the Symptom Intensity Scale score showed closer association with general health than scores on the Health Assessment Questionnaire, a 27-question patient activity scale, the Arthritis Impact Measurement Scale, or the Short Form-36. It also proved to correlate with mood, probability of having diabetes, need for hospitalization, history of or relative time to myocardial infarction, number of comorbidities, rate of disability, and risk of early death (relative risk 1.12, 95% confidence interval 1.10–1.14). The Symptom Intensity Scale is therefore a diagnostic tool as well as a simple measure of general health among all rheumatic disease patients.
IMPLICATIONS OF THE SYMPTOM INTENSITY SCALE
Three arguments provide a strong rationale for using the Symptom Intensity Scale in the outpatient clinic to investigate the biopsychosocial aspects of illness in our patients:
- It is a simple way to measure overall health
- It can uncover comorbid depression
- It can detect fibromyalgia syndrome in patients who have other diseases.
It measures overall health
Unlike instruments intended for a particular disease such as the Disease Activity Score, which measures disease severity only in rheumatoid arthritis, the Symptom Intensity Scale score can also be used as a measure of global health (or disease severity), and the Survey Criteria (8 or more on the Regional Pain Score and 6 or more on the fatigue visual analogue scale) can be used to establish diagnosis. In fact, instruments like the Disease Activity Score essentially ignore biopsychosocial issues that are captured by the Symptom Intensity Scale.27
By detecting fibromyalgia syndrome in our patients, we identify people with symptoms of pain and distress that do not easily fit the prevalent model of organic disease. Measures like the Disease Activity Score are specifically suited as end points in controlled efficacy trials, but if these are the only measures physicians use to estimate a patient’s health in the clinic, they do so at their own and their patient’s peril.27
Because the continuous Symptom Intensity Scale score strongly correlates with patient-perceived pain, depression, and general health, it is an ideal instrument for outpatient evaluation. It complements a complete patient history and physical examination by measuring biopsychosocial factors.
It uncovers comorbid depression
Rheumatologists do a woeful job of recognizing and diagnosing depression in patients with rheumatoid arthritis. Sleath et al28 found that patients with rheumatoid arthritis who were diagnosed with depression in the office were always the ones who initiated the discussion of that diagnosis. Their doctors did not elicit it.
Middleton et al29 found that patients with concomitant fibromyalgia syndrome and systemic lupus erythematosus (SLE) had higher depression scores than did SLE patients without fibromyalgia syndrome. Moussavi et al,30 writing for the World Health Organization about the findings of a 60-country survey, concluded: “The comorbid state of depression incrementally worsens health compared with depression alone, with any of the chronic diseases alone, and with any combination of chronic diseases without depression.”30
Worse health implies earlier death. Ang et al31 reported that a higher average 4-year depression scale score conferred a hazard ratio of 1.35 (P < .001) for earlier death among 1,290 rheumatoid arthritis patients followed for 12 years.
By using a test like the Symptom Intensity Scale to detect fibromyalgia syndrome alone or to detect it in patients with other diseases, we implicitly recognize the high likelihood of simultaneous depression. Recognition and treatment of depression will improve overall health.
It can detect fibromyalgia syndrome in patients with other diseases
Not surprisingly, distress-related fibromyalgia syndrome is more common in patients with chronic rheumatic or arthritic diseases, with a frequency ranging from 5% in osteoarthritis to 47% in Sjögren syndrome.1 When present, fibromyalgia syndrome changes the features of the other disease.
Wolfe and Michaud32 used the Survey Criteria to evaluate 11,866 rheumatoid arthritis patients and found that 17.1% of them also had fibromyalgia syndrome, and those that did had higher levels of pain, greater global severity, higher scores on the Health Assessment Questionnaire and Short Form-36 mental component, and more disability than those without fibromyalgia syndrome.
Urrows et al33 found that the mean tender-joint count correlated with the mean tenderpoint count in 67 patients with rheumatoid arthritis followed for 75 days. Comorbid fibromyalgia syndrome rendered joints more tender, so that an examiner using the tender-joint count as a major indicator of disease severity might overestimate severity and excessively treat a rheumatoid arthritis patient with unrecognized concurrent fibromyalgia syndrome. Because comorbid fibromyalgia syndrome can inflate Health Assessment Questionnaire scores and subjective pain scale scores in rheumatoid arthritis, more appropriate investigation and management decisions should follow recognition.
Concurrent fibromyalgia syndrome can also be troublesome in SLE. Patients with fibromyalgia syndrome had greater disability than patients without fibromyalgia syndrome despite having no worse SLE damage scores.29 Comorbid fibromyalgia syndrome in SLE has also been shown to diminish quality of life as measured by the Short Form-36.34
Fibromyalgia syndrome also has the potential to confound the diagnosis of concomitant diseases. Wolfe et al35 found that 22.1% of 458 patients with SLE also had fibromyalgia syndrome using the Symptom Intensity Scale criteria. At the time of referral to a rheumatologist, patients who met the criteria for fibromyalgia syndrome were more likely to have self-reported a diagnosis of SLE than were patients for whom SLE had been previously physician-confirmed. The authors warned that fibromyalgia syndrome could intrude into the precision of the diagnosis if only a positive antinuclear antibody test and “soft” SLE criteria were used for diagnosis. If we are unaware of fibromyalgia syndrome, spurious diagnoses may ensue.
BOTTOM LINE
I use the Symptom Intensity Scale as part of routine evaluation in my office. Most patients can complete it with no instruction in 2 minutes or less. I believe it should be used in the clinic to confirm a diagnosis of fibromyalgia syndrome in patients with chronic diffuse pain at rest and to identify comorbid distress in patients with other diseases. This test complements a careful patient history and physical examination, and through its symptom and general health correlations facilitates characterization of our patients’ illnesses in line with the biopsychosocial model.
Since the Symptom Intensity Scale has been shown to be an accurate surrogate measure for general health, depression, disability, and death, fibromyalgia syndrome diagnosed using this instrument implies that this illness is not just centrally mediated pain, but that it carries increased medical risk. It can also be used as a research tool to measure the prevalence of fibromyalgia syndrome in other diseases.
Although the Symptom Intensity Scale is not yet recognized by the ACR as part (or the whole) of the classification criteria for fibromyalgia syndrome, it has already been shown in published studies to be a valid research tool, and it will very likely be the cornerstone of the new criteria.
Goodbye to tender points? Get used to it.
- Wilke WS. The clinical utility of fibromyalgia. J Clin Rheumatol 1999; 5:97–103.
- Cohen H, Buskila D, Neumann L, Ebstein RP. Confirmation of an association between fibromyalgia and serotonin transporter region (5-HTTLPR) polymorphism, and relationship to anxiety-related personality traits. Arthritis Rheum 2002; 46:845–847.
- Geisser ME, Glass JM, Rajcevska LD, et al. A psychophysical study of auditory and pressure sensitivity in patients with fibromyalgia and healthy controls. J Pain 2008; 9:417–422.
- Katz DL, Greene L, Ali A, Faridi Z. The pain of fibromyalgia syndrome is due to muscle hypoperfusion induced by regional vasomotor dysregulation. Med Hypotheses 2007; 69:517–525.
- Engel GL. The need for a new medical model: a challenge for biomedicine. Science 1977; 196:129–136.
- Gran JT. The epidemiology of chronic generalized musculoskeletal pain. Best Pract Res Clin Rheumatol 2003; 17:547–561.
- Wolfe F, Ross K, Anderson J, Russell IJ, Hebert L. The prevalence and characteristics of fibromyalgia in the general population. Arthritis Rheum 1995; 38:19–28.
- Lagier R. Nosology versus pathology, two approaches to rheumatic diseases illustrated by Alfred Baring Garrod and Jean-Martin Charcot. Rheumatology (Oxford) 2001; 40:467–471.
