User login
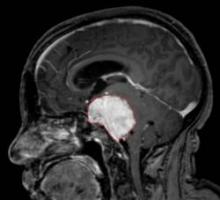
MONTREAL – The tyrosine kinase inhibitors vatalanib and sunitinib may offer alternative approaches to treating patients with recurrent, high-grade meningioma who have failed surgery and radiation therapy, according to two separate phase II studies.
Both drugs significantly improved survival compared with what is generally expected in this patient population, most of whom had grade II or III tumors. Progression-free survival at 6 months occurred in 57% of 25 patients with meningioma who received the investigational drug vatalanib in one study, and in 36% of 36 patients who received sunitinib in another study. Both drugs are inhibitors of receptors for vascular endothelial growth factor and platelet-derived growth factor.
Sunitinib is marketed by the brand name Sutent and has been approved by the Food and Drug Administration for the treatment of advanced renal cell carcinoma and gastrointestinal stromal tumor after disease progression on – or intolerance to – imatinib mesylate (Gleevec).
Progression-free survival (PFS) for patients with recurrent grade II and III tumors is usually considered to occur in fewer than 5%, said Dr. Jeffrey Raizer, who presented the vatalanib study at the annual meeting of the Society for Neuro-Oncology.
"For recurrence of meningioma, treatment options are limited once patients have failed surgery and radiation, and chemotherapies have been very limited to date," said Dr. Raizer, director of medical neuro-oncology at the cancer center of Northwestern University and Northwestern Memorial Hospital, both in Chicago.
Despite the encouraging findings of the two studies, a lack of information on the natural history of these tumors makes it difficult to interpret the studies, said Dr. Michael Vogelbaum, who chaired the session in which the studies were presented.
"While the studies suggest there may be some promise, we don’t really know what to expect from that mix of grade II and III tumors on their own," Dr. Vogelbaum said in an interview. He is chair of neuro-oncology and associate director of the brain tumor and neuro-oncology center at the Cleveland Clinic Neurological Institute.
Dr. Fred Barker of the department of neurosurgery at Massachusetts General Hospital, Boston, echoed Dr. Vogelbaum’s comments. "There’s been very little organized effort to do chemotherapy trials for meningiomas, so these studies are important because they are the first. The results are interesting, but we don’t know exactly what the natural history would be without treatment, so whether there’s a benefit to giving these drugs will require further study."
In Dr. Raizer’s Pfizer-sponsored study of vatalanib in meningioma patients, the median PFS was 5.9 months, and the median overall survival (OS) was 22.3 months. Of the 25 patients in the study, 22 had grade II and III tumors and 14 were men. The PFS at 6 months was comparable between patients with grade II (39%) or grade III disease only (43%).
Dosing of vatalanib began at 250 mg twice daily and increased by 250 mg/day every 7 days until the dosage reached 500 mg twice daily, he said.
For 21 patients with available imaging data, tumors partially responded to treatment in 1, remained stable in 15, and progressed in 5 patients.
The most common adverse events observed were fatigue, rash, and elevated transaminases.
The sunitinib study, sponsored by Novartis, included 36 patients (median age, 62 years) with recurrent grade II and III meningiomas who had exhausted all surgical and radiation options. The patients had a median of five recurrences.
The primary end point of PFS at 6 months was 36% and the median PFS was 5.1 months. The overall survival rate is not known, as only eight patients have died, reported Dr. Thomas Kaley, codirector of the neuro-oncology fellowship program at Memorial Sloan-Kettering Cancer Center in New York.
Imaging was available for 34 patients and showed stable disease in 26, a partial response in 1, and progressive disease in 7 patients, he said.
The dose of sunitinib was 50 mg orally per day on a 4-week-on, 2-week-off cycle.
"The toxicity was high with this drug," Dr. Kaley commented.
A total of 18 patients required dose reductions, and 6 patients withdrew from the study because of toxicity. There was one fatal grade 5 intratumoral hemorrhage and two nonfatal intratumoral hemorrhages (grades 3 and 4). The most common toxicities included myelosuppression, fatigue, headache, and GI side effects.