- Ruhman W. The earliest book on rheumatism. Br J Rheumatol 1940; 2:140–162.
- Gowers WR. A lecture on lumbago: its lessons and analogues. Br Med J 1904; 1:117–121.
- Hench PK. Nonarticular rheumatism. Arthritis Rheum 1976; 19(suppl):1081–1088.
- Smythe HA. “Fibrositis” as a disorder of pain modulation. Clin Rheum Dis 1979; 5:823–832.
- Smythe HA, Moldofsky H. Two contributions to understanding of the “fibrositis” syndrome. Bull Rheum Dis 1977–1978; 28:928–931.
- Yunus M, Masi AT, Calabro JJ, Miller KA, Feigenbaum SL. Primary fibromyalgia (fibrositis): clinical study of 50 patients with matched controls. Semin Arthritis Rheum 1981; 11:151–171.
- Croft P, Rigby AS, Boswell R, Schollum J, Silman A. The prevalence of chronic widespread pain in the general population. J Rheumatol 1993; 20:710–713.
- Wolfe F, Smythe HA, Yunus MB, et al. The American College of Rheumatology 1990 Criteria for Classification of Fibromyalgia. Report of the Multicenter Criteria Committee. Arthritis Rheum 1990; 33:160–172.
- McVeigh JG, Finch MB, Hurley DA, Basford JR, Sim J, Baxter GD. Tender point count and total myalgic score in fibromyalgia: changes over a 28-day period. Rheumatol Int 2007; 27:1011–1018.
- Croft P, Schollum J, Silman A. Population study of tender point counts and pain as evidence of fibromyalgia. BMJ 1994; 309:696–699.
- Wolfe F. The relation between tender points and fibromyalgia symptom variables: evidence that fibromyalgia is not a discrete disorder in the clinic. Ann Rheum Dis 1997; 56:268–271.
- Mease PJ, Arnold LM, Crofford LJ, et al. Identifying the clinical domains of fibromyalgia: contributions from clinician and patient Delphi exercises. Arthritis Rheum 2008; 59:952–960.
- Harth M, Nielson WR. The fibromyalgia tender points: use them or lose them? A brief review of the controversy. J Rheumatol 2007; 34:914–922.
- Macfarlane GJ, Croft PR, Schollum J, Silman AJ. Widespread pain: is an improved classification possible? J Rheumatol 1996; 23:1628–1632.
- White KP, Harth M, Speechley M, Ostbye T. Testing an instrument to screen for fibromyalgia syndrome in general population studies: the London Fibromyalgia Epidemiology Study Screening Questionnaire. J Rheumatol 1999; 26:880–884.
- White KP, Speechly M, Harth M, Osbye T. The London Fibromyalgia Epidemiology Study: comparing the demographic and clinical characteristics in 100 random community cases of fibromyalgia versus controls. J Rheumatol 1999; 26:1577–1585.
- Wolfe F. Pain extent and diagnosis: development and validation of the regional pain scale in 12,799 patients with rheumatic disease. J Rheumatolol 2003; 30:369–378.
- Katz RS, Wolfe F, Michaud K. Fibromyalgia diagnosis. a comparison of clinical, survey, and American College of Rheumatology criteria. Arthritis Rheum 2006; 54:169–176.
- Wolfe F, Rasker JJ. The Symptom Intensity Scale, fibromyalgia, and the meaning of fibromyalgia-like symptoms. J Rheumatol 2006; 33:2291–2299.
- Sleath B, Chewning B, De Vellis BM, et al. Communication about depression during rheumatoid arthritis patient visits. Arthritis Rheum 2008; 59:186–191.
- Middleton GD, McFarlin JE, Lippski PE. The prevalence and clinical impact of fibromyalgia in systemic lupus erythematosus. Arthritis Rheum 1994; 37:1181–1188.
- Moussavi S, Chatterji S, Verdes E, Tandon A, Patel V, Ustun B. Depression, chronic diseases, and decrements in health: results from the World Health Surveys. Lancet 2007; 370:851–858.
- Ang DC, Choi H, Kroenke K, Wolfe F. Comorbid depression is an independent risk factor for mortality in patients with rheumatoid arthritis. J Rheumatol 2005; 32:1013–1019.
- Wolfe F, Michaud K. Severe rheumatoid arthritis (RA), worse outcomes, comorbid illness, and sociodemograghic disadvantage characterize RA patients with fibromyalgia. J Rheumatol 2004; 31:695–700.
- Urrows S, Affleck G, Tennen H, Higgins P. Unique clinical and psychological correlates of fibromyalgia tender points and joint tenderness in rheumatoid arthritis. Arthritis Rheum 1994; 37:1513–1520.
- Gladman DD, Urowitz MB, Gough J, MacKinnon A. Fibromyalgia is a major contributor to quality of life in lupus. J Rheumatol 1997; 24:2145–2148.
- Wolfe F, Petri M, Alarcon GS, et al. Fibromyalgia, systemic lupus erythematosus (SLE) and evaluation of SLE activity. J Rheumatol 2009; 36:.27–33.
A relatively new diagnostic tool, the Symptom Intensity Scale, is an easy, quick way to assess both regional pain and fatigue in a patient. It can be used to establish the diagnosis of fibromyalgia syndrome and measure its severity in daily clinical practice without the need to count tender points. It can also be used to detect fibromyalgia as a comorbidity in other clinical illnesses; by uncovering fibromyalgia, the questionnaire serves as a surrogate measure of depression, anxiety, other serious personality disorders, previous or ongoing abuse, and, when fatigue is the dominant symptom, a consideration of obstructive sleep apnea—all part of the pathoetiology of fibromyalgia in that individual.
This manuscript reviews previous criteria and definitions by which fibromyalgia syndrome was recognized, describes how the new questionnaire was developed, and discusses its implications. It is not meant as a review of the pathogenesis or treatment of fibromyalgia or when to send the patient to the rheumatologist. Each of those topics requires lengthy and complex discussions, which are beyond the scope of this paper.
A COMMON, MULTIFACTORIAL DISEASE
The pathoetiology of fibromyalgia syndrome is rooted in disordered sleep, increased stress, and abnormal neurosensory processing, with secondary endocrine and autonomic dysfunction in those who are genetically predisposed.1–4 Because fibromyalgia is multifactorial, it is best understood from the perspective of an inclusive biopsychosocial model rather than a limited biomedical model.5 Its characteristic signs and symptoms are best understood as emanating from a physiologic state, called central sensitization syndrome, in which the nervous system overresponds to stimuli.1,3 This anomalous state of heightened nervous system response is not confined to the peripheral nervous system, but is also present in the autonomic and central nervous systems.3,4
Fibromyalgia syndrome is common, affecting 0.5% to 5% of the general population,6 and is either the second or third most common diagnosis in a rheumatology practice. Importantly for internists, a diagnosis of fibromyalgia syndrome should be made in 10% to 15% of primary care patients.7 The high prevalence alone demands diagnostic recognition.
KNOWN IN HISTORY AND LITERATURE
Although the designation fibromyalgia syndrome is new, the illness has been with us for as long as we’ve been us. In fact, the word rheumatology may have its origin in fibromyalgia syndrome. Galen (about 180 ad) blamed the symptoms of diffuse pain on the “rheuma,” which has been interpreted as “a great fluxion which races [from the center?] to various parts of the body, and goes from one to another.”8 (Is this the origin of blood-letting as a treatment for diseases?) In 1592, the French physician Guillaume de Baillou introduced the term rheumatism to describe both muscle and joint pain.9
Literature also knows fibromyalgia syndrome. Hans Christian Andersen described a supersensitive princess for whom a pea beneath many mattresses was sufficient to ruin her sleep. In The Fall of the House of Usher, Edgar Allan Poe described Roderick Usher as having an “acute bodily illness and mental disorder that oppressed him.” Usher would wear garments of only soft texture because rough cloth was painful. Light hurt his eyes, forcing him to keep the curtains drawn. Although he had previously played and enjoyed violin music, he could no longer tolerate the sound of the violin. In fact, he suffered such hyperacusis that he could hear his sister moving in her grave many floors below. Other stories by Poe such as Rats in the Wall and The Tell-Tale Heart give more evidence that he was well acquainted with the symptoms of central sensitization syndrome.
HOW THE DEFINITION HAS EVOLVED
To recognize fibromyalgia we need an accurate definition, which has evolved over the years. If we don’t know where we’ve been, it is difficult to understand where we are now or how we got here.
Gowers,10 in 1904, was the first to describe diffuse pain as “fibrositis.” He believed that the pain was due to proliferation or inflammation (or both) of subcutaneous and fibrous tissue, a histopathology that has not been satisfactorily demonstrated to this date. Unfortunately for our purposes, his paper was a descriptive essay that made no attempt at codification. In fact, attempts to clinically define and classify fibromyalgia syndrome have been relatively recent.
Hench11 proposed the first clinical definition in 1976, and it probably did more harm than good. His criteria were two: pain, and no physiologic explanation. The diagnosis was therefore made by ruling out everything else rather than by ruling it in by clinical criteria. Consequently, the diagnosing physician had to investigate the symptom or symptoms by ordering potentially limitless testing, which all had to be normal before the diagnosis could be entertained. I continue to see this phenomenon today as new patients with classic fibromyalgia syndrome arrive carrying reports of normal magnetic imaging of the entire body and serologic testing—a “connective tissue disease workup.”
Counting tender points
Smythe (1979)12 was the first to define and classify fibromyalgia syndrome as a rule-in diagnosis. Smythe’s criteria included tender points in at least 12 of 14 anatomic locations using 4 kg of pressure. In practice, the pressure is approximate—the nail bed blanches in a normotensive examiner with a force of 4 kg. He also described four necessary signs and symptoms: diffuse pain of at least 3 months’ duration, disturbed sleep, skin-roll tenderness at the upper trapezius border, and normal results on laboratory tests. He and Moldofsky13 also found a relationship between disordered slow-wave sleep and the symptoms of fibromyalgia syndrome.
Yunus et al (1981)14 compared signs and symptoms in 50 patients with fibromyalgia syndrome and 50 healthy controls to develop criteria for the disease. Of the resulting criteria, two were mandatory: diffuse pain of at least 3 months’ duration and lack of other obvious causes. The definition also required tenderness in at least 5 of 40 tender points and outlined 10 minor criteria.
Signs and factors that modulate fibromyalgia syndrome and that were derived from these minor criteria are still clinically important today. Factors that aggravate pain include cold or humid weather, fatigue, sedentary state, anxiety, and overactivity. Relieving factors include a hot shower, physical activity, warm dry weather, and massage.
The American College of Rheumatology (1990). Understanding that at any one time approximately 15% of the general population experiences widespread pain,15 a committee of the American College of Rheumatology (ACR) set out to differentiate patients with fibromyalgia syndrome from those with less severe widespread pain. The committee compared signs and symptoms in 293 patients deemed by experts to have fibromyalgia syndrome and 265 control patients matched for age, sex, and concomitant rheumatic disorders. 16
The symptom of widespread pain of at least 3 months’ duration and tenderness in at least 11 of 18 points became the ACR’s diagnostic criteria and provided a sensitivity of 88% and a specificity of 81% compared with the experts’ opinion as the gold standard test.
Low specificity is one of the recognized problems with the ACR criteria: 19% of patients with at least 11 tender points did not have fibromyalgia syndrome. In addition, tender points don’t correlate well with some measures of illness activity, such as the Fibromyalgia Impact Questionnaire.17
Is the tender-point count a good measure?
The best argument for continuing to count tender points as part of the clinical evaluation is that it is a measure of severity. Higher numbers of tender points indicate greater psychological distress and greater severity and frequency of other, closely related fibromyalgia symptoms.18,19 Nearly everyone in the general population has at least a few tender points.16 In fibromyalgia syndrome, the tender-point count is a good status surrogate, a measure of the state of the illness.
But should a state/status measure be used as an illness trait and a criterion for diagnosis? I believe not. Consider, as an analogy, the use of the erythrocyte sedimentation rate in patients with rheumatoid arthritis. An elevated sedimentation rate may indicate increased systemic inflammation, but it is a measure of the status of rheumatoid arthritis, not a trait of this disease. This is why I believe that most rheumatologists would disagree with using some value of the erythrocyte sedimentation rate as a criterion for the diagnosis of rheumatoid arthritis and, by analogy, the tender-point count as a criterion for the diagnosis of fibromyalgia syndrome. Also, the number of tender points, a surrogate for diffuse pain, does not fully capture the essence of the illness, in which accompanying fatigue is often severe and nearly always present.20
A CONTEMPORARY DEFINITION AND ITS VALIDATION
As the concept of fibromyalgia syndrome evolved, a movement away from tender points took hold.21
The Manchester criteria22 used a pain diagram to establish the diagnosis, in which the patient indicated the areas of pain on a simple drawing, obviating the need for tender points. It showed good agreement with the ACR criteria, and in fact identified patients with more severe symptoms.
The London Fibromyalgia Epidemiology Study Screening Questionnaire,23 designed as an epidemiologic tool to estimate the prevalence of the syndrome, was the first test to specifically include both pain and fatigue.
White et al,24 in a very important subsequent study, showed that higher fatigue scores differentiated patients with widespread pain and only a few tender points (7–10) from those with more tender points. This report helped to set the stage for the Symptom Intensity Scale.
What the Symptom Intensity Scale measures
- The Regional Pain Scale score, which is the number of anatomic areas—out of a possible 19—in which the patient feels pain
- A fatigue visual analogue scale score, in which the patient makes a mark somewhere along a 10-cm line to indicate how tired he or she feels. Subsequently, the clinician measures the position of the mark from the left end of the line with a ruler.
How the Symptom Intensity Scale was developed
Wolfe (2003)25 mailed a survey to 12,799 patients who had rheumatoid arthritis, osteoarthritis, or fibromyalgia syndrome. The questionnaire asked respondents if they had pain in 38 articular and nonarticular anatomic regions and to complete a 10-cm fatigue visual analogue scale. He observed that pain in a subset of 19 primarily nonarticular sites differentiated fibromyalgia syndrome from the other two diseases. Calling the number of painful areas of these 19 sites the Regional Pain Scale, he analyzed this measure using Mokken analysis and Rasch analysis to ensure that the questionnaire was statistically valid.
Wolfe also showed that a score of at least 8 points on the Regional Pain Scale, combined with a score of at least 6 cm on the fatigue visual analogue scale, provided the best diagnostic precision consistent with a diagnosis of fibromyalgia syndrome. The combination of these two measures became known as the Survey Criteria.
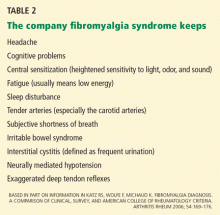
Katz, Wolfe, and Michaud (2006)26 next compared the diagnostic precision of the Survey Criteria, the ACR criteria, and a physician’s clinical diagnosis. The clinicians made their clinical diagnosis by “considering the long-term patient-clinician experience [including] factors related to pain, tenderness, fatigue, sleep disturbance, comorbidity, and psychosocial variables,” or as I call it, the company fibromyalgia syndrome keeps (Table 2).7,14,16,20 The Survey Criteria (8 points or higher on the Regional Pain Scale plus 6 cm or higher on the fatigue visual analogue scale) showed a roughly 75% concordance among all three definitions in 206 patients with fibromyalgia syndrome. In a cohort with clinically diagnosed fibromyalgia syndrome, a Regional Pain Scale score of 8 or more had a sensitivity of 83.2%, a specificity of 87.6%, and a percent correct of 85.4%. The authors reported that a score of 6 cm or more on the fatigue visual analogue scale “was also at the optimum level” for diagnosing fibromyalgia, but they did not provide more information.