The investigators reported having no relevant conflicts of interest.*
* CORRECTION, 1/17/2011: An earlier version of this story included incorrect information about the investigators' disclosures. The error has been corrected.
MONTREAL – The tyrosine kinase inhibitors vatalanib and sunitinib may offer alternative approaches to treating patients with recurrent, high-grade meningioma who have failed surgery and radiation therapy, according to two separate phase II studies.
Both drugs significantly improved survival compared with what is generally expected in this patient population, most of whom had grade II or III tumors. Progression-free survival at 6 months occurred in 57% of 25 patients with meningioma who received the investigational drug vatalanib in one study, and in 36% of 36 patients who received sunitinib in another study. Both drugs are inhibitors of receptors for vascular endothelial growth factor and platelet-derived growth factor.
Sunitinib is marketed by the brand name Sutent and has been approved by the Food and Drug Administration for the treatment of advanced renal cell carcinoma and gastrointestinal stromal tumor after disease progression on – or intolerance to – imatinib mesylate (Gleevec).
Progression-free survival (PFS) for patients with recurrent grade II and III tumors is usually considered to occur in fewer than 5%, said Dr. Jeffrey Raizer, who presented the vatalanib study at the annual meeting of the Society for Neuro-Oncology.
"For recurrence of meningioma, treatment options are limited once patients have failed surgery and radiation, and chemotherapies have been very limited to date," said Dr. Raizer, director of medical neuro-oncology at the cancer center of Northwestern University and Northwestern Memorial Hospital, both in Chicago.
Despite the encouraging findings of the two studies, a lack of information on the natural history of these tumors makes it difficult to interpret the studies, said Dr. Michael Vogelbaum, who chaired the session in which the studies were presented.
"While the studies suggest there may be some promise, we don’t really know what to expect from that mix of grade II and III tumors on their own," Dr. Vogelbaum said in an interview. He is chair of neuro-oncology and associate director of the brain tumor and neuro-oncology center at the Cleveland Clinic Neurological Institute.
Dr. Fred Barker of the department of neurosurgery at Massachusetts General Hospital, Boston, echoed Dr. Vogelbaum’s comments. "There’s been very little organized effort to do chemotherapy trials for meningiomas, so these studies are important because they are the first. The results are interesting, but we don’t know exactly what the natural history would be without treatment, so whether there’s a benefit to giving these drugs will require further study."
In Dr. Raizer’s Pfizer-sponsored study of vatalanib in meningioma patients, the median PFS was 5.9 months, and the median overall survival (OS) was 22.3 months. Of the 25 patients in the study, 22 had grade II and III tumors and 14 were men. The PFS at 6 months was comparable between patients with grade II (39%) or grade III disease only (43%).
Dosing of vatalanib began at 250 mg twice daily and increased by 250 mg/day every 7 days until the dosage reached 500 mg twice daily, he said.
For 21 patients with available imaging data, tumors partially responded to treatment in 1, remained stable in 15, and progressed in 5 patients.
The most common adverse events observed were fatigue, rash, and elevated transaminases.
The sunitinib study, sponsored by Novartis, included 36 patients (median age, 62 years) with recurrent grade II and III meningiomas who had exhausted all surgical and radiation options. The patients had a median of five recurrences.
The primary end point of PFS at 6 months was 36% and the median PFS was 5.1 months. The overall survival rate is not known, as only eight patients have died, reported Dr. Thomas Kaley, codirector of the neuro-oncology fellowship program at Memorial Sloan-Kettering Cancer Center in New York.
Imaging was available for 34 patients and showed stable disease in 26, a partial response in 1, and progressive disease in 7 patients, he said.
The dose of sunitinib was 50 mg orally per day on a 4-week-on, 2-week-off cycle.
"The toxicity was high with this drug," Dr. Kaley commented.
A total of 18 patients required dose reductions, and 6 patients withdrew from the study because of toxicity. There was one fatal grade 5 intratumoral hemorrhage and two nonfatal intratumoral hemorrhages (grades 3 and 4). The most common toxicities included myelosuppression, fatigue, headache, and GI side effects.
The investigators reported having no relevant conflicts of interest.*
* CORRECTION, 1/17/2011: An earlier version of this story included incorrect information about the investigators' disclosures. The error has been corrected.