Wolfe and Rasker (2006),27 using these data, devised the Symptom Intensity Scale, the score of which is calculated as the fatigue visual analogue scale score plus half the Regional Pain Scale score, all divided by 2. The scale is therefore a continuous variable rather than a categorical one, and scores can range from 0 to 9.75.
The authors gave the questionnaire to 25,417 patients who had various rheumatic diseases and found that a score of 5.75 or higher differentiated fibromyalgia syndrome from other rheumatic diseases, identifying 95% of patients who would satisfy the Survey Criteria for fibromyalgia.
In addition, they found a linear relationship between the Symptom Intensity Scale score and key symptoms of fibromyalgia syndrome. Of even greater importance, the Symptom Intensity Scale score showed closer association with general health than scores on the Health Assessment Questionnaire, a 27-question patient activity scale, the Arthritis Impact Measurement Scale, or the Short Form-36. It also proved to correlate with mood, probability of having diabetes, need for hospitalization, history of or relative time to myocardial infarction, number of comorbidities, rate of disability, and risk of early death (relative risk 1.12, 95% confidence interval 1.10–1.14). The Symptom Intensity Scale is therefore a diagnostic tool as well as a simple measure of general health among all rheumatic disease patients.
IMPLICATIONS OF THE SYMPTOM INTENSITY SCALE
Three arguments provide a strong rationale for using the Symptom Intensity Scale in the outpatient clinic to investigate the biopsychosocial aspects of illness in our patients:
- It is a simple way to measure overall health
- It can uncover comorbid depression
- It can detect fibromyalgia syndrome in patients who have other diseases.
It measures overall health
Unlike instruments intended for a particular disease such as the Disease Activity Score, which measures disease severity only in rheumatoid arthritis, the Symptom Intensity Scale score can also be used as a measure of global health (or disease severity), and the Survey Criteria (8 or more on the Regional Pain Score and 6 or more on the fatigue visual analogue scale) can be used to establish diagnosis. In fact, instruments like the Disease Activity Score essentially ignore biopsychosocial issues that are captured by the Symptom Intensity Scale.27
By detecting fibromyalgia syndrome in our patients, we identify people with symptoms of pain and distress that do not easily fit the prevalent model of organic disease. Measures like the Disease Activity Score are specifically suited as end points in controlled efficacy trials, but if these are the only measures physicians use to estimate a patient’s health in the clinic, they do so at their own and their patient’s peril.27
Because the continuous Symptom Intensity Scale score strongly correlates with patient-perceived pain, depression, and general health, it is an ideal instrument for outpatient evaluation. It complements a complete patient history and physical examination by measuring biopsychosocial factors.
It uncovers comorbid depression
Rheumatologists do a woeful job of recognizing and diagnosing depression in patients with rheumatoid arthritis. Sleath et al28 found that patients with rheumatoid arthritis who were diagnosed with depression in the office were always the ones who initiated the discussion of that diagnosis. Their doctors did not elicit it.
Middleton et al29 found that patients with concomitant fibromyalgia syndrome and systemic lupus erythematosus (SLE) had higher depression scores than did SLE patients without fibromyalgia syndrome. Moussavi et al,30 writing for the World Health Organization about the findings of a 60-country survey, concluded: “The comorbid state of depression incrementally worsens health compared with depression alone, with any of the chronic diseases alone, and with any combination of chronic diseases without depression.”30
Worse health implies earlier death. Ang et al31 reported that a higher average 4-year depression scale score conferred a hazard ratio of 1.35 (P < .001) for earlier death among 1,290 rheumatoid arthritis patients followed for 12 years.
By using a test like the Symptom Intensity Scale to detect fibromyalgia syndrome alone or to detect it in patients with other diseases, we implicitly recognize the high likelihood of simultaneous depression. Recognition and treatment of depression will improve overall health.
It can detect fibromyalgia syndrome in patients with other diseases
Not surprisingly, distress-related fibromyalgia syndrome is more common in patients with chronic rheumatic or arthritic diseases, with a frequency ranging from 5% in osteoarthritis to 47% in Sjögren syndrome.1 When present, fibromyalgia syndrome changes the features of the other disease.
Wolfe and Michaud32 used the Survey Criteria to evaluate 11,866 rheumatoid arthritis patients and found that 17.1% of them also had fibromyalgia syndrome, and those that did had higher levels of pain, greater global severity, higher scores on the Health Assessment Questionnaire and Short Form-36 mental component, and more disability than those without fibromyalgia syndrome.
Urrows et al33 found that the mean tender-joint count correlated with the mean tenderpoint count in 67 patients with rheumatoid arthritis followed for 75 days. Comorbid fibromyalgia syndrome rendered joints more tender, so that an examiner using the tender-joint count as a major indicator of disease severity might overestimate severity and excessively treat a rheumatoid arthritis patient with unrecognized concurrent fibromyalgia syndrome. Because comorbid fibromyalgia syndrome can inflate Health Assessment Questionnaire scores and subjective pain scale scores in rheumatoid arthritis, more appropriate investigation and management decisions should follow recognition.
Concurrent fibromyalgia syndrome can also be troublesome in SLE. Patients with fibromyalgia syndrome had greater disability than patients without fibromyalgia syndrome despite having no worse SLE damage scores.29 Comorbid fibromyalgia syndrome in SLE has also been shown to diminish quality of life as measured by the Short Form-36.34
Fibromyalgia syndrome also has the potential to confound the diagnosis of concomitant diseases. Wolfe et al35 found that 22.1% of 458 patients with SLE also had fibromyalgia syndrome using the Symptom Intensity Scale criteria. At the time of referral to a rheumatologist, patients who met the criteria for fibromyalgia syndrome were more likely to have self-reported a diagnosis of SLE than were patients for whom SLE had been previously physician-confirmed. The authors warned that fibromyalgia syndrome could intrude into the precision of the diagnosis if only a positive antinuclear antibody test and “soft” SLE criteria were used for diagnosis. If we are unaware of fibromyalgia syndrome, spurious diagnoses may ensue.
BOTTOM LINE
I use the Symptom Intensity Scale as part of routine evaluation in my office. Most patients can complete it with no instruction in 2 minutes or less. I believe it should be used in the clinic to confirm a diagnosis of fibromyalgia syndrome in patients with chronic diffuse pain at rest and to identify comorbid distress in patients with other diseases. This test complements a careful patient history and physical examination, and through its symptom and general health correlations facilitates characterization of our patients’ illnesses in line with the biopsychosocial model.
Since the Symptom Intensity Scale has been shown to be an accurate surrogate measure for general health, depression, disability, and death, fibromyalgia syndrome diagnosed using this instrument implies that this illness is not just centrally mediated pain, but that it carries increased medical risk. It can also be used as a research tool to measure the prevalence of fibromyalgia syndrome in other diseases.
Although the Symptom Intensity Scale is not yet recognized by the ACR as part (or the whole) of the classification criteria for fibromyalgia syndrome, it has already been shown in published studies to be a valid research tool, and it will very likely be the cornerstone of the new criteria.
Goodbye to tender points? Get used to it.
A relatively new diagnostic tool, the Symptom Intensity Scale, is an easy, quick way to assess both regional pain and fatigue in a patient. It can be used to establish the diagnosis of fibromyalgia syndrome and measure its severity in daily clinical practice without the need to count tender points. It can also be used to detect fibromyalgia as a comorbidity in other clinical illnesses; by uncovering fibromyalgia, the questionnaire serves as a surrogate measure of depression, anxiety, other serious personality disorders, previous or ongoing abuse, and, when fatigue is the dominant symptom, a consideration of obstructive sleep apnea—all part of the pathoetiology of fibromyalgia in that individual.
This manuscript reviews previous criteria and definitions by which fibromyalgia syndrome was recognized, describes how the new questionnaire was developed, and discusses its implications. It is not meant as a review of the pathogenesis or treatment of fibromyalgia or when to send the patient to the rheumatologist. Each of those topics requires lengthy and complex discussions, which are beyond the scope of this paper.
A COMMON, MULTIFACTORIAL DISEASE
The pathoetiology of fibromyalgia syndrome is rooted in disordered sleep, increased stress, and abnormal neurosensory processing, with secondary endocrine and autonomic dysfunction in those who are genetically predisposed.1–4 Because fibromyalgia is multifactorial, it is best understood from the perspective of an inclusive biopsychosocial model rather than a limited biomedical model.5 Its characteristic signs and symptoms are best understood as emanating from a physiologic state, called central sensitization syndrome, in which the nervous system overresponds to stimuli.1,3 This anomalous state of heightened nervous system response is not confined to the peripheral nervous system, but is also present in the autonomic and central nervous systems.3,4
Fibromyalgia syndrome is common, affecting 0.5% to 5% of the general population,6 and is either the second or third most common diagnosis in a rheumatology practice. Importantly for internists, a diagnosis of fibromyalgia syndrome should be made in 10% to 15% of primary care patients.7 The high prevalence alone demands diagnostic recognition.
KNOWN IN HISTORY AND LITERATURE
Although the designation fibromyalgia syndrome is new, the illness has been with us for as long as we’ve been us. In fact, the word rheumatology may have its origin in fibromyalgia syndrome. Galen (about 180 ad) blamed the symptoms of diffuse pain on the “rheuma,” which has been interpreted as “a great fluxion which races [from the center?] to various parts of the body, and goes from one to another.”8 (Is this the origin of blood-letting as a treatment for diseases?) In 1592, the French physician Guillaume de Baillou introduced the term rheumatism to describe both muscle and joint pain.9
Literature also knows fibromyalgia syndrome. Hans Christian Andersen described a supersensitive princess for whom a pea beneath many mattresses was sufficient to ruin her sleep. In The Fall of the House of Usher, Edgar Allan Poe described Roderick Usher as having an “acute bodily illness and mental disorder that oppressed him.” Usher would wear garments of only soft texture because rough cloth was painful. Light hurt his eyes, forcing him to keep the curtains drawn. Although he had previously played and enjoyed violin music, he could no longer tolerate the sound of the violin. In fact, he suffered such hyperacusis that he could hear his sister moving in her grave many floors below. Other stories by Poe such as Rats in the Wall and The Tell-Tale Heart give more evidence that he was well acquainted with the symptoms of central sensitization syndrome.
HOW THE DEFINITION HAS EVOLVED
To recognize fibromyalgia we need an accurate definition, which has evolved over the years. If we don’t know where we’ve been, it is difficult to understand where we are now or how we got here.
Gowers,10 in 1904, was the first to describe diffuse pain as “fibrositis.” He believed that the pain was due to proliferation or inflammation (or both) of subcutaneous and fibrous tissue, a histopathology that has not been satisfactorily demonstrated to this date. Unfortunately for our purposes, his paper was a descriptive essay that made no attempt at codification. In fact, attempts to clinically define and classify fibromyalgia syndrome have been relatively recent.
Hench11 proposed the first clinical definition in 1976, and it probably did more harm than good. His criteria were two: pain, and no physiologic explanation. The diagnosis was therefore made by ruling out everything else rather than by ruling it in by clinical criteria. Consequently, the diagnosing physician had to investigate the symptom or symptoms by ordering potentially limitless testing, which all had to be normal before the diagnosis could be entertained. I continue to see this phenomenon today as new patients with classic fibromyalgia syndrome arrive carrying reports of normal magnetic imaging of the entire body and serologic testing—a “connective tissue disease workup.”
Counting tender points
Smythe (1979)12 was the first to define and classify fibromyalgia syndrome as a rule-in diagnosis. Smythe’s criteria included tender points in at least 12 of 14 anatomic locations using 4 kg of pressure. In practice, the pressure is approximate—the nail bed blanches in a normotensive examiner with a force of 4 kg. He also described four necessary signs and symptoms: diffuse pain of at least 3 months’ duration, disturbed sleep, skin-roll tenderness at the upper trapezius border, and normal results on laboratory tests. He and Moldofsky13 also found a relationship between disordered slow-wave sleep and the symptoms of fibromyalgia syndrome.
Yunus et al (1981)14 compared signs and symptoms in 50 patients with fibromyalgia syndrome and 50 healthy controls to develop criteria for the disease. Of the resulting criteria, two were mandatory: diffuse pain of at least 3 months’ duration and lack of other obvious causes. The definition also required tenderness in at least 5 of 40 tender points and outlined 10 minor criteria.
Signs and factors that modulate fibromyalgia syndrome and that were derived from these minor criteria are still clinically important today. Factors that aggravate pain include cold or humid weather, fatigue, sedentary state, anxiety, and overactivity. Relieving factors include a hot shower, physical activity, warm dry weather, and massage.
The American College of Rheumatology (1990). Understanding that at any one time approximately 15% of the general population experiences widespread pain,15 a committee of the American College of Rheumatology (ACR) set out to differentiate patients with fibromyalgia syndrome from those with less severe widespread pain. The committee compared signs and symptoms in 293 patients deemed by experts to have fibromyalgia syndrome and 265 control patients matched for age, sex, and concomitant rheumatic disorders. 16
The symptom of widespread pain of at least 3 months’ duration and tenderness in at least 11 of 18 points became the ACR’s diagnostic criteria and provided a sensitivity of 88% and a specificity of 81% compared with the experts’ opinion as the gold standard test.
Low specificity is one of the recognized problems with the ACR criteria: 19% of patients with at least 11 tender points did not have fibromyalgia syndrome. In addition, tender points don’t correlate well with some measures of illness activity, such as the Fibromyalgia Impact Questionnaire.17
Is the tender-point count a good measure?
The best argument for continuing to count tender points as part of the clinical evaluation is that it is a measure of severity. Higher numbers of tender points indicate greater psychological distress and greater severity and frequency of other, closely related fibromyalgia symptoms.18,19 Nearly everyone in the general population has at least a few tender points.16 In fibromyalgia syndrome, the tender-point count is a good status surrogate, a measure of the state of the illness.
But should a state/status measure be used as an illness trait and a criterion for diagnosis? I believe not. Consider, as an analogy, the use of the erythrocyte sedimentation rate in patients with rheumatoid arthritis. An elevated sedimentation rate may indicate increased systemic inflammation, but it is a measure of the status of rheumatoid arthritis, not a trait of this disease. This is why I believe that most rheumatologists would disagree with using some value of the erythrocyte sedimentation rate as a criterion for the diagnosis of rheumatoid arthritis and, by analogy, the tender-point count as a criterion for the diagnosis of fibromyalgia syndrome. Also, the number of tender points, a surrogate for diffuse pain, does not fully capture the essence of the illness, in which accompanying fatigue is often severe and nearly always present.20
A CONTEMPORARY DEFINITION AND ITS VALIDATION
As the concept of fibromyalgia syndrome evolved, a movement away from tender points took hold.21
The Manchester criteria22 used a pain diagram to establish the diagnosis, in which the patient indicated the areas of pain on a simple drawing, obviating the need for tender points. It showed good agreement with the ACR criteria, and in fact identified patients with more severe symptoms.