MONTREAL – The tyrosine kinase inhibitors vatalanib and sunitinib may offer alternative approaches to treating patients with recurrent, high-grade meningioma who have failed surgery and radiation therapy, according to two separate phase II studies.
Both drugs significantly improved survival compared with what is generally expected in this patient population, most of whom had grade II or III tumors. Progression-free survival at 6 months occurred in 57% of 25 patients with meningioma who received the investigational drug vatalanib in one study, and in 36% of 36 patients who received sunitinib in another study. Both drugs are inhibitors of receptors for vascular endothelial growth factor and platelet-derived growth factor.
Sunitinib is marketed by the brand name Sutent and has been approved by the Food and Drug Administration for the treatment of advanced renal cell carcinoma and gastrointestinal stromal tumor after disease progression on – or intolerance to – imatinib mesylate (Gleevec).
Progression-free survival (PFS) for patients with recurrent grade II and III tumors is usually considered to occur in fewer than 5%, said Dr. Jeffrey Raizer, who presented the vatalanib study at the annual meeting of the Society for Neuro-Oncology.
"For recurrence of meningioma, treatment options are limited once patients have failed surgery and radiation, and chemotherapies have been very limited to date," said Dr. Raizer, director of medical neuro-oncology at the cancer center of Northwestern University and Northwestern Memorial Hospital, both in Chicago.
Despite the encouraging findings of the two studies, a lack of information on the natural history of these tumors makes it difficult to interpret the studies, said Dr. Michael Vogelbaum, who chaired the session in which the studies were presented.
"While the studies suggest there may be some promise, we don’t really know what to expect from that mix of grade II and III tumors on their own," Dr. Vogelbaum said in an interview. He is chair of neuro-oncology and associate director of the brain tumor and neuro-oncology center at the Cleveland Clinic Neurological Institute.
Dr. Fred Barker of the department of neurosurgery at Massachusetts General Hospital, Boston, echoed Dr. Vogelbaum’s comments. "There’s been very little organized effort to do chemotherapy trials for meningiomas, so these studies are important because they are the first. The results are interesting, but we don’t know exactly what the natural history would be without treatment, so whether there’s a benefit to giving these drugs will require further study."
In Dr. Raizer’s Pfizer-sponsored study of vatalanib in meningioma patients, the median PFS was 5.9 months, and the median overall survival (OS) was 22.3 months. Of the 25 patients in the study, 22 had grade II and III tumors and 14 were men. The PFS at 6 months was comparable between patients with grade II (39%) or grade III disease only (43%).
Dosing of vatalanib began at 250 mg twice daily and increased by 250 mg/day every 7 days until the dosage reached 500 mg twice daily, he said.
For 21 patients with available imaging data, tumors partially responded to treatment in 1, remained stable in 15, and progressed in 5 patients.
The most common adverse events observed were fatigue, rash, and elevated transaminases.
The sunitinib study, sponsored by Novartis, included 36 patients (median age, 62 years) with recurrent grade II and III meningiomas who had exhausted all surgical and radiation options. The patients had a median of five recurrences.
The primary end point of PFS at 6 months was 36% and the median PFS was 5.1 months. The overall survival rate is not known, as only eight patients have died, reported Dr. Thomas Kaley, codirector of the neuro-oncology fellowship program at Memorial Sloan-Kettering Cancer Center in New York.
Imaging was available for 34 patients and showed stable disease in 26, a partial response in 1, and progressive disease in 7 patients, he said.
The dose of sunitinib was 50 mg orally per day on a 4-week-on, 2-week-off cycle.
"The toxicity was high with this drug," Dr. Kaley commented.
A total of 18 patients required dose reductions, and 6 patients withdrew from the study because of toxicity. There was one fatal grade 5 intratumoral hemorrhage and two nonfatal intratumoral hemorrhages (grades 3 and 4). The most common toxicities included myelosuppression, fatigue, headache, and GI side effects.
The investigators reported having no relevant conflicts of interest.*
* CORRECTION, 1/17/2011: An earlier version of this story included incorrect information about the investigators' disclosures. The error has been corrected.