The London Fibromyalgia Epidemiology Study Screening Questionnaire,23 designed as an epidemiologic tool to estimate the prevalence of the syndrome, was the first test to specifically include both pain and fatigue.
White et al,24 in a very important subsequent study, showed that higher fatigue scores differentiated patients with widespread pain and only a few tender points (7–10) from those with more tender points. This report helped to set the stage for the Symptom Intensity Scale.
What the Symptom Intensity Scale measures
- The Regional Pain Scale score, which is the number of anatomic areas—out of a possible 19—in which the patient feels pain
- A fatigue visual analogue scale score, in which the patient makes a mark somewhere along a 10-cm line to indicate how tired he or she feels. Subsequently, the clinician measures the position of the mark from the left end of the line with a ruler.
How the Symptom Intensity Scale was developed
Wolfe (2003)25 mailed a survey to 12,799 patients who had rheumatoid arthritis, osteoarthritis, or fibromyalgia syndrome. The questionnaire asked respondents if they had pain in 38 articular and nonarticular anatomic regions and to complete a 10-cm fatigue visual analogue scale. He observed that pain in a subset of 19 primarily nonarticular sites differentiated fibromyalgia syndrome from the other two diseases. Calling the number of painful areas of these 19 sites the Regional Pain Scale, he analyzed this measure using Mokken analysis and Rasch analysis to ensure that the questionnaire was statistically valid.
Wolfe also showed that a score of at least 8 points on the Regional Pain Scale, combined with a score of at least 6 cm on the fatigue visual analogue scale, provided the best diagnostic precision consistent with a diagnosis of fibromyalgia syndrome. The combination of these two measures became known as the Survey Criteria.
Katz, Wolfe, and Michaud (2006)26 next compared the diagnostic precision of the Survey Criteria, the ACR criteria, and a physician’s clinical diagnosis. The clinicians made their clinical diagnosis by “considering the long-term patient-clinician experience [including] factors related to pain, tenderness, fatigue, sleep disturbance, comorbidity, and psychosocial variables,” or as I call it, the company fibromyalgia syndrome keeps (Table 2).7,14,16,20 The Survey Criteria (8 points or higher on the Regional Pain Scale plus 6 cm or higher on the fatigue visual analogue scale) showed a roughly 75% concordance among all three definitions in 206 patients with fibromyalgia syndrome. In a cohort with clinically diagnosed fibromyalgia syndrome, a Regional Pain Scale score of 8 or more had a sensitivity of 83.2%, a specificity of 87.6%, and a percent correct of 85.4%. The authors reported that a score of 6 cm or more on the fatigue visual analogue scale “was also at the optimum level” for diagnosing fibromyalgia, but they did not provide more information.
Wolfe and Rasker (2006),27 using these data, devised the Symptom Intensity Scale, the score of which is calculated as the fatigue visual analogue scale score plus half the Regional Pain Scale score, all divided by 2. The scale is therefore a continuous variable rather than a categorical one, and scores can range from 0 to 9.75.
The authors gave the questionnaire to 25,417 patients who had various rheumatic diseases and found that a score of 5.75 or higher differentiated fibromyalgia syndrome from other rheumatic diseases, identifying 95% of patients who would satisfy the Survey Criteria for fibromyalgia.
In addition, they found a linear relationship between the Symptom Intensity Scale score and key symptoms of fibromyalgia syndrome. Of even greater importance, the Symptom Intensity Scale score showed closer association with general health than scores on the Health Assessment Questionnaire, a 27-question patient activity scale, the Arthritis Impact Measurement Scale, or the Short Form-36. It also proved to correlate with mood, probability of having diabetes, need for hospitalization, history of or relative time to myocardial infarction, number of comorbidities, rate of disability, and risk of early death (relative risk 1.12, 95% confidence interval 1.10–1.14). The Symptom Intensity Scale is therefore a diagnostic tool as well as a simple measure of general health among all rheumatic disease patients.
IMPLICATIONS OF THE SYMPTOM INTENSITY SCALE
Three arguments provide a strong rationale for using the Symptom Intensity Scale in the outpatient clinic to investigate the biopsychosocial aspects of illness in our patients:
- It is a simple way to measure overall health
- It can uncover comorbid depression
- It can detect fibromyalgia syndrome in patients who have other diseases.
It measures overall health
Unlike instruments intended for a particular disease such as the Disease Activity Score, which measures disease severity only in rheumatoid arthritis, the Symptom Intensity Scale score can also be used as a measure of global health (or disease severity), and the Survey Criteria (8 or more on the Regional Pain Score and 6 or more on the fatigue visual analogue scale) can be used to establish diagnosis. In fact, instruments like the Disease Activity Score essentially ignore biopsychosocial issues that are captured by the Symptom Intensity Scale.27
By detecting fibromyalgia syndrome in our patients, we identify people with symptoms of pain and distress that do not easily fit the prevalent model of organic disease. Measures like the Disease Activity Score are specifically suited as end points in controlled efficacy trials, but if these are the only measures physicians use to estimate a patient’s health in the clinic, they do so at their own and their patient’s peril.27
Because the continuous Symptom Intensity Scale score strongly correlates with patient-perceived pain, depression, and general health, it is an ideal instrument for outpatient evaluation. It complements a complete patient history and physical examination by measuring biopsychosocial factors.
It uncovers comorbid depression
Rheumatologists do a woeful job of recognizing and diagnosing depression in patients with rheumatoid arthritis. Sleath et al28 found that patients with rheumatoid arthritis who were diagnosed with depression in the office were always the ones who initiated the discussion of that diagnosis. Their doctors did not elicit it.
Middleton et al29 found that patients with concomitant fibromyalgia syndrome and systemic lupus erythematosus (SLE) had higher depression scores than did SLE patients without fibromyalgia syndrome. Moussavi et al,30 writing for the World Health Organization about the findings of a 60-country survey, concluded: “The comorbid state of depression incrementally worsens health compared with depression alone, with any of the chronic diseases alone, and with any combination of chronic diseases without depression.”30
Worse health implies earlier death. Ang et al31 reported that a higher average 4-year depression scale score conferred a hazard ratio of 1.35 (P < .001) for earlier death among 1,290 rheumatoid arthritis patients followed for 12 years.
By using a test like the Symptom Intensity Scale to detect fibromyalgia syndrome alone or to detect it in patients with other diseases, we implicitly recognize the high likelihood of simultaneous depression. Recognition and treatment of depression will improve overall health.
It can detect fibromyalgia syndrome in patients with other diseases
Not surprisingly, distress-related fibromyalgia syndrome is more common in patients with chronic rheumatic or arthritic diseases, with a frequency ranging from 5% in osteoarthritis to 47% in Sjögren syndrome.1 When present, fibromyalgia syndrome changes the features of the other disease.
Wolfe and Michaud32 used the Survey Criteria to evaluate 11,866 rheumatoid arthritis patients and found that 17.1% of them also had fibromyalgia syndrome, and those that did had higher levels of pain, greater global severity, higher scores on the Health Assessment Questionnaire and Short Form-36 mental component, and more disability than those without fibromyalgia syndrome.
Urrows et al33 found that the mean tender-joint count correlated with the mean tenderpoint count in 67 patients with rheumatoid arthritis followed for 75 days. Comorbid fibromyalgia syndrome rendered joints more tender, so that an examiner using the tender-joint count as a major indicator of disease severity might overestimate severity and excessively treat a rheumatoid arthritis patient with unrecognized concurrent fibromyalgia syndrome. Because comorbid fibromyalgia syndrome can inflate Health Assessment Questionnaire scores and subjective pain scale scores in rheumatoid arthritis, more appropriate investigation and management decisions should follow recognition.
Concurrent fibromyalgia syndrome can also be troublesome in SLE. Patients with fibromyalgia syndrome had greater disability than patients without fibromyalgia syndrome despite having no worse SLE damage scores.29 Comorbid fibromyalgia syndrome in SLE has also been shown to diminish quality of life as measured by the Short Form-36.34
Fibromyalgia syndrome also has the potential to confound the diagnosis of concomitant diseases. Wolfe et al35 found that 22.1% of 458 patients with SLE also had fibromyalgia syndrome using the Symptom Intensity Scale criteria. At the time of referral to a rheumatologist, patients who met the criteria for fibromyalgia syndrome were more likely to have self-reported a diagnosis of SLE than were patients for whom SLE had been previously physician-confirmed. The authors warned that fibromyalgia syndrome could intrude into the precision of the diagnosis if only a positive antinuclear antibody test and “soft” SLE criteria were used for diagnosis. If we are unaware of fibromyalgia syndrome, spurious diagnoses may ensue.
BOTTOM LINE
I use the Symptom Intensity Scale as part of routine evaluation in my office. Most patients can complete it with no instruction in 2 minutes or less. I believe it should be used in the clinic to confirm a diagnosis of fibromyalgia syndrome in patients with chronic diffuse pain at rest and to identify comorbid distress in patients with other diseases. This test complements a careful patient history and physical examination, and through its symptom and general health correlations facilitates characterization of our patients’ illnesses in line with the biopsychosocial model.
Since the Symptom Intensity Scale has been shown to be an accurate surrogate measure for general health, depression, disability, and death, fibromyalgia syndrome diagnosed using this instrument implies that this illness is not just centrally mediated pain, but that it carries increased medical risk. It can also be used as a research tool to measure the prevalence of fibromyalgia syndrome in other diseases.
Although the Symptom Intensity Scale is not yet recognized by the ACR as part (or the whole) of the classification criteria for fibromyalgia syndrome, it has already been shown in published studies to be a valid research tool, and it will very likely be the cornerstone of the new criteria.
Goodbye to tender points? Get used to it.
- Wilke WS. The clinical utility of fibromyalgia. J Clin Rheumatol 1999; 5:97–103.
- Cohen H, Buskila D, Neumann L, Ebstein RP. Confirmation of an association between fibromyalgia and serotonin transporter region (5-HTTLPR) polymorphism, and relationship to anxiety-related personality traits. Arthritis Rheum 2002; 46:845–847.
- Geisser ME, Glass JM, Rajcevska LD, et al. A psychophysical study of auditory and pressure sensitivity in patients with fibromyalgia and healthy controls. J Pain 2008; 9:417–422.
- Katz DL, Greene L, Ali A, Faridi Z. The pain of fibromyalgia syndrome is due to muscle hypoperfusion induced by regional vasomotor dysregulation. Med Hypotheses 2007; 69:517–525.
- Engel GL. The need for a new medical model: a challenge for biomedicine. Science 1977; 196:129–136.
- Gran JT. The epidemiology of chronic generalized musculoskeletal pain. Best Pract Res Clin Rheumatol 2003; 17:547–561.
- Wolfe F, Ross K, Anderson J, Russell IJ, Hebert L. The prevalence and characteristics of fibromyalgia in the general population. Arthritis Rheum 1995; 38:19–28.
- Lagier R. Nosology versus pathology, two approaches to rheumatic diseases illustrated by Alfred Baring Garrod and Jean-Martin Charcot. Rheumatology (Oxford) 2001; 40:467–471.
- Ruhman W. The earliest book on rheumatism. Br J Rheumatol 1940; 2:140–162.
- Gowers WR. A lecture on lumbago: its lessons and analogues. Br Med J 1904; 1:117–121.
- Hench PK. Nonarticular rheumatism. Arthritis Rheum 1976; 19(suppl):1081–1088.
- Smythe HA. “Fibrositis” as a disorder of pain modulation. Clin Rheum Dis 1979; 5:823–832.
- Smythe HA, Moldofsky H. Two contributions to understanding of the “fibrositis” syndrome. Bull Rheum Dis 1977–1978; 28:928–931.
- Yunus M, Masi AT, Calabro JJ, Miller KA, Feigenbaum SL. Primary fibromyalgia (fibrositis): clinical study of 50 patients with matched controls. Semin Arthritis Rheum 1981; 11:151–171.
- Croft P, Rigby AS, Boswell R, Schollum J, Silman A. The prevalence of chronic widespread pain in the general population. J Rheumatol 1993; 20:710–713.
- Wolfe F, Smythe HA, Yunus MB, et al. The American College of Rheumatology 1990 Criteria for Classification of Fibromyalgia. Report of the Multicenter Criteria Committee. Arthritis Rheum 1990; 33:160–172.
- McVeigh JG, Finch MB, Hurley DA, Basford JR, Sim J, Baxter GD. Tender point count and total myalgic score in fibromyalgia: changes over a 28-day period. Rheumatol Int 2007; 27:1011–1018.
- Croft P, Schollum J, Silman A. Population study of tender point counts and pain as evidence of fibromyalgia. BMJ 1994; 309:696–699.
- Wolfe F. The relation between tender points and fibromyalgia symptom variables: evidence that fibromyalgia is not a discrete disorder in the clinic. Ann Rheum Dis 1997; 56:268–271.
- Mease PJ, Arnold LM, Crofford LJ, et al. Identifying the clinical domains of fibromyalgia: contributions from clinician and patient Delphi exercises. Arthritis Rheum 2008; 59:952–960.
- Harth M, Nielson WR. The fibromyalgia tender points: use them or lose them? A brief review of the controversy. J Rheumatol 2007; 34:914–922.
- Macfarlane GJ, Croft PR, Schollum J, Silman AJ. Widespread pain: is an improved classification possible? J Rheumatol 1996; 23:1628–1632.
- White KP, Harth M, Speechley M, Ostbye T. Testing an instrument to screen for fibromyalgia syndrome in general population studies: the London Fibromyalgia Epidemiology Study Screening Questionnaire. J Rheumatol 1999; 26:880–884.
- White KP, Speechly M, Harth M, Osbye T. The London Fibromyalgia Epidemiology Study: comparing the demographic and clinical characteristics in 100 random community cases of fibromyalgia versus controls. J Rheumatol 1999; 26:1577–1585.
- Wolfe F. Pain extent and diagnosis: development and validation of the regional pain scale in 12,799 patients with rheumatic disease. J Rheumatolol 2003; 30:369–378.
- Katz RS, Wolfe F, Michaud K. Fibromyalgia diagnosis. a comparison of clinical, survey, and American College of Rheumatology criteria. Arthritis Rheum 2006; 54:169–176.
- Wolfe F, Rasker JJ. The Symptom Intensity Scale, fibromyalgia, and the meaning of fibromyalgia-like symptoms. J Rheumatol 2006; 33:2291–2299.
- Sleath B, Chewning B, De Vellis BM, et al. Communication about depression during rheumatoid arthritis patient visits. Arthritis Rheum 2008; 59:186–191.
- Middleton GD, McFarlin JE, Lippski PE. The prevalence and clinical impact of fibromyalgia in systemic lupus erythematosus. Arthritis Rheum 1994; 37:1181–1188.
- Moussavi S, Chatterji S, Verdes E, Tandon A, Patel V, Ustun B. Depression, chronic diseases, and decrements in health: results from the World Health Surveys. Lancet 2007; 370:851–858.
- Ang DC, Choi H, Kroenke K, Wolfe F. Comorbid depression is an independent risk factor for mortality in patients with rheumatoid arthritis. J Rheumatol 2005; 32:1013–1019.
- Wolfe F, Michaud K. Severe rheumatoid arthritis (RA), worse outcomes, comorbid illness, and sociodemograghic disadvantage characterize RA patients with fibromyalgia. J Rheumatol 2004; 31:695–700.
- Urrows S, Affleck G, Tennen H, Higgins P. Unique clinical and psychological correlates of fibromyalgia tender points and joint tenderness in rheumatoid arthritis. Arthritis Rheum 1994; 37:1513–1520.
- Gladman DD, Urowitz MB, Gough J, MacKinnon A. Fibromyalgia is a major contributor to quality of life in lupus. J Rheumatol 1997; 24:2145–2148.
- Wolfe F, Petri M, Alarcon GS, et al. Fibromyalgia, systemic lupus erythematosus (SLE) and evaluation of SLE activity. J Rheumatol 2009; 36:.27–33.
- Wilke WS. The clinical utility of fibromyalgia. J Clin Rheumatol 1999; 5:97–103.
- Cohen H, Buskila D, Neumann L, Ebstein RP. Confirmation of an association between fibromyalgia and serotonin transporter region (5-HTTLPR) polymorphism, and relationship to anxiety-related personality traits. Arthritis Rheum 2002; 46:845–847.
- Geisser ME, Glass JM, Rajcevska LD, et al. A psychophysical study of auditory and pressure sensitivity in patients with fibromyalgia and healthy controls. J Pain 2008; 9:417–422.
- Katz DL, Greene L, Ali A, Faridi Z. The pain of fibromyalgia syndrome is due to muscle hypoperfusion induced by regional vasomotor dysregulation. Med Hypotheses 2007; 69:517–525.
- Engel GL. The need for a new medical model: a challenge for biomedicine. Science 1977; 196:129–136.
- Gran JT. The epidemiology of chronic generalized musculoskeletal pain. Best Pract Res Clin Rheumatol 2003; 17:547–561.
- Wolfe F, Ross K, Anderson J, Russell IJ, Hebert L. The prevalence and characteristics of fibromyalgia in the general population. Arthritis Rheum 1995; 38:19–28.
- Lagier R. Nosology versus pathology, two approaches to rheumatic diseases illustrated by Alfred Baring Garrod and Jean-Martin Charcot. Rheumatology (Oxford) 2001; 40:467–471.
- Ruhman W. The earliest book on rheumatism. Br J Rheumatol 1940; 2:140–162.
- Gowers WR. A lecture on lumbago: its lessons and analogues. Br Med J 1904; 1:117–121.
- Hench PK. Nonarticular rheumatism. Arthritis Rheum 1976; 19(suppl):1081–1088.
- Smythe HA. “Fibrositis” as a disorder of pain modulation. Clin Rheum Dis 1979; 5:823–832.
- Smythe HA, Moldofsky H. Two contributions to understanding of the “fibrositis” syndrome. Bull Rheum Dis 1977–1978; 28:928–931.
- Yunus M, Masi AT, Calabro JJ, Miller KA, Feigenbaum SL. Primary fibromyalgia (fibrositis): clinical study of 50 patients with matched controls. Semin Arthritis Rheum 1981; 11:151–171.
- Croft P, Rigby AS, Boswell R, Schollum J, Silman A. The prevalence of chronic widespread pain in the general population. J Rheumatol 1993; 20:710–713.
- Wolfe F, Smythe HA, Yunus MB, et al. The American College of Rheumatology 1990 Criteria for Classification of Fibromyalgia. Report of the Multicenter Criteria Committee. Arthritis Rheum 1990; 33:160–172.
- McVeigh JG, Finch MB, Hurley DA, Basford JR, Sim J, Baxter GD. Tender point count and total myalgic score in fibromyalgia: changes over a 28-day period. Rheumatol Int 2007; 27:1011–1018.
- Croft P, Schollum J, Silman A. Population study of tender point counts and pain as evidence of fibromyalgia. BMJ 1994; 309:696–699.
- Wolfe F. The relation between tender points and fibromyalgia symptom variables: evidence that fibromyalgia is not a discrete disorder in the clinic. Ann Rheum Dis 1997; 56:268–271.
- Mease PJ, Arnold LM, Crofford LJ, et al. Identifying the clinical domains of fibromyalgia: contributions from clinician and patient Delphi exercises. Arthritis Rheum 2008; 59:952–960.
- Harth M, Nielson WR. The fibromyalgia tender points: use them or lose them? A brief review of the controversy. J Rheumatol 2007; 34:914–922.
- Macfarlane GJ, Croft PR, Schollum J, Silman AJ. Widespread pain: is an improved classification possible? J Rheumatol 1996; 23:1628–1632.
- White KP, Harth M, Speechley M, Ostbye T. Testing an instrument to screen for fibromyalgia syndrome in general population studies: the London Fibromyalgia Epidemiology Study Screening Questionnaire. J Rheumatol 1999; 26:880–884.
- White KP, Speechly M, Harth M, Osbye T. The London Fibromyalgia Epidemiology Study: comparing the demographic and clinical characteristics in 100 random community cases of fibromyalgia versus controls. J Rheumatol 1999; 26:1577–1585.
- Wolfe F. Pain extent and diagnosis: development and validation of the regional pain scale in 12,799 patients with rheumatic disease. J Rheumatolol 2003; 30:369–378.
- Katz RS, Wolfe F, Michaud K. Fibromyalgia diagnosis. a comparison of clinical, survey, and American College of Rheumatology criteria. Arthritis Rheum 2006; 54:169–176.
- Wolfe F, Rasker JJ. The Symptom Intensity Scale, fibromyalgia, and the meaning of fibromyalgia-like symptoms. J Rheumatol 2006; 33:2291–2299.
- Sleath B, Chewning B, De Vellis BM, et al. Communication about depression during rheumatoid arthritis patient visits. Arthritis Rheum 2008; 59:186–191.
- Middleton GD, McFarlin JE, Lippski PE. The prevalence and clinical impact of fibromyalgia in systemic lupus erythematosus. Arthritis Rheum 1994; 37:1181–1188.
- Moussavi S, Chatterji S, Verdes E, Tandon A, Patel V, Ustun B. Depression, chronic diseases, and decrements in health: results from the World Health Surveys. Lancet 2007; 370:851–858.
- Ang DC, Choi H, Kroenke K, Wolfe F. Comorbid depression is an independent risk factor for mortality in patients with rheumatoid arthritis. J Rheumatol 2005; 32:1013–1019.
- Wolfe F, Michaud K. Severe rheumatoid arthritis (RA), worse outcomes, comorbid illness, and sociodemograghic disadvantage characterize RA patients with fibromyalgia. J Rheumatol 2004; 31:695–700.
- Urrows S, Affleck G, Tennen H, Higgins P. Unique clinical and psychological correlates of fibromyalgia tender points and joint tenderness in rheumatoid arthritis. Arthritis Rheum 1994; 37:1513–1520.
- Gladman DD, Urowitz MB, Gough J, MacKinnon A. Fibromyalgia is a major contributor to quality of life in lupus. J Rheumatol 1997; 24:2145–2148.
- Wolfe F, Petri M, Alarcon GS, et al. Fibromyalgia, systemic lupus erythematosus (SLE) and evaluation of SLE activity. J Rheumatol 2009; 36:.27–33.
KEY POINTS
- The Symptom Intensity Scale questionnaire consists of two parts: a list of 19 anatomic areas in which the patient is asked if he or she feels pain (the total number of yes answers being the Regional Pain Scale score), and a visual analogue scale for fatigue.
- According to the Survey Criteria, a diagnosis of fibromyalgia can be entertained if the Regional Pain Scale score is 8 points or higher and the fatigue visual analogue scale score is 6 cm or higher.
- The number of tender points, a surrogate for diffuse pain, does not fully capture the essence of fibromyalgia syndrome, in which accompanying fatigue is often severe and nearly always present.
- The Symptom Intensity Scale is an accurate surrogate measure for general health, depression, disability, and death. Fibromyalgia syndrome diagnosed with this instrument implies that this illness carries increased medical risk.